Abstract
Inflammatory stimuli have been shown to impact brain regions involved in threat detection and emotional processing including amygdala and ventromedial prefrontal cortex (vmPFC), and to increase anxiety. Biomarkers of endogenous inflammation, including inflammatory cytokines and C-reactive protein (CRP), are reliably elevated in a subset of patients with depression and anxiety-related disorders such as post-traumatic stress disorder (PTSD), and have been associated with high anxiety in population studies. We previously reported that plasma CRP and cytokines in patients with depression were negatively correlated with resting-state functional connectivity (FC) between right amygdala and vmPFC, as assessed using both ROI to voxel-wise and targeted FC approaches, in association with symptoms of anxiety, particularly in patients with comorbid anxiety disorders or PTSD. To determine whether relationships between inflammation, right amygdala-vmPFC FC, and anxiety are reproducible across patient samples and research settings, we employed an a priori, hypothesis-driven approach to examine relationships between inflammation, targeted right amygdala-vmPFC FC and anxiety in a cohort of African American (AA) women (n=54) recruited from an inner-city hospital population reliably found to have higher levels of inflammation (median CRP ~4 mg/L) as well as symptoms of anxiety, depression and PTSD. Higher concentrations of plasma CRP were associated with lower right amygdala-vmPFC FC (r=−0.32, p=0.017), and this relationship remained significant when controlling for age, body mass index and number of lifetime trauma events experienced, as well as severity of PTSD and depression symptoms (all p<0.05). This amygdala-vmPFC FC was similarly associated with a composite score of three inflammatory cytokines in a subset of women where plasma was available for analysis (n=33, r=−0.33, p=0.058; adjusted r=−0.43, p=0.026 when controlling for covariates including PTSD and depression symptom severity). Lower right amygdala-vmPFC FC was in turn associated with higher levels of anxiety reported to be generally experienced on the State-Trait Anxiety Inventory, trait component (adjusted r=−0.32, p=0.039 when controlling for covariates). Exploratory analyses also revealed a negative correlation between severity of childhood maltreatment and right amygdala-vmPFC FC (r=−0.32, p=0.018) that was independent of CRP and its association with FC, as well as an association between low amygdala-vmPFC FC and severity of PTSD symptoms, specifically the re-experiencing/intrusive symptom subscale (adjusted r=−0.32, p=0.028 when controlling for covariates). While CRP was not linearly associated with either anxiety or PTSD symptoms, CRP concentrations were higher in women reporting clinically significant anxiety or PTSD symptom severity when these symptoms were considered together (both p<0.05), but with no interaction. These results support our primary hypothesis that higher inflammation was associated with lower amygdala-vmPFC FC, a relationship that was detected using a hypothesis-driven, targeted approach. Findings also support that this phenotype of high CRP and low vmPFC FC was observed in association with anxiety in primary analyses, as well as symptoms of PTSD in exploratory analyses, in a cohort recruited from an inner-city population of AA women enriched for high inflammation, history of trauma exposure, and symptom severity. Larger, longitudinal samples are required to fully tease apart causal relationships between inflammatory biomarkers, FC and PTSD-related symptoms in future studies.
Keywords: Inflammation, trauma, amygdala, functional connectivity, fMRI, C-reactive protein, anxiety
INTRODUCTION
Administration of innate immune stimuli to laboratory subjects and the associated release of inflammatory cytokines has been shown to reliably impact the brain to induce depressive and anxiety behaviors (Dantzer et al., 2008; Raison and Miller, 2013, 2017). While these potentially adaptive behaviors are thought to promote conservation of energy and resources while increasing vigilance and threat sensitivity during recovery from infectious or sterile inflammatory challenge (Dantzer et al., 2008; Raison and Miller, 2013, 2017), chronically elevated levels of inflammation can lead to clinically significant depressive and anxiety symptoms (Felger and Lotrich, 2013) and are reliably observed in subsets of patients with depression and anxiety-related disorders including post-traumatic stress disorder (PTSD) (Michopoulos et al., 2017; Bekhbat et al., 2022). Findings from numerous laboratories across multiple neuroimaging modalities indicate that exogenous administration of inflammatory stimuli reliably affects not only basal ganglia and cortical reward and motor circuits, but also brain regions involved in fear, anxiety, and emotional processing such as the amygdala (Felger, 2018; Harrison, 2019). As a key neural region involved in these processes, as well as in circuits that are disrupted in depression, anxiety disorders, and PTSD (Shin et al., 2006; Tang et al., 2013), the effects of inflammation on the amygdala and its relevant circuity may be involved in and account for mounting evidence of associations between inflammation and transdiagnostic symptoms like anxiety in psychiatric patients and population studies (Liukkonen et al., 2011; Hou et al., 2017; Milaneschi et al., 2021; van Eeden et al., 2021; Kennedy and Niedzwiedz, 2022).
Regarding the impact of inflammatory stimuli on amygdala reactivity and circuitry, low-dose endotoxin administration to healthy subjects, which potently induces interleukin (IL)-6 and tumor necrosis factor (TNF) release as well as depressive and anxiety symptoms (Eisenberger et al., 2010; Lasselin et al., 2016), increased amygdala activity in response to both socially threatening images and negative social feedback (Inagaki et al., 2012; Muscatell et al., 2016). Typhoid vaccination, which also induces IL-6 release and depressive-like behaviors, increased activation of the amygdala during presentation of congruent and incongruent stimuli (Harrison et al., 2009b). In terms of stress sensitivity and neural pathways involved in the inflammatory response to stress, heightened neural activity in the amygdala in response to a psychosocial laboratory stressor was associated with greater stress-induced increases in IL-6 (Muscatell et al., 2015). Thus, greater amygdala sensitivity to threat may lead to higher inflammatory cytokine production, which may in turn affect amygdala activity to create a feed-forward effect of inflammation on neural circuitry relevant to symptoms of depression and anxiety. Finally, evidence of decreased functional connectivity (FC), both at rest and during emotional face processing, involving amygdala and other inflammation-sensitive brain regions including the subgenual anterior cingulate region of the ventromedial prefrontal cortex (vmPFC) (Harrison et al., 2009a; Labrenz et al., 2016) has been observed after typhoid or endotoxin challenge.
Recent studies in patients or healthy individuals have reported similar relationships between higher levels of endogenous inflammation and either enhanced amygdala neural activation or reduced FC with amygdala as that observed following inflammatory challenge (Swartz et al., 2017a; Swartz et al., 2017b; Mehta et al., 2018; Nusslock et al., 2019; Leschak et al., 2020; Miller et al., 2021; Swartz et al., 2021; Gong et al., 2022). For example, using a whole-brain, voxel-wise approach, we found that higher plasma concentrations of the inflammatory marker C-reactive protein (CRP) and cytokines like IL-6 were associated with lower right amygdala-vmPFC FC in medically-stable and medication-free patients with depression (Mehta et al., 2018). While no significant relationships were observed for FC between left amygdala and any brain region, right amygdala-vmPFC FC Z-scores, as identified either by whole-brain analysis or using a targeted right amygdala ROI to a priori defined vmPFC cluster, were in turn associated with symptoms of anxiety, particularly in depressed patients with comorbid anxiety-related disorders including PTSD (Mehta et al., 2018). Low FC in similar amygdala-vmPFC circuitry characterizes individuals with high anxiety (Kim et al., 2011), and has been observed in patients with mood and anxiety-related disorders like depression and PTSD (Stevens et al., 2013; Tang et al., 2013). Indeed, both low amygdala-vmPFC FC and increased neural sensitivity to fearful faces in patients with PTSD in association with symptoms of hyperarousal compared to trauma-exposed controls was found only in the right amygdala (Stevens et al., 2013). Similarly, while inflammatory stimuli have been shown to affect neural sensitivity in both left and right amygdala, decreased FC was only found between right amygdala and vmPFC after typhoid vaccine (Harrison et al., 2009a). A recent study in patients with bipolar depression also reported decreased FC between the right medial amygdala and bilateral medial PFC in association with higher TNF-alpha (Gong et al., 2022). Moreover, cause-and-effect relationships between inflammation and neural activation of amygdala have been highlighted using two clinically-relevant models of cytokine-induced depressive symptoms with interferon-alpha that specifically increased right amygdala reactivity to emotional faces, and the blockade of depressive symptoms with the TNF-antagonist infliximab in inflammatory illness that preferentially decreased right amygdala reactivity (Davies et al., 2021).
