ABSTRACT
Anti-Mullerian hormone is a robust marker of ovarian reserve and ovarian response in in vitro fertilisation (IVF). However, its role extends beyond improving the safety of IVF by aiding in choosing appropriate protocols and dosing. This review looks at the value of pre-treatment anti-Mullerian hormone (AMH) value in choosing the appropriate modality of treatment and its predictive ability for the outcomes of such treatment. It briefly addresses the factors that may modulate AMH levels and make clinical decision-making challenging.
KEYWORDS: Anti-Mullerian hormone, clinical utility of anti-Mullerian hormone, in vitro fertilisation, ovulation induction, polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility in women with PCOS.[1,2] Anti-Mullerian hormone (AMH) is an ovarian growth factor exclusively produced by the granulosa cells of the ovarian follicle.[3,4,5] Circulating concentration of AMH shows a 2–3-fold increase in women with PCOS compared to normo-ovulatory women.[6,7,8] Both increased number of follicles and increased secretion from individual cells contribute to its high serum concentration in PCOS.[9,10] The Rotterdam consensus is the most widely utilised criteria for the diagnosis of PCOS which includes the ultrasound parameter of excess antral follicles or polycystic ovarian morphology (PCOM) as one of its components.[11] AMH has long been proposed as an alternative for PCOM as a diagnostic criterion. The most recent updated evidence-based guidelines recommend that serum AMH could be used for defining PCOM in adults for the diagnosis of PCOS in combination with other criteria.[12]
AMH has an inhibitory effect on early follicular recruitment, preventing the primordial follicles from entering the growing follicular pool and premature exhaustion of the primordial pool.[13,14,15] In addition, AMH reduces follicle sensitivity to follicle-stimulating hormone (FSH) and thus inhibits cyclical follicle recruitment.[16] Understanding the ovarian follicle physiology provides an opportunity to extend the role of AMH in the management of PCOS beyond diagnosis alone.[17] The assay methodology for the measurement of AMH has evolved over the past two decades. Initial semi-automated assays with different antibodies have posed various challenges, despite providing an important understanding of ovarian reserve. These included assay stability under various conditions and interpretation and comparison of AMH values from different assays, thus limiting our ability to understand the clinical utility of AMH in various clinical situations. However, the availability of fully automated tests and attempts at harmonisation of AMH assays have reduced the magnitude of this problem in recent years.[18] This review discusses the important role the analyte plays in decision-making, beyond a reflection of ovarian reserve during the management of infertile women with PCOS.
MATERIALS AND METHODS
Literature search was performed in PubMed, Medline and Google Scholar until August 2023, using the terms: PCOS, anti-Mullerian Hormone, AMH and clinical utility of AMH. Randomised controlled trials and observational studies published in English were included. Case series, case reports and non-English language publications were excluded. Cross-references were manually searched. Zotero was used for reference management. Figure 1 shows the details of the search strategy.
Figure 1.
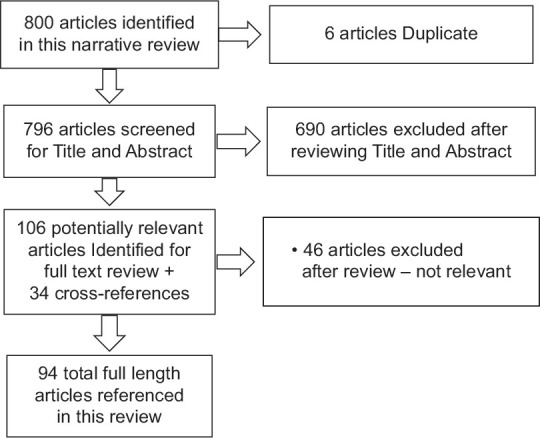
PRISMA flow chart of search strategy
ANTI-MULLERIAN HORMONE AND ITS ROLE IN CLINICAL DECISION-MAKING IN POLYCYSTIC OVARY SYNDROME-RELATED INFERTILITY
The following section provides a comprehensive insight into the role of AMH in decision-making before various treatment modalities for infertility in women with PCOS. In addition, it summarises the factors that may modulate AMH levels and hence pose additional challenges in clinical interpretation.
Anti-Mullerian hormone and in vitro fertilisation
The most established role of AMH in treatment planning is in the context of in vitro fertilisation (IVF). It predicts hyper-response with the highest sensitivity and specificity.[19,20] Women with PCOS are at a high risk of developing ovarian hyperstimulation syndrome (OHSS) while undergoing IVF. Even though titrating the dose of gonadotropin may be challenging in a small proportion of them, a prior knowledge of AMH levels offers a very important opportunity to improve the safety of treatment in the majority.[21] Initial reports using the Diagnostic Systems Laboratories (DSL) assay reported that values above 3.5 ng/l predict excessive response with 88% sensitivity and 70% specificity.[20] A low starting dose of gonadotropin, preference for antagonist protocol and choosing GnRH agonist over human chorionic gonadotropin for oocyte maturation in those with high response are all the strategies that minimise the risk of hyper-response and consequent OHSS in women with PCOS.[22,23,24,25,26]
The role of AMH as a predictor of success in IVF in addition to ovarian response has been explored during the past decade. Considering its strong correlation with ovarian response, it might be expected that a high AMH is predictive of an increased live birth rate (LBR). There is evidence, even though inconclusive, to suggest that AMH is a predictor of live birth in IVF.[24,27,28,29] An improved live birth is noted with increasing AMH in an unselected population which is attributed to the strong correlation between AMH and oocyte yield.[24] The association between AMH and ART outcomes appears to remain despite correcting for age and is more apparent in older women.[30,31] The first meta-analysis addressing the association between live birth and AMH general population of women undergoing IVF reported a weak predictive value of AMH for LBR with IVF and recommended utilising the information for counselling couples before IVF.[28] With changing clinical practice and segmentation of IVF cycles, cumulative LBR (cLBR) is a more meaningful measure for the success of IVF, and AMH shows a weak positive predictive value for cLBR as well.[29]
A prospective study reported a positive correlation between AMH on day 3 of an IVF cycle and implantation and pregnancy rate in women with PCOS. However, the results of this study should be interpreted with caution both due to the criteria used to define PCOS and the AMH levels in the study population. Using the DSL assay, the AMH value at the 75th centile of the study group was relatively low at 3.85 ng/ml, which could be the result of the chosen study population.[32] A further systematic review reported a weaker link between implantation or clinical pregnancy and PCOS compared to those with unspecified ovarian reserve.[33] In addition to the ovarian reserve, factors such as severity of the condition and associated metabolic and endocrine alterations influence the serum values of AMH in PCOS.[34,35,36] Increased number of follicles and variable increase in the secretion from individual follicles are additional confounders.[9,10,37] Any or all of these factors may be contributing to the AMH levels in PCOS and prevent a better understanding of its correlation to clinical outcomes in IVF. However, a high serum AMH concentration can be a useful guide for a positive pre-treatment counselling in young infertile women.
