Abstract
Background
Inefficient migration of dendritic cells (DC) to regional lymph nodes (LN) upon intracutaneous injection is a major obstacle for effective DC vaccination. Intravenous vaccination is unfavorable, because DC cannot migrate directly from the blood into LN.
Methods
To enable human monocyte-derived (mo)DC to enter LN directly from the blood, we manipulated them by RNA electroporation to express a human chimeric E/L-selectin (CD62E/CD62L) protein, which binds to peripheral node addressin expressed on high endothelial venules.
Results
Transfection efficiency exceeded 95%, and high E/L-selectin surface expression was detected for >48 h. E/L-selectin RNA-transfected DC displayed an identical mature DC phenotype as mock-transfected DC. Furthermore, E/L-selectin-transfected DC maintained their normal CCR7-mediated migration capacity, and their ability to prime and expand functional cytotoxic T cells recognizing MelanA. Most importantly, E/L-selectin-RNA-transfected DC gained the capability to attach to and roll on sialyl-LewisX in vitro.
Outlook
The presented strategy can be readily translated into the clinic, as it involves no stable genetic manipulation or viral transformation, and allows targeting of a large number of LN.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-007-0385-1) contains supplementary material, which is available to authorized users.
Keywords: L-Selectin, RNA, Electroporation, Dendritic cells, Homing behavior, Intravenous injection
Introduction
Therapeutic vaccination using dendritic cells (DC) has gained increasing importance in treatment of cancer and infectious diseases. DC can be generated in large quantities from blood-derived monocytes (monocyte-derived DC; moDC), and have been used as an adjuvant in many clinical phase I and phase II trials. In most of these trials, intra- or subcutaneous injection was chosen as the route of application [4, 19, 21, 31, 38]. However, to reach peripheral lymphatic tissue, where antigen-specific T-cell stimulation takes place, DC have to migrate from the site of injection to the draining lymph nodes (LN), where at most 1–2% of the cells arrive [12, 34, 40]. This may contribute to the limited efficacy observed in DC vaccination trials so far [43]. An alternative way of application is the supersonic-guided injection directly into the LN. However, the node may be missed or its structure disturbed, the injectable volume is limited, and special equipment and experience are required. Hence, this method yields very variable results [12, 13].
Intravenous injection of moDC results in their accumulation in the lung, and later in the liver, the spleen, and the bone marrow, but they are unable to transmigrate from the bloodstream into the LN [34]. In humans, this appears to favor a Th2-polarized immune response, which is likely less beneficial in cancer vaccination [20]. However, other immune cells, i.e., T cells, are able to enter the LN from the high endothelial venules (HEV) using the surface proteins L-selectin, CCR7 and LFA-1. Two of them, CCR7 and LFA-1 are expressed on mature moDC, but L-selectin is not. Therefore, the idea was conceived to close that gap by genetic manipulation, thus enabling DC to migrate through the HEV [41]. It was demonstrated that murine DC, retrovirally transduced with a human chimeric E/L-selectin protein, are able to bind to peripheral node addressin (PNAd), the natural ligand of L-selectin, which is highly expressed on HEV. E/L-selectin is a fusion protein composed of the extracellular portion of E-selectin and the transmembrane and intracellular portion of L-selectin. This chimera was constructed to avoid proteolytic shedding from the DC’s surface, which is rich in membrane-bound proteases. Interestingly, the binding specificity of the protein is significantly dependent on the intracellular domains, which define the molecule’s distribution on the cell surface. Accordingly, E/L-selectin expressing murine DC could indeed enter peripheral LN after IV injection in vivo [41].
In the present study we focused on transferring this approach to the human system in a clinically applicable way. Since the application of retrovirally transduced cells is problematic in humans, e.g., due to ethical and regulatory restrictions, and the transfection efficiency is variable and would only be applicable to DC derived from proliferating CD34+ cells, but not at all to the most often used DC derived from non-dividing monocytes, RNA electroporation was used as an alternative method to introduce the protein of choice. This relatively new method of DC transduction results in high transfection rates if an optimized electroporation method is employed (>95%) [47], and ensures that only transient expression will occur since chromosomal integration is impossible. However, RNA electroporation has so far mainly been used as a means of antigen delivery, and sparsely to increase the DC’s stimulatory capacity [1, 11, 22]. So it was unclear and questioned by most investigators whether a sufficient expression level for an adhesion-mediating receptor could at all be achieved by RNA transfection. Here, we show for the first time that introduction of an adhesion molecule by RNA transfection into human moDC is possible, and that the introduced protein is functional in vitro, while the phenotype and the immunologic function of the DC remained unaffected.
Taken together, the technique of RNA electroporation allows for expression of a functional adhesion molecule, avoiding the risks of genetic manipulation, without hampering the DC’s immunogenic functions in vitro. This can provide a new strategy to target DC to LN in cancer vaccination, which may be more efficient than intracutaneous DC injection.
Materials and methods
Antibodies
The following antibodies (Ab) were used for intracellular FACS staining of tumor antigens: mouse-anti-human Melan A (clone A103) (Novocastra, Newcastle upon Tyne, UK), mouse-anti-human MAGE (clone 57b; kindly provided by Dr. Spagnoli, Basel). This Ab detects several members of the MAGE-family, including MAGE-3 [25]. Goat-anti-human survivin, (R&D Systems, Minneapolis, MN, USA). Secondary antibodies used in intracellular staining were goat-anti-mouse PE (BD Pharmingen, Heidelberg Germany) and donkey-anti-goat FITC (Santa Cruz Biotechnology, Santa Cruz, CA, USA). For surface staining the following monoclonal Ab were used: anti-CD83, anti-CD86, anti-CD25, and anti-CD80, anti-HLA-DR (BD Biosciences, Heidelberg, Germany), anti-CD62L, (Beckman Coulter GmbH, Krefeld, Germany), anti-HLA class I (Millipore, Schwalbach, Germany), anti-CD18 (Millipore)—all FITC-labeled—and PE-labeled anti-CD11a (eBioscience, San Diego, CA, USA). Isotype controls were: IgG1-FITC and -PE (BD Pharmingen), and IgG2a-FITC (Millipore). For determining E/L-selectin expression, FITC-labeled anti-human E-Selectin/CD62E mAb (Clone BBIG-E5; R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany) was used. T cell phenotype was determined with: ECD-labeled anti-CD45RA, PC7-labeled anti-CD8 (both from Beckman Coulter), and FITC-labeled anti-CCR7 (R&D Systems).
Human DC generation from leukapheresis and whole blood
moDC were generated essentially as described [5]. In short, peripheral blood mononuclear cells (PBMC) were prepared from leukapheresis products or whole blood of healthy donors (obtained following informed consent and approved by the institutional review board) by density centrifugation using Lymphoprep (Axis-Shield, Oslo, Norway). PBMC were resuspended in autologous medium that consisted of RPMI 1640 (Cambrex, Verviers, Belgium) containing 1% of heat-inactivated human plasma, 2 mM l-Glutamin (Bio-Whittaker, Walkersville, MD, USA), 20 mg/l Gentamicin (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and were transferred to cell-factories (Nunc, Roskilde, Denmark), or for generation of DC in smaller scale to tissue culture dishes [Falcon (BD), Le Pont De Claix, France] at 1.2 × 109 cells/cell-factory, or 3 × 107 cells/dish. Cells were incubated for 1–2 h at 37°C to allow for adherence, the non-adherent fraction was removed and cryopreserved, while 200 ml (cell factory) or 10 ml (dish) of autologous medium was added to the adherent cells. Feeding of cells with medium and cytokines [GM-CSF (Leukine, Berlex, Montville, NJ, USA), and IL-4 (Strathmann, Hamburg, Germany)], and maturation of DC was performed as described previously [47]. After 24 h of maturation, the cells were used for electroporation.
