Abstract
Background
Missing outcome data (MOD) is a common problem in clinical trials and registries, and a potential bias when drawing conclusions from these data. Identifying factors associated with MOD may help to increase follow-up rates and assess the need for imputation strategies. We investigated MOD in a multicenter, prospective registry study of mechanical thrombectomy (MT) in large vessel occlusion ischemic stroke.
Methods
13 082 patients enrolled in the German Stroke Registry-Endovascular Treatment from May 2015 to December 2021 were analyzed with regard to MOD (90 day modified Rankin Scale, mRS). Univariate logistic regression analyses identified factors unbalanced between patients with and without MOD. Subgroup analyses were performed to identify patients for whom increased efforts to perform clinical follow-up after hospital discharge are needed.
Results
We identified 19.7% (2580/13 082) of patients with MOD at the 90 day follow-up. MOD was more common with higher pre-stroke disability (mRS 3–5, 32.2% vs mRS 0–2, 13.7%; P<0.001), absence of bridging intravenous thrombolysis, longer time to treatment, and in patients with high post-stroke disability at discharge (mRS 3–5 vs 0–2: OR 1.234 (95% CI 1.107 to 1.375); P<0.001). In contrast, MOD was less common with futile recanalization (thrombolysis in cerebral infarction (TICI) score of 0–2a, 12.4% vs TICI 2b–3, 15.0%; P=0.001). In patients discharged alive with well documented baseline characteristics, shorter hospital stay (OR 0.992 (95% CI 0.985 to 0.998); P=0.010) and discharge to institutional care or hospital (OR 1.754 (95% CI 1.558 to 1.976); P<0.001) were associated with MOD.
Conclusion
MOD in routine care MT registry data was not random. Increased efforts to perform clinical follow-up are needed, especially in the case of higher pre-stroke and post-stroke disability and discharge to hospital or institutional care.
Trial registration
Keywords: Stroke, Thrombectomy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Missing outcome data is a common problem in registry studies and may bias conclusions, especially in analyses based on complete cases.
WHAT THIS STUDY ADDS
We identified factors associated with missing 90 day follow-up in a large, multicenter registry of mechanical thrombectomy in acute stroke care.
Factors included high pre-stroke and post-stroke disability, absence of bridging intravenous thrombolysis and longer time to treatment, as well as shorter hospital stay and discharge to hospital or institutional care.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
Increased follow-up rates may then also improve the validity and generalizability of findings from routine care mechanical thrombectomy datasets.
Introduction
Mechanical thrombectomy (MT) is a highly effective treatment in large vessel occlusion (LVO) ischemic stroke, which has become the standard of care in acute stroke treatment. The efficacy of MT was initially proven in well defined patient cohorts who were most likely to benefit from MT, selected by clinical and imaging criteria.1 Therefore, certain patient populations were excluded from these trials. Among them were patients with large infarct volume at baseline, patients treated within the extended time window, elderly patients, and patients with relevant pre-stroke disability (modified Rankin Scale (mRS) score ≥2). MT has been studied under trial conditions in some of these populations since then (eg, to conclude on the efficacy of MT beyond 6 hours after symptom onset).2 However, patients treated with MT under real world conditions substantially differ from those fulfilling trial inclusion criteria.3 Multiple multicenter stroke registries have been established and observational data from clinical practice are increasingly used to address questions of MT efficacy and safety in distinct patient populations in order to individualize therapeutic recommendations and maximize patient benefit.4
While real world data collected in stroke registries are especially valuable for evaluation of MT in large cohorts under real world conditions, at the same time they are especially vulnerable to incomplete data. Clinical follow-up is typically conducted after a commonly used 90 day interval after initial stroke treatment. However, multicenter stroke registries report substantial numbers of patients with missing outcome data (MOD) at the 3 month follow-up, ranging between 8% and >20% of all registered cases.5–9 Loss to follow-up has been discussed as a factor limiting the validity and generalizability of trial and registry data in different areas of stroke research. MOD may also contribute to conflicting observations that fail to be replicated in randomized controlled trials or overestimate treatment effects. MOD depicts a potential risk for distortion of data, and different imputation strategies may result in great variance of estimated effect sizes.10–13
It is important to understand the mechanisms behind MOD, especially because missing observations, which are dependent on other variables or the missing variable itself, cannot be as easily addressed by adjusting or imputing strategies as MOD that occurs completely at random.14 Systematic MOD has been described in stroke patients (eg, by analyses reporting that (absence of) consent to follow-up within clinical studies is associated with distinct patient and stroke characteristics within a large longitudinal cohort of patients with carotid artery stenosis).15 We hypothesize that MOD in registry data of acute stroke care does not occur randomly, but needs to be acknowledged when interpreting routine-care MT data.
