Abstract
Anti-NMDA receptor (NMDAR) encephalitis is characterized by a well-defined neuropsychiatric syndrome and CSF antibodies against the GluN1 subunit of the NMDAR. 40% of cases are related to underlying tumors, the vast majority ovarian teratomas (94%). We report a case of anti-NMDAR encephalitis associated with renal cell carcinoma (RCC). A 20-year-old female presented to the ED with behavioral changes, involuntary movements, tachycardia, and alternating obtundation with agitation which progressed over 3 weeks. Involuntary movements were severe, requiring intubation and sedation for control, and were accompanied by rhabdomyolysis. Brain MRI showed bilateral mesiotemporal T2/FLAIR hyperintensities. Anti-NMDAR antibodies were present in the serum (1:640) and CSF (1:320). Malignancy screening revealed a renal mass concerning for RCC, which was confirmed upon resection. She was started on high dose IV methylprednisolone and plasmapheresis, followed by rituximab. Lack of response led to escalating immunotherapy with cyclophosphamide. Clinical course was complicated by prolonged ICU admission, prolonged sedation, severe dysautonomia and bacteremia. Improvement began 2 months after immunotherapy, and she was discharged to rehabilitation 100 days after admission with mild neuropsychiatric symptoms. Repeat malignancy screenings, including whole-body imaging and transvaginal ultrasound were consistently negative. Herein, we describe a case of definite anti-NMDAR encephalitis in the setting of newly diagnosed RCC. This case illustrates how tumors other than ovarian teratomas may act as immunological triggers, as well as the complex and prolonged symptomatic and immunosuppressive therapies required in severe presentations of anti-NMDAR encephalitis.
Keywords: anti-NMDAR encephalitis, autoimmune encephalitis, renal cell carcinoma, case report
Introduction
Anti-N-Methyl-D-Aspartate receptor (NMDAR) encephalitis is characterized by a distinct syndrome of progressive psychiatric symptoms (delusions, hallucinations agitation), with concomitant or subsequent neurological manifestations (disorganized speech, movement disorders, seizures, disorders of consciousness, dysautonomia) accompanied by antibodies against the GluN1 subunit of the NMDAR in the cerebrospinal fluid (CSF). Although originally described in young women with ovarian teratomas, it is now well described in children, men, and those without an underlying teratoma.1,2 Pediatric patients often present with neurological signs such as abnormal movements, while adults tend to manifest psychiatric symptoms first. 40% have an underlying malignancy, most of which are ovarian teratomas. Other tumors have been reported, including lung, breast, and testicular carcinomas and rare instances of renal cell carcinoma (RCC).3-5 Diagnostic criteria have been proposed and depend on clinical presentation and antibody detection in the CSF. Mainstays of therapy are immunotherapy and removal of immunological trigger when identified. Though most patients respond to treatment, recovery times can be months to years.6,7 We report a case of definite anti-NMDAR encephalitis with an underlying RCC. In doing so, we illustrate challenges encountered in the management of these patients, such as symptomatic management, use and timing of immunotherapies, and management during protracted recovery time. Despite these challenges, most treated patients have good outcomes, with more than 80% achieving functional autonomy in 2 years. 6
Case Description
A 20-year-old African American woman with no significant medical history was transferred to our emergency department (ED) for behavioral changes and involuntary movements. Two weeks prior to transfer, she was agitated with ‘tics’ and ‘seizure-like’ movements and received initial diagnoses of anxiety and non-epileptic seizures for which she was prescribed lorazepam and discharged. Over the following week, symptoms progressed with agitation, confusion, insomnia, abnormal movements, and hyperventilation leading to repeat ED evaluation and transfer. On arrival, she was profoundly agitated with generalized choreiform movements. She was hypertensive, tachycardic and tachypneic. Initial work up showed respiratory alkalosis and creatine kinase of >40,000 IU/L. Primary psychiatric etiology was suspected prior to neurology consultation, but she failed to respond to various sedatives and neuroleptics. Neurology consultation yielded concern for anti-NMDAR encephalitis. The patient was transferred to ICU for refractory agitation and worsening rhabdomyolysis, where she was intubated, paralyzed, and sedated.
