Abstract
Purpose:
The benefit of local consolidative therapy (LCT) for oligometastasis across histologies remains uncertain. EXTernal beam radiation to Eliminate Nominal metastatic Disease (EXTEND; NCT03599765) is a randomized phase II basket trial evaluating the effectiveness of LCT for oligometastatic solid tumors. We report here the prospective results of the single arm “lead-in” phase intended to identify histologies most likely to accrue to histology-specific endpoints in the randomized phase.
Methods and Materials:
Eligible histologies included colorectal, sarcoma, lung, head and neck, ovarian, renal, melanoma, pancreatic, prostate, cervix/uterine, breast, and hepatobiliary. Patients received LCT to all sites of active metastatic disease and primary/regional disease (as applicable) plus standard-of-care systemic therapy or observation. The primary endpoint in EXTEND was progression-free survival (PFS), and the primary endpoint of the lead-phase was histology-specific accrual feasibility. Adverse events were graded by Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Results:
From August 2018 through January 2019, 50 patients were enrolled and 49 received definitive LCT. Prostate, breast, and kidney were the highest enrolling histologies and identified for independent accrual in the randomization phase. Most patients (73%) had 1 or 2 metastases, most often in lung or bone (79%), and received ablative radiation (62%). Median follow-up for censored patients was 38 months (range 16–42 months). Median PFS was 13 months (95% CI 9–24), 3-year overall survival rate was 73% (95% CI 57%–83%), and local control rate was 98% (93 of 95 tumors). Two patients (4%) had CTCAE grade 3 toxicity related to LCT; no patient had grade 4 or 5 toxicity.
Conclusions:
The prospective lead-in phase of the EXTEND basket trial demonstrated feasible accrual, encouraging PFS, and low severe-toxicity rates at mature follow-up. The randomized phase is ongoing with histology-based baskets that will provide histology-specific evidence for LCT in oligometastatic disease.
INTRODUCTION
Oligometastasis is theorized to represent a distinct stepwise biological entity that may precede and facilitate development of widespread disseminated disease, through both tumor seeding and promotion of microenvironmental havens favorable for the emergence of clonogens resistant to systemic therapy.1–4 Mitigation of tumor seeding and clonogen resistance through definitive local control, as well as activation of systemic immunity with radiation-induced T-cell priming, may enhance systemic disease control.5,6 Aided by technologic advancements to achieve safe delivery, local consolidative therapy (LCT) has been hypothesized to improve survival outcomes and offer the potential for curing oligometastatic disease.7,8 To date, three randomized trials in non-small cell lung cancer (NSCLC), colorectal cancer, and histology-agnostic solid tumors have demonstrated that LCT can confer an overall survival (OS) benefit.9–12
Although the aforementioned preclinical and randomized clinical evidence are emerging in support of LCT, considerable controversy and debates continue, and many questions remain, especially regarding the histology-specific benefit of LCT.13,14 Therefore, we initiated a multi-institutional, open-label, prospective randomized phase II basket trial, “EXTernal beam radiation to Eliminate Nominal metastatic Disease (EXTEND),” to evaluate the effectiveness of LCT, particularly stereotactic body radiation therapy (SBRT), for various types of oligometastatic solid tumors. The primary objective in EXTEND is to improve progression-free survival (PFS) by using LCT, first assessing individual “baskets” separately, with planned secondary analyses combining baskets. Before the randomized phase of the trial could be opened, a “lead-in” phase was initiated to assess the feasibility of accrual by primary tumor histology to estimate which “histology baskets” are most likely to accrue during the randomized phase. Herein, we report the results of this lead-in phase of EXTEND.
METHODS AND MATERIALS
Study Design and Participants
After institutional review board approval, patients with oligometastatic solid cancer were enrolled in the single-arm, open-label, multicenter, “lead-in” component of the phase II EXTEND trial (NCT03599765). All patients provided informed written consent before study procedures. A copy of the protocol is provided in the Supplement. Patients with pathologically confirmed cancer of colorectal, sarcoma, NSCLC, head and neck, ovarian, kidney, melanoma, pancreatic, prostate, cervix/uterine, breast, and hepatobiliary origin were eligible. Patients were required to have 1–5 active metastatic sites, defined as radiographic evidence of disease activity (Supplement). Patients with oligoprogression or oligorecurrence, as well as synchronous or metachronous oligometastasis, were eligible.15 Additional inclusion and exclusion criteria are described in the Supplement.
Procedures
Patients in the lead-in phase received LCT to all sites of active metastatic disease, including the primary tumor. Surgical resection, cryotherapy, radiofrequency ablation, and radiation were all permitted as LCT if the intent was definitive therapy, consistent with other trials of LCT, as we sought to tailor the method of LCT to each unique clinical scenario in a multidisciplinary setting.9 At least one site was required to be treated with radiation to improve homogeneity in the LCT arm and ensure interpretability of immunostimulatory correlatives.
