Abstract
Malassezia globosa, a lipophilic pathogen, is known to be involved in various chronic skin diseases. Unfortunately, the available treatments have unwanted side effects and microbial drug resistance is evolving. As the antimicrobial activity of propolis is outstanding, this study aimed to examine the potential of propolis from the stingless bee Geniotrigona thoracica against the yeast. Anti-M. globosa growth activity was ascertained in agar well diffusion and broth microdilution assays and the inhibitory concentration value at 50 % (IC50) was determined. Since the yeast cannot synthesize its own fatty acids, extracellular lipase is important for its survival. Here, anti-M. globosa extracellular lipase activity was additionally investigated by colorimetric and agar-based methods. Compared to the crude hexane and crude dichloromethane extracts, the crude methanol partitioned extract (CMPE) exhibited the best anti-M. globosa growth activity with an IC50 of 1.22 mg/mL. After CMPE was further enriched by silica gel column chromatography, fraction CMPE1 (IC50 of 0.98 mM or 184.93 μg/mL) presented the highest activity and was later identified as methyl gallate (MG) by nuclear magnetic resonance analysis. Subsequently, MG was successfully synthesized and shown to have a similar activity, and a minimal fungicidal concentration of 43.44 mM or 8.00 mg/mL. However, lipase assay analysis suggested that extracellular lipase might not be the main target mechanism of MG. This is the first report of MG as a new anti-Malassezia compound. It could be a good candidate for further developing alternative therapeutic agents.
Keywords: Antimicrobial drug, Bee product, Lipase, Natural product, Skin disease
1. Introduction
Mammalian skin diseases caused by infection with fungi or yeast are commonly found worldwide, including in humans. They not only cause physical illness, but also impact on the mental health [1,2]. Recently, there has been growing clinical attention to the skin pathogenic yeast genus Malassezia, the most abundant component of the human mycobiome, which can opportunistically cause skin diseases depending on the age, gender, personal health conditions, and environment. Malassezia spp. are associated with a wide range of chronic skin conditions, including seborrheic dermatitis, atopic dermatitis, folliculitis, pityriasis versicolor, psoriasis, and dandruff. The common species found in patients are M. globosa, M. restricta, M. sympodialis, and M. furfur [[3], [4], [5], [6]]. Azole drugs, such as ketoconazole, fluconazole, and itraconazole, are recognized as primary effective treatments. Unfortunately, both short- and long-term usage can lead to many side effects, including headache, nausea, irritation, hormone-related effects, and liver toxicity. Moreover, resistance in Malassezia to azoles has also been reported [[7], [8], [9], [10]], and so the exploration of alternative remedies should be encouraged.
Natural resources provide a diverse variety of bioactive compounds. Propolis is a bee product that is mainly composed of resin from many plant species. It is harvested by worker bees or stingless bees for constructing or repairing their hives, along with killing pests and pathogens. Propolis contains numerous biological properties, such as antimicrobial, anti-inflammatory, antioxidant, anti-cancer, anti-tumor, and wound healing activities [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. Multiple studies have reported on the strong and wide ranges of antimicrobial activities of propolis. It can inhibit both Gram-positive and Gram-negative bacteria, yeasts, fungi, parasites, and viruses.
Propolis also presents an inhibitory activity against dermatophytes and skin pathogenic yeasts. It effectively inhibits Candida, Trichosporon, Geotrichum, Saccharomyces, Cryptococcus, Rhodotorula, Microsporum, and Trichophyton [[23], [24], [25], [26], [27], [28]]. So far, it has been utilized as a constituent for supplements, cosmetics, and medicines. However, most recent studies have only focused on the inhibitory activity of crude propolis extracts against M. pachydermatis isolated from dogs. Moreover, the mechanism of inhibition remains unknown. According to these limitations, further investigations should give more consideration to the isolates from humans, the main active compound(s), and mechanisms of inhibition. Interestingly, almost all of the Malassezia species are lipid-dependent species that rely on external fatty acid sources. Hence, extracellular lipase activity is important for their growth and pathogenicity. The yeasts secret lipase to hydrolyze lipids on human skin, then uptake only the saturated fatty acids. Accumulation of the remaining unsaturated fatty acids can cause irritation and lead to various skin disorders [[29], [30], [31]]. Thus, determination of the inhibitory activity against extracellular lipases is one of the interesting target mechanisms of these yeast species.
This work aimed to examine the potential of propolis extracts from the stingless bee, G. thoracica, one of the commonly raised species in Thailand, against M. globosa. The study focused on exploration of the bioactive compound(s) in the propolis by extraction and characterization of the propolis components through solvent partitioning and silica gel 60 Å column chromatography (SGCC). The obtained extracts were then screened to determine their activity against M. globosa growth. The most effective fraction was enriched and then analyzed for its chemical structure using spectroscopy. Furthermore, the inhibitory activity against M. globosa extracellular lipase was demonstrated by lipophilic detection and colorimetric lipase assay. The obtained bioactive compound could be developed to an alternative therapeutic agent. Additionally, it can support stingless bee or beekeeping management by adding values to their products, which may lead to protect this important pollinator.
2. Methods
2.1. Sample collection
Raw propolis from the stingless bee, Geniotrigona thoracica, was collected from a local stingless bee apiary in Samut Songkhram province, Thailand in January 2019. It was stored at −20 °C with light protection until used.
2.2. Preparation of propolis extract
The propolis extract was prepared using solvent extraction as previously described [32,33] with slight modification. The raw propolis (145–150 g) was cut into small pieces and then extracted with 900 mL of 80 % (v/v) methanol (MeOH) by shaking at 100 rpm, 25 °C, for 20 h under darkness (SI-23MC, Bioer Technology, China). Next, the soluble part was separated by spinning at 5,488×g (7000 rpm), 4 °C for 15 min (Sorvall RC 6 Plus Centrifuge, SLA-1500 rotor, Thermo Scientific, USA) and collecting the supernatant. The remaining solid was re-extracted twice more with MeOH in the same manner as described and then the obtained supernatants were pooled and evaporated at 40–45 °C under reduced pressure (rotary evaporator, Heidolph, Germany) to yield the crude 80 % MeOH extract (CME) of G. thoracica propolis. The CME was then sequentially partitioned by three organic solvents: hexane, dichloromethane (CH2Cl2), and MeOH, respectively, using a separating funnel.
First, the obtained CME was completely dissolved in MeOH, hexane was then added at the same volume as the MeOH, mixed, and poured into a separating funnel. After phase separation, the upper phase (hexane) was collected while the lower phase (MeOH) was re-partitioned with hexane in the same manner twice more. The hexane phases were pooled and evaporated at 40–45 °C under reduced pressure to obtain the crude hexane partitioned extract (CHPE).
The remaining MeOH part was further partitioned in the same manner three times each with an equal volume of CH2Cl2, except that the CH2Cl2 extract was the lower phase while the MeOH phase was the upper phase. The CH2Cl2 phases were pooled and evaporated as above to yield the crude CH2Cl2 partitioned extract (CDPE), while the residual MeOH phase was evaporated to yield the crude MeOH partitioned extract (CMPE). In all cases, the percentage of yield and appearance were recorded. All crude partitioned extracts were stored in the dark at −20 °C for further primarily screening of anti-M. globosa growth activity using the agar well diffusion assay (section 2.5).
2.3. Bioassay-guided fractionation and compound isolation
2.3.1. Fractionation using SGCC
The SGCC was modified from Khongkarat et al. [34]. A 500-mL glass column (NK Laboratory, Thailand) was packed with silica gel 60 Å (SG; particle size 0.063–0.200 mm for column chromatography) (Merck, Germany). The CMPE extract (4.64 g) was dissolved in 1:1 (v/v) MeOH: CH2Cl2 and mixed with SG. The mixed paste was evaporated to dryness and loaded over the packed SG column. The column was then eluted with the following mobile phases: [i] 2 L of 1:19 (v/v) MeOH: CH2Cl2, [ii] 1 L of 1:4 (v/v) MeOH: CH2Cl2, [iii] 2 L of 1:1 (v/v) MeOH: CH2Cl2, and [iv] 2 L of MeOH, respectively. The eluates obtained from mobile phases [i] and [ii] were collected in 10-mL fractions while those obtained from mobile phases [iii] and [iv] were collected in 200-mL fractions. Fractions showing similar SG thin layer chromatography (TLC) profiles (section 2.3.2) were combined, evaporated to dryness at 40–45 °C under reduced pressure, and stored in the dark at −20 °C.
