Abstract
PURPOSE:
To determine whether macular infarction measured as hyper-reflectivity of the middle and inner retinal layers predicts long-term visual acuity outcomes in participants with central retinal vein occlusion (CRVO) or hemi-retinal vein occlusion (HRVO).
DESIGN:
Clinical cohort study using post hoc secondary analysis of phase 3 clinical trial data.
METHODS:
This post hoc secondary analysis of the phase 3 Study of COmparative Treatments for REtinal Vein Occlusions 2 (SCORE2) clinical trial included 310 of the 362 participants with macular edema secondary to CRVO/HRVO who were randomized to injections of aflibercept or bevacizumab. Month 01 (M01) optical coherence tomography (OCT) images were analyzed using the following grading scheme: no infarction (grade 0), only middle retinal infarction (grade 1), diffuse middle and patchy inner retinal infarction (grade 2), and diffuse middle and inner retinal infarction (grade 3). Visual acuity letter score (VALS), central subfield thickness (CST), and number of anti–vascular endothelial growth factor (anti-VEGF) injections were correlated with the infarction severity grade at month 01.
RESULTS:
More severe macular infarction, with both middle and inner retinal layer hyper-reflectivity (ie, grades 2 and 3), was associated with worse M00 VALS and was predictive of VALS at M01 to M60 (P < .001). More severe infarction was associated with greater CST at presentation; however, after the first anti-VEGF injection, CST decreased and was similar across all grades at all time points (P > .05) with similar number of injections.
CONCLUSIONS:
Participants with more severe macular infarction at M01, as graded with OCT, exhibited worse visual outcomes despite significantly improved macular edema from month 6 to 5 years. This suggests that macular infarction may drive visual acuity after retinal fluid is treated with anti-VEGF.
CENTRAL RETINAL VEIN OCCLUSION (CRVO) IS ONE OF the most common retinal vascular diseases, causing vision loss and blindness due to complications including macular edema and retinal infarction. Dye-based fluorescein angiography is the traditional tool to evaluate retinal non-perfusion and to assess visual and anatomical outcomes in eyes with CRVO. However, this modality requires the intravenous administration of a dye that is invasive, time consuming, and associated with systemic risk, even mortality in rare cases.
Optical coherence tomography (OCT) is a fast, practical, and non-invasive tool that provides depth-resolved, cross-sectional scans of the retina and can be an informative method to assess the severity and anatomic level of macular infarction in eyes with retinal vascular diseases such as CRVO.1–3 The mildest form of acute macular infarction may manifest on OCT first in the middle retinal layers or the inner nuclear layer as hyper-reflective bands and is referred to as paracentral acute middle maculopathy (PAMM). More severe forms of macular infarction can also involve the inner retinal layers, specifically the ganglion cell layer. Progression of acute macular infarction from the middle to the inner retinal levels can even occur in the same eye; it has been referred to as the ischemic cascade and may be related to the predominantly vertical or in series organization of the retinal capillary plexus.1
Although OCT has revolutionized the management of retinal disease, the identification of OCT-based biomarkers that predict visual outcomes in participants with CRVO remains elusive. Intraretinal hyper-reflective foci, disorganization of the inner retinal layers (DRIL), ellipsoid zone (EZ) or external limiting membrane (ELM) disruption, and choroidal thickness analysis are OCT biomarkers that can potentially predict visual acuty.2–23 However, there is significant variation in the predictive power across studies.24–26 This may be because no OCT biomarker measures the fundamental pathophysiological unit of disease in CRVO: namely, macular infarction.
To our knowledge, there is no published OCT-based approach to stratify macular infarction and to predict visual outcomes in eyes with CRVO or HRVO.12–16 The purpose of this study is to determine whether the anatomic level and severity of infarction on OCT, as graded by the extent of hyper-reflectivity involving the middle and inner retinal layers, can predict long-term visual acuity outcomes in eyes with CRVO or HRVO after treatment of macular edema with anti–vascular endothelial growth factor (anti-VEGF) therapy.
METHODS
STUDY PARTICIPANTS:
This is a post hoc secondary analysis of participants from a multicenter, prospective, randomized noninferiority trial (Study of COmparative Treatments for REtinal Vein Occlusion 2, SCORE 2, Clinicaltrials.gov identifier NCT01969708) of eyes with macular edema secondary to CRVO or HRVO comparing intravitreal bevacizumab and aflibercept. The study was approved by the Institutional Review Boards associated with each center or the central Institutional Review Board. All participants provided written informed consent. The study adhered to the tenets of the Declaration of Helsinki.
The SCORE2 design and methods have been described in detail.27 The main inclusion criteria included center-involved macular edema, defined as central subfield thickness on OCT (CST) ≥300 μm (or ≥320 μm if measured on a Heidelberg Spectralis OCT), and a visual acuity letter score (VALS) between 19 and 73 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (∼20/400–20/40). Between September 17, 2014, and November 18, 2015, a total of 362 participants were randomized to receive intravitreal bevacizumab or aflibercept. Participants were excluded from the secondary analysis if an OCT image was unavailable or if the quality of the image precluded adequate grading. Study visits were conducted per protocol with treatment provided per protocol from baseline through month 12 (M12) and then at the discretion of the investigator thereafter. Study visits were conducted monthly from baseline to month 06 (M06). Between M06 and M12, study visits were performed, per protocol, on a variable schedule depending on response to treatment and secondary randomization. After M12, participants were followed off-protocol; study examinations were performed at months 24 (M24), 36 (M36), 48 (M48), and 60 (M60).