Dose and fractionation were determined by the treating radiation oncologist, and stereotactic radiation (defined as ≤5 fractions of ≥7 Gy each) was preferred over non-ablative hypofractionated or conventionally fractionated radiation when feasible. The intent of all treatment was definitive local control. Protocol-recommended regimens ranged from 25 Gy to 50 Gy in 3–5 fractions or 12 Gy to 24 Gy in a single fraction for lesions in the brain, bone, or skull base (Supplement). Normal tissue constraints were prioritized over planning target volume coverage, per the judgement of the treating radiation oncologist, and all plans were reviewed in a quality assurance conference. All patients received standard-of-care systemic therapy or, in the absence of reasonable systemic treatment options, observation. Systemic therapy could be delivered concurrently or as adjuvant therapy, the latter to begin no later than 4 weeks after LCT.
Outcomes
Toxicity was prospectively graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 until time of progression, and all CTCAE grade ≥ 2 events were reported. Details on treatment-related deaths, premature withdrawals for toxicity, and serious adverse events were collected. PFS was defined as the time from enrollment to clinical (symptomatic), radiologic (RECIST 1.1), or biochemical progression (prostate only per Prostate Cancer Working Group 3) or death.16,17 Lesions were imaged with standard of care imaging for the individual disease histology (Supplement). Toxicity was assessed and images were obtained at baseline, every 10–14 weeks after enrollment for up to 2 years, and then every 10–26 weeks thereafter or until disease progression. New lesion development, local failure, and conversion to next-line systemic therapy were prospectively tracked from enrollment until disease progression. The primary endpoint of the randomized phase of EXTEND is to assess PFS, with or without LCT, on a patient-level. Secondary endpoints include OS, time to next-line systemic therapy, time to appearance of new lesion(s), time to local failure, safety/tolerability, and quality of life. The purpose of the lead-in component was to identify “baskets” of primary tumor types that were likely to meet the accrual goals of the randomized trial, which were estimated based on the hypothesis that LCT would improve median PFS from 4 months (no LCT) to 8.5 months (LCT) in each basket. To detect such a difference, each basket (except for the prostate baskets) was assumed to require 40 patients, based on 80% power with a one-sided log-rank test and α of 0.10. The randomization phase was pre-specified (before the lead-in phase) to evaluate six total baskets, including five histology-specific baskets (based on accrual data during the lead-in phase) and an “other histology” basket combining the rest of the histologies together.
Statistical Analysis
As noted, the purpose of the lead-in component was to estimate which “histology baskets” would likely complete accrual during the randomized trial phase. For the lead-in phase, a disease histology was deemed feasible and immediately eligible for randomized enrollment if 8 patients were accrued to the lead-in phase within six months. For all other histologies, the lead-in was closed after six months of enrollment regardless of accrual. After closure of the lead-in phase, the randomized phase was commenced. This accrual rate was selected based on the goal to complete accrual to the randomized phase (i.e., 6 baskets of 40 patients each) within 3 years. Histologies with the highest accrual during the lead-in phase were advanced to the randomized phase in histology-specific baskets. Remaining histologies were grouped in an “other histology” basket. No formal power calculations or pre-planned hypothesis testing were specified for the lead-in phase, as its objective was exploratory.
Data were prospectively collected by using REDCap (Research Electronic Data Capture).18 Exploratory time-to-event outcomes were plotted by the Kaplan–Meier method with Prism v9.0 and analyzed via log-rank test with significance at α of 0.05 (GraphPad Software, San Diego, CA).
RESULTS
A total of 56 patients were assessed for eligibility and approached for study enrollment (Fig. S1). Of those 56 patients, 6 (9%) were excluded as screening failures for lack of active oligometastatic lesions (n=1), enrollment in competing trials (n=1), patient preference for treatment at their local facility (n=2), fluctuating mental status necessary for informed consent (n=1), or personal preference of the patient (n=1). From August 2018 through January 2019, 50 patients were enrolled. One patient had considerable delay in staging procedures, had developed new lesions, and did not receive LCT. Forty-nine enrolled patients were treated with definitive LCT to all disease sites. Data were analyzed as of February 14, 2022.
Patient Characteristics
Median age was 65 years (interquartile range [IQR] 56–70 years), and 23 patients (47%) were female (Table 1). Most patients were white (n=42, 86%) and had a performance status score of 0 (n=36, 73%). Active disease at the primary site, untreated with prior local therapy, was present at the time of enrollment in 9 (18%) patients, and before LCT, most patients had received fewer than 2 lines of systemic therapy (n=29, 59%). The most common types of primary tumor were prostate (n=8, 16%) and breast (n=8, 16%), followed by kidney (n=7, 14%), colorectal (n=6, 12%), sarcoma (n=5, 10%), and head and neck (n=5, 10%).
Table 1.