2.3.2. Fraction evaluation by TLC
The TLC SG F254 plates (Merck, Germany), size 2 cm × 5 cm, were prepared as the stationary phase. A small portion of the combined fractions were dissolved in appropriate solvents, such as MeOH and 1:1 (v/v) MeOH: CH2Cl2, and then spotted on a TLC plate at the starting line (1 cm above the bottom of the TLC plate) using a capillary tube and dried at room temperature. The TLC plate was developed in a glass chamber containing a suitable solvent, including MeOH: CH2Cl2 at 1:4 and 1:19 (v/v), as the mobile phase. After the solvent reached the solvent front (5 cm above the starting line of the TLC plate), the TLC profile was visualized under ultraviolet light (254 nm) followed by dipping in 5 % anisaldehyde reagent. The fractions showing the same TLC (chemical) profile were pooled and tested for anti-M. globosa growth activity using the agar well diffusion assay (section 2.5). After that, they were stored in the dark at −20 °C for further usage.
2.3.3. Chemical structure analysis
The enriched or pure fraction with the highest M. globosa growth inhibition activity (CMPE1) was sent to the Nuclear Magnetic Resonance (NMR) analysis service at the Department of Chemistry, Faculty of Science, Chulalongkorn University for chemical structure analysis. Briefly, CMPE1 (10–20 mg) was completely dissolved in 1.0 mL of methanol-d4 (Eurisotop, Cambridge Isotope Laboratories, Inc., USA). The NMR spectrometer (Jeol JNM-ECZ500R NMR) was operated at 500 MHz and 125 MHz for 13C and 1H, respectively. The NMR spectra, chemical shifts in δ (ppm), and J coupling values (Hz) were reported and compared to reference reports.
2.3.4. Synthesis of MG
The MG was synthesized as reported [35] with slight modification. A methanolic solution (10 mL) containing a mixture of gallic acid (GA; 1.0150 g, 6.0 mmol) and 4 Å molecular sieves was added into concentrated sulphuric acid (1.0 mL) dropwise. The reaction mixture was refluxed until the GA was completely consumed (about 10 h), then cooled down, evaporated to dryness, and dissolved in ethyl acetate. The ethyl acetate soluble portion was extracted twice with water to remove the excess acidity, and the ethyl acetate layer was then dried over anhydrous sodium sulphate (Na2SO4) and evaporated to dryness to yield the MG as a white powder (0.6845 g, 62 %). The synthesized MG was verified by TLC and 1H NMR analyses in comparison to the naturally isolated MG from the CMPE1 propolis extract.
The overall extraction procedure of the G. thoracica propolis extract for anti-M. globosa growth activity is summarized in Fig. 1.
Fig. 1.
Scheme of the bioassay-guided fractionation of the CME G. thoracica propolis extract. The box with color indicates the extract that exhibited anti-M. globosa growth activity in the agar well diffusion assay.
2.4. Culture condition and yeast cell suspension preparation
The M. globosa, Guého & Guillot (1996) isolate used in this study was purchased from the American Type Culture Collection (MYA-4889; ATCC), USA. All experiments were performed under biosafety level 2 guidelines and ATCC recommendations. Modified Leeming-Notman media (MLN) were used as the culture medium, which is composed of 1 % (w/v) bacteriological peptone, 1 % (w/v) D-glucose, 0.2 % (w/v) yeast extract, 0.8 % (w/v) ox bile, 1 % (v/v) glycerol, 0.5 × 10−4 % (w/v) glycerol monostearate, 0.5 % (v/v) Tween 60, and 2 % (v/v) olive oil, plus 1.5 % (w/v) agar for MLN agar medium (HiMedia, India). Culture stock of M. globosa was stored in MLN broth mixed with 18 % (v/v) glycerol at −80 °C. The culture stock was thawed at 25 °C for 2–5 min and then suspended in MLN broth (200–300 μL) and grown on MLN agar using the streak plate technique at 30 ± 2 °C for 5–7 days (Incucell incubator, MMM Medcenter, Germany). After that, a single colony (diameter of 1–2 mm) was picked and transferred to 20 mL of MLN broth. The culture was grown at 30 °C for 7 days with shaking at 200 rpm before use as a working culture (New Brunswick Innova 44 incubator Shaker, Eppendorf, Germany).
To prepare the yeast cell suspensions, yeast cells from the working culture were harvested by centrifugation at 15,322×g (12,000 rpm), room temperature for 5 min (5804R, FA-45-30-11 fixed angle rotor, Eppendorf, Germany). The pellets were collected and washed twice by resuspending in sterile phosphate buffered saline (1× PBS, pH 7.2–7.4). For calibrating the yeast density (cells/mL), the optical density at 600 nm (OD600) was measured using a spectrophotometer (Thermo Scientific, USA) with PBS alone as the blank. The yeast cells were stained with 0.01 % (w/v) methylene blue solution [methylene blue in 2 % (w/v) sodium citrate solution] at 1:1 (v/v) ratio and were counted using a hemocytometer (BOECO, Germany) under a light microscope.
2.5. Agar well diffusion assay
A target sample was tested for its inhibitory activity against M. globosa growth using the agar well diffusion assay modified from Hendi et al. [36]. A small volume of the yeast cell suspension (∼4 × 105 cells/mL final density) was dropped and swabbed with a sterile cotton swab on MLN agar. Each well on the agar was made using a sterile cork borer (diameter 8 mm). A test sample dissolved in 100 dimethyl sulfoxide (DMSO) at the desired target concentration (3.13–400 mg/mL) was added into each well, while DMSO alone was used as the solvent control. The commercial drug, Ketoconazole (KTZ) was used at 2 or 4 μg/mL as a positive control. The agar plates were incubated at 30 ± 2 °C for 7–14 days before observing the results. The area around each well which showed no growth was considered as a zone of inhibition. The diameter of each such zone of inhibition was measured in mm. The percentage of growth inhibition was compared to that for KTZ and then calculated from the following formula [37]:
| 100 |
where A and B is the zone of inhibition (mm) of the test sample and KTZ, respectively.
2.6. Broth microdilution assay
From the obtained samples (CME, CMPE, CMPE1 or MG, and synthetic MG), those that exhibited the best antimicrobial activity in the agar well diffusion assay were selected and their minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) were determined using the broth microdilution method modified from Far et al. [38] and Gucwa et al. [39]. The experiment was performed in a 96-well plate at a final volume of 200 μL in each well, with a final yeast cell density of ∼1–2 x 105 cells/mL. The sample stock in DMSO was diluted to various concentrations (0.031–50 mg/mL) using 2× MLN broth. In some cases, Tween 60 was added as a supplement as required to dissolve the sample. Sterile 2× MLN broth mixed with the appropriate solvent, and KTZ at concentrations of 0.0025–0.8 mg/mL (in the same solvent) were also screened as solvent and positive controls, respectively. In addition, the same tested compositions without inoculation (replaced by 1× PBS) were considered as a negative control within each treatment. The 96-well plates were then cultured at 30 °C for 12 h with shaking at 80 rpm (LSI-3016R, LabTech, Thailand), whereafter 30 μL of 0.02 % (v/w) resazurin was added to each well, gently mixed, and incubated under the same condition for a further 36 h (total 48 h). Finally, the fluorescent intensity was measured using a microplate reader (EnSight Multimode Plate Reader, PerkinElmer, USA) operated at an excitation wavelength of 540 nm and measuring the emission wavelength of 590 nm (A590). The lowest concentration that did not change to pink or did not differ from its negative control was defined as the MIC. The percentage of growth inhibition was calculated using the formula below:
where A, B, a, and b are the A590 values of the solvent control, test treatment, negative solvent control, and the negative test control, respectively.
Subsequently, the IC50 value was calculated using a non-linear regression analysis. A graph between percentage of inhibition (Y-axis) and log concentrations (X-axis) of the tested extracts was plotted, and the IC50 value was determined using GraphPad Prism 8. Furthermore, aliquots (20 μL) from each treatment, ranging in concentration from the MIC to the highest used concentration were dropped onto a MLN agar plate and incubated at 30 ± 2 °C for 5–10 days. The lowest concentration in which no colony growth was detected was defined as the MFC.
2.7. Inhibition of M. globosa lipase activity
2.7.1. Detection of extracellular lipase
A qualitative assay for lipase detection was performed to ensure lipophilic activity. Agar medium plates supplemented with the lipid indicator Vitoria Blue B (TW60-Vic B agar) were prepared as reported [40,41], in addition to agar medium plates without indicator dye (TW60 agar) [42]. The wells in the agar media were made using a sterile cork borer (diameter 4 mm). Then, a M. globosa cell suspension (50 μL at a final density of ∼106 cells/mL) was transferred to each well and incubated at 30 °C for either 1–2 days for TW60-Vic B agar or 10–15 days for TW60 agar. The presence of lipase activity was revealed by a hazy or darker clearance zone in the TW60-Vic B agar or a zone of calcium precipitation in the TW60 agar.
