Abstract
Digital health technologies are being utilized increasingly in the modern management of diabetes. These include tools such as continuous glucose monitoring systems, connected blood glucose monitoring devices, hybrid closed-loop systems, smart insulin pens, telehealth, and smartphone applications (apps). Although many of these technologies have a solid evidence base, from the perspective of a person living with diabetes, there remain multiple barriers preventing their optimal use, creating a digital divide. In this article, we describe many of the origins of these barriers and offer recommendations on widening access to digital health technologies for underserved populations living with diabetes to improve their health outcomes.
Keywords: digital divide, digital health, digital literacy, health equity
1 |. INTRODUCTION
In the United States, the burden of diabetes disproportionately impacts communities facing health disparities.1 These communities also experience barriers in accessing currently available digital health technologies.2,3 The reasons for existing inequities in access to and in the use of digital health technologies to support diabetes self-management are multifactorial, including affordability and challenges related to health and digital literacy. Further, erroneous assumptions leading to bias and discrimination on the part of clinicians have also been recognized.4 The issue of bias appears to be compounded by challenges in accessing professionals who are knowledgeable and interested in digital health technologies and especially by the paucity of endocrinologists and clinicians from these same underserved communities.5 In this article, we will explore the evidence for using digital health technologies and some of the drivers of the ‘digital divide’, and suggest recommendations to narrow this division between the ‘haves’ and ‘have-nots’ regarding digital health and diabetes.
2 |. DRIVERS OF THE DIGITAL DIVIDE
The digital divide can be thought of as the uneven distribution of information and communication technologies encompassing access and usage.6 In diabetes care, the digital divide also includes barriers to US Food and Drug Administration-approved technologies whose use is associated with evidence-based, meaningful clinical benefits. Beyond approved devices, there are also a plethora of other digital health technologies that can be used to support diabetes care, including doit-yourself open-source closed-loop systems, smartphone applications (apps) that allow remote monitoring of glucose data, consumer products to track fitness and well-being, and apps providing diabetes-related information and data-logging features. Challenges in the availability and use of these digital health technologies reinforce or amplify inequalities to cause a persistent knowledge or actionable information gap, especially for marginalized and historically excluded communities, most often communities of colour. Both access to and usage of digital health technologies are influenced primarily by social determinants of health and the social gradient within which people with diabetes (PWD) reside.7 For PWD, the main drivers of the digital divide can be considered financial, informational, and technical (Figure 1).
FIGURE 1.

Requirements for digital inclusion are also stress points for creating a digital divide.
2.1 |. Examples of the financial drivers of the digital divide
Most often, barriers to accessing digital health technologies are due to the financial burden (i.e., cost for the user). This includes the cost of connecting to the Internet. In the United States, access to high-speed internet remains low among Black and Hispanic/Latino individuals and all races and ethnicities living in rural areas.8 Internet connectivity is a ‘super’ social determinant of health because it is a prerequisite for addressing other determinants and is essential in order for PWD to navigate their health system.9 Currently, individuals with low socioeconomic status may use a device with a minimal data plan or lack space to speak privately with their clinician.10 As many existing passive uploads of data from devices can occur with cellular or Wi-Fi connectivity, expanding access to technologies via cellular technology on personal cell phones, independent of using personal data plans instead of relying solely on internet connectivity, could be a potential solution. Recommendation: In discussions around the use of digital health technologies with a person with diabetes, a screening assessment of the user’s potential level of digital connectivity should be performed.11
Trust in digital health technologies is also a fundamental component in evaluating the value of that service. The absence of trust in healthcare is especially challenging for the Black American community based on historical precedents.12 In healthcare, trust can be interpersonal (trust between people), social (trust in a system/institution), and technological (trust in technology)—the three forms of trust are often interrelated. Levels of trust vary from person to person; people with high levels of trust appear to create positive feedback loops with measurable benefits when compared to the people who have low levels of trust in their healthcare providers or systems.13,14 For digital health to be successful in supporting self-efficacy and empowerment, there needs to be both trust in the technology and trust in the professional recommending and/or also using the device, and this needs to be sustained. Recommendation: Specific trust-building mechanisms (e.g., providing timely and appropriate training in using digital health technologies and ensuring cultural congruence of the user interface and user experience) should be implemented as part of a digital health offering.
Another concern related to trust in digital health technologies is the issue of privacy. This includes policies that allow data sharing with third parties, leading to the perception of potential abuse or exploitation by companies taking advantage of personal information.15 Privacy concerns extend to research, as 40% of adults in the United States do not understand clinical trials, and minorities are underrepresented as researchers, research participants, and care providers.16 Further, except for parental caregiving for children with T1D, there is limited research on the use of digital health technologies that has extended beyond the individual with diabetes using the device or software to include their peripheral therapy and support networks.17 Recommendation: By increasing the representation of racial and ethnic minorities as researchers, clinical scientists, and community health workers experienced in the use of digital health technologies, as well as engaging with an individual’s family and/or other support network, there is potential to gain and maintain trust in digital health technologies.18
2.2 |. Informational drivers of the digital divide
Within a clinical consultation and an interaction with digital health technologies, the meaning and context of what is said must be understood in order for a message to be successfully conveyed. PWD may have the technical expertise to perform a prescribed task, but without accurate interpretation, the task may be carried out incorrectly.19 For example, words may not be understood during a telehealth consultation because the physician/trainer uses medical jargon. Further, even if the words are understood, the meaning may not be. The message can be lost if the content does not consider influences outside the usual medical domain—experiential, familial and cultural factors. In other words, information may not be actionable due to outside influences (Figure 2). Recommendation: For any digital health technologies, there is a need to ensure that the interface and experience in using the devices consider the numeracy, literacy, and digital skills of the intended users and require an understanding of their health beliefs20 and to ensure that actionable information is created. Embedding assessments of understanding in the application of digital health technologies is necessary but will require specific training for professionals.
FIGURE 2.