Patient characteristics
| Characteristic | No. of Patients (%) |
|---|---|
| Age, years, median (IQR) Sex |
65 (56–70) |
| Male | 26 (53) |
| Female Race | 23 (47) |
| Asian | 2 (4) |
| Black or African American | 4 (8) |
| White | 42 (86) |
| Other Ethnicity | 1 (2) |
| Hispanic or Latino | 3 (6) |
| Not Hispanic or Latino | 45 (92) |
| Unknown | 1 (2) |
| ECOG Performance Status | |
| 0 | 36 (73) |
| 1 | 11 (22) |
| 2 | 2 (4) |
| Primary Tumor Site | |
| Breast | 8 (16) |
| Cervix / Uterine | 4 (8) |
| Colorectal | 6 (12) |
| Head and Neck | 5 (10) |
| Kidney | 7 (14) |
| Melanoma | 1 (2) |
| Non-Small Cell Lung | 3 (6) |
| Ovarian | 1 (2) |
| Pancreatic | 1 (2) |
| Prostate | 8 (16) |
| Sarcoma | 5 (10) |
| No. of Oligometastases at Enrollment | |
| 1 | 26 (53) |
| 2 | 10 (20) |
| 3 | 7 (14) |
| 4 | 5 (10) |
| 5 | 1 (2) |
| Type of Metastasis | |
| Bone | 22 (26) |
| Brain | 1 (1) |
| Liver | 5 (6) |
| Lung | 46 (53) |
| Lymph Node (M1) | 11 (13) |
| Other | 1 (1) |
| Prior Lines of Systemic Therapy | |
| 0–1 | 29 (59) |
| 2–4 | 20 (41) |
Abbreviations: IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group.
Most patients had one site of metastasis (n=26, 53%), most commonly in lung (n=46, 53%) or bone (n=22, 26%). Eighty-six metastatic lesions were treated with radiation (n=83, 97%) or pulmonary metastatectomy (n=3, 3%). Of the irradiated metastatic tumors, most were treated with SBRT (n=53, 64%), typically delivered in 1–4 fractions (Table 2). The most common SBRT regimen was 50 Gy in 4 fractions. Fewer metastatic lesions were treated with hypofractionated intensity-modulated radiation therapy (n=21, 25%) (i.e., 50–70 Gy in 10–15 fractions) or definitive conventional fractionation (n=8, 10%). One patient with ovarian cancer had metastatic disease to the vaginal cuff, which was treated with conventionally fractionated radiation and high dose rate brachytherapy boost. As part of LCT, 9 patients received local treatment to the primary site, including mastectomy and post-op radiation (n=2), radiation to the cervix (n=2), radiation to the breast (n=1), radiation to the lung (n=2), neoadjuvant radiation followed by esophagectomy (n=1), or radiation to the prostate (n=1).
Table 2.
Radiation treatment characteristics for 83 oligometastatic lesions
| Technique, Incidence, and Frequency | Dose Range, Gy | No. of Fractions |
|---|---|---|
| SRS, SSRS, or SBRT (n=53 [64%]) | 18–24 | 1 |
| 26–36 | 3 | |
| 50–60 | 4 | |
| Hypofractionation (n=21 [25%]) | 50–70 | 10–15 |
| Conventional fractionation (n=8 [10%]) | 50–57.5 | 25–30 |
| Conventional fractionation with brachytherapy boost (n=1 [1%]) | 45 Gy in 25 fractions EBRT + 25 Gy in 5 fractions HDR brachytherapy | |
Abbreviations: SRS, stereotactic radiosurgery; SSRS, spine stereotactic radiosurgery; SBRT, stereotactic body radiation therapy; EBRT, external beam radiation therapy; HDR, high dose rate.
Disease Outcomes
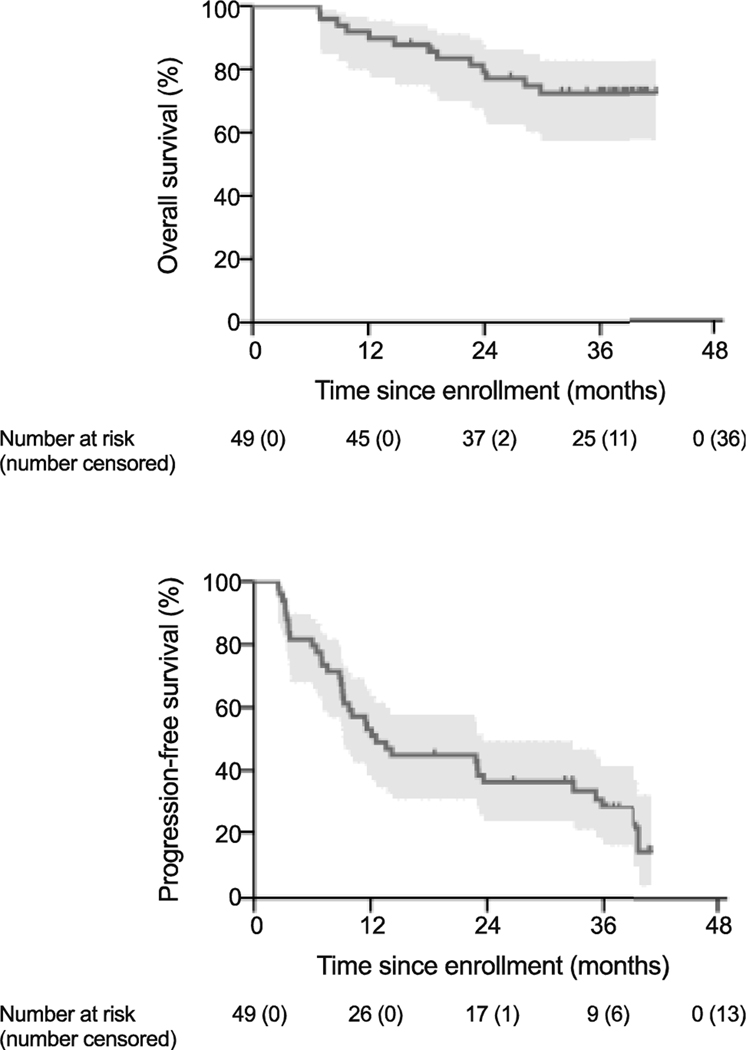
The median follow-up time for censored patients was 38 months (range 16–42 months). Thirteen patients (27%) had died from any cause (Table S1). The median OS was not reached. OS rates were 92% (95% confidence interval [CI] 80%–97%) at 1 year, 79% (95% CI 65%–88%) at 2 years, and 73% (95% CI 57%–83%) at 3 years (Fig. 1A).