SPECTRAL DOMAIN–OCT ACQUISITION AND DATA COLLECTION:
All spectral domain (SD)–OCT volume scans were acquired by study-certified photographers according to the imaging protocol approved by the SCORE2 reading center (Wisconsin Reading Center, University of Wisconsin–Madison) and using the Carl Zeiss Meditec Cirrus (Carl Zeiss Meditec) or Heidelberg Spectralis (Spectralis Heidelberg Engineering) OCT machine. The Zeiss macular volume scans included 6-mm B scans and comprised 512 A-scans and 128 B-scans, and the Heidelberg scans were 20 × 20 degrees and comprised 512 A-scans and 97 B-scans. The VALS was recorded according to the electronic ETDRS protocol at study visits. Demographic data were collected through review of medical records.
SD-OCT ANALYSIS AND GRADING SCHEME:
Central subfoveal OCT B-scans from participants at the baseline and 1-month (M01) follow-up visits were analyzed by 2 independent graders (A.A., D.S.) who were masked to all additional participant data. Images were deemed ungradable if the retinal lamination was not visible on OCT because of significant macular edema or low image quality. Discrepancies in grading were adjudicated after discussion between the 2 graders. The grading scheme for infarction was developed based on our existing knowledge of the ischemic cascade and its relationship to the organization of the retinal capillary plexuses.1
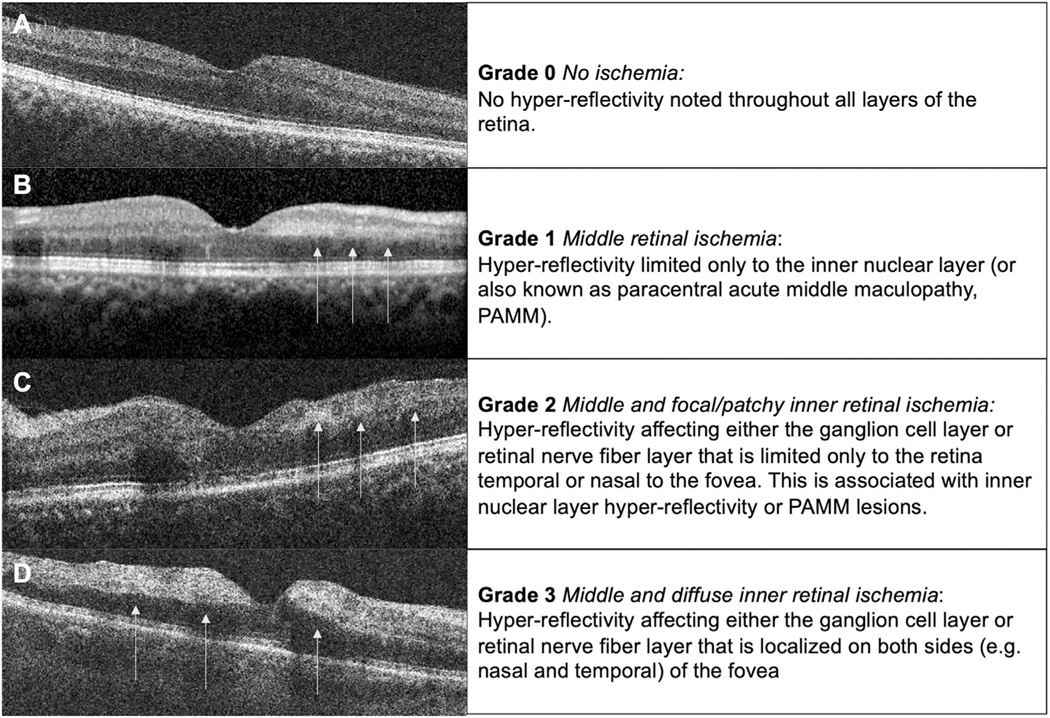
A description of the OCT grading scheme for macular infarction used in this analysis is as follows. The OCT grading scheme includes 4 grades of infarction severity from grade 0 to grade 3, with higher grades denoting increasing severity of infarction (Figure 1). Grade 0 is defined by an OCT macula without any evidence of abnormal hyper-reflectivity or acute infarction of the middle or inner retinal layers (Figure 1, A). Grade 1 macular infarction is defined by the presence of only middle macular infarction with hyper-reflectivity limited only to the inner nuclear layer (INL); this lesion pattern is known as paracentral acute middle maculopathy (PAMM) (Figure 1, B). Grade 2 macular infarction is defined by the presence of middle (ie, INL) retinal layer hyper-reflectivity associated with areas of focal/patchy inner macular infarction (ie, hyper-reflectivity within the ganglion cell layer [GCL] and retinal nerve fiber layer [RNFL]) but limited only to the retina either temporal or nasal to the fovea (Figure 1, C). Grade 3 macular infarction is associated with both middle and diffuse inner macular infarction and hyper-reflectivity present on both sides (eg, nasal and temporal) of the fovea (Figure 1, D).
FIGURE 1.
