Abstract
BACKGROUND
The optimal strategy for thromboprophylaxis in patients with a Fontan circulation is unknown.
OBJECTIVES
The aim of this study was to compare the efficacy and safety of aspirin, warfarin, and nonvitamin K oral anticoagulants (NOACs) in a network meta-analysis.
METHODS
Relevant studies published by February 2022 were included. The primary efficacy outcome was thromboembolic events; major bleeding was a secondary safety outcome. Frequentist network meta-analyses were conducted to estimate the incidence rate ratios (IRRs) of both outcomes. Ranking of treatments was performed based on probability (P) score.
RESULTS
A total of 21 studies were included (26,546 patient-years). When compared with no thromboprophylaxis, NOAC (IRR: 0.11; 95% CI: 0.03–0.40), warfarin (IRR: 0.23; 95% CI: 0.14–0.37), and aspirin (IRR: 0.24; 95% CI: 0.15–0.39) were all associated with significantly lower rates of thromboembolic events. However, the network meta-analysis revealed no significant differences in the rates of major bleeding (NOAC: IRR: 1.45 [95% CI: 0.28–7.43]; warfarin: IRR: 1.38 [95% CI: 0.41–4.69]; and aspirin: IRR: 0.72 [95% CI: 0.20–2.58]). Rankings, which simultaneously analyze competing interventions, suggested that NOACs have the highest P score to prevent thromboembolic events (P score 0.921), followed by warfarin (P score 0.582), aspirin (P score 0.498), and no thromboprophylaxis (P score 0.001). Aspirin tended to have the most favorable overall profile.
CONCLUSIONS
Aspirin, warfarin, and NOAC are associated with lower risk of thromboembolic events. Recognizing the limited number of patients and heterogeneity of studies using NOACs, the results support the safety and efficacy of NOACs in patients with a Fontan circulation.
Keywords: aspirin, nonvitamin K oral anticoagulants, single ventricle, warfarin
The Fontan palliation has transformed the survival perspectives of patients with a functionally univentricular heart, and the vast majority nowadays survive well into adulthood.1–3 Francis Fontan’s concept of rerouting the systemic venous blood directly to the pulmonary vascular bed by bypassing the heart and lacking a subpulmonic pump4 has challenged the physiological concept of a biventricular circulation and comes at the cost of variable short- and long-term complications secondary to chronic low cardiac output, high central venous pressure, and venous stasis.5,6
Thrombus formation and thromboembolic events are adverse complications of the Fontan circulation with important implications including Fontan circuit obstruction and pulmonic and paradoxical emboli. Low-velocity nonlaminar flow, prosthetic material, endothelial dysfunction, blind-ending pouches, atrial arrhythmias, as well as altered procoagulant and anticoagulant factors add to the increased thrombotic risk after the Fontan palliation.7 Although there is wide agreement on the need for any form of antithrombotic treatment in all patients with a Fontan circulation6,8,9 because of the high incidence of thromboembolic events,10 the optimal strategy is unknown. Previous studies and a previous meta-analysis established the benefit of thromboprophylaxis in Fontan.10 The net-benefit of aspirin vs warfarin vs nonvitamin K oral anticoagulants (NOACs), balancing antithrombotic efficacy and bleeding risk, is incompletely characterized. Thus, the aim of this network meta-analysis was to provide an updated analysis of the antithrombotic efficacy and bleeding risk using aspirin, warfarin, or NOAC based on published data.
METHODS
EVIDENCE ACQUISITION.
This systematic review was reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.11
DATA SOURCES AND SEARCH STRATEGY.
A comprehensive search of multiple databases from each database’s inception to February 14, 2022, was conducted. The databases included Medline Epub Ahead of Print, Medline In-Process and Other Non-Indexed Citations, Medline, EMBASE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and Scopus. Only results in English were considered for inclusion. The search strategy was designed and conducted by an experienced researcher (F.A.) trained in meta-analysis methodology with input from the study’s investigators. Controlled vocabulary supplemented with key words was used to search for Fontan anti-coagulation strategy. To identify additional data sources, reference lists from previously reviewed studies were searched. The full search strategy is shown in the Supplemental Appendix.
SELECTION OF STUDIES.
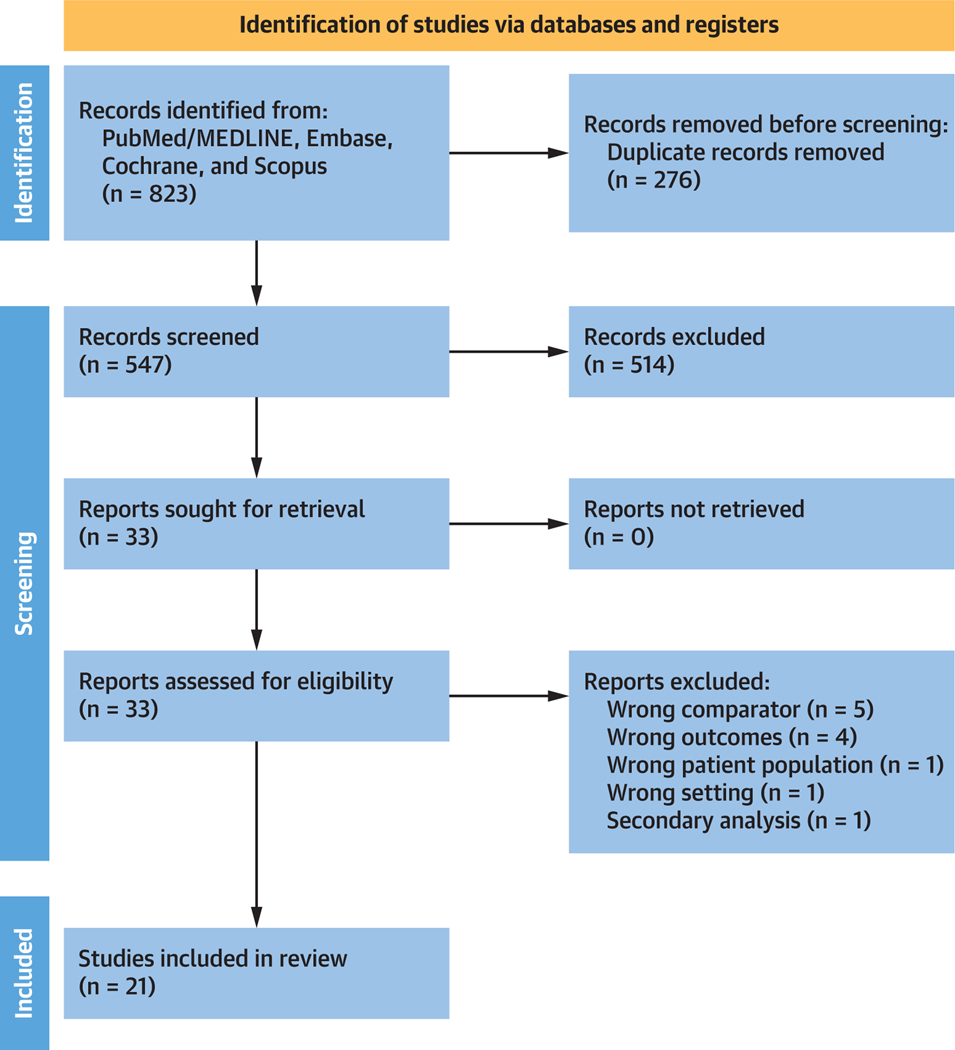
All identified studies were initially reviewed separately by 2 independent reviewers using study title and abstract (M.P. and T.A.). Inclusion criteria were as follows: 1) the study included patients with a Fontan circulation; 2) the study must have included at least 2 interventions of interest, namely aspirin, warfarin, NOAC or no prophylaxis; and 3) the study must have been a comparative randomized or observational study. Reviews, meta-analyses, book chapters, editorials, commentaries, letters, and case reports/series were excluded. Attention was paid to avoid inclusion of studies with patient overlap, unless different studies offered relevant information and supplementary data for the purpose of this meta-analysis. Figure 1 outlines the process of including studies in this meta-analysis.
