Abstract
The infection of woodchucks with woodchuck hepatitis virus (WHV) provides an experimental model to study early immune responses during hepadnavirus infection that cannot be tested in patients. The T-cell response of experimentally WHV-infected woodchucks to WHsAg, rWHcAg, and WHcAg peptides was monitored by observing 5-bromo-2′-deoxyuridine and [2-3H]adenine incorporation. The first T-cell responses were directed against WHsAg 3 weeks after infection; these were followed by responses to rWHcAg including the immunodominant T-cell epitope of WHcAg (amino acids 97 to 110). Maximal proliferative responses were detected when the animals seroconvered to anti-WHs and anti-WHc (week 6). A decrease in the T-cell response to viral antigens coincided with clearance of viral DNA. Polyclonal rWHcAg-specific T-cell lines were established 6, 12, 18, and 24 weeks postinfection, and their responses to WHcAg peptides were assessed. Five to seven peptides including the immunodominant epitope were recognized throughout the observation period (6 months). At 12 months after infection, T-cell responses to antigens and peptides were not detected. Reactivation of T-cell responses to viral antigens and peptides occurred within 7 days after challenge of animals with WHV. These results demonstrate that a fast and vigorous T-cell response to WHsAg, rWHcAg, and amino acids 97 to 110 of the WHcAg occurs within 3 weeks after WHV infection. The peak of this response was associated with viral clearance and may be crucial for recovery from infection. One year after infection, no proliferation of T cells in response to antigens was observed; however, the WHV-specific T-cell response was reactivated after challenge of woodchucks with WHV and may be responsible for protection against WHV reinfection.
Infection with hepatitis B virus (HBV) often results in an acute bout of hepatitis followed by clinical recovery, but progress to chronic infection and disease such as liver cirrhosis and hepatocellular carcinoma is sometimes observed. Several studies indicate that cellular immune responses to HBV proteins have a major influence on the clinical course of HBV infection: vigorous and multispecific T helper (Th)-cell and cytotoxic T-lymphocyte responses to HBV surface protein (HBsAg), core protein (HBcAg), polymerase protein, and X protein (HBxAg) have been associated with recovery from acute HBV infection and viral clearance (4, 11, 12, 17, 18, 21, 26, 29; for reviews, see references 6 and 10). In contrast, low or undetectable T-cell responses to these proteins were associated with viral persistence and chronic hepatitis (3, 27; reviewed in references 6 and 13). However, the exact immunologic mechanisms which contribute to viral elimination or persistence during the natural course of hepadnavirus infection are unknown. Because there are no clinical symptoms immediately after HBV transmission, T-cell-mediated immune responses during the incubation period and the early phase of hepatitis in patients are difficult to analyze. It is also unknown to what extent a long-lasting T-cell response may support protection from viral reinfection after resolution of an acute, self-limited HBV infection (30, 34).
Woodchucks (Marmota monax) infected with woodchuck hepatitis virus (WHV) represent the animal model closest to humans for studying the cell-mediated immune response during hepadnavirus infections. WHV and HBV exhibit a high degree of homology in their nucleotide sequences, genomic organizations, and replication and expression mechanisms (5, 8, 14, 19, 35, 39). Furthermore, the humoral immune responses during the course of acute or chronic infections of HBV-infected humans and WHV-infected woodchucks are similar (31–33, 41).
There are similarities in the cellular immune responses of woodchucks to WHV and the responses of humans infected with HBV. In WHV-infected adult woodchucks, strong T-cell responses to surface protein (WHsAg) and recombinant core protein (rWHcAg) are found (7, 23, 25). The T-cell responses in these woodchucks to WHcAg are predominantly to specific peptides of the antigen, whereas in chronically WHV-infected woodchucks an undetectable or weak T-cell response to these proteins and peptides is observed (23–25). The importance of T-cell responses for the elimination of WHV has been demonstrated by protective immunization with a single WHcAg peptide (amino acids 97 to 110) which contains a major T-cell epitope (23). It was demonstrated in vitro that proliferating cells from these animals were T cells, as shown by staining with a monoclonal antibody against CD3ɛ (23). Although further characterization of T cells in the woodchuck is not yet possible, the proliferation assays were analogous to those used in humans and the use of linear peptides suggested that the T cells were Th cells.
The experimental WHV infection of woodchucks is an important model with which to examine the role of cellular immune responses during the early phase of acute hepadnavirus infection. During this crucial phase, the course of infection resulting in viral elimination or persistence may be determined. This study investigated the kinetics of T-cell responses to WHsAg, rWHcAg, and WHcAg peptides during the early acute phase of self-limited WHV infection. rWHcAg-specific polyclonal T-cell lines were established at different times after infection, and their responses to WHcAg-related peptides were measured. Recognition of rWHcAg and several WHcAg-related peptides was shown to occur over a period of 6 months after inoculation, as was recognition of the immunodominant WHcAg epitope (amino acids 97 to 110) described previously (23). The T-cell responses after convalescence (1 year after infection) and upon challenge with WHV were also examined. No T-cell proliferation in response to WHV antigens could be detected 1 year after infection. Reactivation of the T-cell responses to viral antigens and to the previously recognized WHcAg epitopes was demonstrated after challenge with WHV.
MATERIALS AND METHODS
Animals.
Four WHV-negative woodchucks, trapped in the state of New York, were purchased from North Eastern Wildlife (Ithaca, N.Y.) and maintained in our facility. Before experimentation, all the animals were clinically examined and tested for parasitic infections including intestinal worms. Serologic testing was also performed, and all adult animals, of either sex, were found to be negative for WHV DNA, WHsAg, and antibodies against WHsAg (anti-WHs) and WHcAg (anti-WHc).
Serologic testing, virus detection, and liver function assay.
Serologic testing for markers of WHV replication during the course of acute WHV infection and convalescence and after viral challenge was performed weekly. WHsAg, anti-WHs, and anti-WHc were determined by an enzyme-linked immunosorbent assay (ELISA) as described previously (36, 37). WHV DNA was detected by PCR with two WHV core gene-specific oligonucleotide primers (nucleotides 2015 to 2038 and 2570 to 2595) (23) or by nested PCR with four oligonucleotide primers (nucleotides 2015 to 2038, 2630 to 2656, 2129 to 2148, and 2597 to 2618) after extraction of DNA from serum or from PBMC. Additionally, WHV DNA was detected by a dot blot technique as described previously (36). Sorbitol dehydrogenase (SDH) activity, a marker of acute liver damage in humans (2, 43) and in woodchucks (16), was assessed by a commercial enzyme assay (Sigma, Deisenhofen, Germany).
Monitoring of T-cell proliferation by BrdU and [2-3H]adenine incorporation.