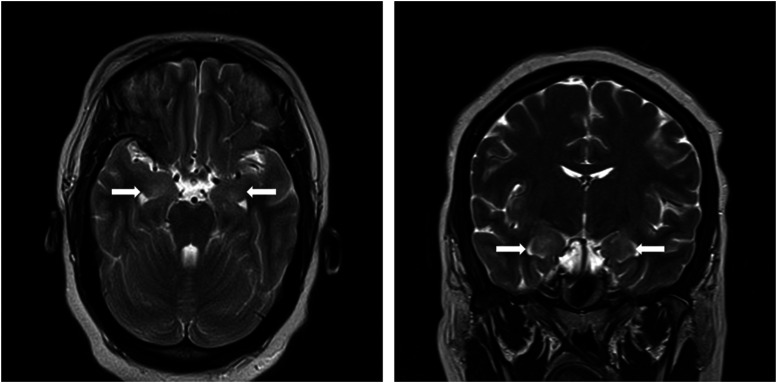
Continuous electroencephalogram monitoring showed diffuse background slowing without epileptiform discharges or seizure. MRI showed asymmetric bitemporal T2 hyperintensities without contrast enhancement (Figure 1). CSF biochemistry and cellularity were unremarkable (no nucleated cells, 2 red cells, protein 21 mg/100 mL, glucose 72 mg/100 mL), infectious meningoencephalitis panel (including HSV PCR) was negative, and while autoimmune encephalopathy panels were pending, she was empirically started on concurrent high-dose methylprednisolone and plasmapheresis. Anti-NMDAR antibodies resulted at 1:320 in the CSF and 1:640 in the serum. Malignancy screening with CT chest, abdomen and pelvis revealed left superior pole renal mass concerning for RCC. There was no evidence of metastatic disease. She underwent total left nephrectomy without complications. Pathology confirmed RCC, WHO/ISUP grade 3. Repeat CT abdomen and pelvis, as well as multiple transvaginal ultrasounds did not show an ovarian teratoma. Initial immunotherapy with steroids and plasmapheresis was followed by 4 weekly doses of rituximab 375 mg/m2. 6 Symptoms remained refractory 4 weeks after initiating rituximab, and immunotherapy was escalated with pulsed IV cyclophosphamide, with proposed five 600 mg/m2 cycles in a 10-day period. The first four doses were well tolerated, but the fifth was withheld due to development of staphylococcal bacteremia.
Figure 1.
Brain MRI showing bilateral asymmetric (right greater than left) mesiotemporal T2 hyperintensities, involving the amygdala (white arrows), on axial and coronal views.
She had a prolonged ICU admission with significant complications. Dysautonomia with sinus pauses and asystole required percutaneous pacemaker placement. Immunosuppression was complicated by multiple infections. Agitation and involuntary movements were refractory to multiple agents, including ketamine, midazolam, and diphenhydramine, before responding to combination of olanzapine, clonazepam, trazodone and clonidine, weeks after initiation of immunotherapy.
Four weeks after cyclophosphamide initiation (8 weeks after rituximab start, 9 weeks after high dose steroids and plasmapheresis) she started to improve. She was extubated, weaned off sedatives and discharged to inpatient rehabilitation 100 days after admission.
At 4-month follow up (7 months from presentation) she was asymptomatic, with a normal exam and a Montreal Cognitive Assessment of 28/30. She discontinued all benzodiazepine medications and neuroleptics and resumed undergraduate studies.
Discussion
Tumor-Associated Anti-NMDA Receptor Encephalitis
We report a case of RCC acting as the likely immunological trigger and highlight several important aspects of anti-NMDAR encephalitis. Roughly 40% of patients have an associated tumor, of which more than 90% are ovarian teratomas. 6 The likelihood of underlying malignancy varies with age and sex, and is less frequent in male and pediatric populations. Despite the strong association with ovarian teratomas, other tumors may trigger anti-NMDAR encephalitis, including carcinomas of the lung, testis, breasts, and pancreas.6,8
RCC is an uncommon malignancy with an estimated incidence of 5.7/100,000 in African American women. 9 Median age is 64, with an extremely low prevalence below 20 years. 10 Between 10%-40% of RCC patients develop paraneoplastic syndromes, most commonly hypercalcemia, hypertension and polycythemia secondary to endocrine production by tumor cells. Less frequently, neurological dysimmune responses have been described, including autoimmune encephalopathies such as autoimmune cerebellar ataxia and limbic encephalitis. 11 Although we could not definitively prove the immunogenicity of the tumor, which would require specific immunohistochemical analysis, the presence of an immunogenic malignancy and absence of other identified trigger argues against coincidence.