2.7.2. Preparation of crude lipase
Crude lipase from M. globosa was prepared from 7-day-old cultures containing M. globosa cell suspensions at an initial OD600 of 2 (∼4 × 106 cells/mL) in 5 mL of MLN broth. After 7 days the inoculated culture was centrifuged at 3,830×g (6000 rpm), 4 °C for 10 min. The supernatant was recovered and filtered through a 0.22 μm polyvinylidene fluoride (PVDF) syringe membrane filter to yield the cell free culture supernatant (CFCS) as a crude lipase source.
2.7.3. Measurement of the inhibition of lipase activity: spectrophotometric analysis
A quantitative assay of lipase inhibition activity was conducted using a modification to the methods of Sivasankar et al. [43] and Honnavar et al. [44]. The reaction was comprised of 1:9 (v/v) of crude lipase: reaction mixture. The reaction mixture was comprised of [i] 1× vol. of substrate solution containing 0.3 % (w/v) p-nitrophenyl palmitate (pNPP) in propanol and [ii] 9× vol. of reaction buffer [0.2 % (w/v) sodium desoxycholate and 0.1 % (w/v) gummi arabicum in 50 mM sodium phosphate (Na2PO4) buffer (pH 8.0)]. The tested sample stocks were prepared in DMSO and were then diluted at various concentrations (0.0008–0.5 mg/mL) in the reaction buffer. In some cases, Tween 60 was supplemented to a maximum final concentration of 0.05 % (v/v).
The assay was performed in a 1.5-mL microcentrifuge tube at a final volume of 100 μL. The tested sample (81 μL) was pre-warmed at 30 °C for 30 min, rotating at 80 rpm, and then mixed with 10 μL of the CFCS (section 2.7.2) as a crude lipase source and incubated at the same condition for another 30 min. After that, the substrate solution (9 μL) was added and the mixture was incubated for 2 h. Subsequently, 2× vol. (200 μL) of 1 M Tris-HCl (pH 8.0) was added to stabilize the pH-dependent p-nitrophenol (pNP), centrifuged at 15,322×g (12,000 rpm), 4 °C, for 5 min, and 200 μL of supernatant was collected and transferred to a 96 well plate to measure its absorbance at 410 nm (A410) using a microplate reader. The reaction containing the reaction mixture mixed with an appropriate solvent was used as the solvent control, while the same composition except without crude lipase (replaced by sterile Type I water) was considered as a negative control within each treatment. The reaction with porcine pancreatic lipase (PPL) at a final concentration of 0.02 mg/mL was performed as a positive control. The percentage of lipase inhibition was calculated from the following formula:
where: A, B, a, and b are defined as the A410 of the solvent control, test treatment, negative control of the solvent control, and the negative control of the test treatment, respectively.
2.7.4. Inhibition of extracellular lipase: plate assay method
Using the same method as in the qualitative assay for lipase detection (section 2.7.1), the most active anti-M. globosa lipase activity fraction (CMPE1) was also tested. At a final volume of 1 mL, an equal volume of CMPE1 in 2× MLN broth (concentrations 0.06–1.00 mg/mL) and yeast cell suspension (density of ∼107 cells/mL) were mixed and incubated at 30 °C for 24 h and 48 h with shaking at 200 rpm.
After reaching the indicated incubated time points, the yeast cells from each treatment were collected by centrifugation at 15,322×g (12,000 rpm), room temperature for 5 min. They were then washed twice with PBS, adjusted in cell density to obtain cell suspensions (50 μL) in each well of TW60-Vic B agar of ∼106 cells/mL final density. The agar medium plates were then incubated at 30 °C for 1–2 days before observing the results as previously described in section 2.7.1.
2.8. Data analysis
All experiments were performed in triplicate. The data are reported as the mean ± one standard error/standard deviation of the mean (mean ± SEM/SD), determined by using Microsoft excel 365. Regression analysis was analyzed using Microsoft excel 365 or GraphPad Prism 8. Statistical significance of the data was calculated by one-way ANOVA using IBM SPSS version 28, in which a p value of ≤0.05 was accepted as a significant difference. Also, evaluations of normality test and homogeneity of variance test were considered.
3. Results
3.1. Crude propolis extract from the stingless bee, G. thoracica
The raw propolis, as a black resin, from the stingless bee, G. thoracica, was collected from the hive entrance, edges, open space, cerumen, and pots (Fig. 2A–D). Extraction of 145–150 g lots of propolis with 80 % (v/v) MeOH gave an average 22.41 g (15.46 %) of a dark brown and sticky resin (CME). Subsequent partitioning of CME with hexane and CH2Cl2 gave CHPE, CDPE, and CMPE at 2.54 g (1.75 %), 7.89 g (5.44 %), and 2.56 g (1.77 %), respectively. All partitioned extracts appeared as a sticky resin, but were different in color, as described in Table 1.
Fig. 2.
Propolis collecting sites: (A) a man-made wooden hive used for meliponiculture, (B) the position of propolis at the entrance, (C) inside of a G. thoracica hive, and (D) raw propolis sample of G. thoracica.
Table 1.
Appearance, TLC pattern, weight (g), and yield (%) of the obtained crude extract, partitioned extracts, and fractions from CMPE.
| Extracts | Appearance | TLC pattern | Weight (g) | Yield[a] (%) |
|---|---|---|---|---|
| CME | Dark brown, sticky resin | N/A [b] | 22.41 | 15.46 |
| CHPE | Yellow, sticky resin | N/A [b] | 2.54 | 1.75 |
| CDPE | Red-brown, sticky resin | N/A [b] | 7.89 | 5.44 |
| CMPE | Dark brown, sticky resin | N/A [b] | 2.56 | 1.77 |
| CMPE1 | Yellow-brown, sticky liquid with white solid | One sharp band | 0.24 | 0.17 |
| CMPE2 | Red-brown, oily liquid | Smear band | 0.68 | 0.47 |
| CMPE3 | Dark-brown, sticky resin with slightly white and oily liquid | Smear bands | 1.98 | 1.37 |
Values were calculated by comparing to 145.0 g of the raw propolis.
N/A indicates the values were not applicable or not tested.
3.2. Primary screening of anti-M. globosa growth activity of the crude and partitioned propolis extracts
The CME, CHPE, CDPE, and CMPE were screened for antimicrobial activity against M. globosa using the agar well diffusion assay. The zone of inhibition from CME was observed at concentrations from and above 200 mg/mL with diameter of 11.83 ± 0.75 mm (56.00 ± 4.31 %) at 200 mg/mL (Fig. 3A). For the crude partitioned extracts, only CMPE exhibited anti-M. globosa activity, with the lowest concentration being 12.5 mg/mL with an inhibition diameter of 9.00 ± 0.52 mm (40.70 ± 2.56 %). Thus, a lower MIC was observed after partitioning. Therefore, the CMPE was chosen for further enrichment.
Fig. 3.
Average diameter of the inhibition zone (mm) from the (A) CME, CHPE, CDPE, and CMPE samples at concentrations of 6.25–400 mg/mL, and (B) CMPE1–3 at concentrations of 3.13–50 mg/mL. Note, KTZ at 2 or 4 μg/mL was used as the positive control within each treatment. Absenting area of bar graph plot (square) indicates the values were not applicable. The SD of each data is shown above the graph bar.
3.3. Bioassay-guided fractionation and enrichment of anti-M. globosa compound(s)
Fractionation of CMPE using SGCC and pooling of the fractions with the same TLC profiles gave three different fractions: CMPE1, CMPE2, and CMPE3 at 0.24 g (0.17 %), 0.68 g (0.47 %), and 1.98 g (1.37 %), respectively, with different appearances as summarized in Table 1. The CMPE1 appeared as crystals, whereas CMPE2 was an oily liquid and CMPE3 was a sticky resin. The antimicrobial activity of CMPE1–3 against M. globosa was preliminarily screened at concentrations of 3.13–50 mg/mL, where no antimicrobial activity against M. globosa was detected with CMPE2, whereas both CMPE1 and CMPE3 exhibited zones of inhibition from concentrations of 12.50 mg/mL at 11.83 ± 1.72 mm (46.36 ± 6.22 %) and 9.67 ± 0.82 mm (37.72 ± 4.05 %), respectively, and above (Fig. 3B). However, CMPE1 had the best antimicrobial activity because the zones of inhibition from CMPE3 were smaller and less stable at increasing concentrations.