Blood was drawn weekly from anesthetized woodchucks via the vena saphena and collected in EDTA monovettes (Sarstedt, Nuembrecht, Germany). Peripheral blood mononuclear cells (PBMC) were separated by Ficoll-Paque (Pharmacia, Freiburg, Germany) density gradient centrifugation and resuspended in 0.9% NaCl. Cell counting was performed with a Thoma hemocytometer or an electronic cell counter (Sysmex, Hamburg, Germany). Triplicate samples of 5 × 104 PBMC were cultured in 96-well microtiter plates (Falcon; Becton Dickinson, Paramus, N.J.) at 37°C in a humidified atmosphere containing 5% CO2. AIM-V medium (Gibco BRL, Eggenstein Leopoldshafen, Germany) (200 μl) supplemented with 4 mM l-glutamine (Sigma), 12.5 mM gentamicin sulfate (Sigma), and 10% fetal calf serum (Gibco) was added to each well (this is referred to below as complete medium). Woodchuck PBMC were stimulated with 2 μg of WHsAg per ml, 1 μg of rWHcAg per ml, or 1 μg of peptide of WHcAg per ml. These concentrations give optimal proliferation results (23).
WHsAg was purified from the sera of chronically WHV-infected woodchucks in the form of small (22-nm-diameter) particles by ultracentrifugation as described previously (15) and resolved in 0.9% NaCl. The purity of WHsAg was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining (purity, about 90%).
rWHcAg was made by cloning the gene coding for WHcAg into pKK 233-2 vector (Clontech, Palo Alto, Calif.). Expression of rWHcAg was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) and isolated to a purity of about 95% (23). The concentrations of WHsAg and rWHcAg were determined by a protein assay (Pierce, Rockford, Ill.), and the particulate nature of the purified proteins was analyzed by electron microscopy.
Synthetic peptides of WHcAg were purchased from Genosys (Cambridge, United Kingdom). Two panels of peptides were used (see Table 2) (23). Panel A contained 16 peptides (overlapping each other by 6 to 10 amino acids) covering the entire WHcAg, and panel B contained 6 peptides (overlapping each other by 3 to 11 amino acids) spanning amino acids 97 to 140 of the WHcAg.
TABLE 2.
PBMC response to WHcAg epitopes during acute WHV infection
| Peptide | PBMC response (mean SI) of woodchucka:
|
|||
|---|---|---|---|---|
| NW7029 | NW7030 | NW7031 | NW7032 | |
| Panel A | ||||
| 1–20 | 7.8 | 8.3 | 2.8 | 8.7 |
| 15–34 | 1.6 | 1.6 | 1.5 | 1.5 |
| 28–47 | 7.3 | 2.1 | 1.9 | 1.4 |
| 38–57 | 6.7 | 7.2 | 2.5 | 2.0 |
| 50–69 | 2.8 | 2.5 | 1.3 | 2.6 |
| 61–80 | 1.4 | 1.4 | 1.8 | 2.3 |
| 70–89 | 6.4 | 6.9 | 2.7 | 1.3 |
| 82–101 | 1.9 | 1.3 | 1.2 | 2.1 |
| 90–109 | 5.9 | 6.1 | 1.4 | 2.3 |
| 100–119 | 8.1 | 8.4 | 8.8 | 7.5 |
| 112–131 | 2.9 | 3.7 | 8.2 | 4.3 |
| 120–139 | 3.7 | 8.0 | 7.0 | 5.2 |
| 131–150 | 2.1 | 2.1 | 1.4 | 1.7 |
| 146–165 | 1.3 | 1.5 | 1.0 | 1.3 |
| 156–175 | 1.7 | 1.8 | 1.7 | 6.2 |
| 169–188 | 2.5 | 2.0 | 1.6 | 6.9 |
| Panel B | ||||
| 97–110 | 8.2 | 9.1 | 8.6 | 8.3 |
| 100–113 | 7.6 | 8.7 | 7.9 | 8.0 |
| 111–124 | 7.1 | 6.2 | 6.0 | 5.9 |
| 120–131 | 3.9 | 5.1 | 4.8 | 4.7 |
| 124–137 | 2.4 | 1.9 | 2.7 | 1.9 |
| 129–140 | 1.7 | 1.4 | 1.8 | 1.4 |
PBMC responses 6 weeks after experimental WHV infection to 1 μg of WHcAg peptides per ml were analyzed by [2-3H]adenine incorporation. PBMC (5 × 104) were obtained from woodchucks NW7029, NW7030, NW7031, and NW7032. Results are presented as mean SI of triplicate determinations. SI values of ≥3.1 are indicated in bold type. The mean cpm for the controls was 2,863 ± 1,397.
Spontaneous proliferation of PBMC in complete medium served as a background control. T-cell responses to WHV antigens were assessed after stimulation for 5 days including a 16- to 20-h pulse with 10 μM bromodeoxyuridine (BrdU) (BrdU cell proliferation ELISA kit [colorimetric] [Boehringer, Mannheim, Germany]) or with 1 μCi of [2-3H]adenine (Amersham, Braunschweig, Germany) as described previously (20, 23–25).
Results for triplicate cultures (5 × 104 PBMC) are presented as the mean stimulation index (SI); for the BrdU assay, the mean total absorption for stimulated PBMC was divided by the mean total absorption for controls (background), and for the [2-3H]adenine assay the mean total counts per minute (cpm) for stimulated PBMC was divided by the mean total cpm for controls (background). An SI of ≥2.1 was considered significant for T-cell proliferation in the BrdU assay (23), and an SI of ≥3.1 was considered significant in the [2-3H]adenine assay. In the BrdU assay, the standard deviations were less than 10% of the mean (range, 5 to 20%), and in the [2-3H]adenine assay, they were less than 30% of the mean (range, 15 to 50%).
For the establishment of rWHcAg-specific polyclonal T-cell lines, woodchuck PBMC were obtained at 6-week intervals after experimental WHV infection (weeks 6, 12, 18, and 24), 1 year after experimental WHV infection, and 1 week after viral challenge. A total of 6 × 106 PBMC (1.5 × 106/ml) were cultured in tissue culture flasks (Becton Dickinson) in the presence of 10 μg of rWHcAg per ml in complete medium. After 1 week, the PBMC were expanded by adding 20 IU of recombinant human interleukin-2 (IL-2; EuroCetus, Ratingen, Germany) per ml for an additional week. The PBMC were then separated on nylon wool columns (Polyscience, Inc., Warrington, Pa.) into adherent and nonadherent lymphocytes. The nonadherent T cells (23) were restimulated weekly over a period of 5 to 6 weeks with 10 μg of rWHcAg per ml plus autologous irradiated (3,000 rads) PBMC (5 × 105/ml) as antigen-presenting cells (APC) in complete medium supplemented with 20 IU of recombinant IL-2 per ml. For further characterization of T-cell lines, T cells were washed four times with 0.9% NaCl to remove IL-2. Subsequently, triplicate samples of 5 × 104 T cells/well were added to a microtiter plate and incubated for 5 days with 105 autologous irradiated (3,000 rads) PBMC in complete medium in the presence of 1 μg of rWHcAg or WHcAg peptides per ml, and T-cell proliferation was assessed by [2-3H]adenine incorporation.