There is concern that women with PCOS with very high AMH (variedly defined as >8.27 ng/ml or >12 ng/ml) may have a lower LBR compared to those with lower levels.[38,39] Various factors have been postulated to be contributing to this problem. Oocyte quality and endometrial aberrations could be two of the important contributors to this not yet fully understood paradox.[40,41,42,43] Different phenotypes and their underlying endocrine abnormalities are other factors which may influence LBR.[38,44,45] However, data from the Society for Assisted Reproductive Technology registry involving more than 2700 patients with PCOS suggest no decline in LBR per completed embryo transfer and attribute the decline in LBR following fresh cycles in women with PCOS to cycle cancellation and segmentation.[46] An analysis of LBR in fresh transfers in women with AMH values of ≥5 ng/ml showed a 3% decrease in the odds of live birth in fresh autologous IVF, with each unit increase in AMH. However, this was due to an increased incidence of fresh embryo transfer cancellation because of concerns regarding OHSS.[46] Hence, AMH plays an important role in pre-treatment counselling of those with very high levels, to alert them to an increased possibility of segmentation of treatment and subsequent frozen embryo transfer.
Anti-Mullerian hormone and ovulation induction
Oral ovulation-inducing agents are the first line of treatment for PCOS-related infertility.
Although letrozole (LET) is now considered the first choice for such a treatment,[12,47,48] clomiphene citrate (CC) continues to be used as an alternative first-line agent for ovulation induction (OI) in PCOS. Clomiphene resistance, seen in up to 25% of women with PCOS and infertility, is diagnosed only after unsuccessful OI with incremental doses of CC in successive cycles.[49] It is important to note that AMH values above 7.7 ng/ml predict a low chance of successful ovulation with CC. Importantly, higher levels of AMH do not predict non-responsiveness to gonadotropins.[50]
A subsequent study reported that an AMH level above 3.4 ng/ml may identify women likely to be resistant to OI.[51] The use of Immunotech (IOT) assays in the initial studies and Uscan in the latter study is attributed to such different discriminatory values of AMH. This also highlights the challenges encountered with different AMH assays in the absence of international standardisation or harmonisation. IOT is considered to yield higher values than Uscan or DSL assays. Another study using AMH Gen II assay found a level of 8.58 ng/ml to have 78% sensitivity and 67% specificity for the prediction of non-response to CC.[52] A small retrospective study further reported an AMH level of 9.78 as the discriminatory level beyond which cumulative ovulation rates were significantly lower.[53] Finally, in a large cohort study of women with PCOS using Gen II AMH assay, it was noted that women with higher AMH levels needed higher doses of CC or LET to achieve ovulation and the chances of ovulation reduced with increasing levels of AMH.[54] In vitro studies involving human granulosa cells suggest that the probable underlying mechanism is related to high AMH, as high AMH inhibits aromatase activity and reduces follicular sensitivity to FSH.[55,56,57] Despite the challenges in inter-assay comparison, it is important to note that anovulatory women with PCOS with very high serum AMH values are less likely to ovulate with CC or LET. Incorporating this into treatment planning can avoid protracted treatment cycles without ovulation and the frustration patients experience thereof.
Anti-Mullerian hormone and intrauterine insemination
There have been some efforts to evaluate whether AMH has any predictive role in intrauterine insemination (IUI). Data from a large retrospective study including more than 700 cycles of IUI show that those with high AMH were less likely to respond to CC or LET, particularly at levels >9.3 ng/ml. Further, this observation was more pronounced with CC compared to LE. However, such an issue was not encountered with exogenous gonadotropin.[58] The authors suggest that in the subgroup of women with very high AMH, gonadotropin may be considered a first-line therapy or alternatively a high starting dose of CC or LE can be considered.[58]
Interestingly, a pilot study evaluating pre-treatment AMH levels in gonadotropin cycles shows a good response in those with AMH ≤4.7 ng/ml. The authors documented a poor response in those above 10.2 ng/ml.[59] This may be a reflection of a very high number of small follicles in such women contributing to excessive AMH levels, which consequently adversely influence the follicular sensitivity to FSH and hence reduced response to exogenous gonadotropins.[9,19,56,59] In addition, ovarian stimulation strategies for IUI are milder than those used in the context of IVF to avoid the risk of high-order multiple pregnancies or OHSS. Currently, there is no evidence evaluating any correlation between AMH values and LBR after IUI.