Figure 1.
Kaplan-Meier plots of (A) overall survival and (B) progression-free survival. Number at risk and number censored are shown below the x axis. Shaded regions denote 95% confidence intervals. Tick marks indicate censoring.
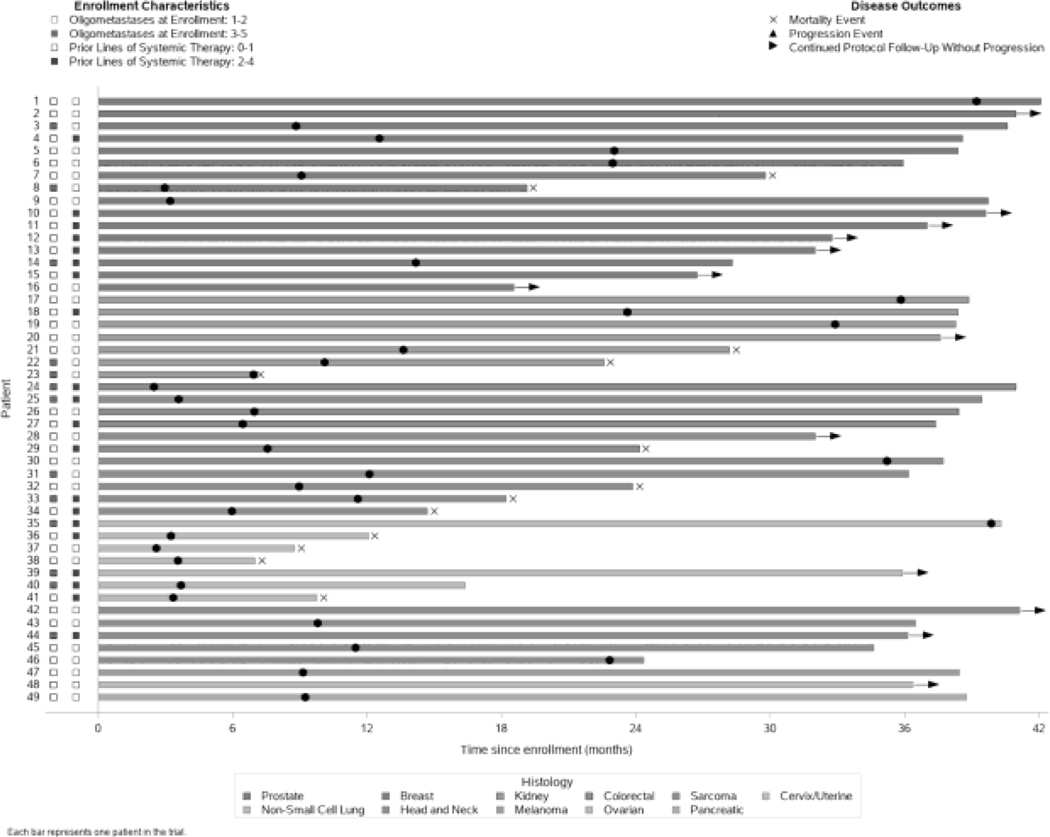
Progression events were observed in 36 patients (73%) (Fig. 2). Median PFS was 13 months (95% CI 9–24 months). The PFS rate at 1 year was 53% (95% CI 38%–66%); at 2 years, 36% (95% CI 23%–50%); and at 3 years, 28% (95% CI 16%–42%) (Fig. 1B). Development of a new lesion outside the irradiated field was the most common progression event (n=30, 83%). Local failure as the first site of progression occurred in 2 patients, yielding an overall local control rate of 98% (93 of 95 treated lesions). The first case of local failure involved progression of a primary cervical mass after conventionally fractionated external-beam radiation and brachytherapy. The second case of local failure involved radiographic progression of metastatic pulmonary lesion from melanoma (by RECIST 1.1) that had been treated with 70 Gy in 10 fractions. One patient had clinical progression due to development of pain associated with left femoral metastasis. Biochemical progression (n=2) and death (n=1) were the remaining events.
Figure 2.
Swimmer plot depicting patient-level primary tumor histology, extent of disease, pre-treatment, and disease outcomes over time.
Systemic therapy was advanced to the next line in 17 patients (35%). The median time to next line systemic therapy was 40 months (95% CI 15 months – not reached) in the whole cohort. For patients with an active primary site treated with LCT, median time to next line systemic therapy was 23 months (95% CI 4 months – not reached), compared to a median time of 40 months (95% CI 15 months – not reached) in patients with a previously treated primary site (log-rank test p=0.31).