FIGURE 1. PRISMA Flow Diagram of Studies Included in Data Search.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for the meta-analysis detailing identification of the included studies.
DATA EXTRACTION.
Data were independently extracted from the included studies by 2 separate authors (J.V.D.E. and M.P.). Data extraction was conducted using a standardized, piloted, internet-based form that was developed specifically for this current protocol.12 The data extracted included demographics of participants, study design, intervention details, and outcomes of interest. Data regarding binary variables were collected as absolute number and the total denominators, whereas continuous variables were collected either as mean ± SD or median (IQR), as reported in the original publication. Outcome data were extracted at the last follow-up reported by each study. All disagreements or differences in extracted data were resolved by consensus.
METHODOLOGIC QUALITY AND RISK OF BIAS ASSESSMENT.
Methodologic quality and risk of bias assessment is reported in the Supplemental Appendix.
OUTCOMES AND EFFECT SUMMARY.
The primary efficacy outcome was thromboembolic events at longest available follow-up. Major bleeding was a secondary safety outcome. Thromboembolic events and major bleeding were defined as reported in the individual studies. The incidence rates (IRs) were considered to account for potentially different follow-up durations between groups. IR was calculated from the following: 1) Kaplan-Meier curves using Web Plot Digitizer software version 4.513; or 2) the reported events and accumulated group-specific person-years of follow-up as previously described.14
STATISTICAL ANALYSIS.
In a first set of analyses, the IRs of thromboembolic events and major bleeding were pooled within each treatment group (“no thromboprophylaxis,” “aspirin,” warfarin,” and “NOAC”) using the “metarate” function in R statistical software. IR with 95% CI and P values were calculated for each study, and were then combined across the studies using a random-effects method (DerSimonian and Laird inverse variance).15 The choice for random-effects models was made based on the assumption that the effect sizes in the individual studies represented samples from a mixing distribution. In addition, the results were reanalyzed using fixed-effects models to explore whether this yielded differences regarding the summary inferences. I2, describing the percentage of total variation across studies that is caused by heterogeneity rather than chance, was calculated to assess the degree of statistical heterogeneity, and its accompanying P value was obtained using the chi-square test of the Cochran’s Q heterogeneity statistic.16 I2 can vary from 0% to 100%, with values >75% generally considered to reflect considerable statistical heterogeneity, meaning differences between studies are more likely caused by clinical factors (eg, differences in age or Fontan type) than merely caused by chance. Forest plots were used to visualize the individual study and summary effect estimates.
Subsequently, a frequentist network meta-analysis was performed using the generic inverse variance method with the “netmeta” package in R as described by Rücker et al.17 Random-effect meta-analysis of the incidence rate ratios (IRR) was reported because it resulted in the lowest inconsistency within the network as assessed based on Cochran’s Q (to evaluate heterogeneity),18 the net splitting method (to assess for statistically significant differences between direct and indirect estimates),19 and net heat maps (to highlight hot spots of inconsistency between specific direct evidence in the whole network).18 The global Cochran’s Q score was further decomposed into its 2 components: within-design heterogeneity (reflecting true effect size differences between studies which included exactly the same conditions), and between-design heterogeneity (suggesting inconsistency between designs). “No thromboprophylaxis” was considered the reference group. Statistical significance was considered when the CIs did not cross the line of neutral effect. Finally, treatments were ranked according to their probability (P) scores. The latter are based on the point estimates and SEs of the network estimates and measure the extent of certainty that a treatment is better than another one, averaged over all competing treatments. Ranks closest to 1 indicate the probability that the treatment group leads to the greatest reduction in the relevant adverse outcome. All analyses were completed with R Statistical Software (version 4.1.1, Foundation for Statistical Computing).
RESULTS
STUDY SELECTION AND CHARACTERISTICS.
A total of 823 citations were identified, of which 21 studies fulfilled our eligibility criteria and were included (Figure 1).20–40 Characteristics of each study and its participants are shown in Table 1. A total of 3,438 participants were included from studies published between 2000 and 2021, encompassing 26,546 patient-years (aspirin: 11,802; warfarin: 11,219; NOAC: 346; no thromboprophylaxis: 3,175). All studies were nonrandomized observational studies, except for 2 randomized controlled trials.34,35 The pooled mean age of participants was 5.5 years (n = 3,306, 20 studies) at the time of Fontan and 9.3 years (n = 3,306, 20 studies) at the time of inclusion. The pooled mean follow-up duration was 8.1 years (n = 3,438, 21 studies).
TABLE 1.
Summary of Study Characteristics
| First Author Year | Study Design | N | Study Period | Age at Fontan (y) | Age at Study Inclusion | Follow-Up (y) | Fontan Type | Study Drug |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Al-Jazairi et al20 2019 | Retrospective | 423 | 1985–2010 | 3.0 (IQR: 2.0) | Following Fontan | 13.6 (IQR: 8.7) | AP (n = 7) LT (n = 307) ECC (n = 107) Other (n = 2) |
Aspirin (n = 22) Warfarin (n = 385) None (n = 24) |
| Attard et al21 2021 | Retrospective | 121 | 1975–2020 | 4.8 (range 3.6–6.6) | 20.8 (range 15.5–28.1) (at least 5 y after Fontan procedure) | 16.2 (range 11.3–22.70) | AP (n = 12) LT (n = 28) ECC (n = 78) Unknown (n = 3) |
Aspirin (n = 54) Warfarin (n = 67) |
| Barker et al22 2005 | Retrospective | 402 | 1975–1998 | 2.2 | Following Fontan | 3.5 | LT (n = 306) ECC (n = 15) Other (n = 81) |
Aspirin (n = 277) Warfarin (n = 39) None (n = 36) Unknown (n = 50) |
| Cheung et al23 2005 | Retrospective | 85 | 1980–2002 | 6.2 ± 4.8 | Following Fontan | 6.6 ± 3.8 | AP (n = 51) LT (n = 19) ECC (n = 15) |
Aspirin (n = 8) Warfarin (n = 46) None (n = 31) |
| d’Udekem et al24 2007 | Retrospective | 215 | 1980–2000 | 4.4 (IQR: 3.0–7.0) | Following Fontan | 12 ± 6 | AP (n = 152) LT (n = 105) ECC (n = 48) (305 patients that underwent a Fontan procedure in the intervention period) |
Aspirin (n = 18) Warfarin (n = 176) None (n = 21) |
| Egbe et al25 2016 | Retrospective | 278 | 1994–2004 | 15 ± 6 | 31 ± 9 | 7.3 ± 1.2 | AP (n = 225) LT (n = 37) ECC (n = 16) |
Aspirin (N=181) Warfarin (n = 88) NOAC (n = 3) None (n = 6) |
| Faircloth et al26 2017 | Retrospective | 89 | 1997–2014 | 3 (IQR: 1.88–3.88) (event) 3.5 (IQR: 3–4.5) (no event) | Following Fontan | 8.3 (IQR: 6.8–11.4) | AP (n = 0) LT (n = 19) ECC (n = 70) |
Not specified |
| Haas et al27 2000 | Retrospective | 45 | 1990–1997 | 4 | Following Fontan | 5.3 | ECC (n = 45) | Aspirin (n = 38) Warfarin (n = 7) |
| Iyengar et al28 2016 | Retrospective | 475 | 1997–2010 | 4.4 (IQR: 3.5–5.6) (<1 y)/5.2 (IQR: 4.4–6.2) (>1 y) | Following Fontan | 7 (IQR: 4.7–9.7) | ECC (n = 475) | At <1 y Aspirin (n = 52) Warfarin (n = 410) None (n = 10) At >1 y Aspirin (n = 157) Warfarin (n = 301) None (n = 17) |