Anti-Mullerian hormone and in vitro maturation
It is well known that a subset of women undergoing IVF is at considerably high risk of developing OHSS with gonadotropin stimulation. In vitro maturation (IVM) is considered a safer option in them as currently IVM is the only strategy with no reported cases of OHSS.[60] Despite its availability since 1935[61] and recent improvements in clinical and laboratory protocols, its acceptance is limited due to lower pregnancy rates compared to conventional IVF.[62,63]
A predictive model utilising AMH and antral follicle count (AFC) has been recommended to choose appropriate candidates for and optimise ovarian response in IVM.[60] An AMH level of 1.63 ng/ml using DSL assay can predict the chances of obtaining five mature oocytes following IVM with a sensitivity of 81% and a specificity of 53%.[64] Further, in women with PCOS, collection of at least eight COCs has been associated with a higher cumulative pregnancy rate. Very high AMH (14.8 ± 10.1 ng/ml) using IOT assay and AFC (38.6 ± 16.1) may have a strong predictive potential (area under the curve = 0.7864) for the number of COCs retrieved. In another cohort study, an AMH level of 8.5 ng/ml or higher was associated with PR comparable to IVF.[65] While the different assays used for AMH may limit the interpretation of the results, a brief description is provided in the discussion section which may be of help in understanding the evolution and interpretation of AMH assays.
Anti-Mullerian hormone and metabolic abnormalities
The negative impact of abnormal metabolic health on maternal and foetal health during pregnancy is increasingly being understood. The prognostic role of AMH has been evaluated in women with PCOS to predict the probability of metabolic syndrome (MetS). The current evidence is limited, conflicting and does not support a prognostic role for AMH in women with PCOS to predict an increased risk of MetS.[66,67,68]
Anti-Mullerian hormone and preterm delivery
Elevated AMH has been reported as a risk factor for an increased incidence of preterm delivery (PTD) in women with PCOS treated for infertility. This appears to be independent from any of its associations with insulin resistance or hyperandrogenemia.[69,70,71,72] There is a need for an increased understanding of this underlying association and awareness of such a risk amongst clinicians providing antenatal care to such women.
Factors modulating anti-Mullerian hormone concentrations
The previous section addresses the important role of AMH in the management of PCOS beyond its diagnosis. However, it is important to consider the common factors which may modulate its values and consequently interfere with its interpretation. The detrimental effect of smoking on ovarian reserve has been previously documented.[73] However, its effect on AMH in PCOS if any has not been documented and hence will not be discussed here. Table 1 summarises the current evidence on the factors that may modulate AMH levels in women with PCOS considering or undergoing treatment for infertility.
Table 1.
Factors with modulatory effect on anti-Mullerian hormone levels in infertile women with polycystic ovary syndrome
| Factors | Effect on AMH | Comments |
|---|---|---|
| Age | Declines with age | - |
| LOD | Declines (normalises) | - |
| Metformin | Declines | - |
| COCP | Declines | Effect seen within 1–3 months of initiating treatment |
| Vitamin D | Effect inconclusive | Current data – inconclusive of the direction of the effect |
| Genital TB | Declines | - |
| Obesity | Declines | Levels remain above non-PCOS |
AMH=Anti-Mullerian hormone, PCOS=Polycystic ovary syndrome, LOD=Laparoscopic ovarian drilling, COCP=Combined oral contraceptive pill, TB=Tuberculosis
Age
Ovarian reserve declines with age and is associated with a decline in pregnancy and livebirth rates in older women.[74] Diagnosis of PCOS may be challenging in older women with lower AMH values compared to younger women. However, a serum AMH above the 75th percentile for age is highly suggestive of PCOS.[75] This criterion would be relevant where population nomogram exists.
There is evidence that the pregnancy rate does not differ in women with different AMH values, in an unselected population of women below the age of 34 years undergoing IVF. However, in older women above 42 years of age, a high AMH does not improve the pregnancy rate underscoring the importance of qualitative decline in oocytes with age.[31] Further, despite a higher oocyte yield, PCOS women do not appear to have an extended reproductive window.[76,77] Hence, AMH may not retain its predictive value for cLBR in older women.
Laparoscopic ovarian drilling
Laparoscopic ovarian drilling (LOD) is considered a second-line therapy for PCOS-related infertility.[12,78] A recent meta-analysis shows a decline in AMH values after LOD, and the results are consistent despite different durations of reported follow-up, different assay kits for AMH used, whether the procedure was performed on both or single ovary and the type of energy used.[79] It is considered normalisation of ovarian reserve rather than any real damage to the ovarian reserve. A reduced AMH following LOD may predict a lowered risk of OHSS in any subsequent IVF and reduced multiple pregnancy following OI in women with PCOS.[80]
Obesity
There is conflicting evidence on the impact of obesity in PCOS on serum AMH values. Obese PCOS women have lower levels of AMH but still higher than non-PCOS women.[74,81] A recent report suggests a lower AMH level in young PCOS with increased cardiometabolic risk, and every unit reduction may indicate a 10% increased risk of MetS.[66]
Pharmacological agents and bariatric surgery play an important role in managing morbid obesity associated with PCOS and contribute towards improved fertility in such women.
The use of sibutramine in PCOS women, while achieving weight loss, leads to a decrease in AMH level which is hypothesised to be due to its direct ovarian action on the endocannabinoid system in the ovary.[82] It is interesting to note that a combination of diet, physical activity and pharmacological (orlistat) induced weight loss in overweight and obese PCOS women leads to improved metabolic status, HA and an increased AMH.[83] A similar increase is noted following sleeve gastrectomy as well.[84] Hence, treatment modality needs to be taken into consideration while interpreting AMH values following any treatment of obesity for an appropriate decision-making.