Immunotherapy, Initial and Second Line Options
Although there are no completed randomized clinical trials in anti-NMDAR encephalitis to date, there is evidence immunotherapy is beneficial based on data from large retrospective cohorts and some prospective descriptive studies. High dose corticosteroids with or without plasmapheresis are considered first line therapy and subsequent treatment with rituximab may reduce relapse risk.6,12 Per published guidelines, newly diagnosed patients are treated with 5-day course of high dose methylprednisolone with concomitant plasmapheresis or intravenous immunoglobulins. This is often immediately followed by second-line therapy with rituximab, depending on severity of presentation and should be considered in all cases referactory to first-line therapies after 2 weeks. The NEOS score 13 (Figure 2), which has been validated to predict functional status 1 year from diagnosis, can be helpful in this setting. Response to rituximab begins 1-3 months after treatment. If at that point clinical improvement has not yet started, then additional second-line treatment with cyclophosphamide should be considered and/or escalation to tocilizumab. Commonly used rituximab regimens include 375 mg/m2, or, alternatively, two 1 g doses 2 weeks apart. Cyclophosphamide dosing varies significantly, most commonly being administered as 600-1000 mg/m2 in monthly doses.2,12 More aggressive induction regimens can be considered in carefully selected situations at the expense of higher infectious risk. Tocilizumab dosing is 12 mg/kg/dose (<30 kg) or 8 mg/kg/dose (≥ 30 kg) given monthly.
Figure 2.
The anti-NMDAR Encephalitis One-Year Functional Status (NEOS) score predicts functional outcome 1 year from presentation. Each item is attributed 1 point. 69% of patients with a score of 4 or 5 points present a modified Rankin Scale ≥3 in 1 year, compared to 3% of patients with zero or 1 point. Our patient had a score of 3, predicting a likely favorable functional outcome in 1 year, which was achieved.
There has been interest in novel immunotherapies for the treatment of rituximab refractory anti-NMDAR encephalitis, particularly tocilizumab. There have been case reports and one retrospective cohort of presumed autoimmune encephalitides which have suggested favorable outcomes when tocilizumab was used as rescue therapy for rituximab refractory cases. Most recently, a single center cohort showed improved 3-month and 1-year outcomes when tocilizumab was added to a regimen of tumor removal, steroids, immunoglobulins, and rituximab. A significant limitation was confounding by short time after rituximab administration prior to tocilizumab. Despite intrinsic limitations of these reports, tocilizumab can be considered as a third line agent in the absence of response to rituximab and cyclophosphamide.14,15
Randomized double-blind placebo-controlled clinical trials are underway in NMDA receptor encephalitis including the use of an anti-CD19 therapy, inebilizumab (ExTINGUISH, NCT04372615) and an anti-IL6 receptor blocking therapy, satralizumab (CIELO, NCT05503264). Recommendations regarding immunotherapy are summarized in Table 1. It is important to stress that removal of immunogenic trigger (frequently ovarian teratoma, in this case, RCC) is essential in the management of anti-NMDAR encephalitis. 6
Table 1.
Immunotherapies Offered as First and Second Lines for the Management of Anti-NMDAR Encephalitis. IVMP – Intravenous Methylprednisolone; IVIg – Intravenous Immunoglobulins.
| 1st Line | IVMP, Plasmapheresis, IVIg |
| 2nd line | Rituximab+/− cyclophosphamide |
| 3rd line | Tocilizumab |
Our patient continued to deteriorate despite steroids, plasmapheresis and rituximab and received cyclophosphamide 4 weeks after initiation of rituximab. The patient received an induction regimen with goal of faster therapeutic response, with an intended regimen of 5 courses of 600 mg/m2 over 10 days, however, the last dose was held due to sepsis. Risks and benefits must be carefully weighed when using cyclophosphamide and should include fertility considerations in young female patients in addition to infectious risks. Additionally, there has been increased interest in tocilizumab as an alternative escalation therapy due to a more favorable perceived safety profile. 12 Our patient’s severe presentation, refractory course, and life-threatening dysautonomia pushed the treating physicians to select a more aggressive cyclophosphamide regimen, as opposed to the more common pulsed monthly dosing of 600-1000mg/m2. We advocate caution with continued escalation of therapy and interventions considering the commonly prolonged recovery periods for these patients. Continued escalation with additional immunotherapies without allowing time for response to rituximab and cyclophosphamide has not shown clear benefit in our experience and incurs in additional risk. The same can be said of empirical oophorectomies, which carry lifelong consequences. Despite prolonged ICU course, our patient ultimately responded to immunotherapies. This illustrates how complicated, prolonged courses do not preclude good outcomes.
