Abstract
Urinary incontinence (UI) is a common health condition that may interfere with the quality of life. A comprehensive evaluation of female UI helps with effective and safe treatments. Ultrasound has gained popularity to explore UI recently because it can collect crucial information for treatment planning and counseling. Translabial and introital approaches are commonly and reliably applied to ultrasound. The images can be obtained using two-dimensional and three-dimensional ultrasounds. Ultrasound is the only modality capable of confirming the presence or absence of a mid-urethral sling (MUS) and is able to demonstrate bulking agents as well. Although some of the ultrasound findings may only be incidental or supplementary to the patient’s symptoms, ultrasound benefits for investigating the pathophysiology of UI and surgical outcomes of MUS procedures. It is anticipated that standardization in terminology, measurement techniques, and reporting can be established in the near future.
Keywords: Bladder neck funneling, sling, sling location, surgical outcomes, urethral hypermobility, urinary incontinence
INTRODUCTION
Urinary incontinence (UI), defined as the involuntary loss of urine, is a common health condition that may interfere with the quality of life, with up to 77% of women suffering from it.[1,2,3,4,5] However, few women seek care despite many effective treatment options.[1] Untreated incontinence is associated with falls and fractures, sleep disturbances, depression, and urinary tract infections (UTIs).[6,7,8]
Among several different categories, stress urinary incontinence (SUI), urge urinary incontinence, and mixed incontinence are the most common.[4,5] A complete evaluation for female UI helps to tailor proper treatment and includes history taking, physical examination, urinalysis, image studies, urodynamic studies, and other optional tests.[1,2,3,4,5] The assessment focuses on elucidating the impact of incontinence on quality of life, the patients’ goals and preferences for management, the outcomes of previous treatments, the presence of concurrent conditions, such as advanced pelvic organ prolapse, urinary infection, or serious underlying pathologies such as cancer or serious neurologic disease.[1,2,3,4,5]
The initial management consists of unsupervised pelvic muscle exercises and lifestyle adjustments suitable to the patients to diminish their symptoms.[1,2,3,4,5] Recommendations for lifestyle adjustments can include weight loss, adequate hydration, avoiding excessive fluids, and scheduled voids.[1,2,3,4,5] For patients not benefiting from the above conservative treatments, medications can be administered for urge incontinence (UUI), and surgeries, principally mid-urethral slings (MUS), can be performed for SUI.[1,2,3,9,10] The MUS is effective with 48%–90% of symptom improvement and safe in low rates of sling complications (<5%).[1,2,3,4]
ULTRASOUND AS THE FIRST-LINE IMAGING TOOL TO EVALUATE URINARY INCONTINENCE
Despite being convenient and useful in gynecological clinical practice, ultrasound has not been recommended as a routine tool to explore uncomplicated UI[2,4] until recently when more and more related studies have been published. The advancements in three-dimensional (3D) [Figure 1] and four-dimensional (4D) technology have provided enhanced diagnostic capabilities as well as dynamic and temporal resolution of the sagittal, axial, and frontal planes of the pelvic structures.[11] Furthermore, polypropylene sling or mesh can only be visualized clearly under ultrasound.[11] Therefore, ultrasound has been accepted by many experts and societies as the first-line imaging tool to evaluate pelvic floor dysfunction.[12,13]
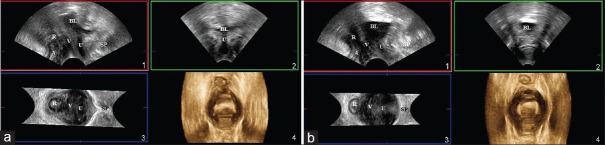
Figure 1.
Three-dimensional introital ultrasound image demonstrating sagittal (panel 1), frontal (panel 2), axial (panel 3), and rendered (panel 4) views. A: Anus, BL: Bladder, R: Rectum, SP: Symphysis pubis, U: Urethra, V: Vagina
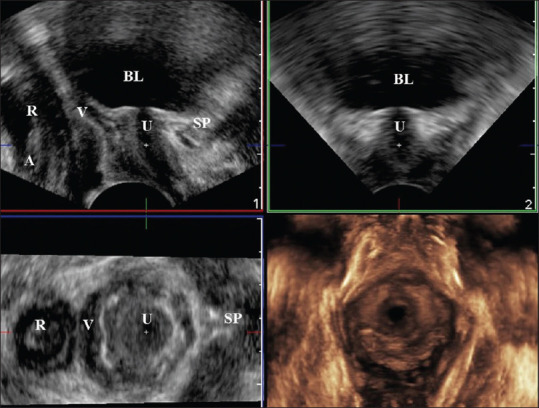
There are several approaches to performing pelvic ultrasounds.[9] The most widely reported method is translabial ultrasound. The approach uses a 3.5 to 6 MHz curved array transducer, which is placed on the perineum. The resulting image includes the symphysis pubis on the left side of the sagittal image, the urethra and bladder neck, the vagina, cervix, rectum, and anal canal.[11] Introital ultrasound[14,15] uses a sector endovaginal probe with a frequency between 5 and 7.5 MHz, which is placed between the labia minora just underneath the external urethral orifice [Figure 2a]. The resulting image includes the symphysis pubis on the right side of the sagittal image, the urethra and bladder neck, the vagina, cervix, rectum, and anal canal [Figure 2b].[15] Introital ultrasound is considered ideal to obtain a dynamic assessment of the anterior compartment, by the tint of minimal intrusiveness, the greatest field of view, the largest frequency of probe bandwidth, and no distortion of the pelvic floor structure.[9]
Figure 2.
