Abstract
INTRODUCTION
New therapies to prevent or delay the onset of symptoms, slow progression, or improve cognitive and behavioral symptoms of Alzheimer's disease (AD) are needed.
METHODS
We interrogated clinicaltrials.gov including all clinical trials assessing pharmaceutical therapies for AD active in on January 1, 2024. We used the Common Alzheimer's Disease Research Ontology (CADRO) to classify the targets of therapies in the pipeline.
RESULTS
There are 164 trials assessing 127 drugs across the 2024 AD pipeline. There were 48 trials in Phase 3 testing 32 drugs, 90 trials in Phase 2 assessing 81 drugs, and 26 trials in Phase 1 testing 25 agents. Of the 164 trials, 34% (N = 56) assess disease‐modifying biological agents, 41% (N = 68) test disease‐modifying small molecule drugs, 10% (N = 17) evaluate cognitive enhancing agents, and 14% (N = 23) test drugs for the treatment of neuropsychiatric symptoms.
DISCUSSION
Compared to the 2023 pipeline, there are fewer trials (164 vs. 187), fewer drugs (127 vs. 141), fewer new chemical entities (88 vs. 101), and a similar number of repurposed agents (39 vs. 40).
Highlights
In the 2024 Alzheimer's disease drug development pipeline, there are 164 clinical trials assessing 127 drugs.
The 2024 Alzheimer's disease drug development pipeline has contracted compared to the 2023 Alzheimer pipeline with fewer trials, fewer drugs, and fewer new chemical entities.
Drugs in the Alzheimer's disease drug development pipeline target a wide array of targets; the most common processes targeted include neurotransmitter receptors, inflammation, amyloid, and synaptic plasticity.
The total development time for a potential Alzheimer's disease therapy to progress from nonclinical studies to FDA review is approximately 13 years.
1. INTRODUCTION
Alzheimer's disease (AD) is an age‐related disorder increasing from approximately 5% of individuals aged 65–74, 13.1% of people aged 75–84, and 33.3% of people aged 85 or older. 1 The aging of the global population anticipated the current marked increase in individuals with this disorder and forecasts a coming surge of affected persons. Between 2015 and 2050 the percentage of the world's population of people over age 60 will nearly double from 12% to 22%, comprising 2.1 billion individuals. 2 In the United States, 17.3% of the population is age 65 or older and approximately 6.5 million have AD dementia. 1 Mild cognitive impairment (MCI) affects approximately 22% of the over‐age 65 group, and AD is the cause of MCI in approximately 50% of cases. 3 , 4 Roughly 22% of those age 65 or older have preclinical AD (e.g., cognitively normal with evidence of AD pathology in the brain reflected in biomarkers). Preclinical AD is age‐related, affecting up to half of persons aged 90 and above. 4 In the United States, Black and Hispanic individuals and persons with lower educational levels are disproportionately affected by AD. 3 The inescapable conclusions to be drawn from these observations are that AD‐type pathology is highly prevalent among older individuals, AD increases with age, and the number of individuals progressing to MCI due to AD and dementia due to AD is high. Prevention, treatment, and scalable public health responses are needed.
After almost two decades of intense pharmacologic and drug development efforts during which no new therapies emerged, recent progress has been made in the development and approval of disease‐modifying therapies (DMTs) and symptomatic treatments for neuropsychiatric syndromes of AD. Two anti‐amyloid monoclonal antibodies (AA‐MABS)—aducanumab and lecanemab—that slow the cognitive decline of AD are approved and another—donanemab—is currently under review. 5 , 6 , 7 Brexpiprazole is approved for the treatment of agitation in dementia associated with AD. 8 These successes have been influential for AD therapeutics including demonstrating the ability to slow disease progression through intervention in key pathological processes of AD, establishing biomarkers as a means of diagnosing AD and following therapeutic responses, providing experience with clinical outcome measures in trials of both DMTs and symptomatic agents, and constructing regulatory standards applicable to the development of DMTs and treatments for symptomatic aspects of AD. The preliminary foundation for future clinical trials for AD therapeutics is in place.
The goal of our annual review of the AD drug development pipeline is to note trends in the therapeutic targets, use of clinical outcome measures, role of biomarkers in clinical trials, and drug mechanisms of action (MoA) of agents being tested in current trials. We assess the trial duration, trial population size, and time to recruit trial participants. The information presented in this review is intended to assist those developing drugs to make evidence‐based decisions; to help patients, families, and practitioners understand what drugs may be moving through the pipeline toward possible clinical application; and to help clinician‐scientists understand the landscape of putative therapeutic interventions for AD.
2. METHODS
The data on which this review is based are derived from a clinical research registry—clinicaltrials.gov—maintained by the US National Library of Medicine of the National Institutes of Health (NIH). The US Food and Drug Administration (FDA) Amendment Act of 2007 (updated 2017) requires that all clinical trials conducted in the United States be registered on clinicaltrials.gov. 9 Registration on clinicaltrials.gov must occur within 21 days of enrolling the first patient in the trial. The legal requirement for registration applies to trials that have at least one site in the United States, are conducted under an FDA Investigational New Drug (IND) or investigational device exemption, or involve a drug, biological or device that is manufactured in the United States or its territories. Failure to comply with the common rule can result in civil penalties or withholding of grant funding in the case of federally funded clinical trials. The International Committee of Medical Journal Editors (ICMJE) requires registration and inclusion of the registration number (National Clinical Trial [NCT]) with reports of clinical trials submitted for publication. This requirement further encourages trial registration on clinicaltrials.gov when clinical trials are initiated. 10 More trials are conducted in the US than in any other global region, and many trials conducted outside of the United States are registered on clinicaltrials.gov though not legally obliged to do so. As a result, most but not all clinical trials are registered on clinicaltrials.gov; the information on which this report is based can be regarded as comprehensive but not exhaustive regarding therapeutic agents in clinical trials.
Data are structured in the clinicaltrials.gov Application Programming Interface (API) and transferred to the database of the Clinical Trial Observatory for analysis and interpretation (regularly updated summary data are available at Alzpipeline.com).
The Index Date for this review is January 1, 2024. Numbers, percentages, and other numerical data provided in this report are true regarding clinical trials on the Index Date. Trials reaching their completion date prior to the Index Date or not yet registered on the Index Date are not included in the review. We include trials labeled as recruiting, active but not recruiting (e.g., trials that have completed recruitment and are continuing with the exposure portion of the trial), enrolling by invitation (e.g., open‐label extensions of trials limited to those participating in the double‐blind portion of the trials), and not yet recruiting (e.g., registered on clincaltrials.gov but no patients have been enrolled). We note whether trials registered on clinicaltrials.gov on the Index Date are labeled as suspended, terminated, completed, withdrawn, or unknown (no status update within the past 2 years). These latter categories of trials are not included in the calculations involving active trials.
We search to include all trials with labels of AD or other terms related to AD (e.g., dementia of the Alzheimer's type) and MCI. Search algorithms allow trials with spelling or grammatical errors to be detected. We do not include trials whose participants have dementia of any cause or in which AD is included with other dementias or other medical conditions not separately identified by inclusion and exclusion criteria. We do not include trials in which the MCI is a manifestation of a non‐AD condition such as MCI of Parkinson's disease.
We include trials in Phase 1, Phase 1/2, Phase 2, Phase 2/3, and Phase 3. When a trial spans two phases, we use the higher number for our calculations. We do not include Phase 0 or Phase 4 clinical trials in this report.
The data resource constructed from the clinicaltrials.gov API is comprehensive and includes the test agent, trial title, NCT number, start date, projected primary end date, duration of treatment exposure, number of arms of the study, whether a biomarker was collected at entry or as an outcome, whether the agent was repurposed (e.g., approved for another indication), whether the trial was a long‐term extension of an earlier clinical trial, and where the trials were performed (United States; ex‐United States with no US sites; global – US and ex‐US sites included in the trial). We calculate the recruitment period as the total trial duration (from the actual start date to the primary completion date) minus the treatment duration.
RESEARCH IN CONTEXT
Systemic review: All clinical trials performed in the United States must be registered on clinicaltrials.gov and most clinical trials conducted globally are entered in this registry. We used this registry to assess the size, duration, and funding of clinical trials for therapies for Alzheimer's disease (AD). We report the therapeutic purpose of the agent, mechanism of action, and biological targets of the agents being assessed.
Interpretation: The number of trials for AD therapeutics and the number of drugs being assessed is somewhat reduced in the 2024 pipeline compared to the 2023 pipeline. There are similar numbers of repurposed agents and fewer new chemical entities in the 2024 pipeline. There are 96 disease‐modifying agents and 31 symptomatic agents in the AD pipeline. The distribution of therapeutic targets as defined by the Common Alzheimer's Disease Research Ontology (CADRO) is diverse with an emphasis on inflammation, synaptic plasticity, transmitter effects, and amyloid ß‐protein.
Future directions: The decrease in the number of trials, drugs, and new chemical entities in the 2024 pipeline suggests that recent successes in the development of disease‐modifying therapies and treatments for neuropsychiatric symptoms is not increasing AD pipeline activity. The greater use of biomarkers, refined target identification, and improved trial conduct may increase the number of successes in AD drug development and attract greater interest and investment in this area where new therapies are urgently needed.
We use the “lead sponsor” designation derived from clinicaltrials.gov to divide the funders into industry or not industry. Most AD trials not funded by industry are funded by the National Institute on Aging (NIA); trials are also funded by other federal agencies such as the Veterans Administration, non‐US governmental agencies, advocacy groups, or philanthropy. Detailed information for public‐private partnerships is not available in the registry.
We note if the trial population is cognitively normal with a biomarker indicative of AD (e.g., a prevention trial), MCI, or mild, moderate, or severe AD dementia. If other trial designations are used such as “early AD” (MCI plus mild AD dementia), we capture the population definition.