As subpopulations of psychiatric or medically ill patients with high inflammation may represent unique groups that could benefit from pharmacological or behavioral interventions targeting inflammation or its effects on the brain, it is important to establish reliable brain biomarkers of inflammation and their relationship with behaviors (Miller et al., 2017). While inflammation has been consistently associated with high neural activation of the amygdala to threat as well as low amygdala-vmPFC FC during rest and in tasks, resting-state fMRI may serve as a reproducible method to detect brain signatures of inflammation with potentially less influence from experimental conditions across labs or learning effects across time for use in translational studies or trials. In this regard, we have recently reported that higher levels of CRP and inflammatory cytokines were associated with lower FC in reward circuitry using a targeted ventral striatum seed to vmPFC ROI approach in association with symptoms of anhedonia in both patients with depression and African American (AA) women recruited from an inner-city hospital (Felger et al., 2016; Mehta et al., 2020). Cohorts recruited from the patients utilizing this hospital are reliably enriched for both high inflammation and symptoms of depression, anxiety, and PTSD due to a myriad of socioeconomic, genetic, lifestyle and environmental risk factors including high rates of exposure to lifetime traumas and childhood maltreatment (Gillespie et al., 2009; Michopoulos et al., 2015a; Michopoulos et al., 2015b), thus providing a unique opportunity to examine whether relationships between inflammation and amygdala-vmPFC FC, and FC and anxiety, are reproducible across patient samples and experimental settings.
Accordingly, we applied our targeted, hypothesis-driven approach (Mehta et al., 2018) to examine relationships between inflammation (CRP and inflammatory cytokines) and amygdala-vmPFC FC in this cohort of AA women in relation to symptoms of anxiety in primary analyses. Primary analyses also focused on right amygdala-vmPFC FC because 1) only right amygdala-vmPFC FC correlated with inflammation and anxiety in our prior work, 2) only right amygdala-vmPFC FC during threat appraisal was reduced after typhoid vaccination or negatively correlated with PTSD symptoms in similar cohorts of women, and 3) neural activation of right amygdala was found to be differentially altered by clinically-relevant interventions that both induce or block the impact of cytokines on the brain, as well as additional support from other studies discussed above (Harrison et al., 2009a; Stevens et al., 2013; Mehta et al., 2018; Davies et al., 2021; Gong et al., 2022). Findings for left amygdala-vmPFC FC was reported in exploratory analyses. Our prior work in depression found that relationships between inflammation and amygdala-vmPFC FC, and FC and symptoms of anxiety, depended on whether patients had comorbid diagnoses including PTSD, and both childhood maltreatment and clinically significant PTSD symptom severity interacted with inflammation to contribute to low FC in reward circuitry in this same cohort of women (Mehta et al., 2018; Mehta et al., 2020). Thus, relationships between inflammation, FC, childhood maltreatment and symptoms of PTSD were also considered in exploratory analyses. The two primary, independent hypotheses were that inflammation would be negatively associated with amygdala-vmPFC FC, and that low FC would in turn be related to higher symptoms of anxiety.
METHODS
Fifty-four AA women (21–65 years) were recruited from the general medical clinics of Grady Memorial Hospital, a publicly funded hospital that serves economically disadvantaged individuals in Atlanta, Georgia through the Grady Trauma Project (GTP) at Emory. High rates of trauma exposure as well as posttraumatic, anxiety and depressive symptoms have been observed in this population (Binder et al., 2008), which is >85% AA. Only subjects self-reporting AA race/ethnicity were studied to enhance homogeneity. Only women were studied because they represent >80% of the Grady patient population, show higher rates of PTSD, anxiety and depression, and to limit variability in circuits involving sexually dimorphic brain regions (Kessler, 2003; Stevens and Hamann, 2012; Stevens et al., 2014). Furthermore, women have been found to be more sensitive to the effects of inflammation on the brain and behavior (Derry et al., 2015). Bipolar disorder, schizophrenia or any other psychotic disorder were exclusionary as determined by Structured Clinical Interview for DSM-IV (SCID-IV) or Mini-International Neuropsychiatric Interview (MINI) administered by trained clinical staff under the supervision of licensed clinicians. Participants were also excluded for neurological disorders, autoimmune disorders, diagnosis of HIV or chronic hepatitis, or treatment with either inflammatory, anticonvulsant or psychotropic medication. Although no restrictions were placed on body mass index (BMI), women were studied if they endorsed being comfortable in the scanner. Pregnancy or use of illegal drug (cocaine, marijuana, opiates, amphetamines, methamphetamines) were exclusionary as per urine tests conducted 24 hours prior to the MRI scan. Additionally, participants were excluded for alcohol abuse within the past year, as assessed by the Alcohol Use Disorders Identification Test and the Drug Abuse Screening Test, and for metal clips or implants or any other contraindication to MRI. Participants with current or history of PTSD or depression were not excluded, and symptom severity was assessed at the time of MRI scans by validated self-report measures (see below and Supplement for details). It should be noted that a lower rate of women with clinically significant depressive symptoms was observed in this study (6 out of 54 women at the time of MRI scan) compared to other samples from GTP (i.e., 37%) (Gillespie et al., 2009), which may be due to the additional exclusion criteria employed in this study (i.e., psychotic disorders, medical comorbidities, substance abuse and potential BMI restrictions due to limitations of scanner size). After screening through GTP, participants were enrolled into studies involving genetic biomarkers, fMRI scans and/or blood collection for plasma isolation. All testing took place at Grady Memorial Hospital and the Emory Center for Systems Imaging. Procedures were approved a priori by the Institutional Review Board of Emory University. All participants provided written informed consent and received monetary compensation for their time.
Inflammatory markers:
Collection.
Blood was collected in the morning after participants had at least 30 minutes of rest. Blood was drawn an average of 57 days (standard deviation [SD]=137 days) prior to scans. Plasma was isolated from whole blood collected into chilled EDTA tubes that were immediately centrifuged at 1000g for 15 minutes at 4°C and stored at −80°C until batched analysis.
Analysis.
The immunoturbidometric method was used to measure high sensitivity CRP with a Beckman AU480 chemistry analyzer and Ultra WR CRP kit (Sekisui Diagnostics) (all patients). Two participants with CRP concentrations >20 mg/L, which were significant outliers by Grubb’s test and >2 standard deviations from the mean (6.0 ± 6.3 mg/L), were excluded from the current analysis to limit the potential influence of extreme values. In participants where additional plasma was available (n=33), high sensitivity inflammatory cytokines interleukin (IL)-6, IL-1beta, and tumor necrosis factor (TNF) were assessed in duplicate using multiplex bead-based assays (R& D Systems) as described (Felger et al., 2020). No inflammatory markers were below the limits of assay detection (see Table S1 for individual assay detection limits), and coefficients of variation (CVs) were reliably <10%. A composite score of inflammation was calculated as the sum of Z scores for concentrations of the inflammatory cytokines IL-6, TNF, IL-1beta because the Z score centers the mean of each marker at 0 ± 1 (mean, SD; Figure S1) (Felger et al., 2020; Mehta et al., 2020). To assess the cytokine effect specifically, as cytokines themselves are thought to affect the brain, CRP was not included in the inflammatory composite score. CRP served as the primary inflammatory marker due to stability, clinical relevance, and previous association with other inflammatory markers in blood and CSF (Felger et al., 2020).