Production of in vitro transcribed RNA
For in vitro transcriptions, the pGEM4Z64A-enhanced GFP (EGFP), pGEM4Z64A-MAGE-3, pGEM4Z64A-MelanA [24], and pGEM4Z64A-survivin (survivin wild-type [2]) plasmids (kindly provided by Dr. I. Tcherepanova, Argos Therapeutics, Durham, NC, USA), were used. For the production of the E/L-selectin encoding RNA, the PSP73 SphA64+EL plasmid that contains the extracellular domain of human E-selectin and the transmembrane and intracellular domain of human L-selectin in one open reading frame was used. All plasmids were transcribed as described before [47] using an Ambion mMESSAGE mMACHINE T7 ULTRA kit, Austin, TX, USA, according to the manufacturer’s instructions.
Electroporation of dendritic cells
Human DC were harvested from the cell-factories or dishes and washed once with pure RPMI 1640 and once with PBS (all at room temperature). The cells were resuspended in OptiMEM without phenol-red (Gibco-BRL, Long Island, NY, USA) at a concentration of 3–6 × 107/ml. RNA was electroporated into DC with a Genepulser Xcell (Bio-Rad, Munich, Germany) machine. The parameters for electroporation were: square wave pulse, 500 V, 1 ms, 4 mm cuvettes, and a volume of 100–600 μl per shot. The RNA-concentration was 150 μg/ml for E/L-selectin RNA, and 50 μg/ml for tumor-antigen encoding RNAs. Immediately after electroporation, the cells were transferred to autologous medium supplemented with the above-indicated concentrations of GM-CSF and IL-4.
Cryopreservation of cells
Cryopreservation was performed as described [18]. In short, cells were taken up in 20% human serum albumin (HSA, Pharmacia&Upjohn, Stockholm, Sweden) at a concentration of 5–10 × 106 cells/ml for DC or 20–50 × 106 cells/ml for the non-adherent fraction, and placed for 10 min on ice. An equal volume of cryopreservation medium, i.e., 55% HSA (20%), 20% dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and 25% Glucose (Glucosteril 40™, Fresenius, Bad Homburg, Germany), was added to the cell suspension. Cells were then frozen at −1°C/min in a cryo-freezing container (Nalgene, Roskilde, Denmark) to −80°C. Thawing was performed by holding cryotubes in a 37°C water-bath till detachment of the cells was visible. Cells were then poured into 10 ml RPMI 1640, washed and added to a cell culture dish containing pre-warmed autologous medium, 250 IU IL-4/ml and 800 IU GM-CSF/ml. Cells were rested for 1–2 h in a 37°C incubator prior to further experiments.
Flow cytometric analysis of DC
For cell surface staining, DC were washed and, thereafter, suspended at 1 × 105 cells in 100 μl of cold FACS solution [DPBS (Bio Whittaker) containing 0.1% sodium azide (Sigma-Aldrich) and 0.2% HSA (Octapharma, Langenfeld, Germany)] and incubated with mAb or appropriate isotype controls for 30 min. Cells were then washed twice and resuspended in 100 μl of cold FACS solution. Stained cells were analyzed for immunofluorescence with a FACSstar cell analyzer (Becton-Dickinson, Heidelberg, Germany). Cell debris was eliminated from the analysis using a gate on forward and side scatter. A minimum of 104 cells was analyzed for each sample of surface-stained cells. Results were evaluated using the Cellquest software (Becton-Dickinson).
Transwell migration assay
Transwell migration assays were performed using transwell inserts (Costar, London, UK) with a pore size of 5 μm, and CCL19 (100 ng/ml, tebu-bio GmbH, Offenbach, Germany) as described previously [47].
Cytotoxic T Cell (CTL) induction assay
Dendritic cells were pretreated as indicated. A part of the DC were pulsed for 1 h at 37°C with 10 μg/ml of the MelanA-derived HLA-A2-binding analogue peptide ELAGIGILTV for comparison. The non-adherent fraction from the same healthy donor was used as source for generating CD8+ T-cell using MACS (Miltenyi Biotech, Bergisch-Gladbach, Germany) according to the manufacturer’s instructions. CD8+ cells were then co-cultivated with DC at final concentrations of 1 × 106/ml and 1 × 105/ml, respectively, in RPMI supplemented with 10% pooled-serum (Cambrex), 10 mM Hepes, 1 mM sodiumpyruvate, 1% MEM non-essential aminoacids (100×), 2 mM l-glutamine, 20 mg/l Gentamycin, and 20 U/ml of IL-7. Twenty IU/ml of IL-2 and 20 U/ml of IL-7 were added on days 2 and 4. On day 7, the cells were harvested and used for readout.
Tetramer staining and phenotyping of antigen-specific CD8+ T cells
HLA-A2-MelanA (ELAGIGILTV) tetramer staining (Beckman Coulter GmbH) and T cell phenotyping using anti-CCR7, anti-CD45RA and anti-CD8 antibodies were performed as described previously [47]. Cells were analyzed on a CYTOMICS FC500 from Beckman Coulter.
Cytotoxicity assay
Cytotoxicity was tested in standard 4 h 51Cr release assays as described previously [47].
E/L-selectin-induced in vitro migration assay
Dendritic cells, electroporated with or without E/L-selectin RNA, were resuspended at a concentration of 1 × 106 cells/ml. Rectangular cover slips (24 × 60 mm2) were coated with 5 μg biotinylated polyacrylamide bound to sialyl-Lewisx and sulphated tyrosine (s-Lex/TS-PAA; Lectinity Holdings Inc., Moscow, Russia) and air-dried. All cover slips were incubated for at least 30 min with 0.5% bovine serum albumin (in PBS) to prevent non-specific binding. Transparent flow chambers with a slit depth of 50 μm and a slit width of 500 μm, equipped with coated or uncoated cover slips were briefly rinsed with Hank’s balanced salt solution (HBSS) supplemented with 2 mM CaCl2 prior to connection to a syringe containing 1 ml cell suspension. Perfusion was performed at 20°C using a pulse-free pump at a shear rate of 1.04 dyne/s2. During perfusion, microscopic phase-contrast images were recorded in real time. After 10 min of perfusion, four different microscopic fields of vision (10× objective) were recorded and the numbers of adhering cells were counted for each field. Image analysis was performed off-line using MetaView Imaging software (Universal Imaging Corporation, Downington, PA, USA). For statistical analysis, a paired, one-tailed Student’s t-test was chosen.