We derive the target of the test agent based on the descriptive categories of the Common Alzheimer's Disease Research Ontology (CADRO). 11 CADRO categories include amyloid beta; tau; apolipoprotein E (ApoE), lipids, and lipoprotein receptors; transmitter receptors; neurogenesis; inflammation; oxidative stress; cell death; proteostasis/proteinopathies; metabolism and bioenergetics; vasculature; growth factors and hormones; synaptic plasticity/neuroprotection; gut‐brain axis; circadian rhythm; environmental factors; epigenetic regulators; multitarget; unknown target; other. Within each of the CADRO categories, such as synaptic plasticity or inflammation, agents can have one or several specific MoAs. The MoAs presented in the tables are derived from clinicaltrials.gov, available literature, or informational websites such as alzforum.org. Based on MoA information, we classify agents as DMTs or as symptomatic therapies depending on whether the declared therapeutic purpose is to slow cognitive decline or to improve symptoms present at baseline in the trial (cognitive impairment or neuropsychiatric symptoms). We acknowledge that this classification can be ambiguous; agents may have multiple therapeutic mechanisms; and drugs may have both DMT and symptomatic properties. Trials are typically designed to show one therapeutic property of a drug; trials for symptomatic agents are smaller, shorter in duration, and have less reliance on biomarkers, whereas demonstrating disease modification typically requires larger numbers of participants, longer exposures, and more reliance on biomarkers. Trial features are used to classify drugs as DMTs or symptomatic agents. We divide DMTs into biologics (e.g., monoclonal antibodies, vaccines, antisense oligonucleotides [ASOs], gene therapy, etc) and small molecules (e.g., drugs typically taken orally and less than 500 Daltons in molecular weight).
To determine if a drug in the AD pipeline is repurposed, we compare the agents in the pipeline to the currently available version of DrugBank (https://go.drugbank.com/). Most, but not all repurposed agents, are generic. A few in the pipeline such as brexpiprazole are repurposed agents with proprietary status.
In this report, we do not include trials of non‐pharmacologic therapeutic approaches such as exercise trials, lifestyle interventions, cognitive behavior therapies, caregiver interventions, supplements, medical foods, or devices. We do not include biomarker trials if no intervention is being assessed.
3. RESULTS
3.1. Overview
On the Index Date of January 1, 2024, there were 164 clinical trials for AD (prevention, MCI, AD dementia) assessing 127 drugs. This included 48 trials testing 32 drugs in Phase 3; 90 trials assessing 81 drugs in Phase 2; and 26 trials testing 25 drugs in Phase 1 (Figure 1). Of the 164 trials, 11 are long‐term extensions of agents in prior trials.
FIGURE 1.
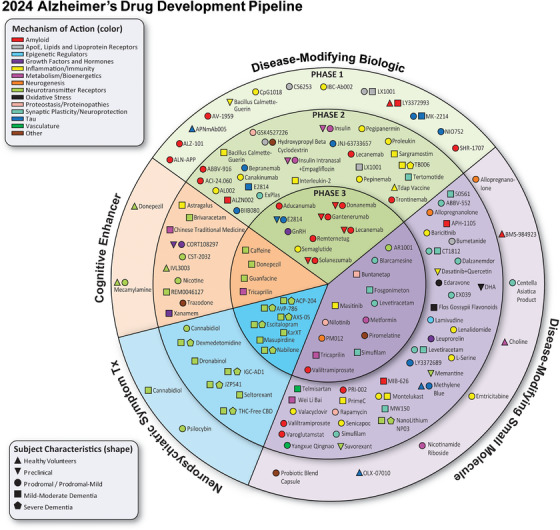
Agents in clinical trials for treatment of Alzheimer's disease on the Index Date of January 1, 2024, as recorded on clinicaltrials.gov. The inner ring shows Phase 3 agents; the middle ring includes Phase 2 agents; the outer ring presents Phase 1 therapies. Agents in green areas are biologics; those in purple areas are disease‐modifying small molecules; agents in orange areas are symptomatic agents addressing cognitive enhancement; and those in the blue sections of the figure target behavioral and neuropsychiatric symptoms. The shape of the icon denotes the population of the trial; the color of the icon denotes the CADRO‐based class of the agent (© J Cummings; M de la Fleur, PhD, Illustrator).
Most of the drugs in the AD drug development pipeline are DMTs. In total there are 96 DMTs representing 76% of drugs in clinical trials. Twelve percent of the pipeline (15 agents) target cognitive enhancement as their therapeutic purpose, and 13% (16 agents) are proposed treatments for neuropsychiatric symptoms.
Of the 96 disease‐modifying agents, 53 (55%) are small molecules and 43 (45%) are biologics. Sixty‐six percent (21 agents) in Phase 3 are DMTS; 78% (63 agents) in Phase 2 are DMTs; 84% (21 agents) in Phase 1 are DMTs.
Agents in the pipeline address nearly all the processes of the CADRO classification (Figure 2). Twenty‐eight drugs (22% of all drugs in the pipeline) target neurotransmitter receptors; 25 agents (20%) target neuroinflammation; 23 therapies (18%) target amyloid beta protein (Aß) processes; 15 drugs (12%) address synaptic plasticity/neuroprotection; 11 agents (9%) target tau‐related processes; 8 agents (6%) address metabolism and bioenergetics; 5 (4%) drugs target ApoE, lipids, and lipoprotein receptors; 4 drugs (3%) each address proteostasis/proteinopathy and growth factors and hormones; 3 therapies (2%) target oxidative stress, neurogenesis, and circadian rhythm disturbances; 2 drugs (2%) target vasculature factors; and there is 1 agent (1%) for each of the categories of gut‐brain axis and epigenetic regulators.
FIGURE 2.
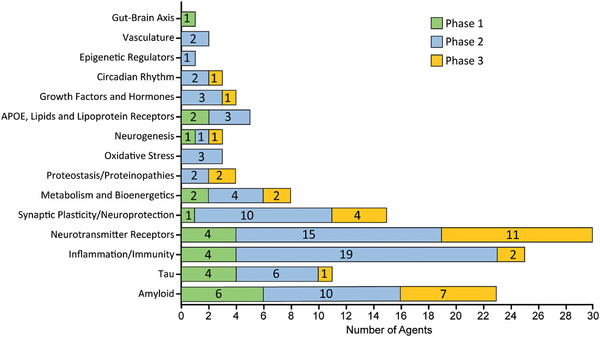
Alzheimer‐related processes as categorized by the Common Alzheimer's Disease Research Ontology (CADRO) for agents in each phase of the Alzheimer's drug development pipeline (© J Cummings; M de la Flor, PhD, Illustrator).
Of the 164 current AD trials, 35 (21%) are new since the last Index Date (January 1, 2023) including 9 trials in Phase 3, 17 trials in Phase 2, and 9 trials in Phase 1. In the past year, 37 clinical trials were completed, 10 were terminated, 4 were withdrawn, 1 was suspended, and 7 became of “unknown” status.
There are 39 repurposed agents in 52 trials in the pipeline comprising 31% of current drugs and 32% of current trials. Among repurposed agents, 15% (6 agents) are DMT biologics, 54% (21 agents) are DMT small molecules, 15% (6 agents) target cognitive enhancement, and 15% (6 drugs) are being assessed for the treatment of neuropsychiatric symptoms. Repurposed agents account for 14% of DMT biologics, 40% of DMT small molecules, 40% of cognitive enhancing agents, and 38% of neuropsychiatric agents in the pipeline.
Currently active trials require 51,398 participants including 36,998 in Phase 3; 13,138 in Phase 2; and 1,262 in Phase 1. DMTs account of 79% of all participants required for current trials. Forty‐four percent of all trials are conducted in the United States without non‐US sites. Four trials in the pipeline are prevention trials of cognitively normal individuals at risk for symptomatic AD; 26% of trials (42) are assessing participants with MCI (with or without confirmation of AD pathology); 30% (49 trials) involve participants with early AD (MCI or mild AD dementia); and 75 trials (46%) include participants with mild, moderate, or severe AD dementia.
The pharmaceutical industry accounts for funding of 60% (98 of 164) of trials.
3.2. Phase 3 trials
There are 48 Phase 3 trials assessing 32 drugs (Table 1; Figure 3). Sixty‐six percent of agents (N = 21) in Phase 3 are DMTs including nine biologics (43% of DMTs) and 12 small molecules (57%). There are four (12% of Phase 3 agents) cognitive enhancing agents and seven (22% of Phase 3 agents) neuropsychiatric agents in Phase 3. There are nine new trials assessing eight drugs since the Index Date of January 1, 2023. There are nine (28% of Phase 3 agents) repurposed agents in Phase 3; four (44%) of repurposed agents are DMTs (one biologic [25% of repurposed DMTs]; three small molecules [75% of repurposed DMTs]), three (33% of Phase 3 repurposed agents) cognitive enhancing agents, and two (22% of Phase 3 repurposed agents) drugs addressing neuropsychiatric symptoms. Five trials are long‐term extensions of drugs that have completed a prior trial. There are two prevention trials in Phase 3 enrolling individuals who are cognitively normal and at risk for progressing to symptomatic AD.
TABLE 1.
