Abstract
Background
Postdural puncture headache has been traditionally viewed as benign, self-limited, and highly responsive to epidural blood patching (EBP) when needed. A growing body of data from patients experiencing unintended dural puncture (UDP) in the setting of attempted labor epidural placement suggests a minority of patients will have more severe and persistent symptoms. However, the mechanisms accounting for the failure of EBP following dural puncture remain obscure. An understanding of these potential mechanisms is critical to guide management decisions in the face of severe and persistent cerebrospinal fluid (CSF) leak.
Case presentation
We report the case of a peripartum patient who developed a severe and persistent CSF leak unresponsive to multiple EBPs following a UDP during epidural catheter placement for labor analgesia. Lumbar MRI revealed a ventral rather than dorsal epidural fluid collection suggesting that the needle had crossed the thecal sac and punctured the ventral dura, creating a puncture site not readily accessible to blood injected in the dorsal epidural space. The location of this persistent ventral dural defect was confirmed with digital subtraction myelography, permitting a transdural surgical exploration and repair of the ventral dura with resolution of the severe intracranial hypotension.
Conclusions
A ventral rather than dorsal dural puncture is one mechanism that may contribute to both severe and persistent spinal CSF leak with resulting intracranial hypotension following a UDP.
Keywords: Post-Dural Puncture Headache; Multimodal Imaging; analgesia; Injections, Spinal
Introduction
Neuraxial analgesia primarily through epidural analgesia is used by over 70% of patients undergoing labor in the USA, and epidural catheter placement is complicated by unintended dural puncture (UDP) in 0.5%–1% of obstetric patients.1 2 Following UDP, acute postdural puncture headache (PDPH) rates range from 50% to 80%. Many physicians mistakenly believe that the cerebrospinal fluid (CSF) leak following UDP is a benign, self-limited condition that typically remits spontaneously, and rarely causes long-term complications. However, the American Society of Anesthesiologists closed claims registry suggests that the third most common cause of lawsuits against anesthesiologists by obstetric patients was headache (approximately 12%) which may speak to the severity, impact, and indeed persistence of long-term head pain following accidental dural puncture.3 4
In addition, accumulating data suggests long-term headache following UDP may lead to persistent symptoms in up to a third of such patients despite epidural blood patch (EBP).5–9 The different potential mechanisms contributing to a chronic CSF leak following UDP despite technically successful EBP, are incompletely described in the literature. Here, we present the case of a patient with a UDP with persistent severe symptoms despite repeated EBP. In this patient, the mechanism, a persistent ventral dural puncture (rather than expected dorsal dural puncture), was definitively established, leading to effective care. The patient provided informed consent for the publication of her case, and to further preserve her anonymity all identifying information has been removed.
Case report
An otherwise healthy woman in her first pregnancy gave birth via an uncomplicated vaginal delivery at 39 weeks at an outside institution. Prior to her pregnancy the patients had a history of episodic tension type headaches approximately once per month, rated as 3 out 10 in severity, and responsive to treatment with ibuprofen. The patients was five foot five inches, 72 kg (body mass index of 26.4), did not have scoliosis, prior back surgery or other definable risk for difficult placement of the epidural. There was no trainee involved in the placement. Analgesia for her delivery was provided by placement of an epidural catheter requiring two attempts, and a dural puncture was not recognized. The patient did report dense numbness in her legs and lower body with the epidural and reported no sensation at all of her delivery including no sensation of pain. Headache was not reported at the time of the epidural placement, and the epidural was removed shortly after delivery. On the second postpartum day she reported a postural headache rated as 10 out of 10 in severity. The headache was treated with intravenous fluids, acetaminophen, intravenous hydromorphone, and caffeine and she was discharged home on the third day after her delivery. Five days postpartum, she returned to an outside emergency room with postural headache, dizziness, and neck pain. Imaging of her brain at that time demonstrated bilateral subdural hygromas (figure 1). She was given the first of two bedside epidural blood patches without imaging guidance. The patient was then admitted to an outside hospital intensive care unit for bedrest, a repeat bedside EBP on postpuncture day nine and multiple nerve blocks (bilateral occipital and sphenopalatine ganglion). She experienced no relief of symptoms for any amount of time with either blood patch. Her hospitalization was complicated by a generalized tonic clonic seizure for which she was started on levetiracetam. The patient was discharged on hospital day 5. She returned to the emergency room 1 week later with worsening complaints; imaging at that time demonstrated slight enlargement of her subdural collections, now with a small amount of acute blood products. The patient was admitted for observation and discharged the next day. The patient returned again to the emergency room 3 days later and was discharged again after pain control. Finally, almost 1 month after her delivery, she returned to the emergency room for severe headaches and nausea and a repeat CT scan of her head showed progressive enlargement of her subdural collections with increased hyperdense component. The patient was transferred to our institution at that time for a higher level of care.