Grading scheme of macular infarction illustrated with optical coherence tomography (OCT). The infarction grading scale was developed based on the infarction cascade and the predominant vertical organization of the retinal capillary plexus.1 Briefly, the retinal capillary plexus is proposed to be a predominantly in serial organization in which the deep retinal capillary plexus is the primary venous outflow track. In mild retinal vascular occlusion, infarction may first be detected in the retinal layer closest to the venous pole where oxygen tension is lowest. This manifests as inner nuclear layer (INL) hyper-reflectivity on OCT and is commonly described as paracentral acute middle maculopathy. With more severe vascular occlusion, infarction progresses anterior to involve the inner retinal layer, that is, the ganglion cell layer (GCL) and retinal nerve fiber layer (RNFL), which are supplied by the superficial retinal capillary plexus. Our OCT grading scheme of macular infarction is therefore as follows. (A) Grade 0 is defined by the absence of macular infarction without any hyper-reflectivity detected on OCT. (B) Grade 1 is defined by isolated hyper-reflectivity to the middle retinal layer, for example, paracentral acute middle maculopathy (PAMM) with hyper-reflectivity limited only to the INL on OCT (arrows). (C) Grade 2 macular infarction is defined by middle retinal hyper-reflectivity and infarction associated with focal or patchy inner retinal infarction and hyper-reflectivity noted in the GCL and RNFL (arrows) and limited to 1 side of the fovea (ie, nasal or temporal). (D) Grade 3 is defined by middle and diffuse inner macular infarction with hyper-reflectivity of GCL and RNFL on both sides of the fovea (arrows) associated with middle layer hyper-reflectivity on OCT.
Non-perfusion was measured within the ETDRS and Networc grid, and foveal avascular zone (FAZ) integrity was also measured, in the available 94 patients with FA at baseline.28 If the quality of the image prevented analysis, the patient was not included. The FA non-perfusion measurements were measured on either a superimposed ETDRS grid (28.3 mm2) over the macula placed on the central 35 degrees or a superimposed Networc grid (860 mm2) that incorporated most of the visible retina.28 FAZ integrity was based on the greatest linear dimension (GLD) from 1 edge of the fovea to the other edge (μm). Intact FAZ showed no evidence of FAZ enlargement with a GLD of 500 μm. An abnormally enlarged FAZ was separated into 3 groups based on the GLD: group 1, <900 μm; group 2, <1800 μm but >900 μm; group 3, >1800 μm.
STATISTICAL ANALYSIS:
Statistical analyses were performed using R version 3.5.0 (www.r-project.org). Interval variables were reported as means and standard deviations (VALS, change in VALS, CST, number of anti-VEGF injections). The χ2 test was performed to compare categorical variables (sex, history of anemia, anemia at presentation, history of renal disease, renal disease at presentation, FAZ integrity). The Shapiro–Wilk normality test showed a non-parametric distribution for all interval variables. Therefore, the Kruskal–Wallis analysis of variance and post hoc pairwise Wilcoxon rank sum tested were performed comparing interval variables (VALS, change in VALS, CST, number of anti-VEGF injections) among the 4 infarction grades. Inter-rater reliability correlation kappa was used to determine the consistency of infarction grade measurements between D.S. and A.A. The Pearson correlation and Kendall correlation were performed to correlate VALS and age and non-perfusion within ETDRS and Networc grid based on infarction gradings.28 Non-perfusion within ETDRS and Networc grid and FAZ integrity were analyzed by Kendall rank correlation. A P value <.05 was considered statistically significant, except when Bonferroni correction was performed for both of the analyses shown in the Table (P < .008; 0.05/6). The Bonferroni correction of 6 was based on the total possible variations of comparison between grade 0 and grade 3. Where any post hoc pairwise Wilcoxon rank sum test was performed, Bonferroni correction was applied.
TABLE.
Comparison Between Number of Anti-VEGF Injections Between Ischemia Groups
| Follow-up Interval (Months) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Group | 0–6 | 6–12 | 12–24 | 24–36 | 36–48 | 48–60 |
| Total | 5.9 ± 0.3 | 4.9 ± 1.6 | 3.9 ± 3.3 | 3.8 ± 3.3 | 3.2 ± 3.5 | 2.4 ± 3.5 |
| 0 (n = 46) | 5.9 ± 0.4 | 4.8 ± 1.3 | 3.6 ± 3.3 | 3.2 ± 2.9 | 3.3 ± 3.3 | 1.8 ± 2.9 |
| 1 (n = 5) | 6.0 ± 0.0 | 4.8 ± 1.6 | 2.6 ± 3.7 | 1.8 ± 2.5 | 2.2 ± 3.2 | 2.2 ± 3.5 |
| 2 (n = 189) | 5.9 ± 1.0 | 5.0 ± 2.0 | 3.9 ± 3.3 | 3.9 ± 3.4 | 3.3 ± 3.6 | 2.8 ± 3.8 |
| 3 (n = 70) | 5.9 ± 1.4 | 4.9 ± 2.2 | 4.1 ± 3.4 | 3.9 ± 3.4 | 2.8 ± 3.7 | 1.6 ± 2.9 |
| P value | .79 | .60 | .74 | .43 | .74 | .31 |
Anti-VEGF = anti–vascular endothelial growth factor.
Values are reported in mean ± SD.