Design of experimental WHV infection and viral challenge in woodchucks.
Infection of woodchucks was performed by intravenous inoculation with 105 50% woodchuck infectious doses (ID50) of a WHV serum pool of known titer (37). Blood samples for proliferation assays and for detection of viral DNA, WHsAg, anti-WHs, and anti-WHc were drawn weekly over a total of 12 weeks. One year after experimental WHV infection, woodchucks were challenged by inoculation with 200 μl of autologous serum, containing 109 woodchuck ID50. This serum was obtained during the highly viremic phase of acute, self-limited WHV infection (i.e., 4 to 5 weeks after experimental infection).
RESULTS
Kinetics of WHV serological markers, WHV DNA, and T-cell responses to viral antigens during acute, self-limited WHV infection.
Markers of WHV infection and T-cell proliferation in response to WHV antigens were analyzed in four experimentally WHV-infected adult woodchucks (NW7029, NW7030, NW7031, and NW7032) to characterize the appearance and duration of humoral and cellular immune responses to WHsAg, rWHcAg, and WHcAg-related peptides during the incubation period and the early phase of acute WHV infection. Humoral and cellular immune responses were determined weekly for a total of 12 weeks.
Markers of WHV infection.
Woodchucks NW7029, NW7030, and NW7032 showed a similar pattern of viral markers, i.e., appearance and duration of viremia (WHV DNA, WHsAg) and onset of convalescence (anti-WHs and anti-WHc). WHV DNA and WHsAg were detected in the serum from week 2 or 3 to week 7 (Fig. 1). Anti-WHc and anti-WHs were detected in the serum beginning at week 4 or weeks 5 to 6, respectively, and continued throughout the study (more than 52 weeks). A positive signal for anti-WHc at week 1 is a result of infection with an inoculum containing a high titer of anti-WHc. The SDH level in serum increased from normal values (86 to 182 IU) beginning at week 4 and reached its peak value at week 7 (1,185 to 1,325 IU). The SDH activity subsequently decreased and returned to normal values by week 9.
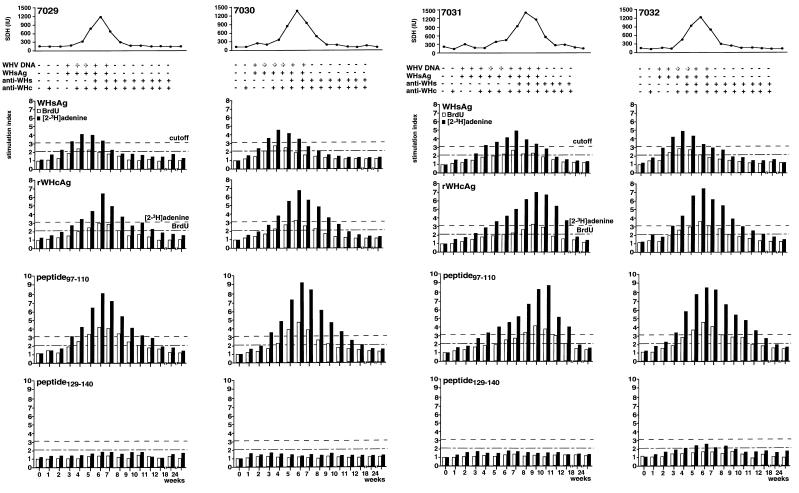
FIG. 1.
Humoral and cellular immune responses of woodchucks NW7029, NW7030, NW7031, and NW7032 during an acute, self-limited WHV infection. T-cell responses to WHsAg, rWHcAg, peptide 97–110, and peptide 129–140 were monitored during WHV infection with 105 woodchuck ID50. WHsAg, anti-WHs, and anti- WHc in the sera were detected by ELISA. SDH levels were assessed by a commercial enzyme assay. Viremia was detected by DNA dot blot (boldface +) and PCR (lightface +). T-cell responses (5 × 104 PBMC) after stimulation with 2 μg of WHsAg per ml or 1 μg of rWHcAg or peptides per ml were analyzed weekly after infection by BrdU and [2-3H]adenine incorporation. Results are presented as mean SI of triplicate determinations. The mean values (optical density at 450 nm) for the controls were 0.22 ± 0.08. The mean cpm for the controls was 3,213 ± 1,722.
The pattern of viremia and increase in SDH levels were different in woodchuck NW7031 from those in the other three animals (Fig. 1). Although the onset of viremia was similar, WHV DNA and WHsAg were detected in the serum of NW7031 for an additional 3 weeks. Anti-WHc was detected in serum beginning at week 4, but anti-WHs was not detected until week 8. Both antibodies were then detected throughout the remainder of the study. SDH levels began to increase at weeks 5 to 6 (212 IU) to a peak level at week 8 (1,219 IU) and declined to normal values by week 11.
Cellular immune responses to WHV antigens. (i) T-cell response to WHsAg.
By using both a BrdU assay and a [2-3H] adenine incorporation assay, the first T-cell response detected was directed against WHsAg in woodchucks NW7029, NW7030, and NW7032 (Fig. 1). This T-cell response was detected 3 weeks after experimental infection and correlated with the detection of WHV DNA and WHsAg in the serum as well as with an increase in SDH levels. A maximum T-cell response to WHsAg was observed at week 4. The mean SI was 2.3 to 2.6 in the BrdU assay and 4.2 to 4.9 in the [2-3H]adenine assay. By weeks 5 to 6, the T-cell response to WHsAg decreased, and it was below the cutoff value by week 8. The decrease in the T-cell response to WHsAg coincided with the detection of anti- WHs in serum (weeks 5 to 6). Clearance of WHV DNA (week 8) and normalization of SDH levels (week 9) followed shortly. Some differences in the course of the T-cell response to WHsAg were evident between woodchuck NW7031 and the other three animals (Fig. 1). Depending on the assay, a T-cell response to WHsAg was detected 1 to 3 weeks later in the course of infection beginning on weeks 4 to 6. The T-cell response reached its maximum level 7 weeks after infection (mean SI, 2.5 in the BrdU assay and 4.9 in the [2-3H]adenine assay). Similar to the other three animals, the T-cell response decreased afterwards (weeks 8 and 9), coincident with the detection of anti-WHs (week 8). Clearance of WHV DNA and normalization of SDH levels were observed at week 11.
As a control for the observed specific T-cell response to WHsAg that was purified from the sera of chronically WHV-infected woodchucks, the studied animals were tested in parallel for their response to pooled serum from WHV-negative woodchucks. Neither the four woodchucks (NW7029, NW7030, NW7031, and NW7032 [data not shown]) nor several WHV-negative woodchucks (Table 1) showed a T-cell response to these serum in the [2-3H]adenine assay. Stimulation of PBMC from WHV-negative woodchucks with WHsAg led to no proliferative response (Table 1).
TABLE 1.