Assessments:
Subjects completed self-report clinical assessments prior to MRI scans. Trauma Exposure. The Traumatic Events Inventory (TEI), a 14-item screen for lifetime history of traumatic events (Schwartz et al., 2005), was used to assesses the total number of traumatic events experienced or witnessed (e.g., witness of murder of friend or family member) across the different types of trauma. As two TEI questions assess physical abuse and sexual abuse occurring before age 14, the TEI score was also reported after removing the contribution of abuse during childhood. In prior GTP studies, childhood abuse and other lifetime traumas reported by TEI, either separately or in combination, predicted adult PTSD and depressive symptomatology, and lifetime traumas most frequently reported by women included serious accident or injury, followed by interpersonal violence and sexual assault (Gillespie et al., 2009; Powers et al., 2016; Gluck et al., 2021). Childhood Maltreatment. The Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 1994) was used to assess the childhood maltreatment as both a continuous measure of severity and to classify the presence or absence of at least one moderate to severe form of childhood abuse or neglect (see Supplement for details). Symptom Severity. Severity of anxiety generally experienced was assessed by the State-Trait Anxiety Inventory (STAI) Trait component (Spielberger et al., 1979), and depressive symptoms severity was assessed by Beck Depression Inventory (BDI) (Beck et al., 1988). The 17 item modified PTSD symptom scale (PSS) was used to define overall PTSD symptom severity and severity of three symptom subscales: re-experiencing/intrusive (i.e., recurrent and intrusive distressing recollections of the event, items 1–5), avoidance/numbness (i.e., efforts to avoid thoughts, feelings, or conversations associated with the event, items 6–12), and hyperarousal (i.e., difficulty falling or staying asleep, items 13–17) (Foa and Tolin, 2000). No scores for PSS or BDI were exclusionary. Symptom severity for each scale was used both as a continuous variable in linear regression models and to classify whether or not participants reported clinically significant symptoms, as described in detail in the Supplement.
fMRI FC:
Wakeful resting-state fMRI images were acquired on a 3T Magnetom Trio scanner (Siemens, USA) with a 12-channel head coil using a Z-saga pulse sequence (Heberlein and Hu, 2004) to minimize signal loss due to inhomogeneous susceptibility (Felger et al., 2016). Each scan volume contained 30 axially acquired 4mm thick images with an in-plane resolution of 3.44 × 3.44 mm2 utilizing the parameters: pulse repetition time 3000ms, volumes=150, matrix=64 × 64, echo time 1=30ms, echo time 2=67ms, at a flip angle of 90 deg. Structural images were acquired using a gradient-echo, T1-weighted pulse sequence (TR=2600ms, TE=3.02ms; 1mm×1mm×1mm voxel size). Analysis of functional connectivity was conducted with AFNI (http://afni.nimh.nih.gov/). Preprocessing steps included outlier detection (~5.5 times median absolute deviation), despiking, slice timing correction, motion correction, anatomy-to-functional image co-registration, nuisance signal (head motion parameters and derivatives, cerebral spinal fluid, and white matter) regression, band pass filtering (0.009<f<0.08 Hz) and 5mm full-width half-maximum spatial smoothing. Time points with more than 10% of voxels as temporal outliers as well as with excessive head motion (framewise displacement >0.3 mm) were excluded (or in other words, censored/scrubbed) from the subsequent FC analysis (Power et al., 2014). Excluded time points were less than 23 (15%) in all the participants analyzed. Preprocessed 4D data were transformed into the standard Montreal Neurological Institute (MNI) space with an isotropic resolution of 2mm3. The right amygdala (Cullen et al., 2014; Mehta et al., 2018) was individually derived from FreeSurfer (https://surfer.nmr.mgh.harvard.edu/) and then registered to preprocessed fMRI data for primary analyses. Left amygdala was also derived for exploratory analyses (see Introduction for details). A spherical vmPFC ROI identified by meta-analysis (Diekhof et al., 2012) and used in our previous work (Felger et al., 2016; Yin et al., 2019; Goldsmith et al., 2020; Mehta et al., 2020), including right amygdala-vmPFC FC (Mehta et al., 2018), was defined as MNI x=0, y=44, z=−8, volume=1408mm3. Mean time series of voxels in these ROIs were extracted for cross-correlation, and subject-level FC correlations were Fisher’s Z transformed for use in subsequent linear regressions at group level. Exploratory right amygdala ROI to voxel-wise whole brain analyses as a function of CRP were used to identify other brain regions whereby resting-state FC was associated with inflammation (see Supplement).
Statistics:
Patient characteristics were summarized using mean and standard deviation for continuous variables and percent for categorical variables. Primary analyses tested two independent hypotheses: 1) that inflammatory markers (with CRP as the primary inflammatory measure) would be negatively correlated with right amygdala-vmPFC FC, and 2) that right amygdala-vmPFC FC would in turn be negatively associated with symptoms of anxiety (see Introduction above). Subject-level FC Z-scores (as dependent variable) were entered into linear regression models to assess relationships with inflammatory markers (independent variables) with and without covariates that may influence or confound relationships between inflammation and neural circuitry including age, body mass index (BMI) (Felger et al., 2016; Yin et al., 2019) as well as lifetime trauma exposure (TEI scores), an exposure variable frequently controlled for in GTP studies to assess whether trauma, or other unmeasured factors associated with trauma, confound or influence relationships between physiologic or neuroimaging biomarkers and symptoms, e.g., (Stevens et al., 2017; Lin et al., 2018). To examine whether relationships between FC and inflammatory markers were independent of PTSD and depression symptom severity, and consistent with our prior work in major depression or GTP studies (Spann et al., 2014; Felger et al., 2016; Stevens et al., 2017; Mehta et al., 2020), PSS and/or BDI scores were also controlled. To determine which cytokine from the composite score was most significantly associated with FC, linear regression models were conducted with backward and forward selection including covariates. To determine whether relationships between anxiety symptoms (dependent variable) and FC (independent variable) were independent of or confounded by inflammation, linear regression models were conducted with and without covariates including CRP (Felger et al., 2016; Yin et al., 2019; Fani et al., 2020). All results were reported with and without adjustment with covariates. Exploratory analyses assessed 1) the potential influence of childhood maltreatment on relationships between inflammation and FC using multiple linear regression including interaction terms for CTQ and CRP, 2) potential associations between CRP and left amygdala-vmPFC FC, and 3) unadjusted Pearson’s correlations between CRP, right amygdala-vmPFC FC, and symptoms of PTSD (along with other study variables) followed by multiple linear regression models as described above for findings of interest. Significance was two-tailed, α<0.05, with statistics conducted in IBM SPSS 25.0.
RESULTS
See Table 1 for sample characteristics. Consistent with previous GTP studies, subjects reported experiencing on average approximately five lifetime traumatic events on the TEI, and about 4 when removing the contribution of childhood abuse (TEI no child). Only 3 women did not report experiencing any childhood or lifetime trauma on TEI. The mean age of the women was ~40 years. On average, their CRP was >5 (median ~4) mg/L and BMI was >30. On the scan day, 19 participants were considered to have clinically significant PTSD symptoms as assessed by PSS, and 6 of these 19 participants also exhibited symptom severity consistent with moderate to severe depression as assessed by BDI (see Supplement for details).
Table 1.
Demographic, clinical and inflammatory variables in the study sample of African American women recruited from an inner-city hospital (n=54).
| Variable | Mean (SD) |
|---|---|
|
| |
| Demographic | |
| Age (years) | 39.8 (11.9) |
| BMI (kg/m2)^ | 32.2 (6.2) |
|
| |
| Clinical | |
| Number of Lifetime Traumas Experienced (TEI) | 4.7 (2.6) |
| TEI, No Child (Excluding Childhood Abuse) | 3.9 (2.2) |
| Childhood Maltreatment Severity (CTQ) | 39.2 (14.5) |
| CTQ+, ≥1 Moderate to Severe form of Abuse or Neglect (n,%) | 33 (61.1) |
| Depressive Symptom Severity (BDI) | 10.3 (8.3) |
| Significant Depressive Symptoms (n,%) | 6 (11.1) |
| PTSD Symptom Severity (PSS) | 10.7 (9.9) |
| Significant PTSD Symptoms (n,%) | 19 (35.2) |
| Anxiety Symptom Severity (STAI)‡ | 46.1 (5.4) |
|
| |
| Inflammatory | |
| CRP (mg/L) | 5.2 (4.5) |
| TNF (pg/ml)§ | 4.5 (2.3) |
| IL-6 (pg/ml)§ | 1.0 (0.7) |
| IL-lbeta (pg/ml)§ | 0.7 (0.4) |
p<0.05
p<0.01.