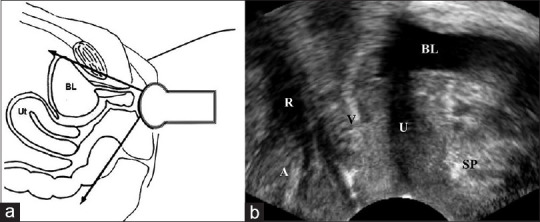
Illustrations of performance (a) and resulting image (b) of introital ultrasound. A: Anus, BL: Bladder, R: Rectum, SP: Symphysis pubis, U: Urethra, V: Vagina
The application of ultrasound in pelvic function can be creative and versatile for clinical or research purposes. This review focuses on the clinical applications for women with UI.
ULTRASOUND IN CLINICAL EVALUATIONS FOR WOMEN REPORTING URINARY INCONTINENCE
Ultrasound is convenient and ideal for estimating postvoid residual (PVR),[2,5] which is crucial and basic in evaluating patients who describe symptoms suggestive of urinary retention, bladder outlet obstruction, or overflow incontinence.[3] In general, a PVR of <50 mL is regarded as normal, while those of more than 200 mL may suggest insufficient bladder emptying.[3,16] The bladder volume (mL) can be estimated by transabdominal ultrasound via the formula of H (bladder height) × D (bladder depth) × W (bladder width) × 0.7 (correction factor for the nonspherical bladder shape) as well as by transvaginal ultrasound via the formula of H (bladder height) × D (bladder depth) × 5.9 – 14.6 (95% confidence limits around ± 37 mL).[17]
Ultrasound is feasible, reliable, and minimally invasive to explore the pathophysiology of UI.[18] Table 1 lists the differential diagnosis of UI.[3] SUI is defined as the involuntary leakage of urine with increased intra-abdominal pressure, such as activities including physical exertion, coughing, laughing, or sneezing.[19] SUI occurs due to an inability to maintain the normal pressure gradient between the bladder and the urethra during the increased intra-abdominal pressure.[3,20] SUI in women has often been associated with pelvic floor weakness resulting from vaginal delivery, trauma, or surgeries.[3,16,20]
Table 1.
Differential diagnosis of female urinary incontinence[3]
| Differential diagnosis of female urinary incontinence |
|---|
| Stress urinary incontinence |
| Urge incontinence |
| Mixed incontinence |
| Overflow incontinence |
| Urethral diverticulum |
| Genitourinary fistulas |
| Anatomic lesions |
| Congenital urethral anomalies |
| Functional/transient abnormalities |
| Infection |
| Medications |
| Reduced mobility |
| Dementia/delirium |
| Excessive urine production associating medical morbidities, such as diabetes |
SUI is traditionally categorized into two subtypes: urethral hypermobility [Figure 3] and intrinsic urethral sphincter.[18,21,22] Urethral hypermobility is the most common morphological feature of SUI. The commonly accepted methods to assess urethral mobility include the Q-tip test, radiographic tests, and ultrasound.[18] Ultrasound has the advantage of evaluating the dynamic movement of the urethra and bladder neck during the Valsalva maneuver and can be used as an alternative to the Q-tip test.[18,21] The German Association of Urogynecology has recommended three methods for the measurement of bladder neck position: from one distance and one angle, from two distances, or from the height of the bladder neck with reference to a horizontal line drawn at the lower border of the symphysis pubis.[23,24]
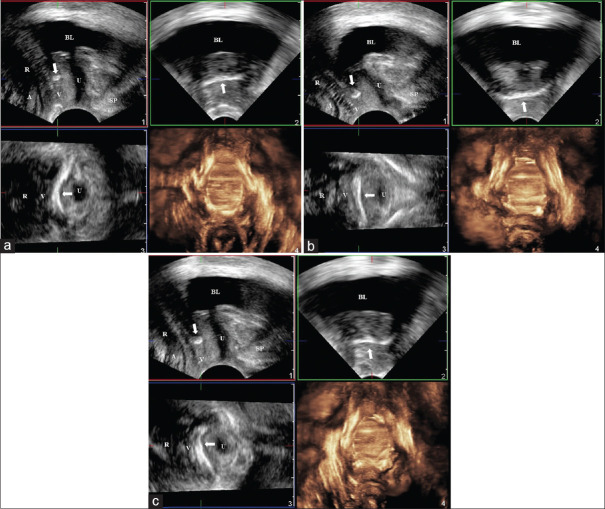
Figure 3.