| Kaulitz et al29 2005 | Retrospective | 142 | 1988–2002 | 5.5 | Following Fontan | 7.6 | LT (n = 121) ECC (n = 21) |
Aspirin (n = 86) Warfarin (n = 11) None (n = 45) |
| Kawamatsu et al30 2021 | Retrospective | 139 | 2015–2018 | 13 ± 10 | 27 ± 7 | 7.9 ± 5.3 | AP (n = 9) LT (n = 48) ECC (n = 81) Unknown (n = 1) |
Antiplatelet (n = 43) Warfarin (n = 54) NOAC (n = 37) None (n = 5) |
| Lin et al31 2016 | Retrospective | 57 | 1992–2014 | 5.4 (IQR: 3.1–6.8) | 17.3 (IQR: 11.1–20.8) | 8.1 (IQR: 3.25–12.9) | AP (n = 35) LT (n = 17) ECC (n = 2) Other (n = 3) |
Not specified |
| Rationale for the Study Drug | Atrial Arrhythmiasa | Thromboembolic Event Rates (per 100 Patient-y) | Types of Thromboembolic Eventsb | Major Bleeding Event Rates (per 100 Patient-y) |
|---|---|---|---|---|
|
| ||||
| Not mentioned | 109/423 (26%) | Aspirin: 0.6, Warfarin: 0.8, NOAC: NA, None: NA | Not mentioned | Aspirin: 0.4, Warfarin: 0.3, NOAC: NA, None: NA |
| Not mentioned | Not mentioned | Aspirin: 0.5, Warfarin: 0.3, NOAC: NA, None: NA | 7 ischemic strokes, 2 PEs, 4 intracardiac thrombi | Aspirin: NA, Warfarin: NA, NOAC: NA, None: NA |
| Not mentioned | Not mentioned | Aspirin: 0.1, Warfarin: 0.0, NOAC: NA, None: 1.4 | Not mentioned | Aspirin: NA, Warfarin: NA, NOAC: NA, None: NA |
| Not mentioned | 8/77 (10%) with symptomatic cardiac arrhythmias | Aspirin: 0.0, Warfarin: 0.8, NOAC: NA, None: 0.8 | 2 strokes, 1 thrombus in blind ending PA stump, 1 unclear (2 were on warfarin, 1 had IART/PLE) | Aspirin: 0.0, Warfarin: 0.0, NOAC: NA, None: 0.0 |
| Not mentioned | 62/257 (24%) | Aspirin: 0.0, Warfarin: 0.7, NOAC: NA, None: 0.0 | 9 PEs, 1 stroke, 2 TIAs, 1 renal infarct (all were on warfarin at the time of the event) | Aspirin: NA, Warfarin: NA, NOAC: NA, None: NA |
| Not mentioned | 100% | Aspirin: 4.4, Warfarin: 2.2, NOAC: NA, None: 43.2 | 33 Fontan conduits/RA, 32 PEs, 14 intracardiac thrombi, 15 ischemic strokes, 2 renal infarctions, 1 splenic infarction | Aspirin: NA, Warfarin: NA, NOAC: NA, None: NA |
| Not mentioned | 30/89 (34%) | Aspirin: 1.9, Warfarin: 2.0, NOAC: NA, None: NA | 2 deep vein thromboses, 1 innominate vein/SVC, 1 PE, 3 Fontan conduits/RA, 1 single ventricle (2 were on warfarin) | Aspirin: NA, Warfarin: NA, NOAC: NA, None: NA |
| Absence of sinus rhythm or suboptimal hemodynamics (not further specified) | 6/45 (13%) had transient supraventricular tachycardia in the early postoperative phase | Aspirin: 0.0, Warfarin: 0.0, NOAC: NA, None: NA | - | Aspirin:, Warfarin:, NOAC:, None: |
| Center preference | Not mentioned | Aspirin: 0.4, Warfarin: 1.2, NOAC: NA, None: 2.4 | 6 strokes, 2 TIAs, 3 watershed infarcts, 17 Fontan conduit thromboses, 5 PEs, 1 renal embolism | Aspirin: 0.1, Warfarin: 0.5, NOAC: NA, None: 0.0 |
| Based on surgical method, preoperative parameters and early post-operative functional result (not further specified) |
18/142 (13%) | Aspirin: 0.2, Warfarin: 0.0, NOAC: NA, None: 2.4 | 2 strokes, 8 systemic venous thrombi (8 were on heparin, 1 on warfarin, 1 had no TE prophylaxis) | Aspirin: 0.0, Warfarin: 0.0, NOAC: NA, None: 0.0 |
| Not specified | 29/139 (21%) | Aspirin: 0.7, Warfarin: 1.7, NOAC: 0.0, None: 4.3 | Not specified | Aspirin: 0.7, Warfarin: 2.0, NOAC: 0.6, None: 0.0 |
| Aspirin (n = 13) Warfarin (n = 36) None (n = 8) | 100% | Aspirin: 3.8, Warfarin: 1.0, NOAC: NA, None: 7.7 | 12 strokes | Aspirin: NA, Warfarin: NA, NOAC: NA, None: NA |
| First Author Year | Study Design | N | Study Period | Age at Fontan (y) | Age at Study Inclusion | Follow-Up (y) | Fontan Type | Study Drug |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mahnke et al32 2005 | Retrospective | 132 | 1976–2001 | Not mentioned | Following Fontan | 7.6 | AP (n = 40) LT (n = 74) ECC (n = 18) |
Aspirin (n = 87) Warfarin (n = 28) None (n = 17) |
| Manlhiot et al33 2012 | Retrospective | 162 | 2000–2009 | 3.4 | Following Fontan | 2.3 | LT (n = 35) ECC (n = 124) Other (n = 3) |
Aspirin (n = 26) Warfarin (n = 126) None (n = 10) |
| McCrindle et al34 2021 | RCT | 112 | 2016–2020 | 4.0 ± 1.6 | Following Fontan | 1 | Not reported | Aspirin (n = 34) NOAC (n = 78) NOAC were used in prophylactic doses |
| Monagle et al35 2011 | RCT | 111 | 1998–2003 | 4.8 | Following Fontan | 2 | ECC (n = 95) LT (n = 16) |
Aspirin (n = 57) Warfarin (n = 54) |
| Potter et al36 2013 | Retrospective | 210 | 1973–1991 | 8.5 | Following Fontan | 14.5 | AP (n = 102) LT (n = 81) ECC (n = 4) Other (n = 23) |
Aspirin (n = 51) Warfarin (n = 54) None (n = 105) |
| Seipelt et al37 2002 | Retrospective | 101 | 1986–1998 | 7.3 | Following Fontan | 5.7 | AP (n = 40) LT (n = 61) |
Aspirin (n = 14) Warfarin (n = 26) None (n = 45) |
| Small et al38 2018 | Retrospective | 52 | 2004–2017 | 8.2 (median) | 25.9 (range 16.2–39.4) | 2.8 | AP (n = 52) | Aspirin (n = 13) Warfarin (n = 13) None (n = 2) Other (n = 5) |
| Takawira et al39 2008 | Retrospective | 13 | 1997–2002 | 4.9 | Following Fontan | 5.2 | ECC | Aspirin (n = 10) Warfarin (n = 2) None (n = 1) |
| Yang et al40 2019 | Retrospective | 74 | 2014–2019 | 1.4±0.9 y | 32 ± 10 | 1.2 (median) | AP (n = 26) TCPC (n = 48) |
NOAC (n = 74) |
| Rationale for the Study Drug | Atrial Arrhythmiasa | Thromboembolic Event Rates (per 100 Patient-y) | Types of Thromboembolic Eventsb | Major Bleeding Event Rates (per 100 Patient-y) |
|---|---|---|---|---|
|
| ||||
| Prior PE, prosthetic valve or physician preference caused by ECC | 17/132 (13%) | Aspirin: 0.3, Warfarin: 0.0, NOAC: NA, None: 0.6 | 3 strokes (none had atrial arrhythmias) | Aspirin: 0.0, Warfarin: 0.0, NOAC: NA, None: 0.0 |
| Not mentioned | Not mentioned | Aspirin: 12.9, Warfarin: 4.5, NOAC: NA, None: 21.7 | Not mentioned | Aspirin: 0.0, Warfarin: 0.3, NOAC: NA, None: 0.0 |
| Randomization to Aspirin vs Rivaroxaban | Not mentioned | Aspirin: 8.8, Warfarin: NA, NOAC: 2.6, None: NA | 1 PE, 1 stroke, 2 venous thromboses | Aspirin: 0.0, Warfarin: NA, NOAC: 1.3, None: NA |
| Randomization to Aspirin vs Warfarin | Not mentioned (patients with a recognized indication for anticoagulation were a priori excluded) | Aspirin: 10.5, Warfarin: 12.0, NOAC: NA, None: NA | 20 within the Fontan connection, 4 PEs, 7 other venous sites (7 patients had thromboses identified in multiple locations) | Aspirin: 0.9, Warfarin: 0.9, NOAC: NA, None: NA |
| Not mentioned | Not mentioned | Aspirin: 0.6, Warfarin: 0.8, NOAC: NA, None: 2.2 | 25 RA, 3 PA, 2 SVC, 3 pulmonary venous atrium, 7 with missing information | Aspirin: NA, Warfarin: NA, NOAC: NA, None: NA |
| Before 1995 all patients received no treatment or Aspirin, after 1995 all received Warfarin | 18/85 (21%) | Aspirin: 1.6, Warfarin: 1.1, NOAC: NA, None: 4.2 | 2 Strokes, 2 SVC/PA thrombus (1 of which was followed by fatal PE), 1 PE, 1 innominate vein thrombus (with chylothorax), 4 RA thrombus, 3 residual PA trunk | Aspirin: 0.0, Warfarin: 0.0, NOAC: NA, None: 0.0 |
| Inconsistent (atrial arrhythmia was the cause to choose warfarin or aspirin) | 100% (none had a history of thromboembolic events at the beginning of the study period) | Aspirin: 16.5, Warfarin: 2.8, NOAC: NA, None: NA | Not mentioned | Aspirin: 2.7, Warfarin: 8.5, NOAC: NA, None: NA |
| Not mentioned | 0/13 (0%) | Aspirin: 0.0, Warfarin: 0.0, NOAC: NA, None: 0.0 | - | Aspirin: NA, Warfarin: NA, NOAC: NA, None: NA |
| Not mentioned | 52/74 (70%) | Aspirin: 6.0, Warfarin: 1.5, NOAC: 2.9, None: NA | 2 PEs, 1 stroke | Aspirin: 0.0, Warfarin: 0.8, NOAC: 2.9, None: NA |
The denominator might be different from the number of subjects included in the meta-analysis depending on the way the data is reported in the individual studies.