Medications
Metformin co-treatment is often an integral part of the management of PCOS-related infertility. A recent meta-analysis shows that obese women who are on metformin for more than 2 months may experience a 30% decline in AMH and this decline could be more pronounced in lean PCOS women.[85]
Oral contraceptive pills (OCPs) are often used in women with PCOS to treat certain endocrine abnormalities before active fertility treatment. AMH is reduced in current OCP users, and the decline is experienced in <3 months of use and can be as high as 60%–70% in both non-PCOS and PCOS women.[85,86] Hence, decision-making based on AMH values in current or recent OCP users may be affected.
Vitamin D3 deficiency
While there appears to be a cause–effect relationship between Vitamin D levels and AMH, the current evidence is inconclusive regarding the direction of effect either for its deficiency or for supplementation on serum AMH values.[87,88,89]
Genital infections
Genital tuberculosis (GTB) is a common cause of infertility and may co-exist with PCOS.[90] It is known that GTB is associated with reduced ovarian reserve.[90,91] It is to be noted that both PCOS and GTB are prevalent in many of the low- and middle-income countries and such co-existence may mask the diagnosis of PCOS. In addition, it may also affect the clinical utility of AMH for treatment planning and add to failures of treatment.
DISCUSSION
This review provides a comprehensive insight into the prognostic role of AMH while strategising various treatments in infertile women with PCOS. It is well known that AMH is a robust alternative for PCOM in the diagnosis of PCOS. However, it should be used in conjunction with either oligo-ovulation or clinical/biochemical hyperandrogenism to arrive at the diagnosis.[12] The role of this powerful analyte is not limited to the estimation of ovarian reserve alone, and the current evidence shows its ability to influence treatment decisions in PCOS-related infertility. The evidence supports its prognostic role in addition to the endocrine or metabolic markers commonly seen in PCOS.[92] Translating this insight into clinical practice offers an opportunity for effective pre-treatment counselling and planning. It may help avoid protracted courses of medications and timely recourse to effective treatment strategies with added emotional and financial benefits. Hence, the most important role of AMH in PCOS-related infertility is its clinical utility as a predictive and prognostic tool. Majority of the women with PCOS conceive with simple interventions, and awareness of pre-treatment AMH values will help in deciding the choice of treatment modality.
A high pre-treatment AMH merits initiating OI with higher doses of CC or LET than used in ovulatory women.[50,51,52,53,54] Alternatively, gonadotropins may be more effective in inducing ovulation in such women. In the context of IUI, very high levels of AMH >10 ng/ml may predict a poor response even with gonadotropins.[59] The prognostic role of AMH in predicting ovarian response in IVF is well established. This has led to the adoption of individualised controlled ovarian stimulation strategies which have improved the safety of IVF in PCOS by reducing the incidence of significant OHSS.[26] AMH may also guide in decision-making and identify women in whom IVM may be a safer option than IVF without compromising pregnancy rates.[64,65] In addition, AMH has a weak predictive ability for LBR except in women with advanced maternal age. Another predictive role of AMH that is not widely acknowledged is its ability to predict an increased risk of PTD in women with high AMH who conceive following treatment for PCOS-related infertility.[69,70,71,72] It is important to incorporate the impact of any factors that may modulate the AMH values into the decision-making process.
An important and ongoing challenge in AMH estimation, which has prevented comparison between clinics or populations and thus limited the clinical utility, has been the laboratory issues with various assays used. The initial assays including DSL, IOT and Gen II utilise enzyme-linked immunosorbent assay (ELISA) methodology. The use of different antibodies, the differential effect of various sample storage and processing on results, lack of internationally standardised material for uniform calibration and lack of awareness of the differential performance of various assays have all limited the clinical utility of AMH.[93] There are no universally accepted values of conversion factors for inter-assay comparison. Similar issues exist with the currently available tests of ultrasensitive ELISA (Ansh) or fully automated chemi-immunoluminiscene (Elecsys and Access), which has prevented harmonisation of results.[18,94] Access assay-measured values of AMH may be 5%–15% higher than Elecsys.[18] This has important clinical implications including wrong dosing and compromised safety of treatment. Sample storage and batch processing is a common practice in clinical laboratories. With all the three assays currently available, there is a difference in the AMH values obtained between the fresh and the frozen-thawed serum samples.[95] Both Elecsys and Access AMH assays show a very small decrease in the AMH value in frozen-thawed samples in comparison to fresh samples, considered to be of little clinical importance. Contrarily, higher values are noted in the frozen-thawed samples compared to the fresh samples with ultrasensitive ELISA (Ansh) assay, and the magnitude of difference may be of clinical importance.[95] This is another factor to be considered while incorporating AMH values for clinical decision-making.
CONCLUSION
The role of AMH as a quantitative marker of ovarian reserve has long been established. This review looks at its value beyond the diagnostic role towards its predictive and prognostic role in various treatment options available for infertile women with PCOS. Current evidence does not support AMH as an additional diagnostic marker in those with an established diagnosis of PCOS or its evaluation routinely before the first-line therapy of oral OI. However, AMH may play an important role and assist in clinical decision-making in those resistant to or fail to conceive with oral ovulogens, to avoid protracted treatment cycles. Laboratory issues related to different AMH assays have been a limiting factor in understanding and utilising this powerful analyte to the fullest extent. An awareness of the clinical scenarios that may influence the AMH values is important for the effective use of this tool in decision-making and treatment planning.