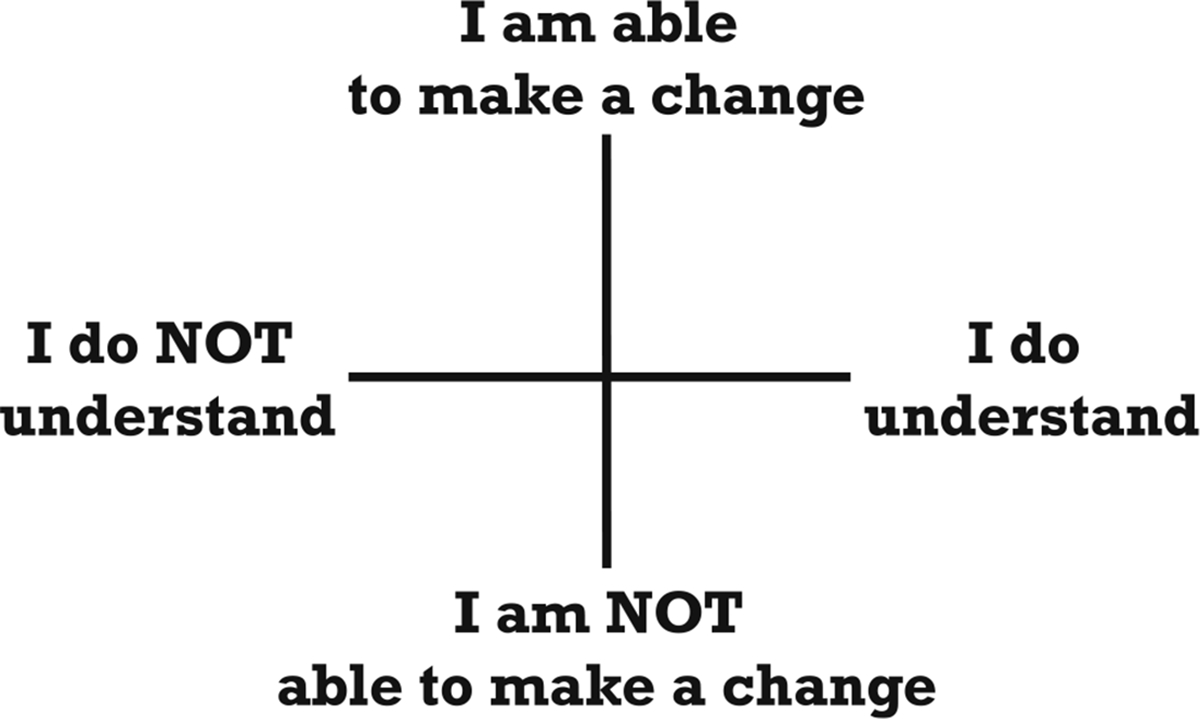
Domains of understanding and use of actionable information associated with the use of digital health.
2.3 |. Technical drivers of the digital divide
The central driver for inventing any technology is reduction in human burden through making things easier with technology compared to the same task without technology. All technology that is perceived as leading to a net increase of burden will likely not become adopted; for example, we charge mobile phones every day and pay bills every month, but as the net benefit is so high, we are willing to take on the additional burden. As the above discussion highlights, even when accessible and affordable, digital health technologies can be viewed as a net increase in personal burden. One of those burdens is tracking and understanding data, which is often designed to be shared but can be presented to the user in a format that is beyond their level of training or expertise. Recommendation: Technologies should be designed to deliver interventions that only engage with the user when essential or desired and only add additional layers of information if a user desires.
To achieve that goal, it is essential to design data science methodologies that extract insights from data that (i) rely on a user’s own data, (ii) tell a cohesive ‘story’ that allows the user to understand one’s progress and (iii) have the ability to dial-up/dial-down engagement with the user. Because diabetes outcomes are impacted by multiple factors, including physical activity, diet, and medications, any insights and storytelling must account for all factors relevant to the user. Recent work has demonstrated the ability to extract patterns from user data that take complex streams of data and convert them into relevant bio-behavioural information for the users,21 for example, helping users decide on how much postprandial physical activity will lead to desirable mealtime glycaemic outcomes. Recommendation: New data summarization and storytelling techniques should be tested in PWD (and those with prediabetes) to determine the ability of this approach to reach diverse populations at their health literacy levels and present culturally appropriate, actionable information.
2.4 |. Telehealth, mhealth, and the digital divide
Telehealth is defined as the use of electronic information and telecommunication technologies to support long-distance clinical healthcare, patient and professional health-related education, health administration, and public health, and mHealth is the application of mobile phones, mobile applications, and wireless devices.22,23 Given the ability of telehealth to overcome barriers to accessing care, including transportation and distance to specialty care, it has been suggested as a strategy to address healthcare disparities.24 As with other digital health technologies, telehealth and mHealth have been demonstrated to be beneficial for PWD but are also less likely to be used by underserved individuals with diabetes.25,26 It is also noteworthy that many studies of telehealth interventions have not specifically enrolled medically underserved populations. Where data are available, a recent meta-analysis of telehealth interventions27 reported that among nine randomized controlled trials in the United States that enrolled >50% Black and Hispanic participants, the majority of interventions were delivered at the individual patient level by telephone call or text message and were effective in reducing glycated haemoglobin (HbA1c) by −0.47% (confidence interval −0.65 to −0.28). Recommendation: Telehealth is a viable alternative to traditional face-to-face clinic visits and should be encouraged in underserved communities for diabetes technology initiation and ongoing support.28,29 Future studies of telehealth should ensure adequate representation of communities previously underrepresented. Telehealth is also a helpful tool that increases convenience for pregnant women with diabetes, especially those in remote areas, as it offers economic savings related to fewer clinical visits and reduces the time away from usual activities for both the patient and endocrinologist.30–32 Recommendation: Telehealth should continue to be made available to pregnant women with all forms of diabetes.
In addition to telehealth for patients, telehealth for professional education and mentoring (tele-mentoring) is an innovative care delivery model that seeks to overcome workforce issues to improve health disparities. The diabetes ECHO tele-mentoring programmes have been shown to improve primary care clinician knowledge and confidence in managing diabetes. Patients treated by ECHO-trained physicians were more likely to have an HbA1c concentration less than 64 mmol/mol (8%) than those who were not.33 Going forward, advances in the application of machine learning and artificial intelligence to support remote patient monitoring have the potential for identification of high-risk patients for targeted intervention and may serve as a mechanism by which to reduce inequities in health outcomes. Recommendation: Tele-mentoring should be considered as a means to upskill clinicians involved in diabetes care.
mHealth can also be leveraged to support diabetes self-management. Multiple studies have demonstrated that mHealth interventions can improve glycaemic control and adherence to medication.34 Studies of mHealth interventions among socially disadvantaged adults with type 2 diabetes (T2D) have demonstrated improved glycaemic outcomes, acute care use, and multiple patient-reported measures, including diabetes distress, self-efficacy, and self-management behaviours.35 As mHealth interventions can be linguistically and culturally tailored to ensure relevancy and extend the reach of healthcare into the homes and daily lives of PWD, they hold the potential to reduce inequities in diabetes care. Recommendation: When considering mHealth interventions, clinicians should consider reach, uptake, patient preferences, and the context of the intervention when deciding on the mode of delivery of any mHealth as well as the ‘dose’ of the intervention.36
Finally, policy-level changes are necessary to reduce the digital divide for PWD. Supporting the importance of policy change in closing the digital divide, it has been observed that when Medicaid programmes are modified and technology costs are subsidized, disparities in digital health technologies use can be ameliorated and glycaemic control improved.37 Recommendation: Improving awareness of and use of federal programmes to improve the affordability of internet services38 as well as supporting additional governmental funding to improve the availability of services,39 particularly in rural areas, will be critical to enable access to digital health technologies for underserved PWD to avoid perpetuating the existing digital divide.