Acute treatment strategies for thromboembolic events have not been reported universally. Heparin has been used in several reported cases for the acute management of thromboembolic events.
AP = atriopulmonary; ECC = extracardiac conduit; LT = lateral tunnel; NA = not applicable/assessed; NOAC = nonvitamin K oral anticoagulant; PA = pulmonary artery; PE = pulmonary embolism; RA = right atrium; TCPC = total cavopulmonary connection; TIA = transient ischemic attack.
INCIDENCE RATES OF THROMBOEMBOLIC EVENTS AND MAJOR BLEEDING FOR EACH TREATMENT GROUP.
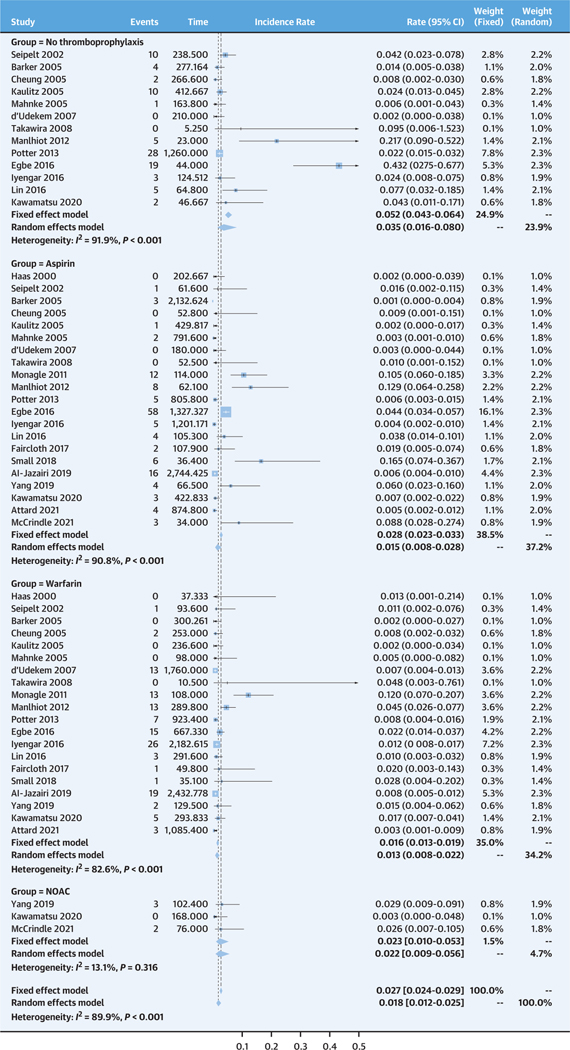
According to the random-effects models, the pooled incidence rate of thromboembolic events for the no thromboprophylaxis group was 3.5 per 100 patient-years (95% CI: 1.6–8.0 per 100 patient-years), and the aspirin, warfarin, and NOAC groups had incidence rates of 1.5 per 100 patient-years (95% CI: 0.8–2.8 per 100 patient-years), 1.3 per 100 patient-years (95% CI: 0.8–2.2 per 100 patient-years), and 2.2 per 100 patient-years (95% CI: 0.9–5.6 per 100 patient-years), respectively (Figure 2). The fixed-effects model revealed overall comparable estimates, although it estimated slightly higher incidence rates compared with the random-effects models for the no thromboprophylaxis and aspirin group (5.2 per 100 patient-years [95% CI: 4.3–6.4 per 100 patient-years] and 2.8 per 100 patient-years [95% CI: 2.3–3.3 per 100 patient-years], respectively).
FIGURE 2. Pooled Incidence Rates of Thromboembolic Events for Each Treatment Group.
The top represents no treatment, followed by aspirin, warfarin, and nonvitamin K oral anticoagulant (NOAC).
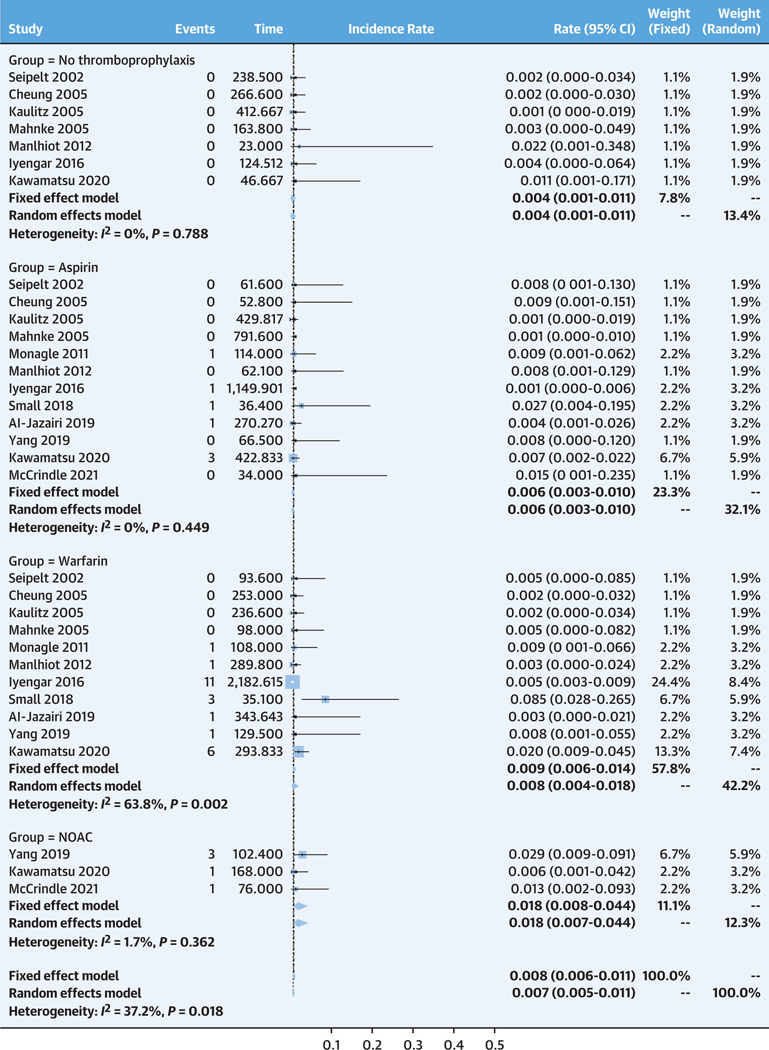
The incidence rates of major bleeding were very low for the no thromboprophylaxis, aspirin, and warfarin groups at 0.4 per 100 patient-years (95% CI: 0.1–1.1 per 100 patient-years), 0.6 per 100 patient-years (95% CI: 0.3–1.0 per 100 patient-years), and 0.8 per 100 patient-years (95% CI: 0.4–1.8 per 100 patient-years), respectively (Figure 3). The incidence rate of major bleeding tended to be higher in the NOAC group at 1.8 per 100 patient-years (95% CI: 0.7–4.4 per 100 patient-years). The fixed-effects model revealed similar estimates compared with those from the random-effects model.