PBMC response of WHV-negative woodchucks to serum, WHV antigens, and WHcAg peptides
| Peptide | PBMC response (mean SI) of WHV-negative woodchucka:
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| WHV-negative serum | 1.1 | 1.1 | 1.0 | 1.2 |
| WHsAg | 1.3 | 1.2 | 1.0 | 1.1 |
| rWHcAg | 1.6 | 1.7 | 2.0 | 2.5 |
| Peptide 97–110 | 1.7 | 1.5 | 1.5 | 1.6 |
| Peptide 129–140 | 1.5 | 1.3 | 1.5 | 1.4 |
Responses to medium supplemented with 10% pooled serum from WHV-negative woodchucks, 2 μg of WHsAg per ml, 1 μg of rWHcAg per ml, 1 μg of peptide 97–110 per ml, and 1 μg of peptide 129–140 per ml were analyzed by [2-3H]adenine incorporation. PBMC (5 × 104) were obtained from WHV-negative woodchucks. The results are presented as mean SI of triplicate determinations. The mean cpm for the controls was 2,615 ± 1,473.
(ii) T-cell response to rWHcAg.
The T-cell responses to rWHcAg in woodchucks NW7029, NW7030, and NW7032 were detected 1 week after the T-cell response to WHsAg (Fig. 1). The T-cell response to rWHcAg reached maximal levels at week 6 and decreased thereafter (weeks 7 to 9). The maximum mean SI was 2.9 to 3.3 in the BrdU assay and 6.8 to 7.5 in the [2-3H]adenine assay. The maximum T-cell response appeared 2 weeks later but was stronger than the T-cell response to WHsAg. It appeared that the increase, maximum, and decrease in the T-cell response to WHcAg coincided with the detection of anti-WHc in the serum beginning at week 4. The maximum T-cell response to rWHcAg in woodchuck NW7031 occurred later during the course of acute infection (week 9) than did the response to WHsAg (Fig. 1). However, the maximum T-cell response was similar to that in the other three woodchucks (SI = 3.2 in the BrdU assay and 7.3 in the [2-3H]adenine assay) and a decrease in the T-cell response to rWHcAg was observed at weeks 10 to 12. As with the other animals, anti-WHc was detected in the serum beginning at a point coincident with the maximum rWHcAg-specific T-cell response.
Stimulation of PBMC from WHV-negative woodchucks with rWHcAg led to no significant proliferative response (Table 1).
(iii) T-cell response to WHcAg-derived peptides.
In addition to the T-cell responses to WHsAg and rWHcAg, the T-cell response to the peptide from amino acids 97 to 110 (peptide 97–110) representing an immunodominant epitope of the WHcAg (23), was monitored. As a control for the peptide-specific proliferative response, based on previous experiments (23), the WHcAg peptide 129–140 was used. Stimulation of PBMC from WHV-negative woodchucks with peptide 97–110 or 129–140 resulted in no T-cell response (Table 1).
T-cell responses to peptide 97–110 were detected in woodchucks NW7029, NW7030, and NW7032 3 to 4 weeks after infection (Fig. 1). A maximum T-cell response was seen at week 6 and decreased thereafter (weeks 7 to 10). The maximum mean SI was 4.2 to 4.9 in the BrdU assay and 8.2 to 9.1 in the [2-3H]adenine assay.
A T-cell response to peptide 97–110 in woodchuck NW7031 became detectable at weeks 4 to 6 (Fig. 1). The maximum T-cell response was seen at weeks 9 to 10 (mean SI = 4.2 in the BrdU assay and 8.6 in the [2-3H]adenine assay). As expected, stimulation with peptide 129–140 did not result in T-cell proliferation in the tested animals at any time during acute WHV infection (Fig. 1). Based on these results, we decided to assess T-cell responses in the following experiments, e.g., of polyclonal T-cell lines or after WHV reinoculation, only by [2-3H]adenine incorporation.
Recognition of WHcAg epitopes during acute WHV infection and convalescence.
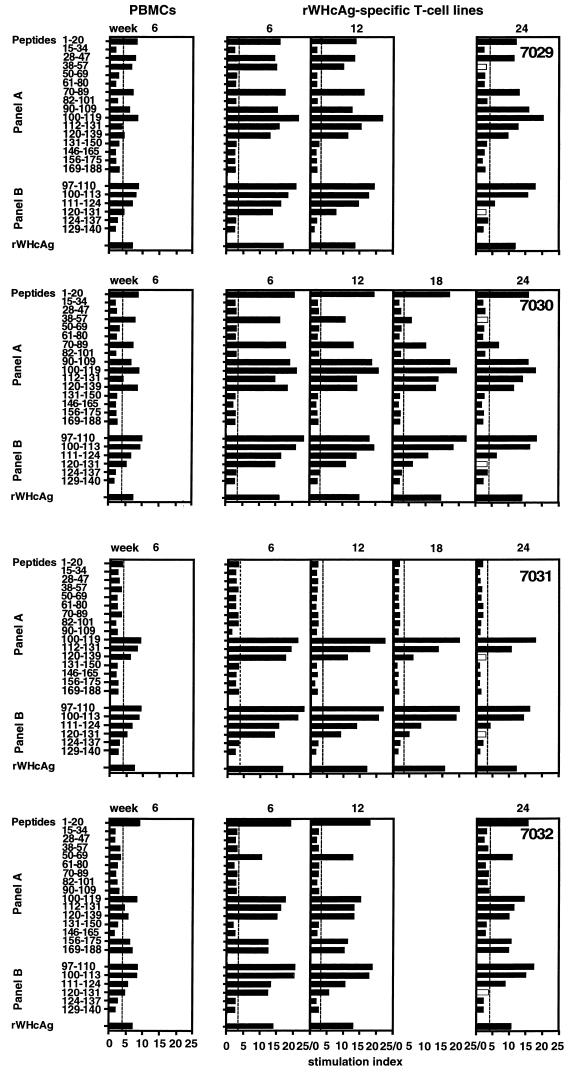
Epitopes recognized by Th cells are major histocompatibility complex (MHC) class II restricted (42), and it has previously been shown that T cells of outbred woodchucks recognize different epitopes of WHcAg (23). However, one epitope located between amino acids 97 and 110 appeared promiscuous, since it has so far been recognized by all the woodchucks during acute self-limited WHV infections. PBMC from experimentally WHV-infected animals were stimulated with rWHcAg-related peptides 6 weeks after infection. It was found that PBMC from woodchucks NW7029, NW7030, and NW7032 recognized six or seven WHcAg peptides of panel A (peptides 1–20, 28–47, 28–57, 70–89, 90–109, 100–119, 112–131 and 120–139) whereas woodchuck NW7031 showed PBMC proliferation only to three peptides of panel A (Table 2). Four peptides of panel B (peptides 97–110, 100–113, 111–124, and 120–131) induced proliferation of PBMC from the four woodchucks tested, whereas several peptides from panel A (peptides 15–34, 50–69, 61–80, 82–101, 131–150, and 146–165) were not stimulatory for PBMC from any of these animals.