Figure 1.
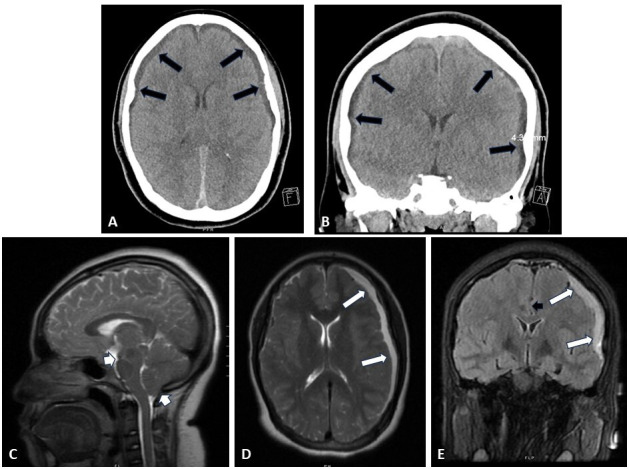
Cranial imaging both at initial presentation to an outside hospital emergency room (A and B) and 1 month later at our institution (C–E). (A and B) Axial (A) and coronal (B) CT imaging of the patient’s head on demonstrated bilateral subdural collections of heterogenous density, thicker on the left than the right (black arrows). (C–E) Midline sagittal (C), axial (D), and coronal (E) T2-weighted MRI of the patient’s brain on arrival to our institution demonstrated increased left sided subdural collection (white arrows) with midline shift (black arrowhead) as well as significant cerebellar and midbrain sagging (white arrowheads).
On arrival, the patient was neurologically intact, but in obvious discomfort. She was maintained on flat bedrest and her levetiracetam was increased to appropriate weight-based dosing. MRI of the patient’s brain and spine were obtained after arrival, demonstrating stable cranial subdural collections, and identifying a ventral epidural collection spanning multiple levels of her lumbar spine (figure 2A,B). To identify the exact location of the leak for surgical repair, the patient underwent a prone digital subtraction myelogram (DSM), which confirmed a site of ventral leakage at L3 (figure 2D,E). Given her previous failed EBP, progressing subdural collections with seizures, and confirmed ventral leak location, the decision was made to purse a surgical repair rather than further patching. A transdural repair of her ventral CSF leak was accomplished via a posterior transdural approach. Following an L3 laminectomy, the posterior dura was opened, and the ventral dura was inspected. A small elliptical defect in the ventral dura approximately 1–2 mm in length was noted which appeared consistent with a needle puncture site (figure 3). This site was sutured primarily with 6-0 Gore-Tex in a figure-of-eight suture without further placement of a graft over the defect. Postoperative brain MRIs showed improvement of her subdural collections and brain sagging (figure 4). She had gradual resolution of headache, with improvement first noted on postoperative day 5. She was discharged on postoperative day 7 without return of her preoperative headache. One month postoperatively the patient had no recurrence of positional headaches. At 2-year follow-up the patient reported some persistent lower extremity numbness dating from the time of the initial epidural placement but was otherwise functioning well.