As CMPE1 revealed the best antimicrobial activity against M. globosa and was likely to be almost pure due to the presence of a single sharp band on the TLC plate (Supplement 1), the chemical structure of CMPE1 was further analyzed by NMR, with the results as follows: 1H NMR (500 MHz, MeOH-d4) 7.03 (s, 2H) and 3.81 (s, 3H); 13C NMR (125 MHz, MeOH-d4) 169.03, 146.51, 139.77, 121.44, 110.02, and 52.26 (Supplement 2). Comparison of the obtained information with recorded data [45,46], revealed that the structure of the bioactive compound in CMPE1 was methyl 3,4,5-trihydroxybenzoate (C8H8O5) or MG (Fig. 4).
Fig. 4.

Chemical structure of MG [47].
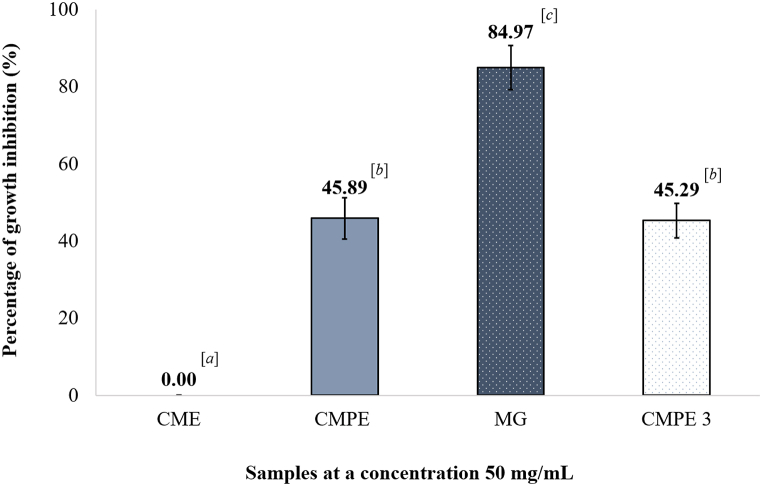
Considering the results at the same concentration of 50 mg/mL from Fig. 5, MG caused a significantly higher percentage of growth inhibition than the CME, CMPE, and CMPE3. The percentage inhibition by CMPE3 was numerically slightly, but not significantly (p > 0.05), lower than CMPE. Thus, further enrichment steps isolated and improved the activity of the extract, with MG having the best antimicrobial activity. The zone of inhibition from the most potent samples from each extraction step is illustrated in Fig. 6A and B.
Fig. 5.
Percentage growth inhibition of CME, CMPE, MG, and CMPE3 at 50 mg/mL in the agar well diffusion assay. Values were calculated relative to the zone of inhibition of KTZ from each treatment. Data are shown as the mean ± 1 SD. Means with a different superscript letter are significantly different (p ≤ 0.05; One-way ANOVA, Dunnett's T3).
Fig. 6.
Representative results from the agar well diffusion assay for (A) CMPE and (B) MG. The symbols “+2”, “+4” and “–” represent the positive control (2 or 4 g/mL KTZ) and solvent control (DMSO), respectively. The numbers represent the extract concentrations (mg/mL).
3.4. Determination of the MIC and MFC: broth microdilution assay
After primary screening, the potent anti-M. globosa extracts, including CME, CMPE, and MG were further determined for their MIC and IC50 values. Since the amount of MG purified from the propolis was small, further MG was then successfully synthesized (Supplement 1). The IC50 values of both the purified MG (0.98 mM) and synthetic MG (1.19 mM) were close, and so they could be used to replace each other. The obtained results are summarized in Table 2 and Fig. 7, along with those for KTZ (positive control) using appropriate solvent control conditions. The MIC value for KTZ was 0.80 mg/mL in 10 % (v/v) DMSO with or without 5 % (v/v) Tween 60, while it was 0.03 mg/mL in 1 % or 2 % (v/v) DMSO.
Table 2.
The MIC and IC50 values against M. globosa growth from samples in different solvents.
| Solvent control | Tested samples | MIC (mg/mL) | IC50 (mg/mL) |
|---|---|---|---|
| 10 % DMSO and 5 % Tween 60 (or 10 % DMSO) | CME | 3.13 | 2.21 |
| CMPE | 1.56 | 1.22 | |
| KTZ | 0.80 or 1.51 mM | 0.77 or 1.45 mM | |
| 1 % DMSO (or 2 % DMSO) | Purified MG | ≥0.13 or 0.71 mM | 0.18 or 0.98 mM |
| Synthetic MG | ≥0.13 or 0.71 mM | 0.22 or 1.19 mM | |
| KTZ | 0.03 or 0.06 mM | 0.03 or 0.06 mM |
Fig. 7.
Percentage growth inhibition of M. globosa in the broth microdilution assay by (A) CME, CMPE, and MG along with (B) their representative results in the resazurin assay and colony pattern. For CME and CMPE, the positive control was KTZ at 0.80 mg/mL and the solvent control was 10 % (v/v) DMSO/5 % (v/v) Tween 60. For MG, the positive control was KTZ at 0.03 mg/mL and the solvent control was 1 % (v/v) DMSO were used. Data are shown as the mean ± 1 SEM.
With respect to the extracts, the MIC values decreased after more enrichment stages (more potent inhibition), in accord with the results from the agar well diffusion assay. The MIC of CME, CMPE, and MG were 25.00, 6.25, and ≥0.13 mg/mL, respectively, and the percentage of inhibition was dose-dependent in each case (Fig. 7A). At the same concentrations, 0.78–50.00 mg/mL, the percentage of growth inhibition induced by CME (33.64–83.62 %) and CMPE (42.43–91.49 %) were not significantly different (p > 0.05, Dunnett's T3) (Fig. 7A). The estimated IC50 value of CME was 2.21 mg/mL (R2 = 0.9569), while those for CMPE and MG were lower at 1.22 mg/mL (R2 = 0.90) and 0.18 mg/mL (R2 = 0.89), respectively.
Due to the poor solubility of the crude and partitioned extracts, the MFC of CME and CMPE were not determined. However, a reduced colony growth after treatment with CME and CMPE was clearly detected at 50 mg/mL in both cases (Fig. 7B). The colony growth pattern was visually decreased after treatment with MG at 16.29 mM (3.00 mg/mL) and dramatically reduced at 6.00 mg/mL. The MFC values were indicated at 43.33 mM (8.00 mg/mL) of MG, where no colony growth was evident (Fig. 8).
Fig. 8.
Representative results of the resazurin assay (upper panel) and colony growth pattern of M. globosa (lower panel) after treatment with MG at concentrations of 2.72–54.30 mM (0.50–10.00 mg/mL). Abbreviation: “rep.” represents technical replicate.
3.5. Evaluation of M. globosa extracellular lipase inhibitory activity
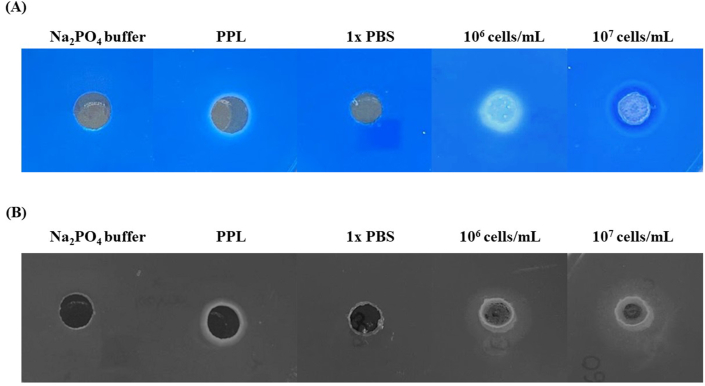
In this work, the extracellular lipase activity from M. globosa was evaluated on TW60 agar and TW60-Vic B agar media to ensure extracellular lipase secretion of M. globosa in these media. The extracellular lipase activities from TW60 agar and TW60-Vic B agar were detected as opaque halos zones and darker transparent or hazy zones, respectively, after adding the yeast cells at a concentration of at least 106 cells/mL (Fig. 9A and B).
Fig. 9.
Detection of the extracellular lipase activity from M. globosa on (A) agar medium supplemented with indicator dye (TW60-Vic B agar) and (B) agar medium without indicator dye (TW60 agar). Note, PPL was used as a positive control along with M. globosa at a cell density of 106–107 cells/mL, while Na2PO4 buffer and 1× PBS were used as negative solvent controls when examining PPL and the yeast cells, respectively.