Compared to patients with all other cancers, breast cancer patients had prolonged PFS after LCT (log-rank test p=0.03). Patients receiving hormonal therapy had longer PFS after LCT compared to patients treated with other systemic therapies (log-rank test p=0.04), and there was no difference in overall survival (log-rank test p=0.09) (Fig. S2). Patients who had received multiple lines of systemic therapy before LCT did not have significantly different PFS compared to patients who did not (log-rank test p=0.61). There were no significant differences in PFS (log-rank test p=0.64) or OS (log-rank test p=0.43) between patients treated to primary and metastatic sites during LCT versus patients treated to metastatic sites only during LCT (Fig. S3).
Safety and Toxicity
Adverse events are reported in Table 3. No grade 4 or 5 toxicities were observed. Eight patients (17%) experienced grade ≥2 toxicities at least possibly related to LCT, and 2 patients (4%) experienced grade 3 toxicity. In these 8 patients, 12 grade 2 toxicities and 5 grade 3 toxicities occurred. Eight grade 2 toxicities were related to radiation LCT to sites of metastasis, two grade 2 toxicities and one grade 3 toxicity were related to radiation LCT to the primary site, and two grade 2 toxicities and four grade 3 toxicities were related to surgical LCT to primary and metastatic sites.
Table 3.
Grade ≥2 toxicity definitely, probably, or possibly related to LCT at the patient level. Toxicities occurred in relationship to LCT at the primary site for patient 1 and 2 and in relationship to LCT at sites of metastases for patients 1, 3, 4, 5, 6, 7, and 8. Patient 1 and 2 are discussed in detail in the manuscript.
| ID | Treatment | Adverse Event(s) | Grade | Timepoint | Attribution |
|---|---|---|---|---|---|
| 1 | Neoadjuvant IMRT, | Chest pain - cardiac | 2 | During LCT | Possibly Related |
| Esophagectomy, | Fever | 2 | After LCT | Probably Related | |
| Decortication, | Non-cardiac chest pain | 3 | During LCT | Definitely Related | |
| Partial pleurectomy, | Lung infection | 3 | After LCT | Possibly Related | |
| Wedge resection | Chylothorax | 3 | After LCT | Definitely Related | |
| Pleural effusion | 3 | After LCT | Definitely Related | ||
|
| |||||
| 2 | EBRT to primary cervical tumor | Diarrhea | 2 | During LCT | Possibly Related |
| Anxiety | 2 | During LCT | Definitely Related | ||
| Vaginal hemorrhage | 3 | During LCT | Possibly Related | ||
|
| |||||
| 3 | Thoracic Hypofractionated IMRT | Cough | 2 | After LCT | Possibly Related |
|
| |||||
| 4 | Thoracic SBRT | Back pain | 2 | During LCT | Probably Related |
|
| |||||
| 5 | Thoracic SBRT | Nausea | 2 | After LCT | Possibly Related |
| Cough | 2 | After LCT | Possibly Related | ||
|
| |||||
| 6 | Thoracic Hypofractionated IMRT | Esophagitis | 2 | After LCT | Probably Related |
|
| |||||
| 7 | Spine Stereotactic Radiosurgery | Back pain | 2 | During LCT | Definitely Related |
| Pain in extremity | 2 | After LCT | Possibly Related | ||
|
| |||||
| 8 | Thoracic Hypofractionated IMRT | Pneumonitis | 2 | After LCT | Possibly Related |
Abbreviations: LCT, local consolidative therapy; EBRT, external-beam radiation therapy; IMRT, intensity-modulated radiation therapy; SBRT, stereotactic body radiation therapy.
The first case of grade 3 toxicity occurred in a patient following surgical LCT. This patient presented with synovial sarcoma of the distal esophagus metastatic to the right lower lobe and right middle lobe of the lung. The esophageal primary site was treated with neoadjuvant intensity-modulated radiotherapy to 50.4 Gy in 28 fractions followed by partial esophagectomy and thoracic esophagogastrostomy. Sites of metastasis were concurrently treated with decortication and partial pleurectomy, right lower lobe wedge resection, and right middle lobe wedge resection. After surgical LCT, the patient developed grade 2 non-cardiac chest pain, grade 2 fever, grade 3 chylothorax requiring embolization of the thoracic duct, grade 3 pleural effusion, grade 3 non-cardiac chest pain, and grade 3 lung infection associated with an approximately 1-month period of hospitalization. The second case of grade 3 toxicity occurred during radiation LCT to the primary site, a vaginal hemorrhage in a patient with cervical cancer receiving conventionally fractionated radiation.
DISCUSSION
This lead-in phase of the open-label, multi-institutional randomized phase II trial EXTEND showed that accrual was feasible for a multi-histology basket study of LCT for oligometastatic solid tumors. At mature follow-up 3 years following LCT, PFS for the entire cohort is promising, and there were low rates of severe (grade 3) toxicity. Almost all of the LCT given in this lead-in phase was radiation therapy, delivered mostly by ablative stereotactic techniques, and our findings contribute additional prospective data to the increasing support for using LCT for oligometastatic disease. The randomization phase is nearing accrual completion, and its findings will be reported subsequently for each basket, as specified by the protocol.