BMI available in n=53
STAI n=49
Inflammatory cytokines n=33. BDI - Beck Depression Inventory; BMI - body mass index; CRP - high sensitivity C-reactive protein; CTQ - Childhood Trauma Questionnaire; IL -interleukin; PSS - PTSD Symptom Scale; PTSD - post traumatic stress disorder; SD - standard deviation; TEI-Traumatic Events Inventory; SD - standard deviation; TNF - tumor necrosis factor
1. Relationships between inflammatory markers and amygdala-vmPFC FC
1.1. Primary Analyses.
1.1a. Relationships between CRP and right amygdala-vmPFC FC.
Plasma CRP concentrations showed a negative relationship with right amygdala-vmPFC FC (r=−0.32, p=0.017), which was strengthened after controlling for age, BMI, and trauma exposure (radj=−0.38, p=0.008; Figure 1) and remained significant after further adjusting for time (number of days) between the blood draw and MRI scan (radj=−0.37, p=0.012) or for PTSD and depression symptom severity (radj=−0.31, p=0.042).
Figure 1.
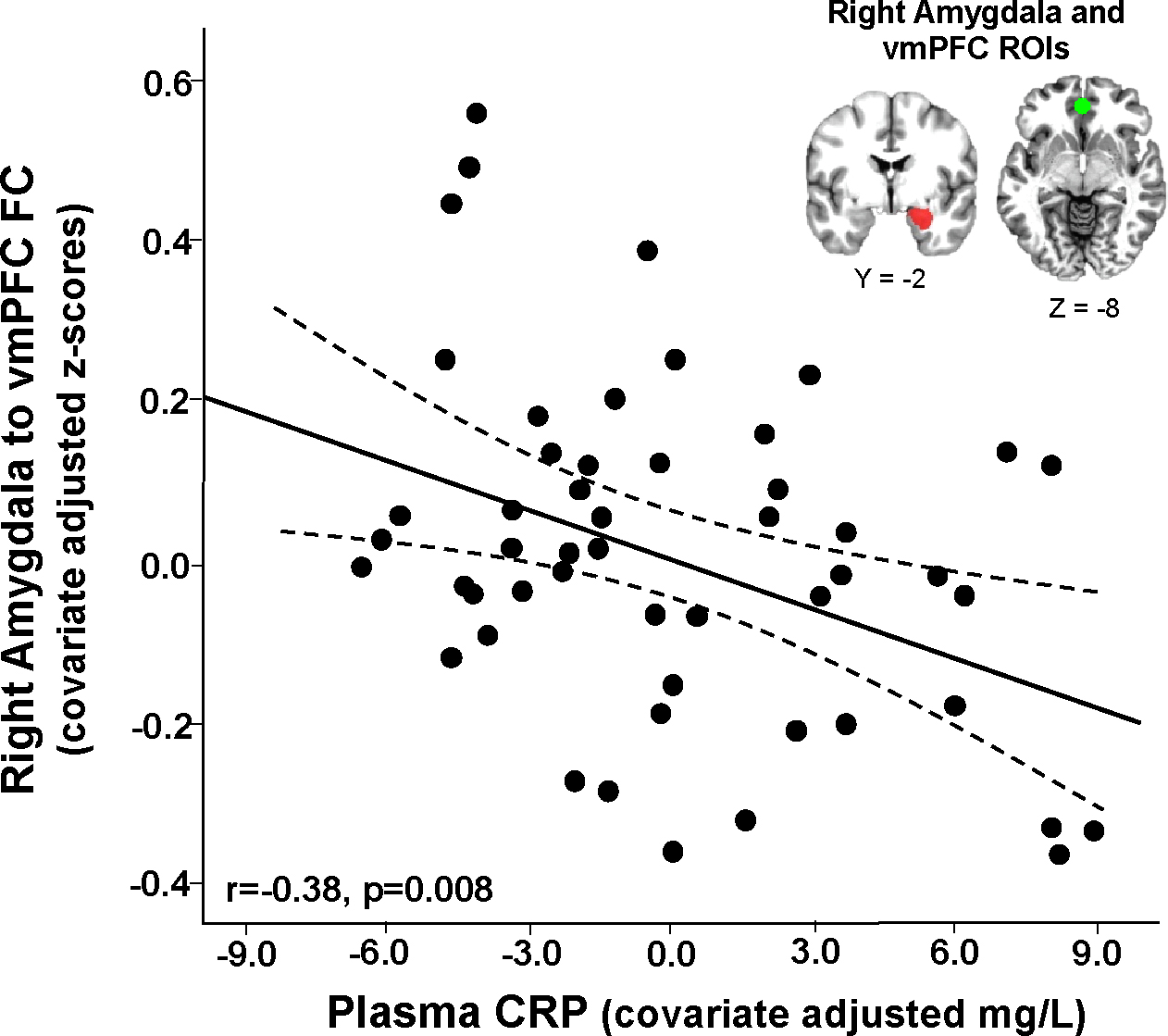
Plasma C-reactive protein (CRP) was negatively associated with right amygdala-ventromedial prefrontal cortex (vmPFC) functional connectivity (FC) in African American women recruited from an inner-city hospital (data presented as partial correlations adjusted for covariates - age, body mass index and lifetime trauma exposure).
1.1b. Relationships between inflammatory cytokines and right amygdala-vmPFC FC.
In participants where plasma was available to measure cytokines (n=33), the cytokine composite score was marginally associated with right amygdala-vmPFC FC (r=−0.33, p=0.058) and only significant when controlling for PTSD and depression symptom severity (radj=−0.40, p=0.026; p=0.044 when adjusting for time between blood draw and MRI scan). Of note, PSS and BDI scores were not significant predictors of FC in models including the cytokine composite score (both p>0.15), and while there was no interaction between the composite score and PSS on the relationship with right amygdala-vmPFC FC (p=0.350), there was a significant interaction for BDI (p=0.046). While only 4 of the women with samples available for cytokine analysis had clinically significant depressive symptoms, there was a much stronger effect size (n=4, r=−0.73, p=0.266) for the relationship between cytokines and FC in these women than in those without depressive significant symptoms (n=29, r=−0.30, p=0.114). Although no individual cytokine significantly correlated with FC, IL-6 was the final predictor in backward and forward linear regression models including covariates and using the same selection criteria (r=−0.336, p=0.06).
1.2. Exploratory Analyses.
1.2a. Relationships between childhood maltreatment, CRP and right amygdala-vmPFC FC.
Total CTQ score was negatively associated with right amygdala-vmPFC FC (r=−0.32, p=0.018), but did not correlate with CRP (see Table S2). Multiple linear regression was used to determine whether CRP influenced the relationship between childhood maltreatment and right amygdala-vmPFC FC, or vice versa. While CRP and CTQ scores continued to predict FC when in a model together with and without covariates (both p<0.05), there was no CRP by CTQ interaction (radj=0.12, p=0.416), suggesting that CRP and CTQ exhibited independent relationships with right amygdala-vmPFC FC. For example, similar negative associations between CRP and right amygdala-vmPFC FC were observed in both women who did (CTQ+, (n=33, radj=−0.39, p=0.031) and did not (CTQ-, n=21, radj=−0.32, p=0.173) report experiencing at least one moderate to severe form of childhood maltreatment (Figure S2).
1.2b. Relationships between CRP and left amygdala-vmPFC FC.
Primary analyses were focused on right amygdala FC (see above). As hypothesized and consistent with our prior work (Mehta et al., 2018), exploratory analyses showed that left amygdala-vmPFC FC was not significantly associated with CRP (r=−0.14, p=0.325) or the cytokine composite score (n=33, r=−0.37, p=0.071) and this was not modified by inclusion of covariates (radj=−0.18, p=0.226 for CRP; radj=−0.31, p=0.103 for cytokine composite) or PSS and BDI scores (radj=−0.14, p=0.342 for CRP; radj=−0.37, p=0.056 for cytokine composite).
1.2c. Exploratory right amygdala to whole brain FC analysis.