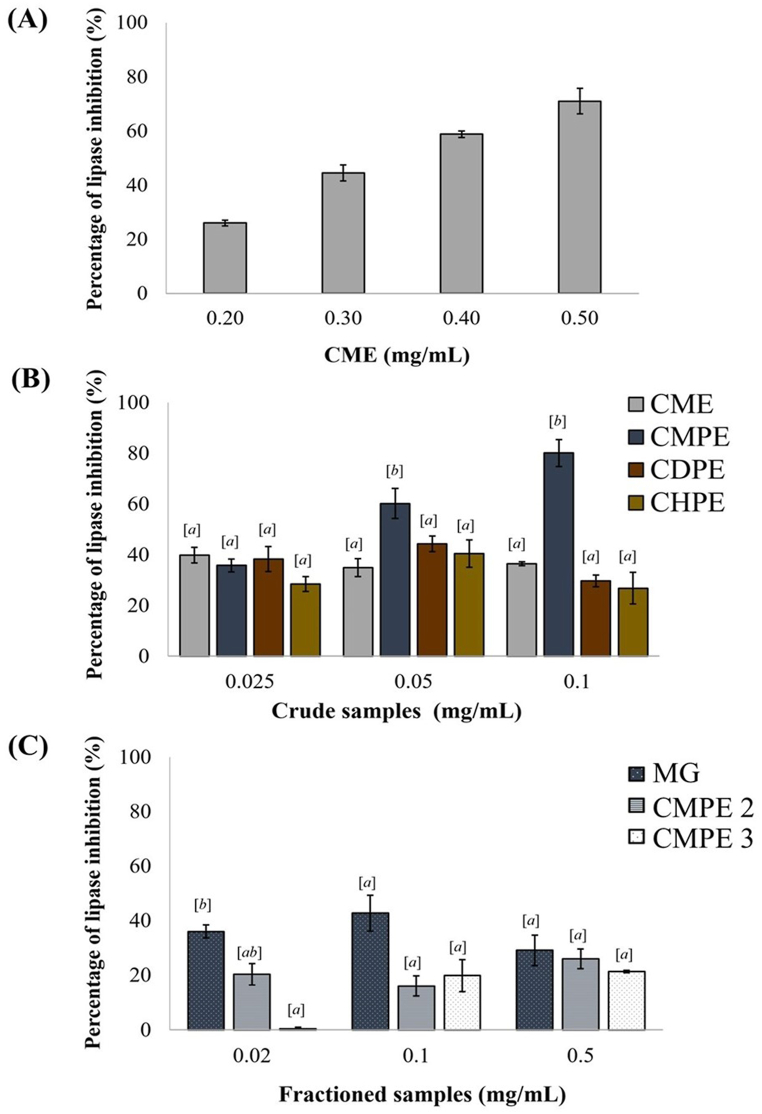
Next, the samples were quantitatively assayed for lipase inhibition activity colorimetrically using pNPP as the substrate. Lipases can hydrolyze pNPP to release pNP giving a yellowish coloration that can be measured at 405–410 nm in an alkaline buffer. This colorimetric lipase assay revealed that CME exhibited lipase inhibition at 26.01–70.98 % in a dose-dependent manner at concentrations of 0.20–0.50 mg/mL (Fig. 10A). The partitioned extracts, CMPE, CDPE, and CHPE were then examined in comparison to CME at 0.025–0.1 mg/mL (Fig. 10B). All the crude and partitioned extracts revealed a clear lipase inhibition activity at these concentrations, but the lipase inhibition activity of CME (34.95–39.80 %), CDPE (29.30–44.25 %), and CHPE (26.81–40.49 %) were neither clearly dose-dependent over this tested concentration range nor significantly different (p > 0.05). On the other hand, CMPE exhibited the highest lipase inhibition at 35.74–81.11 % in a dose-dependent manner. The lipase inhibitory activities of the CMPE1 and CMPE2 samples were lower than those of CME and CMPE, but were not significantly different (p > 0.05), whereas CMPE3 showed the lowest activity at 0.02 mg/mL (Fig. 10C). Overall, it was clearly observed that the inhibition of M. globosa lipase activity was decreased with further enrichment of the crude extracts.
Fig. 10.
Percentage lipase inhibition of (A) CME at 0.20–0.50 mg/mL, (B) CME, CMPE, CDPE, and CHPE at 0.025–0.1 mg/mL, and (C) MG, CMPE2, and CMPE3 at 0.02–0.50 mg/mL. Data are shown as the mean ± 1 SEM. Means with a different superscript letter are significantly different (p ≤ 0.05; One-way ANOVA, Tukey HSD).
Finally, the inhibition of lipase activity by MG was tested at higher concentrations by both quantitative and qualitative methods. From the quantitative lipase assay results, the percentage of lipase inhibition by MG was 30.25–33.79 % at concentrations of 0.06–1.00 mg/mL or 0.33–5.43 mM (Fig. 11A). The detection of lipase activity in the TW60-Vic B agar medium (Fig. 11B) after treatment of M. globosa with MG for 24 h clearly revealed a reduced intensity of the hazy zone in a dose-dependent manner. Similar results were obtained when M. globosa were treated with MG for 48 h, except for the inhibition zone at 0.5 mg/mL (2.72 mM) that was slightly more intense, but was still in accord with the previous quantitative lipase assay results, where the percentage of inhibition decreased at that point (Fig. 11A). The control 48-h treatment had a more intense hazy zone as the longer time of incubation led to an increased yeast growth and enhanced lipase activity, which can affect the inhibitory activity between the two time points. However, the results from both different incubation times could better clarify the effect of MG on M. globosa extracellular lipase.
Fig. 11.
The (A) percentage lipase inhibition and (B) M. globosa lipase activity on TW60-Vic B agar plate from yeast after treatment with MG at 0.06–1.00 mg/mL (0.33–5.43 mM). Data are shown as the mean ± 1 SEM. Abbreviations; “PPL” and “Rep.” refer to porcine pancreatic lipase (2 mg/mL) and replicate, respectively.
4. Discussion
Propolis is one of the most important bee products displaying strong antimicrobial activities [48,49]. It is a fascinating product that has been utilized as a traditional medicine since ancient times. Initially, methanol was found to be a suitable extraction solvent for anti-M. globosa activity, as seen in the CME and CMPE. Alcoholic extraction is the most popular approach and has been commonly used in medical and food developments [50,51]. Moreover, it is a traditional propolis extraction method that is simple and effective, yielding an extract that is low in wax but rich in bioactive compounds, especially polyphenolic components like flavonoids, phenolic acids, and esters [52,53].
In this work, both agar well diffusion and broth microdilution assays were used to investigate the M. globosa growth inhibition activity, while many previous works have used only a single assay [[54], [55], [56], [57]]. There are many factors that might affect the sensitivity of a given antimicrobial method, such as species and strain, inoculum size, culture medium type, interactions between the components and solvent or diluent, formation of emulsion, time, and condition of incubation [58]. Thus, there is currently no specifically validated procedure for determining the antifungal susceptibility of Malassezia sp., and the guidelines recommended by the CLSI and EUCAST do not cover slowing the growth of the yeast [59]. However, the performance of both antimicrobial methods in this study confirmed the anti-M. globosa growth activity of the G. thoracica propolis extracts because the overall trends of both assays coincided with each other.
The crude extract of propolis from Apis mellifera has previously been reported to have potential anti-Malassezia activity. For example, the ethanolic extract or propolis tincture from A. mellifera exhibited the highest activity against 14 strains of M. pachydermatis compared to that with other bee products, such as honey and royal jelly. Furthermore, the ethanolic extract of Brazilian A. mellifera propolis exhibited a MIC50 of 2.6 mg/mL and MFC90 of 5.3 mg/mL against M. pachydermatis isolated from dogs with canine otitis [60]. Moreover, the supercritical and ethanolic extracts of three types (green, red, and brown) of Brazilian A. mellifera propolis could inhibit M. pachydermatis from both normal and resistant clinical isolates [61]. In addition, M. globosa, M. slooffiae, and M. pachydermatis isolated from onychomycosis patients were susceptible to the ethanolic extract of Iranian propolis from A. mellifera at a MIC80 of 2–6 μg/mL [54].