Results
Electroporation of E/L-selectin encoding RNA into mature human DC resulted in high transfection efficiency and good survival
Our goal was to enable DC to enter LN directly from the bloodstream through HEV by introduction of a chimeric E/L-selectin molecule by RNA electroporation. Since this would require high and long-lasting expression of the functional protein, we further optimized the electroporation of mature DC with RNA [47]. For this, we used GFP RNA in initial experiments, and E/L-selectin RNA in the subsequent ones. We decided to use a square wave pulse in accordance to the protocol published by Sæbøe-Larssen et al. [44]. In these preceding experiments, we found that at a voltage of 500 V in a 4 mm cuvette, the expression of the protein, encoded by the introduced RNA, is almost proportional to the pulse length between 0 and 1 ms. Longer pulse times resulted in increased cell death and reduced expression levels (data not shown). This was by and large in line with previously published data [44]. The voltage of 500 V was chosen for technical reasons, since higher voltages are not possible with the gene pulser Xcell (in 4 mm cuvettes). A reduction of the voltage did not increase the cell survival, but reduced the expression levels (data not shown). We also found that between 100 and 600 μl, the volume had no influence on the transfection efficiency and cell survival, as long as the RNA concentration was kept constant (data not shown). Increasing the RNA concentration almost proportionally increased expression of the encoded protein, up to an RNA concentration of 150 μg/ml ([47] and data not shown). DC concentrations above 6 × 106/ml resulted in reduced transfection efficiency (not shown). With respect to the level and kinetics of E/L-selectin expression, the optimal electroporation conditions were defined to be 500 V, 1 ms pulse length and a RNA concentration of 150 μg/ml.
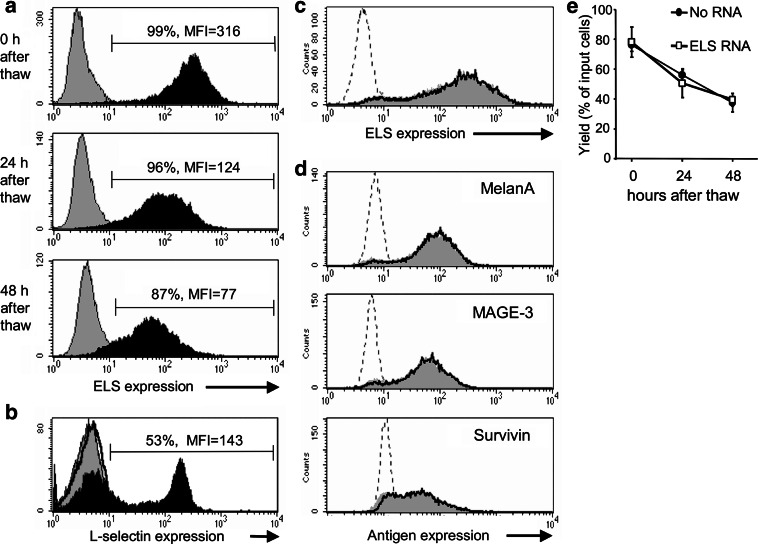
To demonstrate substantial and prolonged expression of E/L-selectin, DC were electroporated with the above described conditions and cryopreserved 4 h later. As shown in Fig. 1a, high and homogeneous expression of E/L-selectin was observed even 24 and 48 h after thawing, proving that a prolonged expression of E/L-selectin was obtained. Exclusion of dead cells by PI-staining at the 48 h time-point did not alter the expression profile (data not shown). For comparison, CD8+ T cells were stained for E-selectin and L-selectin expression. As expected, only L-selectin expression was observed (Fig. 1b).
Fig. 1.
High E/L-selectin expression, no competition with other RNAs, and high DC yield after E/L-selectin RNA electroporation. a Mature DC were electroporated with or without E/L-selectin (ELS) RNA and cryopreserved after 4 h. ELS expression of ELS-RNA-electroporated DC (black histogram) and mock-electroporated DC (gray histrogram) was determined by FACS analysis 0, 24, or 48 h after thawing. The percentage of ELS-positive cells and mean fluorescence intensity (MFI) are depicted in the histogram. b For comparison, purified CD8+ T cells were stained for E-selectin (thick black line) and for L-selectin expression (black histogram); gray histogram: isotype control. c and d Mature DC were electroporated without RNA, with ELS RNA, with RNA encoding the three TAAs MelanA, MAGE-3, and surviving, or with the combination of E/L-selectin RNA and RNA of all three TAAs. Four hours later they were cryopreserved. Directly after thawing, ELS-expression of mock-electroporated (dotted line), and of DC electroporated with ELS RNA only (gray histograms) or with both ELS and TAAs RNA (thick line) was determined by FACS analysis (c). DC transfected without RNA (dotted line), with the TAAs RNA alone (gray histogram), or in combination with the ELS RNA (thick line) were stained intracellularly for the three TAAs and analyzed by FACS (d). The data shown in a, b, c, and d are representative for three independent standardized experiments. DC yield (e) of ELS-RNA-electroporated DC (white square) and mock-electroporated DC (black circle) was determined by trypan-blue staining and cell counting 0, 24, or 48 h after thawing. Yield was expressed as percent surviving cells of input cells before electroporation The average ± standard error of the mean (SEM) of three standardized experiments is shown
The introduction of large quantities of different mRNAs could lead to a competition for the translation machinery of the cell. To examine this, we co-electroporated 150 μg/ml of E/L-selectin RNA with RNA encoding the three tumor-associated antigens (TAAs) MelanA, MAGE-3, and survivin (50 μg/ml RNA each). Four hours after electroporation, DC were cryoconserved, and thawed to be stained and analyzed by FACS immediately. Neither the E/L-selectin-expression was negatively influenced when the three TAAs were present (Fig. 1c), nor was the expression of the TAAs reduced by co-electroporation of the E/L-selectin RNA (Fig. 1d). From this we conclude that no competition for translation factors occurred under our electroporation conditions.
Cell survival after transfection is critical to obtain enough DC for vaccination, and gives an indication for survival capacity of electroporated DC in vivo. Harmful effects of the electroporation procedure and of the introduction of E/L-selectin into DC had to be excluded. Hence, we determined yield by counting in trypan-blue (i.e., percent surviving cells after electroporation and freeze/thaw relative to the number of DC before electroporation) of DC electroporated without RNA and E/L-selectin RNA-electroporated DC 0, 24, and 48 h after freeze/thaw (for the 24 and 48 h time-point, cells were cultured in autologous medium with IL-4 and GM-CSF at 37°C). Survival of E/L-selectin RNA-electroporated DC directly after freeze/thaw was ∼80%, and decreased slowly from 60 to 47% over 2 days not differing from mock-electroporated DCs (Fig. 1e). These data are in line with the yield obtained with non-electroporated DC ([47], and data not shown). Thus, the introduction of E/L-selectin RNA had no toxic effect on transfected DC.