Agents in Phase 3 Alzheimer's disease drug development (clinicaltrials.gov accessed January 1, 2024).
| Agent | Therapeutic purpose | CADRO target | Mechanism of action | Clinical trial | Lead sponsor | Start Date | Estimated primary completion date |
|---|---|---|---|---|---|---|---|
| ACP‐204 | Neuropsychiatric symptom | Neurotransmitter Receptors | Selective antagonist/inverse agonist of 5‐hydroxytryptamine (serotonin) receptor subtype 2A | NCT06159673 | ACADIA Pharmaceuticals Inc. | Nov 2023 | Jan 2028 |
| Aducanumab | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody directed at plaques and oligomers |
NCT04241068 NCT05310071 |
Biogen Biogen |
Mar 2020 Jun 2022 |
Aug 2023 Dec 2025 |
| AR1001 | Disease‐modifying small molecule | Neurotransmitter receptors | PDE5 inhibitor that reduces amyloid production and decreases inflammation in animal models of AD | NCT05531526 | AriBio Co., Ltd. | Dec 2022 | Dec 2025 |
| AVP‐786 | Neuropsychiatric symptom | Neurotransmitter receptors | NMDA receptor antagonist, sigma 1 receptor agonist; serotonin and norepinephrine transporter inhibitor |
NCT02446132 NCT03393520 NCT04408755 NCT04464564 |
Otsuka Pharmaceutical Development & Commercialization, Inc. |
Dec 2015 Oct 2017 Jul 2020 Sep 2020 |
Jul 2025 Dec 2023 Dec 2024 Dec 2024 |
| AXS‐05 | Neuropsychiatric symptom | Neurotransmitter receptors | NMDA receptor antagonist, sigma 1 receptor agonist; serotonin and norepinephrine transporter inhibitor |
NCT04947553 NCT05557409 |
Axsome Therapeutics, Inc. |
Jun 2021 Sep 2022 |
Jun 2023 Jun 2025 |
| Blarcamesine | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Sigma‐1 receptor agonist, M2 autoreceptor antagonist | NCT04314934 | Anavex Life Sciences Corp. | Oct 2019 | Jul 2024 |
| Buntanetap | Disease‐modifying small molecule | Proteostasis/proteinopathies | Decrease protein translation | NCT05686044 | Annovis Bio Inc. | Apr 2023 | Feb 2024 |
| Caffeine | Cognitive enhancement | Neurotransmitter receptors | Adenosine antagonist; non‐specific phosphodiesterase inhibitor | NCT04570085 | University Hospital, Lille | Mar 2021 | Nov 2024 |
| Donanemab | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody specific for pyroglutamate plaque amyloid |
NCT04437511 NCT05026866 NCT05508789 NCT05738486 |
Eli Lilly and Company |
Jun 2020 Aug 2021 Oct 2022 Feb 2023 |
Apr 2023 Oct 2027 Apr 2027 Mar 2024 |
| Donepezil | Cognitive enhancement | Neurotransmitter receptors | Acetylcholinesterase inhibitor; adipokine modulation |
NCT04661280 NCT05592678 |
Assistance Publique—Hôpitaux de Paris The University of Texas Health Science Center at San Antonio |
Feb 2022 Mar 2024 |
Aug 2026 Nov 2028 |
| E2814 | Disease‐modifying biologic | Tau | Anti‐tau monoclonal antibody |
NCT01760005 NCT05269394 |
Washington University School of Medicine |
Dec 2012 Dec 2021 |
Oct 2027 Jul 2027 |
| Escitalopram | Neuropsychiatric symptom | Neurotransmitter receptors | Selective serotonin reuptake inhibitor | NCT03108846 | JHSPH Center for Clinical Trials | Jan 2018 | May 2024 |
| Fosgonimeton | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Hepatocyte growth factor (HGF); activates signaling via the hepatocyte growth factor (HGF)/MET receptor system; promotes survival of neurons, enhances hippocampal synaptic plasticity |
NCT04488419 NCT04886063 |
Athira Pharma |
Sep 2020 Jun 2021 |
Jul 2024 Jan 2027 |
| Gantenerumab | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody | NCT01760005 | Washington University School of Medicine | Dec 2012 | Oct 2027 |
| GnRH | Disease‐modifying biologic | Growth factors and hormones | Anti‐aging | NCT04390646 | Nelly Pitteloud | Aug 2020 | Dec 2028 |
| Guanfacine | Cognitive enhancement | Neurotransmitter receptors | Alpha‐2 adrenergic agonist | NCT03116126 | Imperial College London | Jan 2019 | Dec 2022 |
| KarXT | Neuropsychiatric symptom | Neurotransmitter receptors | Muscarinic cholinergic agonist with peripheral anticholinergic |
NCT05511363 NCT05980949 NCT06126224 |
Karuna Therapeutics |
Aug 2022 Jul 2023 Dec 2023 |
Mar 2025 Apr 2026 Jul 2025 |
| Lecanemab | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody directed at amyloid protofibrils and amyloid plaques |
NCT01760005 NCT03887455 NCT04468659 NCT05269394 |
Washington University School of Medicine Eisai Inc. Eisai Inc. Washington University School of Medicine |
Dec 2012 Mar 2019 Jul 2020 Dec 2021 |
Oct 2027 Sep 2027 Oct 2027 Jul 2027 |
| Levetiracetam | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Modulator of the synaptic vesicle protein (SV2A) to reduce aberrant neuronal hyperactivity | NCT05986721 | AgeneBio | Dec 2024 | Jul 2028 |
| Masitinib | Disease‐modifying small molecule | Inflammation | Tyrosine kinase inhibitor; exhibits neuroprotection via inhibition of mast cell and microglia/macrophage activity | NCT05564169 | AB Science | Jan 2024 | Dec 2026 |
| Masupirdine | Neuropsychiatric symptom | Neurotransmitter receptors | 5HT6 receptor antagonist | NCT05397639 | Suven Life Sciences Limited | Nov 2022 | Jan 2025 |
| Metformin | Disease‐modifying small molecule | Metabolism and bioenergetics | Insulin sensitizer | NCT04098666 | Columbia University | Mar 2021 | Mar 2026 |
| Nabilone | Neuropsychiatric symptom | Neurotransmitter receptors | Synthetic cannabinoid; cannabinoid (receptor agent); antiemetic | NCT04516057 | Sunnybrook Health Sciences Center | Feb 2021 | Oct 2025 |
| Nilotinib BE | Disease‐modifying small molecule | Proteostasis/Proteinopathies | Abl tyrosine kinase inhibitor; autophagy enhancer | NCT05143528 | KeifeRx, LLC | Feb 2022 | Dec 2025 |
| Piromelatine | Disease‐modifying small molecule | Circadian rhythm | Melatonin and serotonin receptor agonist | NCT05267535 | Neurim Pharmaceuticals Ltd. | May 2022 | May 2024 |
| PM012 | Disease‐modifying small molecule | Neurogenesis | Upregulation of BDNF | NCT05811000 | Mediforum Ltd., Co. | Nov 2020 | Feb 2021 |
| Remternetug | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody | NCT05463731 | Eli Lilly and Company | Aug 2022 | Oct 2025 |
| Semaglutide | Disease‐modifying biologic | Inflammation | GLP‐1 agonist; anti‐inflammatory and insulin sensitivity effects |
NCT04777396 NCT04777409 NCT05891496 |
Novo Nordisk A/S |
May 2021 May 2021 Jun 2023 |
Sep 2025 Sep 2025 May 2024 |
| Simufilam | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Filamin A conformation stabilizer; disrupts the interaction of filamin A with the alpha 7 nicotinic acetylcholine receptor to reduce tau hyperphosphorylation and neurodegeneration; dependent on A‐beta's signaling via the alpha 7 pathway |
NCT04994483 NCT05026177 NCT05575076 |
Cassava Sciences, Inc. |
Nov 2021 Nov 2021 Nov 2022 |
Oct 2024 May 2025 Jul 2026 |
| Solanezumab | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody | NCT01760005 | Washington University School of Medicine | Dec 2012 | Oct 2027 |
| Tricaprilin | Cognitive enhancement | Metabolism and bioenergetics | Caprylic acid is metabolized to ketone bodies to create ketosis and stimulate mitochondria | NCT05809908 | Cerecin | Jan 2024 | Jan 2026 |
| Valiltramiprosate | Disease‐modifying small molecule | Amyloid beta | Prodrug of tramiprostate | NCT04770220 | Alzheon Inc. | May 2021 | May 2024 |
FIGURE 3.
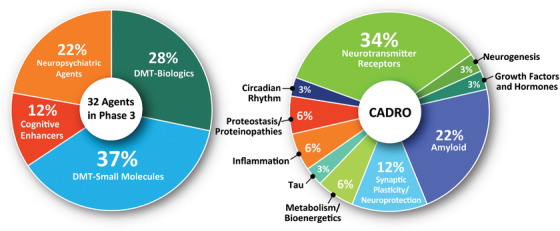
Mechanisms of action of agents in Phase 3 Alzheimer clinical trials as classified using 4 categories of therapeutic purpose (left) or the Common Alzheimer's Disease Research Ontology (CADRO) approach (right)(figure © J Cummings; M de la Flor, PhD, Illustrator).
The CADRO categories of agents in Phase 3 include 11 (34%) targeting transmitter systems; 7 (22%) targeting amyloid‐related processes; 4 (12%) addressing synaptic plasticity/neuroprotection; 2 (6%) each addressing metabolic and bioenergetic targets, inflammation, and proteostatsis/proteinopathy; and 1 (3%) each directed at tau, neurogenesis, growth factors and hormones, and circadian rhythm‐related processes. In total, 10 biological process categories are being addressed by agents in Phase 3 trials.
Thirty‐eight trials in Phase 3 (79%) are funded by biopharmaceutical industry sponsors. Twenty‐one percent are funded primarily through non‐pharmaceutical sources including the NIH (especially the NIA), other federal agencies, non‐US governmental agencies, philanthropies (e.g., Alzheimer's Drug Discovery Foundation), and advocacy groups (e.g., Alzheimer's Association). Pharmaceutical/non‐pharmaceutical collaborations are common; the API of clinicaltrials.gov does not allow specific identification of public‐private partnerships.
There are two Phase 3 prevention trials, both involving biological agents. The mean anticipated recruitment time for prevention trials is 2.9 years. On average, DMT Phase 3 trials (not including prevention trials) of biologics will require 3.5 years and small molecules will require 2.2 years to recruit the needed number of patients. Recruitment for cognitive enhancers takes on average 3.4 years and for trials of neuropsychiatric symptoms, 3.7 years. Treatment duration varies by therapeutic class, Phase 3 prevention trials of biologics have mean treatment periods of 3.8 years. Non‐prevention, Phase 3 trials of biologics have a mean treatment period of 1.5 years; non‐prevention Phase 3 trials of small molecules have a mean period of 1 year. On average, cognitive enhancer treatment exposure periods and treatment periods of drugs for neuropsychiatric agents in Phase 3 are 4.5 and 4.8 months, respectively.