Three-dimensional introital ultrasound image showing urethral rotational descent from resting (a) to straining (b) in sagittal (panel 1), frontal (panel 2), axial (panel 3), and rendered (panel 4) views. A: Anus, BL: Bladder, R: Rectum, SP: Symphysis pubis, U: Urethra, V: Vagina
In women reporting SUI, bladder neck funneling [Figure 4] on ultrasound implies the potential coexistence of poor anatomic support and an intrinsic sphincter defect, which requires urodynamic investigation to verify.[22] The high negative predictive value of bladder neck funneling is useful in excluding the presence of low leak point pressure.[22]
Figure 4.
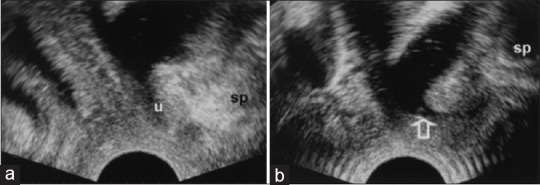
Introital ultrasound displaying bladder neck funneling (arrow) from resting (a) to straining (b). SP: Symphysis pubis, U: Urethra
For women with recurrent or persistent SUI and complex histories of pelvic repairs, ultrasound is able to demonstrate and localize the previously interposed vaginal polypropylene grafts.[9,25] On ultrasound, a retropubic MUS is a U-shaped echogenic structure with its arms sharply traversing toward the pubic bone. A transobturator MUS typically appears V-shaped with its arms obtusely crossing toward the obturator foramen.[9,26] Transvaginal meshes or meshes applied through sacrocolpopexy are possibly delineated by ultrasound, with regard to the position and number of anchoring arms and mesh shapes.[9] Elucidating the above histories is useful in planning further treatment for complex patients.
Urethral diverticulum [Figure 5] is not rare in women with SUI. While magnetic resonance imaging (MRI) is the most accurate method to diagnose urethral diverticula,[27,28,29] it is less accessible and more expensive. Nevertheless, ultrasound is convenient and inexpensive[28,29] and can be assisted as an initial test.
Figure 5.
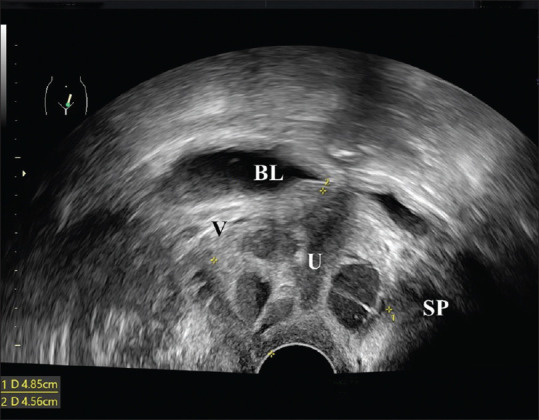
Introital ultrasound revealing a 4.85 cm × 4.56 cm complex lesion surrounding the urethra (U). BL: Bladder, SP: Symphysis pubis, V: Vagina
Genitourinary fistulas are abnormal communications between the urinary tract and genital organs. Vesicovaginal fistula (VVF) is an abnormal passage between the bladder and the vagina, leading to continuous urine leakage through the vagina.[30] VVF is among the most distressing and socially devastating conditions in women and commonly develops due to obstetrical or gynecological injury.[30] A suspicion of VVF arises when urine leakage develops between the 7 and 12 days after pelvic surgery, obstructed labor, or even years after radiation.[30] This probably resulted from necrosis followed by obstructed prolonged labor and tissue taken up in stitches in pelvic surgery.[30] Cystoscopy and contrast studies are common tools for the diagnosis and management of genitourinary fistulas. Contrast studies such as intravenous urogram or cystogram are essential to rule out associated ureterovaginal fistula, although they might not benefit in showing genital abnormality.[30] On the other hand, a color Doppler ultrasound [Figure 6] is convenient and feasible in proving the existence of the fistula by revealing a jet phenomenon through the lower urinary tract toward the vagina.[31]
Figure 6.