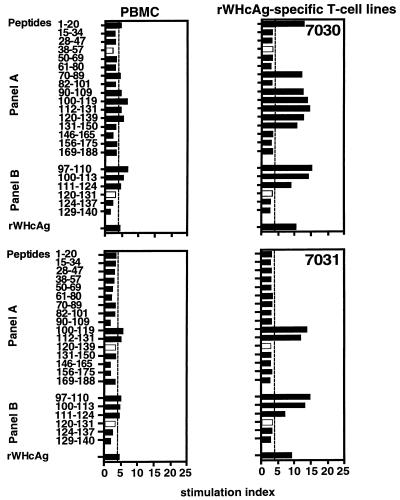
Comparing epitopes which were recognized by PBMC and by T cells of rWHcAg-specific polyclonal T-cell lines 6 weeks after experimental infection, we demonstrated that the number of peptides recognized did not differ generally (Fig. 2). Two exceptions were found: the T cells of woodchucks NW7029 and NW7032 recognized only peptide 112–131 and 50–69, respectively.
FIG. 2.
T-cell responses of PBMC 6 weeks after experimental WHV infection and of rWHcAg-specific T-cell lines established at 6, 12, 18, and 24 weeks postinfection to 1 μg of rWHcAg or WHcAg peptides per ml were analyzed by [2-3H]adenine incorporation. PBMC (5 × 104) were obtained from woodchucks NW7029, NW7030, NW7031, and NW7032 6 weeks after infection. T cells (5 × 104) were derived from polyclonal T-cell lines established at weeks 6, 12, 18, and 24 by continuous stimulation with rWHcAg. Results are presented as mean SI of triplicate determinations. The mean cpm for the controls was 7,043 ± 3,368.
To test whether the repertoire of epitopes recognized during the incubation period, the acute phase, and convalescence varied, rWHcAg-specific polyclonal T-cell lines were established at weeks 6, 12, 18 (at this time only from woodchucks NW7030 and NW7031), and 24 after experimental WHV infection. These lines were stimulated with peptides of both panel A and B, and it was found that all stimulatory peptides recognized by rWHcAg-specific T cells of woodchucks NW7029, NW7030, and NW7032 at week 6 also induced T-cell responses during the following weeks, although their stimulatory effects decreased during convalescence. Only two peptides (peptides 38–57 and 120–131) did not induce the stimulation of T-cell lines from these woodchucks at week 24.
rWHcAg-specific T cells derived from woodchuck NW7031 were stimulated only by peptides 100–119 and 112–131 at weeks 12 to 24 (Fig. 2). The stimulatory effects of peptides 120–131 and 120–139 observed at week 6 induced no specific T-cell responses at week 24.
As expected, rWHcAg was always stimulatory for T cells derived from all woodchucks throughout the course of the acute, self-limited WHV infection (Fig. 2), and WHsAg or BSA induced no stimulatory effects on these rWHcAg-specific T-cell lines (data not shown).
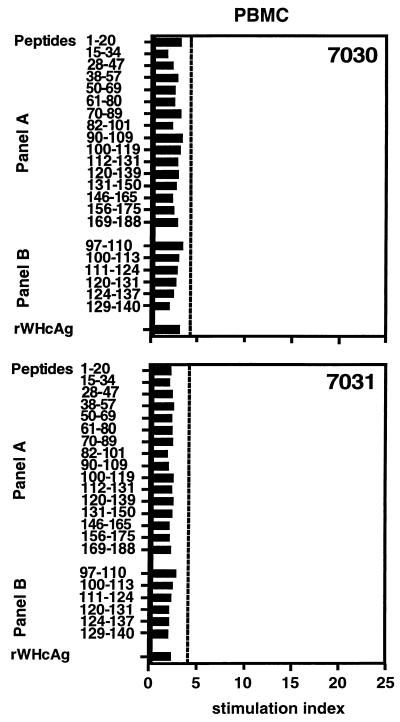
T-cell response to WHV antigens 1 year after infection.
During the acute phase of WHV infection (week 6), PBMC of all woodchucks recognized WHsAg, rWHcAg, and certain WHcAg epitopes. To determine whether these woodchucks possess a long-lasting cellular immune response, PBMC from woodchucks NW7030 and NW7031 were stimulated with viral proteins and WHcAg peptides 1 year after the experimental WHV infection. At this time, both animals were positive for anti-WHs and anti-WHc and negative for WHsAg. WHV DNA was detectable neither in the sera nor in the PBMC by nested PCR (data not shown). Furthermore, PBMC of both animals were not specifically stimulated by any of the previous recognized epitopes or rWHcAg (SI ≤ 3.1) (Fig. 3).
FIG. 3.
T-cell responses of PBMC 1 year after experimental WHV infection. PBMC (5 × 104) were obtained from woodchucks NW7030 and NW7031 and stimulated with 1 μg of rWHcAg or WHcAg peptides per ml. Proliferation was determined by [2-3H]adenine incorporation. Results are presented as mean SI of triplicate determinations. The mean cpm for the controls was 3,015 ± 1,968.
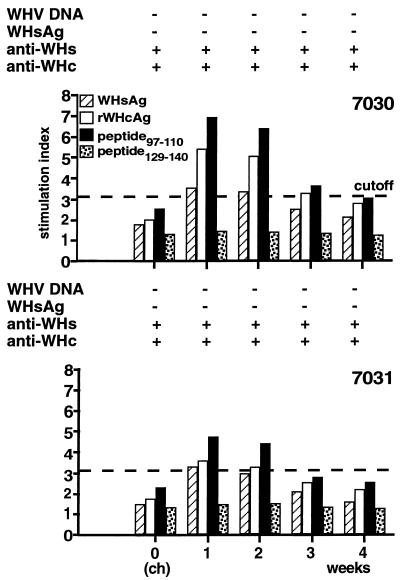
T-cell response to WHV antigens after viral challenge.
At 1 year after experimental WHV infection, woodchucks NW7030 and NW7031 were challenged with a higher dose of WHV than for the primary experimental infection to analyze whether reinfection and/or reactivation of a cellular immune response to WHV antigens and WHcAg epitopes may occur. Before challenge (week zero), the animals showed no specific T-cell responses to WHsAg, rWHcAg, or peptide 97–110 (Fig. 4). At 1 week after WHV challenge, T-cell responses to WHsAg, rWHcAg, and peptide 97–110 were detected. For woodchuck NW7030 the SI was 3.4 for WHsAg, 5.3 for rWHcAg, and 6.9 for peptide 97–110, and for woodchuck NW7031 the SI was 3.2, 3.4, and 4.7, respectively. Control peptide 129–140 did not stimulate T cells from either animal. T-cell responses were detected for 2 weeks and decreased thereafter, approaching cutoff values by week 4. At the time of viral challenge, both woodchucks were positive for anti-WHs and anti-WHc and negative for WHsAg. WHV DNA and WHsAg were not detectable in the sera by nested PCR or ELISA, respectively, at the time of challenge and thereafter.