Phase 3 trials required a total of 36,998 participants to populate all trials currently in progress. DMT biologics have a total of 18,232 participants; DMT small molecules have, in total, 10,439 participants; trials of cognitive enhancers require 1,383 participants, and trials of agents for neuropsychiatric symptoms require 6,944 participants. The mean number of participants per trial in Phase 3 trials is 1215.5 for DMT biologic trials, 695.9 for DMT small molecules, 276.6 for cognitive enhancers, and 534.2 for trials of neuropsychiatric agents.
3.3. Phase 2 trials
There are 90 Phase 2 trials assessing 81 drugs (Table 2; Figure 4). Seventy‐eight percent of agents (N = 63) in Phase 2 are DMTs including 26 biologics (41% of DMTs) and 37 small molecules (59% of DMTs). There are 10 (12%) cognitive enhancing agents and 8 (10%) neuropsychiatric agents in Phase 2. There are 17 new trials in Phase 2 assessing 17 drugs since the Index Date of January 1, 2023. There are 33 Phase 2 trials assessing repurposed 28 agents and representing 35% of the Phase 2 pipeline. Twenty‐two (79%) of repurposed agents are DMTs (5 biologics (23% of DMTs); 17 small molecules (77% of DMTs)); 2 (7% of Phase 2 repurposed agents) cognitive enhancing agents, and 4 (14% of Phase 2 repurposed agents) drugs addressing neuropsychiatric symptoms. Five trials are long‐term extensions of trials of drugs that have completed the double bind phase of a prior trial. There is one prevention trial in Phase 2.
TABLE 2.
Agents in Phase 2 Alzheimer's disease drug development (clinicaltrials.gov accessed January 1, 2024).
| Agent | Therapeutic purpose | CADRO target | Mechanism of action | Clinical trial | Lead sponsor | Start date | Estimated primary completion date |
|---|---|---|---|---|---|---|---|
| 50561 | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | RAC1 inhibitor (RAC family small GTPase inhibitors) enhance dendritic spine morphogenesis and synaptic plasticity | NCT05811442 | Beijing Joekai Biotechnology LLC | Apr 2023 | May 2024 |
| ABBV‐552 | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Synaptic vesicle glycoprotein 2A (SV2A) modulator | NCT05771428 | AbbVie | Apr 2023 | Jun 2024 |
| ABBV‐916 | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid antibody | NCT05291234 | AbbVie | Aug 2022 | Jan 2030 |
| ACI‐24.060 | Disease‐modifying biologic | Amyloid beta | Vaccine stimulates antibodies against amyloid beta protein | NCT05462106 | AC Immune SA | Jun 2022 | Jun 2026 |
| AL002 | Disease‐modifying biologic | Inflammation | Monoclonal antibody targeting TREM2 receptors |
NCT04592874 NCT05744401 |
Alector Inc. |
Jan 2021 Jan 2023 |
Sep 2024 Sep 2025 |
| Allopregnanolone | Disease‐modifying small molecule | Neurogenesis | Allosteric modulator of GABA‐A Receptors | NCT04838301 | University of Arizona | Aug 2023 | Apr 2025 |
| ALZN002 | Disease‐modifying biologic | Amyloid beta | Autologous Beta‐Amyloid Mutant Peptide‐pulsed Dendritic Cells | NCT05834296 | Alzamend Neuro, Inc. | Jul 2023 | Mar 2028 |
| APH‐1105 | Disease‐modifying small molecule | Amyloid beta | Alpha‐secretase modulator (amyloid precursor protein secretase modulator) | NCT03806478 | Aphios | Jun 2023 | Sep 2024 |
| Astragalus | Cognitive enhancement | Inflammation | Undisclosed | NCT05647473 | Fujian Medical University Union Hospital | Feb 2024 | May 2025 |
| Bacillus Calmette‐Guerin | Disease‐modifying biologic | Inflammation | Vaccine to stimulate resilience to Alzheimer‐related processes | NCT05004688 | Steven E Arnold, MD | Mar 2022 | Oct 2023 |
| Baricitinib | Disease‐modifying small molecule | Inflammation | Janus kinase (JAK) inhibitor | NCT05189106 | Massachusetts General Hospital | Dec 2022 | Jul 2024 |
| Bepranemab | Disease‐modifying biologic | Tau | Anti‐tau monoclonal antibody binding to central region of tau | NCT04867616 | UCB Biopharma SRL | Jun 2021 | May 2024 |
| BIIB080 | Disease‐modifying biologic | Tau | Antisense oligonucleotide that inhibits translation of tau mRNA into the tau protein | NCT05399888 | Biogen | Aug 2022 | Nov 2027 |
| Brivaracetam | Cognitive enhancement | Neurotransmitter receptors | Anticonvulsant with high affinity for synaptic vesicle protein 2A | NCT05899764 | University of California, Los Angeles | Jun 2023 | Jun 2028 |
| Bumetanide | Disease‐modifying small molecule | ApoE, lipids and lipoprotein receptors | Reversal of ApoE‐specific AD signatures | NCT06052163 | Stanford University | Oct 2023 | Oct 2025 |
| Canakinumab | Disease‐modifying biologic | Inflammation | Anti‐IL‐1‐beta monoclonal antibody | NCT04795466 | Novartis Pharmaceuticals | Oct 2021 | Mar 2024 |
| Cannabidiol | Neuropsychiatric symptom | Neurotransmitter receptors | Endocannabinoid receptor agonist | NCT05822362 | University of Colorado, Denver | Jan 2024 | Apr 2028 |
| Chinese Traditional Medicine | Cognitive enhancement | Metabolism and bioenergetics | Three herbs (Rhizoma Acori Tatarinowii, Poria cum Radix Pini, Radix Polygalae); mechanism unknown | NCT05538507 | Peking Union Medical College Hospital | Jun 2022 | Jun 2024 |
| CORT108297 | Cognitive enhancement | Growth factors and hormones | Selective glucocorticoid receptor antagonist | NCT04601038 | Johns Hopkins University | Jun 2021 | Jun 2025 |
| CST‐2032 | Cognitive enhancement | Neurotransmitter receptors | Noradrenergic agonist | NCT05104463 | CuraSen Therapeutics, Inc. | Apr 2022 | Nov 2023 |
| CT1812 | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Sigma 2 receptor antagonist; binds to sigma‐2/PGRMC1 receptor and regulates Aβ oligomer‐mediated synaptic toxicity |
NCT03507790 NCT05531656 |
Cognition Therapeutics |
Oct 2018 Jun 2023 |
Jul 2024 Apr 2027 |
| Dalzanemdor | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Enhnaces synaptic function through NMDA receptor blockade | NCT05619692 | Sage Therapeutics | Dec 2022 | Dec 2024 |
| Dasatinib + Quercetin | Disease‐modifying small molecule | Inflammation | Dasatinib induces apoptosis in senescent cells to allow their removal; quercetin is a flavonoid |
NCT04685590 NCT04785300 NCT05422885 |
Wake Forest University Health Sciences James L. Kirkland, MD, PhD Lewis Lipsitz |
Dec 2021 Jul 2022 May 2022 |
Jan 2025 Dec 2023 Jun 2024 |
| Dexmedetomidine | Neuropsychiatric symptom | Neurotransmitter receptors | Presynaptic alpha‐2 adrenoceptor agonist to inhibit release of norepinephrine | NCT06052254 | Teikoku Pharma USA, Inc. | Dec 2023 | Dec 2024 |
| DHA | Disease‐modifying small molecule | Oxidative stress | Omega 3 fatty acid; reduce amyloid production; improve synaptic function; antioxidant | NCT03613844 | University of Southern California | Sep 2018 | May 2024 |
| Dronabinol | Neuropsychiatric symptom | Neurotransmitter receptors | CB1 and CB2 endocannabinoid receptor partial agonist | NCT02792257 | Johns Hopkins University | Mar 2017 | May 2024 |
| Dronabinol + PEA | Neuropsychiatric symptom | Neurotransmitter receptors | Cannibinoid | NCT05239390 | The Israeli Medical Center for Alzheimer's | Dec 2021 | Jun 2023 |
| E2814 | Disease‐modifying biologic | Tau | Anti‐tau monoclonal antibody | NCT04971733 | Eisai Inc. | Jun 2021 | Jul 2025 |
| Edaravone | Disease‐modifying small molecule | Oxidative stress | Pyrazolone free‐radical scavenger | NCT05323812 | Treeway B.V. | Mar 2023 | Jan 2024 |
| EX039 | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Inhibits D‐amino acids oxidate to increase N‐methyl‐D‐aspartate receptor activity | NCT05413655 | Excelsior | Aug 2022 | Aug 2025 |
| ExPlas | Disease‐modifying biologic | Synaptic plasticity/neuroprotection | Plasma transfusion from exercise‐trained donors | NCT05068830 | Norwegian University of Science and Technology | Sep 2021 | Sep 2024 |
| Flos gossypii flavonoids | Disease‐modifying small molecule | Oxidative stress | Antioxidant; anti‐inflammatory | NCT05269173 | Capital Medical University | Oct 2020 | Jun 2024 |
| GSK4527226 | Disease‐modifying biologic | Proteostasis/proteinopathies | Monoclonal antibody to sortilin (SORT1) to improve lysosomal function | NCT06079190 | GlaxoSmithKline | Oct 2023 | Dec 2026 |
| Hydroxypropyl beta‐cyclodextrin | Disease‐modifying biologic | ApoE, lipids and lipoprotein receptors | Modulates cholesterol transportation with secondary effects on amyloid, tau, and oxidative e stress | NCT05607615 | Cyclo Therapeutics, Inc. | Sep 2022 | Mar 2024 |
| IGC‐AD1 | Neuropsychiatric symptom | Neurotransmitter receptors | Cannabinoid | NCT05543681 | IGC Pharma LLC | Oct 2022 | Jun 2025 |
| Insulin | Disease‐modifying biologic | Metabolism and bioenergetics | Decreases glucose resistance; increase insulin signaling in the brain | NCT05006599 | Wake Forest University Health Sciences | May 2025 | May 2029 |
| Insulin + Empagliflozin | Disease‐modifying biologic | Metabolism and bioenergetics | SGLT2 inhibitor (empagliflozin) and insulin combination therapy; decrease glucose resistance and increase insulin signaling in the brain | NCT05081219 | Wake Forest University Health Sciences | Oct 2021 | Oct 2026 |