The prostate and breast groups met the prespecified criteria (that is, accrual of at least 8 patients within 6 months) for histology-specific accrual and endpoint analysis in the randomized phase. Because of expected differences in time to biochemical progression between patients treated with intermittent versus continuous androgen deprivation therapy for prostate cancer, we subsequently separated prostate into two distinct baskets. The kidney group was the next highest accrual histology and therefore was assigned an independent basket for randomization. Because no other group met the prespecified criteria, the remaining histologies are being monitored for accrual and the final histology-specific basket will be determined based on enrollment characteristics during the randomized phase as specified in the study protocol.
Notably, our trial indicates that patients with diverse and currently understudied oligometastatic tumor types can be accrued rapidly onto modern LCT protocols.19 Two previous multicenter landmark trials of LCT for oligometastasis accrued 49 patients over 3 years and 99 patients over 4.5 years.9,12 Our multi-institutional trial, initiated in 2018 after publication of the aforementioned trials in 2016 and 2017, successfully enrolled 49 patients during a 6-month period, although accrual to randomized studies is often much more challenging than single-arm trials. This observation likely reflects an evolving interest in and awareness of the LCT paradigm by patients, caregivers, and oncologists, and a favorable environment for research on LCT for oligometastatic disease for the expedient generation of high-level, high-quality clinical evidence.20
PFS in our trial is comparable to those of other studies including diverse histologies (Table 4). For example, the median PFS in SABR-COMET was 12 months (95% CI 6.9–30.4 months),12 which is nearly identical to that in our trial (median PFS 13 months, 95% CI 9–24 months). Median PFS in the Single-Fraction vs Multifraction Stereotactic Ablative Radiotherapy for Pulmonary Oligometastases (SAFRON II) study was 14 months for the single-fraction arm and 13 months in the multifraction arm.21 A meta-analysis of 21 studies that involved 943 patients and 1290 metastases treated by SBRT revealed a 1-year PFS rate of 51.4% (95% CI 42.7%–60.1%), which again mirrors the findings of our study (1-year PFS rate 53%, 95% CI 38%–66%).22 Such reproducibility across histologic tumor types, practice settings, institutions, and countries strengths the plausibility of our current findings and the argument for using LCT in well-selected cases of oligometastatic disease.
Table 4.
Summary of published prospective trials evaluating local consolidation therapy for histology-agnostic oligometastasis
| Trial/Sponsor | Reference | No. of Patients | Grade 3+ Toxicity | Median FU time, months | Median PFS time, months | Median OS time, years | Local Control Rate |
|---|---|---|---|---|---|---|---|
| Erasmus MC Cancer Institute | Nuyttens, 201531 | 30 | 17% | 36 | ~8 (NR) | ~3.3 (NR) | 77% |
| University of Chicago | Wong, 201632 | 61 | 3% | 28 | 5 | 2.4 | 44% |
| University of Pittsburgh Medical Center | Sutera, 201933 | 147 | 2% | 41 | NR | 3.5 | 75% |
| SABR-COMET | Palma, 202034 | 66 | 11% | 51 | 12 | 4.2 | 63% |
| SAFRON II | Siva, 202121 | 87 | 3% | 37 | 13, 14 | Not reached | 85% |
| NRG-BR001 | Chmura, 202135 | 35 | 20% | 23 | NR | Not reached | NR |
| Multi-center | Zelefsky, 202136 | 117 | NR | 52 | NR | NR | 85% |
| DESTROY | Mercier, 202124 | 90 | 0% | 17 | 15 | NR | 96% |
| EXTEND Lead-In | Present study | 49 | 4% | 38 | 13 | Not reached | 98% |
Abbreviations: NR, not reported; FU, follow-up; PFS, progression-free survival; OS, overall survival.
Grade 3 toxicity was seen in two patients (4%), a reassuringly low rate. In the first case, grade 3 toxicity occurred in a patient undergoing surgical LCT to the primary and metastatic sites including esophagectomy, decortication and partial pleurectomy, and multiple wedge resections of the lung. In the second case, a patient with cervical cancer experienced vaginal hemorrhage during conventionally fractionated radiation to the primary site. No grade 3 events were related to SBRT or radiation delivered to metastasis. Similarly, a retrospective investigation of 317 patients with 406 pulmonary metastases treated with SBRT revealed grade ≥3 toxicity in 5 patients (1.6%).23 Investigators for the phase I dose-escalation trial “DESTROY” reported no grade ≥3 toxicity among 90 patients with 1–3 lesions treated by SBRT.24 In the randomized phase II trial Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer (ORIOLE), none of 36 patients with 1–3 metastases treated with SBRT experienced grade ≥3 side effects.25 Finally, the previously mentioned meta-analysis revealed acute grade 3–5 toxicity in 1.2% of cases (95% CI 0%–3.8%).22 Notably, no patient in our present study experienced grade 4 or 5 toxicity. Our protocol prioritized meeting normal tissue constraints over target volume coverage, which remains a critical aspect of caring for patients with metastatic disease and reflects our observations of toxicity in the current trial. Thus, our prospective study demonstrates that LCT, particularly SBRT, seems to be well tolerated, with few serious toxic effects.22
Although these trials provide evidence that LCT prolongs PFS (and perhaps OS) in oligometastatic disease without significant incidence of serious toxicity, LCT may have additional benefits as well. In the setting of LCT, PFS may correlate directly with quality of life, as LCT is often utilized to defer systemic therapy and/or allow a “drug holiday” in patients with burdensome symptoms.26,27 In a single-arm phase II study, 30 patients with oligometastatic renal cell carcinoma received definitive LCT with SBRT.28 At a median follow-up time of 17.5 months, the median PFS was 22.7 months (95% CI 10.4 to not reached). Moreover, 82% of patients (95% CI 70%–98%) exhibited systemic therapy–free survival at 1 year, and the median interval of systemic therapy–free survival was not reached (95% CI 16.1 to not reached). Secondary endpoints of EXTEND are to compare time to next line systemic therapy with and without LCT and patient-reported quality life, measured by the Center for Epidemiologic Studies Depression Scale, Short Form health Survey 12, Economic StraiN and Resilence in Cancer, and MD Anderson Symptom Inventory.
