Abstract
Tumors are highly complex and heterogenous ecosystems where malignant cells interact with healthy cells and the surrounding extracellular matrix (ECM). Solid tumors contain large ECM deposits that can constitute up to 60% of the tumor mass. This supports the survival and growth of cancerous cells and plays a critical role in the response to immune therapy. There is untapped potential in targeting the ECM and cell-ECM interactions to improve existing immune therapy and explore novel therapeutic strategies. The most abundant proteins in the ECM are the collagen family. There are 28 different collagen subtypes that can undergo several post-translational modifications (PTMs), which alter both their structure and functionality. Here, we review current knowledge on tumor collagen composition and the consequences of collagen PTMs affecting receptor binding, cell migration and tumor stiffness. Furthermore, we discuss how these alterations impact tumor immune responses and how collagen could be targeted to treat cancer.
Introduction
The extracellular matrix (ECM) is a constantly evolving structure that is produced, modified, remodeled and maintained by the cells residing within it. The ECM is dynamic and responds to changes in the local and systemic environment, making it a central player in tissue physiology and pathology such as cancer [1]. Tissues have unique ECM compositions tailored to their specific mechanical and structural demands which can be further modified in pathological conditions. The main component of the ECM are collagens consisting of 28 different types [2]. During homeostasis, the biophysical properties of collagen are critical for maintaining tissue integrity. Collagen stiffness is influenced by the size of its fibers; short fibers offer a greater range of orientation possibilities, enhancing tissue permeability, while long fibers align closely, resulting in cells organizing in the same orientation within the tissue [3]. This close alignment facilitates the formation of collagen crosslinks contributing to increased ECM stiffness [4]. In the context of tissue repair, additional collagen crosslinks serve as a protective mechanism to aid wound closure.
Cancer is viewed as a form of excessive wound-healing as similar pathways are activated in wound healing and tumorigenesis [5]. For instance, dense ECM that forms part of the scar tissue in wound healing is similar to collagen deposits known as desmoplasia in cancer, which correlate with poor prognosis [5, 6]. During tumor development, desmoplasia is induced by cancer-associated fibroblasts (CAF), macrophages and tumor cells and the produced collagen is resistant to enzymatic degradation [7–11]. CAFs are the main players in collagen remodeling in cancer. These are highly activated fibroblasts and which, in contrast to normal activated fibroblasts after tissue repair, do not undergo apoptosis or return to resting state [12]. CAFs are a heterogeneous population of cells implicated in different stages of tumor development, from primary growth to metastasis, in different tumor types [11, 12]. However, a number of studies propose an anti-tumor role for CAFs, as ablation of CAFs results in more aggressive phenotypes [13].
A hallmark of cancer is the epithelial-to-mesenchymal transition (EMT). Epithelial cells and endothelial cells secrete a laminin-rich ECM while mesenchymal cells secrete a collagen-rich ECM [14, 15]. Both EMT and desmoplasia enhance tumor stiffness by increasing the mechanical strength, density and crosslinking of collagen [16, 17]. Cells sense the mechanical properties of their surroundings and in stiffer matrices, pathways promoting proliferation, survival and invasiveness of tumor cells are triggered.
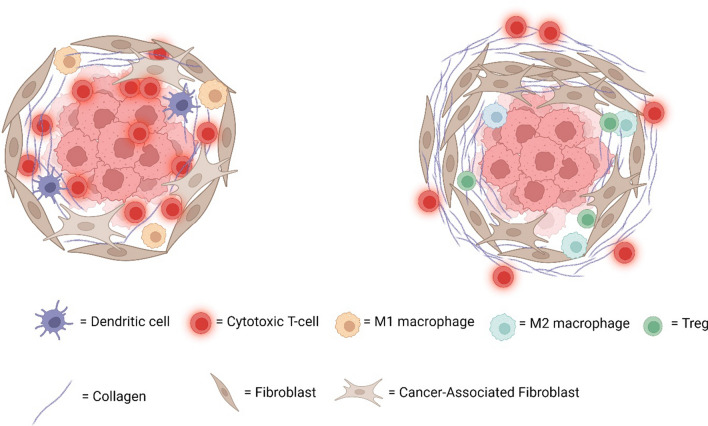
The ECM also influences the migration of immune cells that promote or prevent tumor growth, depending on tumor type and disease stage [7, 9, 18, 19]. T-cell migration is reduced in dense, stiff matrices compared to less dense, more flexible matrices. T cells preferentially migrate along long collagen fibers using integrin-independent migration while in disorganized collagen structures they use integrin-dependent migration [20]. Solid tumors can be classified into inflamed “hot” tumors and non-inflamed “cold” (Fig. 1). Immune inflamed tumors have a high infiltration of cytotoxic T-cells and in general are responsive to immunotherapy. Non-inflamed tumors are characterized by an absence or low numbers of infiltrated T-cells, increased collagen deposition and the presence of a stromal barrier, abnormal vasculature, lack of chemokines, hypoxia or activated oncogenic pathways [21–24]. Non-inflamed tumors have increased resistance to immunotherapy and a higher chance of disease recurrence within five years [24]. The mechanism behind the resistance is not fully understood but there is an increasing interest in the role of the ECM in immune cell infiltration of tumors. For example, the ability of T-cells to reach the tumor core of lung and ovarian tumors is hindered by the collagen alignment in the tumor periphery [25].
Fig. 1.
Inflamed (left) and non-inflamed tumors respond differently to immune checkpoint blockade therapy. Inflamed tumors contain more T cells, antigen presenting cells and inflammatory M1 macrophages compared to non-inflamed tumors. In non-inflamed tumors, T cells are mainly present at the tumor border and have difficulties infiltrating into the tumor. These are also characterized by more tumor-suppressing immune cells such as M2-macrophages and T-regulatory cells and high abundance of collagen produced by fibroblasts and cancer-associated fibroblasts
Collagen expression differs between different cancer types but in general tumors maintain the collagen lineament of the tissue of origin [26].However, collagens can undergo extensive post-translational modifications (PTM) [27], which can result in an almost infinite array of matrices. Manipulation of specific PTMs could provide new therapeutic possibilities to inhibit or correct localized pathological alterations to the ECM that occur in cancer or tissue fibrosis. Here, we review current knowledge on the impact of collagen and collagen PTMs on the antitumor response and their potential as therapeutic target.
Collagen-receptor interactions affect tumor growth and anti-tumor immune responses
Cells can interact with collagens through at least six different groups of receptors, namely: (1) integrins, (2) the Discoidin Domain Receptor family (DDR1 and DDR2), (3) the mannose receptor family (4) Glycoprotein VI (GPVI) (5) Osteoclast-associated receptor (OSCAR), (6) Leukocyte Associated Immunoglobulin Like Receptor 1 (LAIR-1) [28]. The interaction of collagen with these receptors regulates diverse responses, encompassing cell adhesion, matrix metalloprotease (MMP) activity, thrombus formation, cell survival and proliferation, cytokine production, immune effector function and collagen remodeling. (Table 1).
Table 1.
Groups of collagen receptors
| Receptors | Expression | Function upon binding collagen |
|---|---|---|
| Integrins | All cells | Regulation of cell adhesion [29] |
| Discoidin domain Receptor family | Epithelial cells and mesenchymal cells | Regulation of cell migration, differentiation, proliferation, survival, ECM remodeling [30] |
| Mannose receptor family | Macrophages and fibroblasts | Endocytosis of collagen for lysomal degradation [31] |
| Glycoprotein VI | Platelets | Platelet activation [32] |
| Osteoclast-associated receptor | Osteoclasts, myeloid cells | Osteoclast differentiation, Monocyte survival [33] |
| Leukocyte Associated Immunoglobulin Like Receptor 1 | Immune cells | Inhibition of immune cell activity [34] |
Both immune and tumor cells bind to collagens via integrins such as α1β1, α2β1, α3β1, α4β1, α10β1, and α11β1 for migration [29, 35, 36]. Integrins expressed by tumors interact with collagen and overcomes tumor dormancy by increasing tumor cell motility. In contrast, collagen-integrin interaction on immune cells is anti-tumorigenic and promotes migration of T-cells and natural killer cells into the tumor [37]. Integrin a3 (itga3) mRNA expression is increased by PDAC tumor cells and negatively correlates with T-cell presence which is associated with poor prognosis [38].
The DDR family consists of receptor tyrosine kinases that bind to collagen and induce MMP secretion and regulate cellular functions [30]. DDR1 expression is positively correlated with tumor stage and promotes tumor cell proliferation, migration and invasion [39, 40]. In PDAC, the DDR1–NF-κB–p62–NRF2 cascade can be activated by cleaved collagen I which limits metabolism and growth of tumours [41]. In contrast, cleavage resistant collagen I induces proteasomal degradation of DDR1. Binding of DDR1-ectodomain to collagen mechanistically aligns collagen fibers, independent of receptor activation [42]. Tight fiber alignment can prevent immune cells from infiltrating into the tumor in breast cancer. DDR2 is overexpressed on CAFs and regulates force-mediated collagen fiber remodeling that results in a stiffer tumor microenvironment [43, 44].
The uPAR-associated protein (uPARAP/Endo180, encoded by MRC2) is an endocytic transmembrane receptor for collagen of the mannose receptor family [31]. uPARAP facilitates the degradation of collagen and therefore plays a crucial role in ECM homeostasis, tissue remodeling, and turnover. Macrophages and fibroblasts remodel collagens via uPARAP by targeting them to lysosomal degradation. Lower expression of uPARAP/Endo180 in metastatic melanoma and advanced urothelial cancer results in increased responsiveness to immune checkpoint blockade therapy [45].
Both GPVI and LAIR-1 recognize Glycine-Proline-Hydroxyproline motif repeats in collagens but have opposing effects on immune activation. GPVI signals through an immunoreceptor tyrosine-based activation motif (ITAM) to activate platelets resulting in thrombus formation [32]. Platelets can interact with tumor cells shielding them from shear stress in the circulation and preventing recognition by natural killer cells [46, 47]. In contrast, LAIR-1 is an immune inhibitory receptor that signals through an immunoreceptor tyrosine-based inhibition motif (ITIM) and is broadly expressed on immune cells, including T cells [34, 48]. Collagens can set a threshold for immune cell activation through LAIR-1. Collagen deposition in tumors could therefore protect tumor cells from the immune system through LAIR-1 [49].