| Interleukin‐2 | Disease‐modifying biologic | Inflammation | Restore function of regulatory T cells | NCT06096090 | The Methodist Hospital Research Institute | Mar 2023 | Dec 2025 |
| IVL3003 | Cognitive enhancement | Neurotransmitter receptors | Cholinesterase inhibitor | NCT05345509 | Inventage Lab., Inc. | Apr 2023 | Mar 2024 |
| JNJ‐63733657 | Disease‐modifying biologic | Tau | Monoclonal antibody targeted at soluble tau (mid‐region of tau) | NCT04619420 | Janssen Research & Development, LLC | Jan 2021 | Mar 2025 |
| JZP541 | Neuropsychiatric symptom | Neurotransmitter receptors | Cannabinoid receptor agonists of the endocannabinoid system | NCT06014424 | Sunnybrook Health Sciences Center | Sep 2023 | Dec 2026 |
| Lamivudine | Disease‐modifying small molecule | Epigenetic regulators | Human immunodeficiency virus nucleoside analog reverse transcriptase inhibitor | NCT04552795 | Bess Frost, PhD | Feb 2021 | May 2023 |
| Lecanemab | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody directed at amyloid protofibrils and amyloid plaques | NCT01767311 | Eisai Inc. | Dec 2012 | Feb 2025 |
| Lenalidomide | Disease‐modifying small molecule | Inflammation | Anti‐inflammatory and immunomodulatory originally approved to treat multiple myeloma |
NCT04032626 NCT06177028 |
St. Joseph's Hospital and Medical Center, Phoenix |
Jul 2020 Jan 2024 |
Sep 2023 Jan 2026 |
| Leuprorelin | Disease‐modifying small molecule | Growth factors and hormones | Gonadotropin releasing hormone (GnRH) receptor agonist | NCT03649724 | Weill Medical College of Cornell University | Nov 2020 | Feb 2025 |
| Levetiracetam | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | SV2A modulator enhancing synaptic plasticity |
NCT03875638 NCT04004702 |
Beth Israel Deaconess Medical Center Walter Reed National Military Medical Center |
Aug 2019 Jan 2020 |
Aug 2023 Dec 2024 |
| L‐Serine | Disease‐modifying small molecule | Inflammation | Naturally‐occurring dietary amino acid; inhibits toxic misfolding | NCT03062449 | Aleksandra Stark | Mar 2017 | Dec 2022 |
| LX1001 | Disease‐modifying biologic | ApoE, lipids and lipoprotein receptors | Adeno‐associated virus (AAV) gene transfer vector expressing the cDNA coding for human apolipoprotein E2 (APOE2) directly to the CNS/CSF of APOE4 homozygotes | NCT03634007 | Lexeo Therapeutics | Nov 2019 | Nov 2024 |
| LY3372689 | Disease‐modifying small molecule | Tau | O‐GlcNAcase enzyme inhibitor | NCT05063539 | Eli Lilly and Company | Sep 2021 | Jul 2024 |
| Memantine | Disease‐modifying small molecule | Neurotransmitter receptors | NMDA receptor antagonist | NCT05063851 | University of Virginia | Oct 2021 | Dec 2025 |
| Methylene Blue | Disease‐modifying small molecule | Tau | Tau protein aggregation inhibitor | NCT02380573 | The University of Texas Health Science Center at San Antonio | Jul 2015 | Apr 2022 |
| MIB‐626 | Disease‐modifying small molecule | Amyloid beta | Sirtuin‐nicotinamide adenine dinucleotide stimulator to enhance alpha‐secretase | NCT05040321 | Brigham and Women's Hospital | Dec 2021 | Apr 2024 |
| Montelukast buccal film | Disease‐modifying small molecule | Inflammation | Leukotriene receptor antagonist (LTRA); anti‐inflammatory effects | NCT03402503 | IntelGenx Corp. | Nov 2018 | Feb 2024 |
| MW150 | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | p38 alpha MAPK kinase inhibitor | NCT05194163 | Neurokine Therapeutics | May 2022 | Aug 2024 |
| NanoLithium NP03 | Disease‐modifying small molecule | Neurotransmitter receptors | Ion with effects on amyloid, oxidation, and inflammation | NCT05423522 | Medesis Pharma SA | May 2022 | Jan 2024 |
| Nicotine transdermal patch | Cognitive enhancement | Neurotransmitter receptors | Nicotinic acetylcholine receptor agonist | NCT02720445 | University of Southern California | Jan 2017 | Aug 2025 |
| Pegipanermin | Disease‐modifying biologic | Inflammation | Neutralizes TNF‐alpha |
NCT05318976 NCT05522387 |
Inmune Bio, Inc. |
Feb 2022 Feb 2023 |
Dec 2024 May 2026 |
| Pepinemab | Disease‐modifying biologic | Inflammation | Monoclonal antibody directed at semaphorin 4D; reduces inflammatory cytokine release | NCT04381468 | Vaccinex Inc. | Jul 2021 | Jun 2023 |
| PRI‐002 | Disease‐modifying small molecule | Amyloid beta | Interferes with oligomerization of A‐beta 42 to prevent formation and enhance reduction of A‐beta oligomers | NCT06182085 | PRInnovation GmbH | Dec 2023 | Apr 2026 |
| PrimeC | Disease‐modifying small molecule | Inflammation | Combined product targeting inflammation, iron accumulation, impaired RNA regulation | NCT06185543 | NeuroSense Therapeutics Ltd. | Nov 2023 | Nov 2025 |
| Proleukin | Disease‐modifying biologic | Inflammation | IL‐2 immunomodulator | NCT05468073 | Center Hospitalier St Anne | Oct 2022 | Sep 2025 |
| Rapamycin | Disease‐modifying small molecule | Proteostasis/proteinopathies | Autophagy enhancer; MTOR inhibitor; immunomodulator |
NCT04629495 NCT06022068 |
The University of Texas Health Science Center at San Antonio Karolinska Institutet |
Aug 2021 Sep 2023 |
Dec 2023 Jan 2025 |
| REM0046127 | Cognitive enhancement | Neurotransmitter receptors | Modulates Orai calcium (Ca2+) channel activity to normalize neuronal Ca2+ homeostasis | NCT05478031 | reMYND | Jun 2022 | Jun 2023 |
| Sargramostim | Disease‐modifying biologic | Inflammation | Hematopoietic growth factor granulocyte macrophage colony stimulating factor; anti‐inflammatory | NCT04902703 | University of Colorado, Denver | Jun 2022 | Jul 2024 |
| Seltorexant | Neuropsychiatric symptom | Circadian rhythm | Dual orexin receptor antagonist | NCT05307692 | Janssen Research & Development, LLC | May 2022 | Oct 2023 |
| Senicapoc | Disease‐modifying small molecule | Inflammation | Calcium‐activated potassium channel inhibitor | NCT04804241 | University of California, Davis | Mar 2022 | Dec 2024 |
| Simufilam | Disease‐modifying small molecule | Synaptic plasticity/neuroprotection | Filamin A conformation stabilizer; disrupts the interaction of filamin A with the alpha 7 nicotinic acetylcholine receptor to reduce tau hyperphosphorylation and neurodegeneration; dependent on A‐beta's signaling via the alpha 7 pathway | NCT05352763 | Cassava Sciences, Inc. | May 2022 | Oct 2025 |
| Suvorexant | Disease‐modifying small molecule | Neurotransmitter receptors | Dual orexin receptor antagonist | NCT04629547 | Washington University School of Medicine | May 2022 | May 2026 |
| TB006 | Disease‐modifying biologic | Inflammation | Monoclonal antibody targeting galactose‐specific lectin (galectin) 3, a β‐galactosidase‐binding protein that activates macrophages; anti‐inflammatory | NCT05476783 | TrueBinding, Inc. | Sep 2022 | Oct 2024 |
| Tdap | Disease‐modifying biologic | Inflammation | Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine to stimulate inflammatory protection | NCT05183516 | Mindful Diagnostics and Therapeutics, LLC | May 2023 | Dec 2023 |
| Telmisartan | Disease‐modifying small molecule | Vasculature | Angiotensin II receptor blocker | NCT02085265 | Sunnybrook Health Sciences Center | Mar 2014 | Sep 2023 |
| Tertomotide | Disease‐modifying biologic | Synaptic plasticity/neuroprotection | Human telomerase reverse transcriptase (hTERT) mimic | NCT05189210 | GemVax & Kael | Oct 2022 | Jul 2023 |
| THC‐free cannabidiol | Neuropsychiatric symptom | Neurotransmitter receptors | Cannabinoid | NCT04436081 | Eastern Virginia Medical School | Feb 2021 | Mar 2024 |
| Trazodone | Cognitive enhancement | Circadian rhythm | Serotonin reuptake inhibitor | NCT05282550 | Johns Hopkins University | Jan 2023 | Mar 2027 |
| Trontinemab | Disease‐modifying biologic | Amyloid beta | Monoclonal antibody directed at plaques and oligomers; “brain‐shuttle” gantenerumab | NCT04639050 | Hoffmann‐La Roche | Mar 2021 | Sep 2027 |
| Valacyclovir | Disease‐modifying small molecule | Inflammation | Anti‐viral against HSV‐1 and −2; reduces vira‐related ‘seeding’ of amyloid plaque deposition | NCT03282916 | Columbia University | Feb 2018 | Dec 2024 |
| Valiltramiprosate | Disease‐modifying small molecule | Amyloid beta | Aggregation Inhibitor | NCT04693520 | Alzheon Inc. | Sep 2020 | Jul 2023 |
| Varoglutamstat | Disease‐modifying small molecule | Amyloid beta | Glutaminyl cyclase (QC) enzyme inhibitor to reduce production of pyroglutamate Aβ |
NCT03919162 NCT04498650 |
Vivoryon Therapeutics N.V. |
Nov 2021 Jul 2020 |
Nov 2023 Jan 2024 |
| Wei Li Bai | Disease‐modifying small molecule | Metabolism and bioenergetics | Not specified; reported to regulate metabolism, improve blood circulation, and exert anti‐inflammatory and antioxidant effects | NCT05670912 | Capital Medical University | Oct 2022 | Nov 2024 |
| Xanamem | Cognitive enhancement | Growth factors and hormones | 11‐beta‐hydroxysteroid dehydrogenase type 1 inhibitor | NCT06125951 | Actinogen Medical | Dec 2023 | Dec 2025 |
| Yangxue Qingnao pills | Disease‐modifying small molecule | Vasculature | Cerebral blood flow enhancer; traditional Chinese herbal medicine | NCT04780399 | Dongzhimen Hospital, Beijing | Nov 2021 | Mar 2024 |
FIGURE 4.