RESULTS
All 362 SCORE2 study participants were initially evaluated, and 52 participants were excluded because of an absent or ungradable M01 OCT for macular infarction (14.3%). Detailed demographic data of the SCORE2 population have been published previously.27 Of the 310 remaining participants whose M01 OCT of the central macula was graded, 46 were grade 0, 5 were grade 1, 189 were grade 2, and 70 were grade 3. Inter-rater reliability correlation kappa between the graders (A.A., D.S.) was 0.89 (95% CI = 0.87–0.91). There was no difference between patients in terms of age, sex, or concomitant history or diagnosis of systemic disease such as anemia (P ≥ .05) (Supplemental Table 1). Of note, there were fewer patients with a history of or diagnosis of renal disease at presentation in higher infarction grades compared to lower infarction grades (P = .02 and P = .01, respectively), but this is likely a spurious correlation related to small sample size.
VISUAL ACUITY OUTCOMES:
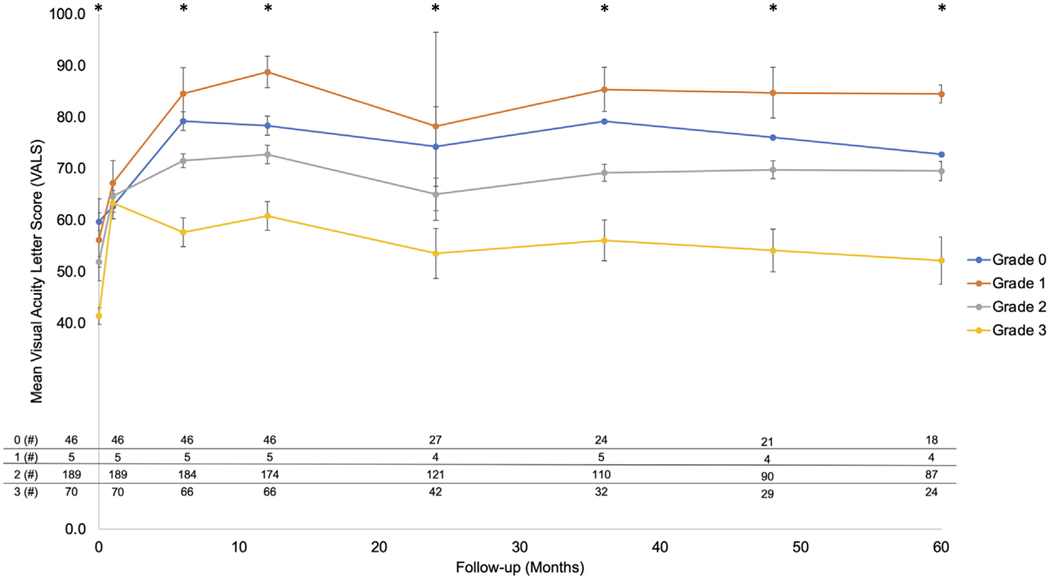
A summary of the VALS across grades over 5 years is detailed in Figure 2. Notably, the number of participants with available VALS is presented at the bottom of Figure 2. Of the 310 included participants, 291 (93.9%) at M12, 194 (62.6%) at M24, 171 (55.2%) at M36, 144 (46.5%) at M48, and 133 (42.9%) at M60 successfully completed follow-up.
FIGURE 2.
Visual acuity letter score (VALS) over time as correlated with the 4 optical coherence tomography (OCT) infarction grades. The mean VALS of the 4 infarction grades was graphed on the y-axis, with the follow-up interval on the x-axis. There is an initial increase in the visual acuity across all grades that correlates with resolution of macular edema, most notably at month 1 when VALS normalizes across all grades (P = .81). However, at baseline and every subsequent visit, higher grades of macular infarction, as assessed with OCT, are correlated with a graded and statistically significant decrease in VALS compared to other grades with less severe macular infarction on OCT (P < .001). Error bars represent standard error of the mean. The number of participants at baseline and at each follow-up visit (M01, M06, M12, M24, M36, M48, M60) are shown in the table at the bottom to the left or right of the Y-axis, respectively. Asterisks indicate statistically significant difference across infarction grades (P < .001).
Baseline (M00) VALS was significantly higher among grade 0 (59.7 ± 11.8) compared with grade 2 participants (51.9 ± 14.3, P < .008), and participants in grades 0, 1, and 2 exhibited a higher mean baseline (M00) VALS compared to participants in grade 3 (41.4 ± 14.0, P < .008). At M01 (when OCTs were graded), all 4 grades demonstrated a similar VALS with an overall mean of 64.1 ± 15, P = .88). At M06, grade 0 (79.2 ± 12.3) continued to exhibit a higher mean VALS compared to grade 2 (71.5 ± 18.5, P < .008), whereas all grades (84.6 ± 11.3) except grade 1 VALS exhibited a significantly higher mean VALS than grade 3 (57.7 ± 23.2, P < .008). At M12, all grades demonstrated a significantly higher mean VALS than grade 3 (60.8 ± 23.1, P < .008). Grades 0 and 2 continued to have a higher VALS compared to grade 3 at M24, M36, and M48 (P < .008), whereas there were insufficient participants in the grade 1 cohort to meet statistical significance. At M60, although there was a trend toward higher VALS in lower grades, only grade 2 (69.5 ± 18.1) showed a significantly higher VALS than grade 3 (52.2 ± 22.5, P < .008), likely because of substantial attrition in the grade 0 cohort.
Infarction grading was correlated with VALS at M12 (R = –0.37, P < .001), M24 (R = –0.35, P < .001), M36 (R = –0.44, P < .001), M48 (R = –0.40, P < .001), and M60 (R = –0.36, P < .001).