Expression of E/L-selectin did not influence CCR7-mediated migratory capacity and LFA-1 expression
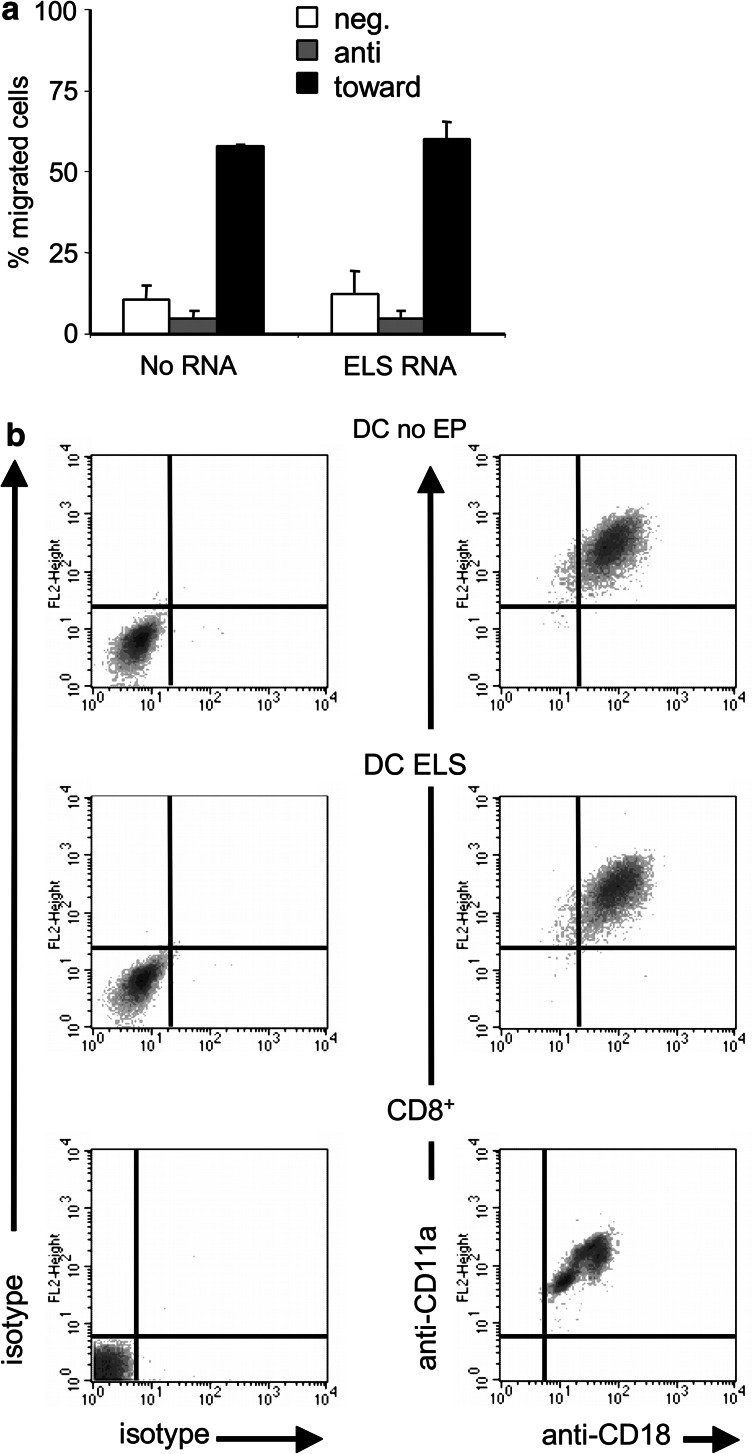
Migration mediated by the chemokine receptor CCR7 is critical for normal DC movement from the skin into the LN, but also for the intranodal orientation. Furthermore, functional CCR7 expression is also necessary for rolling on HEV and the subsequent transmigration. Although it was previously shown that electroporation per se does not reduce CCR7-mediated migration of DC [17, 47], we still had to exclude any interference of the E/L-selectin expression with CCR7-mediated migration. Therefore, mock-electroporated and E/L-selectin RNA-electroporated DC were compared in a standard transwell in vitro migration assay [46]. DC were allowed to migrate for 2 h either against (i.e., chemokine in upper well together with DC) or toward (i.e., chemokine in lower well and DC in upper well) a CCL19 gradient. Both mock-electroporated and E/L-selectin RNA-electroporated DC did not migrate against the CCL19 gradient (Fig. 2a). Most importantly, both DC populations showed an identical migration capacity of ∼60% toward CCL19 (Fig. 2a). This is similar to the migrational capacity of not electroporated mature DC [17, 47]. As a negative control, cells were incubated in transwells without CCL19. These data indicate, that the E/L-selectin expressing DC were still capable of CCR7-mediated migration, which is a prerequisite to extravasate into the LN.
Fig. 2.
Electroporation with E/L-selectin-encoding RNA does not influence CCR7-mediated migration and LFA-1 expression. a Mature DC were electroporated with or without E/L-selectin (ELS) RNA and cryopreserved after 4 h. After thawing, cells were tested for their CCR7-mediated migratory capacity toward medium containing CCL19 in a standard transwell migration assay. To measure spontaneous migration, cells were incubated in a transwell without CCL19 in the upper or lower compartment (neg.), or with CCL19 in the upper compartment (anti). The average ± standard error of the mean (SEM) of three standardized experiments is shown. b Mature DC were electroporated (EP) with E/L-selectin (ELS) RNA or were only replated (DC no EP). After resting for 4 h, they were cryopreserved, thawed and stained for CD11a and CD18 expression (which form LFA-1 as a heterodimer). As a control for the staining, the LFA-1 expression of CD8+ cells was determined. Isotype-controls are depicted in the left panel. The data shown are representative for three independent standardized experiments
Another surface molecule that is required to enter the LN through the HEV is the integin LFA-1, a heterodimer that consists of CD11a and CD18. To exclude negative effects of the electroporation on LFA-1 expression, we compared E/L-selectin RNA-electroporated DC with mock-electroporated DC and with CD8+ cells by double-staining for both CD11a and CD18 and subsequent FACS analysis. The LFA-1 expression was not influenced by the E/L-selectin expression, and was similar to that of CD8+ T cells (Fig. 2b). From this, we conclude that sufficient LFA-1 expression is present on the electroporated DC, to allow for transmigration into the LN.