In summary, to narrow the digital divide in diabetes care, creators of digital health devices and connected digital ecosystems should consider eight features that influence digital equity (Table 1).36,40 In the next sections, we will consider recommendations to close the digital divide for specific groups of PWD.
TABLE 1.
Eight features that influence digital equity.
| Feature | Description |
|---|---|
| Accessibility | Components of the digital ecosystem, such as internet access, hardware, and software, are available with no implicit bias by the prescriber. |
| Affordability | Components of the digital ecosystem, such as internet access, hardware, and software, are affordable in terms of financial cost and time burden. |
| Concordance | Digital user interface and user experience are culturally appropriate and understandable for the target group's sociodemographic characteristics. |
| Connectivity | Device and/or connected ecosystem is reliably connected for >95% of the time and no immediate harm occurs with loss of connection. |
| Empathy | User experience is sincere, non-judgemental, and patient- (vs. professional-) centric. |
| Existentialism | Device and/or ecosystem does not have a deleterious impact on other social and biological determinants of health and does not require continuous input from the user. |
| Trustworthiness | Device and/or connected ecosystem inspires user trust as cyber-secure and confidential, minimizing privacy/security threats, inaccurate data, and obsolete information. |
| Validity | Efficacy of the device and/or connected ecosystem has been proven through participation of target groups during research and development and has the appropriate level of evidence for clinicians, payors, and people with diabetes. |
3 |. IMPROVING ACCESS TO DIGITAL HEALTH TECHNOLOGIES FOR PEOPLE WITH TYPE 1 DIABETES
Underserved people with type 1 diabetes (T1D) comprise ethnic-racial minorities, those living in rural communities, and individuals with low socioeconomic status.41 Furthermore, it is noteworthy that both the prevalence and incidence of T1D among racial and ethnic minority groups are increasing.42,43 At present, underserved people with T1D are less likely to use technologies such as continuous glucose monitoring (CGM) and insulin pump therapy.44,45 There are numerous contributors to the digital divide in T1D,46–54 as illustrated in Figure 3.
FIGURE 3.

Swiss cheese model of contributing factors to inequities for people with type 1 diabetes.
The need to address digital health technologies inequities in T1D has been highlighted by the T1D Exchange Quality Improvement (T1DX-QI) network, based on longitudinal data from 2016 to 2022, covering over 48 000 people with T1D. Data from this network have demonstrated that, despite overall improvement in glycaemic outcomes, there remain persistent inequities mediated by race, ethnicity and lack of affordable health insurance.55
T1DX-QI is one of the largest US population health research and improvement networks, with 55 T1D centres and 10 T2D datasharing centres across 22 US states.56,57 In this section, we share insights and recommendations inspired by T1DX-QI network efforts to address inequities in T1D.58
3.1 |. Crossing the digital divide in T1D
In the last year, there have been improvements in public health insurance coverage for digital health technologies,59 especially for CGM at the state and federal levels. Unfortunately, even among those with access to insurance coverage, inequities in digital health technologies use persist.60–62 Recommendation: To fully transform the digital health technologies landscape, insurance payors must remove any administrative barriers and simplify the processes to improve access for people with T1D. Administrative burdens, such as documentation of the frequency of blood glucose checks and hypoglycaemic episodes and prior authorizations, limit access and widen equity gaps.63 Other administrative burdens related to challenging insurance-related decisions also contribute to diabetes providers’ insurance-mediated biases against recommending digital health technologies.
In a 2022 T1DX-QI study, investigators found that 34% of the study cohort were less likely to recommend digital health technologies to people of minority race and ethnicity, while 66% were less likely to recommend digital health technologies to people with T1D on public insurance.46 While health equity training to reduce bias is a good start, evidence suggests that this is not fully effective in closing equity gaps.52 T1DX-QI centres are testing electronic health record (EHR) clinical decision support systems and shared decision making as potential opportunities to reduce the impact of provider implicit bias. Recommendation: Diabetes stakeholders need to explore additional innovative solutions that can automate digital health technologies prescriptions and encourage more active participation from people with T1D in their treatment discussions, including the need to call out bias.
Despite improved coverage for digital health technologies, there remain significant variations in their use across similar diabetes centres and clinics. T1DX-QI investigators found the overall median CGM use among 21 paediatric centres to be 80%, but there were significant centre-to-centre variations ranging from 35% to 90%,53 with similar trends observed in the use of insulin pumps. It will be beneficial to promote public reporting on these variations and encourage peer-to-peer sharing with an overall goal to reduce variation. Recommendation: The T1DX-QI encourages benchmarking efforts using an online EHR system to identify equity trend variations and share best practices.64 Similar programmes can be encouraged for primary care organizations, rural practices, and other major healthcare systems. Insurance payors can also use available claims data to identify variations in access, with the goal of promoting strategies to support broader access for underserved individuals with T1D.
People with T1D with effective training and coaching on digital health technologies can make significant differences in outcomes. Reviewing data patterns from digital health technologies and making appropriate adjustments have been described as one of the six critical habits necessary to improve outcomes, even among underserved communities.65
Quality improvement (QI) principles can be used to promote digital health technologies.66,67 T1DX-QI centres have used QI to increase use of digital health technologies,68–70 reduce equity gaps, and promote screening of social determinants of health. Recommendation: Clinics providing care for adults and children with T1D should consider adopting evidence-based QI principles.
Inequities begin early in the trajectory of T1D71 and the time of initiation of diabetes technologies following diagnosis has a significant impact on the long-term trajectory of outcomes.72,73 Studies have demonstrated that supporting underserved communities to begin diabetes devices early, even by using a trial version, can promote their engagement and support them to maximize the benefit of the diabetes devices. Centres that begin CGM for all newly diagnosed people with T1D have reported significant improvement in outcomes and sustained benefits.74 Recommendation: Consider the use of digital health technologies early after diagnosis of T1D.