FIG. 4.
Humoral and cellular immune responses of woodchucks NW7030 and NW7031 after viral challenge. T-cell responses to WHsAg, rWHcAg, peptide 97–110, and peptide 129–140 were analyzed after challenge with 109 woodchuck ID50. WHsAg, anti-WHs, and anti-WHc in the sera was detected by ELISA. Viremia was tested by nested PCR (top). T-cell responses (5 × 104 PBMC) to stimulation with 2 μg of WHsAg per ml or 1 μg of rWHcAg or peptides per ml were assayed each week postchallenge (ch) by [2-3H]adenine incorporation (bottom). The mean cpm for the controls was 2,976 ± 1,711.
Peptide stimulation of PBMC and T cells of rWHcAg-specific T-cell lines obtained 1 week after viral challenge confirmed the reactivation of the cellular immune response and also showed a loss of recognition of certain WHcAg epitopes (peptides 38–57, 120–131, and 120–139) (Fig. 5). T cells obtained from both animals after viral challenge recognized the same subset of WHcAg epitopes that were recognized during the period of an acute WHV infection (Fig. 2). Furthermore, the same WHcAg peptides, which no longer induced T-cell proliferation during the convalescence period (week 24 [Fig. 2]), also remained unstimulatory after challenge with WHV (Fig. 5).
FIG. 5.
T-cell responses of PBMC (5 × 104) and rWHcAg-specific T-cell lines (5 × 104) 1 week after viral challenge to 1 μg of rWHcAg or WHcAg peptides per ml were analyzed by [2-3H]adenine incorporation. PBMC were obtained from woodchucks NW7030 and NW7031 1 week postchallenge. T cells were derived from polyclonal T-cell lines established 1 week after viral challenge by continuous stimulation with rWHcAg. Results are presented as mean SI of triplicate determinations. The mean cpm for the controls was 4,691 ± 2,352.
DISCUSSION
Appropriate Th and cytotoxic T-lymphocyte responses during the early phase of an acute HBV infection are regarded as crucial for determining either recovery from infection or progression to chronicity (6, 13). However, studies on the earliest immunological events occurring after HBV infection in humans are limited because monitoring first becomes possible only at the onset of symptoms, not immediately after exposure. Thus, studies in animal models are required to examine the asymptomatic incubation period of hepadnavirus infection. The experimental WHV infection of woodchucks is an excellent model with which to study cellular and humoral immune responses during the earliest phase after inoculation and to determine their role in disease progression.
In this study, T-cell responses to viral proteins and peptides after experimental WHV infection of adult woodchucks were examined. T-cell responses were assessed by BrdU and [2-3H] adenine incorporation (20, 23) due to low [3H]thymidine uptake by woodchuck PBMC (22). The molecular mechanism by which BrdU that is used as a thymidine analog in proliferation assays is incorporated into cellular DNA has not yet been characterized and needs further investigation (25). For the kinetics of T-cell responses after infection, we used both assays in parallel to compare their sensitivity (25). The response of woodchuck T-cells to WHsAg, rWHcAg, and WHcAg peptides could be monitored by both assays (Fig. 1). However, the [2-3H]adenine assay had higher sensitivity in that the T-cell responses were detected up to 3 weeks earlier and up to 2 weeks later than by the BrdU assay (Fig. 1).
The first T-cell responses were directed against WHsAg and were detected 3 weeks after WHV inoculation (Fig. 1). The maximum T-cell response to WHsAg occurred when WHsAg was detected in serum and decreased upon seroconversion to anti-WHs. The development of anti-WHs, which is regarded as a virus-neutralizing antibody (40), was associated with the elimination of WHsAg from serum. These findings suggest that T-cell responses to WHsAg occur prior to liver cell damage, as shown by the presence of normal SDH levels in serum (Fig. 1). WHsAg may be secreted at high abundance early after infection and therefore is the first antigen presented to T cells by MHC class II molecules of APC. In addition, the differential abundance of WHsAg and WHcAg could explain why T-cell responses to rWHcAg and the corresponding immunodominant epitope (peptide 97–110) became detectable later than the WHsAg-specific T-cell response (Fig. 1).
The T-cell response in woodchucks to recombinant WHV core protein was similar to the response observed in HBV-infected patients, whose HBcAg-specific T-cell responses were stronger and occurred later during the course of infection than did the HBsAg-specific T-cell responses (12, 17, 21). This was likely for the stronger T-cell proliferation to major epitopes of the HBcAg compared to the entire rHBcAg. In all four woodchucks, stimulation of T cells with peptide 97–110 resulted in a greater proliferation than with rWHcAg. The maximum T-cell responses to rWHcAg and peptide 97–110 coincided with the peaks of SDH activities.
The more pronounced T-cell response to rWHcAg than to WHsAg may depend on the higher immunogenicity of this internal viral protein (23, 28). The vigorous T-cell response to WHcAg epitopes (e.g., peptide 97–110) is probably based on their linear features facilitating internalization and presentation by MHC class II molecules of APC.
The T-cell responses to the peptides described previously (23) and used in this study indicate that a variety of epitopes, located throughout the entire core protein, are recognized. The subset of WHcAg epitopes recognized by each animal was different, however (Table 2) (23). One reason for this may be that the animals used in these studies were outbred and unrelated to each other in terms of MHC. This was confirmed by a one-way mixed lymphocyte reaction (data not shown) and characterization of MHC class I patterns (unpublished results). Peptide 97–110 was recognized by all four woodchucks tested in this study, strengthening previous findings that this epitope is promiscuous and immunodominant (23). So far, epitopes covering amino acids 15 to 34, 50 to 69, 61 to 80, 82 to 101, 131 to 150, and 146 to 165 were not recognized (Table 2).
Proliferation of PBMC and of T cells from rWHcAg-specific polyclonal T-cell lines to rWHcAg- and WHcAg-related peptides demonstrated that the rWHcAg, the immunodominant epitope (peptide 97–110), and other epitopes (peptides 100–119, 112–131, and 120–139) were commonly recognized during the early phase of acute WHV infection by all woodchucks included in this study (week 6) (Fig. 2; Table 2). However, the pattern of peptide-induced T-cell response and its magnitude varied among all the woodchucks tested (Table 2). T-cell responses of woodchucks NW7029, NW7030, and NW7032 were directed against five to eight WHcAg epitopes, whereas woodchuck NW7031 showed responses to only three epitopes (Fig. 2). In the woodchuck model, immunological factors such as MHC class II patterns, MHC restriction, and cytokine expression have not been defined; therefore, mechanisms that may have led to the delay in the humoral and cellular immune response in woodchuck NW7031 but resulted in the resolution of WHV infection remain unknown.