For several decades, many active compounds of a known chemical structure have been reported from propolis and their identification allows quality control in drug development, and examination of the mechanism of action [62,63]. Here, SGCC fractionation was used to further enrich the solvent extracts and two fractions, CMPE1 and CMPE3, were found to also present anti-M. globosa growth activity. However, the inhibitory activity of CMPE3 appeared to be lower and unstable. Perhaps, further enrichment of CMPE3 may lead to an improved inhibitory activity. Overall, the data suggests that propolis from G. thoracica likely contains more than one potential anti-M. globosa compound.
The chemical composition of propolis varies depending on many factors, such as the bee species, harvesting period, plant source, geography, and environmental conditions. For example, propolis harvested in Brazil was different from propolis harvested in Europe, North America, New Zealand, and Asia [[64], [65], [66]]. In this work, CMPE1 was found to be MG, a polyphenolic compound that is an important ester of GA. It has been used in traditional Chinese medicine due to its variety of biological activities, including antioxidant, antitumor, anti-inflammatory, anti-spasmodic, anti-atherogenic, and antimicrobial activities [67,68]. Although MG has been widely reported to inhibit the growth of many microbes, such as Shigella dysenteriae, Streptococcus mutans, Bacillus cereus, Escherichia coli, Candida albicans, Vibrio cholera, and herpes simplex virus type 2 [[69], [70], [71]], surprisingly, the anti-M. globosa growth activity of MG has not been recorded. Besides propolis, which has plant resins as its main component, MG can be derived from plants directly, such as Galla Rhois (Rhus chinensis L.), Toona sinensis, Rhus glabra, and Glochidion superbum [72]. In this study, propolis from G. thoracica was collected from Samut Songkhram province, Thailand, which includes many natural features and different seasons. Also, stingless bees have been recognized as an important and economic insect. The likely plant origins for the propolis used in this study was not determined but is likely to be a mixture of resin from various plant species. Nonetheless, the isolation of MG from mango (Mangifera indica) twigs has been recorded [73] and mango trees were found abundantly in the sample collecting area.
As almost all Malassezia species are lipid-dependent yeasts, the activities of extracellular lipases are vital for their survival and pathogenesis roles. Thus, we investigated the efficiency of the propolis extracts to inhibit M. globosa lipase activity. The application of more enrichment steps in this study appeared to decrease the lipase inhibitory activity. This could be a consequence of separating synergistic components, since natural extracts are comprised of a variety of molecules leading to complex interactions between the constituents [74]. The results also suggested that the lipase inhibition by MG might not be related to the inhibition of M. globosa growth. At a low MG concentration, the growth inhibition by MG decreased in a dose-dependent manner, which might imply that MG could still help to reduce the pathogenicity of this yeast at low concentrations. In accord with the anti-M. globosa lipase activity by MG reported here, an anti-pancreatic lipase activity by MG was previously reported [[75], [76], [77]].
The mechanism of MG on anti-M. globosa activity is still unresolved, while other mechanisms of action by MG on bacteria have been demonstrated, including membrane damaging, membrane hyperpolarization, DNA gyrase inhibition, and ATPase inhibition [[78], [79], [80]]. Hence, the mechanism of MG on anti-M. globosa activity should be further investigated. Further evaluation such as toxicity, ex vivo or in vivo examination and also standardization in the case of crude extract usage are encouraged for future drugs development, supplements, and additives in cosmetic or other products.
5. Conclusion
Thai propolis from G. thoracica is a potential effective source for searching new anti-Malassezia agents. The constituents in the propolis extracts from G. thoracica appeared to display a synergistic mode against M. globosa extracellular lipase activity. A potent lipase inhibition activity was present in both the CME and CMPE. However, MG, the main active compound, does not relate to extracellular lipase inhibition. Since MG reduced the pathogenicity of M. globosa at very low concentrations, it could be useful in avoiding side effects at higher doses. This is the first report of MG as a potential anti-M. globosa compound from a natural resource. In the future, it is possible that MG could be further developed as an alternative new anti-Malassezia drug.
Ethics
This work did not involve with ethical considerations or approval.
Data accessibility
Data will be made available on request.
CRediT authorship contribution statement
Kawisara Konsila: Writing – original draft, Resources, Methodology, Formal analysis. Wanchai Assavalapsakul: Writing – review & editing, Supervision, Investigation. Preecha Phuwapraisirisan: Writing – review & editing, Supervision, Investigation. Chanpen Chanchao: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), Toray Science Foundation, Plant Genetic Conservation Project under the Royal Initiative of Her Royal Highness Princess Maha Chakri Sirindhorn - Chulalongkorn University, and National Science and Technology Development Agency.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29421.
Contributor Information
Kawisara Konsila, Email: kawisara.ks23@gmail.com.
Wanchai Assavalapsakul, Email: wanchai.a@chula.ac.th.
Preecha Phuwapraisirisan, Email: preecha.p@chula.ac.th.
Chanpen Chanchao, Email: chanpen.c@chula.ac.th.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplement 1.
Representative TLC images showing the compound profile of extracted MG (lane 1A), synthetic MG (lane 1B), and pure/commercial MG as control (lane 1A and 1B) under UV light. The mobile phase was 2:23 MeOH: CH2Cl2.
Supplement 2.
1H NMR (A) and 13C NMR (B) peak data of the CMPE1 fraction as methyl gallate.
References
- 1.Hay R.J., et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J. Invest. Dermatol. 2014;134:1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]
- 2.Tuckman A. The potential psychological impact of skin conditions. Dermatol. Ther. 2017;7:53–57. doi: 10.1007/s13555-016-0169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batra R., Boekhout T., Guého E., Cabanes F.J., Dawson Jr TL., Gupta A.K. Malassezia Baillon, emerging clinical yeasts. FEMS Yeast Res. 2005;5:1101–1113. doi: 10.1107/S160053680900112310.1016/j.femsyr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Cabañes F.J. Malassezia yeasts: how many species infect humans and animals. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorch J., Palmer J., Vanderwolf K., Schmidt K., Verant M., Weller T., Blehert D. Malassezia vespertilionis sp. nov.: a new cold-tolerant species of yeast isolated from bats. Pers.: Mol. Phylogeny Evol. Fungi. 2018;41:56–70. doi: 10.3767/persoonia.2018.41.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seifert K.A. The human microbiome project: fungi on human skin. IMA Fungus. 2013;4:16–18. doi: 10.1038/nature12171. [DOI] [Google Scholar]
- 7.Angileri M., Pasquetti M., De Lucia M., Peano A. Azole resistance of Malassezia pachydermatis causing treatment failure in a dog. Med. Mycol. Case Rep. 2019;23:58–61. doi: 10.1107/S160053680900112310.1016/j.mmcr.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benitez LL, Carver PL. 2019 Adverse effects associated with long-term administration of azole antifungal agents. Drugs. 79, 833–853. (doi:10.1007/s40265-019-01127-8). [DOI] [PubMed]
- 9.Leong C., Chan J.W.K., Lee S.M., Lam Y.I., Goh J.P., Ianiri G., Dawson Jr TL. Azole resistance mechanisms in pathogenic Malassezia furfur. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.01975-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas F.D., Sosa MdeL., Fernandez M.S., Cattana M.E., Cordoba S.B., Giusiano G.E. Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Sabouraudia. 2014;52:641–646. doi: 10.1093/mmy/myu010. [DOI] [PubMed] [Google Scholar]