The present study had some important limitations. The lead-in phase of the trial was not planned for hypothesis testing or powered for efficacy endpoints, and as such survival estimates from this study must be interpreted carefully. The specific dose, fractionation schedule, and systemic therapy regimens used in this study varied and were at the discretion of the treating oncologists. Histology-level analysis was not planned for this lead-in phase, and thus aggregating several solid primary tumor types, with varying biology and rates of distant spread, into a single estimate of survival undoubtedly introduced bias. Several lesions were treated surgically, introducing heterogeneity. However, this is also an important strength of our protocol, as LCT requires a multidisciplinary approach to provide optimal and tailored treatment for each patient, broadening our study’s generalizability and consistent with the approach to LCT of previous trials.9 Nonetheless, almost all lesions in the present study were treated with radiation, and we have described the specific impact of LCT method on outcomes. Because median OS was not reached in the current study, longer follow-up is needed to assess efficacy and late-term toxicity. Finally, these patients represent a well-selected subset of cases with respect to disease and performance status, and the generalizability of our results may be limited.29 Caution is therefore recommended when extrapolating the results of emerging data to routine clinical practice outside the context of a clinical trial. Because patient selection remains a challenge, EXTEND includes the collection of translational specimens to investigate the “circulome” and patient immunophenotype for exploratory analysis based on prior reported data.30 Notwithstanding these limitations, the strengths of the current analysis include its prospective design and data collection as well as rapid accrual. The feasibility and benefits of a basket trial design, increasingly popular in medical oncology trials, are showcased here in a radiation oncology trial. Further, the probability of bias from overrepresentation of any one disease histology in this lead-in phase is reduced by our limiting each basket to 8 patients. Finally, the follow-up time in our study is among the longest reported thus far, providing an important benchmark for toxicity measures.
In summary, this prospective lead-in phase of the phase II trial EXTEND has provided evidence of the feasibility of accruing tumors of several histologic types for a basket study of LCT in patients with oligometastatic solid tumors. Toxicities and PFS seem promising and mirror the findings of other prospective trials. The randomized phase of EXTEND, currently in accrual, will provide further histology-specific information on the role and value of LCT for oligometastatic disease.
Supplementary Material
Acknowledgments:
The authors thank Christine F. Wogan, MS, ELS, from the Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center, for editing the manuscript.
Funding:
Cancer Prevention and Research Institute of Texas (CPRIT) Grant RP200669 and the National Cancer Institute, National Institutes of Health (P30CA016672).
Disclosures:
Dr. Chun reports consulting fees from AstraZeneca and Norton Healthcare, honoraria from Hong Kong International Oncology Symposium and Japan Team Oncology Program, and leadership roles in the American Radium Society, NRG Oncology, American College of Radiation Oncology, and the International Association for the Study of Lung Cancer. Dr. Jhingran reports consulting fees from Genentech and patents issued for Adaptive Intracavitary Brachytherapy Applicator for Cervical Cancer MDA04-056. Dr. Gomez reports grants from Merck and AstraZeneca, consulting fees from AstraZeneca, Olympus, and Grail, and honoraria from BMS, Merck, Varian, Research to Practice, and Med Learning Group. Dr. Tang reports royalties from Wolters Kluwer Health, licenses from the Stanford Office of Technology for US patent #9175079, and honoraria from AstraZeneca. The other authors report no disclosures.
Footnotes
Authors responsible for statistical analyses: Alexander Sherry, MD, Suyu Liu, PhD, Bryan M. Fellman, MS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability:
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.