T-cell responses to rWHcAg and several epitopes were present beginning 6 weeks after experimental WHV infection and continued to be detected throughout convalescence (weeks 12, 18, and 24). Similar to results with rWHcAg, peptide 97–110 and peptides 100–113, 100–119, and 112–131 were recognized by the T cells of all woodchucks throughout this study. Further WHcAg epitopes, e.g., peptides 1–20, 70–89, and 90–109, which were recognized by woodchucks NW7029, NW7030, or NW7032 at week 6 were also stimulatory at week 24. T-cell proliferation in response to additional WHcAg epitopes during the follow-up was not observed. However, several peptides, e.g., peptides 38–57, 120–131, and 120–139 which induced a strong T-cell response at weeks 6 to 18, were no longer stimulatory for T cells after convalescence (week 24). Consistent responses to different epitopes during a 24-week follow-up have not been detected in studies on human patients (9, 17).
Recent studies have shown a long-term persistence of HBV-specific Th-cell and CTL responses in patients after recovery from HBV infection (30, 34). These results may be due to T-cell memory after initial development of a vigorous T-cell response which led to resolution of HBV infection. They may also be due to the continuous stimulation by residual virus, replicating at very low levels in extrahepatic sites, as demonstrated by the detection of HBV DNA in the sera and/or PBMC of some patients. In contrast to these findings, WHV-specific T-cell responses or WHV DNA were not detected 1 year after the acute self-limited WHV infection in the four woodchucks tested (Fig. 3). This could be due to the short recovery period after infection or to the limited number of animals. However, a decrease in the Th-cell responses to HBV nucleocapsid antigens has been observed in acutely HBV-infected patients simultaneously with or shortly after resolution of infection (11, 12). Especially if HBV DNA was not detected in the serum, the Th-cell and CTL responses to HBV proteins were low (30, 34). These results are explained by the downregulation of the frequency of effector cells toward the end of a successful immune response (1, 38).
The woodchuck model presents a unique opportunity to investigate the cellular immune response upon reexposure to hepadnavirus infection. To test for the presence of memory T cells, which may become reactivated upon challenge with the virus, two animals were inoculated with 109 woodchuck ID50 1 year after recovery from the initial infection. T-cell proliferation in response to WHV antigens was demonstrated 1 week after challenge in both animals (Fig. 4). This T-cell response was detected for 2 to 3 weeks and decreased thereafter. Although circulating anti-WHs may have neutralized most virus particles, a small number of hepatocytes could have been infected, resulting in a limited and low-level viral replication that was responsible for the reactivation of T cells. Interestingly, the reactivated T-cell responses were directed against the same WHcAg epitopes observed during convalescence after the primary WHV infection (Fig. 2 and 5). The similar pattern of T-cell responses found in these woodchucks suggests a hierarchy of specificity in epitope recognition that is maintained unchanged after convalescence until reexposure to the virus. In conclusion, these results demonstrate the development of T-cell responses in the woodchuck directed against WHsAg, rWHcAg, and distinct epitopes of the WHcAg during the early phase of infection and show that they are closely associated with viral clearance and are retained after convalescence, contributing to long-lasting immunity.
ACKNOWLEDGMENTS
We gratefully acknowledge the helpful discussion and critical comments of M.-C. Jung (University of Munich, Munich, Germany) and of J. R. Jacob and B. C. Tennant (Cornell University, Ithaca, N.Y.).
This work was supported by ‘Deutsche Forschungsgemeinschaft’ grant Ro 687/6-1.
REFERENCES
- 1.Akbar A N, Salmon M, Savill J, Janossy G. A possible role for bcl-2 in regulating T-cell memory: a “balancing act” between cell death and survival. Immunol Today. 1993;14:526–532. doi: 10.1016/0167-5699(93)90181-J. [DOI] [PubMed] [Google Scholar]
- 2.Asada M, Galambos J T. Sorbitol dehydrogenase and hepatocellular injury: an experimental and clinical study. Gastroenterology. 1963;44:578–584. [Google Scholar]
- 3.Bertoletti A, Sette A, Chisari F V, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T-cells. Nature (London) 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 4.Bertoletti A, Ferrari C, Fiaccadori F, Penna A, Margolskee R, Schlicht H J, Fowler P, Guilhot S, Chisari F V. HLA class-I human cytotoxic T-cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci USA. 1991;88:10445–10449. doi: 10.1073/pnas.88.23.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buendia M A. Hepatitis B viruses and liver cancer: the woodchuck model. Symp Soc Gen Microbiol. 1994;51:183–187. [Google Scholar]
- 6.Chisari F V, Ferrari C. Viral hepatitis. In: Nathanson N, et al., editors. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 745–778. [Google Scholar]
- 7.Cote P J, Gerin J L. In vitro activation of woodchuck lymphocytes measured by radiopurine incorporation and interleukin-2 production: implications for modeling immunity and therapy in hepatitis B virus infection. Hepatology. 1995;22:687–699. [PubMed] [Google Scholar]
- 8.Cote P J, Gerin J L. The woodchuck as a model of hepadnavirus infection, pathogenesis and therapy. FORUM Trends Exp Clin Med. 1996;6:131–159. [Google Scholar]
- 9.Diepolder H M, Jung M-C, Wierenga E, Hoffmann R M, Zachoval R, Gerlach T J, Scholz S, Heavner G, Riethmueller G, Pape G R. Anergic TH1 clones specific for hepatitis B virus (HBV) core-specific CD4+ T cells in vitro. J Virol. 1996;70:7540–7548. doi: 10.1128/jvi.70.11.7540-7548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feitelson M A, Duan L-X. Hepatitis B virus x antigen in the pathogenesis of chronic infections and the development of hepatocellular carcinoma. Am J Pathol. 1997;150:1141–1157. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari C, Bertoletti A, Penna A, Cavalli A, Valli A, Missale G, Pilli M, Fowler P, Giuberti T, Chisari F V, Fiaccadori F. Identification of immunodominant T-cell epitopes of the hepatitis B virus nucleocapsid antigen. J Clin Invest. 1991;88:214–222. doi: 10.1172/JCI115280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari C, Penna A, Bertoletti A, Valli A, Degli Antoni A, Giuberti T, Cavalli A, Petit M A, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- 13.Franco A, Ferrari C, Sette A, Chisari F V. Viral mutations, TCR antagonism and escape from the immune response. Curr Opin Immunol. 1995;7:524–531. doi: 10.1016/0952-7915(95)80098-0. [DOI] [PubMed] [Google Scholar]