FIGURE 3. Pooled Incidence Rates of Major Bleeding for Each Treatment Group.
The top represents no treatment, followed by aspirin, warfarin and nonvitamin K oral anticoagulant (NOAC).
NETWORK META-ANALYSIS.
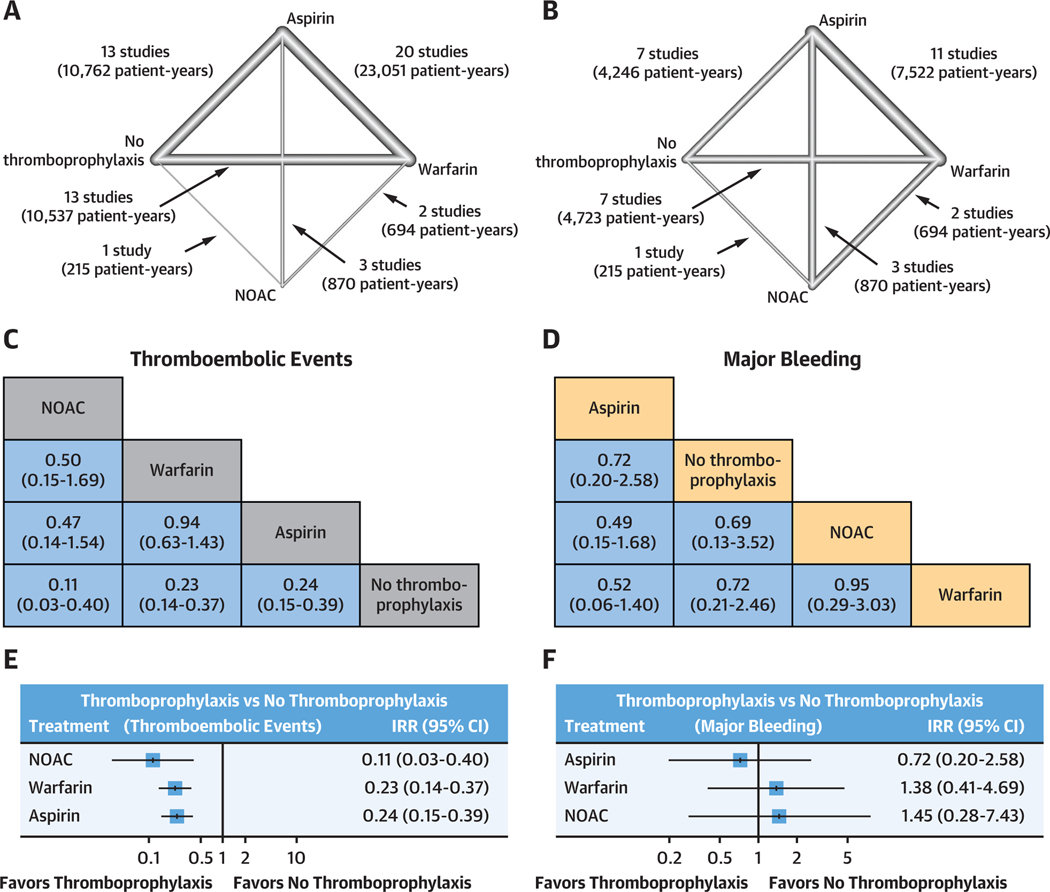
The evidence networks for thromboembolic events and major bleeding are shown in Figures 4A and 4B, respectively. League plots summarizing the results of the network meta-analyses for both outcomes are given in Figures 4C and 4D, respectively, and corresponding forest plots and funnel plots are given in Figures 4E to 4H. As shown in Supplemental Figures 1 and 2, both networks were based mostly on direct evidence (comparisons of treatments arms within the same studies, rather than of treatment arms in different studies), and none of the comparisons had a mean path length >2 (number of steps along with the shortest paths for all possible pairs of nodes in the network), supporting robustness of the networks.
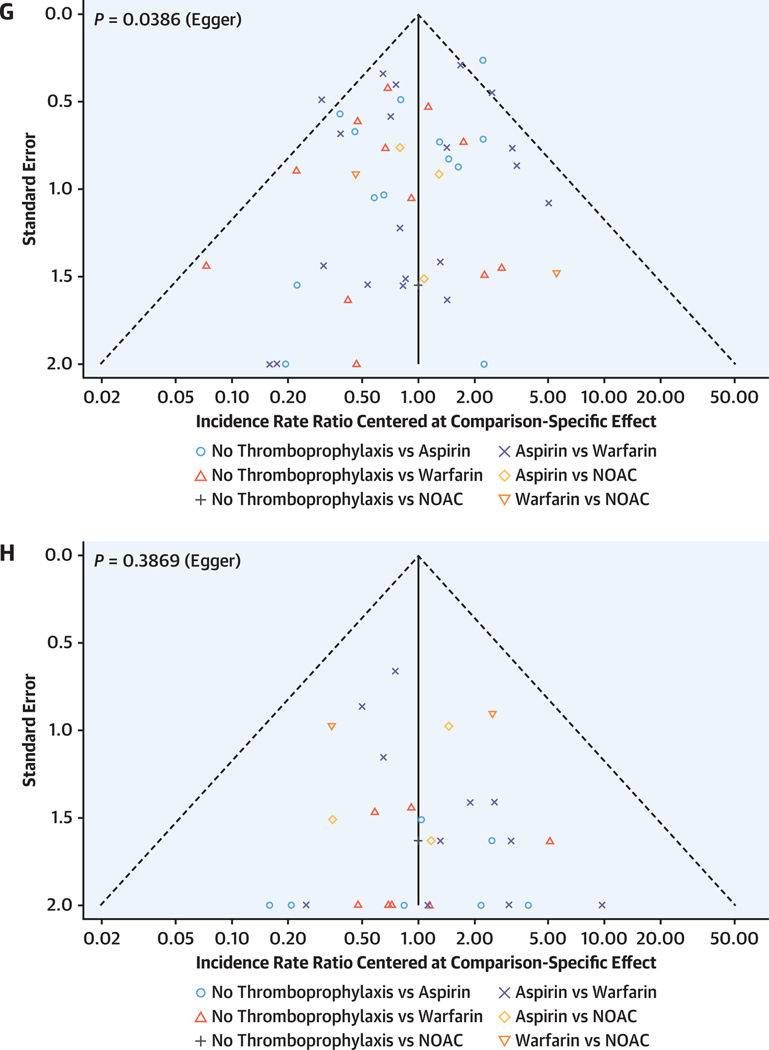
FIGURE 4. Results of the Random-Effects Network Meta-Analysis.
(A and B) Network plots showing the strategies that have been tested for their efficacy in preventing thromboembolic events (A) and safety in terms of major bleeding (B). Lines are weighted according to the number of studies comparing the 2 connected strategies. (C and D) League plots representing the results of the network meta-analyses comparing the effect of all strategies. The estimates (incidence rate ratio [IRR], 95% CI) are presented for each comparison; IRR >1 favors the row-defining treatment, and IRR <1 favors the column-defining treatment. (E and F) Forest plots summarizing the network estimates for each thromboprophylaxis strategy compared with no thromboprophylaxis. (G and H). All strategies have a lower risk for thrombosis compared with no treatment and no increased risk of bleeding. Funnel plots for thromboembolic events (G) and major bleeding (H). The results resemble a symmetrical inverted funnel suggesting low between study heterogeneity. NOAC = nonvitamin K oral anticoagulant.