Exploratory right amygdala ROI-to-voxel analysis revealed decreased FC with a cluster in vmPFC (BA11, Figure S3) as well as eight additional clusters representing five other cortical structures including the precentral gyrus (BA6, Table S2), a region whereby lower FC with right amygdala was also observed as a function of increasing plasma CRP concentrations in patients with depression in our prior work (Mehta et al., 2018).
2. Relationships between inflammatory markers, amygdala-vmPFC FC and symptoms of anxiety or PTSD
2.1. Primary Analyses.
2.1a. Relationship between right amygdala-vmPFC FC and anxiety.
Based on our previous work, we hypothesized that right amygdala-vmPFC FC would be negatively corelated with symptoms of anxiety. While right amygdala-vmPFC FC marginally correlated with STAI scores (r=−0.273, p=0.057), this relationship was significant when excluding one participant who experienced disruptions during the resting-state fMRI scan (task instructions appeared on the screen, r=−0.29, p=0.046), and in the whole group when controlling for covariates including CRP (radj=−0.32, p=0.039; Figure 2A).
Figure 2.
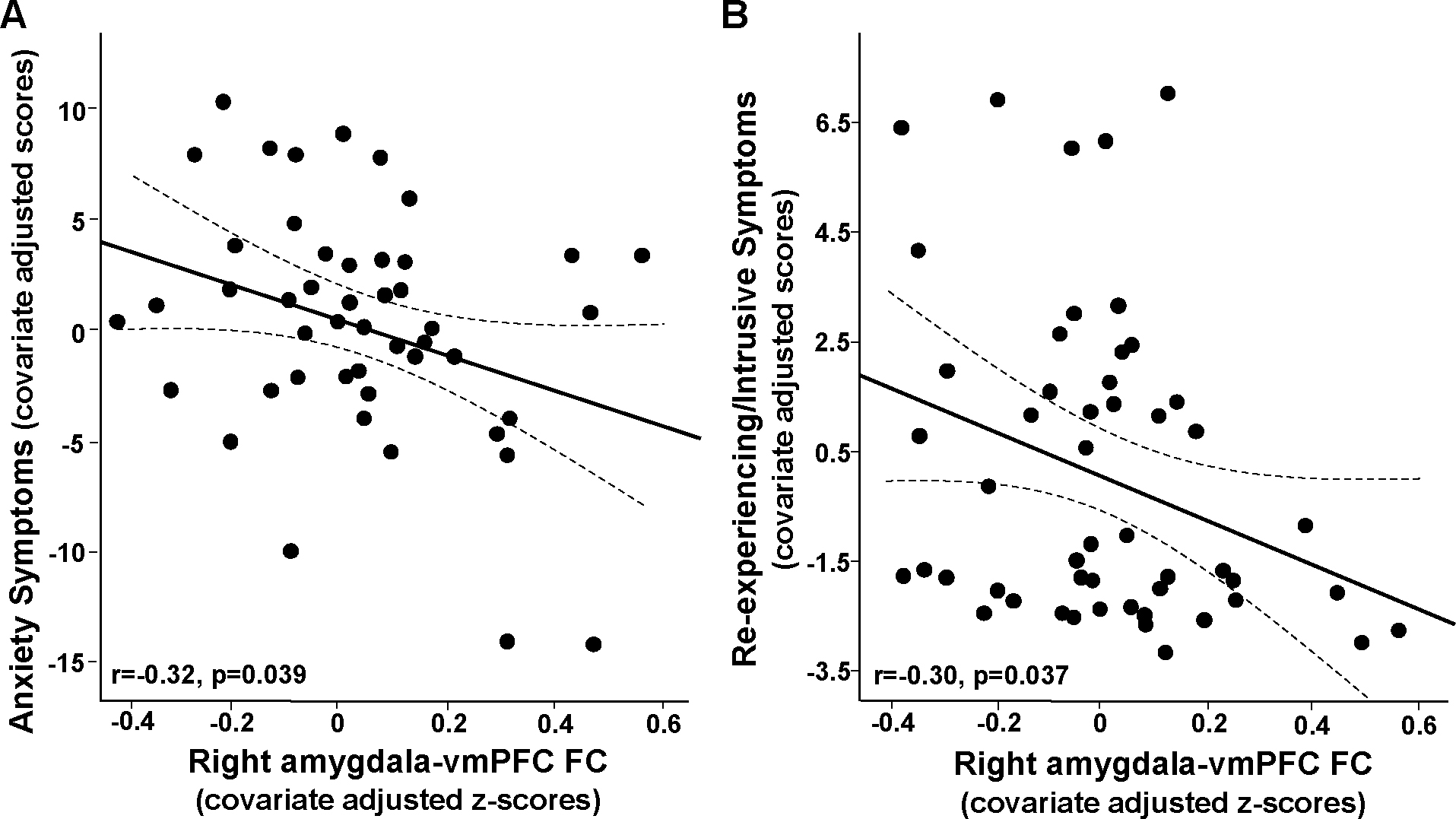
Right amygdala-ventromedial prefrontal cortex (vmPFC) functional connectivity (FC) in African American women recruited from an inner-city hospital negatively associated with symptoms of anxiety in primary analyses (anxiety generally experienced as reported by STAI trait; A), and re-experiencing/intrusive symptoms of PTSD in exploratory analyses (subscale from the PSS; B) (data presented as partial correlations adjusted for covariates - age, body mass index and lifetime trauma exposure).
2.2. Exploratory Analyses.
Relationships between CRP, right amygdala-vmPFC FC, and symptoms of PTSD.
Relationships between the primary variables of interest, CRP and amygdala-vmPFC, with numerous other study variables including symptoms of PTSD were first explored as unadjusted Pearson’s correlations (Table S3). In addition to relationships with CRP, childhood maltreatment, and anxiety as described above, right amygdala-vmPFC FC also exhibited a trend to negatively corelate with PTSD symptom severity and particularly the PSS Re-experiencing/Intrusive Symptom Subscale (r=−0.25, p=0.064). This relationship was significant when controlling for age, BMI, and trauma exposure (radj=−0.30, p=0.037; Figure 2B) as well as depressive symptom severity (radj=−0.32, p=0.027), but not CRP (radj=−0.22, p=0.141). Despite this potentially confounding effect of CRP, and significant negative correlation between CRP and this right amygdala-vmPFC FC that associated with symptoms, CRP was not significantly linearly associated with either anxiety or PTSD symptoms (Table S3). Thus, potential relationships between CRP, anxiety and PTSD symptom severity were further explored using categorical variables. As both mean and median CRP concentrations (~5 and 4 mg/L, respectively) in the women were greater than the commonly used AHA cut-point for high CRP of 3 mg/L (Ridker, 2003), established cut-points for clinically significant anxiety and PTSD symptoms (see Supplement for details) were used to examine relationships with CRP. While there were no differences between women with versus without significant anxiety or PTSD when considered alone (both p>0.43), and no significant anxiety by PTSD interaction (F[1,45]=0.0, p=0.996), CRP concentrations were increased in women with significant anxiety and in women with significant PTSD symptoms when considered in a model together (main effects of anxiety F[1,45]=201.3, p=0.045 and PTSD F[1,45]=224.1, p=0.042; see Figure S4).
Discussion
Using a hypothesis-driven targeted approach, our primary analyses found that plasma CRP and inflammatory cytokines negatively correlated with right amygdala-vmPFC resting-state FC, which in turn associated with higher levels of self-reported general anxiety symptoms. These results are most important in the context of our goal towards determining whether relationships between inflammation, amygdala-vmPFC FC and anxiety are generalizable across patient samples and research settings, and whether they can be reproducibly detected by our hypothesis-driven, targeted approach using a priori established ROIs (Mehta et al., 2018). The primary results of this work also contribute to a growing body of literature showing relationships between higher endogenous inflammation and altered activity of and/or FC between brain regions involved in threat detection and emotional processing like amygdala and vmPFC (Swartz et al., 2017a; Swartz et al., 2017b; Mehta et al., 2018; Nusslock et al., 2019; Leschak et al., 2020; Miller et al., 2021; Swartz et al., 2021; Gong et al., 2022), and which support and extend prior work showing the effects of endogenous inflammation on the brain as discussed in detail in the Introduction.