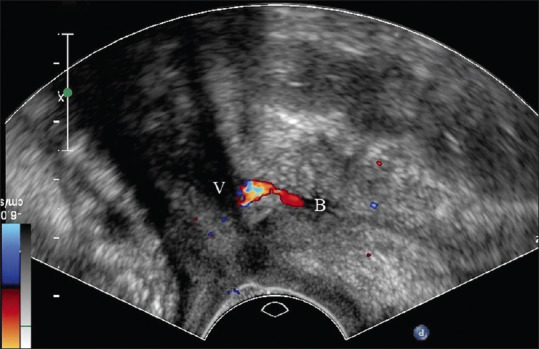
Introital ultrasound disclosing a vesicovaginal fistula with urine flow between the bladder (B) and the vagina (V) seen on color Doppler. BL: Bladder, V: Vagina
The lifetime risk of bladder cancer is approximately 0.27% in women.[32] The presentations of bladder cancer can be gross hematuria, microscopic hematuria, irritative voiding symptoms, or a tumor incidentally unveiled on imaging.[32] Bladder cancers are typically diagnosed with cystoscopy.[33] Updated diagnostic evaluation for hematuria is based on the potential risks of bladder cancer, given the relatively low frequency of malignancy, invasive nature of cystoscopy, radiation exposure from cross-sectional imaging, and associated health-care costs.[32] Ultrasound is reported to be 63% sensitive and 99% specific for the detection of bladder cancer.[33] On ultrasound, bladder cancer most commonly appears as an immobile polypoid heterogeneous mass arising from the bladder wall [Figure 7].[33] The presence of flow identified on color Doppler helps to differentiate the solid tissue of a tumor from a blood clot or debris.[33]
Figure 7.
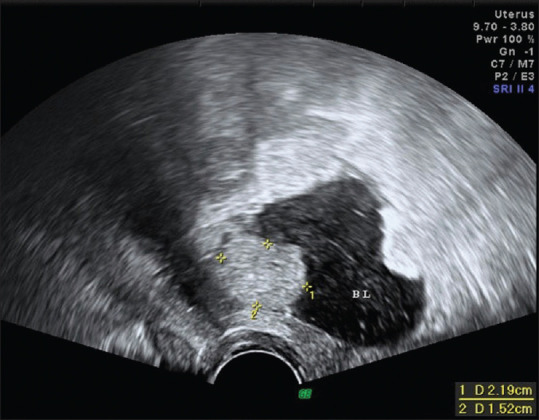
Introital ultrasound unveiling a 2.19 cm × 1.52 cm bladder mass arising from the posterior wall of the bladder (BL). BL: Bladder
ULTRASOUND IN POSTOPERATIVE SURVEILLANCE FOR WOMEN WHO HAVE UNDERGONE OPERATIONS
Currently, the MUS has become the gold standard surgery for treating SUI.[9,10] Ultrasound is feasible in dynamically visualizing MUS [Figure 8] and surveilling surgical effectiveness and adverse events.[9,25,34,35,36] On ultrasound, the MUS appears a highly echogenic linear or curved structure.[9,15] In addition to qualitative assessments, ultrasound also can reliably and validly provide quantitative evaluations.[9,15,37,38,39,40,41,42,43] Tapping into 3D or 4D technology, the MUS can be explored in different aspects of planes. The mid-sagittal images allow for the evaluation of the MUS location relative to the length of the urethra.[9,15] Coronal and axial images can provide the visualization of the full distribution of the MUS including symmetry, curling, or folding.[9,15]
Figure 8.