There are several reasons why people, particularly those in underserved communities, might discontinue diabetes technologies. Device cost, alarm fatigue, skin irritation, and interruptions in insurance coverage are among them. Data have shown that outcomes worsen for people who discontinue CGM.75 There are also psychosocial reasons and the burden of T1D management, which should be frequently monitored and managed. Community health workers and peer navigators can be especially influential in providing the needed support for people with T1D.76–78 Recommendation: Professionals involved in diabetes care should monitor engagement and identify any potential reasons that might lead to early discontinuation of digital health technologies.
With collaboration, innovation, and persistence, we can continue to drive improvement in outcomes and close equity gaps for people living with T1D.
4 |. IMPROVING ACCESS TO DIGITAL HEALTH TECHNOLOGIES FOR PEOPLE WITH T2D
The use of digital health technologies in T2D care has grown rapidly in recent years. However, multiple drivers of inequitable access to digital health technologies have widened disparities in health outcomes between the most and least socially privileged.
4.1 |. Crossing the digital divide in T2D
Multiple clinical trials and real-world evidence studies of CGM have demonstrated improvement in glycaemic control, acute care use, and quality-of-life outcomes for adults with T2D.79–82 Furthermore, in a recent study of intermittent short-term use of real-time CGM, this appears to be an effective method for glucose control in adults with T2D treated with oral agents, especially when combined with traditional self-monitoring of blood glucose.83 CGM use in adults with T2D is now recommended within authoritative clinical guidelines.84,85 In routine clinical practice, as evidence of clinical efficacy and effectiveness has mounted, rates of CGM use have increased over time, although CGM remains under-prescribed to those with T2D using insulin. As the evidence for benefit of CGM in broad groups with T2D grows, CGM coverage may become a more attractive economic option for payors.
Similarly to T1D, advances in insulin delivery systems, including hybrid closed-loop (HCL) insulin pump therapy that adjusts insulin delivery based on CGM data, and smart insulin pens (SIPs) that use mobile applications to assist with dosing decisions, hold promise for people living with T2D. SIPs offer the ability to monitor insulin dosing behaviour, assist with insulin dosing decisions, and remind users to administer insulin without having to wear an on-body device. Studies of the impact of SIPs demonstrate that fewer missed insulin boluses are associated with improved glycaemia.86 Despite these benefits, clinicians at endocrine and diabetes specialty centres have reported low awareness of the potential benefits of SIPs and limited training on their use.87
Although racial and ethnic inequity in CGM and insulin pump use has been well demonstrated in T1D, inequity in device use has been relatively understudied in T2D. Data from centers for medicare & medicaid services have demonstrated that Black and Hispanic/Latino adults with T2D are prescribed CGM at lower rates than White adults.88 In a study of CGM use in T2D at a large academic medical centre, prescription of CGM was associated with younger age, private insurance, basal-bolus insulin use, endocrinologist visits, and higher HbA1c levels.89 Similarly, younger age and endocrinologist visits have been associated with greater likelihood of insulin pump initiation in T2D.90 Recommendation: Both HCL systems and SIPs are currently understudied in T2D, and additional research on inequities in use and how the use of these devices may improve disparities in outcomes is necessary. Further research is also required to evaluate the health and economic impact of the use of intermittent CGM use on both insulin-treated and non-insulin-treated T2D.
As previously described, drivers of digital health inequities exist at multiple levels, which can be understood according to the domains of the socio-ecological model91 (Figure 4). Given the evidence that device prescription has historically been associated with endocrinology specialty visits,88 increasing primary care clinician knowledge of devices will be essential as the majority of people with T2D continue to be cared for in primary care.92 It has been shown that clinician training and resources to support patient education and insurance navigation serve as facilitators to prescribing digital health technologies in primary care. Recommendation: Health systems that care for minoritized and socially disadvantaged adults with T2D should consider investing in clinician training and resources to increase the uptake of digital health technologies.93 These types of intervention address both individual and interpersonal level drivers of inequities as studies have identified that, for Black individuals with T2D, shared decision making94 about diabetes care is limited and that racial differences in referrals for specialist care have been observed, with fewer Black patients referred to specialty care than White patients.95 For PWD, peer support may also help to encourage initial and persistent device use.77
FIGURE 4.

Factors influencing technology use for adults with type 2 diabetes based on the socio-ecological model.
5 |. IMPROVING ACCESS TO DIGITAL HEALTH TECHNOLOGIES FOR PREGNANT WOMEN WITH DIABETES
Approximately 1% of all pregnancies are affected by pre-existing diabetes.96 Gestational diabetes (GDM), referred to as new-onset diabetes during pregnancy, has significantly increased in prevalence over the last 20 years, with current incidence rates of 1.7% to 15.7%, depending on the ethnic origin, maternal age, and diagnostic criteria.97
Over recent years, the frequency of adverse maternal and foetal outcomes associated with gestational diabetes has been increasing in the United States, with greater risks seen among women from racial and ethnic minorities.98,99 Similarly, adverse maternal and foetal outcomes for women with T1D and T2D remain significantly higher than for their non-diabetic counterparts.99,100
5.1 |. Crossing the digital divide for pregnant women with diabetes
During pregnancy, digital health technologies have been shown to provide educational and behavioural support for women with diabetes.101 For example, recent meta-analyses of digital health technologies have shown that web-based interventions improve glycaemic control during pregnancy and improve several maternal and foetal outcomes including lower C-section rates and macrosomia.102–104 Despite these benefits of digital health technologies for pregnant women, inequities in access to and use of digital health technologies remains an issue adversely impacting the woman and her unborn child. Recommendation: There is an urgent need to develop culturally tailored digital health technologies for diverse populations of women of child-bearing age, including exploring options for subsidizing or providing low-cost digital health technologies to those in need, to reduce health inequities associated with diabetes and pregnancy.