In this study, our aim was to investigate MOD in a nationwide, multicenter, prospective registry study of MT in clinical practice in Germany. We describe differences in baseline, stroke, and treatment characteristics between patients with a 90 day clinical follow-up available and those with MOD. Hence we aim to identify aspects in which registry data selected by complete case analysis may not fully depict real world care, which should be taken into account when drawing conclusions. We also provide factors associated with MOD in order to be acknowledged within the analysis plan of registry studies, to be accounted for by study design, or to consider appropriate methods of imputation.
To ensure conclusive and scientifically valid analyses of routine care MT data, MOD should be kept to a minimum. Therefore, our second aim was to identify factors associated with MOD at the 90 day follow-up in a subgroup of patients who were followed up until being discharged alive from the treating hospital. This could help to identify patient groups for whom increased effort to perform clinical follow-up is needed and so to facilitate higher follow-up rates, decreasing sources of bias.
Methods
Study population
Study protocols and procedures were conducted in compliance with the Declaration of Helsinki. The German Stroke Registry-Endovascular Treatment (GSR-ET) is an ongoing academic, independent, prospective, multicenter, observational registry study. Thirty certified German stroke centers consecutively enroll adult patients diagnosed with acute ischemic stroke due to LVO with the intention of being treated with MT. Baseline demographics, comorbidities, clinical and procedural information, as well as clinical follow-up after 90 days are recorded. Centers enrolling ≥90% of their MT treated patients could access the data for analysis. Queries were sent to the centers to increase follow-up rates. More detailed information on the registry’s study protocol and variables has been published previously.16 We included all 13 082 patients enrolled in the GSR-ET from July 2015 to December 2021 in our primary analysis.
Comparison groups and outcome parameters
MOD was defined as the main clinical outcome parameter (mRS at the 90 day follow-up) not being available in the database. Group comparison was performed for patients with MOD versus patients with mRS at the 90 day follow-up available (see also online supplemental figure 1). For the subgroup analysis, we excluded 2247 patients with documented inhouse mortality, 545 patients with an unknown discharge mRS score who had 90 day functional outcome available, and 874 patients with missing 90 day clinical outcome who had incomplete baseline documentation on basic patient, stroke, and treatment characteristics (age, sex, admission National Institutes of Health Stroke Scale (NIHSS) score, intravenous thrombolysis (IVT), and thrombolysis in cerebral infarction (TICI) scale score following mechanical thrombectomy) or were not followed up until discharge (mRS). These were compared with patients who had 90 day mRS available.
jnis-2023-020435supp001.pdf (185.4KB, pdf)
Statistical analysis
Data are presented as mean±SD, median (IQR), or proportions (categorical variables), unless indicated otherwise. Group comparison on the univariate level was performed with the Mann–Whitney U test or χ2 test as appropriate. In the subgroup analysis, binary logistic regression analyses were conducted to evaluate associations with higher odds of MOD after discharge from the treating hospital despite well documented baseline characteristics. A significant difference was considered as P<0.05. Statistical analyses were performed using SPSS (V.29, IBM, Armonk, New York, USA).
Results
Univariate group comparison of patients with missing versus available 90 day clinical outcome data
Baseline and stroke characteristics
A total of 13 082 patients were included in our analysis (median age 76.0 years, 51.4% women). Of these, 19.7% (n=2580) had MOD. In the univariate group comparison, patients with MOD were slightly younger (median age 76 (IQR 63.25–82) vs 76 (65–83) years; P=0.001) and more often had relevant premorbid disability, measured by a pre-stroke mRS score of >2 (32.2% (629/1,955) vs 13.7% (1,393/10,195); P<0.001, figure 1A). Stroke severity, measured by the NIHSS on admission, was lower in patients with MOD (mean NIHSS 13.8±7.1 vs 14.4±7.5; P=0.005). In patients with MOD, occlusion of the middle cerebral artery in the M1 segment was more frequent (55.8% (1366/2448) vs 51.1% (5242/10 252); P<0.001), whereas occlusion of the anterior cerebral artery was less frequent (1.8% (44/2448) vs 2.9% (294/10 252); P=0.003). All other vessel territories were equally common. Stroke etiology was unbalanced, with more strokes due to large artery atherosclerosis (33.9% (676/1997) vs 24.8% (2553/10 284)) and less cardioembolism (48.5% (968/1997) vs 50.6% (5204/10 284); P<0.001) in patients with MOD (table 1).