Symptomatic Therapies in the Acute Setting
This case illustrates many of the difficulties in symptomatic management. Severe agitation and involuntary movements led to rhabdomyolysis and ultimately required sedation and paralysis. Long-term control was only achieved after immunotherapy and with a combination of olanzapine, clonazepam, trazodone, and clonidine. Management of involuntary movements related to anti-NMDAR AE in the critical care setting has been reviewed. 18 We recommend empirical trials of anticholinergics, benzodiazepines, dopamine receptor blockers and beta blockers for managing hyperkinetic movements. Dysautonomia may limit the use of these medications, and adequate dosing may only be possible after pacemaker placement, as occurred with our patient. Severe refractory movements may require sedation with IV benzodiazepines, propofol and neuromuscular blockade. A proposed approach is outlined in Table 2. Though neuroleptic resistance is characteristic of NMDAR encephalitis, with a high incidence of neuroleptic malignant syndrome (NMS), 13 our patient presented rhabdomyolysis before anti-psychotic use. However, physicians should be vigilant for NMS, especially with increasing anti-psychotic doses. 16 , 17
Table 2.
Proposed Framework for the Symptomatic Management of Hyperkinetic Movement Disorders in Anti-NMDAR Encephalitis.
| Phenotype | Pharmacological Classes | Agents | Regimens |
|---|---|---|---|
| Tremor, myoclonus | Beta-blockers | Propranolol | 40-320 mg/day |
| Nadolol | 40-320 mg/day | ||
| Anticholinergics | Trihexyphenidyl | 1-15 mg/day | |
| Benztropine | 1-6 mg/day | ||
| Diphenhydramine | 10-400 mg/day | ||
| Dystonia, dyskinesias, chorea | Benzodiazepines | Midazolam | 1-2 μg/kg/min |
| Clonazepam | 3-40 mg/day | ||
| Dopamine receptor blockers | Haloperidol | 1-15 mg/day | |
| Risperidone | .5-6 mg/day | ||
| Quetiapine | 12.5-400 mg/day | ||
| Olanzapine | 5-20 mg/day | ||
| Dopamine depleters | Tetrabenazine | 12.5-100 mg/day | |
| Deutetrabenazine | 6-48 mg/day | ||
| Alpha adrenergic agents | Clonidine | .1-2.4 mg/day | |
| Anticholinergics | Trihexyphenidyl | 1-15 mg/day | |
| Benztropine | 1-6 mg/day | ||
| Diphenhydramine | 10-400 mg/day | ||
| Refractory hyperkinetic movements | Benzodiazepines | Midazolam | 1-2 μg/kg/min |
| Clonazepam | 3-40 mg/day | ||
| Sedatives | Dexmedetomidine | .15-1.5 μg/kg/h | |
| Propofol | .3-3.0 mg/kg/h | ||
| Neuromuscular junction blockers | Vecuronium | ||
| Pancuronium |
Despite a majority of good outcomes, patients usually have a protracted recovery, and prolonged ICU admissions are common. NMDAR encephalitis presents specific ICU challenges such as differentiating movement disorders from seizures, differentiating fever due to infections from dysautonomia, as well as complications related to prolonged stays in intensive care. Orofacial dyskinesias and agitation may lead to displacement and loss of airway devices, and bleeding due to tongue biting. Dysautonomia may lead to bradyarrhythmias requiring pacemaker placement.19,20 Nosocomial infections are common due to prolonged hospitalization, intubation, and immunosuppression. Our patient experienced bradyarrhythmias and sinus pauses, requiring pacemaker placement, as well as developed sepsis during therapy with cyclophosphamide. Both complications resolved with appropriate therapies.
In summary, this case of definite anti-NMDAR AE associated with RCC highlights several challenges in the diagnosis and management of these patients and serves as a reminder that prolonged and complicated courses can still have an excellent outcome.