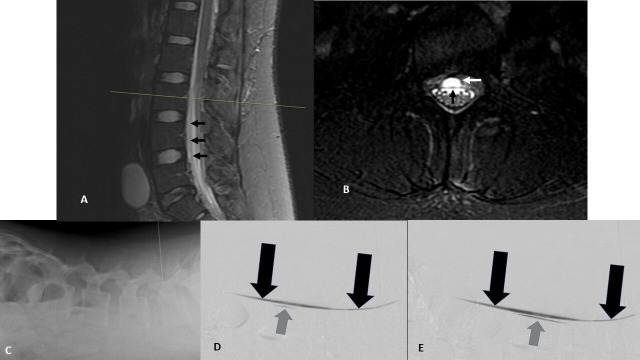
Figure 2.
Lumbar imaging confirming persistent ventral cerebrospinal fluid leak. (A and B) Midline sagittal (A) and axial (B, lumbar L3 level) T2-weighted fat suppressed MRIs. Black arrows show ventral dura (A and B). White arrow shows ventral epidural fluid collection (B). (C–E) Prone digital subtraction myelogram: (C) lateral image of lumbar spine with patient prone on cath lab table showing needle placement and demonstrating site of contrast injection. (D) Early postcontrast injection images show pooling of unmixed intrathecal contrast along the ventral dura (black arrows) and ventral extravasation of contrast (gray arrow) at L3. (E) Seconds-delayed postcontrast injection image shows further spread of ventral epidural contrast extending from the ventral dural defect (gray arrow).
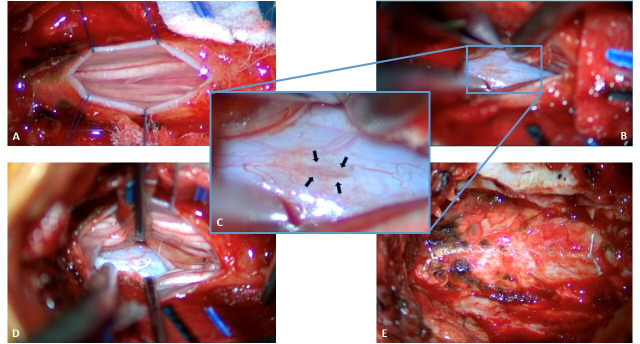
Figure 3.
Intraoperative imaging. (A) Dorsal dura has been opened in the midline and tacked open with 6-0 prolene sutures after an L3 laminectomy was performed. Cauda equina is visualized. (B) After careful inspection of visualizable ventral dura within the extent of our exposure, nerve roots have been retracted laterally and a defect is identified in the ventral dura (blue rectangle). (C) Magnified view of the ventral elliptical dural defect (black arrows show edges). (D) A 6-0 Gore-Tex suture has been used to primarily repair the ventral dural defect. (E) The dorsal dura has been repaired primarily with 6-0 Gore-Tex suture.
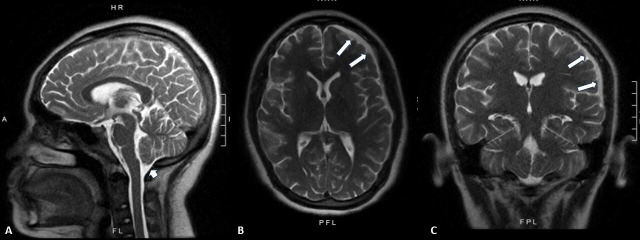
Figure 4.
Postoperative imaging. (A–C) Midline sagittal (A), axial (B), and coronal (C) T2-weighted MRI of the patient’s brain 1 week postoperatively demonstrating decreased left sided subdural collection (B and C: white arrows) with significantly decreased cerebellar sag (A: white arrowhead).
Discussion
Two complications of UDP have been increasingly appreciated: (1) the risk of early severe complications including subdural hematoma, cerebral venous thrombosis, and bacterial meningitis; and (2) the later risk of persistent headache and other symptoms of ongoing CSF leak.5–11 This patient experienced subdural hematomas, a generalized tonic clonic seizure, as well as severe ongoing headache in a persistent, progressive and deteriorating course for a month following UDP despite repeated intralaminar EBP. Seizure has been previously reported in both spontaneous CSF leak and PDPH but is most often reported to be associated with cerebral venous thrombosis which we did not detect in this case. Her condition continued to decline until a persistent ventral dural puncture was identified as the clear cause of her ongoing symptoms and resistance to treatment.