Despite the solid benefit of CGM for pregnant women with T1D, evidence suggests that the majority of reproductive-age women with T1D still do not use it and those who did not use CGM were more likely to be those at greatest risk of adverse pregnancy outcomes.105 In the CONCEPT Trial where improvements in maternal-foetal outcomes were seen in pregnant women with T1D randomized to CGM, most participants were of European or Mediterranean origin.106 Currently, there is a paucity of trials specifically recruiting non-White pregnant women with diabetes. Possible reasons for existing disparities in CGM use in pregnancy include systemic racism, diminished access to healthcare, psychosocial stressors, cost, provider bias, inadequate patient–provider communication, and strict eligibility criteria that add additional hurdles to use for those who experience more unmet social needs. Recommendation: CGM should be offered to all women with T1D considering pregnancy or who become pregnant. Even though there is lack of sufficient evidence to strongly support the benefit of CGM in pregnancies impacted by T2D and GDM, there are emerging data that support the safety and efficacy of CGM in these pregnancies as well.106–109
Despite the clear benefits of continuous subcutaneous insulin infusion (CSII) outside of pregnancy, in pregnancies complicated by T1D, the use of CSII has been associated with conflicting evidence. Outcomes ranging from better glycaemic control and less maternal hypoglycaemia to no glycaemic benefit have been reported and, similarly, improvement of maternal–foetal outcomes to worsening of outcomes have also been found. HCL systems that provide automated, glucose-responsive, basal insulin delivery with manual, self-administered, premeal insulin doses are emerging as promising treatment options in the management of T1D in pregnancy.110 However, most HCL systems are currently not approved for use in pregnancy and the gestational changes in insulin requirements could alter the effectiveness and safety of HCL systems that use algorithms derived from non-pregnant populations. These devices are also costly and often not easily accessible. To facilitate the real-world adoption of HCL systems in pregnant women with T1D, presenting HCL devices to pregnant women and healthcare professionals as a pillar of a three-party collaboration might help to promote optimal use. However, for broad implementation of HCL use in pregnancy, improved equity in access to HCL systems worldwide is also needed. Recommendation: In future studies of HCL systems in pregnancy, efforts should be made to ensure adequate representation beyond non-Hispanic White participants.
6 |. CONCLUSIONS
Within diabetes care, for a connected digital health ecosystem to be equitable, successful and sustainable, specific features need to be considered by healthcare professionals in their discussions with PWD. This includes ensuring evidence is generated covering the key domains of user and provider experience, cost-effectiveness, data governance, and interoperability in addition to health equity.111 Further, to close the digital divide, stakeholders need to move beyond considering diabetes as ‘types’ to test interventions that take into consideration the heterogeneity of diabetes, eventually down to the personal level. Within digital health technologies, data and algorithms alone are insufficient and often do not account for human behaviour. With the growing application of artificial intelligence for healthcare, it is paramount that populations and individuals facing a disproportionate burden of diabetes and living with health disparities are represented at all stages from the development of a technology through algorithm development, data acquisition, validation, and implementation. At present, there is certainty that digital health technologies will improve health outcomes and reduce costs but there remains considerable uncertainty that digital health technologies will deliver health equity, narrowing the gap between what is and what ought to be.112
ACKNOWLEDGMENTS
Thank you to Susan Lane at the Journal for the excellent editorial assistance. This article was commissioned by the Editor as part of a special Supplement made possible by an Educational Grant from Roche Diabetes Care GmbH. Sponsor identity was not disclosed to the authors prior to publication.
Footnotes
CONFLICT OF INTEREST STATEMENT
Osagie Ebekozien is a member of the Medtronic Diabetes and Sanofi Advisory Board, and has received research support from Medtronic Diabetes, MannKind Pharmaceutical, Dexcom, Eli Lilly Diabetes, Abbott, Vertex Pharmaceutical and Janssen Pharmaceutical, and consultation and speaker fees from Medtronic Diabetes, Sanofi and Vertex. All financial support from industry has been though his organization, T1D Exchange. David Kerr has received consultancy fees from Sanofi, Abbott Rapid Diagnostics, Proteomics, Evidation Health, Better Therapeutics, owns share options in Glooko, and has received research support from Abbott Diabetes Care. All other authors have no relevant disclosures.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1111/dom.15470.
DATA AVAILABILITY STATEMENT
No data are available.
REFERENCES
- 1.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanbour S, Jones M, Abusamaan MS, et al. Racial disparities in access and use of diabetes technology among adult patients with type 1 diabetes in a U.S. Academic Medical Center. Diabetes Care. 2023;46(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang M, Johnson CM, D’Eramo-Melkus G, Vorderstrasse AA. Participation of racial and ethnic minorities in technology-based interventions to self-manage type 2 diabetes: a scoping review. J Transcult Nurs. 2018;29(3):292–307. [DOI] [PubMed] [Google Scholar]
- 4.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffman J, Calimlim E, Fix M. 2021 Edition—California Physicians. A portrait of practice. California Health Care Almanac. 2021. [Google Scholar]
- 6.Schweitzer EJ. Digital Divide. Encyclopedia Britannica; 2023. [Google Scholar]
- 7.Kerr D, Aram M, Crosby KM, Glantz N. A persisting parallel universe in diabetes care within America’s capital. EClinicalMedicine. 2022;43:101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atske A, Perrin A. Home broadband adoption, computer ownership vary by race, ethnicity in the U.S. Pew Research Center. 2021. [Accessed 11/21/2023]. Available from: https://www.pewresearch.org/short-reads/2021/07/16/home-broadband-adoption-computer-ownership-vary-by-race-ethnicity-in-the-u-s/
- 9.Sieck CJ, Sheon A, Ancker JS, Castek J, Callahan B, Siefer A. Digital inclusion as a social determinant of health. NPJ Digit Med. 2021;4(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia JF, Fogel J, Reid M, Bisno DI, Raymond JK. Telehealth for young adults with diabetes: addressing social determinants of health. Diabetes Spectr. 2021;34(4):357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klonoff DC, Shang T, Zhang JY, Cengiz E, Mehta C, Kerr D. Digital connectivity: the sixth vital sign. J Diabetes Sci Technol. 2022;16(5):1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washington HA. Medical Apartheid: the dark history of medical experimentation on Black Americans from Colonial Times to the Present. Knopf Doubleday Publishing Group; 2008. [Google Scholar]
- 13.Montague EN, Winchester WW, Kleiner BM. Trust in medical technology by patients and health care providers in obstetric work systems. Behav Inf Technol. 2010;29(5):541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belfrage S, Helgesson G, Lynøe N. Trust and digital privacy in healthcare: a cross-sectional descriptive study of trust and attitudes towards uses of electronic health data among the general public in Sweden. BMC Med Ethics. 2022;23:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr D, Sabharwal A. Principles for virtual health care to deliver real equity in diabetes. Lancet Diabetes Endocrinol. 2021;9(8):480–482. [DOI] [PubMed] [Google Scholar]
- 16.Lack of Patient Awareness = Lack of Participation = Delays & Higher Costs Coalition for Clinical Trials Awareness. 2016. [Accesed 11/21/2023]. Available from. https://cctawareness.org/about-us/ [Google Scholar]
- 17.Gunn KL, Seers K, Posner N, Coates V. Somebody there to watch over you’: the role of the family in everyday and emergency diabetes care. Health Soc Care Community. 2012;20(6):591–598. [DOI] [PubMed] [Google Scholar]
- 18.Morales J, Glantz N, Larez A, et al. Understanding the impact of five major determinants of health (genetics, biology, behavior, psychology, society/environment) on type 2 diabetes in U.S. Hispanic/Latino families: mil Familias—a cohort study. BMC Endocr Disord. 2020;20(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reach G Linguistic barriers in diabetes care. Diabetologia. 2009; 52(8):1461–1463. [DOI] [PubMed] [Google Scholar]
- 20.Rovner BW, Casten RJ. Discordant health beliefs and telehealth use in African Americans with diabetes. J Am Geriatr Soc. 2021;69(6):1684–1686. [DOI] [PubMed] [Google Scholar]
- 21.Pai A, Santiago R, Glantz N, et al. Multimodal digital phenotyping of diet, physical activity, and glycemia in Hispanic/Latino adults with or at risk of type 2 diabetes. NPJ Digital Med. 2024;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Director General. mHealth. Use of appropriate digital technologies for public health. World Health Organization; March 26, 2018:A71/20. [Google Scholar]
- 23.What is Telehealth? HRSA. 2022. Available from: https://www.hrsa.gov/telehealth/what-is-telehealth#:~:text=Telehealth%20is%20defined%20as%20the,health%20administration%2C%20and%20public%20health [Accessed 11/21/2023].