Changes in collagen composition during tumor progression
Collagens form a diverse family of proteins with multiple subtypes, each of which has its specific structural and functional characteristics (Table 2). The general structure of collagens consists of three polypeptide α-chains that fold into a triple helix, improving the thermal stability of the collagen. The human genome encodes for 44 different forms of α-chains to produce a total of 28 types of collagens [50]. Depending on the collagen type, this triple-helix is a homotrimer or mixture of two or three different α-chains [51]. The most common motif within α-chains is a (Gly-X-Y)n-repeat in which every third amino acid is a glycine followed by two non-glycines. The small size of glycine is crucial for the folding of the triple helix. While X–Y can be all amino acids, they most commonly are proline and hydroxyproline, respectively.
Table 2.
Collagens organized by their subtype and tissue of origin [52]
| Subtype | Collagen | Tissue of origin |
|---|---|---|
| Fibril-forming |
I II III V XI XXIV XXVII |
Bone, cartilage, skin, tendon Cartilage, vitreous body Blood vessels, bone, skin Blood vessels, bone, cornea, placenta, skin, tendon Cartilage, placenta, tendon Bone, cornea Cartilage |
| Network-forming |
IV VIII X |
Basement membrane, blood vessels, connective tissue Calcifying cartilage |
| Beaded-filament |
VI XXVI XXVIII |
Bone, cartilage, cornea, skin, Ovary, testis Basement membrane |
| Anchoring | VII | Basement membrane |
| Transmembrane |
XIII XVII |
Cell junctions Hemidesmosomes |
| FACITs |
IX XII XIV XVI XIX XX XXI XXII |
Cartilage, vitreous body Connective tissue Connective tissue Cartilage, papillary dermis, placenta Basement membrane, Widespread Widespread Tissue junctions |
Fibrillar collagens
The classical fibrillar or fibril-forming collagens include collagen I, II, III, V, and XI, with collagen I as the most abundant collagen throughout the body. They form long and highly organized fibrils and are the dominant component of the ECM and important contributors to cancer progression if mutated or exceedingly present [10, 53]. Long aligned fibrils provide an easy route for tumor cells to migrate out of the tumor nest while excluding immune cells [42, 54]. Pancreatic tumor cells produce unique collagen I homotrimers (a1/a1/a1) instead of the normal collagen I heterotrimers (a1/a2/a1), enhancing resistance to MMP degradation and tumor progression [38]. Homotrimeric collagen I increases proliferation of tumor cells through DDR1 and signaling through ITGA3 compared to heterotrimeric collagen I [38]. In mice models, deletion of homotrimeric collagen I or suppression of ITGA3 in tumor cells improved overall survival and tumor T-cell infiltration.
While collagen XI is a minor collagen and preferentially expressed in cartilage in homeostasis, several studies report it to be present in tumors and propose to use it as cancer-biomarker [55]. In ovarian cancer, increased expression of collagen I and XI is associated with disease progression. In non-small lung cancer, collagen XI expression in the tumor induces a negative feedback loop reducing CAF-mediated collagen remodeling and CAF migration as collagen XI sterically interferes with collagen I- integrin-binding [55].
In several cancer types, collagen V is over-expressed in non-inflamed tumors compared to inflamed tumors, in metastatic tumors compared to primary tumors and in patients resistant to cytotoxic drugs [56–60]. In contrast to most fibrillar collagens, collagen III plays a role in suppressing rather than promoting the metastatic processes such as adhesion, migration and invasion of tumor cells in a murine breast cancer model [61]. In human head and neck squamous cell carcinomas, collagen III is the most abundant collagen type in patients with dormant tumors compared to tumors from patients with additional lymph node metastases [62]. The collagen architecture of dormant tumors is characterized by wavy collagen fibers and low degree of linear organization compared to proliferative tumors [62].
Basement membrane collagens
Network-forming collagens such as collagen IV, -VIII and -X form open network structures instead of fibers. Collagen IV is an essential part of the basement membrane and upregulated in several types of cancer promoting cell proliferation, migration, and invasion [63]. Network-collagens also play an important role in mediating platelet interaction with tumor cells and thereby enhance metastasis [47]. Collagen VIII is normally expressed in vascular smooth muscle cells (SMC) and plays an important role in vascular remodeling. High expression of collagen VIII in tumors is associated with poor prognosis, likely through SMC survival and migration, enhancing angiogenesis [64]. Lastly, expression of collagen X is high in immune-excluded triple-negative breast cancers that are resistant to anti- programmed cell death-1 (PD-1) ICB therapy [60].
Minor collagens
Although minor collagens are less abundant in human body, they do play a crucial role in collagen structures. Beaded-filament-forming collagens such as collagen VI are closely related to basement membrane collagens. Breast cancer adipocytes upregulate collagen VI expression during tumorigenesis [65, 66]. Collagen VI is also found near vascular structures and increased in colorectal cancer [67].
Anchoring such as collagen VII and Transmembrane collagens such as XIII and XVII have a role in spatial compartmentalization and enhancing cell–cell and cell–matrix interaction, respectively [2, 68–70]. In breast cancer, collagen XIII activates the Tumor Growth Factor-β (TGF-β) pathway through B1 integrin, promoting cancer progression and metastasis [71]. In epithelial cancers, overexpression and increased ectodomain shedding of the transmembrane collagen XVII leads to tumorigenesis and is associated with poor prognosis [72]. Fibril-associated Collagens with Interrupted Triple Helices (FACIT) are important mediators in the organization of the collagen fibrils and the density of the ECM. In breast cancer FACIT collagens are highly present and inhibit fibril fusion [73, 74].
Collagen post-translational modifications and their impact on the anti-tumor immune response
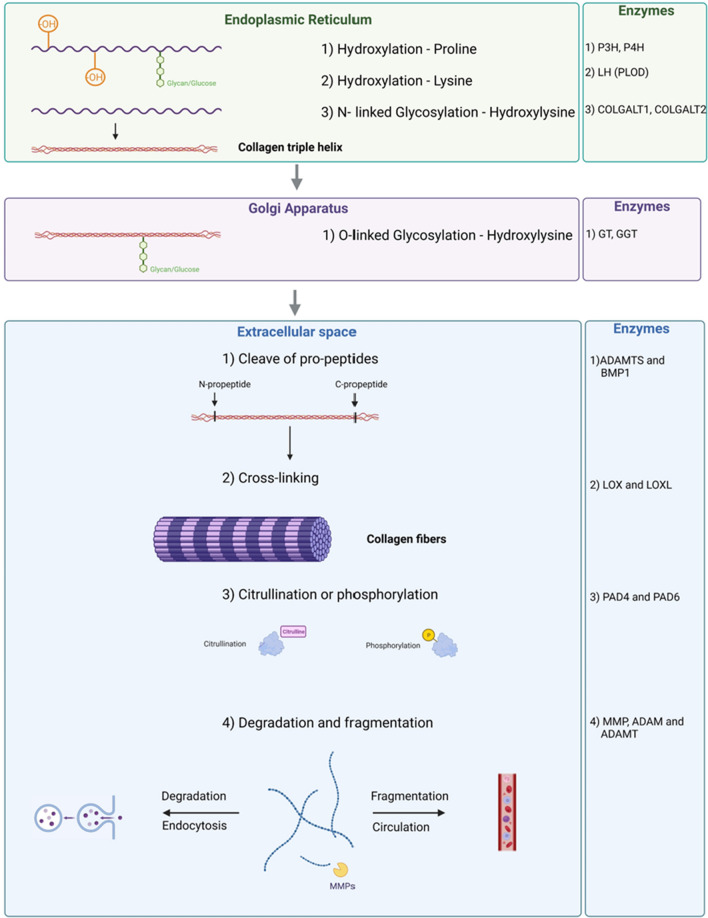
During collagen biosynthesis, the collagen structure undergoes several PTMs. PTMs can modify protein function by altering protein structure, protein–protein interactions, and degradation. PTMs take place intra- and extracellularly and once collagen is in its triple helical form, further PTMs such as hydroxylation and glycosylation will not occur [75, 76] (Fig. 2).
Fig. 2.
Schematic overview of collagen post-translational modifications. Hydroxylation of proline and lysine takes place in the endoplasmic reticulum while N-linked glycosylation and O-linked glycosylation of hydroxylysine take place in the endoplasmic Reticulum and golgi apparatus, respectively. The collagen triple helix is formed intracellularly and in most subtypes of collagen N- and C-propeptides are cleaved off after secretion before the collagen can be crosslinked to form collagen fibers. Collagen can also be modified by citrullination or phosphorylation. During collagen remodeling, collagen is normally degraded while in cancer also fragmentation can take place leaving collagen fragments in the circulation [2]
Proline hydroxylation
The synthesis of fibrillar collagen begins with the formation of procollagen in the endoplasmic reticulum followed by proline and lysine hydroxylation and glycosylation [77]. Proline hydroxylation on fibrillar collagen by prolyl-4-hydroxylases (P4HA1, P4HA2 and P4HB) is the most frequent PTM and improves stability of the collagen triple helix by forming strong electronegative bonds. Despite the commonality of proline hydroxylation, each collagen helix exhibits a distinctive hydroxylation pattern [78]. This variability in hydroxyproline localization within the collagen structure impacts protein folding and triple helical configuration and affects protease access to the collagen structure [79–81]. Hydroxylated proline sites are important for cells to bind and interact with collagen via integrins and DDR receptors. Hence, changes in hydroxylated proline sites impact adhesion, proliferation, and cell migration [82]. Hydroxyproline and P4HA1 stabilize Hypoxia-inducible factor 1-alpha (HIF-α) enhancing the hypoxia cycle, proline synthesis and collagen deposition [83, 84] (Table 3). In The Cancer Genome Atlas (TCGA) database, high frequency of mutations in P4HA1 are associated with lower progression free survival [85]. P4HB is overexpressed in bladder and colon cancer, increasing cell proliferation, migration and reducing apoptosis and in hepatocellular carcinoma inducing EMT [86]. For healthy collagen IV it is important to undergo PTMs such as 3-proline hydroxylation as the absence of this modification leads to platelet aggregation, which supports tumors [63].