It is important for providers to develop a mechanism-based differential diagnosis for the persistence of CSF leak symptoms following UDP despite a properly done epidural blood patch. We and others have recently reported that a small persistent pseudomeningocele (dural bleb) can develop at the site of UDP and cause chronic CSF leak.12–14 In this situation, the punctured dura never heals in its prepuncture anatomic conformation. Instead, a friable inner arachnoid membrane effectively herniates through a persistent defect in the outer-tougher dura forming a millimeter-sized persistent aneurysmal defect of the thecal sac which may require surgery rather than EBP for definitive sealing. Another reported mechanism that can lead to a persistent postpuncture CSF leak is the formation of a CSF venous fistula, in which the puncture establishes a durable connection between the intrathecal space and an adjacent epidural vein.14 This allows escape of CSF directly into the venous system and is especially pernicious because there is no epidural collection on spine MRI to suggest ongoing CSF leak. In this report, we highlight a ventral dural puncture as a third mechanism for a persistent postpuncture CSF leak despite appropriate EBP following UDP. Collectively, these three potential mechanisms constitute the beginning of a differential diagnosis for persistent CSF leak symptoms following a dural puncture despite a properly done EBP and this differential diagnosis can guide efforts at diagnosis and treatment.
In this case, lumbar MRI revealed a T2 hyperintense collection in the ventral epidural space—anterior to the thecal sac rather than posterior to it—suggesting but not proving that the puncturing needle had traversed not only the dorsal dura, but crossed the thecal sac and punctured the ventral dura. A ventral collection on MRI suggested a puncture site on the ventral dura not readily accessible to blood administered in the dorsal epidural space during an intralaminar EBP which may stay confined to the dorsal epidural space.15 The risk of a ventral dural puncture leading to chronic leak symptoms has not gone completely unnoticed by experts in the field of CSF leak, and Schievink and Maya have reported on the occurrence of such ventral CSF leaks in a handful of patients with prolonged CSF leak following deliberate dural puncture in two separate reports.14 16 However, the occurrence of persistent ventral dural puncture following a UDP from an epidural has not been previously reported.
Given the severe nature of the patient’s symptoms, and progressive subdural collections despite repeated EBP, we felt the patient would be best served by definitive surgical closure of her CSF leak, rather than a further targeted blood or fibrin patch as described in the literature for less fulminant cases.14 However, a neurosurgeon cannot readily inspect the ventral dura to find a puncture site without first opening the dorsal dura to gain access to the ventral dura. DSM is a technique most commonly used to identify the location of ventral CSF leaks in patients with spontaneous CSF leaks, and proved critical in this case of iatrogenic CSF leak.17 The DSM, in which unmixed hyperdense contrast was allowed to pool intrathecally on the ventral dura while obtaining lateral video fluoroscopy using digital subtraction readily identified contrast escaping the thecal sac into the ventral epidural space. It both proved the occurrence of a ventral leak and also revealed the exact spinal location of that persistent dural defect. Confirmation of a ventral dural puncture and its location provided the surgical team with a compelling reason to go further than simply exposing, inspecting, and repairing the dorsal dura for any puncture sites, but rather to proceed with the more invasive and, otherwise, risky operation in which the dorsal dura was opened to allow inspection and repair of the ventral dura as well.
Conclusion
The present case demonstrates the workup, management, and surgical intervention of a persistent ventral dural puncture as a previously undescribed mechanism of severe and persistent CSF leak after epidural anesthesia. In the case of EBP failure, refractory cases should be more extensively investigated, as neurosurgical intervention may be indicated in the setting of ventral dural puncture to avoid deteriorating neurological status and function.