REFERENCES
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- 2.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell 2009;139:1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015;520:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norton L, Simon R. Growth curve of an experimental solid tumor following radiotherapy. J Natl Cancer Inst 1977;58:1735–41. [DOI] [PubMed] [Google Scholar]
- 5.Patel RR, Verma V, Barsoumian HB, et al. Use of Multi-Site Radiation Therapy for Systemic Disease Control. Int J Radiat Oncol Biol Phys 2021;109:352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498–509. [DOI] [PubMed] [Google Scholar]
- 7.Farooqi A, Ludmir EB, Mitchell KG, et al. Increased biologically effective dose (BED) to the primary tumor is associated with improved survival in patients with oligometastatic NSCLC. Radiother Oncol 2021;163:114–8. [DOI] [PubMed] [Google Scholar]
- 8.Beckham TH, Yang TJ, Gomez D, Tsai CJ. Metastasis-directed therapy for oligometastasis and beyond. Br J Cancer 2021;124:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez DR, Blumenschein GR Jr., Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruers T, Van Coevorden F, Punt CJ, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed]
- 12.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051–8. [DOI] [PubMed] [Google Scholar]
- 13.Kamran SC, Zietman AL. Curing Metastatic Disease With Ablative Radiation Therapy: Separating Truth From Wish. Int J Radiat Oncol Biol Phys 2020;107:433–6. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi A, Timmerman R. Curing Metastatic Disease with Radiation Therapy: Myth or Reality?-Arguing for Reality. Int J Radiat Oncol Biol Phys 2020;107:429–32. [DOI] [PubMed] [Google Scholar]
- 15.Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18–e28. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Morris MJ, Stadler WM, et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016;34:1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrigan KL, Yoder A, De B, et al. Long-term survival following definitive radiation therapy for recurrence or oligometastases in gynecological malignancies: A landmark analysis. Gynecol Oncol 2022;164:550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiontov SI, Pitroda SP, Tran PT, Weichselbaum RR. (Oligo)metastasis as a Spectrum of Disease. Cancer Res 2021;81:2577–83. [DOI] [PubMed] [Google Scholar]
- 21.Siva S, Bressel M, Mai T, et al. Single-Fraction vs Multifraction Stereotactic Ablative Body Radiotherapy for Pulmonary Oligometastases (SAFRON II): The Trans Tasman Radiation Oncology Group 13.01 Phase 2 Randomized Clinical Trial. JAMA Oncol 2021;7:1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrer EJ, Singh R, Wang M, et al. Safety and Survival Rates Associated With Ablative Stereotactic Radiotherapy for Patients With Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2021;7:92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasalic D, Lu Y, Betancourt-Cuellar SL, et al. Stereotactic ablative radiation therapy for pulmonary metastases: Improving overall survival and identifying subgroups at high risk of local failure. Radiother Oncol 2020;145:178–85. [DOI] [PubMed] [Google Scholar]
- 24.Mercier C, Claessens M, Buys MA, et al. Stereotactic Ablative Radiation Therapy to All Lesions in Patients With Oligometastatic Cancers: A Phase 1 Dose-Escalation Trial. Int J Radiat Oncol Biol Phys 2021;109:1195–205. [DOI] [PubMed] [Google Scholar]
- 25.Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020;6:650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446–53. [DOI] [PubMed] [Google Scholar]
- 27.De B, Venkatesan AM, Msaouel P, et al. Definitive radiotherapy for extracranial oligoprogressive metastatic renal cell carcinoma as a strategy to defer systemic therapy escalation. BJU Int 2021. [DOI] [PMC free article] [PubMed]
- 28.Tang C, Msaouel P, Hara K, et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-centre, feasibility, phase 2 trial. Lancet Oncol 2021;22:1732–9. [DOI] [PubMed] [Google Scholar]
- 29.Jasper K, Stiles B, McDonald F, Palma DA. Practical Management of Oligometastatic Non-Small-Cell Lung Cancer. J Clin Oncol 2022:JCO2101719. [DOI] [PubMed]
- 30.Tang C, Lee WC, Reuben A, et al. Immune and Circulating Tumor DNA Profiling After Radiation Treatment for Oligometastatic Non-Small Cell Lung Cancer: Translational Correlatives from a Mature Randomized Phase II Trial. Int J Radiat Oncol Biol Phys 2020;106:349–57. [DOI] [PubMed] [Google Scholar]
- 31.Nuyttens JJ, van der Voort van Zyp NC, Verhoef C, et al. Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys 2015;91:337–43. [DOI] [PubMed] [Google Scholar]
- 32.Wong AC, Watson SP, Pitroda SP, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 2016;122:2242–50. [DOI] [PubMed] [Google Scholar]
- 33.Sutera P, Clump DA, Kalash R, et al. Initial Results of a Multicenter Phase 2 Trial of Stereotactic Ablative Radiation Therapy for Oligometastatic Cancer. Int J Radiat Oncol Biol Phys 2019;103:116–22. [DOI] [PubMed] [Google Scholar]
- 34.Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol 2020;38:2830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chmura S, Winter KA, Robinson C, et al. Evaluation of Safety of Stereotactic Body Radiotherapy for the Treatment of Patients With Multiple Metastases: Findings From the NRG-BR001 Phase 1 Trial. JAMA Oncol 2021;7:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelefsky MJ, Yamada Y, Greco C, et al. Phase 3 Multi-Center, Prospective, Randomized Trial Comparing Single-Dose 24 Gy Radiation Therapy to a 3-Fraction SBRT Regimen in the Treatment of Oligometastatic Cancer. Int J Radiat Oncol Biol Phys 2021;110:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.