Table 3.
Amino acids in collagen with their corresponding post-translation modification, added group and enzyme involved. The outcome of overexpression of each modification is described in the last column
| Amino acids | PTM | Group | Enzymes | Outcome if increased |
|---|---|---|---|---|
| Proline | Hydroxylation (ER) | –OH | P4Hs, P3Hs | Hypoxia |
| Lysine | Hydroxylation (ER) | –OH | LH1, LH2, LH3 | Increased glycosylation, crosslinking and fibrosis |
| Hydroxylysine |
N-Linked (ER) O-linked (GA) Glycosylation |
Glucose or galactose |
COLGALT1, COLGALT2, GT, GGT |
Increased crosslinking, matrix stiffness |
| Lysine/Hydroxylysine | Oxidation (ES) | –O | LOX, LOXL1, LOXL2, LOXL3, LOXL4 | Fibrosis, matrix stiffness |
| Arginine | Citrullination (ES) |
Citrulline H2O to NH4 + |
PAD4 |
Role in protein folding, apoptosis, TGF-β Pathway, receptor binding |
Loss of P3H2 expression is found in breast cancer and enhances cell proliferation and is therefore pro-tumorigenic [87]. Taken together, a pattern of increased hydroxylation by prolyl-4-hydroxylases but decreased hydroxylation of 3-proline enhances tumorigenesis. This suggests that specific prolines within the collagen structure may be more susceptible to hydroxylation under pathological conditions, supporting that post-translational changes to collagen structure by specific enzymes could be used as biomarkers [82].
Lysine hydroxylation
Besides proline, lysine also undergoes hydroxylation which stabilizes collagen triple helixes, increases the stiffness and reduces the sensitivity of collagen to proteases [88, 89]. Hydroxylation of lysine is catalyzed by lysyl hydroxylase (LH) and most commonly occurs at lysine residues in the Y-position of the Gly-X-Y sequence repeat. The α2-chains have a higher content of this repeat compared to the α1-chains resulting in more hydroxylated lysine in heterotrimeric collagens compared to homotrimeric collagens [76, 90]. Whether a lysine is hydroxylated in collagen depends on the specific amino acid sequence, activity of the hydroxylation enzyme in the collagen-producing cells and/or the collagen conformation during its exposure to the enzyme. For example, hydroxylation by LH3 is especially important in collagen IV as LH3 deficient cell lines accumulate intracellular collagen IV, have reduced secretion and form instable triple helices [91]. Increased hydroxylation of lysine residues within telopeptides by LH2 is associated with fibrotic conditions by increasing collagen crosslinking and stiffness, protecting the collagen from degradation [89] (Table 3). This supports tumor cells by serving as a physical barrier for therapeutics and promoting metastasis [88, 89, 92]. In contrast, hypoxia decreases hydroxylation of lysine residues [78, 93]. In some tumors, mutations in human LH2 (D689A) lead to loss of LH activity reduction of tumor cell migration [94, 95].
Glycosylation and glycation
Glycosylation and glycation are enzymatic and non-enzymatic reactions, respectively, of glucose, glucose metabolites and other reducing sugars with different substrates, such as proteins, lipids, and nucleic acids. Similar to other modifications, there is high variability in glycosylation patterns between different types of collagen [90]. Glycosylation is most common in less organized collagens such as collagen IV. N-linked glycosylation takes place in the endoplasmic reticulum by Collagen Beta(1-O)Galactosyltransferases. For O-glycosylation of collagens there are two glycosyltransferases that catalyze this process: hydroxylysyl galactosyltransferase (GT) and galactosylhydroxylysyl glucosyltransferase (GGT) [75]. These enzymes add glucose and galactose to the -OH group of hydroxylysine in the Golgi apparatus. The glycosylation of hydroxylysine is crucial in collagen IV and VI to assemble basement membrane. Defects in LH3 prevents intracellular tetramerization of collagen VI and its secretion [90]. Non-enzymatic glycation can also occur on fibrillar collagens, resulting in a lower number of crosslinks and reduced collagen stiffness [35]. Adding sugar molecules to collagen fibrils also impacts theirs functionality as it prohibits cell adhesion by blocking integrin-binding places on the structure [35]. Although tumor cells carry many mutations, documented mutations of genes encoding glycosyltransferases are relatively rare in tumor cells highlighting their importance for collagen stability. Overall, further mechanistic investigation is required to understand the role of collagen glycosylation in cancer and assess its potential as a novel therapeutic target.
Propeptide cleavage
After the procollagen is released to the extracellular space, the N- and C-propeptides of fibrillar collagens are cleaved off. Cleaving of the C-propeptides of collagen I by bone morphogenetic protein 1 (BMP1) impacts the fibril formation and thus the orientation of the collagen structures. Mutations in BMP1 are documented in individuals with gastroschisis and osteogenesis imperfecta and might potentially function as a therapeutic biomarker for individuals with cancer [96–99]. However, in case of collagen IV only the N-propeptides are cleaved off. The C-propeptides in the molecules bind head-to-head to form a network, with covalent intra- and intermolecular cross-linking into the subtype structure [77, 100]. The cleaved off N-propeptide is a non-collagenous fragment that is also known as arresten which acts as angiogenesis inhibitor [101, 102]. Arresten inhibits endothelial cell proliferation, migration and tube formation and reduces subcutaneous tumor growth in mice and suppresses squamous cell carcinoma invasion [101–103].
Crosslinking affects matrix stiffness
Important enzymes in the regulation of fibril collagen are lysyl oxidase (LOXs) and lysyl oxidase-like (LOXL) that catalyze oxidation of lysine and hydroxylysine in a copper-dependent way [100, 104, 105]. LOX can only catalyze lysine after removal of the C-propeptides which prevents collagen from becoming anionic. High concentrations of copper ions in tumors promote LOX secretion [104]. The hypoxic TME increases tumor cell expression of LOX and promotes collagen covalent crosslinking, which increases matrix stiffness [20, 106–109] (Table 3). LOXL2 suppression in lung tumors mice, increases cytotoxic T-cell infiltration and decreases cytotoxic T-cell exhaustion [22].
Mutations in LOX are associated with colon tumor pathogenesis [110]. Despite the wealth of information available on the overexpression of LOXL2 in tumors, there are scarce data regarding the presence of genetic mutations in LOXL2. Mutations in LOXL2 are identified in skin cutaneous melanoma and uterine corpus endometrial carcinoma. However, LOXL2 mutational burden does not impact the fitness of human tumors, although it is possible that specific mutations could be important in specific types of tumors [111].
Citrullination and receptor binding
Citrullination changes an arginine residue into a citrulline residue, which is a none-standard amino acid [112]. Intracellular protein arginine deiminases (PADs) catalyse this process and typically become active when calcium levels exceed the normal physiological concentration, for instance during apoptosis [112]. Transitioning arginines to citrullines, the reduces the positive charge of the collagen molecule, increasing hydrophobicity. PAD4 citrullinates collagen and is increasingly expressed in several types of cancer, particularly in metastases [112, 113]. PAD4 is mainly produced by neutrophils and deletion of this enzyme in mouse tumor models, results in lower neutrophil infiltration in tumors and reduced tumor progression [114]. In Rheumatoid arthritis citrullinated collagen can bind LAIR-1 as decoy ligand impairing the immunosuppressive function of LAIR-1 on T cells [115]. In cancer, impaired LAIR-1 mediated inhibition could lead to inflammation in inappropriate sites, depending on the citrullinated collagen location within the tumor. Additionally, citrullinated collagen decreases integrin-mediated cell adhesion, potentially reducing the capacity of immune cells to migrate into the tumor [116] (Table 3). A genome-wide SNP study showed a significant correlation between cutaneous-basal cell carcinoma risk and mutations in the PAD4/PAD6 locus at 1p36 [117].
Phosphorylation
Network-forming collagens, short-chains collagens and FACIT collagens such as collagen III, IV, V, VI, XVII XXVII can be phosphorylated [118]. Phosphorylation of collagen XVII by ecto-CK2 blocks its ectodomain shedding by Tumor necrosis factor alpha (TNF alpha)-converting enzyme (TACE), affecting the adhesion and motility of epithelial cells [119]. In squamous cell carcinoma, shed collagen XVII is suggested to promote tumor progression and invasion [72]. Therefore, it is tempting to speculate that collagen phosphorylation has a protective role in epithelial cancers but further research has to be conducted to elucidate this.
MMP degradation and collagen fragments
The ECM undergoes constant remodeling involving collagen cleavage by proteases such as matrix metalloproteases (MMPs), a disintegrin and metalloproteinases (ADAMs) and ADAM with thrombospondin motifs (ADAMTs) [120, 121]. These enzymes directly influence the biological characteristics and functions of collagen by uncovering cryptic sites, releasing collagen-bound growth factors and degrading collagen [122]. Compared to intact collagen fragmented collagens are unstable and therefore more prone to degradation. However, collagen fragments still have a bioactive role by binding to cell surface receptors regulating numerous biological processes in physiological and pathological situations [123, 124]. MMP-1, 8 and 13 also known as collagenase 1, 2 and 3 have a pro-tumorigenic role by cleaving fibrillar collagens and enhancing tumor cell motility. MMP-2 and MMP-9 cleavage activates latent TGF- β and produces collagen fragments which in turn induces TGF-β secretion. TGF-β has an inhibitory effect on cell proliferation in early stages of cancer and is also a key factor in fibrosis [125, 126]. Collagen I fragments cleaved by MMP1, 2, and 14 activate the DDR-1 receptor enhancing tumor growth in pancreatic cancer, thereby reducing patient survival [41]. Collagen I fragments cleaved by MMP-1 and MMP-9 have an inhibitory effect on T-cell receptor activation and IFN-y secretion through LAIR-1 signaling [127].