Footnotes
Contributors: All authors contributed to this case report, navigating the clinical decisions described and the research putting it into the context of the current literature. All provided input and critique on the final manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Butwick AJ, Bentley J, Wong CA, et al. United States state-level variation in the use of neuraxial analgesia during labor for pregnant women. JAMA Netw Open 2018;1:e186567. 10.1001/jamanetworkopen.2018.6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berger CW, Crosby ET, Grodecki W. North American survey of the management of dural puncture occurring during labour epidural analgesia. Can J Anaesth 1998;45:110–4. 10.1007/BF03013247 [DOI] [PubMed] [Google Scholar]
- 3. Sachs A, Smiley R. Post-dural puncture headache: the worst common complication in obstetric anesthesia. Semin Perinatol 2014;38:386–94. 10.1053/j.semperi.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 4. Davies JM, Posner KL, Lee LA, et al. Liability associated with obstetric anesthesia: a closed claims analysis. Anesthesiology 2009;110:131–9. 10.1097/ALN.0b013e318190e16a [DOI] [PubMed] [Google Scholar]
- 5. Webb CA-J, Weyker PD, Zhang L, et al. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg 2012;115:124–32. 10.1213/ANE.0b013e3182501c06 [DOI] [PubMed] [Google Scholar]
- 6. Ranganathan P, Golfeiz C, Phelps AL, et al. Chronic headache and Backache are long-term sequelae of unintentional dural puncture in the obstetric population. J Clin Anesth 2015;27:201–6. 10.1016/j.jclinane.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 7. Niraj G, Mushambi M, Gauthama P, et al. Persistent headache and low back pain after accidental dural puncture in the obstetric population: a prospective, observational, Multicentre cohort study. Anaesthesia 2021;76:1068–76. 10.1111/anae.15491 [DOI] [PubMed] [Google Scholar]
- 8. Niraj G, Critchley P. Management and outcomes of persistent headache after accidental dural puncture in the obstetric population: A 9-year prospective audit. Headache 2023;63:71–8. 10.1111/head.14447 [DOI] [PubMed] [Google Scholar]
- 9. Binyamin Y, Heesen P, Orbach-Zinger S, et al. Chronic pain in Parturients with an accidental dural puncture: A case-controlled prospective observational study. Acta Anaesthesiol Scand 2021;65:959–66. 10.1111/aas.13816 [DOI] [PubMed] [Google Scholar]
- 10. Lim G, Zorn JM, Dong YJ, et al. Subdural Hematoma associated with labor epidural analgesia: A case series. Reg Anesth Pain Med 2016;41:628–31. 10.1097/AAP.0000000000000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guglielminotti J, Landau R, Li G. Major neurologic complications associated with Postdural puncture headache in obstetrics: A retrospective cohort study. Anesth Analg 2019;129:1328–36. 10.1213/ANE.0000000000004336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callen AL, Lennarson P, Carroll IR. A causative role for remote dural puncture and resultant Arachnoid Bleb in New daily persistent headache: A case report. Headache 2023;63:981–3. 10.1111/head.14584 [DOI] [PubMed] [Google Scholar]
- 13. Roytman M, Ulrich CT, Chazen JL. Post-dural puncture Pseudomeningocele (‘Arachnoid Bleb’): an Underrecognized etiology of spontaneous intracranial hypotension Symptomatology. Clin Imaging 2021;80:377–81. 10.1016/j.clinimag.2021.08.023 [DOI] [PubMed] [Google Scholar]
- 14. Schievink WI, Maya MM, Moser FG. Digital subtraction Myelography in the investigation of post-dural puncture headache in 27 patients: technical NOTE. J Neurosurg Spine 2017;26:760–4. 10.3171/2016.11.SPINE16968 [DOI] [PubMed] [Google Scholar]
- 15. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA 2006;295:2286–96. 10.1001/jama.295.19.2286 [DOI] [PubMed] [Google Scholar]
- 16. Schievink WI, Maya MM. Ventral spinal cerebrospinal fluid leak as the cause of persistent post–dural puncture headache in children: report of 3 cases. J Neurosurg Pediatr 2013;11:48–51. 10.3171/2012.10.PEDS12353 [DOI] [PubMed] [Google Scholar]
- 17. Hoxworth JM, Patel AC, Bosch EP, et al. Localization of a rapid CSF leak with Digital subtraction Myelography. AJNR Am J Neuroradiol 2009;30:516–9. 10.3174/ajnr.A1294 [DOI] [PMC free article] [PubMed] [Google Scholar]