Author’s contributions
DS – Concept, manuscript preparation, editing and review. PRJ – Concept, literature search, manuscript preparation, editing and review.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Data availability statement
No original data used.
REFERENCES
- 1.Franks S, Mason H, Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163:49–52. doi: 10.1016/s0303-7207(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 2.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 3.Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril. 2002;77:141–6. doi: 10.1016/s0015-0282(01)02944-2. [DOI] [PubMed] [Google Scholar]
- 4.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary:Potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 5.Seifer DB, Maclaughlin DT. Mullerian inhibiting substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88:539–46. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Fleming R, Kelsey TW, Anderson RA, Wallace WH, Nelson SM. Interpreting human follicular recruitment and antimüllerian hormone concentrations throughout life. Fertil Steril. 2012;98:1097–102. doi: 10.1016/j.fertnstert.2012.07.1114. [DOI] [PubMed] [Google Scholar]
- 7.Sova H, Unkila-Kallio L, Tiitinen A, Hippeläinen M, Perheentupa A, Tinkanen H, et al. Hormone profiling, including anti-müllerian hormone (AMH), for the diagnosis of polycystic ovary syndrome (PCOS) and characterization of PCOS phenotypes. Gynecol Endocrinol. 2019;35:595–600. doi: 10.1080/09513590.2018.1559807. [DOI] [PubMed] [Google Scholar]
- 8.Fleming R. Anti-müllerian hormone:A personal view of the empowering Analyte. J Hum Reprod Sci. 2020;13:257–60. doi: 10.4103/jhrs.JHRS_231_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome:Relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–62. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 10.Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–5. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 11.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 12.Teede HJ, Tay CT, Laven J, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2023;120:767–93. doi: 10.1016/j.fertnstert.2023.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome?A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab. 2013;98:3332–40. doi: 10.1210/jc.2013-1393. [DOI] [PubMed] [Google Scholar]
- 14.Themmen AP. Anti-müllerian hormone:Its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr. 2005;34:18–21. doi: 10.1093/jncimonographs/lgi026. [DOI] [PubMed] [Google Scholar]
- 15.Visser JA, Themmen AP. Anti-müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–6. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function:The role of anti-Müllerian hormone. Reproduction. 2002;124:601–9. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 17.Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, et al. Anti-müllerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005;90:5536–43. doi: 10.1210/jc.2005-0907. [DOI] [PubMed] [Google Scholar]
- 18.Punchoo R, Bhoora S. Variation in the measurement of Anti-Müllerian hormone –What are the laboratory issues? Front Endocrinol (Lausanne) 2021;12:719029. doi: 10.3389/fendo.2021.719029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941–5. doi: 10.1210/jc.2005-2076. [DOI] [PubMed] [Google Scholar]
- 20.Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92:1586–93. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 21.La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–30. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 22.Doldi N, Persico P, Di Sebastiano F, Marsiglio E, Ferrari A. Gonadotropin-releasing hormone antagonist and metformin for treatment of polycystic ovary syndrome patients undergoing in vitro fertilization-embryo transfer. Gynecol Endocrinol. 2006;22:235–8. doi: 10.1080/14767050600761893. [DOI] [PubMed] [Google Scholar]
- 23.Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol:A systematic review and meta-analysis. Hum Reprod Update. 2006;12:159–68. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- 24.Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH:Prediction of live birth and extremes of response in stimulated cycles –Implications for individualization of therapy. Hum Reprod. 2007;22:2414–21. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 25.Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, et al. Anti-müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24:867–75. doi: 10.1093/humrep/den480. [DOI] [PubMed] [Google Scholar]
- 26.Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750. doi: 10.1002/14651858.CD001750.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Marca A, Nelson SM, Sighinolfi G, Manno M, Baraldi E, Roli L, et al. Anti-müllerian hormone-based prediction model for a live birth in assisted reproduction. Reprod Biomed Online. 2011;22:341–9. doi: 10.1016/j.rbmo.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Iliodromiti S, Kelsey TW, Wu O, Anderson RA, Nelson SM. The predictive accuracy of anti-Müllerian hormone for live birth after assisted conception:A systematic review and meta-analysis of the literature. Hum Reprod Update. 2014;20:560–70. doi: 10.1093/humupd/dmu003. [DOI] [PubMed] [Google Scholar]
- 29.Peigné M, Bernard V, Dijols L, Creux H, Robin G, Hocké C, et al. Using serum anti-Müllerian hormone levels to predict the chance of live birth after spontaneous or assisted conception:A systematic review and meta-analysis. Hum Reprod. 2023;38:1789–806. doi: 10.1093/humrep/dead147. [DOI] [PubMed] [Google Scholar]
- 30.Brodin T, Hadziosmanovic N, Berglund L, Olovsson M, Holte J. Antimüllerian hormone levels are strongly associated with live-birth rates after assisted reproduction. J Clin Endocrinol Metab. 2013;98:1107–14. doi: 10.1210/jc.2012-3676. [DOI] [PubMed] [Google Scholar]