Large-scale genomic studies have delved into the potential genetic alterations of MMPs across a spectrum of human malignant tumors from diverse origins. These studies have specifically revealed MMP8 as a frequently mutated gene in human melanoma [128]. Functional analysis of the identified mutations verified that all mutations result in loss-of-function of MMP8, contributing to melanoma progression. These findings conclusively establish MMP8 as a tumor-suppressor gene. Additionally, parallel studies have expanded these observations to other MMP-related metalloproteinases, such as ADAMTS15 that is genetically inactivated in human colorectal cancer [129].
Collagen post-translational modifications as potential novel therapeutical targets in cancer
Numerous potential treatments, including antibodies and small molecule inhibitors, are currently studied for their ability to target enzymes and PTMs involved in ECM remodeling in tumors (Table 4). Targeting intracellular PTMs could inhibit collagen secretion and deposition, reduce stiffness and change the collagen architecture, thereby improving immune cell migration and penetration into the tumor mass. Various rate-limiting steps in collagen deposition were explored, including the targeting of proline hydroxylases. Knocking down P4HA1, P4HA2, and HIF-α reduces collagen deposition in primary breast cancer tumors, consequently preventing metastases [130]. Additionally, small molecules targeting P4HA1 reduce tumor growth in colorectal cancer models possibly through inhibition of MMP1 [131]. Aspirin targets P4HA2 by decreasing its gene transcription which results in reduced collagen deposition and tumor growth in hepatocellular carcinoma [132].
Table 4.
inhibitors and drugs targeting collagen modifications
| Therapy target | Name drug | Drug format | Disease | Research stage |
|---|---|---|---|---|
| P4HA1 | Diethyl-pythiDC | Small molecule inhibitor | Colorectal cancer | Preclinical [131] |
| P4HA2 | Aspirin | Small molecule inhibitor | Hepatocellular carcinoma | Preclinical [132] |
| P4H | EDHB | Small molecule inhibitor | Breast cancer | Preclinical [160] |
| BMP1.3 | Anti-BMP1.3 | mAb | Myocardial infarction | Preclinical [134] |
| LOX |
BAPN (β-aminopropionitrile) PXS-5505 |
Irreversible inhibitor Small molecule inhibitor |
Cancer Pancreatic cancer |
Preclinical [139] Preclinical [161] |
| LOXL2 |
Simtuzumab GS341 |
mAb pAb |
Fibrosis, Cancer |
Phase II [162] |
| LOXL2 |
PXS-S1A PXS-S2A PAT-1251 PXS-5382A Epigallocatechin gallate (EGCG) |
Small molecule inhibitors | Fibrosis, heart failure, glaucoma, oncological and angiogenic diseases |
Phase II [135] Phase II [135] Phase I [135] Phase I [166] |
| Copper |
tetrathiomolybdate (TM)I d-penicillamine (D-pen) |
Copper chelators |
Cancer Breast cancer |
|
| Dual LOX/LOXL |
PXS-5153A CCT365623 |
Inhibitor |
Fibrosis Cancer |
Preclinical [169] |
|
MMP9 MMP9 MMP14 MMP1, MMP2, MMP3 |
Andecaliximab (GS-5745) AB0041, AB0046, DX-2400 Single Chain Fragment Variables |
mAb mAb mAb mAb |
Cancer, Colorectal cancer Breast, melanoma, sarcoma Breast cancer |
Preclinical [175] Preclinical [176] |
|
PAD4 PAD4 PAD4 PAD4 |
TDFA TDCA Cl-amidine F-amidine GSK199, GSK484 JBI-589 |
Selective Irreversible small molecule inhibitors Reversible inhibitors Small molecule |
Inflammatory disorders and Cancer Cancer |
Preclinical [177] Preclinical [177] Preclinical [114] |
Collagen fibers in tumors are characterized as linear and compact due to the high level of deposition and post-translational crosslinking. This physical restructuring of collagen progressively stiffens the ECM leading to extensive biochemical and biomechanical changes, affecting cell signaling and tumor tissue three-dimensional architecture [133]. Therefore, targeting collagen crosslinking might be a good anticancer therapeutic strategy. In mice, anti-BMP1.3 treatment reduces expression of collagen I, LOX and TGF-β leading to a reduced overall scar size and improved cardiac function in a model of cardiac fibrosis [134]. This therapy shows significant potential in preventing fibrosis with minimal adverse effects. Investigating its potential effects on already established fibrotic tumors or in preventing metastases would be of interest. LOX/LOXL inhibitors, specifically LOXL2 inhibitors, are used in cancer and fibrosis to prevent collagen crosslinking [135]. In mice and clinical studies, LOXL targeting results in low toxicity and adverse effects, but yielded limited clinical benefits [135]. In preclinical cancer models, inhibiting LOXL2 does result in a reduction in metastasis but not in reduced primary tumor size [136]. In the clinic, LOXL2 inhibitors are used before surgical intervention to reduce metastasis [137]. Given that only the crystal structure of LOXL2 is solved, the potential of inhibitors targeting other LOXL enzymes has yet to be explored. Inhibitors of LOX enzymatic activity such as beta-aminopropionitrile (BAPN) were tested in combination with PD-1 treatment in mouse models leading to tumor reduction and increased T-cell infiltration [138], however the clinical use of BAPN is impeded by concerns regarding toxicities [139]. Another approach to reduce LOX/LOXL activity is to target copper which is an important cofactor for LOXL functionality [140]. Inhibiting copper results in anti-angiogenic, anti-fibrotic activities, however, the mechanism of LOXL-regulation by copper is poorly understood [141]. In preclinical mouse models, treatment with a copper chelator reduces the levels of myeloid-derived suppressor cells and increases CD4 + T-cell infiltration in tumors [140].
Extensive experimental and clinical data associate MMPs with tumor invasion, neo angiogenesis, and metastasis, positioning MMPs as promising pharmacologic targets for cancer therapy [122]. Numerous MMP inhibitors demonstrated promise as anti-cancer treatments in pre-clinical studies [142]. Unfortunately, none of them progressed significantly in clinical trials due to severe adverse effects, including musculoskeletal pain and inflammation [143]. MMP inhibitors that lacked specificity did not succeed in clinical trials, but current efforts are focused on developing more specific antibodies and inhibitors [144].
Of note, most inhibitors are still clinically tested in metastatic cancer while it is hypothesized that MMP inhibition would be more effective in early stages of tumor progression [144]. Since then, the understanding regarding the diversity of MMPs, the intricacy of their mechanisms, and the cross-reactivity of certain inhibitors with the ADAM and ADAMTS families has increased. Endogenous MMP inhibitors such as Thrombospondin-1 (TSP-1) regulate MMP-2 and MMP-9 activity reducing tumor growth in pre-clinical tumor models [145]. However, the function of TSP-1 in angiogenesis and tumor progression remains disputable in certain cancers and may be organ specific [146]. While TSP-1 is identified as an inhibitor of both processes, while in others, it is characterized as a stimulator [145, 147–151]. Additionally, MMPs can modulate the immune system by regulating chemokines and altering their activity [152]. MMP9 plays a pivotal role in promoting tumorigenesis across various cancer types. Inhibiting MMP9 leads to enhanced chemotaxis through elevated expression of CXCL10, coupled with increased T-cell activation triggered by higher levels of IL12p70 and IL-18 expression [153]. In preclinical models, the combined administration of anti-MMP9 and anti-PDL1 results in increased intra-tumoral T-cell diversity characterized by larger CD4/CD8 memory and effector cell populations, along with an enhanced Th1 responses [153].
The increasing body of evidence for PADs in cancer progression [154] has resulted in a growing interest towards targeting PADs and citrullination as potential therapeutic targets. Tumor cells can produce PAD4 and high PAD4 expression is found in patients’ blood and malignant tumor tissue [155]. In mice, PAD4 deletion in combination with ICB therapy results in increased presence and activation of CD8+ T cells, reduced tumor growth and lung metastasis compared to ICB treatment only [114]. Whether this effect is due to PAD4-mediated collagen citrullination has not yet been investigated in tumors.
Another approach to improve cancer treatments based on tumoral ECM characteristics, is using fusion proteins with a collagen binding domain (CBD) carrying bioactive-inhibiting cues, immune chemoattracts or radioactive substances. For example, recombinant protein containing the EGFR binding fragment of cetuximab improved by a CBD resulted in specific targeting to and penetration into squamous carcinoma A431 cell xenografts [156]. A similar approach was used with CBDs fused to immune checkpoint inhibitor antibodies and to IL-2 [157]. Both CBD-fused IL2 and CBD-conjugated checkpoint inhibitors showed enhanced antitumor efficacy and reduced associated toxicity compared with their unmodified counterparts in several tumor models. In addition, CBD fusion to IL-12 is described as result in systemic toxicity reduction and synergy with immune checkpoint inhibitor therapy [158]. This targeting strategy could also leverage collagen PTMs making this approach more tumor specific. Specific ECM components and PTMs are highly expressed in areas of active tumor invasion and thus could be used as targets. This strategy has the potential to augment the efficacy of radiation, chemotherapy, or targeted therapy by concentrating drugs, or antitumor biologics specifically at active tumor sites, thereby reducing their dispersion in healthy tissues [159].
Conclusion and future perspectives
Immune therapy revolutionized cancer treatment options. However, not every tumor responds well to this treatment, especially tumors with high desmoplasia and low immune cell infiltration are resistant to therapy. Collagen deposition in tumors acts as a physical barrier to therapeutic treatment. This barrier is not only passive, keeping immune cells out, but can also actively protect the tumor cells specially when altered by certain PTMs. To enhance cancer treatment for non-responders, immune therapy could be combined with therapies targeting the ECM of tumors.