- 11.Abdullah N.A., Zullkiflee N., Zaini S.N.Z., Taha H., Hashim F., Usman A. Phytochemicals, mineral contents, antioxidants, and antimicrobial activities of propolis produced by Brunei stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J. Biol. Sci. 2020;27:2902–2911. doi: 10.1016/j.sjbs.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos J.F., et al. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless bee Tetragonisca fiebrigi (Jataí). Evid.-based Complement. Altern. Med. 2015;2015 doi: 10.1155/2015/296186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desamero M.J., et al. Tumor-suppressing potential of stingless bee propolis in in vitro and in vivo models of differentiated-type gastric adenocarcinoma. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-55465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutra R.P., Bezerra J.L., da Silva M.C.P., Batista M.C.A., Patrício F.J.B., Nascimento F.R.F., Ribeiro M.N.S., Guerra R.N.M. Antileishmanial activity and chemical composition from Brazilian geopropolis produced by stingless bee Melipona fasciculata. Rev. Bras. Farmacog. 2019;29:287–293. doi: 10.1016/j.bjp.2019.02.009. [DOI] [Google Scholar]
- 15.Ibrahim N., Niza N., Rodi M.M., Zakaria A.J., Ismail Z., Mohd K.S. Chemical and biological analyses of Malaysian stingless bee propolis extracts. Malays. J. Anal. Sci. 2016;20:413–422. doi: 10.1016/j.sjbs.2021.07.049. [DOI] [Google Scholar]
- 16.Jongjitaree S., Koontongkaew S., Niyomtham N., Yingyongnarongkul B., Utispan K. The oral wound healing potential of Thai propolis based on its antioxidant activity and stimulation of oral fibroblast migration and proliferation. Evid.-based Complement. Altern. Med. 2022;2022 doi: 10.1155/2022/3503164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolkemper S., Reichling J., Sensch K.H., Schnitzler P. Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine. 2010;17:132–138. doi: 10.1016/j.phymed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Sforcin J.M. Biological properties and therapeutic applications of propolis. Phytother Res. 2016;30:894–905. doi: 10.1002/ptr.5605. [DOI] [PubMed] [Google Scholar]
- 19.Umthong S., Phuwapraisirisan P., Puthong S., Chanchao C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Compl. Alternative Med. 2011;11:1–8. doi: 10.1186/1472-6882-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xool-Tamayo J., et al. In vitro and in vivo anti-inflammatory properties of Mayan propolis. Eur. J. Inflamm. 2020;18:1–11. doi: 10.1177/20587392209352. [DOI] [Google Scholar]
- 21.Zullkiflee N., Hashim F., Taha H., Usman A. Antifungal and antiamoebic activities, cytotoxicity, and toxicity of aqueous and ethanolic extracts of propolis produced by Brunei stingless bees. Jordan J. Biol. Sci. 2023;16:259–266. doi: 10.54319/jjbs/160210. [DOI] [Google Scholar]
- 22.Zullkiflee N., Taha H., Usman A. Propolis: its role and efficacy in human health and diseases. Molecules. 2022;27:6120. doi: 10.3390/molecules27186120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batac M.C.R., Sison M.A.C., Cervancia C.R., Nicolas M.E.O. Honey and propolis have antifungal property against select dermatophytes and Candida albicans. Acta Med. Philipp. 2020;54:11–14. doi: 10.47895/amp.v54i1.1084. [DOI] [Google Scholar]
- 24.Gharib A.A., Taha M. Antimicrobial activity of propolis against some bacteria and fungi. Zagazig Vet. J. 2013;41:81–97. doi: 10.21608/zvjz.2013.94459. [DOI] [Google Scholar]
- 25.Gur N., Bayrak N., Topdemir A. Determination of antimicrobial activity and some biochemical properties of honey and propolis in Turkish markets. Prog. Nutr. 2020;22 doi: 10.23751/pn.v22i3.9166. [DOI] [Google Scholar]
- 26.Oliveira A.C.P., Shinobu C.S., Longhini R., Franco S.L., Svidzinski T.I.E. Antifungal activity of propolis extract against yeasts isolated from onychomycosis lesions. Mem. Inst. Oswaldo Cruz. 2006;101:493–497. doi: 10.1590/s0074-02762006000500002. [DOI] [PubMed] [Google Scholar]
- 27.Shehu A., Ismail S., Rohin M.A.K., Harun A., Abd Aziz A., Haque M. Antifungal properties of Malaysian Tualang honey and stingless bee propolis against Candida albicans and Cryptococcus neoformans. J. Appl. Pharmaceut. Sci. 2016;6:44–50. doi: 10.7324/JAPS.2016.60206. [DOI] [Google Scholar]
- 28.Veiga F.F., et al. Propolis extract for onychomycosis topical treatment: from bench to clinic. Front. Microbiol. 2018;9:779. doi: 10.3389/fmicb.2018.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park M., Park S., Jung W.H. Skin commensal fungus Malassezia and its lipases. J. Microbiol. Biotechnol. 2021;31:637–644. doi: 10.4014/jmb.2012.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White T.C., Findley K., Dawson T.L., Scheynius A., Boekhout T., Cuomo C.A., Xu J., Saunders C.W. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014;4 doi: 10.1101/cshperspect.a019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wijaya W.H., Timotius K.H., Wijaya J.K. Extracellular lipase of Malassezia as anti dandruff drug target: a review. Sys. Rev. Pharm. 2020;11:446–451. doi: 10.31838/SRP.2020.8.64. [DOI] [Google Scholar]
- 32.Yang S., Peng L., Su X., Chen F., Cheng Y., Fan G., Pan S. Bioassay-guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chem. 2011;127:210–215. doi: 10.1016/j.foodchem.2010.12.011. [DOI] [Google Scholar]
- 33.Boonsai P., Phuwapraisirisan P., Chanchao C. Antibacterial activity of a cardanol from Thai Apis mellifera propolis. Int. J. Med. Sci. 2014;11:327–366. doi: 10.7150/ijms.7373. 0.7150/ijms.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khongkarat P., Ramadhan R., Phuwapraisirisan P., Chanchao C. Safflospermidines from the bee pollen of Helianthus annuus L. exhibit a higher in vitro antityrosinase activity than kojic acid. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maximo da Silva M., Comin M., Santos Duarte T., Foglio M.A., De Carvalho J.E., do Carmo Vieira M., Nazari Formagio A.S. Synthesis, antiproliferative activity and molecular properties predictions of galloyl derivatives. Molecules. 2015;20:5360–5373. doi: 10.3390/molecules20045360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendi N.K., Naher H.S., Al-Charrakh A.H. In vitro antibacterial and antifungal activity of Iraqi propolis. J. Med. Plants Res. 2011;5:5058–5066. [Google Scholar]
- 37.Khalid A., Ullah H., Ul-Islam M., Khan R., Khan S., Ahmad F., Khan T., Wahid F. Bacterial cellulose-TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017;7:47662–47668. doi: 10.1039/C7RA06699F. [DOI] [Google Scholar]
- 38.Far F., Al-Obaidi M., Desa M. Efficacy of modified Leeming-Notman media in a resazurin microtiter assay in the evaluation of in-vitro activity of fluconazole against Malassezia furfur ATCC 14521. J. Mycol. Med. 2018;28:486–491. doi: 10.1016/j.mycmed.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Gucwa K., Kusznierewicz B., Milewski S., Van Dijck P., Szweda P. Antifungal activity and synergism with azoles of polish propolis. Pathogens. 2018;7:56–82. doi: 10.3390/pathogens7020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samad M.Y.A., Razak C.N.A., Salleh A.B., Yunus W.Z.W., Ampon K., Basri M. A plate assay for primary screening of lipase activity. J. Microbiol. Methods. 1989;9:51–56. doi: 10.1016/0167-7012(89)90030-4. [DOI] [Google Scholar]
- 41.Cania A., Oetari A., Sjamsuridzal W. Year detection of olive oil and Tween 80 utilization by Rhizopus azygosporus UICC 539 at various temperatures. AIP Conf. Proc. 2020;2242 doi: 10.1063/5.0007873. [DOI] [Google Scholar]
- 42.Ramnath L., Sithole B., Govinden R. Identification of lipolytic enzymes isolated from bacteria indigenous to Eucalyptus wood species for application in the pulping industry. Biotechnol. Rep. 2017;15:114–124. doi: 10.1016/j.btre.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivasankar C., Gayathri S., Bhaskar J.P., Krishnan V., Pandian S.K. Evaluation of selected Indian medicinal plants for antagonistic potential against Malassezia spp. and the synergistic effect of embelin in combination with ketoconazole. Microb. Pathog. 2017;110:66–72. doi: 10.1016/j.micpath.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 44.Honnavar P., Chakrabarti A., Prasad G.S., Joseph J., Dogra S., Handa S., Rudramurthy S.M. The lipase activities of Malassezia species isolated from seborrhoeic dermatitis/dandruff patients. J. Clin. Diagn. Res. 2018;12:DC17–DC19. doi: 10.7860/JCDR/2018/31303.11535. [DOI] [Google Scholar]
- 45.Hernández-García E., García A., Avalos-Alanís F.G., Rivas-Galindo V.M., Delgadillo-Puga C., Camacho-Corona M.D.R. Nuclear magnetic resonance spectroscopy data of isolated compounds from Acacia farnesiana (L) Willd fruits and two esterified derivatives. Data Brief. 2019;22:255–268. doi: 10.1016/j.dib.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan Y.P., Chan E.W.C., Lim C.S.Y. Potent quorum sensing inhibition by methyl gallate isolated from leaves of Anacardium occidentale L. (cashew) Chiang Mai J. Sci. 2015;42:650–656. [Google Scholar]