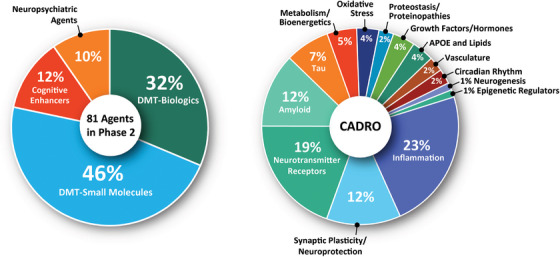
Mechanisms of action of agents in Phase 2 Alzheimer clinical trials as classified using 4 categories of therapeutic purpose (left) or the Common Alzheimer's Disease Research Ontology (CADRO) approach (right)(figure © J Cummings; M de la Flor, PhD, Illustrator).
The CADRO categories of agents in Phase 2 include 19 (23% of Phase 2 agents) agents addressing inflammation; 15 (19%) agents target transmitter receptors; 10 drugs (12%) target synaptic plasticity/neuroprotection; 10 (12%) target amyloid‐related processes; 6 (7%) have tau targets; 4 (5%) have metabolic and bioenergetic targets; 3 (4%) address ApoE and lipids, oxidative stress, or growth factors and hormones; 2 (2%) target proteostasis/proteinopathies, vasculature, and circadian rhythm each; 1 (1%) each address neurogenesis and epigenetic regulators. Fourteen biological process categories are being addressed by agents in Phase 2 trials.
Together all Phase 2 trials require 13,138 participants. Phase 2 DMT trials require 5286 and 5751 for biologic and small molecule studies, respectively. Current cognitive enhancer trials require 1185 participants and trials of drugs for neuropsychiatric symptoms require 916 participants. The mean number of participants for Phase 2 DMT biological agents is 188.8 and for DMT small molecule trials, 130.7. There are an average of 118.5 participants in Phase 2 cognitive enhancer trials and 114.5 participants in Phase 2 trials of neuropsychiatric agents.
Forty‐six trials in Phase 2 (51%) are funded by biopharmaceutical industry sponsors with the other 49% funded by a variety of NIH, US federal, non‐US governmental, advocacy, and philanthropic non‐industry organizations.
On average, DMT Phase 2 trials (not prevention) of biologics and small molecules will each require 2.3 years to recruit the needed number of patients. Recruitment for cognitive enhancers and neuropsychiatric agents will take on average 2.6 years and 2.8 years, respectively. Treatment duration varies by therapeutic class. Non‐prevention, Phase 2 trials of biologics have a mean treatment period of 1.2 years; non‐prevention, Phase 2 trials of small molecules have a mean period of 0.8 year (10 months). On average, cognitive enhancer treatment exposure periods in Phase 2 trials are 5.9 months and for trials of neuropsychiatric agents, 2.5 months.
3.4. Phase 1 trials
There are 26 Phase 1 trials assessing 25 drugs (Table 3). Eighty‐four percent of agents (N = 21) in Phase 1 are DMTs including 13 biologics (62% of DMTs) and eight small molecules (38%). There are two (8% of Phase 1 drugs) cognitive enhancing agents and two (8% of Phase 1 drugs) neuropsychiatric agents in Phase 1. There are nine new trials assessing eight drugs since the Index Date of January 1, 2023.
TABLE 3.
Agents in Phase 1 Alzheimer's disease drug development (clinicaltrials.gov accessed January 1, 2024).
| Agent | Therapeutic purpose | CADRO target | Mechanism of action | Clinical trial | Lead sponsor | Start date | Estimated primary completion date |
|---|---|---|---|---|---|---|---|
| Allopregnanolone | Disease‐modifying small molecule | Neurogenesis | Allosteric modulator of GABA‐A Receptors | NCT03748303 | University of Arizona | Oct 2019 | Dec 2022 |
| ALN‐APP | Disease‐modifying biologic | Amyloid beta | RNAi to decrease APP and downstream Aβ‐related events | NCT05231785 | Alnylam Pharmaceuticals | Feb 2022 | Jul 2025 |
| ALZ‐101 | Disease‐modifying biologic | Amyloid beta | Amyloid beta‐directed vaccine | NCT05328115 | Alzinova AB | Sep 2021 | Dec 2023 |
| APNmAb005 | Disease‐modifying biologic | Tau | Anti‐tau antibody | NCT05344989 | APRINOIA Therapeutics, LLC | May 2022 | Mar 2024 |
| AV‐1959 | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid vaccine | NCT05642429 | Institute for Molecular Medicine | Feb 2023 | Feb 2026 |
| Bacillus Calmette‐Guerin | Disease‐modifying biologic | Inflammation | Vaccine to stimulate resilience to Alzheimer‐related processes | NCT06078891 | Tamir Ben‐Hur | Jul 2023 | Jul 2024 |
| BMS‐984923 | Disease‐modifying small molecule | Amyloid beta | Silent allosteric modulator (SAM) of mGluR5 |
NCT05804383 NCT05817643 |
Allyx Therapeutics |
Mar 2023 Jan 2023 |
Oct 2024 Feb 2023 |
| Cannabidiol | Neuropsychiatric symptom | Neurotransmitter Receptors | Cannabinoid | NCT04075435 | Mclean Hospital | Jan 2021 | Sep 2024 |
| Centella asiatica product | Disease‐modifying small molecule | Synaptic Plasticity/Neuroprotection | Antioxidant and anti‐inflammatory agent with synaptic and neuroprotective effects | NCT05591027 | Oregon Health and Science University | Dec 2022 | Nov 2024 |
| Choline | Disease‐modifying small molecule | Metabolism and Bioenergetics | Stabilizes the lipid metabolism and concomitantly restoring normal cell function by increasing phosphatidylcholine activity via the Kennedy pathway | NCT05880849 | Paul E Schulz | Jun 2023 | Jun 2025 |
| CpG1018 | Disease‐modifying biologic | Inflammation | Toll‐like receptor nine agonist leading to reduced Aβ plaques and tau pathology | NCT05606341 | NYU Langone Health | Mar 2023 | Nov 2024 |
| CS6253 | Disease‐modifying biologic | ApoE, Lipids and Lipoprotein Receptors | Adenosine triphosphate‐binding cassette transporter A1 (ABCA1) transfers lipids to ApoE, and increases clearance of A‐beta from the brain | NCT05965414 | Artery Therapeutics, Inc. | Oct 2023 | Sep 2024 |
| Donepezil | Cognitive enhancement | Neurotransmitter Receptors | Cholinesterase inhibitor | NCT06127368 | G2GBio, Inc. | Jan 2024 | Sep 2024 |
| Emtricitabine | Disease‐modifying small molecule | Inflammation | Nucleoside reverse transcriptase inhibitor (NRTI) | NCT04500847 | Butler Hospital | Dec 2021 | Mar 2024 |
| IBC‐Ab002 | Disease‐modifying biologic | Inflammation | Anti‐programmed death‐ligand 1 (PD‐L1) immune checkpoint inhibitor | NCT05551741 | Immunobrain Checkpoint | Feb 2023 | Oct 2024 |
| LX1001 | Disease‐modifying biologic | ApoE, Lipids and Lipoprotein Receptors | Adeno‐associated virus (AAV) gene transfer vector expressing the cDNA coding for human apolipoprotein E2 (APOE2) directly to the CNS/CSF of APOE4 homozygotes | NCT05400330 | Lexeo Therapeutics | May 2023 | Nov 2028 |
| Mecamylamine | Cognitive enhancement | Neurotransmitter Receptors | Nicotinic antagonist | NCT04129060 | University of Vermont | Mar 2020 | Mar 2024 |
| MK‐2214 | Disease‐modifying biologic | Tau | Anti‐tau monoclonal antibody | NCT05466422 | Merck Sharp & Dohme LLC | Sep 2022 | May 2025 |
| Nicotinamide Riboside | Disease‐modifying small molecule | Metabolism and Bioenergetics | Mitochondrial function enhancer and antioxidant | NCT04430517 | Mclean Hospital | Mar 2022 | Apr 2025 |
| NIO752 | Disease‐modifying biologic | Tau | Anti‐tau antisense oligonucleotide | NCT05469360 | Novartis Pharmaceuticals | Feb 2023 | Oct 2024 |
| OLX‐07010 | Disease‐modifying small molecule | Tau | Inhibits tau self‐aggregation | NCT05696483 | Oligomerix, Inc | Jan 2023 | Dec 2024 |
| Probiotic Blend Capsule | Disease‐modifying small molecule | Gut‐Brain Axis | Inflammation/immunity | NCT06181513 | University of Nicosia | Dec 2022 | Jul 2024 |
| Psilocybin | Neuropsychiatric symptom | Neurotransmitter Receptors | Psychedelic | NCT04123314 | Johns Hopkins University | Mar 2021 | Dec 2024 |
| Remternetug | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody | NCT04451408 | Eli Lilly and Company | Jul 2020 | Aug 2024 |
| SHR‐1707 | Disease‐modifying biologic | Amyloid beta | Anti‐amyloid monoclonal antibody | NCT06114745 | Atridia Pty Ltd. | Jan 2024 | Nov 2025 |
The CADRO categories of agents in Phase 1 include six (24% of the Phase 1 pipeline) for amyloid; four (16%) each for transmitter receptors, tau, and inflammation; two (8%) each for ApoE/lipids and metabolism and bioenergetics; and one (4%) each for neurogenesis, synaptic plasticity/neuroprotection, and gut‐brain axis. Nine CADRO processes are included among the targets of Phase 1 compounds.