To implement ECM targets in future treatment of cancer patients, more studies should focus on when the ECM changes from being tumor suppressive to tumor promoting and which PTMs play an important role in this process. Promoting increased immune cell infiltration through the breakdown of the ECM may also create an opportunity for tumor cells to disseminate throughout the body. Hence, the course of treatment and the tumor stage should be meticulously assessed and determined. Characterizing different types of collagens, PTMs and the abundance of PTM associated enzymes could aid in stratifying patients who may benefit from ICB alone or in combination with ECM targeted therapies. Targeting collagens and collagen-modifying enzymes for oncological purposes is intricate, given the widespread presence of collagen throughout the body. However, understanding the spatial heterogeneity and temporal dynamics of collagen PTMs in different types of solid tumors has the potential to refine the selective targeting of tumor stroma and bolster anti-tumor immune responses.
Acknowledgements
We thank our colleagues Michiel van der Vlist and Enrique Andres Sastre for their critical feedback on the manuscript.
Abbreviations
- ECM
Extracellular matrix
- ICB
Immune checkpoint blockade
- PTM
Post-translational modification
- CAF
Cancer-associated Fibroblasts
- EMT
Epithelial-to-mesenchymal transition
- DDR
Discoidin domain receptor
- GPVI
Glycoprotein VI
- OSCAR
Osteoclast-associated receptor
- LAIR-1
Leukocyte Associated Immunoglobulin Like Receptor 1
- MMP
Matrix metalloproteases
- ITAM
Immunoreceptor tyrosine-based activating motif
- ITIM
Immunoreceptor tyrosine-based inhibiting motif
- uPARAP
UPAR-associated protein
- SMC
Smooth muscle cells
- PD-1
Programmed cell death-1
- TGF-β
Transforming growth factor beta
- FACIT
Fibril-associated Collagens with Interrupted Triple Helices
- P4H
Prolyl-4-hydroxylases
- P3H
Prolyl-3-hydroxylases
- HIF-α
Hypoxia-inducible factor 1-alpha
- TCGA
The cancer genome atlas
- LH
Lysyl hydroxylase
- GT
Galactosyltransferase
- GGT
Galactosylhydroxylysyl glucosyltransferase
- BMP-1
Bone morphogenic
- LOX
Lysyl oxidase
- LOXL
Lysyl oxidase-like
- PAD
Protein arginine deiminases
- TNF-α
Tumor necrosis factor alpha
- TACE
Tumor necrosis factor alpha (TNF alpha)-converting enzyme
- ADAM
A disintegrin and metalloproteinases
- ADAMT
A disintegrin and metalloproteinases with thrombospondin motifs
- BAPN
Beta-aminopropionitrile
- TSP-1
Thrombospondin-1
- CBD
Collagen binding domain
- mAb
Monoclonal antibody
Author contributions
Conceptualization IR LM. Literature search RB. Discussion IR LM RB. Wrote first draft RB. Edited manuscript LM IR.
Funding
KWF.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to the publication of this manuscript.
Competing interests
LM’s lab has received funding for investigator-initiated studies on LAIR-1-collagen interaction from NextCure, NGM Biopharmaceuticals and Boehringer Ingelheim. The other authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Henke E, Nandigama R, Ergün S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2020;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Liu GY, Agarwal R, Ko KR, Ruthven M, Sarhan HT, Frampton JP. Templated assembly of collagen fibers directs cell growth in 2D and 3D. Sci Rep. 2017;7(1):9628. doi: 10.1038/s41598-017-10182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brereton CJ, Yao L, Davies ER, Zhou Y, Vukmirovic M, Bell JA, et al. Pseudohypoxic HIF pathway activation dysregulates collagen structure-function in human lung fibrosis. Elife. 2022;1:11. doi: 10.7554/eLife.69348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundaram GM, Quah S, Sampath P. Cancer: the dark side of wound healing. FEBS J. 2018;285:4516–4534. doi: 10.1111/febs.14586. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3(1):1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11(4):M111.014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Januchowski R, Zawierucha P, Ruciński M, Nowicki M, Zabel M. Extracellular matrix proteins expression profiling in chemoresistant variants of the A2780 ovarian cancer cell line. Biomed Res Int. 2014;2014:1–9. doi: 10.1155/2014/365867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naba A, Clauser KR, Lamar JM, Carr SA, Hynes RO. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. Elife. 2014;3:e01308. doi: 10.7554/eLife.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 12.Yang D, Liu J, Qian H, Zhuang Q. Cancer-associated fibroblasts: from basic science to anticancer therapy. Exp Mol Med. 2023 doi: 10.1038/s12276-023-01013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804. doi: 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 15.Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22(7):521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Vidal L, Murdica V, Venegoni C, Pederzoli F, Bandini M, Necchi A, et al. Causal contributors to tissue stiffness and clinical relevance in urology. Commun Biol. 2021;4:1011. doi: 10.1038/s42003-021-02539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novikov NM, Zolotaryova SY, Gautreau AM, Denisov EV. Mutational drivers of cancer cell migration and invasion. Br J Cancer. 2021;124:102–114. doi: 10.1038/s41416-020-01149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadjadi Z, Zhao R, Hoth M, Qu B, Rieger H. Migration of cytotoxic t lymphocytes in 3D collagen matrices. Biophys J. 2020;119(11):2141–2152. doi: 10.1016/j.bpj.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai SI, Cesano A, Marincola FM. The paradox of cancer immune exclusion: immune oncology next frontier. In: Lee PP, Marincola FM, editors. Cancer treatment and research. Berlin: Springer; 2020. pp. 173–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng DH, Rodriguez BL, Diao L, Chen L, Wang J, Byers LA, et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat Commun. 2020;11(1):4520. doi: 10.1038/s41467-020-18298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trujillo JA, Sweis RF, Bao R, Luke JJ. T cell–inflamed versus Non-T cell–inflamed tumors: a conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol Res. 2018;6(9):990–1000. doi: 10.1158/2326-6066.CIR-18-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rømer AMA, Thorseth ML, Madsen DH. Immune modulatory properties of collagen in cancer. Front Immunol. 2021;12:791453. doi: 10.3389/fimmu.2021.791453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo KS, Brodsky AS. Tumor collagens predict genetic features and patient outcomes. NPJ Genom Med. 2023;8(1):15. doi: 10.1038/s41525-023-00358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leeming DJ, Bay-Jensen AC, Vassiliadis E, Larsen MR, Henriksen K, Karsdal MA. Post-translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers. 2011;16(3):193–205. doi: 10.3109/1354750X.2011.557440. [DOI] [PubMed] [Google Scholar]
- 28.Elango J, Hou C, Bao B, Wang S, Maté Sánchez de Val JE, Wenhui W. The molecular interaction of collagen with cell receptors for biological function. Polymers. 2022;14:876. doi: 10.3390/polym14050876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. In: Jeon KW, editor. International review of cell and molecular biology. Amsterdam: Elsevier Inc.; 2014. pp. 39–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gucciardo F, Pirson S, Baudin L, Lebeau A, Noël A. uPARAP/Endo180: a multifaceted protein of mesenchymal cells. Cell Mol Life Sci. 2022;79:255. doi: 10.1007/s00018-022-04249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135:1146–1160. doi: 10.1182/blood.2019002649. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Hinerman JM, Blaszczyk M, Miller JLC, Conrady DG, Barrow AD, et al. Structural basis for collagen recognition by the immune receptor OSCAR. Blood, J Am Soc Hematol. 2016;127(5):529–37. http://ashpublications.org/blood/article-pdf/127/5/529/1394873/529.pdf. [DOI] [PMC free article] [PubMed]
- 34.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7(2):283–290. doi: 10.1016/S1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 35.Bansode S, Bashtanova U, Li R, Clark J, Müller KH, Puszkarska A, et al. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci Rep. 2020;10(1):3397. doi: 10.1038/s41598-020-60250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boraschi-Diaz I, Wang J, Mort JS, Komarova SV. Collagen type i as a ligand for receptor-mediated signaling. Front Phys. 2017;5:12. doi: 10.3389/fphy.2017.00012. [DOI] [Google Scholar]
- 37.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Yang S, Tavormina J, Tampe D, Zeisberg M, Wang H, et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. 2022;40(8):818–834.e9. doi: 10.1016/j.ccell.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moll S, Desmoulière A, Moeller MJ, Pache JC, Badi L, Arcadu F, et al. DDR1 role in fibrosis and its pharmacological targeting. Biochim Biophys Acta Mol Cell Res. 2019;1866:118474. doi: 10.1016/j.bbamcr.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Hu Y, Pan Y, Xiong Y, Zhang Y, Han M, et al. DDR1 promotes hepatocellular carcinoma metastasis through recruiting PSD4 to ARF6. Oncogene. 2022;41(12):1821–1834. doi: 10.1038/s41388-022-02212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H, Yang F, Fu R, Trinh B, Sun N, Liu J, et al. Collagenolysis-dependent DDR1 signalling dictates pancreatic cancer outcome. Nature. 2022;610(7931):366–372. doi: 10.1038/s41586-022-05169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X, Wu B, Chiang HC, Deng H, Zhang X, Xiong W, et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature. 2021;599(7886):673–678. doi: 10.1038/s41586-021-04057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barcus CE, Hwang PY, Morikis V, Brenot A, Pence P, Clarke M, et al. Tyrosine kinase-independent actions of DDR2 in tumor cells and cancer-associated fibroblasts influence tumor invasion, migration and metastasis. J Cell Sci. 2021;134(19):jcs258431. doi: 10.1242/jcs.258431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vh Bayer S, Grither WR, Brenot A, Hwang PY, Barcus CE, Ernst M, et al. DDR2 controls breast tumor stiffness and metastasis by regulating integrin mediated mechanotransduction in CAFs. Elife. 2019 doi: 10.7554/eLife.45508.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Z, Yang Y, Liu Z, Chen H, Guan X, Jiang Z, et al. Prognostic and immunotherapeutic significance of mannose receptor C type II in 33 cancers: an integrated analysis. Front Mol Biosci. 2022;14:9. doi: 10.3389/fmolb.2022.951636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2:1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130:2747–2760. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 48.Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305) J Leukoc Biol. 2008;83(4):799–803. doi: 10.1189/jlb.0907609. [DOI] [PubMed] [Google Scholar]