Rankings that simultaneously analyze competing interventions were used. Ranks closest to 1 indicate the probability that the treatment group leads to greatest reduction in the relevant adverse outcome. The rankings suggested that NOAC has the highest P score to prevent thromboembolic events (P score 0.921), followed by warfarin (P score 0.582), aspirin (P score 0.498), and no thromboprophylaxis (P score 0.001). When compared with no thromboprophylaxis, NOAC (IRR: 0.11; 95% CI: 0.03–0.40), warfarin (IRR: 0.23; 95% CI: 0.14–0.37), and aspirin (IRR: 0.24; 95% CI: 0.15–0.39) were all associated with significantly lower rates of thromboembolic events (Figures 4C and 4E). The funnel plot revealed mild asymmetry, and Egger’s test was significant (P = 0.039), meaning that small-study effects (publication bias) cannot be entirely excluded (Figure 4G). Net splitting analysis (Supplemental Table 1) revealed that estimates from direct and indirect evidence were comparable for all comparisons except for aspirin vs warfarin (IRRs: 1.15 and 0.07, respectively; P = 0.027); however, indirect evidence contributed only 3% to the latter comparison, such that its overall estimate (IRR: 1.06) followed the direction of the direct evidence.
In terms of major bleeding, aspirin was ranked as the best strategy (P score 0.842), followed by no thromboprophylaxis (P score 0.559), NOAC (P score 0.307), and warfarin (P score 0.292). However, the network meta-analysis revealed no significant differences in the rates of major bleeding between any of the strategies (Figures 4D and 4F). The funnel plot revealed no asymmetry, and Egger’s test was not significant (P = 0.387), meaning that small-study effects (publication bias) were not likely to be present in this analysis (Figure 4H). Net splitting analysis (Supplemental Table 2) revealed that estimates from direct and indirect evidence were comparable for all comparisons.
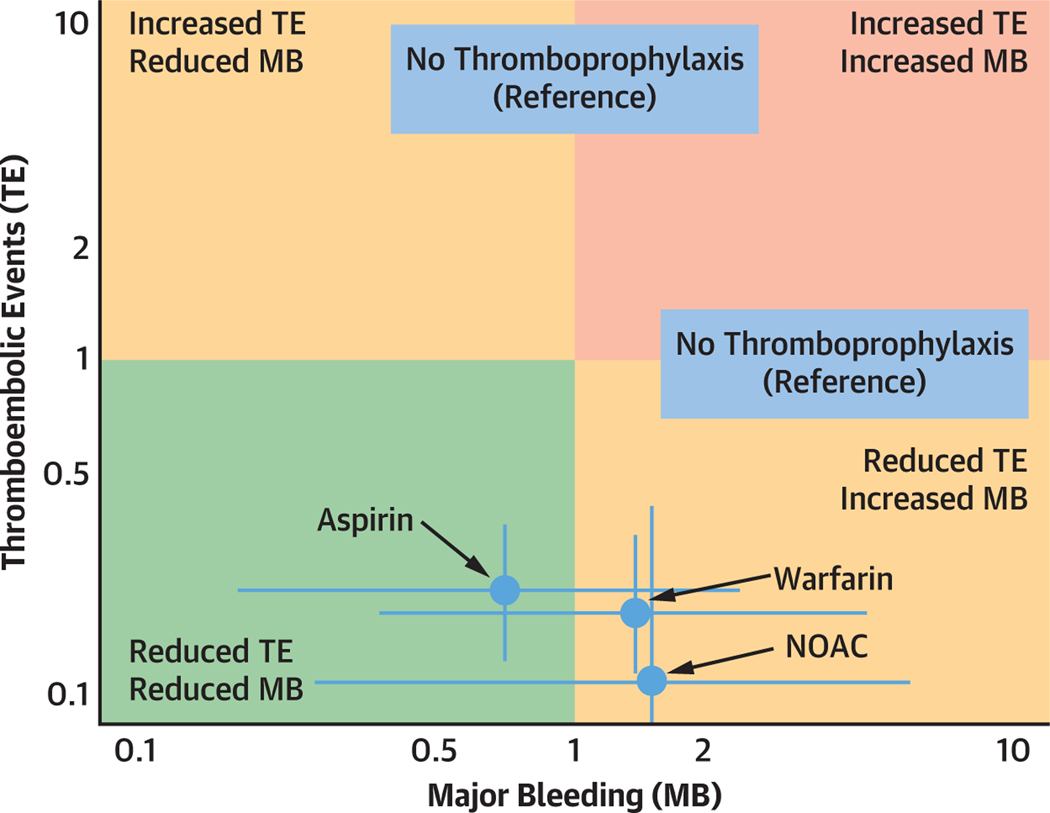
A bivariate analysis of thromboembolic events and major bleeding for aspirin, warfarin, and NOAC, using no thromboprophylaxis as a reference, is shown in the Central Illustration. NOACs were associated with the greatest reduction in thromboembolic events, yet also had the greatest risk of major bleeding. On the other hand, aspirin tended to have the most favorable overall profile, with both a significantly reduced risk of thromboembolic events and a tendency toward lower risk of major bleeding.
CENTRAL ILLUSTRATION. Treatment Effects of Different Antithrombotic Strategies in the Fontan Circulation.
Treatment effects of aspirin, warfarin, and nonvitamin K oral anticoagulants (NOACs) were compared using no thromboprophylaxis as a reference in a bivariate analysis. Incidence rate ratios (IRRs) of thromboembolic events (TEs) are reported on the y-axis and IRRs of major bleeding (MB) on x-axis. No thromboprophylaxis as a reference has a neutral effect on TE and MB, which equals 1 on the x- and y-axes. NOAC were associated with the greatest reduction in thromboembolic events, yet the greatest risk of major bleeding. Aspirin tended to have the most favorable overall profile, with both a significantly reduced risk of thromboembolic events and a tendency toward lower risk of MB.
SENSITIVITY ANALYSIS.
The sensitivity analysis based on fixed-effects models revealed results that were consistent with those of the random-effects models (Supplemental Tables 1 and 2, Supplemental Figure 3).
RISK OF BIAS ASSESSMENT.
The risk of bias in the included studies is summarized in Supplemental Figures 4 and 5. The risk of bias was assessed as low to moderate in most of the studies.
DISCUSSION
In this network meta-analysis, we analyzed the antithrombotic efficacy and bleeding risk using aspirin, warfarin, or NOAC in patients with a Fontan circulation. The main findings are the following (Central Illustration): 1) aspirin, warfarin, and NOAC all lower the risk of thromboembolic events compared with no antithrombotic therapy; 2) NOAC seems to be most effective in the prevention of thromboembolic events compared with aspirin and warfarin (highest P score); and 3) aspirin tended to have the most favorable overall profile, with both a significantly reduced risk of thromboembolic events and a tendency toward lower risk of major bleeding.
Given the high incidence of thromboembolic complications,10 there is a general consensus on the use of any form of antithrombotic therapy for primary prevention in patients with a Fontan circulation. However, the optimal antithrombotic strategy remains unclear. Several retrospective studies assessing the antithrombotic efficacy of aspirin vs warfarin demonstrated similar antithrombotic potential.7,36 Further evidence comes from a randomized trial that demonstrated equal antithrombotic effects of aspirin and warfarin in the primary prevention of thromboembolic events.35 Those results are supported by a previous10 and the current meta-analysis, where we were able to demonstrate similar antithrombotic efficacy of both aspirin and warfarin.