E/L-selectin RNA-electroporated human DC displayed a mature phenotype and were efficient inducers of MelanA-specific CTL
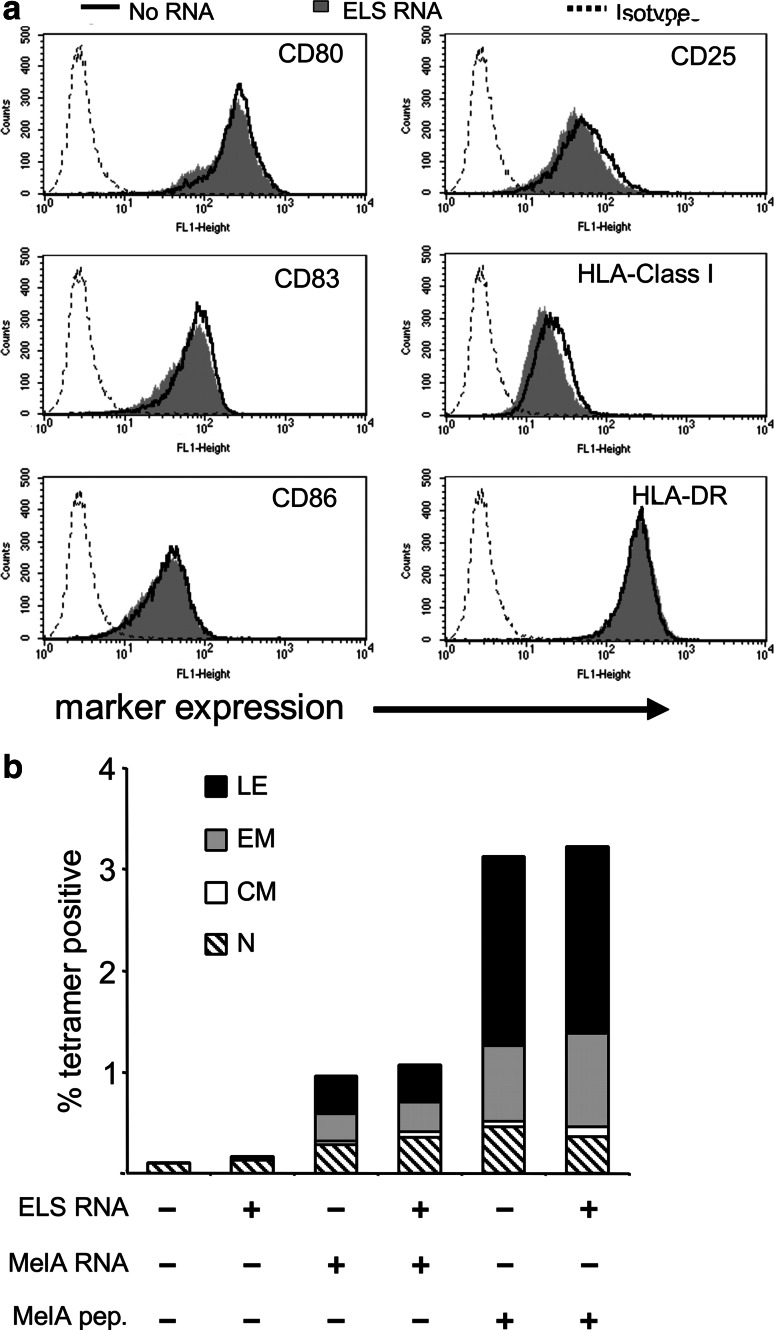
In case of using E/L-selectin-expressing DC as a vaccine against cancer, it is essential that the DC can process and present antigen to induce TAA-specific CTL. This requires a mature phenotype. To exclude that the E/L-selectin expression affects the DC’s mature phenotype, we compared mock-electroporated DC with E/L-selectin RNA-electroporated DC by determining the surface expression of CD80, CD83, CD86, CD25, HLA class I and HLA-DR molecules on these DC. Both DC populations exhibited a mature phenotype, and no difference was observed between the two (Fig. 3a). From these data, it can be concluded that the expression of E/L-selectin on the surface of the DC does not change their mature phenotype.
Fig. 3.
E/L-selectin transfected DC retain mature phenotype and their capacity to stimulate naive CD8+ T-cells. a Mature DC were electroporated with (gray histogram) or without (black line) E/L-selectin (ELS) RNA, rested for 4 h, cryopreserved and stained for the characteristic maturation-surface markers CD25, CD80, CD83, CD86, HLA class I, and HLA-DR after thawing. Interrupted lines represent respective isotype controls. b DC were electroporated without RNA, with E/L-selectin RNA (ELS RNA) alone, with MelanA RNA (MelA RNA) alone, or with MelanA in combination with E/L-selectin RNA, and were used to stimulate autologous naive CD8+ cells. In addition, mock-electroporated and E/L-selectin RNA-electroporated DC were loaded with MelanA-derived analogue peptide (MelA pep.) and used for stimulation of naïve CD8+ T cells. After 1 week of stimulation, the percentage of MelanA/A2-tetramer-binding CD8+ T cells, and their phenotype were determined. Assignment of functional T cell phenotype was as follows: lytic effectors (LE): CD45RA+/CCR7−; effector memory (EM): CD45RA−/CCR7−; central memory (CM) CD45RA−/CCR7+; naïve (N): CD45RA+/CCR7+. Both a and b show one representative out of three independent standardized experiments
The DC’s ability to process and present antigen to prime CTL was tested in an autologous CD8+ T cell stimulation assay, with the melanoma-associated antigen MelanA. After one stimulation for one week, the number of MelanA-specific CD8+ T cells was determined by tetramer staining, and their phenotype (i.e., naïve, central memory, effector memory, or lytic effector) by CCR7 and CD45RA staining [45]. We compared the induction capacity of DC loaded with MelanA alone, with the induction capacity of MelanA-loaded DC electroporated with E/L-selectin RNA. The antigen-loading was performed either by co-electroporation of RNA coding for the natural MelanA or by pulsing with a MelanA-derived HLA-A2 restricted analogue peptide. DC electroporated with MelanA RNA alone or with the combination of MelanA RNA and E/L-selectin RNA expanded the MelanA-specific T cells to the same extent, with a strong bias toward the effector population (Fig. 3b). Mock-electroporated and E/L-selectin RNA-electroporated DC served as negative controls (Fig. 3b). The MelanA analogue peptide-loaded DC expectedly stimulated the MelanA peptide-specific T cells even stronger, and again no substantial difference in stimulation capacity between the mock-electroporated and the E/L-selectin RNA-electroporated DC was observed (Fig. 3b).
The generated T cells were checked for their cytolytic capacity in a standard Cr51-release assay, using T2 cells loaded with the specific analogue peptide as target cells. This reflected the results of the tetramer staining: the E/L-selectin expression on the DC had no influence on the T cells lytic capacity (Suppl. Fig. 1), while both the pulsing of the DC with analogue peptide, and the electroporation with MelanA RNA resulted in T cells capable to specifically lyse the targets. As expected, lysis was stronger after stimulation with the peptide. In summary, these data demonstrate that E/L-selectin co-expression does not alter the DC’s function to induce specific CTL by presenting a tumor-associated Ag.