- 49.Ramos MIP, Tian L, de Ruiter EJ, Song C, Paucarmayta A, Singh A, et al. Cancer immunotherapy by NC410, a LAIR-2 Fc protein blocking human LAIR-collagen interaction. Elife. 2021;10:e62927. doi: 10.7554/eLife.62927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Liu Z, Lin L, Wu Z, Gao X, Cai X, et al. Collagen-related gene expression level predicts the prognosis and immune therapy response. Gastric Cancer. 2023;26(6):891–903. doi: 10.1007/s10120-023-01416-y. [DOI] [PubMed] [Google Scholar]
- 51.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 52.Byron A, Humphries JD, Humphries MJ. Defining the extracellular matrix using proteomics. Int J Exp Pathol. 2013;94:75–92. doi: 10.1111/iep.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su H, Karin M. Collagen architecture and signaling orchestrate cancer development. Trends Cancer. 2023;9:764–773. doi: 10.1016/j.trecan.2023.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Zeltz C, Khalil M, Navab R, Tsao MS. Collagen type XI inhibits lung cancer-associated fibroblast functions and restrains the integrin binding site availability on collagen type I matrix. Int J Mol Sci. 2022;23(19):11722. doi: 10.3390/ijms231911722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mlynska A, Vaišnorė R, Rafanavičius V, Jocys S, Janeiko J, Petrauskytė M, et al. A gene signature for immune subtyping of desert, excluded, and inflamed ovarian tumors. Am J Reprod Immunol. 2020;84(1):e13244. doi: 10.1111/aji.13244. [DOI] [PubMed] [Google Scholar]
- 57.Liu Z, Beach JA, Agadjanian H, Jia D, Aspuria PJ, Karlan BY, et al. Suboptimal cytoreduction in ovarian carcinoma is associated with molecular pathways characteristic of increased stromal activation. Gynecol Oncol. 2015;139(3):394–400. doi: 10.1016/j.ygyno.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bignotti E, Tassi RA, Calza S, Ravaggi A, Bandiera E, Rossi E, et al. Gene expression profile of ovarian serous papillary carcinomas: identification of metastasis-associated genes. Am J Obstet Gynecol. 2007;196(3):245.e1–245.e11. doi: 10.1016/j.ajog.2006.10.874. [DOI] [PubMed] [Google Scholar]
- 59.Fischer H, Stenling R, Rubio C. Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis. 2001;22:875. doi: 10.1093/carcin/22.6.875. [DOI] [PubMed] [Google Scholar]
- 60.Hammerl D, Martens JWM, Timmermans M, Smid M, Trapman-Jansen AM, Foekens R, et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer. Nat Commun. 2021;12(1):5668. doi: 10.1038/s41467-021-25962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brisson BK, Mauldin EA, Lei W, Vogel LK, Power AM, Lo A, et al. Type III collagen directs stromal organization and limits metastasis in a murine model of breast cancer. Am J Pathol. 2015;185(5):1471–1486. doi: 10.1016/j.ajpath.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Martino JS, Nobre AR, Mondal C, Taha I, Farias EF, Fertig EJ, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. 2022;3(1):90–107. doi: 10.1038/s43018-021-00291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Liu J, Zhang H, Wang J, Hua H, Jiang Y. The role of network-forming collagens in cancer progression. Int J Cancer. 2022;151:833–842. doi: 10.1002/ijc.34004. [DOI] [PubMed] [Google Scholar]
- 64.Lopes J, Adiguzel E, Gu S, Liu SL, Hou G, Heximer S, et al. Type VIII collagen mediates vessel wall remodeling after arterial injury and fibrous cap formation in atherosclerosis. Am J Pathol. 2013;182(6):2241–2253. doi: 10.1016/j.ajpath.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22(41):6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 66.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Investig. 2012;122(11):4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, et al. TEM8 interacts with the cleaved C5 domain of collagen 3(VI) Cancer Res. 2004;64:817. doi: 10.1158/0008-5472.CAN-03-2408. [DOI] [PubMed] [Google Scholar]
- 68.Kuivaniemi H, Tromp G, Prockop DJ. Mutations in fibrillar collagens (types I, II, III, and XI), fibril- associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of disease of bone, cartilage, and blood vessels. Hum Mutat. 1997;9(4):300–315. doi: 10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 69.Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28:93–105. doi: 10.1016/j.det.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heikkinen A, Tu H, Pihlajaniemi T. Collagen XIII: a type II transmembrane protein with relevance to musculoskeletal tissues, microvessels and inflammation. Int J Biochem Cell Biol. 2012;44:714–717. doi: 10.1016/j.biocel.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Fredericks T, Xiong G, Qi Y, Rychahou PG, Da LJ, et al. Membrane associated collagen XIII promotes cancer metastasis and enhances anoikis resistance. Breast Cancer Res. 2018;20(1):1–4. doi: 10.1186/s13058-018-1030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones VA, Patel PM, Gibson FT, Cordova A, Amber KT. The role of collagen XVII in cancer: squamous cell carcinoma and beyond. Front Oncol. 2020;10:352. doi: 10.3389/fonc.2020.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339:247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papanicolaou M, Parker AL, Yam M, Filipe EC, Wu SZ, Chitty JL, et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat Commun. 2022;13(1):4587. doi: 10.1038/s41467-022-32255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hennet T. Collagen glycosylation. Curr Opin Struct Biol. 2019;56:131–138. doi: 10.1016/j.sbi.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 76.Kivirikko KI, Ryhanen L, Anttinen H, Bornstein P, Prockop DJ. Further hydroxylation of lysyl residues in collagen by protocollagen lysyl hydroxylase in vitro. Biochemistry. 1971;12:4966. doi: 10.1021/bi00748a023. [DOI] [PubMed] [Google Scholar]
- 77.Kannicht C. Post-translational Modifications of Proteins. Humana Press; 2008.
- 78.van Huizen NA, Burgers PC, van Rosmalen J, Doukas M, IJzermans JNM, Luider TM. Down-regulation of collagen hydroxylation in colorectal liver metastasis. Front Oncol. 2020;10:557737. doi: 10.3389/fonc.2020.557737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bella J. Collagen structure: new tricks from a very old dog. Biochem J. 2016;473:1001–1025. doi: 10.1042/BJ20151169. [DOI] [PubMed] [Google Scholar]
- 81.Krane SM. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids. 2008;35:703–710. doi: 10.1007/s00726-008-0073-2. [DOI] [PubMed] [Google Scholar]
- 82.Sipila KH, Drushinin K, Rappu P, Jokinen J, Salminen TA, Salo AM, et al. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J Biol Chem. 2018;293(20):7645–7658. doi: 10.1074/jbc.RA118.002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geng P, Qin W, Xu G. Proline metabolism in cancer. Amino Acids. 2021;53:1769–1777. doi: 10.1007/s00726-021-03060-1. [DOI] [PubMed] [Google Scholar]
- 84.Hudson DM, Eyre DR. Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification? Connect Tissue Res. 2013;54:245–251. doi: 10.3109/03008207.2013.800867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y, Ge YZ, Qian Y, Chen K, Zhao F, Qin Z, et al. The role of P4HA1 in multiple cancer types and its potential as a target in renal cell carcinoma. Front Genet. 2022;23:13. doi: 10.3389/fgene.2022.848456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia W, Zhuang J, Wang G, Ni J, Wang J, Ye Y. P4HB promotes HCC tumorigenesis through downregulation of GRP78 and subsequent upregulation of epithelial-to-mesenchymal transition. Oncotarget. 2017;8(5):8512. doi: 10.18632/oncotarget.14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah R, Smith P, Purdie C, Quinlan P, Baker L, Aman P, et al. The prolyl 3-hydroxylases P3H2 and P3H3 are novel targets for epigenetic silencing in breast cancer. Br J Cancer. 2009;100(10):1687–1696. doi: 10.1038/sj.bjc.6605042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adams JC. Passing the post: roles of posttranslational modifications in the form and function of extracellular matrix. Am J Physiol-Cell Physiol. 2023;324:C1179–C1197. doi: 10.1152/ajpcell.00054.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Der Slot AJ, Zuurmond AM, Van Den Bogaerdt AJ, Ulrich MMW, Middelkoop E, Boers W, et al. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 2004;23(4):251–257. doi: 10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Sipilä L, Ruotsalainen H, Sormunen R, Baker NL, Lamande SR, Vapola M, et al. Secretion and assembly of type IV and VI collagens depend on glycosylation of hydroxylysines. J Biol Chem. 2007;282(46):33381–33388. doi: 10.1074/jbc.M704198200. [DOI] [PubMed] [Google Scholar]
- 91.Ishikawa Y, Taga Y, Coste T, Tufa SF, Keene DR, Mizuno K, et al. Lysyl hydroxylase 3–mediated post-translational modifications are required for proper biosynthesis of collagen α1α1α2(IV) J Biol Chem. 2022;298(12):102713. doi: 10.1016/j.jbc.2022.102713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sato K, Parag-Sharma K, Terajima M, Musicant AM, Murphy RM, Ramsey MR, et al. Lysyl hydroxylase 2-induced collagen cross-link switching promotes metastasis in head and neck squamous cell carcinomas. Neoplasia. 2021;23(6):594–606. doi: 10.1016/j.neo.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res. 2006;312(10):1693–1702. doi: 10.1016/j.yexcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Guo HF, Tsai CL, Terajima M, Tan X, Banerjee P, Miller MD, et al. Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe2+-binding. Nat Commun. 2018;9(1):512. doi: 10.1038/s41467-018-02859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eisinger-Mathason TSK, Zhang M, Qiu Q, Skuli N, Nakazawa MS, Karakasheva T, et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3(10):1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Komuro H, Mori M, Hayashi Y, Fukagawa M, Makino SI, Takahara K, et al. Mutational analysis of the BMP-1 gene in patients with gastroschisis. J Pediatr Surg. 2001;36(6):885–887. doi: 10.1053/jpsu.2001.23961. [DOI] [PubMed] [Google Scholar]