Three-dimensional introital ultrasound image demonstrating a mid-urethral sling (arrow) during resting (a), straining (b), and coughing (c) on sagittal image in Panel 1, frontal image in Panel 2, axial image in Panel 3, and rendered image in Panel 4. A, Anus, BL: Bladder, R: Rectum, SP: Symphysis pubis, U: Urethra, V: Vagina
Several qualitative and quantitative parameters have been explored for assessing MUS functionality.[9,37,38,39,40,41,42,43,44,45,46,47] The dynamic change in MUS shape from flat at rest to C-shaped on Valsalva is associated with optimal surgical outcomes.[38] A MUS location at 40%–70% of the urethral length is considered optimal.[38] However, some investigators found MUS locations relative to pubic symphysis or the urethral length unrelated to surgical outcomes.[37,45]
The distances between the MUS and the urethra or the symphysis pubis are another widely explored parameter.[34,38,41,42,46] A shorter distance between the MUS and the symphysis pubis is associated with a higher satisfaction rate[34,38,42,47] while more with obstruction complications after MUS procedures.The distance between MUS and the urethral lumen is considered more reflective in MUS efficacy than that between MUS and urethral rhabdosphincter, given that the thickness of the urethral walls could vary.[41,42,46]
Urethral bulking agent injection is another method to treat SUI. Ultrasound is also suitable to explore the relative position of injected urethral bulking to the urethra or bladder neck.[9,26] The types of bulking agents could be differentiated based on the echogenicity of the material.[9] For example, Bulkamid® injections appear less echogenic in comparison with Macroplastique®.[9]
ULTRASOUND IN EXPLORING POSTOPERATIVE ADVERSE EVENTS
Despite being highly effective procedures, there is still concern surrounding the use of a sling for incontinence.[9,48] Management of MUS-associated complications requires an understanding of the position, morphology, and shape of the synthetic implant.[9] Ultrasound is deemed most useful for assessing the urethrovaginal or vaginal wall space[9,35,36] because it is the only modality that can clearly demonstrate the polypropylene implant at this location owing to the high echogenicity.[9,25] The dynamic assessment via ultrasound can help to explore the nature of surgical complications and to select appropriate conservative or surgical management for postoperative adverse events.[9,35,49]
Too proximal MUS location at the proximal half of the urethra has been found in 74.8% of failed cases.[42,44] A shorter distance between MUS and urethra longitudinal smooth muscle is associated with a higher probability of voiding dysfunction[42] and recurrent UTI, while a long distance between MUS and urethra longitudinal smooth muscle is associated with an increased possibility of sling exposure.[34]
Severe pain, particularly associated with voiding dysfunction, bleeding, or decreased hemoglobin levels, immediately postoperatively could be due to a hematoma formation [Figure 9]. Ultrasound allows the visualization of hematoma to confirm the diagnosis.[50,51] Nevertheless, most conditions causing pain or dyspareunia such as nerve entrapment can hardly be defined by ultrasound.[9] Most of the time, ultrasound can only provide reassurance of the absence of an abscess or hematoma and correct sling placement.[9] Hypoechogenic areas surrounding a MUS on ultrasound raise the suspicions of areas of inflammation, infection, or fluid collection.[9] An MRI is recommended since it outperforms the ultrasound in assessing the status and extent of inflammation, particularly when intraabdominal or retropubic involvement is suspected[9] or removal of MUS is considered. In the meantime, the association between the positioning of the MUS in the urethral rhabdosphincter and postoperative pain remains inconclusive.[9]
Figure 9.
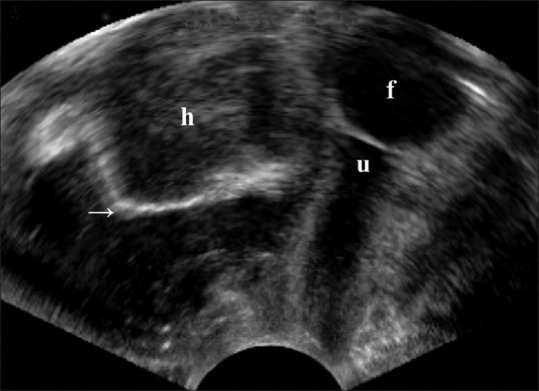
A vaginal three-dimensional ultrasound showed a 5.7 cm × 4.8 cm vaginal hematoma (h) beneath the urethra (u) and Foley catheter (f) and a dorsally deviated vaginal mesh (arrow) in the sagittal plane. H: Hematoma, U: Urethra, F: Foley catheter
Unfortunately, studies have not been able to consistently report any parameters that correlate with clinical outcomes, partly because patients’ perception of surgical failure is always multifactorial.[9,37,38,39,40,41,42,43,44,45]
CONCLUSIONS
Ultrasound is applicable in the evaluation of women with SUI and those having undergone MUS placement. Ultrasound is the only modality capable of reliably demonstrating MUS. For the time being, there still lacks substantial agreement on optimal MUS interposition and standardized methods for measurements and reporting. Ultrasound findings must be cautiously interpreted when correlating them with symptoms, surgical outcomes, and postoperative adverse events, as some ultrasound findings may only be incidental or supplementary to the patient’s symptoms, rather than causative.
Financial support and sponsorship
This study was supported by Cathay General Hospital (CGH-MR-A11105).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The author would like to thank the participating hospital and institution for their support to this study.