Fourteen (54%) Phase 1 trials are funded by biopharmaceutical industry sponsors.
Phase 1 trials require 1,262 participants for all ongoing trials. DMT trials will enroll 1054 participants, 705 for biologics and 349 for small molecules. Phase 1 cognitive enhancer trials require 176 participants, and trials of drugs for neuropsychiatric symptoms require 32 participants. DMT biologic trials have a mean of 54 participants; DMT small molecules have, on average, 39 participants; trials of cognitive enhancers have a mean trial size of 88 participants; and trials of agents for neuropsychiatric symptoms include 16 participants.
On average, DMT Phase 1 trials of biologics will require 1.5 years and small molecules will require 1.7 years to recruit the needed number of patients. Recruitment for cognitive enhancers and neuropsychiatric agents takes on average 2.2 and 3.6 years, respectively. Phase 1 trials of biologics have a mean treatment period of 12.7 months; Phase 1 trials of small molecules have a mean treatment period of 3.0 months. On average, cognitive enhancer treatment exposure periods in Phase 1 trials are 1.8 months and trials for neuropsychiatric agents have a 1‐month treatment period.
3.5. Biomarkers in trials
In Phase 3 trials, amyloid PET (11 trials) cerebrospinal fluid (CSF) amyloid (9 trials), CSF amyloid/tau ratios (2 trials) are collected at entry. Tau PET (1 trial), CSF p‐tau (4 trials), and CSF tau (2 trials) are collected at baseline. Plasma amyloid (3 trials), plasma p‐tau (1 trial), and amyloid/tau ratio (1 trial) were collected in other trials. Among DMT trials, 4 trials (all studying small molecules) did not include a biomarker as part of the entry criteria as listed on clinicaltrials.gov.
In Phase 2 trials amyloid PET (30 trials) CSF amyloid (25 trials), CSF amyloid/tau ratios (3 trials) are collected at entry. Tau PET (5 trials), CSF tau (6 trials), and CSF p‐tau (6 trials) are collected at baseline. Plasma amyloid (4 trials) and plasma p‐tau (1 trial) are collected at baseline in Phase 2 trials. Eleven DMT Phase 2 did not include biomarker collection at baseline as registered on clinicaltrials.gov.
CSF plays a key role in Phase 1 with CSF amyloid collected in four trials, tau in two trials, p‐tau in two trials, and amyloid/tau ratios in two trials. Amyloid and tau PET are used in three and one trials, respectively. Plasma p‐tau is collected in one as shown on clinicaltrials.gov. Eleven Phase 1 trials including seven DMTs do not describe collecting biomarkers at baseline as shown on clinicaltrials.gov.
Forty‐eight trials in the pipeline do not collect biomarkers at baseline. Seventeen of Phase 3, 20 of Phase 2, and 11 of Phase 1 trials do not collect biomarkers at trial entry. Six trials of DMT biologics (11% of DMT biologic trials), 16 (24% of trial of DMT small molecules) trials of DMT small molecules, 6 (35%) trials of cognitive enhancing agents, and 20 (87%) of trials of drugs addressing neuropsychiatric symptoms do not collect biomarkers at the time of trial initiation.
3.6. Trial participants
Considered together all current trials require 51,398 participants. To populate all current Phase 3 trials, 36,998 participants are required. Of these, 28,671 are needed for DMT trials (18,232 for trials of biological agents; 10,439 for small molecule trials), 1383 are needed for trials of cognitive enhancing agents, and 6944 for trials of drugs to treat neuropsychiatric symptoms. Populating all current Phase 2 trials, requires 13,138 participants. Of these, 11,037 are needed for DMT trials (5,286 for biologic trials; 5,751 for small molecule trials); 1185 are needed for trials of cognitive enhancing agents; and 916 for trials of drugs to treat neuropsychiatric symptoms. Current Phase 1 trials require 1,262 participants. Of these, 1,054 are needed for DMT trials (705 for biologic trials; 349 for small molecule trials), 176 are needed for trials of cognitive enhancing agents, and 32 for trials of drugs to treat neuropsychiatric symptoms.
3.7. Global trial distribution
Of the 45 trials in Phase 3 whose location is recorded, 56% (25 trials) are global with both North American and non‐North American sites. Twenty‐nine percent (13 trials) are performed in North America only and 16% (7 trials) are being performed only in non‐North American sites. Of the 85 trials in Phase 2 whose location is recorded, 25% (21 trials) are global with both North American and non‐North American site. Fifty‐one percent (43 trials) are performed in North America only, and 25% percent (21 trials) are being performed only in non‐North American sites. Of the 25 trials in which the Phase 1 trial location is included in the registration, 64% (N = 16) are conducted in North America only; 7 (28%) include only non‐North American sites; and 2 (8%) include both North American and non‐North American sites. In total, of the trials with location recorded, 31% are conducted globally with both North American and non‐North American sites; 46% are conducted in North America only; and 23% are conducted outside of North America with no North American sites participating.
3.8. Repurposed agents
The 39 repurposed agents comprise 31% of drugs in the AD drug development pipeline. They comprise nine agents in Phase 3; 28 agents of Phase 2, and seven agents of Phase 1. Six agents (15%) of repurposed agents are DMT biologics, 21 (54%) are DMT small molecules, 6 (15%) are cognitive enhancers, and 6 (15%) are drugs for NPS. The 21 repurposed DMT small molecules represent 40% of DMT small molecules in the AD pipeline.
The number of new chemical entities (NCEs) in the AD drug development pipeline declined disproportionately. There were 101 NCEs in the 2023 pipeline and there are 88 NCEs in the 2024 pipeline, representing a 13% drop.
3.9. Trial funders
Sixty percent of clinical trials are industry funded including 79% of Phase 3 trials, 51% of Phase 2 trials, and 54% of Phase 1 trials.
The funding pattern for trials with repurposed agents differs from that of most trials in the pipeline. Seventy‐seven percent of repurposed trials are funded by non‐industry sources. Fifty‐eight percent of Phase 3 trials, 82% of Phase 2 trials, and 86% of Phase 1 trials are funded through non‐industry sources.
For NCEs, 77% are industry funded including 92% of Phase 3 NCE trials, 70% of Phase 2 NCE trials, and 68% of Phase 1 NCE trials.
3.10. Combination therapies
Combination therapies are present in the 2024 AD drug development pipeline. One pharmacodynamic combination is Tdap, a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine associated with a reduction in the occurrence of AD in epidemiologic studies. 12 The Dominantly Inherited Alzheimer's Disease—Treatment Unit (DIAN‐TU) will include assessment of the combination of lecanemab, an anti‐amyloid agent, and E2814 and anti‐tau agent. Several trials are assessing the combination of dasatinib and quercetin for its senolytic activity in AD, and a combination of insulin and empagliflozin addresses metabolic and bioenergetic pathways. A combination of dronabinol (tetrahydrocannabinol) and palmitoylethanolamide (an endogenous cannabinoid with putative anti‐inflammatory properties) is being used in a trial to treat agitation in AD.
Pharmacokinetic combinations are also evident in the pipeline. AVP‐786 is a putative anti‐agitation agent consisting of dextromethorphan and quinidine 13 ; AXS‐05 is being assessed as an anti‐agitation agent consisting of dextromethorphan and bupropion 14 ; and KarXT, consisting of a combination of this xanomeline and trospium, is an being tested as an antipsychotic agent.
Other types of combinations include trontinemab, a combination of gantenerumab and a transferrin‐based brain‐shuttle, 15 and a combination of aducanumab with focused ultrasound to increase the entry of aducanumab into the brain through the blood‐brain barrier. 16
3.11. Total study duration and implications for drug development time requirements
Examination of the duration of each phase of drug development provides insight into the average time required to advance a drug through the pipeline. The study time on clinicaltrials.gov is comprised of the total of the recruitment time plus the treatment exposure time. The number represents the period from the initiation of the study to primary completion date of the study. As shown in Table 4, the mean durations of the three phases of drug development for a DMT biologic sum to 12.7 years, for a DMT small molecule 9.7 years, for a cognitive enhancing agent 10 years, and for a drug for neuropsychiatric symptoms 11.1 years.
TABLE 4.
Minimal, mean, and maximal study durations (treatment plus recruitment) for Phase 1, Phase 2, and Phase 3 trials of DMT biologics, DMT small molecules, cognitive enhancing agents, and drugs for neuropsychiatric syndromes (prevention trials not included).
| Therapeutic purpose | Relative duration | Phase 1 | Phase 2 | Phase 3 | Total; weeks | Total; years |
|---|---|---|---|---|---|---|
| DMT; biologic | Minimal | 49.0 | 47.9 | 104.3 | 201.2 | 3.9 |
| Mean | 141.9 | 218.9 | 302.3 | 663.1 | 12.7 | |
| Maximal | 286.3 | 635.0 | 773.9 | 1695.2 | 32.5 | |
| DMT; small molecule | Minimal | 74.6 | 58.6 | 43.7 | 176.9 | 3.4 |
| Mean | 124.7 | 184.5 | 195.8 | 505.0 | 9.7 | |
| Maximal | 191.3 | 495.9 | 291.7 | 978.9 | 18.8 | |
| Cognitive enhancing agent | Minimal | 53.9 | 52.1 | 156.6 | 262.6 | 5 |
| Mean | 136.8 | 178.9 | 206.7 | 522.4 | 10 | |
| Maximal | 219.7 | 446.0 | 243.7 | 909.4 | 17.4 | |
| Drugs for NPS | Minimal | 191.9 | 52.3 | 79.0 | 323.2 | 6.2 |
| Mean | 194.3 | 165.6 | 219.5 | 579.4 | 11.1 | |
| Maximal | 196.7 | 378.3 | 500.0 | 1075.0 | 20.6 |
Abbreviations: DMT, Disease‐modifying therapy; NPS, neuropsychiatric symptoms.