Univariate regression modeling confirmed that the M01 infarction grade was predictive of VALS at M12 (β = 0.14, P < .001), M24 (β = 0.08, P < .001), M36 (β = 0.15, P < .001), M48 (β = 0.14, P < .001), and M60 (β = 0.11, P < .001).
To reduce the risk of outlier bias, change in VALS at each follow-up time point compared to baseline was calculated. Comparison of the change in VALS across all grades at M00 and M06, M12, M24, M36, M48, M60 revealed no differences (P > .23) (Supplemental Table 2).
RETINAL THICKNESS OUTCOMES:
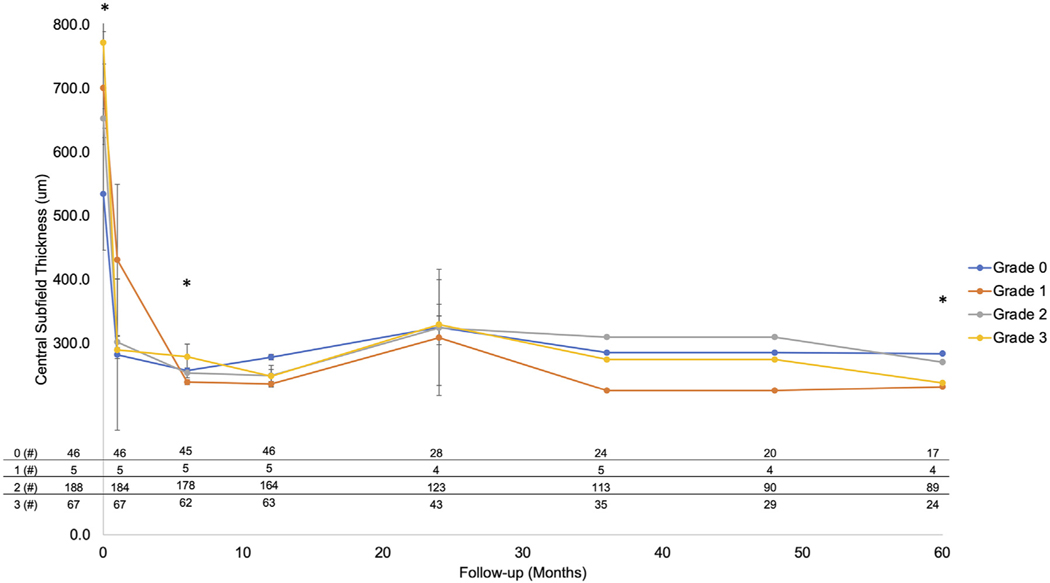
A summary of CST across grades over 5 years is shown in Figure 3. Again, the number of participants with available CST is presented at the bottom of Figure 3. Of the 310 participants with a gradable OCT at baseline, CST was available in 306 (98.7%) at M00, 302 (97.4%) at M01, 290 (93.5%) at M06, 278 (89.7%) at M12, 198 (63.9%) at M24, 177 (57.1%) at M36, 143 (46.1%) at M48, and 134 (43.2%) at M60. At the M00 visit, a larger CST was correlated with increasing grades of infarction severity as measured at M01, with a statistically significant increase in CST comparing grade 0 (534.5 ± 172.5) vs grade 2 (652.9 ± 215.5, P = .003) and comparing grade 0 and grade 2 vs grade 3 (771.5 ± 278.3, P < .002). At M12, grade 0 exhibited a significantly greater CST (278.5 ± 118.3) compared to grade 2 (249.6 ± 125.8) and grade 3 (248.7 ± 138.3) (P < .003). At all other time points, there was no significant difference in CST among the infarction grades (P > .05).
FIGURE 3.
Central subfield thickness (CST) over time as correlated with the 4 optical coherence tomography (OCT) infarction grades. The mean CST of the 4 infarction grades was graphed on the y-axis with the follow-up interval on the x-axis. There is a statistically significant increase in CST in the grades with greater macular infarction as graded with OCT (eg, grades 2 and 3) at baseline. However, after the first anti–vascular endothelial growth factor (anti-VEGF) injection, CST decreases dramatically in all 4 grades and is equivalent at all additional follow-up visits (P > .14) across all grades. Error bars represent standard error of the mean. The number of participants at baseline and at each follow-up visit (M01, M06, M12, M24, M36, M48, M60) are shown in the table at the bottom to the left or right of the Y-axis, respectively. Asterisks indicate statistically significant difference across infarction grades (P < .001).
NUMBER OF ANTI-VEGF INJECTIONS:
The number of anti-VEGF injections received between 2 timepoints are summarized in Table 1. Across all grades, the mean number of anti-VEGF injections received was as follows: M00 to M06 was 5.9 ± 0.3, M06 to M12 was 4.9 ± 1.6, M12 to M24 was 3.9 ± 3.3, M24 to M36 was 3.8 ± 3.3, M36 to M48 was 3.2 ± 3.5, and M48 to M60 was 2.4 ± 3.5. Despite a gradual decrease in the number of anti-VEGF injections over time, at no time point was there a significant difference among OCT infarction grades in terms of the number of injections (P > .31).
Of note, we found a statistically significant but low correlation between OCT macular infarction and non-perfusion on macular ETDRS (R= 0.26, P = .03, Tau-B = 0.29, Tau-B P = .006) and peripheral Networc grid (R= 0.26, P = .03, Tau-B = 0.24, Tau-B P = .02) (Supplemental Table 3). In addition, increasing grades of infarction severity correlated with a greater degree of disrupted FAZ integrity (Tau-B = 0.22, P = .06) (Supplemental Table 3).