- 14.Gerin J L, Cote P J, Korba B E, Miller R H, Purcell R H, Tennant B C. Hepatitis B virus and liver cancer: the woodchuck as an experimental model of hepadnavirus induced liver cancer. In: Hollinger F B, Lemon S M, Margolis H, editors. Viral hepatitis and liver disease. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 556–559. [Google Scholar]
- 15.Gerin J L, Faust R M, Holland P V. Biophysical characterization of the adr subtype of hepatitis B antigen and preparation of anti-r sera in rabbits. J Immunol. 1975;115:100–105. [PubMed] [Google Scholar]
- 16.Hornbuckle W E, Graham E S, Roth L, Baldwin B H, Wickenden C, Tennant B C. Laboratory assessment of hepatic injury in the woodchuck (Marmota monax) Lab Anim Sci. 1985;35:376–381. [PubMed] [Google Scholar]
- 17.Jung M-C, Diepolder H M, Spengler U, Wierenga E A, Zachoval R, Hoffmann R M, Eichenlaub D, Frösner G, Will H, Pape G R. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J Virol. 1995;69:3358–3368. doi: 10.1128/jvi.69.6.3358-3368.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung M-C, Spengler U, Schraut W, Hoffmann R, Zachoval R, Eisenburg J, Eichenlaub D, Riethmüller G, Paumgartner G, Ziegler-Heitbrock H W L, Will H, Pape G R. Hepatitis B virus antigen specific T-cell activation in patients with acute and chronic hepatitis B. J Hepatol. 1991;13:310–317. doi: 10.1016/0168-8278(91)90074-l. [DOI] [PubMed] [Google Scholar]
- 19.Kajino K, Jilbert A R, Saputelli J, Aldrich C E, Cullen J, Mason W S. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994;68:5792–5803. doi: 10.1128/jvi.68.9.5792-5803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreuzfelder E, Menne S, Ferencik S, Roggendorf M, Grosse-Wilde H. Assessment of peripheral blood mononuclear cell proliferation by 2[3H]adenine uptake in the woodchuck model. Clin Immunol Immunopathol. 1996;78:223–227. doi: 10.1006/clin.1996.0033. [DOI] [PubMed] [Google Scholar]
- 21.Löhr H F, Weber W, Schlaak J, Goergen B, Meyer zum Büschenfelde K-H, Gerken G. Proliferative response of CD4+ T-cells and hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology. 1995;22:61–68. doi: 10.1002/hep.1840220110. [DOI] [PubMed] [Google Scholar]
- 22.Maschke J, Menne S, Kreuzfelder E, Roggendorf M, Grosse-Wilde H. First evidence of low thymidine kinase activity in the woodchuck. Immunobiology. 1996;196:128. [Google Scholar]
- 23.Menne S, Maschke J, Tolle T K, Lu M, Roggendorf M. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J Virol. 1997;71:65–74. doi: 10.1128/jvi.71.1.65-74.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menne S, Maschke J, Klaes R, Grosse-Wilde H, Roggendorf M. Cellular immune response of woodchucks to woodchuck hepatitis virus surface protein during acute WHV infection. In: Rizzetto M, Purcell R H, Gerin J L, Verme G, editors. Viral hepatitis and liver disease. Turin, Italy: Edizioni Minerva Medica; 1997. pp. 453–457. [Google Scholar]
- 25.Menne S, Maschke J, Tolle T K, Kreuzfelder E, Grosse-Wilde H, Roggendorf M. Determination of peripheral blood mononuclear cell responses to mitogens and woodchuck hepatitis virus core antigen in woodchucks by 5-bromo-2′-deoxyuridine or 2[3H]adenine incorporation. Arch Virol. 1997;142:511–521. doi: 10.1007/s007050050097. [DOI] [PubMed] [Google Scholar]
- 26.Milich D R, Jones J E, Hughes J L, Maruyama T. Role of T-cell tolerance in the persistence of hepatitis B virus infection. J Immunother. 1993;14:223–226. doi: 10.1097/00002371-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Milich D R, Jones J E, Hughes J L, Price J, Raney A K, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci USA. 1990;487:6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milich D R, McLachlan A, Thornton G B, Hughes J L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T-cell site. Nature (London) 1987;329:547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- 29.Nayersina R, Fowler P, Guilhot S, Missale G, Cerny A, Schlicht H-J, Vitiello A, Chesnut R, Person J L, Redeker A G, Chisari F V. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993;150:4659–4671. [PubMed] [Google Scholar]
- 30.Penna A, Artini M, Cavalli A, Levrero M, Bertoletti A, Pilli M, Chisari F V, Rehermann B, Del Prete G, Fiaccadori F, Ferrari C. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest. 1996;98:1185–1194. doi: 10.1172/JCI118902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponzetto A, Cote P J, Ford E C, Purcell R H, Gerin J L. Core antigen and antibody in woodchucks after infection with woodchuck hepatitis virus. J Virol. 1984;52:70–76. doi: 10.1128/jvi.52.1.70-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popper H, Roth L, Purcell R H, Tennant B C, Gerin J L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci USA. 1987;84:866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popper H, Shih J W-K, Gerin J L, Wong D C, Hoyer B H, London W T, Sly D L, Purcell R H. Woodchuck hepatitis and hepatocellular carcinoma: correlation of histologic with virologic observations. J Hepatol. 1981;1:19–28. doi: 10.1002/hep.1840010202. [DOI] [PubMed] [Google Scholar]
- 34.Rehermann B, Ferrari C, Pasquinelli C, Chisari F V. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 35.Roggendorf M, Tolle T K. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology. 1995;38:100–112. doi: 10.1159/000150418. [DOI] [PubMed] [Google Scholar]
- 36.Roos S, Fuchs K, Roggendorf M. Protection of woodchucks from infection with woodchuck hepatitis virus (WHV) by immunization with recombinant core protein. J Gen Virol. 1989;70:2087–2095. doi: 10.1099/0022-1317-70-8-2087. [DOI] [PubMed] [Google Scholar]
- 37.Schödel F, Neckermann G, Peterson D, Fuchs K, Fuller S, Will H, Roggendorf M. Immunization with recombinant woodchuck hepatitis virus nucleocapsid antigen or hepatitis B virus nucleocapsid antigen protects woodchucks from woodchuck hepatitis virus infection. Vaccine. 1993;11:624–628. doi: 10.1016/0264-410x(93)90307-j. [DOI] [PubMed] [Google Scholar]
- 38.Sprent J. T and B cell memory. Cell. 1994;76:315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 39.Tennant B C, Gerin J L. The woodchuck model of hepatitis B virus infection. In: Arias I M, et al., editors. The liver: biology and pathobiology. New York, N.Y: Raven Press; 1994. pp. 1455–1466. [Google Scholar]
- 40.Tyler G V. Natural and experimental infections of woodchuck hepatitis virus. Ph.D. thesis. University of Pennsylvania; 1984. [Google Scholar]
- 41.Tyler G V, Snyder R L, Summers J. Experimental infection of the woodchuck (Marmota monax monax) with woodchuck hepatitis virus. Lab Invest. 1986;55:51–55. [PubMed] [Google Scholar]
- 42.Weiss S, Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991;64:767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- 43.Wiesner I S, Rawnsley H M, Brooks F P, Senior J R. Sorbitol dehydrogenase in the diagnosis of liver disease. Am J Dig Dis. 1965;10:147–151. doi: 10.1007/BF02236665. [DOI] [PubMed] [Google Scholar]