REFERENCES
- 1.Lukacz ES, Santiago-Lastra Y, Albo ME, Brubaker L. Urinary incontinence in women:A review. JAMA. 2017;318:1592–604. doi: 10.1001/jama.2017.12137. [DOI] [PubMed] [Google Scholar]
- 2.Hu JS, Pierre EF. Urinary incontinence in women:Evaluation and management. Am Fam Physician. 2019;100:339–48. [PubMed] [Google Scholar]
- 3.Trowbridge ER, Hoover EF. Evaluation and treatment of urinary incontinence in women. Gastroenterol Clin North Am. 2022;51:157–75. doi: 10.1016/j.gtc.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Sussman RD, Syan R, Brucker BM. Guideline of guidelines:Urinary incontinence in women. BJU Int. 2020;125:638–55. doi: 10.1111/bju.14927. [DOI] [PubMed] [Google Scholar]
- 5.ACOG practice bulletin no. 155:Urinary incontinence in women. Obstet Gynecol. 2015;126:e66–81. doi: 10.1097/AOG.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 6.Brown JS, McGhan WF, Chokroverty S. Comorbidities associated with overactive bladder. Am J Manag Care. 2000;6:S574–9. [PubMed] [Google Scholar]
- 7.Wagner TH, Hu TW, Bentkover J, LeBlanc K, Stewart W, Corey R, et al. Health-related consequences of overactive bladder. Am J Manag Care. 2002;8:S598–607. [PubMed] [Google Scholar]
- 8.Gibson W, Hunter KF, Camicioli R, Booth J, Skelton DA, Dumoulin C, et al. The association between lower urinary tract symptoms and falls:Forming a theoretical model for a research agenda. Neurourol Urodyn. 2018;37:501–9. doi: 10.1002/nau.23295. [DOI] [PubMed] [Google Scholar]
- 9.Taithongchai A, Sultan AH, Wieczorek PA, Thakar R. Clinical application of 2D and 3D pelvic floor ultrasound of mid-urethral slings and vaginal wall mesh. Int Urogynecol J. 2019;30:1401–11. doi: 10.1007/s00192-019-03973-2. [DOI] [PubMed] [Google Scholar]
- 10.Paraiso MF, Walters MD, Karram MM, Barber MD. Laparoscopic Burch colposuspension versus tension-free vaginal tape:A randomized trial. Obstet Gynecol. 2004;104:1249–58. doi: 10.1097/01.AOG.0000146290.10472.b3. [DOI] [PubMed] [Google Scholar]
- 11.Dietz HP. Ultrasound in the investigation of pelvic floor disorders. Curr Opin Obstet Gynecol. 2020;32:431–40. doi: 10.1097/GCO.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 12.Bahrami S, Khatri G, Sheridan AD, Palmer SL, Lockhart ME, Arif-Tiwari H, et al. Pelvic floor ultrasound:When, why, and how? Abdom Radiol (NY) 2021;46:1395–413. doi: 10.1007/s00261-019-02216-8. [DOI] [PubMed] [Google Scholar]
- 13.Ram R, Jambhekar K, Glanc P, Steiner A, Sheridan AD, Arif-Tiwari H, et al. Meshy business:MRI and ultrasound evaluation of pelvic floor mesh and slings. Abdom Radiol (NY) 2021;46:1414–42. doi: 10.1007/s00261-020-02404-x. [DOI] [PubMed] [Google Scholar]
- 14.Tunn R, Petri E. Introital and transvaginal ultrasound as the main tool in the assessment of urogenital and pelvic floor dysfunction:An imaging panel and practical approach. Ultrasound Obstet Gynecol. 2003;22:205–13. doi: 10.1002/uog.189. [DOI] [PubMed] [Google Scholar]
- 15.Huang WC, Yang JM, Chen HF. Five-year clinical and imaging outcomes of primary transobturator midurethral sling procedures for uncomplicated urodynamic stress incontinence. Maturitas. 2020;138:42–50. doi: 10.1016/j.maturitas.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Carr R. Urinary incontinence. In: South-Paul JE, Matheny SC, Lewis EL, editors. Current Diagnosis and Treatment:Family Medicine. 5th ed. New York: McGraw-Hill; 2020. [Google Scholar]
- 17.Haylen BT. Residual urine volumes in a normal female population:Application of transvaginal ultrasound. Br J Urol. 1989;64:347–9. doi: 10.1111/j.1464-410x.1989.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 18.Turkoglu A, Coskun AD, Arinkan SA, Vural F. The role of transperineal ultrasound in the evaluation of stress urinary incontinence cases. Int Braz J Urol. 2022;48:70–7. doi: 10.1590/S1677-5538.IBJU.2020.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21:5–26. doi: 10.1007/s00192-009-0976-9. [DOI] [PubMed] [Google Scholar]