DISCUSSION
In this post hoc analysis of SCORE2 data, we define an OCT grading system of macular infarction, based on the level and extent of retinal layer hyper-reflectivity, to stratify disease severity and to predict visual acuity in participants treated with anti-VEGF for macular edema secondary to CRVO or HRVO. We graded OCT images at M01 after the baseline visit (ie, 1 month after the first anti-VEGF injection) because the presence of significant edema and hemorrhage at baseline typically limits OCT grading of macular infarction. We found that more severe OCT grades of macular infarction (ie, grades 2 and 3 with both middle and inner retinal layer hyper-reflectivity) at M01 were associated with lower (ie, poorer) VALS at baseline (M00) and were predictive of lower VALS at M06-, M12-, M24-, M36-, M48-, and M60-month follow-up. However, all infarction grades achieved similar gains from baseline (M00) in VALS without a difference in the number of anti-VEGF injections given in each grade. Although macular edema assessed by CST was greatest in the grades with more severe OCT graded macular infarction at M00, there was no difference in macular thickness at any time point after anti-VEGF therapy was started (ie, there was a large reduction in all infarction grades). This suggests that macular infarction, as graded with OCT at month 01, might be the key factor (not CST or some other biomarker) driving vision and can be measured to help predict visual acuity outcomes.
It is true that retinal non-perfusion as assessed with dye-based fluorescein angiography (FA) can predict visual outcomes.29–31 In the Central Vein Occlusion Study (CVOS), the presence of ischemic CRVO, defined as 10 disc areas of non-perfusion, or macular non-perfusion with an enlarged foveal avascular zone as graded with FA, were correlated with worse visual acuity outcomes.32 However, FA, unlike OCT, is invasive, is time consuming, and does not provide depth resolution. These FA studies, therefore, were unable to capture participants with mild CRVO, such as those with isolated PAMM, as FA cannot accurately identify eyes with only deep retinal capillary non-perfusion causing isolated middle retinal layer infarction.33 In addition, FA identifies non-perfusion or absence of blood flow to retinal tissue, whereas OCT captures infarction, or damage to the retinal tissue as a consequence of non-perfusion. Three studies have since incorporated OCT grading systems of macular infarction to evaluate long-term visual acuity outcomes; however, all of these analyses were limited by small sample sizes.34–36 Two of these studies developed and used a semi-automated algorithm to measure ganglion cell layer (GCL) reflectivity and found that the heterogeneity of reflectivity within the GCL identified at M01 was predictive of visual acuity at year 01.35,36 The current analysis is unique, however, in that the OCT macular infarction grading scale that we have developed is uncomplicated and practical and can easily be implemented and applied by clinicians, without the use of an automated algorithm or other complex methodology. In addition, the current analysis used a large cohort of eyes followed up to 5 years and included a standardized protocol to record visual acuity as well as certified technicians to acquire the OCT images.
Other OCT biomarkers may be predictive of visual acuity in CRVO and HRVO. These include intraretinal hyper-reflective foci, DRIL, EZ or ELM integrity, and choroidal thickness.12–29 Of these, EZ integrity was reported as predictive of visual acuity outcomes in 2 clinical trial cohorts (SCORE2 and LEAVO). Specifically, Sen et al reported that participants with intact EZ exhibited a best-corrected VALS >70 at 100 weeks, and Etheridge et al reported that absence of the EZ inside the central subfield identified at month 1 was predictive of worse 2 year VALS in multivariate analysis including DRIL.13,15 Although the integrity of the EZ is an important biomarker for visual potential, EZ loss is not a direct measure of macular infarction. Furthermore, 35.0% of images were ungradable for EZ assessment at M01 as compared to an ungradable rate of 14.3% using our OCT classification system in this study.15 It is likely that the high ungradable rate for EZ assessment in eyes with CRVO or HRVO will be common in future datasets because of the inherent nature of CRVO and HRVO that are associated with significant retinal edema and hemorrhage rendering the EZ difficult to assess. In contrast, OCT hyper-reflectivity provides a predictor specifically related to underlying macular infarction and can be detected even in scenarios in which the EZ integrity is difficult to assess. Moreover, severe macular infarction as detected with OCT at M01 can be present even in the presence of an intact EZ band.
STRENGTHS AND LIMITATIONS:
Strengths of our study include analysis of a carefully characterized cohort from a large multicenter clinical trial with standardized OCT parameters and treatment protocols. The injection regimen after 1 year represents real-world treatment and outcomes. Limitations include a substantial decrease in participant retention after 2 years. In addition, the clinical trial required macular edema for enrollment, and therefore results from the current analysis can only be extrapolated to such eyes. The cohort comprising participants with grade 1 macular infarction (ie, isolated PAMM-only lesions) was, in fact, very small, and this limited the power of our analysis. It is very likely, however, that cases with only PAMM and no evidence of macular edema represent the mildest forms of retinal vein occlusion with the best visual outcomes. In addition, our proposed OCT-based system of macular infarction requires grading at month 01 when macular edema is improved or resolved and does not confound analysis of hyper-reflectivity. Finally, no study has directly correlated OCT infarction and FA non-perfusion, in part because of the poor visualization of the deep capillary plexus by FA.33 In addition, proper co-localization of FA non-perfusion and OCT infarction would require en face OCT, which was not performed in the initial clinical trial. Although we found a correlation between OCT macular infarction grading with non-perfusion graded within the ETDRS and Networc grid and integrity of the foveal avascular zone (unpublished data), further validation studies are necessary.28
CONCLUSIONS:
Our study supports a practical OCT grading system of macular infarction, based on the anatomic level and extent of retinal layer hyper-reflectivity, to predict visual acuity in eyes with CRVO or HRVO. Specifically, eyes with worse macular infarction, as represented by hyper-reflectivity within the middle and inner retina on OCT at month 1, are more likely to have worse baseline visual acuity and worse long-term visual outcomes. This OCT grading scheme can therefore be used to predict long-term visual outcomes, and indicates that a key factor with an impact on long-term visual acuity may be the level of macular infarction after retinal fluid is treated with anti-VEGF.