- 97.Syx D, Guillemyn B, Symoens S, Sousa AB, Medeira A, Whiteford M, et al. Defective proteolytic processing of fibrillar procollagens and prodecorin due to biallelic BMP1 mutations results in a severe, progressive form of osteogenesis imperfecta. J Bone Miner Res. 2015;30(8):1445–1456. doi: 10.1002/jbmr.2473. [DOI] [PubMed] [Google Scholar]
- 98.Slattery ML, Lundgreen A, Herrick JS, Kadlubar S, Caan BJ, Potter JD, et al. Genetic variation in bone morphogenetic protein and colon and rectal cancer. Int J Cancer. 2012;130(3):653–664. doi: 10.1002/ijc.26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park SW, Hur SY, Yoo NJ, Lee SH. Somatic frameshift mutations of bone morphogenic protein receptor 2 gene in gastric and colorectal cancers with microsatellite instability. APMIS. 2010;118(11):824–829. doi: 10.1111/j.1600-0463.2010.02670.x. [DOI] [PubMed] [Google Scholar]
- 100.Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem. 2012;52(1):113–133. doi: 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aikio M, Alahuhta I, Nurmenniemi S, Suojanen J, Palovuori R, Teppo S, et al. Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma. PLoS ONE. 2012;7(12):e51044. doi: 10.1371/journal.pone.0051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sudhakar A, Boosani CS. Signaling mechanisms of endogenous angiogenesis inhibitors derived from type IV collagen. Gene Regul Syst Biol. 2007 doi: 10.4137/GRSB.S345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gajjar DU, Vasavada AR, Patel P, Praveen MR, Shah SR. Evaluation of collagen derived antiangiogenic factors and matrix metalloproteinases in anterior lens epithelial cells of pediatric eyes with persistent fetal vasculature. Indian J Ophthalmol. 2019;67:1618–1622. doi: 10.4103/ijo.IJO_185_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng F, Peng G, Lu Y, Wang K, Ju Q, Ju Y, et al. Relationship between copper and immunity: The potential role of copper in tumor immunity. Front Oncol. 2022;12:1019153. doi: 10.3389/fonc.2022.1019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Ann Rev Biochem. 1984;53:717. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 106.Cox TR, Bird D, Baker AM, Barker HE, Ho MWY, Lang G, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73(6):1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shanbhag V, Jasmer-McDonald K, Zhu S, Martin AL, Gudekar N, Khan A, et al. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc Natl Acad Sci USA. 2019;116(14):6836–6841. doi: 10.1073/pnas.1817473116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bignon M, Pichol-Thievend C, Hardouin J, Malbouyres M, Bréchot N, Nasciutti L, et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood. 2011;118(14):3979–3989. doi: 10.1182/blood-2010-10-313296. [DOI] [PubMed] [Google Scholar]
- 109.Wong CCL, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011;108(39):16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Csiszar K, Fong SFT, Ujfalusi A, Krawetz SA, Salvati EP, Mackenzie JW, et al. Somatic mutations of the lysyl oxidase gene on chromosome 5q23.1 in colorectal tumors. Int J Cancer. 2002;97(5):636–642. doi: 10.1002/ijc.10035. [DOI] [PubMed] [Google Scholar]
- 111.Cano A, Eraso P, Mazón MJ, Portillo F. LOXL2 in cancer: a two-decade perspective. Int J Mol Sci. 2023;24:14405. doi: 10.3390/ijms241814405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Witalison EE, Thompson PR, Hofseth LJ. Protein arginine deiminases and associated citrullination: physiological functions and diseases associated with dysregulation. Curr Drug Targets. 2015;16(7):700–710. doi: 10.2174/1389450116666150202160954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yuzhalin AE, Gordon-Weeks AN, Tognoli ML, Jones K, Markelc B, Konietzny R, et al. Colorectal cancer liver metastatic growth depends on PAD4-driven citrullination of the extracellular matrix. Nat Commun. 2018;9(1):4783. doi: 10.1038/s41467-018-07306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deng H, Lin C, Garcia-Gerique L, Fu S, Cruz Z, Bonner EE, et al. A novel selective inhibitor JBI-589 targets PAD4-mediated neutrophil migration to suppress tumor progression. Cancer Res. 2022;82(19):3561–3572. doi: 10.1158/0008-5472.CAN-21-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Myers LK, Ouyang YX, Patel JR, Odens HH, Woo-Rasberry V, Park J, et al. Role of citrullinated collagen in autoimmune arthritis. Int J Mol Sci. 2022;23(17):9833. doi: 10.3390/ijms23179833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sipilä K, Haag S, Denessiouk K, Käpylä J, Peters EC, Denesyuk A, et al. Citrullination of collagen II affects integrin-mediated cell adhesion in a receptor-specific manner. FASEB J. 2014;28(8):3758–3768. doi: 10.1096/fj.13-247767. [DOI] [PubMed] [Google Scholar]
- 117.Stacey SN, Gudbjartsson DF, Sulem P, Bergthorsson JT, Kumar R, Thorleifsson G, et al. Common variants on 1p36 and 1q42 are associated with cutaneous basal cell carcinoma but not with melanoma or pigmentation traits. Nat Genet. 2008;40(11):1313–1318. doi: 10.1038/ng.234. [DOI] [PubMed] [Google Scholar]
- 118.Acevedo-Jake AM, Ngo DH, Hartgerink JD. Control of collagen triple helix stability by phosphorylation. Biomacromol. 2017;18(4):1157–1161. doi: 10.1021/acs.biomac.6b01814. [DOI] [PubMed] [Google Scholar]
- 119.Zimina EP, Fritsch A, Schermer B, Bakulina AY, Bashkurov M, Benzing T, et al. Extracellular phosphorylation of collagen XVII by ecto-casein kinase 2 inhibits ectodomain shedding. J Biol Chem. 2007;282(31):22737–22746. doi: 10.1074/jbc.M701937200. [DOI] [PubMed] [Google Scholar]
- 120.Malemud CJ. Inhibition of MMPs and ADAM/ADAMTS. Biochem Pharmacol. 2019;165:33–40. doi: 10.1016/j.bcp.2019.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Z, Li W, Chen S, Tang XX. Role of ADAM and ADAMTS proteases in pathological tissue remodeling. Cell Death Discov. 2023;9(1):447. doi: 10.1038/s41420-023-01744-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mustafa S, Koran S, AlOmair L. Insights into the role of matrix metalloproteinases in cancer and its various therapeutic aspects: a review. Front Mol Biosci. 2022;9:896099. doi: 10.3389/fmolb.2022.896099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kisling A, Lust RM, Katwa LC. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019;228:30–34. doi: 10.1016/j.lfs.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 124.Ricard-Blum S, Vallet SD. Fragments generated upon extracellular matrix remodeling: Biological regulators and potential drugs. Matrix Biol. 2019;75–76:170–189. doi: 10.1016/j.matbio.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 125.Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 126.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-and promotes tumor invasion and angiogenesis. Genes Dev. 2000 doi: 10.1101/gad.14.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vijver SV, Singh A, Mommers-Elshof ETAM, Meeldijk J, Copeland R, Boon L, et al. Collagen fragments produced in cancer mediate T cell suppression through leukocyte-associated immunoglobulin-like receptor 1. Front Immunol. 2021;7:12. doi: 10.3389/fimmu.2021.733561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, et al. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet. 2009;41(5):518–520. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Viloria CG, Obaya AJ, Moncada-Pazos A, Llamazares M, Astudillo A, Capellá G, et al. Genetic inactivation of ADAMTS15 metalloprotease in human colorectal cancer. Cancer Res. 2009;69(11):4926–4934. doi: 10.1158/0008-5472.CAN-08-4155. [DOI] [PubMed] [Google Scholar]
- 130.Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73(11):3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Agarwal S, Behring M, Kim HG, Bajpai P, Chakravarthi BVSK, Gupta N, et al. Targeting P4HA1 with a small molecule inhibitor in a colorectal cancer PDX model. Transl Oncol. 2020;13(4):100754. doi: 10.1016/j.tranon.2020.100754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang T, Fu X, Jin T, Zhang L, Liu B, Wu Y, et al. Aspirin targets P4HA2 through inhibiting NF-κB and LMCD1-AS1/let-7g to inhibit tumour growth and collagen deposition in hepatocellular carcinoma. EBioMedicine. 2019;1(45):168–180. doi: 10.1016/j.ebiom.2019.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mieulet V, Garnier C, Kieffer Y, Guilbert T, Nemati F, Marangoni E, et al. Stiffness increases with myofibroblast content and collagen density in mesenchymal high grade serous ovarian cancer. Sci Rep. 2021;11(1):4219. doi: 10.1038/s41598-021-83685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vukicevic S, Colliva A, Kufner V, Martinelli V, Moimas S, Vodret S, et al. Bone morphogenetic protein 1.3 inhibition decreases scar formation and supports cardiomyocyte survival after myocardial infarction. Nat Commun. 2022;13(1):81. doi: 10.1038/s41467-021-27622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferreira S, Saraiva N, Rijo P, Fernandes AS. Loxl2 inhibitors and breast cancer progression. Antioxidants. 2021;10:1–16. doi: 10.3390/antiox10020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, et al. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71(5):1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rachman-Tzemah C, Zaffryar-Eilot S, Grossman M, Ribero D, Timaner M, Mäki JM, et al. Blocking surgically induced lysyl oxidase activity reduces the risk of lung metastases. Cell Rep. 2017;19(4):774–784. doi: 10.1016/j.celrep.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nicolas-Boluda A, Vaquero J, Vimeux L, Guilbert T, Barrin S, Kantari-Mimoun C, et al. Tumor stiffening reversion through collagen crosslinking inhibition improves t cell migration and anti-pd-1 treatment. Elife. 2021;1:10. doi: 10.7554/eLife.58688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci Transl Med. 2018;10:eaao047. doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- 140.Liu YL, Bager CL, Willumsen N, Ramchandani D, Kornhauser N, Ling L, et al. Tetrathiomolybdate (TM)-associated copper depletion influences collagen remodeling and immune response in the pre-metastatic niche of breast cancer. NPJ Breast Cancer. 2021;7(1):108. doi: 10.1038/s41523-021-00313-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cox TR, Gartland A, Erler JT. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res. 2016;76:188–192. doi: 10.1158/0008-5472.CAN-15-2306. [DOI] [PubMed] [Google Scholar]