A secondary analysis of the above-mentioned randomized trial35 demonstrated that suboptimal international normalized ratio (INR) levels with a time in therapeutic range of <30% increased the thromboembolic risk significantly in patients with a Fontan circulation.41 In line with these results, Faircloth et al42 were able to demonstrate that greater time spent in the therapeutic range reduced both thromboembolic and bleeding events. Overcoming the issues of suboptimal INR levels and the need for frequent monitoring, NOACs represent an attractive alternative to warfarin. Although there is striking evidence of the superiority of NOACs over warfarin in patients with atrial fibrillation from many clinical trials, patients with congenital heart disease were excluded a priori from those trials.43 Because there is still lack of evidence from randomized controlled trials on the use of NOACs in patients with a Fontan circulation, the current American44 and European9 guidelines on adult congenital heart disease do not recommend NOAC as the first line treatment in patients with a Fontan circulation who have an indication for anticoagulation. Nevertheless, the many potential advantages of NOACs over vitamin K antagonists, such as the greater ease of use because of the lack of need of frequent INR monitoring, more predictable pharmacokinetics, fewer drug–drug and food–drug interactions, and an attractive risk-to-benefit profile, render them an attractive alternative in patients with congenital heart disease. This is also reflected by the increased use of NOACs in real-world data over the last few years in the treatment of patients with congenital heart disease.45 However, extrapolating data from noncongenital to congenital heart disease patients—and in particular to patients with complex lesions such as univentricular hearts with a Fontan palliation—does not substitute clinical research on the use of NOAC in patients with congenital heart disease and a Fontan circulation. Although randomization of patients with a Fontan circulation in a double-blinded fashion to NOAC or aspirin would offer the most robust data, such trials are challenging given the low number of patients, long expected follow-up period, and high costs. To improve the current understanding on the efficacy and safety of NOACs in patients with a Fontan circulation, we thus performed this meta-analysis to help guide clinicians in their choice on the type of antithrombotic therapy. Despite the low number of patients (1.2% of the total patient-years) on NOAC and the heterogeneity of the studies, this meta-analysis supports the use of NOAC in patients with a Fontan circulation with no added risk of major bleeding. NOAC even seem to have superior antithrombotic efficacy compared with aspirin and vitamin K antagonists.
Although these results are encouraging for the use of NOACs in the setting of a Fontan circulation, cautious interpretation of the results is necessary. The number of patients on NOACs included in this meta-analysis is low (1.2% of the total patient-years) with heterogenous application in the different studies. Only 3 studies included patients on NOACs. Although 2 of those studies investigated adult patients on standard doses of NOAC,30,40 the UNIVERSE (Rivaroxaban, a direct Factor Xa inhibitor, versus acetylsalicylic acid as thromboprophylaxis in children post–Fontan procedure) study by McCrindle et al34 evaluated the efficacy of low-dose rivaroxaban in children that underwent the Fontan procedure within the last 4 months. In the UNIVERSE study, patients were randomized in an open-label fashion to either rivaroxaban or aspirin for a study period of 12 months. The results demonstrated similar safety profiles and fewer, albeit not statistically significant, thromboembolic events in the NOAC arm. In the international multicenter NOTE (non-vitamin K antagonist oral anticoagulants for thromboembolic prevention in patients with congenital heart disease) registry published by Yang et al,40 74 adult patients on NOACs for primary or secondary indication were prospectively followed for a median of 1.2 years. The authors reported a low incidence of thromboembolic and major bleeding events (2.9 events for each outcome per 100 patient-years). When compared with the incidence of historical thromboembolic events of a subgroup of patients who were on vitamin K antagonists before initiation of NOAC, similar rates of thromboembolic and major bleeding events were reported. In the retrospective cohort study by Kawamatsu et al,30 efficacy of different antithrombotic regimens was assessed in Fontan patients over the age of 15 years. The study demonstrated that patients taking NOAC had a lower rate of thromboembolic and major bleeding events. Although the study by Kawamatsu et al30 reports a relatively high incidence of clinically relevant abnormal uterine bleeds (11 of a total of 18 bleeding events), the current data do not allow any gender-specific differentiation in the choice of the optimal antithrombotic regimen. Nevertheless, the side effect of uterine bleeding should be taken into consideration when using NOAC.
Optimal dosing of NOACs in patients with a Fontan circulation for prevention of thromboembolic events is currently unknown. Although standard full doses of NOAC were used in the 2 NOAC studies conducted in adults,30,40 the pediatric dose of rivaroxaban chosen in the UNIVERSE study34 was equivalent to 10 mg once daily in adults, which is an effective dose for thromboembolism prophylaxis in adults. Further studies defining the optimal dose of NOAC with favorable efficacy and safety in patients with a Fontan circulation—particularly in the setting of primary prevention—are needed.
In summary, respecting its limitations, this meta-analysis supports the safety and efficacy of NOAC in the treatment of patients with a Fontan circulation. However, the choice of the antithrombotic regimen in patients with a Fontan circulation needs to be individualized. Continued data collection of patients using different treatment strategies is important to gain further evidence in the management of this complex patient population.
STUDY LIMITATIONS.
Several important limitations of this meta-analysis must be pointed out and need to be considered in the interpretation of the presented results. First, the follow-up periods vary markedly between studies. Second, the number of patients on NOACs is markedly lower compared with the numbers of patients on aspirin or vitamin K antagonists. Furthermore, given the low number of patients on NOACs, the absolute numbers of thromboembolic and bleeding events were markedly lower in the NOAC group. Moreover, 2 of the 3 studies that included an NOAC arm were conducted in adults. Considering that adults might have an overall higher risk of thromboembolic events, this might have affected our estimates on the relative efficacy of NOAC compared with other strategies (ie, no thromboprophylaxis, aspirin, warfarin). Indeed, our meta-analysis might still have underestimated the efficacy of NOAC. Third, different types of NOAC might have different profiles in terms of antithrombotic efficacy and bleeding risk. The evaluation of different NOAC agents was not possible given the lack of specific information and limited number of events. Fourth, several clinical factors, such as age, Fontan type, underlying anatomy, and comorbidities, might modulate the baseline risk of thromboembolic events in complex ways, and thus, the strategy of thromboprophylaxis might have changed in different age groups in the included studies; this was also suggested by the high statistical heterogeneity (I2 = 90%) in our meta-analysis of thromboembolic events, meaning that differences between studies are more likely caused by clinical factors (eg, differences in age or Fontan type) than merely because of chance. Finally, a notoriously difficult issue in studies evaluating thromboembolic events in patients with a Fontan circulation remains frequency and variety of tests that are used for screening of thromboembolic events. Although some studies only include clinically manifest thromboembolic events, others report the incidence of both symptomatic and clinically asymptomatic thromboembolic events as assessed by different imaging modalities, which results in markedly different rates of thromboembolic events in different studies.
CONCLUSIONS
In this network meta-analysis, aspirin, warfarin, and NOAC were all effective antithrombotic regimens in patients with a Fontan circulation, with NOAC showing the strongest antithrombotic effect. Major bleeding events were comparable in the different treatment regimens. Recognizing the limited number of patients and heterogeneity of studies using NOAC, the results support the safety and efficacy of NOAC in the treatment of patients with a Fontan circulation.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Aspirin, warfarin, and target-specific oral anticoagulants reduce the risk of thromboembolic events in patients with Fontan circulation. Network meta-analysis identified no significant differences between these strategies in rates of major bleeding.