Supplementary Material
Funding/Support:
This work was supported by the National Eye Institute (National Institutes of Health, Department of Health and Human Services) grants U10EY023529, U10EY023533, and U10EY023521. Support also provided in part by Regeneron, Inc and Allergan, Inc through donation of investigational drug. This work was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. to the University of Wisconsin Madison Department of Ophthalmology and Visual Sciences and to the Stein Eye Institute (DS) and Doheny Eye Institute, Department of Ophthalmology at the University of California Los Angeles.
Footnotes
Financial Disclosures: All authors report no financial disclosures or conflicts of interest. All authors attest that they meet the current ICMJE criteria for authorship.
Supplemental Material available at AJO.com.
REFERENCES
- 1.Bakhoum MF, Freund KB, Dolz-Marco R, et al. Paracentral acute middle maculopathy and the ischemic cascade associated with retinal vascular occlusion. Am J Ophthalmol. 2018;195:143–153. doi: 10.1016/j.ajo.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Willoughby AS, Vuong VS, Cunefare D, et al. Choroidal changes after suprachoroidal injection of triamcinolone acetonide in eyes with macular edema secondary to retinal vein occlusion. Am J Ophthalmol. 2018;186:144–151. doi: 10.1016/j.ajo.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rayess N, Rahimy E, Ying G, et al. Baseline choroidal thickness as a predictor for treatment outcomes in central retinal vein occlusion. Am J Ophthalmol. 2016;171:47–52. doi: 10.1016/j.ajo.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Berry D, Thomas AS, Fekrat S, Grewal DS. Association of disorganization of retinal inner layers with ischemic index and visual acuity in central retinal vein occlusion. Ophthalmol Retin. 2018;2(11):1125–1132. doi: 10.1016/j.oret.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babiuch AS, Han M, Conti FF, Wai K, Silva FQ, Singh RP. Association of disorganization of retinal inner layers with visual acuity response to anti–vascular endothelial growth factor therapy for macular edema secondary to retinal vein occlusion. JAMA Ophthalmol. 2019;137(1):38. doi: 10.1001/jamaophthalmol.2018.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimouni M, Segev O, Dori D, Geffen N, Flores V, Segal O. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol. 2017;182:160–167. doi: 10.1016/j.ajo.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Chan EW, Eldeeb M, Sun V, et al. Disorganization of retinal inner layers and ellipsoid zone disruption predict visual outcomes in central retinal vein occlusion. Ophthalmol Retin. 2019;3(1):83–92 Published online. doi: 10.1016/j.oret.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Cui Y, Vingopoulos F, et al. Disorganisation of retinal inner layers is associated with reduced contrast sensitivity in retinal vein occlusion. Br J Ophthalmol. 2022;106(2):241–245. doi: 10.1136/bjophthalmol-2020-317615. [DOI] [PubMed] [Google Scholar]
- 9.Mo B, Zhou HY, Jiao X, Zhang F. Evaluation of hyperreflective foci as a prognostic factor of visual outcome in retinal vein occlusion. Int J Ophthalmol. 2017;10(4):605–612 Published online. doi: 10.18240/ijo.2017.04.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang HS, Chae JB, Kim JY, Kim DY. Association between hyperreflective dots on spectral-domain optical coherence tomography in macular edema and response to treatment. Invest Ophthalmol Vis Sci. 2017;58(13):5958–5967 Published online. doi: 10.1167/iovs.17-22725. [DOI] [PubMed] [Google Scholar]
- 11.Kang J-W, Chung H, Chan Kim H. Correlation of optical coherence tomographic hyperreflective foci with visual outcomes in different patterns of diabetic macular edema. Retina. 2016;36(9):1630–1639. doi: 10.1097/IAE.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 12.Hayreh SS, Zimmerman MB. Fundus changes in central retinal vein occlusion. Retina. 2015;35(1):29–42 Published online. doi: 10.1097/IAE.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen P, Gurudas S, Ramu J, et al. Predictors of visual acuity outcomes after anti–vascular endothelial growth factor treatment for macular edema secondary to central retinal vein occlusion. Ophthalmol Retin. 2021;5(11):1115–1124. doi: 10.1016/j.oret.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto M, Yamashita M, Sakamoto T, Ogata N. Choroidal blood flow and thickness as predictors for response to anti-vascular endothelial growth factor therapy in macular edema secondary to branch retinal vein occlusion. Retina. 2018;38(3):550–558 Published online. doi: 10.1097/IAE.0000000000001566. [DOI] [PubMed] [Google Scholar]