- 24.Martinez M, Perle JG. Reaching the Latino population: a brief conceptual discussion on the use of telehealth to address healthcare disparities for the large and growing population. J Technol Behav Sci. 2019;4(3):267–273. [Google Scholar]
- 25.Ballesta S, Chillaron JJ, Inglada Y, et al. Telehealth model versus in-person standard care for persons with type 1 diabetes treated with multiple daily injections: an open-label randomized controlled trial. Front Endocrinol (Lausanne). 2023;14:1176765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeStourgeon L, Bergner E, Datye K, Streisand R, Jaser S. Evaluation of study engagement with an mHealth intervention (THR1VE) to treat diabetes distress in teens with type 1 diabetes: randomized clinical trial. JMIR Pediatr Parent. 2023;6:e47089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson A, O’Connell SS, Thomas C, Chimmanamada R. Telehealth interventions to improve diabetes management among black and hispanic patients: a systematic review and meta-analysis. J Racial Ethn Health Disparities. 2022;9(6):2375–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JM, Ospelt E, Noor N, et al. Institutional barriers to the successful implementation of telemedicine for type 1 diabetes care. Clin Diabetes. 2023;42:cd230056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalfrà MG, Nicolucci A, Lapolla A. The effect of telemedicine on outcome and quality of life in pregnant women with diabetes. J Telemed Telecare. 2009;15(5):238–242. [DOI] [PubMed] [Google Scholar]
- 31.Kruger DF, White K, Galpern A, et al. Effect of modem transmission of blood glucose data on telephone consultation time, clinic work flow, and patient satisfaction for patients with gestational diabetes mellitus. J Am Acad Nurse Pract. 2003;15(8):371–375. [DOI] [PubMed] [Google Scholar]
- 32.Snoswell CL, Caffery LJ, Haydon HM, Wickramasinghe SI, Crumblin K, Smith AC. A cost-consequence analysis comparing patient travel, outreach, and telehealth clinic models for a specialist diabetes service to indigenous people in Queensland. J Telemed Telecare. 2019;25(9):537–544. [DOI] [PubMed] [Google Scholar]
- 33.Ehrhardt N, Bouchonville M, Peek ME, et al. Telementoring with project ECHO: a new era in diabetes-related continuing education for primary care to address health disparities. J Diabetes Sci Technol. 2023;17(4):916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chong CJ, Bakry MM, Hatah E, Mohd Tahir NA, Mustafa N. Effects of mobile apps intervention on medication adherence and type 2 diabetes mellitus control: a systematic review and meta-analysis. J Telemed Telecare. 2023;1357633X231174933. [DOI] [PubMed] [Google Scholar]
- 35.Mayberry LS, Lyles CR, Oldenburg B, Osborn CY, Parks M, Peek ME. mHealth interventions for disadvantaged and vulnerable people with type 2 diabetes. Curr Diab Rep. 2019;19(12):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moschonis G, Siopis G, Jung J, et al. Effectiveness, reach, uptake, and feasibility of digital health interventions for adults with type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Lancet Digit Health. 2023;5(3):e125–e143. [DOI] [PubMed] [Google Scholar]
- 37.Ni K, Tampe CA, Sol K, Richardson DB, Pereira RI. Effect of CGM access expansion on uptake among patients on medicaid with diabetes. Diabetes Care. 2023;46(2):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Federal Communications Commission. Affordable Connectivity Program. 2023. Available from: https://www.fcc.gov/acp [Accessed 11/21/2023].
- 39.Government US. Broadband Equity Access and Deployment Program: BroadbandUSA. Available from https://broadbandusa.ntia.doc.gov/funding-programs/broadband-equity-access-and-deployment-bead-program#:~:text=The%20Broadband%20Equity%2C%20Access%2C%20and,and%20the%20Commonwealth%20of%20the [Accessed 11/21/2023]. [Google Scholar]
- 40.Kerr D, Glantz N. Access to and understanding of health information in the era of telehealth. In: Klonoff DC, Kerr D, Weitzman ER, eds. Diabetes Digital Health and Telehealth. Elsevier Science; 2022. [Google Scholar]
- 41.Department of Health and Human Services. Minority Health and Health Disparities: Definitions and Parameters US. 2023. Available from https://www.nimhd.nih.gov/about/strategic-plan/nih-strategic-plan-definitions-and-parameters.html [Accessed 11/21/2023].