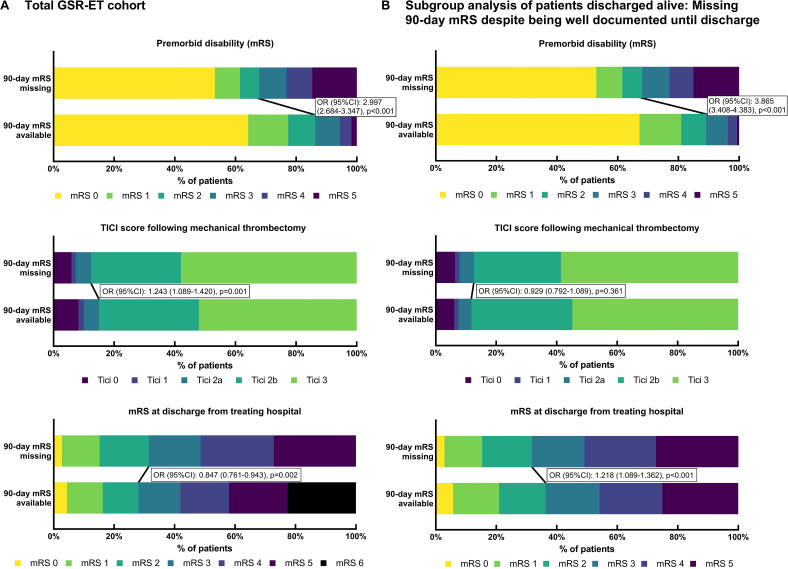
Figure 1.
Distribution of premorbid disability, recanalization status, and clinical outcome at discharge in cohorts of patients with 90 day missing clinical outcome data (MOD) versus those with data available. (A) Total German Stroke Registry-Endovascular Treatment (GSR-ET) cohort: MOD was associated with higher premorbid disability. Premorbid modified Rankin Scale (mRS) score was available in 10 195/10 502 (97.1%) patients with 90 day outcome available and in 1995/2580 (77.3%) patients with MOD. MOD was associated with successful recanalization (thrombolysis in cerebral infarction (TICI) scale score 2b–3); TICI was available in 10 173/10 502 (96.9%) patients with 90 day outcome available and in 2404/2580 (93.2%) patients with MOD. MOD was inversely associated with higher disability at discharge from the treating hospital as of mRS 3–6 versus 0–2; mRS at discharge was available in 9957/10 502 (94.8%) patients with 90 day outcome available and in 1858/2580 (72.0%) patients with MOD. (B) Subgroup analysis of patients discharged from hospital alive: comparison of patients with clinical outcome at 90 day follow-up available (n=7710) versus patients with MOD despite complete baseline documentation (age, sex, admission National Institutes of Health Stroke Scale score, intravenous thrombolysis, TICI, and mRS at discharge) (n=1706). MOD was associated with higher premorbid disability; premorbid mRS was available in 7606/7710 (98.7%) patients discharged from hospital alive with 90 day outcome available and in 1665/1706 (97.6%) patients with MOD despite complete baseline documentation. MOD was not significantly associated with successful recanalization (TICI 2b–3); TICI was available in 7537/7710 (97.8%) patients discharged from hospital alive with 90 day outcome available and in 1706/1706 (100.0%) patients with MOD despite complete baseline documentation. MOD was associated with higher disability at discharge from the treating hospital as of mRS 3–5 versus 0–2.
Table 1.