- 20.Tarnay CM, Medendorp AR, Cohen SA, Mwesigwa PJ. Urinary incontinence and pelvic floor disorders. In: DeCherney AH, Nathan L, Laufer N, Roman AS, editors. Current Diagnosis and Treatment:Obstetrics and Gynecology. 12th ed. New York: McGraw-Hill; 2019. [Google Scholar]
- 21.Yang JM, Yang SH, Huang WC. Dynamic morphological changes in the anterior vaginal wall before and after laparoscopic Burch colposuspension in primary urodynamic stress incontinence. Ultrasound Obstet Gynecol. 2005;25:289–95. doi: 10.1002/uog.1838. [DOI] [PubMed] [Google Scholar]
- 22.Huang WC, Yang JM. Bladder neck funneling on ultrasound cystourethrography in primary stress urinary incontinence:A sign associated with urethral hypermobility and intrinsic sphincter deficiency. Urology. 2003;61:936–41. doi: 10.1016/s0090-4295(02)02558-x. [DOI] [PubMed] [Google Scholar]
- 23.Schaer G, Koelbl H, Voigt R, Merz E, Anthuber C, Niemeyer R, et al. Recommendations of the German Association of Urogynecology on functional sonography of the lower female urinary tract. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:105–8. doi: 10.1007/BF01902383. [DOI] [PubMed] [Google Scholar]
- 24.Huang WC, Yang JM, Yang SH. Applications of ultrasonography in female lower urinary tract symptoms:Diagnosis and intervention. Taiwanese J Obstet Gynecol. 2004;43:125–35. [Google Scholar]
- 25.Schuettoff S, Beyersdorff D, Gauruder-Burmester A, Tunn R. Visibility of the polypropylene tape after tension-free vaginal tape (TVT) procedure in women with stress urinary incontinence:Comparison of introital ultrasound and magnetic resonance imaging in vitro and in vivo. Ultrasound Obstet Gynecol. 2006;27:687–92. doi: 10.1002/uog.2781. [DOI] [PubMed] [Google Scholar]
- 26.Denson L, Shobeiri SA. Three-dimensional endovaginal sonography of synthetic implanted materials in the female pelvic floor. J Ultrasound Med. 2014;33:521–9. doi: 10.7863/ultra.33.3.521. [DOI] [PubMed] [Google Scholar]
- 27.Greenwell TJ, Spilotros M. Urethral diverticula in women. Nat Rev Urol. 2015;12:671–80. doi: 10.1038/nrurol.2015.230. [DOI] [PubMed] [Google Scholar]
- 28.Crescenze IM, Goldman HB. Female urethral diverticulum:Current diagnosis and management. Curr Urol Rep. 2015;16:71. doi: 10.1007/s11934-015-0540-8. [DOI] [PubMed] [Google Scholar]
- 29.Huang WC, Yang JM. A urethral diverticulum presenting with pure stress urinary incontinence. Taiwan J Obstet Gynecol. 2022;61:1058–60. doi: 10.1016/j.tjog.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Malik MA, Sohail M, Malik MT, Khalid N, Akram A. Changing trends in the etiology and management of vesicovaginal fistula. Int J Urol. 2018;25:25–9. doi: 10.1111/iju.13419. [DOI] [PubMed] [Google Scholar]
- 31.Volkmer BG, Kuefer R, Nesslauer T, Loeffler M, Gottfried HW. Colour Doppler ultrasound in vesicovaginal fistulas. Ultrasound Med Biol. 2000;26:771–5. doi: 10.1016/s0301-5629(00)00210-6. [DOI] [PubMed] [Google Scholar]
- 32.Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer:A review. JAMA. 2020;324:1980–91. doi: 10.1001/jama.2020.17598. [DOI] [PubMed] [Google Scholar]
- 33.Wentland AL, Desser TS, Troxell ML, Kamaya A. Bladder cancer and its mimics:A sonographic pictorial review with CT/MR and histologic correlation. Abdom Radiol (NY) 2019;44:3827–42. doi: 10.1007/s00261-019-02276-w. [DOI] [PubMed] [Google Scholar]
- 34.Ghanbari Z, Ayati E, Bastani P, Pourali L, Amini E. Ultrasound evaluation of mid-urethral sling position:A potential predictor of outcomes and adverse effects. Gynecol Obstet Invest. 2022;87:344–51. doi: 10.1159/000526506. [DOI] [PubMed] [Google Scholar]
- 35.Manonai J, Rostaminia G, Denson L, Shobeiri SA. Clinical and ultrasonographic study of patients presenting with transvaginal mesh complications. Neurourol Urodyn. 2016;35:407–11. doi: 10.1002/nau.22725. [DOI] [PubMed] [Google Scholar]