Consistent with our prior work in a primarily female, majority AA sample of medically-stable depressed patients assessing relationships between inflammation and amygdala-vmPFC FC (Mehta et al., 2018), exploratory analyses did not find any significant relationships between CRP and left amygdala FC, and only a trend for correlations with cytokines. These data suggests that a larger sample would be needed to uncover significant relationships with left amygdala, and that right amygdala-vmPFC FC, at least with our targeted methods, may serve as a more sensitive marker for capturing relationships between inflammation and resting-state FC in this circuit in future studies. Additionally, this targeted ROI approach of deriving independent FC relationships from each patient allows for hypothesis testing of associations with other variables outside of inflammatory markers, such as behaviors and other exposure variables, without risk of “double-dipping,” or violating the assumption of independence of data derived from whole-brain analyses of the same dataset. That being said, results from the integration of this targeted FC with the inflammatory markers and anxiety into exploratory analyses to consider relationships with early life adversity and/or PTSD symptom severity were not as clear as in our prior work.
Indeed, one difference between our prior work and the findings revealed by exploratory analyses herein was that relationships between inflammation and FC were not modified by the presence of either significant childhood maltreatment or PTSD symptoms. Interactions (but no independent relationships) between both CTQ scores and PTSD severity and inflammatory markers on their relationships with FC were observed in our prior investigation of reward circuitry in this GTP cohort, as well as between PTSD and amygdala-vmPFC FC on the relationship with symptoms of anxiety in our depression study (Mehta et al., 2018; Mehta et al., 2020). Conversely, in this study, amygdala-vmPFC FC exhibited a significant correlation with childhood maltreatment that was independent of and not influenced by inflammation, and vice versa (Figure S2). It should be noted, and as mentioned above in the introduction, while inflammation is known to impact brain regions like amygdala to drive behavioral symptoms, neural reactivity to stress involving amygdala and relevant circuitry is also associated with release of inflammatory cytokines (Muscatell et al., 2014) and, for example via neuroendocrine and autonomic responses, serves as one potential mechanisms of increased inflammation that has been previously associated with chronic stress and early-life adversity (Danese et al., 2008; Irwin and Cole, 2011). While neither the number of lifetime traumas nor the severity of childhood maltreatment was associated with CRP or cytokines in this cohort, higher levels of inflammation observed in these women could be due to numerous genetic, environmental, and lifestyles factors and exposures outside of or in addition to the severe traumatic events and childhood abuse/neglect that were assessed herein, such as obesity and metabolic disturbances, dietary sources and shifts in microbiota, environmental toxins, and everyday psychological and psychosocial stresses or microaggressions, to name a few (Furman et al., 2019). Thus, bidirectional relationships involving this amygdala-vmPFC circuit and inflammation, in that it can both contribute to release of inflammatory cytokines (and subsequent downstream release of CRP) and be affected by higher inflammation arising from many other sources, may set the stage for complex interactions between these measures and many of the other variables assessed in this study. Accordingly, our results did not support clear directional pathways that would permit the use of methods such as mediation analysis to potentially isolate causation in cross-sectional studies, as we were able to do in our more homogenous samples of patients with depression (Felger et al., 2016; Mehta et al., 2018). This highlights the need for longitudinal and interventional studies in patients with high inflammation and symptoms of anxiety, which may benefit from reproducible neuroimaging markers to define brain signatures of high inflammation such as amygdala-vmPFC FC.
Our exploratory analyses did reveal an interesting association between amygdala-vmPFC FC and PTSD symptoms specific to those related to re-experiencing/intrusive thoughts. It was surprising, however, given associations between this amygdala-vmPFC FC and anxiety that a similar relationship was not observed for PTSD symptoms of hyperarousal, which have been found to correlate with low amygdala-vmPFC FC in similar cohorts of women from GTP (Stevens et al., 2013). It is also interesting note that some items on the PSS probing PTSD symptoms have significant overlap with depressive symptoms and items on the BDI specifically for subscales of hyperarousal (e.g., irritability, agitation, insomnia, concentration) and avoidance/numbing (e.g., loss of interest in activities, feeling as if hopes and plans will not come true), but not the re-experiencing/intrusive subscale that asks only about symptoms or reactions specific to the remembering or re-experiencing of traumatic events. Despite only a handful of women endorsing clinically significant depressive symptoms consistent with moderate depression, the BDI scores highly correlated with CTQ (r=0.53) and significantly associated with all CTQ subcomponents of abuse and neglect (see Table S3 and Supplemental Results), while PSS was not as strongly correlated with CTQ (r=0.42) and only correlated with components related to abuse but not neglect. Of its subscales, the PSS re-experiencing/intrusive subscale only significantly correlated with emotional and sexual but not physical abuse, and with smaller effect sizes than the hyperarousal and avoidance/numbness subscales (e.g., r=0.37 versus 0.45 for emotional abuse). Interestingly, right amygdala-vmPFC FC followed the same pattern as the re-experiencing/intrusive subscale and was only significantly (albeit negatively) correlated with severity of the emotional and sexual abuse subcomponents of CTQ with moderate effect sizes (r=−0.32 to −0.34) and did not correlate with BDI. While CRP was not correlated with CTQ or TEI, the cytokines and particularly IL-6 alone did have moderate effect sizes for correlation with the lifetime trauma scores, particularly when removing the contribution of childhood abuse (TEI no child; r=0.29). Although not statistically significant, CRP did also have a moderate positive correlation coefficient for PSS and specifically the re-experiencing/intrusive subscale (r=0.21). While quite complex and potentially confounded by intercorrelations amongst both the biomarkers and self-report scales, together these preliminary findings suggest that to some extent inflammation, but certainly this inflammation-associated amygdala-vmPFC FC, was more strongly relate to symptoms that were both more specific to PTSD versus depression and to aspects of childhood maltreatment related to abuse than neglect.
Regarding the relationship of inflammation-associated right amygdala-vmPFC FC and both anxiety and PTSD symptoms, PSS scores did not correlate with anxiety in this cohort as has been previously shown (Foa et al., 1993; Beck et al., 2004; Atik et al., 2017), and CRP was also not linearly associated with anxiety (Table S2). Therefore, it was interesting that in a follow-up exploratory analysis, higher CRP was observed in patients reporting significant anxiety or PTSD symptoms only in models that accounted for the other symptom, and this effect was additive but not interactive (Figure S3). This is somewhat consistent with recent findings that relationships between early life maltreatment (as PTSD or its symptom severity were not measured) and IL-6 were dependent on the severity of social anxiety symptoms (Carlton et al., 2021). Of note, no significant interactions were observed to indicate that the relationships between FC and anxiety or FC and PSS re-experiencing/intrusive symptoms were modified by severity of the other symptom (see Supplement). Thus, despite the above mentioned potential confounds of interrelatedness of behavioral scales, bidirectional relationships between the inflammatory and imaging biomarkers, and a number of contributing methodologic limitations (as discuss ed in detail below), a pattern emerged whereby patients with high inflammation and/or early life maltreatment had lower right amygdala-vmPFC FC, and lower right amygdala-vmPFC FC was in turn is associated with anxiety and/or re-experiencing/intrusive symptoms of PTSD. It should be noted that these exploratory findings are extremely preliminary and would require a much larger patient sample, and more controlled and/or longitudinal or interventional experimental design (see discussion below), to fully tease apart causal relationships between the assessed exposures, biomarkers, and behavioral outcomes in a more conclusive and meaningful way. Nevertheless, exploratory findings for childhood maltreatment and PTSD symptoms should not discount the primary result of reproducible relationships between inflammation and right amygdala-vmPFC FC.