- 47.Bebout D., Pagola S. Methyl gallate. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2009;65:o317–o318. doi: 10.1107/S1600536809001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo X., Dong Y., Gu C., Zhang X., Ma H. Processing technologies for bee products: an overview of recent developments and perspectives. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.727181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasupuleti V.R., Sammugam L., Ramesh N., Gan S.H. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/1259510. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pobiega K., Kraśniewska K., Derewiaka D., Gniewosz M. Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J. Food Sci. Technol. 2019;56:5386–5395. doi: 10.1007/s13197-019-04009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sultana B., Anwar F., Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gómez-Caravaca A., Gómez-Romero M., Arráez-Román D., Segura-Carretero A., Fernández-Gutiérrez A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006;41:1220–1234. doi: 10.1016/j.jpba.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Kucukates E. Candida and Candidiasis. IntechOpen; London, Greater London: 2022. Antifungal activity of propolis against Candida species: propolis and antifungal action; pp. 2–20. [DOI] [Google Scholar]
- 54.Khosravi A.R., Shokri H., Nikaein D., Mansouri P., Erfanmanesh A., Chalangari R., Katalin M. Yeasts as important agents of onychomycosis: in vitro activity of propolis against yeasts isolated from patients with nail infection. J. Alternative Compl. Med. 2013;19:57–62. doi: 10.1089/acm.2011.0722. [DOI] [PubMed] [Google Scholar]
- 55.Mishra R.K., Mishra V., Pandey A., Tiwari A.K., Pandey H., Sharma S., Pandey A.C., Dikshit A. Exploration of anti-Malassezia potential of Nyctanthes arbor-tristis L. and their application to combat the infection caused by Mala s1 a novel allergen. BMC Compl. Alternative Med. 2016;16:1–14. doi: 10.1186/s12906-016-1092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nazeri M., Ata-Bakhshian R., Taghizadeh M., Talaee R., Mahboubi M. Antifungal activity of herbal extracts against Malassezia species. Iran. J. Dermatol. 2015;18:10–15. [Google Scholar]
- 57.Wu T., Chen M., Zhou L., Lu F., Bie X., Lu Z. Bacillomycin D effectively controls growth of Malassezia globosa by disrupting the cell membrane. Appl. Microbiol. Biotechnol. 2020;104:3529–3540. doi: 10.1007/s00253-020-10462-w. [DOI] [PubMed] [Google Scholar]
- 58.David V., Andrea A.N., Aleksandr K., Lourdes J.A., Eugenia P., Nancy C., Isabel W., Jessica C., León-Tamariz F. Validation of a method of broth microdilution for the determination of antibacterial activity of essential oils. BMC Res. Notes. 2021;14:1–7. doi: 10.1186/s13104-021-05838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chebil W., Haouas N., Eskes E., Vandecruys P., Belgacem S., Belhadj Ali H., Babba H., Van Dijck P. In vitro assessment of azole and amphotericin B susceptibilities of Malassezia spp. isolated from healthy and lesioned skin. J. Fungi. 2022;8:959–968. doi: 10.3390/jof8090959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cardoso R.L., Maboni F., Machado G., Alves S.H., de Vargas A.C. Antimicrobial activity of propolis extract against Staphylococcus coagulase positive and Malassezia pachydermatis of canine otitis. Vet. Microbiol. 2010;142:432–434. doi: 10.1016/j.vetmic.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 61.Deegan K.R., et al. Susceptibility of Malassezia pachydermatis clinical isolates to allopathic antifungals and Brazilian red, green, and brown propolis extracts. Front. Vet. Sci. 2019;6:460. doi: 10.3389/fvets.2019.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amit Koparde A., Doijad R.C., Magdum C.S. Pharmacognosy - Medicinal Plants. IntechOpen; London, Greater London: 2018. Natural products in drug discovery; pp. 2–20. [DOI] [Google Scholar]
- 63.Schenone M., Dančík V., Wagner B.K., Clemons P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013;9:232–240. doi: 10.1038/nchembio.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badiazaman A.A.M., Zin N.B.M., Annisava A.R., Nafi N.E.M., Mohd K.S. Phytochemical screening and antioxidant properties of stingless bee Geniotrigona thoracica propolis. Malays. J. Fundam. Appl. Sci. 2019;15:330–335. doi: 10.1107/S160053680900112310.11113/mjfas.v15n2-1.1557. [DOI] [Google Scholar]
- 65.Bankova V.S., de Castro S.L., Marcucci M.C. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. doi: 10.1051/apido:2000102. [DOI] [Google Scholar]
- 66.Salatino A., Salatino M.L.F., Negri G. How diverse is the chemistry and plant origin of Brazilian propolis? Apidologie. 2021;52:1075–1097. doi: 10.1007/s13592-021-00889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang C.Y., Chang Y.J., Wei P.L., Hung C.S., Wang W. Methyl gallate, gallic acid-derived compound, inhibit cell proliferation through increasing ROS production and apoptosis in hepatocellular carcinoma cells. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazurova J., Kukla R., Rozkot M., Lustykova A., Slehova E., Sleha R., Lipensky J., Opletal L. Use of natural substances for boar semen decontamination. Vet. Med. 2015;60:235–247. doi: 10.17221/8175-VETMED. [DOI] [Google Scholar]
- 69.Muhammad M.T., Fayyaz N., Tauseef S., Razaq U., Versiani M.A., Ahmad A., Faizi S., Rasheed M. Antibacterial activity of flower of Melia azedarach Linn. and identification of its metabolites. J. Korean Soc. Appl. Biol. Chem. 2015;58:219–227. doi: 10.1007/s13765-015-0029-7. [DOI] [Google Scholar]
- 70.Sánchez E., Heredia N., Camacho-Corona MdelR., García S. Isolation, characterization and mode of antimicrobial action against Vibrio cholerae of methyl gallate isolated from Acacia farnesiana. J. Appl. Microbiol. 2013;115:1307–1316. doi: 10.1111/jam.12328. [DOI] [PubMed] [Google Scholar]
- 71.Zheng D., Xu Y., Yuan G., Wu X., Li Q. Bacterial ClpP protease is a potential target for methyl gallate. Front. Microbiol. 2021;11 doi: 10.3389/fmicb.2020.598692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmed M.D., Taher M., Maimusa A.H., Rezali M.F., Mahmud M.I.A.M. Antimicrobial activity of methyl gallate isolated from the leaves of Glochidion superbum against hospital isolates of methicillin resistant Staphylococcus aureus. Nat. Prod. Sci. 2017;23:5–8. doi: 10.1107/S160053680900112310.20307/nps.2017.23.1.5. [DOI] [Google Scholar]
- 73.Subramanian R., Chandra M., Yogapriya S., Aravindh S., Ponmurugan K. Isolation of methyl gallate from mango twigs and its anti-biofilm activity. J. Biol. Act. Prod. Nat. 2016;6:383–392. doi: 10.1080/22311866.2016.1268066. [DOI] [Google Scholar]
- 74.Caesar L.K., Cech N.B. Synergy and antagonism in natural product extracts: when 1+ 1 does not equal 2. Nat. Prod. Rep. 2019;36:869–888. doi: 10.1039/c9np00011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaradat N., Zaid A.N., Hussein F., Zaqzouq M., Aljammal H., Ayesh O. Anti-lipase potential of the organic and aqueous extracts of ten traditional edible and medicinal plants in Palestine; a comparison study with orlistat. Medicines. 2017;4:89–101. doi: 10.3390/medicines4040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwon O.J., Bae J.S., Lee H.Y., Hwang J.Y., Lee E.W., Ito H., Kim T.H. Pancreatic lipase inhibitory gallotannins from Galla Rhois with inhibitory effects on adipocyte differentiation in 3T3-L1 cells. Molecules. 2013;18:10629–10638. doi: 10.3390/molecules180910629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sapkota B.K., Khadayat K., Aryal B., Bashyal J., Jaisi S., Parajuli N. LC-HRMS-based profiling: antibacterial and lipase inhibitory activities of some medicinal plants for the remedy of obesity. Sci. Pharm. 2022;90:55–71. doi: 10.3390/scipharm90030055. [DOI] [Google Scholar]
- 78.Choi J.G., Mun S.H., Chahar H.S., Bharaj P., Kang O.H., Kim S.G., Shin D.W., Kwon D.Y. Methyl gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang M.S., Oh J.S., Kang I.C., Hong S.J., Choi C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008;46:744–750. doi: 10.1007/s12275-008-0235-7. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Xu Y., Liu Z. A review of plant antipathogenic constituents: source, activity and mechanism. Pestic. Biochem. Physiol. 2022;188 doi: 10.1016/j.pestbp.2022.105225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.