4. DISCUSSION
In 2023, the FDA approved ‐ 55 NCEs and 18 biologics—across all therapeutic areas. One drug—the anti‐amyloid MAB, lecanemab—was approved for AD. 7 Another agent, brexpiprazole, was approved by the FDA for the treatment of agitation associated with dementia due to AD. It is not a novel agent since it is approved for other indications; it is the first drug approved for agitation or any neuropsychiatric syndrome associated with AD. There were approvals of treatments for two other neurodegenerative diseases including toferson—an antisense oligonucleotide (ASO)—to treat amyotrophic lateral sclerosis in adults who have a superoxide dismutase 1 (SOD1) gene mutation and omaveloxolone to treat Friedreich's ataxia. This trend suggests that new means of intervening in neurodegenerative disorders are being discovered.
The 164 trials and 127 drugs in the current AD drug development pipeline compares to 187 trials assessing 141 drugs for AD in 2023. There is a disproportionate decrease in the number of NCEs in the pipeline (e.g., from 101 to 88, representing a 13% decrease). NCEs are more likely to be sponsored by industry and more likely to be advanced to Phase 3 if early phase trials are promising. Repurposed agents are disproportionately funded through non‐industry sources and are less likely to advance to Phase 3.
Currently, there are 24 agents targeting neuroinflammation in the pipeline, comprising 19% of all therapeutic agents in the pipeline. The canonical targets of amyloid, tau, and neurodegeneration (ATN), 17 have 20 (16% of the pipeline agents), 10 (8%), and 0 active agents, respectively, in the pipeline representing 24% of agents. Within each of the CADRO categories, nearly every agent being studied has a different MoA and a different approach to modulating the target process.
Recruitment of a sufficient number of trial participants is a major issue for conduct of clinical trials in a timely way. On average, it takes 2.1 years to recruit the populations for a Phase 1 trial, 2.5 years to recruit enough subjects for a Phase 2 trial, and 3.2 years for recruitment of subjects for a Phase 3 trial. These recruitment time frames are largely applicable across therapeutic areas. Although many fewer patients are required for clinical trials assessing agents for the treatment of neuropsychiatric syndromes, enrolling participants with AD and exhibiting specific behavioral features is more challenging than recruiting participants with AD and no required neuropsychiatric symptoms. The approval of lecanemab and the possible approval of donanemab may affect recruitment if patients choose approved over experimental therapies. Identifying strategies to increase interest in trial participation and improve trial recruitment could have a major impact on accelerating drug development for AD.
Study of the mean, minimal, and maximal duration of trials for each of the phases for drugs of different therapeutic classes provides insight into the total duration of drug development programs for AD. DMT small molecules are the most common agent in the AD pipeline. On average, these drugs require 9.7 years to complete one trial in each of the three development phases. There is typically 1 year between phases for data review, next phase planning, and regulatory interactions. This would add 2 years to the total development time. In addition, most trials require at least 1 year of nonclinical studies prior to first‐in‐human exposures. An agent that goes for regulatory review following a Phase 3 trial will add 12–18 months to the total program time. Beginning with 9.7 years as the mean time for one trial in each phase; adding 2 years for between trial decisions and 1 year for nonclinical testing would result in an average development time of 13 years. A total of 6‐12 months of additional time would be required if the Phase 3 results are positive and FDA review of possible approval is required (total 13.5‐14 years). This observation is similar to the conclusion reached by Scott and colleagues in a 2014 study of the AD drug development pipeline. 18 This might suggest that the increased technological complexity involved in current AD trials is not increasing the drug development duration and that greater experience in clinical trial conduct is not decreasing the time required for AD drug development.
Combination therapies including pharmacodynamic combinations, pharmacokinetic combinations and combinations aimed at enhancing penetration of the blood brain barrier are evident in the 2024 AD drug development pipeline. Combinations of experimental agents with approved anti‐amyloid monoclonal antibodies are anticipated as patients being treated with these agents participate in clinical trials of emerging therapeutics.
AD therapeutics have made remarkable progress. The approval of aducanumab in 2021 ended a 17 year period during which no new AD therapeutics were approved. 5 The accelerated approval of lecanemab followed by its standard approval in 2023 was based on growing evidence that reduction of amyloid plaque as demonstrated by amyloid PET was associated with slowing of cognitive decline. 7 Donanemab is currently under review by the FDA and interrogation of the trial data will provide more insight into the relationship between slowing of cognitive decline and findings on amyloid and tau PET. 6 The atypical antipsychotic, brexpiprazole, was approved in 2023 for the treatment of agitation in dementia associated with AD, becoming the first approved therapy for any neuropsychiatric syndrome in AD. 8 Data are emerging that may allow plasma biomarkers to replace amyloid PET and CSF studies of AD markers for diagnosis. 19 This will facilitate patient recruitment and improve trial quality by confirming the presence of the target disease. Learnings about appropriate biological targets, effective pharmacology, biomarkers to guide diagnosis and clinical trials, and new trial designs and analysis are positioned to accelerate drug development for AD. Continued investment from governmental sources, advocacy groups, philanthropies, and biotechnology and pharmaceutical companies is critical to capitalize on this growing knowledge base and advance therapeutics for patients requiring new therapies.
CONFLICT OF INTEREST STATEMENT
J.C. has provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, ALZpath, Aprinoia, AriBio, Artery, Biogen, Biohaven, BioVie, BioXcel, Bristol‐Myers Squib, Cassava, Cerecin, Diadem, Eisai, GAP Foundation, GemVax, Janssen, Jocasta, Karuna, Lighthouse, Lilly, Lundbeck, LSP/eqt, Mangrove Therapeutics, Merck, NervGen, New Amsterdam, Novo Nordisk, Oligomerix, ONO, Optoceutics, Otsuka, Oxford Brain Diagnostics, Prothena, ReMYND, Roche, Sage Therapeutics, Signant Health, Simcere, sinaptica, Suven, TrueBinding, Vaxxinity, and Wren pharmaceutical, assessment, and investment companies. J.C. owns the copyright of the Neuropsychiatric Inventory. J.C. has stocks/options in Artery, Vaxxinity, Behrens, Alzheon, MedAvante‐Prophase, Acumen. GL is a full‐time employee of Eisia Co, Ltd. KZ is CEO of CNS Innovations. Y.Z. and J.F. declare no competing interests. F.C. declare no other competing interests. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Not applicable. All data are from an anonymized publicly available clinical trial registry (clinicaltrials.gov). No individual patient‐level data is available on the registry.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
J.C. is supported by NIA grant R35AG71476; NIA R25 AG083721‐01; Alzheimer's Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; Joy Chambers‐Grundy Endowment. F.C. is supported by the National Institute of Neurological Disorders and Stroke (NINDS) under Award Number RF1NS133812, and the Alzheimer's Association award (ALZDISCOVERY‐1051936) to F.C. The funding sources had no role in the preparation of this manuscript.
Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer's disease drug development pipeline: 2024. Alzheimer's Dement. 2024;10:e12465. 10.1002/trc2.12465
REFERENCES
- 1. 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598‐1695. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization, Health and Ageing. https://www.who.int/news‐room/fact‐sheets/detail/ageing‐and‐health
- 3. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022;79:1242‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta‐analysis. JAMA. 2015;313:1924‐1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9:197‐210. [DOI] [PubMed] [Google Scholar]
- 6. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER‐ALZ 2 randomized clinical trial. JAMA. 2023;330:512‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. [DOI] [PubMed] [Google Scholar]
- 8. Lee D, Slomkowski M, Hefting N, et al. Brexpiprazole for the treatment of agitation in Alzheimer dementia: A randomized clinical trial. JAMA Neurol. 2023;80(12):1307‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burris JF, Puglisi JT. Impact of federal regulatory changes on clinical pharmacology and drug development: the Common Rule and the 21st Century Cures Act. J Clin Pharmacol. 2018;58:281‐285. [DOI] [PubMed] [Google Scholar]
- 10. Al‐Durra M, Nolan RP, Seto E, et al. Prospective registration and reporting of trial number in randomised clinical trials: global cross sectional study of the adoption of ICMJE and Declaration of Helsinki recommendations. BMJ. 2020;369:m982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Refolo LM, Snyder H, Liggins C, et al. Common Alzheimer's disease research ontology: national institute on aging and Alzheimer's association collaborative project. Alzheimers Dement. 2012;8:372‐375. [DOI] [PubMed] [Google Scholar]
- 12. Harris K, Ling Y, Bukhbinder AS, et al. The impact of routine vaccinations on Alzheimer's disease risk in persons 65 years and older: a claims‐based cohort study using propensity score matching. J Alzheimers Dis. 2023;95:703‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khoury R, Marx C, Mirgati S, et al. AVP‐786 as a promising treatment option for Alzheimer's disease including agitation. Expert Opin Pharmacother. 2021;22:783‐795. [DOI] [PubMed] [Google Scholar]
- 14. Ward K, Citrome L. AXS‐05: an investigational treatment for Alzheimer's disease‐associated agitation. Expert Opin Investig Drugs. 2022;31:773‐780. [DOI] [PubMed] [Google Scholar]
- 15. Grimm HP, Schumacher V, Schafer M, et al. Delivery of the Brainshuttle amyloid‐beta antibody fusion trontinemab to non‐human primate brain and projected efficacious dose regimens in humans. MAbs. 2023;15:2261509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rezai AR, D'Haese PF, Finomore V, et al. Ultrasound blood‐brain barrier opening and aducanumab in Alzheimer's disease. N Engl J Med. 2024;390:55‐62. [DOI] [PubMed] [Google Scholar]
- 17. Jack CR, Jr., Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scott TJ, O'Connor AC, Link AN, et al. Economic analysis of opportunities to accelerate Alzheimer's disease research and development. Ann N Y Acad Sci. 2014;1313:17‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mila‐Aloma M, Ashton NJ, Shekari M, et al. Plasma p‐tau231 and p‐tau217 as state markers of amyloid‐beta pathology in preclinical Alzheimer's disease. Nat Med. 2022;28:1797‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