E/L-selectin RNA-electroporated human DC rolled by binding to sialyl-Lewisx
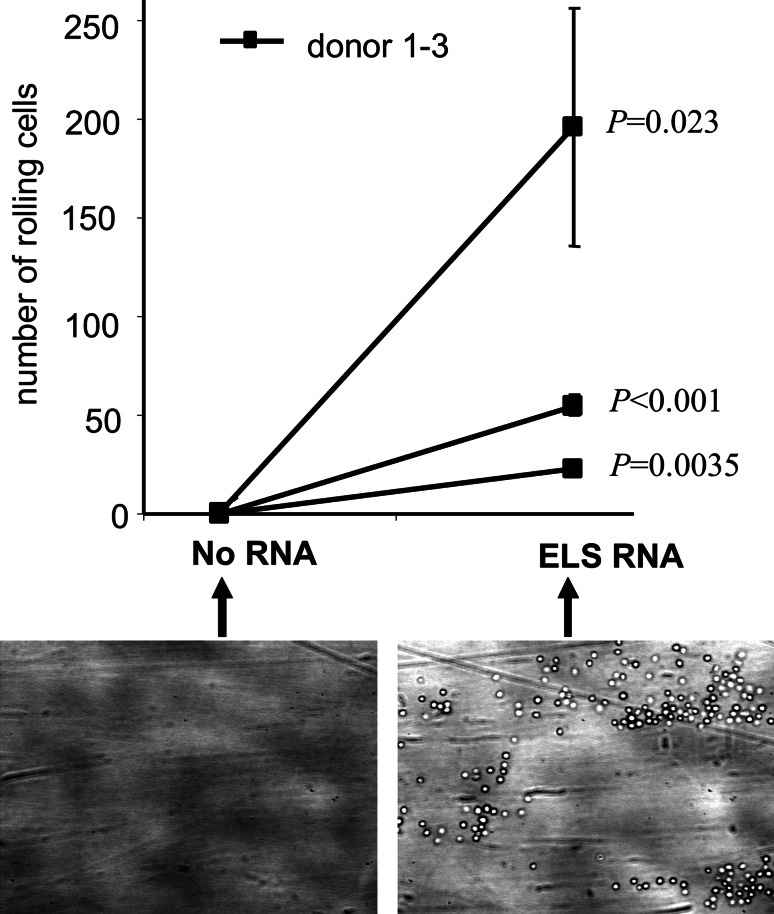
The functionality of the introduced chimeric E/L-selectin protein was determined in an in vitro rolling assay using a surface coated with sialyl-Lewisx and sulphated tyrosine (s-Lex/TS-PAA) in a parallel plate flow chamber [28]. The shear force in the assay approximated the one in HEV (1.04 dyne/s2). As shown in Suppl. Fig. 2a, mock-electroporated DC did not roll on, or adhere to s-Lex/TS-PAA-coated slides. In contrast, E/L-selectin RNA-electroporated DC clearly rolled on the s-Lex/TS-PAA-coated surface with a slow rolling velocity (Suppl. Fig. 2b). Both DC populations did not roll on or adhere to uncoated slides (data not shown). After 10 min of flow, pictures of four random fields were taken, and cells were counted. Figure 4 summarizes this quantification for three different donors. DC electroporated without RNA did not adhere to or roll on the s-Lex/TS-PAA-coated slides at all (Fig. 4), while E/L-selectin RNA-electroporated DC of all three donors showed adherence and rolling (Fig. 4). Since the s-Lex/TS-PAA-coating of the slides is not a standardized process, large variations between the donors were observed, however, a statistical analysis revealed that the observed differences were significant for each individual donor (P < 0.05). Taken together, these data show that the E/L-selectin protein that was expressed following E/L-selectin-RNA transfection into DC was functional and mediated in vitro rolling of these DC. Previous studies in other tissues have shown that a slow rolling velocity facilitates integrin activation [27, 29]. Therefore, it is likely that the slow rolling velocity of the DC could allow their extravasation through the HEV into the LN.
Fig. 4.
E/L-selectin transfected DC gain the ability to roll on sialyl-Lewisx/TS-PAA. Human DC (three donors) were electroporated without RNA (No RNA) or with E/L-selectin RNA (ELS RNA) and cryopreserved. Their ability to tether and roll was analyzed in a parallel plate flow chamber using slides coated with sialyl-Lewisx/TS-PAA directly after thawing. Perfusion was performed at 20°C using a pulse-free pump at a shear rate of 1.04 dyne/s2. During perfusion, microscopic phase-contrast images were recorded in real time. After 10 min of perfusion, four different randomly chosen microscopic fields (one representative is depicted) (10× objective) were recorded and the numbers of adhering cells were counted for each field. An average (±SEM) of cell numbers of the four fields and the P-value as determined by unpaired, one-tailed Student’s t-test is shown for each donor
In aggregate, these data prove that RNA electroporation is a possible way to introduce functional E/L-selectin into DC with high efficiency and without influencing their immunogenic functions. This could make the IV injection of DC in humans in vaccination studies feasible, and shows that RNA-electroporation is a possibility to provide DC with new functions, they cannot fulfill by themselves.
Discussion
This paper describes the introduction of a functional chimeric molecule, E/L-selectin, into human moDC. A high transfection efficiency (>95%) and yield (>75%) was obtained after electroporation and subsequent cryopreservation (Fig. 1). This high transfection efficiency of E/L-selectin RNA into DC was in line with introduction of RNA encoding tumor antigens [47]. Other groups also expressed functional proteins in DC via RNA transfection [1, 10, 11, 22]. However, in these studies, lower expression levels were observed, or the cellular expression was not determined, but the introduced proteins (signaling receptors or proteins involved in certain signaling cascades) nevertheless exerted their function. The group of Sæbøe-Larssen previously reported high expression for both functional and non-functional proteins [44]. For our purpose, also high expression of E/L-selectin on the DC surface was critical for functional rolling to occur. Therefore, we optimized the RNA-electroporation protocol in line with the above mentioned publication, which resulted in such high and prolonged expression levels. For this, we used GFP RNA in initial experiments, and E/L-selectin RNA in the subsequent ones. With respect to the level and kinetics of E/L-selectin expression, the optimal electroporation conditions were defined to be 500 V, 1 ms pulse length and an RNA concentration of 150 μg/ml.
Hence, this paper shows for the first time that functional adhesion receptors can be introduced into human moDC by RNA electroporation, since the E/L-selectin transfected DC gained the capability to tether and roll on slides coated with the natural ligand of E/L-selectin, proving the functional introduction of this chimeric molecule in vitro (Fig. 4 and Suppl. Fig. 2).
Functional introduction of E/L-selectin in bone marrow-derived murine DC was previously shown [41]. However, retroviral transduction was used in this study to insert E/L-selectin. RNA electroporation has several considerable advantages as compared to retroviral transduction. First, the most important drawback of using retroviral transduction is that the provirus integrates at random in the genome of the transduced cells. Thus, it can also integrate in genes involved in cell cycle control, and subsequently disturb cell growth (i.e., insertional mutagenesis) [7, 23, 32]. The problem of possible insertional mutagenesis is completely absent when RNA is electroporated in cells, and for applications where only transient expression of certain molecules is necessary, such as targeting of DC from blood to LN, RNA electroporation is a good alternative. Therefore, our E/L-selectin RNA transfection of DC is generally safer for clinical application, with respect to mutagenesis, than retroviral transduction of the chimeric E/L-selectin gene into DC.
Second, retroviral transduction can only be performed in dividing cells, such as bone marrow-derived DC. In principle, human CD34+ stem cells could be used to provide a pool of DC precursors that divide, which can be retrovirally transduced. However, isolation of CD34+ stem cells is a time-consuming and complicated procedure and is additionally a burden for the patient, because of the necessary pretreatment with hematopoietic growth factors such as G-CSF. Therefore, almost exclusively moDC are used. These cells do not divide, and cannot be retrovirally transduced. Non-dividing DCs can be transduced using other viruses, such as lentivirus, although, in contrast to RNA electroporation, this results in heterogeneous expression of the introduced molecules [6, 16, 48], which is undesired for targeting of all DC to the LN, or adenovirus [26, 37, 39], which also results in heterogeneous expression of the introduced molecule [9]. Moreover, adenovirally transduced DC may even induce regulatory T cells instead of an efficient anti-tumor response [30]. In addition, viral transduction is still classified as gene therapy, and is hampered by many regulatory restrictions and ethical considerations. Here we show that RNA transfection is a promising alternative for the introduction of the chimeric E/L-selectin molecule into moDC, because it does not change the phenotypical and functional characteristics of the transfected cells (Figs. 2, 3).