- 31.Wang JG, Douglas NC, Nakhuda GS, Choi JM, Park SJ, Thornton MH, et al. The association between anti-Müllerian hormone and IVF pregnancy outcomes is influenced by age. Reprod Biomed Online. 2010;21:757–61. doi: 10.1016/j.rbmo.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 32.Kaya C, Pabuccu R, Satıroglu H. Serum antimüllerian hormone concentrations on day 3 of the in vitro fertilization stimulation cycle are predictive of the fertilization, implantation, and pregnancy in polycystic ovary syndrome patients undergoing assisted reproduction. Fertil Steril. 2010;94:2202–7. doi: 10.1016/j.fertnstert.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Tal R, Tal O, Seifer BJ, Seifer DB. Antimüllerian hormone as predictor of implantation and clinical pregnancy after assisted conception:A systematic review and meta-analysis. Fertil Steril. 2015;103:119–30.e3. doi: 10.1016/j.fertnstert.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 34.La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Mullerian-inhibiting substance in women with polycystic ovary syndrome:Relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970–2. doi: 10.1016/j.fertnstert.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity:Relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–43. doi: 10.1152/ajpendo.90684.2008. [DOI] [PubMed] [Google Scholar]
- 36.Lin YH, Chiu WC, Wu CH, Tzeng CR, Hsu CS, Hsu MI. Antimüllerian hormone and polycystic ovary syndrome. Fertil Steril. 2011;96:230–5. doi: 10.1016/j.fertnstert.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Arkfeld C, Han E, Tal R, Seifer DB. AMH predicts miscarriage in non-PCOS but not in PCOS related infertility ART cycles. Reprod Biol Endocrinol. 2023;21:35. doi: 10.1186/s12958-023-01087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tal R, Seifer CM, Khanimov M, Seifer DB, Tal O. High serum antimullerian hormone levels are associated with lower live birth rates in women with polycystic ovarian syndrome undergoing assisted reproductive technology. Reprod Biol Endocrinol. 2020;18:20. doi: 10.1186/s12958-020-00581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su N, Zhan J, Xie M, Zhao Y, Huang C, Wang S, et al. High anti-Mullerian hormone level is adversely associated with cumulative live birth rates of two embryo transfers after the first initiated cycle in patients with polycystic ovary syndrome. Front Endocrinol (Lausanne) 2023;14:1123125. doi: 10.3389/fendo.2023.1123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel SS, Carr BR. Oocyte quality in adult polycystic ovary syndrome. Semin Reprod Med. 2008;26:196–203. doi: 10.1055/s-2008-1042958. [DOI] [PubMed] [Google Scholar]
- 41.Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome:Impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33. doi: 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tal R, Seifer DB, Arici A. The emerging role of angiogenic factor dysregulation in the pathogenesis of polycystic ovarian syndrome. Semin Reprod Med. 2015;33:195–207. doi: 10.1055/s-0035-1552582. [DOI] [PubMed] [Google Scholar]
- 43.Rosas C, Oróstica L, Poblete C, Carvajal R, Gabler F, Romero C, et al. Hyperandrogenism decreases GRP78 protein level and glucose uptake in human endometrial stromal cells. Reprod Sci. 2016;23:761–70. doi: 10.1177/1933719115618283. [DOI] [PubMed] [Google Scholar]
- 44.Tal R, Seifer DB, Khanimov M, Malter HE, Grazi RV, Leader B. Characterization of women with elevated antimüllerian hormone levels (AMH):Correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol. 2014;211:8.e1–8. doi: 10.1016/j.ajog.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–6. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- 46.Acharya KS, Harris BS, Weber JM, Truong T, Pieper C, Eaton JL. Impact of increasing antimüllerian hormone level on in vitro fertilization fresh transfer and live birth rate. F S Rep. 2022;3:223–30. doi: 10.1016/j.xfre.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2018;5:CD010287. doi: 10.1002/14651858.CD010287.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang R, Li W, Bordewijk EM, Legro RS, Zhang H, Wu X, et al. First-line ovulation induction for polycystic ovary syndrome:An individual participant data meta-analysis. Hum Reprod Update. 2019;25:717–32. doi: 10.1093/humupd/dmz029. [DOI] [PubMed] [Google Scholar]
- 49.Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J Clin Endocrinol Metab. 2002;87:569–74. doi: 10.1210/jcem.87.2.8261. [DOI] [PubMed] [Google Scholar]
- 50.Xi W, Yang Y, Mao H, Zhao X, Liu M, Fu S. Circulating anti-mullerian hormone as predictor of ovarian response to clomiphene citrate in women with polycystic ovary syndrome. J Ovarian Res. 2016;9:3. doi: 10.1186/s13048-016-0214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahran A, Abdelmeged A, El-Adawy AR, Eissa MK, Shaw RW, Amer SA. The predictive value of circulating anti-Müllerian hormone in women with polycystic ovarian syndrome receiving clomiphene citrate:A prospective observational study. J Clin Endocrinol Metab. 2013;98:4170–5. doi: 10.1210/jc.2013-2193. [DOI] [PubMed] [Google Scholar]