- 15.Etheridge T, Blodi B, Oden N, et al. Spectral domain OCT predictors of visual acuity in the Study of COmparative Treatments for REtinal Vein Occlusion 2: SCORE 2 report 15. Ophthalmol Retin. 2021;5(10):991–998. doi: 10.1016/j.oret.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lent-Schochet D, Avaylon J, Nguyen M, et al. OCT predictors of treatment discontinuation in eyes with retinal vein occlusion and macular edema. Ophthalmol Retin. 2022;7(5):462–464 Published online. doi: 10.1016/j.oret.2022.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Moon BG, Cho AR, Kim YN, Kim J-G. Predictors of refractory macular edema after branch retinal vein occlusion following intravitreal bevacizumab. Retina. 2018;38(6):1166–1174. doi: 10.1097/IAE.0000000000001674. [DOI] [PubMed] [Google Scholar]
- 18.Chatziralli I, Theodossiadis G, Chatzirallis A, Parikakis E, Mitropoulos P, Theodossiadis P. Ranibizumab for retinal vein occlusion. Retina. 2018;38(3):559–568. doi: 10.1097/IAE.0000000000001579. [DOI] [PubMed] [Google Scholar]
- 19.Banaee T, Singh RP, Champ K, et al. Ellipsoid zone mapping parameters in retinal venous occlusive disease with associated macular edema. Ophthalmol Retin. 2018;2(8):836–841. doi: 10.1016/j.oret.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitamura Y, Fujihara-Mino A, Inomoto N, Sano H, Akaiwa K, Semba K. Optical coherence tomography parameters predictive of visual outcome after anti-VEGF therapy for retinal vein occlusion. Clin Ophthalmol. 2016;10:1305–1313. doi: 10.2147/OPTH.S110793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balaratnasingam C, Inoue M, Ahn S, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123(11):2352–2367 Published online. doi: 10.1016/j.ophtha.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Yiu G, Welch RJ, Wang Y, Wang Z, Wang PW, Haskova Z. Spectral-domain OCT predictors of visual outcomes after ranibizumab treatment for macular edema resulting from retinal vein occlusion. Ophthalmol Retin. 2020;4(1):67–76 Published online. doi: 10.1016/j.oret.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etheridge T, Dobson ETA, Wiedenmann M, et al. A semi-automated machine-learning based workflow for ellipsoid zone analysis in eyes with macular edema: SCORE2 pilot study. PLoS One. 2020;15(4):e0232494. doi: 10.1371/journal.pone.0232494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Călugăru D, Călugăru M. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol. 2017;184:190–191. doi: 10.1016/j.ajo.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Eldeeb M, Chan EW, Sun V, Chen JC. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol. 2018;186:167–168. doi: 10.1016/j.ajo.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt-Erfurth U, Michl M. Disorganization of retinal inner layers and the importance of setting boundaries. JAMA Ophthalmol. 2019;137(1):46. doi: 10.1001/jamaophthalmol.2018.4516. [DOI] [PubMed] [Google Scholar]
- 27.Scott IU, VanVeldhuisen PC, Ip MS, et al. SCORE2 Report 2. Ophthalmology. 2017;124(2):245–256. doi: 10.1016/j.ophtha.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacCormick IJC, Maude RJ, Beare NAV, et al. Grading fluorescein angiograms in malarial retinopathy. Malar J. 2015;14(1):367. doi: 10.1186/s12936-015-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima VC, Yeung L, Castro LC, Landa G, Rosen RB. Correlation between spectral domain optical coherence tomography findings and visual outcomes in central retinal vein occlusion. Clin Ophthalmol. 2011;5:299–305. doi: 10.2147/OPTH.S16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laatikainen L, Kohner EM. Fluorescein angiography and its prognostic significance in central retinal vein occlusion. Br J Ophthalmol. 1976;60(6):411–418. doi: 10.1136/bjo.60.6.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daien V, Navarre S, Fesler P, Vergely L, Villain M, Schneider C. Visual acuity outcome and predictive factors after bevacizumab for central retinal vein occlusion. Eur J Ophthalmol. 2012;22(6):1013–1018. doi: 10.5301/ejo.5000162. [DOI] [PubMed] [Google Scholar]
- 32.Central Vein Occlusion Study GroupA randomized clinical trial of early panretinal photocoagulation for ischermic central vein occlusion: Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102(10):1443–1444 Published online. doi: 10.1016/S0161-6420(95)30848-2. [DOI] [PubMed] [Google Scholar]
- 33.Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis). J Neurosci. 1992;12(4):1169–1193 Published online. doi: 10.1523/JNEUROSCI.12-04-01169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Browning DJ, Punjabi OS, Lee C. Assessment of ischemia in acute central retinal vein occlusion from inner retinal reflectivity on spectral domain optical coherence tomography. Clin Ophthalmol. 2017;11:71–79. doi: 10.2147/OPTH.S122683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta N, Lavinsky F, Gattoussi S, et al. Increased inner retinal layer reflectivity in eyes with acute CRVO correlates with worse visual outcomes at 12 months. Invest Opthalmol Vis Sci. 2018;59(8):3503. doi: 10.1167/iovs.18-24153. [DOI] [PubMed] [Google Scholar]
- 36.Greenlee TE, Cutler NE, Mehta N, et al. Inner retinal layer reflectivity as predictor of retinal vein occlusion visual acuity outcomes. Ophthalmol Retin. 2020;4(3):343–344. doi: 10.1016/j.oret.2019.09.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.