As mentioned in the introduction, the number of DC reaching the LN after intravenous injection is very low [12, 34, 40]. Intravenous injection of E/L-selectin-targeted DC might be more efficient than intracutaneous injection of DC, i.e., a higher number of DC may reach the LN. Furthermore, by targeting of DC from the blood directly into the LN, not only the draining LN is reached by the DC, but in principle all LN. This might result in a more efficient stimulation of antigen-specific T cells, because the quantity of T cell-DC contacts is increased.
Evidence is increasing that T cells in LN draining different organs acquire a homing pattern that targets these T cells to the respective organ after contact with the DC [8, 14, 15, 42, 50]. Moreover, it was described that in vitro stimulation of T cells with skin-derived DC, and later restimulation with gut-derived DC, results in a replacement of skin-homing molecules with gut-homing molecules [33], proving the plasticity of T cell homing. By intracutaneous injection and subsequent stimulation of T cells in the draining LN, preferentially T cells with a skin homing pattern are generated which migrate to cutaneous effector sites. Indeed, it was shown that DC vaccination strategies, in which DC were injected intracutaneously or intralymphaticly, resulted in effective clearance of mainly cutaneous metastases [3, 35, 49]. Using the vaccination strategy described in this paper, it is possible that after intravenous injection, by targeting DC to several peripheral LN, T cells with different homing patterns will be generated. This could be of great advantage for the induction of an immune response directed against metastases within various organs and tissues. Alternatively, it is also possible, that the T cells acquire no homing pattern, because the DC did not migrate to the LN via the natural route. Which alternative holds true, will be subject of future in vivo experiments analyzing T cell location, and homing receptors.
Although the question whether the E/L-selectin transfected DC will be capable of entering the LN from the blood stream in vivo, and will initiate a strong immune response there, can be discussed here only on a theoretical level, we are confident that this approach will be successful. Monocyte-derived mature DC express LFA-1 and CCR7, and this is not diminished by the introduction of E/L-selectin (Fig. 2). The DC’s capability to migrate through a 5 μm mesh toward a CCL19 gradient (i.e., a ligand of CCR7) was also shown (Fig. 2). Furthermore, the functionality of the introduced E/L-selectin was shown in a flow chamber assay in vitro. The biological relevance of this assay had been shown before with murine DC, retrovirally transduced with E/L-selectin, which gained the ability to tether and roll in this in vitro assay, but also were able to enter the T cell areas in the LN in vivo [41]. In addition, blocking experiments also showed that the in vitro assay correlates with in vivo homing, i.e., agents that blocked lymphocytes binding in the in vitro assay also inhibited rolling and homing to peripheral LN in vivo [36].
Since the human, RNA-electroporated DC were equipped with all three molecules needed for extravasation from the HEV to the LN (i.e., CCR7, LFA-1, and E/L-selectin), they should be able to extravasate in vivo, in analogy to the murine DC described above. As T cells enter the LN by the very same route, DC/T cell encounters are very likely. In addition, the DC still possess their CCR7-mediated ability to orientate in the LN. As the DC display a high immunogenicity in vitro, T cell stimulation in the natural environment of the LN is very probable. On the other hand, it is also possible that the human DC, although expressing LFA-1, are not able to respond to LFA-1 signaling as T cells do, or do not possess the mechanical functions to transmigrate through the endothelium of the HEV. It could also be that the DC need to receive special signals for optimal function during their natural path of migration toward the LN, which would make our approach less efficient. When we balance these arguments, our expectation is that the described approach will work, although proof definitely requires in vivo experiments.
Taken together, the introduction of a chimeric E/L-selectin molecule into human non-dividing moDC is now possible without genetic manipulation. This may target the DC from the blood directly into the LN, without changing their phenotypical and functional characteristics. For the first time a functional adhesion molecule was introduced into cells by RNA electroporation, thus bypassing the serious problems related to viral introduction of these molecules, which constricted the clinical use of this vaccination approach. These DC might provide a quantitatively and qualitatively more efficient antigen-specific T cell stimulation, which is critical to induce effective immune resistance to cancer. This hypothesis will be tested in a clinical trial in the near future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Manfred Lutz for fruitful discussions. We thank Drs. Sandy King and Robert Fuhlbrigge for performing pilot rolling experiments. We thank Susanne Rößner and Stefanie Baumann for technical assistance. This study was supported by grants to G.S. from the DFG—German Research Foundation (Collaborative Research Centre SFB643, Project C1), the European Union (DCVACC, contract no.: 503037), and by the Cancer Immunotherapy (CIMT) EU Integrated Project, WP02.02.
Footnotes
Jan Dörrie and Niels Schaft contributed equally to this work.
References
- 1.Abdel-Wahab Z, Cisco R, Dannull J, Ueno T, Abdel-Wahab O, Kalady MF, Onaitis MW, Tyler DS, Pruitt SK. Cotransfection of DC with TLR4 and MART-1 RNA induces MART-1-specific responses. J Surg Res. 2005;124:264–273. doi: 10.1016/j.jss.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 4.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–274. doi: 10.1016/S0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 5.Berger TG, Feuerstein B, Strasser E, Hirsch U, Schreiner D, Schuler G, Schuler-Thurner B. Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J Immunol Methods. 2002;268:131–140. doi: 10.1016/S0022-1759(02)00189-8. [DOI] [PubMed] [Google Scholar]
- 6.Breckpot K, Dullaers M, Bonehill A, Van Meirvenne S, Heirman C, De Greef C, Van Der BP, Thielemans K. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med. 2003;5:654–667. doi: 10.1002/jgm.400. [DOI] [PubMed] [Google Scholar]
- 7.Buckley RH. Gene therapy for SCID—a complication after remarkable progress. Lancet. 2002;360:1185–1186. doi: 10.1016/S0140-6736(02)11290-6. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho HI, Kim HJ, Oh ST, Kim TG. In vitro induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses. Vaccine. 2003;22:224–236. doi: 10.1016/S0264-410X(03)00569-3. [DOI] [PubMed] [Google Scholar]
- 10.Cisco RM, Abdel-Wahab Z, Dannull J, Nair S, Tyler DS, Gilboa E, Vieweg J, Daaka Y, Pruitt SK. Induction of human dendritic cell maturation using transfection with RNA encoding a dominant positive toll-like receptor 4. J Immunol. 2004;172:7162–7168. doi: 10.4049/jimmunol.172.11.7162. [DOI] [PubMed] [Google Scholar]
- 11.Dannull J, Nair S, Su Z, Boczkowski D, DeBeck C, Yang B, Gilboa E, Vieweg J. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood. 2005;105:3206–3213. doi: 10.1182/blood-2004-10-3944. [DOI] [PubMed] [Google Scholar]