Limitations and future directions
As numerous variables can influence inflammatory markers and FC as well as behavior, it is worthy to discuss methodologic concerns within this dataset. Primary issues included dissociation of timing between blood collection and MRI, no control for timing of scans, only one scale for anxiety. While laboratory testing was not used to rule out undiagnosed or uncontrolled medical illness, medical status and history of the women were evaluated by a clinician during routine screening for GTP studies, and this cohort of imaged patients reflects a higher level of medical scrutiny both with respect to their medical status at that time (see Methods for exclusions) and also that participation in MRI scans at the clinical research facilities in campus would preclude emergent symptomatic illness. Medical history was also reviewed by study staff prior to the scan. Another potential concern is that assessment of clinically significant symptoms of PTSD and depression were based on symptom severity scales collected on the day of scanning. This method was preferred in this study and our previous work in order to more accurately reflect behavior of the participants in the days leading up to the study visit and improve correlations with neuroimaging (Stevens et al., 2013; Stevens et al., 2014). Finally, as exploratory analyses could only provide preliminary associations of inflammation and FC as a signature of adult or early life trauma or its functional outcomes (e.g., PTSD symptom severity), larger studies with better control of experimental parameters and assessment of not only the number but also severity of trauma experienced in adulthood are required to fully understand the potential role of inflammation and/or amygdala-vmPFC FC. Despite these weaknesses, and especially the lag between measurement of CRP and cytokines and the collection of MRI and self-report data, we still observed significant relationships between inflammation and our targeted right amygdala-vmPFC FC that survived correction for multiple covariates including time between blood draw and MRI scan, as well as weak relationships between inflammation and the behaviors that were related to the FC (as to be expected per associations between the inflammatory and brain biomarkers and their relationships with behavior in our prior work) (Felger et al., 2016; Haroon et al., 2018; Mehta et al., 2018; Mehta et al., 2020). Importantly, this suggests that these primary relationships between inflammation and right amygdala-vmPFC FC and FC and anxiety may be not only be reproducible across heterogenous samples, but also potentially stable over time and these methods could be indicated for use in future translational studies.
Supplementary Material
Inflammatory stimuli can affect brain regions involved in depression, anxiety and PTSD
CRP negatively associated with amygdala-vmPFC functional connectivity in inner-city AA women
Low amygdala-vmPFC connectivity in turn associated with symptoms of anxiety and PTSD
Funding sources:
This work was supported by funds from the National Institute of Mental Health grants R01MH109637, R61MH121625, R01MH096764, R01MH099211, R01MH071537, R01AT011267, F31MH119745, the National Institute of Health/National Cancer Institute under award number P30CA138292, and the National Natural Science Foundation of China (grant number 31671169, 31530031 and 31920103009 to ZL).
Footnotes
Financial Disclosure: All authors declare no conflicts of interest. Dr. Felger recently consulted for Cello Health BioConsulting on a topic unrelated to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atik D, Neşe A, Çakir M, Ünal A & Yüce UÖ (2017) ‘Post traumatic stress and anxiety in patients with acute coronary syndrome’, 2017, 3(8), p. 7. [Google Scholar]
- Beck AT, Steer RA & Carbin MG (1988) ‘Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation’, Clinical Psychology Review, 8(1), pp. 77–100. [Google Scholar]
- Beck JG, Coffey SF, Palyo SA, Gudmundsdottir B, Miller LM & Colder CR (2004) ‘Psychometric Properties of the Posttraumatic Cognitions Inventory (PTCI): a replication with motor vehicle accident survivors’, Psychol Assess, 16(3), pp. 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Treadway MT & Felger JC (2022) ‘Inflammation as a Pathophysiologic Pathway to Anhedonia: Mechanisms and Therapeutic Implications’, Curr Top Behav Neurosci. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E & Ruggiero J (1994) ‘Initial reliability and validity of a new retrospective measure of child abuse and neglect’, Am J Psychiatry, 151(8), pp. 1132–1136. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF & Ressler KJ (2008) ‘Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults’, JAMA, 299(11), pp. 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton CN, Garcia KM, Sullivan-Toole H, Stanton K, McDonnell CG & Richey JA (2021) ‘From childhood maltreatment to adult inflammation: Evidence for the mediational status of social anxiety and low positive affect’, Brain Behav Immun Health, 18, p. 100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE & Lim KO (2014) ‘Abnormal amygdala resting-state functional connectivity in adolescent depression’, JAMA Psychiatry, 71(10), pp. 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R & Caspi A (2008) ‘Elevated inflammation levels in depressed adults with a history of childhood maltreatment’, Arch Gen Psychiatry, 65(4), pp. 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW & Kelley KW (2008) ‘From inflammation to sickness and depression: when the immune system subjugates the brain’, Nat Rev Neurosci, 9(1), pp. 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KA, Cooper E, Voon V, Tibble J, Cercignani M & Harrison NA (2021) ‘Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms’, Mol Psychiatry, 26(9), pp. 5150–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry HM, Padin AC, Kuo JL, Hughes S & Kiecolt-Glaser JK (2015) ‘Sex Differences in Depression: Does Inflammation Play a Role?’, Curr Psychiatry Rep, 17(10), p. 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P & Gruber O (2012) ‘The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing’, Neuropsychologia, 50(7), pp. 1252–1266. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM & Irwin MR (2010) ‘Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood’, Brain Behav Immun, 24(4), pp. 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Jain J, Hudak LA, Rothbaum BO, Ressler KJ & Michopoulos V (2020) ‘Post-trauma anhedonia is associated with increased substance use in a recently-traumatized population’, Psychiatry Res, 285, p. 112777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC (2018) ‘Imaging the Role of Inflammation in Mood and Anxiety-related Disorders’, Curr Neuropharmacol, 16(5), pp. 533–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, Le NA, Feinberg R, Tansey MG & Miller AH (2020) ‘What does plasma CRP tell us about peripheral and central inflammation in depression?’, Mol Psychiatry, 25(6), pp. 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X & Miller AH (2016) ‘Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression’, Mol Psychiatry, 21(10), pp. 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC & Lotrich FE (2013) ‘Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications’, Neuroscience, 246, pp. 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV & Rothbaum BO (1993) ‘Reliability and validity of a brief instrument for assessing post-traumatic stress disorder’, J Trauma Stress, 6(4), pp. 459–473. [Google Scholar]
- Foa EB & Tolin DF (2000) ‘Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale’, J Trauma Stress, 13(2), pp. 181–191. [DOI] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N & Slavich GM (2019) ‘Chronic inflammation in the etiology of disease across the life span’, Nat Med, 25(12), pp. 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF & Ressler KJ (2009) ‘Trauma exposure and stress-related disorders in inner city primary care patients’, Gen Hosp Psychiatry, 31(6), pp. 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck RL, Hartzell GE, Dixon HD, Michopoulos V, Powers A, Stevens JS, Fani N, Carter S, Schwartz AC, Jovanovic T, Ressler KJ, Bradley B & Gillespie CF (2021) ‘Trauma exposure and stress-related disorders in a large, urban, predominantly African-American, female sample’, Arch Womens Ment Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Bekhbat M, Le NA, Chen X, Woolwine BJ, Li Z, Haroon E & Felger JC (2020) ‘Protein and gene markers of metabolic dysfunction and inflammation together associate with functional connectivity in reward and motor circuits in depression’, Brain Behav Immun, 88, pp. 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Chen G, Chen F, Zhong S, Chen P, Zhong H, Lai S, Tang G, Wang J, Luo Z, Qi Z, Jia Y, Huang L & Wang Y (2022) ‘Association between resting-state functional connectivity of amygdala subregions and peripheral pro-inflammation cytokines levels in bipolar disorder’, Brain Imaging Behav. [DOI] [PubMed] [Google Scholar]
- Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, Felger JC & Miller AH (2018) ‘Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia’, Transl Psychiatry, 8(1), p. 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA (2019) ‘Disentangling the Effects of Peripheral Inflammatory Markers on Brain Functional Connectivity’, Biol Psychiatry, 86(2), pp. 84–86. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A & Critchley HD (2009a) ‘Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity’, Biol Psychiatry, 66(5), pp. 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ & Critchley HD (2009b) ‘Neural origins of human sickness in interoceptive responses to inflammation’, Biol Psychiatry, 66(5), pp. 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein KA & Hu X (2004) ‘Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA’, Magn Reson Med, 51(1), pp. 212–216. [DOI] [PubMed] [Google Scholar]