TRANSLATIONAL OUTLOOK:
Randomized trials are needed to confirm the optimum antithrombotic management of patients with Fontan circulation.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- IR
incidence rate
- IRR
incidence rate ratio
- NOAC
nonvitamin K oral anticoagulant
- P
probability
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Pundi KN, Johnson JN, Dearani JA, et al. 40-year follow-up after the Fontan operation: long-term outcomes of 1,052 patients. J Am Coll Cardiol. 2015;66:1700–1710. [DOI] [PubMed] [Google Scholar]
- 2.Atz AM, Zak V, Mahony L, Uzark K, et al. Longitudinal outcomes of patients with single ventricle after the Fontan procedure. J Am Coll Cardiol. 2017;69:2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis M, Zannino D, du Plessis K, et al. Clinical outcomes in adolescents and adults after the Fontan procedure. J Am Coll Cardiol. 2018;71: 1009–1017. [DOI] [PubMed] [Google Scholar]
- 4.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gewillig M, Brown SC, van de Bruaene A, Rychik J. Providing a framework of principles for conceptualising the Fontan circulation. Acta Paediatr. 2020;109:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rychik J, Atz AM, Celermajer DS, et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation. 2019;140: e234–e284. 10.1161/CIR.0000000000000696 [DOI] [PubMed] [Google Scholar]
- 7.Attard C, Huang J, Monagle P, Ignjatovic V. Pathophysiology of thrombosis and anti-coagulation post Fontan surgery. Thromb Res. 2018;172:204–213. 10.1016/j.thromres.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 8.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12): 1494–1563. 10.1016/j.jacc.2018.08.1028 [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. [DOI] [PubMed] [Google Scholar]
- 10.Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR. Strategies for thromboprophylaxis in Fontan circulation: a meta-analysis. Heart. 2015;101(21):1731–1737. [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covidence. Accessed November 29, 2022. https://www.covidence.org/ [Google Scholar]
- 13.Web Plot Digitizer. Accessed November 29, 2022. https://automeris.io/WebPlotDigitizer/ [Google Scholar]
- 14.Yanagawa B, Verma S, Jüni P, et al. A systematic review and meta-analysis of in situ versus composite bilateral internal thoracic artery grafting. J Thorac Cardiovasc Surg. 2017;153:1108–1116.e16. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rücker G, Schwarzer G, Krahn U, König J. netmeta: network meta-analysis using frequentist methods. R Package Version 08–0; 2015. [Google Scholar]
- 18.Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol. 2013;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. [DOI] [PubMed] [Google Scholar]
- 20.Al-Jazairi AS, Al Alshaykh HA, Di Salvo G, De Vol EB, Alhalees ZY. Assessment of late thromboembolic complications post-Fontan procedure in relation to different antithrombotic regimens: 30-years’ follow-up experience. Ann Pharmacother. 2019;53:786–793. [DOI] [PubMed] [Google Scholar]
- 21.Attard C, Monagle PT, d’Udekem Y, et al. Long-term outcomes of warfarin versus aspirin after Fontan surgery. J Thorac Cardiovasc Surg. 2021;162:1218–1228.e3. [DOI] [PubMed] [Google Scholar]
- 22.Barker PC, Nowak C, King K, Mosca RS, Bove EL, Goldberg CS. Risk factors for cerebrovascular events following Fontan palliation in patients with a functional single ventricle. Am J Cardiol. 2005;96:587–591. [DOI] [PubMed] [Google Scholar]
- 23.Cheung YF, Chay GW, Chiu CS, Cheng LC. Long-term anticoagulation therapy and thromboembolic complications after the Fontan procedure. Int J Cardiol. 2005;102:509–513. [DOI] [PubMed] [Google Scholar]
- 24.d’Udekem Y, Iyengar AJ, Cochrane AD, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007;116(11 Suppl):I157–I164. [DOI] [PubMed] [Google Scholar]
- 25.Egbe AC, Connolly HM, McLeod CJ, et al. Thrombotic and embolic complications associated with atrial arrhythmia after Fontan operation: role of prophylactic therapy. J Am Coll Cardiol. 2016;68:1312–1319. [DOI] [PubMed] [Google Scholar]
- 26.Faircloth JM, Roe O, Alsaied T, Palumbo JS, Vinks A, Veldtman GR. Intermediate term thrombotic risk in contemporary total cavo-pulmonary connection for single ventricle circulations. J Thromb Thrombolysis. 2017;44:275–280. [DOI] [PubMed] [Google Scholar]
- 27.Haas GS, Hess H, Black M, Onnasch J, Mohr FW, van Son JA. Extracardiac conduit Fontan procedure: early and intermediate results. Eur J Cardiothorac Surg. 2000;17:648–654. [DOI] [PubMed] [Google Scholar]
- 28.Iyengar AJ, Winlaw DS, Galati JC, et al. No difference between aspirin and warfarin after extracardiac Fontan in a propensity score analysis of 475 patients. Eur J Cardiothorac Surg. 2016;50: 980–987. [DOI] [PubMed] [Google Scholar]
- 29.Kaulitz R, Ziemer G, Rauch R, et al. Prophylaxis of thromboembolic complications after the Fontan operation (total cavopulmonary anastomosis). J Thorac Cardiovasc Surg. 2005;129:569–575. [DOI] [PubMed] [Google Scholar]
- 30.Kawamatsu N, Ishizu T, Machino-Ohtsuka T, et al. Direct oral anticoagulant use and outcomes in adult patients with Fontan circulation: a multicenter retrospective cohort study. Int J Cardiol. 2021;327:74–79. [DOI] [PubMed] [Google Scholar]
- 31.Lin JH, Kean AC, Cordes TM. The risk of thromboembolic complications in Fontan patients with atrial flutter/fibrillation treated with electrical cardioversion. Pediatr Cardiol. 2016;37:1351–1360. [DOI] [PubMed] [Google Scholar]
- 32.Mahnke CB, Boyle GJ, Janosky JE, Siewers RD, Pigula FA. Anticoagulation and incidence of late cerebrovascular accidents following the Fontan procedure. Pediatr Cardiol. 2005;26:56–61. [DOI] [PubMed] [Google Scholar]
- 33.Manlhiot C, Brandão LR, Kwok J, et al. Thrombotic complications and thromboprophylaxis across all 3 stages of single ventricle heart palliation. J Pediatr. 2012;161:513–519.e3. [DOI] [PubMed] [Google Scholar]
- 34.McCrindle BW, Michelson AD, Van Bergen AH, et al. Thromboprophylaxis for children post-fontan procedure: insights from the UNIVERSE study. J Am Heart Assoc. 2021;10:e021765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monagle P, Cochrane A, Roberts R, et al. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J Am Coll Cardiol. 2011;58: 645–651. [DOI] [PubMed] [Google Scholar]
- 36.Potter BJ, Leong-Sit P, Fernandes SM, et al. Effect of aspirin and warfarin therapy on thromboembolic events in patients with univentricular hearts and Fontan palliation. Int J Cardiol. 2013;168:3940–3943. [DOI] [PubMed] [Google Scholar]
- 37.Seipelt RG, Franke A, Vazquez-Jimenez JF, et al. Thromboembolic complications after Fontan procedures: comparison of different therapeutic approaches. Ann Thorac Surg. 2002;74(2):556–562. [DOI] [PubMed] [Google Scholar]
- 38.Small AJ, Aboulhosn JA, Lluri G. Thromboprophylaxis in adults with atrio-pulmonary Fontan. World J Pediatr Congenit Heart Surg. 2018;9: 504–508. [DOI] [PubMed] [Google Scholar]
- 39.Takawira F, Ayer JG, Onikul E, et al. Evaluation of the extracardiac conduit modification of the Fontan operation for thrombus formation using magnetic resonance imaging. Heart Lung Circ. 2008;17:407–410. [DOI] [PubMed] [Google Scholar]
- 40.Yang H, Veldtman GR, Bouma BJ, et al. Non-vitamin K antagonist oral anticoagulants in adults with a Fontan circulation: are they safe. Open Heart. 2019;6:e000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCrindle BW, Manlhiot C, Cochrane A, et al. Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. 2013;61:346–353. [DOI] [PubMed] [Google Scholar]
- 42.Faircloth JM, Miner KM, Alsaied T, et al. Time in therapeutic range as a marker for thrombotic and bleeding outcomes in Fontan patients. J Thromb Thrombolysis. 2017;44:38–47. [DOI] [PubMed] [Google Scholar]
- 43.Stalikas N, Doundoulakis I, Karagiannidis E, et al. Non-vitamin K oral anticoagulants in adults with congenital heart disease: a systematic review. J Clin Med. 2020;9(6):1794. 10.3390/jcm9061794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease. J Am Coll Cardiol. 2019;73(12):e81–e192. 10.1016/j.jacc.2018.08.1029 [DOI] [PubMed] [Google Scholar]
- 45.Freisinger E, Gerß J, Makowski L, et al. Current use and safety of novel oral anticoagulants in adults with congenital heart disease: results of a nationwide analysis including more than 44 000 patients. Eur Heart J. 2020;41:4168–4177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.