Footnotes
Author Contributions: Carlos Eduardo Vervloet Sollero: manuscript elaboration, review, and editing. Amanda L Piquet: manuscript review and editing. Christopher Robinson: manuscript review and editing. Tirisham V Gyang: manuscript review and editing. Aaron Carlson: manuscript elaboration, review, and editing.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Sollero has nothing to disclose. Dr Robinson has nothing to disclose. Dr Piquet reports grants from University of Colorado and Rocky Mountain MS Center; consulting fees from Genentech/Roche, Alexion, UCB and EMD Serono; and honorarium from Medlink publication royalties from Springer as co-editor of a textbook. Dr. Piquet also serves in an unpaid position the Autoimmune Encephalitis Alliance (AEA) Medical Advisory Board. Dr Gyang has served as consultant and received compensation for working with Genentech, Horizon Therapeutics Inc, EMD Serono and Greenwich Biosciences, Inc. Dr Carlson serves in an unpaid position for the American Academy of Neurology on the Health Services Research Subcommittee. He is an editorial board member for The Neurohospitalist, and recused himself from review of this manuscript. He has previously served as a consultant for Sanofi-Genzyme.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Carlos Eduardo Vervloet Sollero https://orcid.org/0000-0001-8538-6552
Aaron Carlson https://orcid.org/0000-0001-7520-7864
References
- 1.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. December 2008;7:1091-1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Tüzün E, Wu H-y, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Li B, Li X, Lai Z. Anti-N-Methyl-D-Aspartate receptor encephalitis associated with clear cell renal carcinoma: a case report. Front Oncol. 2020;10:350. Published 2020 Mar 27. doi: 10.3389/fonc.2020.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashton C, Dunuwille J. EBV and NMDA receptor antibody positive opsoclonus-myoclonus syndrome in an immunocompromised patient with renal clear cell carcinoma: a case report. BMJ Neurology Open. 2021;3:65. doi: 10.1136/bmjno-2021-ANZAN.65. [DOI] [Google Scholar]
- 5.Miyagi H, Le C, Dennis M, Crispen P. A case of anti-N-methyl-D-aspartate receptor encephalitis in renal-cell carcinoma. J Clin Urol. Published online September. 2020;30:205141582096190. [Google Scholar]
- 6.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. February 2013;12(2). 157-165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. April 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florance NR, Davis RLCL, Szperka C, et al. Anti-N-Methyl-D-Aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. July 2009;66(1):11-18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow W-H, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. May 2010;7(5):245-257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuch B, Srinivas V, Ricketts CJ, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J Clin Oncol: Official Journal of the American Society of Clinical Oncology. 2014;32(5):431-437. doi: 10.1200/JCO.2013.50.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegemann M, Kroeger N, Stenzl A, Bedke J. Rare and changeable as a chameleon: paraneoplastic syndromes in renal cell carcinoma. World J Urol. 2018. Jun;36(6):849-854. doi: 10.1007/s00345-018-2215-9. [DOI] [PubMed] [Google Scholar]
- 12.Nosadini M, Thomas T, Eyre M, et al. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021. Jul 22;8(5):e1052. doi: 10.1212/NXI.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. 2019;92(3):e244-e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smets I, Titulaer MJ. Antibody therapies in autoimmune encephalitis. Neurotherapeutics. 2022;19:823-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee WJ, Lee ST, Shin YW, et al. Teratoma removal, steroid, ivig, rituximab and tocilizumab (T-sirt) in anti-nmdar encephalitis. Neurotherapeutics. 2021;18(1):474-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lejuste F, Thomas L, Picard G, et al. Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurology - Neuroimmunology Neuroinflammation. October 2016;3(5):e280. doi: 10.1212/NXI.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti- N -methyl- d -aspartate receptor encephalitis. JAMA Neurol. 2013;70(9):1133. doi: 10.1001/jamaneurol.2013.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali F, Wijdicks EF. Treatment of movement disorder emergencies in autoimmune encephalitis in the neurosciences icu. Neurocritical Care. 2020;32(1):286-294. [DOI] [PubMed] [Google Scholar]
- 19.Montmollin Ede, Demeret S, Brulé N, et al. Anti-N-Methyl-d-Aspartate receptor encephalitis in adult patients requiring intensive care. Am J Respir Crit Care Med. 2017;195(4):491-499. doi: 10.1164/rccm.201603-0507OC. [DOI] [PubMed] [Google Scholar]
- 20.Howard CM, JosephKass SVDPB, Guntupalli KK. Challenges in providing critical care for patients with anti-N-Methyl-D-Aspartate receptor encephalitis. Chest. May 2014;145(5):1143-1147. doi: 10.1378/chest.13-1490. [DOI] [PubMed] [Google Scholar]