- 42.Lado JJ, Lipman TH. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45(2):453–461. [DOI] [PubMed] [Google Scholar]
- 43.Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebekozien O, Hardison H, Shah V. The promise of diabetes technologies. Clin Diabetol. 2023;12(1):1–3. [Google Scholar]
- 46.Implicit racial–ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022;24(9):619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellis DA, Cutchin MP, Templin T, et al. Effects of family and neighborhood risks on glycemic control among young black adolescents with type 1 diabetes: findings from a multi-center study. Pediatr Diabetes. 2021;22(3):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howe CJ, Morone J, Hawkes CP, Lipman TH. Racial disparities in technology use in children with type 1 diabetes: a qualitative content analysis of parents’ perspectives. Sci Diabetes Self Manag Care. 2023;49(1):55–64. [DOI] [PubMed] [Google Scholar]
- 49.Miyazaki B, Ebekozien O, Rompicherla S, et al. Association between health insurance type and adverse outcomes for children and young adults with type 1 diabetes and coronavirus disease 2019. Diabetes Spectr. 2023;36(4):398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeSalvo DJ, Lanzinger S, Noor N, et al. Transatlantic comparison of pediatric continuous glucose monitoring use in the diabetes-Patienten-Verlaufsdokumentation initiative and type 1 diabetes exchange quality improvement Collaborative. Diabetes Technol Ther. 2022;24(12):920–924. [DOI] [PubMed] [Google Scholar]
- 51.Gallagher M-P, Noor N, Ebekozien O. Variations in clinic staffing for adult and pediatric diabetes centers in the United States: data from T1D exchange. Endocr Pract. 2023;29(8):678–679. [DOI] [PubMed] [Google Scholar]
- 52.Addala A, Mungmode A, Ospelt E, et al. Current practices in operationalizing and addressing racial equity in the provision of type 1 diabetes care: insights from the T1DX-QI health equity advancement lab. Endocr Pract. 2024;30(1):41–48. [DOI] [PubMed] [Google Scholar]
- 53.Prahalad P, Hardison H, Odugbesan O, et al. Benchmarking diabetes technology use among 21 United States pediatric diabetes centers. Clin Diabetes. 2024;42:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puckett C, Wong JC, Daley TC, Cossen K. How organizations shape medical technology allocation: insulin pumps and pediatric patients with type 1 diabetes. Soc Sci Med. 2020;249:112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebekozien O, Mungmode A, Sanchez J, et al. Longitudinal trends in glycemic outcomes and technology use for over 48,000 people with type 1 diabetes (2016–2022) from the type 1 diabetes exchange quality improvement collaborative. Diabetes Technol Ther. 2023; 25(11):765–773. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal S, Rioles N, Majidi S, Rapaport R, Ebekozien O, for the T1DX-QI Collaborative. Commentary on the T1D exchange quality improvement collaborative learning session November 2022 abstracts. J Diabetes. 2022;14(11):780–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majidi S, Rioles N, Agarwal S, Ebekozien O, Collaborative TDEQI. Evolution of the T1D exchange quality improvement collaborative (T1DX-QI): using real-world data and quality improvement to advance diabetes outcomes. Clin Diabetes. 2022;41(1):32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D exchange QI collaborative. J Diabetes. 2022;14(1):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howe G, Chavis J. Expanding Medicaid Access To Continuous Glucose Monitors. Center for Health Care Strategies 2022. https://www.chcs.org/resource/expanding-medicaid-access-to-continuous-glucose-monitors/ [Accessed 01/04/2024].
- 60.Ravi SJ, Coakley A, Vigers T, Pyle L, Forlenza GP, Alonso T. Pediatric medicaid patients with type 1 diabetes benefit from continuous glucose monitor technology. J Diabetes Sci Technol. 2021;15(3):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kommareddi M, Wherry K, Vigersky RA. Racial/ethnic inequities in use of diabetes technologies among medicare advantage beneficiaries with type 1 diabetes. J Clin Endocrinol Metab. 2023;108(7):e388–e395. [DOI] [PubMed] [Google Scholar]
- 62.DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D exchange real-world observational study. J Diabetes Sci Technol. 2023;17(2):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Center for Health Care Strategies. Continuous Glucose Monitor Access For Medicaid Beneficiaries Living With Diabetes: State-By-State Coverage. 2023. Available from: https://www.chcs.org/resource/continuous-glucose-monitor-access-for-medicaid-beneficiaries-living-with-diabetes-state-by-state-coverage/ [Accessed 11/21/2023].
- 64.Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health improvement using the T1D exchange quality improvement portal. Clin Diabetes. 2022;41(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JM, Rusnak A, Garrity A, et al. Feasibility of electronic health record assessment of 6 pediatric type 1 diabetes self-management habits and their association with glycemic outcomes. JAMA Netw Open. 2021;4(10):e2131278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebekozien O, Odugbesan O, Rioles N, Majidi S, Jones N-HY, Kamboj M. Equitable post-COVID-19 care: a practical framework to integrate health equity in diabetes management. J Clin Outcomes Manag. 2020;27(6):256–259. [Google Scholar]
- 68.Prahalad P, Ebekozien O, Alonso GT, et al. Multi-clinic quality improvement initiative increases continuous glucose monitoring use among adolescents and young adults with type 1 diabetes. Clin Diabetes. 2021;39(3):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lyons SK, Ebekozien O, Garrity A, et al. Increasing insulin pump use among 12- to 26-year-olds with type 1 diabetes: results from the T1D exchange quality improvement collaborative. Clin Diabetes. 2021;39(3):272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Odugbesan O, Mungmode A, Rioles N, et al. Increasing continuous glucose monitoring use for non-hispanic black and hispanic people with type 1 diabetes: results from the T1D exchange quality improvement collaborative equity study. Clin Diabetes. 2024;42(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tremblay ES, Liu E, Laffel LM. Health disparities likely emerge early in the course of type-1 diabetes in youth. J Diabetes Sci Technol. 2022;16(4):929–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin T, Manfredo JA, Illesca N, et al. Improving continuous glucose monitoring uptake in underserved youth with type 1 diabetes: the IMPACT study. Diabetes Technol Ther. 2023;25(1):13–19. [DOI] [PubMed] [Google Scholar]
- 73.Mulinacci G, Alonso GT, Snell-Bergeon JK, Shah VN. Glycemic outcomes with early initiation of continuous glucose monitoring system in recently diagnosed patients with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):6–10. [DOI] [PubMed] [Google Scholar]
- 74.Mathias P, Mahali LP, Agarwal S. Targeting technology in underserved adults with type 1 diabetes: effect of diabetes practice transformations on improving equity in CGM prescribing behaviors. Diabetes Care. 2022;45(10):2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noor N, Norman G, Sonabend R, et al. An observational crossover study of people using real-time continuous glucose monitors versus self-monitoring of blood glucose: real-world evidence using EMR data from more than 12,000 people with type 1 diabetes. J Diabetes Sci Technol. 2023;19322968231178017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker AF, Graham S, Maple-Brown L, et al. Interventions to address global inequity in diabetes: international progress. Lancet. 2023;402(10397):250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walker AF, Addala A, Sheehan E, et al. Using peer power to reduce health disparities: implementation of a diabetes support coach program in federally qualified health centers. Diabetes Spectr. 2022; 35(3):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker AF, Haller MJ, Gurka MJ, et al. Addressing health disparities in type 1 diabetes through peer mentorship. Pediatr Diabetes. 2020; 21(1):120–127. [DOI] [PubMed] [Google Scholar]