- 52.Hestiantoro A, Negoro YS, Afrita Y, Wiweko B, Sumapradja K, Natadisastra M. Anti-müllerian hormone as a predictor of polycystic ovary syndrome treated with clomiphene citrate. Clin Exp Reprod Med. 2016;43:207–14. doi: 10.5653/cerm.2016.43.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo Z, Chen S, Chen Z, Hu P, Hao Y, Yu Q. Predictors of response to ovulation induction using letrozole in women with polycystic ovary syndrome. BMC Endocr Disord. 2023;23:90. doi: 10.1186/s12902-023-01336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mumford SL, Legro RS, Diamond MP, Coutifaris C, Steiner AZ, Schlaff WD, et al. Baseline AMH level associated with ovulation following ovulation induction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101:3288–96. doi: 10.1210/jc.2016-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossman MP, Nakajima ST, Fallat ME, Siow Y. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89:1364–70. doi: 10.1016/j.fertnstert.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 56.Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, Brincat M, et al. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril. 2011;96:1246–51.e1. doi: 10.1016/j.fertnstert.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Chang HM, Klausen C, Leung PC. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril. 2013;100:585–92.e1. doi: 10.1016/j.fertnstert.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 58.Vagios S, Sacha CR, Hammer KC, Dimitriadis I, James KE, Bormann CL, et al. Response to ovulation induction treatments in women with polycystic ovary syndrome as a function of serum anti-Müllerian hormone levels. J Assist Reprod Genet. 2021;38:1827–33. doi: 10.1007/s10815-021-02217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amer SA, Mahran A, Abdelmaged A, El-Adawy AR, Eissa MK, Shaw RW. The influence of circulating anti-Müllerian hormone on ovarian responsiveness to ovulation induction with gonadotrophins in women with polycystic ovarian syndrome:A pilot study. Reprod Biol Endocrinol. 2013;11:115. doi: 10.1186/1477-7827-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guzman L, Ortega-Hrepich C, Polyzos NP, Anckaert E, Verheyen G, Coucke W, et al. Aprediction model to select PCOS patients suitable for IVM treatment based on anti-Mullerian hormone and antral follicle count. Hum Reprod. 2013;28:1261–6. doi: 10.1093/humrep/det034. [DOI] [PubMed] [Google Scholar]
- 61.Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro:I. The activation of ovarian eggs. J Exp Med. 1935;62:665–75. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilchrist RB, Smitz J. Oocyte in vitro maturation:Physiological basis and application to clinical practice. Fertil Steril. 2023;119:524–39. doi: 10.1016/j.fertnstert.2023.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries:A comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30:88–96. doi: 10.1093/humrep/deu248. [DOI] [PubMed] [Google Scholar]
- 64.Kedem A, Yerushalmi GM, Maman E, Hemi R, Hanochi M, Hourvitz A. What is the optimal threshold of serum Anti-Müllerian Hormone (AMH) necessary for IVM treatments? J Assist Reprod Genet. 2013;30:745–51. doi: 10.1007/s10815-013-9996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seok HH, Song H, Lyu SW, Kim YS, Lee DR, Lee WS, et al. Application of serum anti-Müllerian hormone levels in selecting patients with polycystic ovary syndrome for in vitro maturation treatment. Clin Exp Reprod Med. 2016;43:126–32. doi: 10.5653/cerm.2016.43.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feldman RA, O'Neill K, Butts SF, Dokras A. Antimüllerian hormone levels and cardiometabolic risk in young women with polycystic ovary syndrome. Fertil Steril. 2017;107:276–81. doi: 10.1016/j.fertnstert.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Wiweko B, Handayani LK, Harzif AK, Pratama G, Muharam R, Hestiantoro A, et al. Correlation of anti-Müllerian hormone levels with metabolic syndrome events in polycystic ovary syndrome:A cross-sectional study. Int J Reprod Biomed. 2020;18:187–92. doi: 10.18502/ijrm.v18i3.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitra S, Saharia GK, Jena SK. Correlation between serum AMH levels and cardiometabolic indices in PCOS women. Indian J Endocrinol Metab. 2021;25:545–50. doi: 10.4103/ijem.ijem_421_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu JY, James KE, Bormann CL, Donahoe PK, Pépin D, Sabatini ME. Müllerian-inhibiting substance/anti-müllerian hormone as a predictor of preterm birth in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:4187–96. doi: 10.1210/jc.2018-01320. [DOI] [PubMed] [Google Scholar]
- 70.Hu KL, Liu FT, Xu H, Li R, Qiao J. High antimüllerian hormone levels are associated with preterm delivery in patients with polycystic ovary syndrome. Fertil Steril. 2020;113:444–52.e1. doi: 10.1016/j.fertnstert.2019.09.039. [DOI] [PubMed] [Google Scholar]
- 71.Kaing A, Jaswa EA, Diamond MP, Legro RS, Cedars MI, Huddleston HG. Highly elevated level of antimüllerian hormone associated with preterm delivery in polycystic ovary syndrome patients who underwent ovulation induction. Fertil Steril. 2021;115:438–46. doi: 10.1016/j.fertnstert.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Abdelsalam WA, Harb OA, Shazly SA. Antimullerian hormone levels and association with abortion and preterm delivery in patients with polycystic ovary syndrome who conceived with assisted reproductive techniques. J Obstet Gynaecol India. 2022;72:295–8. doi: 10.1007/s13224-021-01506-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oladipupo I, Ali T, Hein DW, Pagidas K, Bohler H, Doll MA, et al. Association between cigarette smoking and ovarian reserve among women seeking fertility care. PLoS One. 2022;17:e0278998. doi: 10.1371/journal.pone.0278998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tzeng CR, Huang Z, Asada Y, Zhang C, Ho MT, Li RH, et al. Factors affecting the distribution of serum anti-müllerian hormone levels among infertile Asian women:A multi-nation, multi-centre, and multi-ethnicity prospective cohort study. Hum Reprod. 2023;38:1368–78. doi: 10.1093/humrep/dead081. [DOI] [PubMed] [Google Scholar]
- 75.Tremellen K, Zander-Fox D. Serum anti-Mullerian hormone assessment of ovarian reserve and polycystic ovary syndrome status over the reproductive lifespan. Aust N Z J Obstet Gynaecol. 2015;55:384–9. doi: 10.1111/ajo.12366. [DOI] [PubMed] [Google Scholar]