Patient, stroke, and treatment characteristics of patients with missing clinical outcome data and patients with clinical outcome data available
| Variable | mRS at 90 day follow-up available (n=10 502) |
mRS at 90 day follow-up missing (MOD) (n=2580) |
P value |
| Age (years) | 73.5±13.1 76 (65–83) |
72.4±13.7 76 (63.25–82) |
0.001 |
| Women | 51.4 (5389/10 494) | 51.6 (1329/2574) | 0.800 |
| Premorbid disability (mRS 3–5) | 13.7 (1393/10 195) | 32.2 (629/1955) | <0.001 |
| Cardiovascular risk factors | |||
| Arterial hypertension | 77.1 (7976/10 344) | 77.5 (1524/1966) | 0.691 |
| Diabetes mellitus | 22.7 (2339/10 311) | 21.7 (408/1876) | 0.372 |
| Dyslipidemia | 41.9 (4309/10 289) | 36.0 (660/1834) | <0.001 |
| Atrial fibrillation | 41.5 (4274/10 292) | 42.1 (784/1862) | 0.642 |
| Smoker (current) | 16.2 (1561/9654) | 18.9 (310/1636) | 0.005 |
| Baseline medication | |||
| Anticoagulation | 24.4 (2487/10 186) | 26.0 (458/1760) | 0.149 |
| Platelet inhibition | 30.5 (3111/10 186) | 31.0 (545/1760) | 0.722 |
| Stroke characteristics | |||
| NIHSS on admission | 14.4±7.5 14 (9–19) |
13.8±7.1 14 (9–18) |
0.005 |
| Location of occlusion | |||
| Carotid artery | 25.7 (2635/10 252) | 25.5 (625/2448) | 0.862 |
| ACA | 2.9 (294/10 252) | 1.8 (44/2448) | 0.003 |
| MCA M1 | 51.1 (5242/10 252) | 55.8 (1366/2448) | <0.001 |
| MCA M2 | 22.3 (2288/10 252) | 21.6 (528/2448) | 0.423 |
| PCA | 3.1 (315/10 252) | 2.5 (62/2448) | 0.157 |
| VB | 10.3 (1056/10 252) | 9.4 (231/2448) | 0.203 |
| Stroke etiology | <0.001 | ||
| Large artery atherosclerosis | 24.8 (2553/10 284) | 33.9 (676/1997) | |
| Cardioembolism | 50.6 (5204/10 284) | 48.5 (968/1997) | |
| Dissection | 1.8 (181/10 284) | 1.3 (25/1997) | |
| Other | 4.8 (490/10 284) | 4.5 (89/1997) | |
| Undetermined | 18.0 (1856/10 284) | 12.0 (239/1997) | |
| Treatment characteristics | |||
| Intravenous thrombolysis | 48.4 (5027/10 393) | 44.3 (1110/2507) | <0.001 |
| Primary admission at MT site | 60.0 (6013/10 015) | 61.8 (1481/2397) | 0.117 |
| Symptom onset/time to recognition-to-groin puncture (min) | 391.3±405.8 245 (165–450) |
421.2±651.4 275 (185–517) |
<0.001 |
| Door-to-groin puncture (min) | 100.8±158.6 70 (46–100) |
102.80±151.2 74 (48–105) |
0.009 |
| Outcome parameters | |||
| Successful reperfusion (TICI 2b–3) | 85.0 (8651/10 173) | 87.6 (2106/2404) | 0.001 |
| Good outcome at discharge (mRS 0–2) | 28.0 (2790/9957) | 31.5 (585/1858) | 0.002 |
| Disabled at discharge (mRS 3–5) | 49.4 (4920/9957) | 68.5 (1273/1858) | <0.001 |
| Inhouse mortality (mRS 6) at discharge | 22.6 (2247/9957) | N/A | |
| Duration of hospital stay (days) | 8 (5–13) | 8 (5–14) | 0.287 |
| Discharge to home or neurorehabilitation vs hospital or institutional care | 64.0 (6629/10 363) | 70.1 (1399/1997) | <0.001 |
Data are percentage (number/absolute number) except for age, NIHSS on admission, and door-to-groin puncture (mean±SD, median (IQR)), and duration of hospital stay (median (IQR)).
ACA, anterior cerebral artery; MCA, middle cerebral artery; MOD, missing outcome data; mRS, modified Rankin Scale; MT, mechanical thrombectomy; N/A, not applicable; NIHSS, National Institutes of Health Stroke Scale; PCA, posterior cerebral artery; TICI, thrombolysis in cerebral infarction; VB, vertebrobasilar artery.
Treatment and outcome parameters
Treatment of LVO less often contained bridging IVT in patients with MOD (44.3% (1110/2507) vs 48.4% (5027/10 393); P<0.001). Onset-to-groin puncture time was longer in patients with MOD (median 275 (IQR 185–517) vs 245 (165–450) min; P<0.001). Futile recanalization (TICI 0–2a) was less common in patients with MOD (12.4% (298/2404) vs 15.0% (1522/10 173); P=0.001, figure 1A). With regard to functional outcome, patients with 90 day clinical outcome data available had a lower odds of good outcome, as shown by an mRS score of 0–2 compared with 3–6 at discharge from the treating hospital (OR 0.847 (95% CI 0.761 to 0.943); P=0.002, figure 1A). At the same time, a discharge mRS score of 3–5 compared with 0–2 was significantly associated with MOD (OR 1.234 (95% CI 1.107 to 1.375); P<0.001). In patients with MOD, discharge from the treating hospital to home or to neurorehabilitation (compared with discharge to hospital or institutional care) was more frequent than in patients with 90 day mRS available (70.1% (1399/1997) vs 64.0% (6629/10 363); P<0.001).