- 79.Lu J, Ying Z, Wang P, Fu M, Han C, Zhang M. Effects of continuous glucose monitoring on glycaemic control in type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2024;26:362–372. [DOI] [PubMed] [Google Scholar]
- 80.Reaven PD, Newell M, Rivas S, Zhou X, Norman GJ, Zhou JJ. Initiation of continuous glucose monitoring is linked to improved glycemic control and fewer clinical events in type 1 and type 2 diabetes in the veterans health administration. Diabetes Care. 2023;46(4):854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;25(22):2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gavin JR, Bailey CJ. Real-world studies support use of continuous glucose monitoring in type 1 and type 2 diabetes independently of treatment regimen. Diabetes Technol Ther. 2021;23(S3):S19–s27. [DOI] [PubMed] [Google Scholar]
- 83.Moon SJ, Kim KS, Lee WJ, Lee MY, Vigersky R, Park CY. Efficacy of intermittent short-term use of a real-time continuous glucose monitoring system in non-insulin-treated patients with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2023;25(1):110–120. [DOI] [PubMed] [Google Scholar]
- 84.ElSayed NA, Aleppo G, Aroda VR, et al. 7. Diabetes technology: standards of care in diabetes—2023. Diabetes Care. 2022;46-(Supplement_1):S111–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29(5):305–340. [DOI] [PubMed] [Google Scholar]
- 86.MacLeod J, Im GH, Smith M, Vigersky RA. Shining the spotlight on multiple daily insulin therapy: real-world evidence of the InPen™ smart insulin pen. Diabetes Technol Ther. 2023;26:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ospelt E, Noor N, Sanchez J, et al. Facilitators and barriers to smart insulin pen use: a mixed-method study of multidisciplinary stakeholders from diabetes teams in the United States. Clin Diabetes. 2022;41(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Isaacs D, Bellini NJ, Biba U, Cai A, Close KL. Health care disparities in use of continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S3):S81–S87. [DOI] [PubMed] [Google Scholar]
- 89.Mayberry LS, Guy C, Hendrickson CD, McCoy AB, Elasy T. Rates and correlates of uptake of continuous glucose monitors among adults with type 2 diabetes in primary care and endocrinology settings. J Gen Intern Med. 2023;38(11):2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hankosky ER, Katz ML, Fan L, et al. Predictors of insulin pump initiation among people with type 2 diabetes from a US claims database using machine learning. Curr Med Res Opin. 2023;39(6):843–853. [DOI] [PubMed] [Google Scholar]
- 91.National Center for Injury Prevention and Control DoVP. The Social-Ecological Model: A Framework for Prevention Centers for Disease Control. 2022. Accessed 11/21/2023. Available from: https://www.cdc.gov/violenceprevention/about/social-ecologicalmodel.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fviolenceprevention%2Fpublichealthissue%2Fsocial-ecologicalmodel.html
- 92.Vigersky RA, Fish L, Hogan P, et al. The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab. 2014;99(9):3112–3121. [DOI] [PubMed] [Google Scholar]
- 93.Warman M, Fillippi M, Manning B, et al. Continuous glucose monitoring for primary care patients with diabetes: barriers, facilitators, & resources to support access. Ann Fam Med. 2022;20(20 Suppl 1):2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peek ME, Odoms-Young A, Quinn MT, Gorawara-Bhat R, Wilson SC, Chin MH. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Landon BE, Onnela JP, Meneades L, O’Malley AJ, Keating NL. Assessment of racial disparities in primary care physician specialty referrals. JAMA Netw Open. 2021;4(1):e2029238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes during pregnancy: a maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. A clinical review. Int J Mol Sci. 2021;22(6):2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kong L, Nilsson IAK, Gissler M, Lavebratt C. Associations of maternal diabetes and body mass index with offspring birth weight and prematurity. JAMA Pediatr. 2019;173(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venkatesh KK, Lynch CD, Powe CE, et al. Risk of adverse pregnancy outcomes among pregnant individuals with gestational diabetes by race and ethnicity in the United States, 2014–2020. JAMA. 2022; 327(14):1356–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.American Diabetes Association. 14. Management of Diabetes in pregnancy: standards of medical Care in Diabetes—2021. Diabetes Care. 2020;44(Supplement_1):S200–S210. [DOI] [PubMed] [Google Scholar]
- 100.National Diabetes Statistics Report. Centers for Disease Control; 2020. [Google Scholar]
- 101.Immanuel J, Simmons D. Apps and the woman with gestational diabetes mellitus. Diabetes Care. 2021;44(2):313–315. [DOI] [PubMed] [Google Scholar]
- 102.Guo P, Chen D, Xu P, et al. Web-based interventions for pregnant women with gestational diabetes mellitus: systematic review and meta-analysis. J Med Internet Res. 2023;25:e36922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leblalta B, Kebaili H, Sim R, Lee SWH. Digital health interventions for gestational diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. PLOS Digit Health. 2022; 1(2):e0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie W, Dai P, Qin Y, Wu M, Yang B, Yu X. Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: an updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth. 2020;20(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Venkatesh KK, Powe CE, Buschur E, et al. Disparities in continuous glucose monitoring use among women of reproductive age with type 1 diabetes in the T1D exchange. Diabetes Technol Ther. 2023;25(3):201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multi-centre international randomised controlled trial. Lancet. 2017; 390(10110):2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang VYX, Tan YL, Ang WHD, Lau Y. Effects of continuous glucose monitoring on maternal and neonatal outcomes in perinatal women with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2022;184:109192. [DOI] [PubMed] [Google Scholar]
- 108.García-Moreno RM, Benítez-Valderrama P, Barquiel B, et al. Efficacy of continuous glucose monitoring on maternal and neonatal outcomes in gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabet Med. 2022;39(1):e14703. [DOI] [PubMed] [Google Scholar]
- 109.Wilkie G, Melnik V, Brainard L, et al. Continuous glucose monitor use in type 2 diabetes mellitus in pregnancy and perinatal outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2023;5(7):100969. [DOI] [PubMed] [Google Scholar]
- 110.Lee TTM, Collett C, Bergford S, et al. Automated insulin delivery in women with pregnancy complicated by type 1 diabetes. N Engl J Med. 2023;389(17):1566–1578. [DOI] [PubMed] [Google Scholar]
- 111.Silberman J, Wicks P, Patel S, et al. Rigorous and rapid evidence assessment in digital health with the evidence DEFINED framework. NPJ Digit Med. 2023;6(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kerr D, Glantz N. Diabetes, like COVID-19, is a wicked problem. Lancet Diabetes Endocrinol. 2020;8(11):873–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.