- 12.de Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, Adema GJ, Punt CJ, Figdor CG. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 13.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, Bulte JW, Scheenen TW, Punt CJ, Heerschap A, Figdor CG. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 14.Dudda JC, Lembo A, Bachtanian E, Huehn J, Siewert C, Hamann A, Kremmer E, Forster R, Martin SF. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- 15.Dudda JC, Simon JC, Martin S. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J Immunol. 2004;172:857–863. doi: 10.4049/jimmunol.172.2.857. [DOI] [PubMed] [Google Scholar]
- 16.Dullaers M, Breckpot K, Van Meirvenne S, Bonehill A, Tuyaerts S, Michiels A, Straetman L, Heirman C, De Greef C, Van Der BP, Thielemans K. Side-by-side comparison of lentivirally transduced and mRNA-electroporated dendritic cells: implications for cancer immunotherapy protocols. Mol Ther. 2004;10:768–779. doi: 10.1016/j.ymthe.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Erdmann M, Dörrie J, Schaft N, Strasser E, Hendelmeier M, Kämpgen E, Schuler G, Schuler-Thurner B. Effective clinical-scale production of dendritic cell vaccines by monocyte elutriation directly in medium, subsequent culture in bags and final antigen loading using peptides or RNA transfection. J Immunother. 2007;30:663–674. doi: 10.1097/CJI.0b013e3180ca7cd6. [DOI] [PubMed] [Google Scholar]
- 18.Feuerstein B, Berger TG, Maczek C, Roder C, Schreiner D, Hirsch U, Haendle I, Leisgang W, Glaser A, Kuss O, Diepgen TL, Schuler G, Schuler-Thurner B. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J Immunol Methods. 2000;245:15–29. doi: 10.1016/S0022-1759(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 19.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 20.Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166:4254–4259. doi: 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- 21.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–263. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 22.Grunebach F, Kayser K, Weck MM, Muller MR, Appel S, Brossart P. Cotransfection of dendritic cells with RNA coding for HER-2/neu and 4-1BBL increases the induction of tumor antigen specific cytotoxic T lymphocytes. Cancer Gene Ther. 2005;12:749–756. doi: 10.1038/sj.cgt.7700842. [DOI] [PubMed] [Google Scholar]
- 23.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, Thrasher AJ, Wulffraat N, Sorensen R, Dupuis-Girod S, Fischer A, Davies EG, Kuis W, Leiva L, Cavazzana-Calvo M. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 24.Heiser A, Dahm P, Yancey DR, Maurice MA, Boczkowski D, Nair SK, Gilboa E, Vieweg J. Human dendritic cells transfected with RNA encoding prostate-specific antigen stimulate prostate-specific CTL responses in vitro. J Immunol. 2000;164:5508–5514. doi: 10.4049/jimmunol.164.10.5508. [DOI] [PubMed] [Google Scholar]
- 25.Kocher T, Schultz-Thater E, Gudat F, Schaefer C, Casorati G, Juretic A, Willimann T, Harder F, Heberer M, Spagnoli GC. Identification and intracellular location of MAGE-3 gene product. Cancer Res. 1995;55:2236–2239. [PubMed] [Google Scholar]
- 26.Korokhov N, de Gruijl TD, Aldrich WA, Triozzi PL, Banerjee PT, Gillies SD, Curiel TJ, Douglas JT, Scheper RJ, Curiel DT. High Efficiency Transduction of Dendritic Cells by Adenoviral Vectors Targeted To DC-SIGN. Cancer Biol Ther. 2005;4:289–294. doi: 10.4161/cbt.4.3.1499. [DOI] [PubMed] [Google Scholar]
- 27.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-D. [DOI] [PubMed] [Google Scholar]
- 29.Ley K, Allietta M, Bullard DC, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- 30.Lundqvist A, Palmborg A, Pavlenko M, Levitskaya J, Pisa P. Mature dendritic cells induce tumor-specific type 1 regulatory T cells. J Immunother. 2005;28:229–235. doi: 10.1097/01.cji.0000158854.15664.c2. [DOI] [PubMed] [Google Scholar]
- 31.Markiewicz MA, Kast WM. Progress in the development of immunotherapy of cancer using ex vivo-generated dendritic cells expressing multiple tumor antigen epitopes. Cancer Invest. 2004;22:417–434. doi: 10.1081/CNV-200029072. [DOI] [PubMed] [Google Scholar]
- 32.Marshall E. Clinical research. Gene therapy a suspect in leukemia-like disease. Science. 2002;298:34–35. doi: 10.1126/science.298.5591.34. [DOI] [PubMed] [Google Scholar]
- 33.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 35.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 36.Oostingh GJ, Ludwig RJ, Enders S, Grüner S, Harms G, Boehncke W-H, Nieswandt B, Tauber R, Schön MP. Diminished lymphocyte adhesion and alleviation of allergic responses by small-molecule- or antibody-mediated inhibition of l-selectin functions. J Invest Dermatol. 2007;127:90–97. doi: 10.1038/sj.jid.5700504. [DOI] [PubMed] [Google Scholar]
- 37.Ophorst OJ, Kostense S, Goudsmit J, De Swart RL, Verhaagh S, Zakhartchouk A, Van Meijer M, Sprangers M, Van Amerongen G, Yuksel S, Osterhaus AD, Havenga MJ. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine. 2004;22:3035–3044. doi: 10.1016/j.vaccine.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Paczesny S, Ueno H, Fay J, Banchereau J, Palucka AK. Dendritic cells as vectors for immunotherapy of cancer. Semin Cancer Biol. 2003;13:439–447. doi: 10.1016/j.semcancer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Rea D, Havenga MJ, van Den AM, Sutmuller RP, Lemckert A, Hoeben RC, Bout A, Melief CJ, Offringa R. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J Immunol. 2001;166:5236–5244. doi: 10.4049/jimmunol.166.8.5236. [DOI] [PubMed] [Google Scholar]
- 40.Ridolfi R, Riccobon A, Galassi R, Giorgetti G, Petrini M, Fiammenghi L, Stefanelli M, Ridolfi L, Moretti A, Migliori G, Fiorentini G. Evaluation of in vivo labelled dendritic cell migration in cancer patients. J Transl Med. 2004;2:27. doi: 10.1186/1479-5876-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robert C, Klein C, Cheng G, Kogan A, Mulligan RC, von Andrian UH, Kupper TS. Gene therapy to target dendritic cells from blood to lymph nodes. Gene Ther. 2003;10:1479–1486. doi: 10.1038/sj.gt.3302008. [DOI] [PubMed] [Google Scholar]
- 42.Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341:1817–1828. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sæbøe-Larssen S, Fossberg E, Gaudernack G. mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT) J Immunol Methods. 2002;259:191–203. doi: 10.1016/S0022-1759(01)00506-3. [DOI] [PubMed] [Google Scholar]
- 45.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 46.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 47.Schaft N, Dorrie J, Thumann P, Beck VE, Muller I, Schultz ES, Kampgen E, Dieckmann D, Schuler G. Generation of an optimized polyvalent monocyte-derived dendritic cell vaccine by transfecting defined RNAs after rather than before maturation. J Immunol. 2005;174:3087–3097. doi: 10.4049/jimmunol.174.5.3087. [DOI] [PubMed] [Google Scholar]
- 48.Sumimoto H, Tsuji T, Miyoshi H, Hagihara M, Takada-Yamazaki R, Okamoto S, Ikeda Y, Takahashi T, Kawakami Y. Rapid and efficient generation of lentivirally gene-modified dendritic cells from DC progenitors with bone marrow stromal cells. J Immunol Methods. 2002;271:153–165. doi: 10.1016/S0022-1759(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 49.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den DP, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.