- Hou R, Garner M, Holmes C, Osmond C, Teeling J, Lau L & Baldwin DS (2017) ‘Peripheral inflammatory cytokines and immune balance in Generalised Anxiety Disorder: Case-controlled study’, Brain Behav Immun, 62, pp. 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW & Eisenberger NI (2012) ‘Inflammation selectively enhances amygdala activity to socially threatening images’, Neuroimage, 59(4), pp. 3222–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR & Cole SW (2011) ‘Reciprocal regulation of the neural and innate immune systems’, Nat Rev Immunol, 11(9), pp. 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E & Niedzwiedz CL (2022) ‘The association of anxiety and stress-related disorders with C-reactive protein (CRP) within UK Biobank’, Brain Behav Immun Health, 19, p. 100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2003) ‘Epidemiology of women and depression’, J Affect Disord, 74(1), pp. 5–13. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC & Whalen PJ (2011) ‘Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest’, Cereb Cortex, 21(7), pp. 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrenz F, Wrede K, Forsting M, Engler H, Schedlowski M, Elsenbruch S & Benson S (2016) ‘Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men’, Brain Behav Immun, 54, pp. 17–26. [DOI] [PubMed] [Google Scholar]
- Lasselin J, Elsenbruch S, Lekander M, Axelsson J, Karshikoff B, Grigoleit JS, Engler H, Schedlowski M & Benson S (2016) ‘Mood disturbance during experimental endotoxemia: Predictors of state anxiety as a psychological component of sickness behavior’, Brain Behav Immun, 57, pp. 30–37. [DOI] [PubMed] [Google Scholar]
- Leschak CJ, Dutcher JM, Haltom KEB, Breen EC, Bower JE & Eisenberger NI (2020) ‘Associations between amygdala reactivity to social threat, perceived stress and C-reactive protein in breast cancer survivors’, Soc Cogn Affect Neurosci, 15(10), pp. 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Michopoulos V, Powers A, Wingo AP, Schwartz A, Bradley B, Ressler KJ & Gillespie CF (2018) ‘Affect, inflammation, and health in urban at-risk civilians’, J Psychiatr Res, 104, pp. 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liukkonen T, Räsänen P, Jokelainen J, Leinonen M, Järvelin MR, Meyer-Rochow VB & Timonen M (2011) ‘The association between anxiety and C-reactive protein (CRP) levels: results from the Northern Finland 1966 birth cohort study’, Eur Psychiatry, 26(6), pp. 363–369. [DOI] [PubMed] [Google Scholar]
- Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z & Felger JC (2018) ‘Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results’, Brain Behav Immun, 73, pp. 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V & Felger JC (2020) ‘Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women’, Soc Cogn Affect Neurosci, 15(10), pp. 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ & Jovanovic T (2017) ‘Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond’, Neuropsychopharmacology, 42(1), pp. 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Moore C, Villarreal S, Ressler KJ & Bradley B (2015a) ‘The mediating role of emotion dysregulation and depression on the relationship between childhood trauma exposure and emotional eating’, Appetite, 91, pp. 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF & Ressler KJ (2015b) ‘Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma’, Am J Psychiatry, 172(4), pp. 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, Burgess S, Penninx B & Khandaker GM (2021) ‘Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts’, Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E & Felger JC (2017) ‘Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation’, Neuropsychopharmacology, 42(1), pp. 334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, White SF, Chen E & Nusslock R (2021) ‘Association of Inflammatory Activity With Larger Neural Responses to Threat and Reward Among Children Living in Poverty’, Am J Psychiatry, 178(4), pp. 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, Irwin MR & Eisenberger NI (2014) ‘Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress’, Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, Irwin MR & Eisenberger NI (2015) ‘Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress’, Brain Behav Immun, 43, pp. 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, Irwin MR & Eisenberger NI (2016) ‘Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback’, Brain Behav Immun, 57, pp. 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Brody GH, Armstrong CC, Carroll AL, Sweet LH, Yu T, Barton AW, Hallowell ES, Chen E, Higgins JP, Parrish TB, Wang L & Miller GE (2019) ‘Higher Peripheral Inflammatory Signaling Associated With Lower Resting-State Functional Brain Connectivity in Emotion Regulation and Central Executive Networks’, Biol Psychiatry, 86(2), pp. 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL & Petersen SE (2014) ‘Methods to detect, characterize, and remove motion artifact in resting state fMRI’, Neuroimage, 84, pp. 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A, Michopoulos V, Conneely K, Gluck R, Dixon H, Wilson J, Jovanovic T, Pace TW, Umpierrez GE, Ressler KJ, Bradley B & Gillespie CF (2016) ‘Emotion Dysregulation and Inflammation in African-American Women with Type 2 Diabetes’, Neural Plast, 2016, p. 8926840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL & Miller AH (2013) ‘The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D)’, Mol Psychiatry, 18(1), pp. 15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL & Miller AH (2017) ‘Pathogen-Host Defense in the Evolution of Depression: Insights into Epidemiology, Genetics, Bioregional Differences and Female Preponderance’, Neuropsychopharmacology, 42(1), pp. 5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM (2003) ‘Clinical application of C-reactive protein for cardiovascular disease detection and prevention’, Circulation, 107(3), pp. 363–369. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley RL, Sexton M, Sherry A & Ressler KJ (2005) ‘Posttraumatic stress disorder among African Americans in an inner city mental health clinic’, Psychiatr Serv, 56(2), pp. 212–215. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL & Pitman RK (2006) ‘Amygdala, medial prefrontal cortex, and hippocampal function in PTSD’, Ann N Y Acad Sci, 1071, pp. 67–79. [DOI] [PubMed] [Google Scholar]
- Spann SJ, Gillespie CF, Davis JS, Brown A, Schwartz A, Wingo A, Habib L & Ressler KJ (2014) ‘The association between childhood trauma and lipid levels in an adult low-income, minority population’, Gen Hosp Psychiatry, 36(2), pp. 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL & Lushene R (1979) Manual of the State-Trait Anxiety Inventory, Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, Reiser E, Ely TD, Dhanani R, Glover EM, Jovanovic T & Ressler KJ (2014) ‘PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus’, Proc Natl Acad Sci U S A, 111(8), pp. 3158–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS & Hamann S (2012) ‘Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies’, Neuropsychologia, 50(7), pp. 1578–1593. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B & Ressler KJ (2013) ‘Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder’, J Psychiatr Res, 47(10), pp. 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, Hudak LA, Jovanovic T, Rothbaum BO & Ressler KJ (2017) ‘Amygdala Reactivity and Anterior Cingulate Habituation Predict Posttraumatic Stress Disorder Symptom Maintenance After Acute Civilian Trauma’, Biological Psychiatry, 81(12), pp. 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carranza AF, Tully LM, Knodt AR, Jiang J, Irwin MR & Hostinar CE (2021) ‘Associations between peripheral inflammation and resting state functional connectivity in adolescents’, Brain Behav Immun, 95, pp. 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Prather AA, Di Iorio CR, Bogdan R & Hariri AR (2017a) ‘A Functional Interleukin-18 Haplotype Predicts Depression and Anxiety through Increased Threat-Related Amygdala Reactivity in Women but Not Men’, Neuropsychopharmacology, 42(2), pp. 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Prather AA & Hariri AR (2017b) ‘Threat-related amygdala activity is associated with peripheral CRP concentrations in men but not women’, Psychoneuroendocrinology, 78, pp. 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Kong L, Wu F, Womer F, Jiang W, Cao Y, Ren L, Wang J, Fan G, Blumberg HP, Xu K & Wang F (2013) ‘Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study’, Psychol Med, 43(9), pp. 1921–1927. [DOI] [PubMed] [Google Scholar]
- van Eeden WA, El Filali E, van Hemert AM, Carlier IVE, Penninx B, Lamers F, Schoevers R & Giltay EJ (2021) ‘Basal and LPS-stimulated inflammatory markers and the course of anxiety symptoms’, Brain Behav Immun, 98, pp. 378–387. [DOI] [PubMed] [Google Scholar]
- Yin L, Xu X, Chen G, Mehta ND, Haroon E, Miller AH, Luo Y, Li Z & Felger JC (2019) ‘Inflammation and decreased functional connectivity in a widely-distributed network in depression: Centralized effects in the ventral medial prefrontal cortex’, Brain, Behavior, and Immunity, 80, pp. 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