- 76.Guan Y, Kong P, Xiao Z, Zhang J, He J, Geng W, et al. Independent variables for determining the cumulative live birth rates of aged patients with polycystic ovary syndrome or tubal factor infertility:A retrospective cohort study. Front Endocrinol (Lausanne) 2021;12:728051. doi: 10.3389/fendo.2021.728051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalra SK, Ratcliffe SJ, Dokras A. Is the fertile window extended in women with polycystic ovary syndrome?Utilizing the society for assisted reproductive technology registry to assess the impact of reproductive aging on live-birth rate. Fertil Steril. 2013;100:208–13. doi: 10.1016/j.fertnstert.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 78.Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89:505–22. doi: 10.1016/j.fertnstert.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 79.Amer SA, Shamy TT, James C, Yosef AH, Mohamed AA. The impact of laparoscopic ovarian drilling on AMH and ovarian reserve:A meta-analysis. Reproduction. 2017;154:R13–21. doi: 10.1530/REP-17-0063. [DOI] [PubMed] [Google Scholar]
- 80.Bordewijk EM, Ng KY, Rakic L, Mol BW, Brown J, Crawford TJ, et al. Laparoscopic ovarian drilling for ovulation induction in women with anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;2:CD001122. doi: 10.1002/14651858.CD001122.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kataoka J, Larsson I, Lindgren E, Kindstrand LO, Schmidt J, Stener-Victorin E. Circulating anti-müllerian hormone in a cohort-study of women with severe obesity with and without polycystic ovary syndrome and the effect of a one-year weight loss intervention. Reprod Biol Endocrinol. 2022;20:153. doi: 10.1186/s12958-022-01022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vosnakis C, Georgopoulos NA, Armeni AK, Papadakis E, Roupas ND, Katsikis I, et al. Sibutramine administration decreases serum anti-Müllerian hormone (AMH) levels in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2012;163:185–9. doi: 10.1016/j.ejogrb.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Vosnakis C, Georgopoulos NA, Rousso D, Mavromatidis G, Katsikis I, Roupas ND, et al. Diet, physical exercise and Orlistat administration increase serum anti-Müllerian hormone (AMH) levels in women with polycystic ovary syndrome (PCOS) Gynecol Endocrinol. 2013;29:242–5. doi: 10.3109/09513590.2012.736557. [DOI] [PubMed] [Google Scholar]
- 84.Buyukkaba M, Turgut S, Ilhan MM, Ekinci I, Yaylım İ, Zeybek SU, et al. Anti-mullerian hormone levels increase after bariatric surgery in obese female patients with and without polycystic ovary syndrome. Horm Metab Res. 2022;54:194–8. doi: 10.1055/a-1756-4798. [DOI] [PubMed] [Google Scholar]
- 85.Yin WW, Huang CC, Chen YR, Yu DQ, Jin M, Feng C. The effect of medication on serum anti-müllerian hormone (AMH) levels in women of reproductive age:A meta-analysis. BMC Endocr Disord. 2022;22:158. doi: 10.1186/s12902-022-01065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shah A, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Gnatuk CL, et al. Effects of oral contraception and lifestyle modification on incretins and TGF-ßsuperfamily hormones in PCOS. J Clin Endocrinol Metab. 2021;106:108–19. doi: 10.1210/clinem/dgaa682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong HY, Li HW, Lam KS, Tam S, Shek CC, Lee CY, et al. Independent association of serum Vitamin D with anti-Mullerian hormone levels in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2018;89:634–41. doi: 10.1111/cen.13816. [DOI] [PubMed] [Google Scholar]
- 88.Cappy H, Giacobini P, Pigny P, Bruyneel A, Leroy-Billiard M, Dewailly D, et al. Low Vitamin D3 and high anti-Müllerian hormone serum levels in the polycystic ovary syndrome (PCOS):Is there a link?Ann Endocrinol (Paris) 2016;77:593–9. doi: 10.1016/j.ando.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Moridi I, Chen A, Tal O, Tal R. The association between Vitamin D and anti-müllerian hormone:A systematic review and meta-analysis. Nutrients. 2020;12:1567. doi: 10.3390/nu12061567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jirge PR, Chougule SM, Keni A, Kumar S, Modi D. Latent genital tuberculosis adversely affects the ovarian reserve in infertile women. Hum Reprod. 2018;33:1262–9. doi: 10.1093/humrep/dey117. [DOI] [PubMed] [Google Scholar]
- 91.Richa S, Anjali K, Sonal J, Akrati J. Analysis of the effect of female genital tuberculosis on ovarian reserve parameters. J Hum Reprod Sci. 2023;16:125–31. doi: 10.4103/jhrs.jhrs_36_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah D, Patil M. Infertility Management in Lean Versus Obese PCOS. In: Genazzani AR, Ibáñez L, Milewicz A, Shah D, editors. Impact of Polycystic Ovary, Metabolic Syndrome and Obesity on Women Health. Volume 8:Frontiers in Gynecological Endocrinology. Cham: Springer International Publishing; 2021. [[Last accessed on 2023 Sep 08]]. pp. 105–27. (ISGE Series) Available from: https://doi.org/10.1007/978-3-030-63650-0_9 . [Google Scholar]
- 93.Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. The measurement of anti-Müllerian hormone:A critical appraisal. J Clin Endocrinol Metab. 2014;99:723–32. doi: 10.1210/jc.2013-3476. [DOI] [PubMed] [Google Scholar]
- 94.Li HW, Robertson DM, Burns C, Ledger WL. Challenges in measuring AMH in the clinical setting. Front Endocrinol (Lausanne) 2021;12:691432. doi: 10.3389/fendo.2021.691432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li HW, Wong BP, Ip WK, Yeung WS, Ho PC, Ng EH. Comparative evaluation of three new commercial immunoassays for anti-Müllerian hormone measurement. Hum Reprod. 2016;31:2796–802. doi: 10.1093/humrep/dew248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data used.