- 142.Fields GB. Mechanisms of action of novel drugs targeting angiogenesis-promoting matrix metalloproteinases. Front Immunol. 2019;10:442290. doi: 10.3389/fimmu.2019.01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002 doi: 10.1126/science.106710. [DOI] [PubMed] [Google Scholar]
- 144.Winer A, Adams S, Mignatti P. Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther. 2018;17:1147–1155. doi: 10.1158/1535-7163.MCT-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodríguez-Manzaneque JC, Lane TF, Ortega MAN, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98:12485. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tanaka K, Sonoo H, Kurebayashi J, Nomura T, Ohkubo S, Yamamoto Y, et al. Inhibition of infiltration and angiogenesis by thrombospondin-1 in papillary thyroid carcinoma 1. http://aacrjournals.org/clincancerres/article-pdf/8/5/1125/2084958/df0502001125.pdf. [PubMed]
- 147.Grossfeld GD, Ginsberg DA, Stein JP, Bochner BH, Esrig D, Groshen S, et al. Thrombospondin-1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. 1997. https://academic.oup.com/jnci/article/89/3/219/2526687. [DOI] [PubMed]
- 148.Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, et al. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;55(2):441–452. doi: 10.1016/S0002-9440(10)65140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fontanini G, Boldrini L, Calcinai A, Chinè S, Lucchi M, Mussi A, et al. Thrombospondins I and II messenger RNA expression in lung carcinoma: relationship with p53 alterations, angiogenic growth factors, and vascular density. Clin Cancer Res. 1999;5(1):155–161. [PubMed] [Google Scholar]
- 150.Yamashita Y, Kurohiji T, Tuszynski GP, Sakai T, Shirakusa T. Plasma thrombospondin levels in patients with colorectal carcinoma. Cancer. 1998;82(4):632–638. doi: 10.1002/(SICI)1097-0142(19980215)82:4<632::AID-CNCR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 151.Straume O, Akslen LA. Expresson of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159(1):223–235. doi: 10.1016/S0002-9440(10)61688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 153.Juric V, O’Sullivan C, Stefanutti E, Kovalenko M, Greenstein A, Barry-Hamilton V, et al. MMP-9 inhibition promotes anti-tumor immunity through disruption of biochemical and physical barriers to T-cell trafficking to tumors. PLoS ONE. 2018;13(11):e020725. doi: 10.1371/journal.pone.0207255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yuzhalin AE. Citrullination in cancer. Cancer Res. 2019;79:1274–1284. doi: 10.1158/0008-5472.CAN-18-2797. [DOI] [PubMed] [Google Scholar]
- 155.Chang X, Han J, Pang L, Zhao Y, Yang Y, Shen Z. Increased PADI4 expression in blood and tissues of patients with malignant tumors. BMC Cancer. 2009;9:1. doi: 10.1186/1471-2407-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liang H, Li X, Wang B, Chen B, Zhao Y, Sun J, et al. A collagen-binding EGFR antibody fragment targeting tumors with a collagen-rich extracellular matrix. Sci Rep. 2016;6:18205. doi: 10.1038/srep18205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ishihara J, Ishihara A, Sasaki K, Seung-Young Lee S, Williford JM, Yasui M, et al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci Transl Med. 2019;11:eaau3259. doi: 10.1126/scitranslmed.aau3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mansurov A, Ishihara J, Hosseinchi P, Potin L, Marchell TM, Ishihara A, et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat Biomed Eng. 2020;4(5):531–543. doi: 10.1038/s41551-020-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pondé N, Aftimos P, Piccart M. Antibody-drug conjugates in breast cancer: a comprehensive review. Curr Treat Options Oncol. 2019;20(5):1–22. doi: 10.1007/s11864-019-0633-6. [DOI] [PubMed] [Google Scholar]
- 160.Vasta JD, Andersen KA, Deck KM, Nizzi CP, Eisenstein RS, Raines RT. Selective inhibition of collagen prolyl 4-hydroxylase in human cells. ACS Chem Biol. 2016;11(1):193–199. doi: 10.1021/acschembio.5b00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chitty JL, Yam M, Perryman L, Parker AL, Skhinas JN, Setargew YFI, et al. A first-in-class pan-lysyl oxidase inhibitor impairs stromal remodeling and enhances gemcitabine response and survival in pancreatic cancer. Nat Cancer. 2023;4(9):1326–1344. doi: 10.1038/s43018-023-00614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Benson AB, Wainberg ZA, Hecht JR, Vyushkov D, Dong H, Bendell J, et al. A phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma. Oncologist. 2017;22(3):241–e15. doi: 10.1634/theoncologist.2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Grossman M, Ben-Chetrit N, Zhuravlev A, Afik R, Bassat E, Solomonov I, et al. Tumor cell invasion can be blocked by modulators of collagen fibril alignment that control assembly of the extracellular matrix. Cancer Res. 2016;76(14):4249–58. doi: 10.1158/0008-5472.CAN-15-2813. [DOI] [PubMed] [Google Scholar]
- 164.Rowbottom MW, Bain G, Calderon I, Lasof T, Lonergan D, Lai A, et al. Identification of 4-(aminomethyl)-6-(trifluoromethyl)-2-(phenoxy) pyridine derivatives as potent, selective, and orally efficacious inhibitors of the copper-dependent amine oxidase, lysyl oxidase-like 2 (LOXL2) J Med Chem. 2017;60(10):4403–23. doi: 10.1021/acs.jmedchem.7b00345. [DOI] [PubMed] [Google Scholar]
- 165.Luangmonkong T, Parichatikanond W, Olinga P. Targeting collagen homeostasis for the treatment of liver fibrosis: opportunities and challenges. Biochem Pharmacol. 2023;215:115740. doi: 10.1016/j.bcp.2023.115740. [DOI] [PubMed] [Google Scholar]
- 166.Wei Y, Dong W, Jackson J, Ho TC, Le Saux CJ, Brumwell A, et al. Blocking LOXL2 and TGFβ1 signalling induces collagen I turnover in precision-cut lung slices derived from patients with idiopathic pulmonary fibrosis. Thorax. 2021;76(7):729–32. doi: 10.1136/thoraxjnl-2020-215745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chan N, Willis A, Kornhauser N, Mward M, Lee SB, Nackos E, et al. Influencing the tumor microenvironment: a Phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin Cancer Res. 2017;23(3):666–76. doi: 10.1158/1078-0432.CCR-16-1326. [DOI] [PubMed] [Google Scholar]
- 168.Setargew YFI, Wyllie K, Grant RD, Chitty JL, Cox TR. Targeting lysyl oxidase family meditated matrix cross-linking as an anti-stromal therapy in solid tumours. Cancers. 2021;13:1–26. doi: 10.3390/cancers13030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schilter H, Findlay AD, Perryman L, Yow TT, Moses J, Zahoor A, et al. The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis. J Cell Mol Med. 2019;23(3):1759–70. doi: 10.1111/jcmm.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leung L, Niculescu-Duvaz D, Smithen D, Lopes F, Callens C, McLeary R, et al. Anti-metastatic inhibitors of lysyl oxidase (LOX): design and structure-activity relationships. J Med Chem. 2019;62(12):5863–84. doi: 10.1021/acs.jmedchem.9b00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tang HR, Leung L, Saturno G, Viros A, Smith D, Di Leva G, et al. Lysyl oxidase drives tumour progression by trapping EGF receptors at the cell surface. Nat Commun. 2017;8:14909. doi: 10.1038/ncomms14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bendell J, Sharma S, Patel MR, Windsor KS, Wainberg ZA, Gordon M, et al. Safety and efficacy of andecaliximab (GS-5745) plus gemcitabine and nab-paclitaxel in patients with advanced pancreatic adenocarcinoma: results from a phase I study. Oncologist. 2020;25(11):954–62. doi: 10.1634/theoncologist.2020-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Marshall DC, Lyman SK, McCauley S, Kovalenko M, Spangler R, Liu C, et al. Selective allosteric inhibition of MMP9 is efficacious in preclinical models of ulcerative colitis and colorectal cancer. PLoS ONE. 2015;10(5):e0127063. doi: 10.1371/journal.pone.0127063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Levin M, Udi Y, Solomonov I, Sagi I. Next generation matrix metalloproteinase inhibitors—novel strategies bring new prospects. Biochim Biophys Acta Mol Cell Res. 2017;1864:1927–39. doi: 10.1016/j.bbamcr.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 175.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69(4):1517–26. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 176.Pfaffen S, Hemmerle T, Weber M, Neri D. Isolation and characterization of human monoclonal antibodies specific to MMP-1A, MMP-2 and MMP-3. Exp Cell Res. 2010;316(5):836–47. doi: 10.1016/j.yexcr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 177.Jones JE, Slack JL, Fang P, Zhang X, Subramanian V, Causey CP, et al. Synthesis and screening of a haloacetamidine containing library to identify PAD4 selective inhibitors. ACS Chem Biol. 2012;7(1):160–5. doi: 10.1021/cb200258q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Witalison EE, Cui X, Causey CP, Thompson PR, Hofseth LJ. Molecular targeting of protein arginine deiminases to suppress colitis and prevent colon cancer. Oncotarget. 2015;6:36053. doi: 10.18632/oncotarget.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luo Y, Knuckley B, Lee YH, Stallcup MR, Thompson PR. A fluoroacetamidine-based inactivator of protein arginine deiminase 4: deiminase 4: design, synthesis, and in vitro and in vivo evaluation. J Am Chem Soc. 2006 doi: 10.1021/ja0576233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.