- 36.Khatri G, Carmel ME, Bailey AA, Foreman MR, Brewington CC, Zimmern PE, et al. Postoperative imaging after surgical repair for pelvic floor dysfunction. Radiographics. 2016;36:1233–56. doi: 10.1148/rg.2016150215. [DOI] [PubMed] [Google Scholar]
- 37.Dietz HP, Mouritsen L, Ellis G, Wilson PD. How important is TVT location? Acta Obstet Gynecol Scand. 2004;83:904–8. doi: 10.1111/j.0001-6349.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 38.Kociszewski J, Rautenberg O, Kolben S, Eberhard J, Hilgers R, Viereck V. Tape functionality:Position, change in shape, and outcome after TVT procedure –Mid-term results. Int Urogynecol J. 2010;21:795–800. doi: 10.1007/s00192-010-1119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang JM, Yang SH, Huang WC. Dynamic interaction involved in the tension-free vaginal tape obturator procedure. J Urol. 2008;180:2081–7. doi: 10.1016/j.juro.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 40.Yang JM, Yang SH, Huang WC. Correlation of morphological alterations and functional impairment of the tension-free vaginal tape obturator procedure. J Urol. 2009;181:211–8. doi: 10.1016/j.juro.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 41.Yang JM, Yang SH, Huang WC, Tzeng CR. Reliability of a new method for assessing urethral compression following midurethral tape procedures using four-dimensional ultrasound. Ultrasound Obstet Gynecol. 2011;38:210–6. doi: 10.1002/uog.9003. [DOI] [PubMed] [Google Scholar]
- 42.Yang JM, Yang SH, Huang WC, Tzeng CR. Correlation of tape location and tension with surgical outcome after transobturator suburethral tape procedures. Ultrasound Obstet Gynecol. 2012;39:458–65. doi: 10.1002/uog.10086. [DOI] [PubMed] [Google Scholar]
- 43.Huang WC, Yang SH, Yang JM, Tzeng CR. Functional and morphological differences following Monarc and TVT-O procedures. Ultrasound Obstet Gynecol. 2012;40:699–705. doi: 10.1002/uog.10153. [DOI] [PubMed] [Google Scholar]
- 44.Bogusiewicz M, Monist M, Stankiewicz A, Woźniak M, Wieczorek AP, Rechberger T. Most of the patients with suburethral sling failure have tapes located outside the high-pressure zone of the urethra. Ginekol Pol. 2013;84:334–8. doi: 10.17772/gp/1585. [DOI] [PubMed] [Google Scholar]
- 45.Ng CC, Lee LC, Han WH. Use of three-dimensional ultrasound scan to assess the clinical importance of midurethral placement of the tension-free vaginal tape (TVT) for treatment of incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:220–5. doi: 10.1007/s00192-004-1245-6. [DOI] [PubMed] [Google Scholar]
- 46.Dietz HP, Mouritsen L, Ellis G, Wilson PD. Does the tension-free vaginal tape stay where you put it? Am J Obstet Gynecol. 2003;188:950–3. doi: 10.1067/mob.2003.268. [DOI] [PubMed] [Google Scholar]
- 47.Chantarasorn V, Shek KL, Dietz HP. Sonographic appearance of transobturator slings:Implications for function and dysfunction. Int Urogynecol J. 2011;22:493–8. doi: 10.1007/s00192-010-1306-y. [DOI] [PubMed] [Google Scholar]
- 48.Blaivas JG, Purohit RS, Benedon MS, Mekel G, Stern M, Billah M, et al. Safety considerations for synthetic sling surgery. Nat Rev Urol. 2015;12:481–509. doi: 10.1038/nrurol.2015.183. [DOI] [PubMed] [Google Scholar]
- 49.Kociszewski J, Kolben S, Barski D, Viereck V, Barcz E. Complications following tension-free vaginal tapes:Accurate diagnosis and complications management. Biomed Res Int. 2015;2015:538391. doi: 10.1155/2015/538391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bogusiewicz M. Ultrasound imaging in urogynecology –State of the art 2016. Prz Menopauzalny. 2016;15:123–32. doi: 10.5114/pm.2016.63060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang WC, Yang JM. Voiding dysfunction related to a vaginal hematoma after a Perigee™procedure. Ultrasound Obstet Gynecol. 2013;41:230–1. doi: 10.1002/uog.12275. [DOI] [PubMed] [Google Scholar]