Subgroup analysis: factors associated with missing 90 day clinical outcome data after discharge
Baseline and stroke characteristics
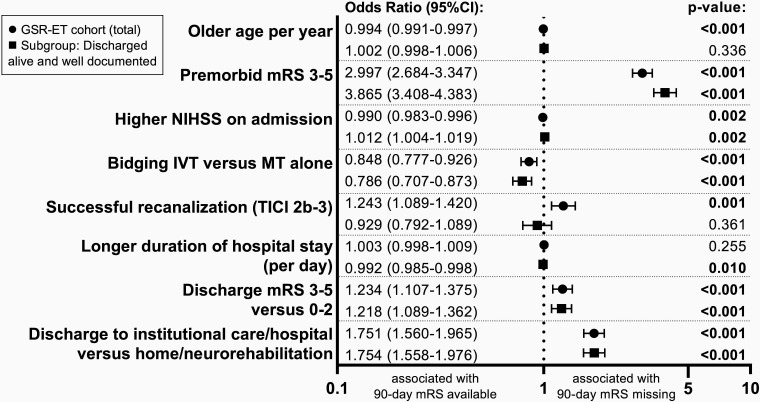
We analyzed 9416 patients in a subgroup analysis of factors associated with MOD after hospital discharge despite complete inhouse documentation. We found significantly higher odds for MOD in patients with higher pre-stroke disability (mRS 3–5: OR 3.865 (95% CI 3.408 to 4.383); P<0.001). Also, higher stroke severity, displayed by admission NIHSS, was associated with an increased risk of MOD (OR 1.012 (95% CI 1.004 to 1.019); P=0.002). Of all of the vessel territories, only occlusion of the middle cerebral artery in the M1 segment was significantly associated with MOD (OR 1.120 (95% CI 1.007 to 1.246); P=0.036), while we found a tendency for carotid artery occlusions towards MOD and a tendency for vertebrobasilar occlusions towards available 90 day outcome data. Large artery atherosclerosis etiology of LVO increased the odds of MOD by 52% (OR 1.521 (95% CI 1.359 to 1.702); P<0.001).
Treatment and outcome parameters
Patients treated with bridging IVT had lower rates of MOD (OR 0.786 (95% CI 0.707 to 0.873); P<0.001), while recanalization status did not significantly predict availability of 90 day clinical outcome. We observed increased odds for MOD with longer onset-to-groin time (OR per 10 min 1.001 (95% CI 1.000 to 1.003); P=0.038) as well as with unfavorable functional outcome at discharge from the treating hospital, for both discharge mRS 3–5 (OR 1.218 (95% CI 1.089 to 1.362); P<0.001) and discharge to institutional care or hospital (OR 1.754 (95% CI 1.558 to 1.976); P<0.001). Patients with a longer duration of hospital stay had lower odds of MOD (OR 0.992 (95% CI 0.985 to 0.998); P=0.010) (table 2 and figure 1B). A comparative illustration of associations with MOD in the whole GSR-ET cohort and the subgroup of patients discharged from hospital alive with MOD at the 90 day follow-up despite well documented baseline characteristics is depicted in figure 2.
Table 2.
Subgroup analysis: factors associated with missing clinical outcome data at 90 day follow-up despite complete baseline documentation in patients discharged from hospital alive.
| Variable | OR | 95% CI | P value |
| Older age | 1.002 | 0.997 to 1.006 | 0.336 |
| Female sex | 1.069 | 0.962 to 1.187 | 0.215 |
| Premorbid disability (mRS 3–5) | 3.865 | 3.408 to 4.383 | <0.001 |
| Cardiovascular risk factors | |||
| Arterial hypertension | 1.125 | 0.990 to 1.279 | 0.071 |
| Diabetes mellitus | 1.025 | 0.900 to 1.167 | 0.713 |
| Dyslipidemia | 0.734 | 0.656 to 0.822 | <0.001 |
| Atrial fibrillation | 1.108 | 0.993 to 1.236 | 0.065 |
| Smoker (current) | 1.087 | 0.936 to 1.263 | 0.274 |
| Baseline medication | |||
| Anticoagulation | 1.160 | 1.021 to 1.319 | 0.023 |
| Platelet inhibition | 1.047 | 0.927 to 1.183 | 0.459 |
| Stroke characteristics | |||
| Higher NIHSS on admission | 1.012 | 1.004 to 1.019 | 0.002 |
| Location of occlusion | |||
| Carotid artery | 1.129 | 1.000 to 1.275 | 0.050 |
| Anterior cerebral artery | 0.838 | 0.582 to 1.206 | 0.341 |
| Middle cerebral artery M1 segment | 1.120 | 1.007 to 1.246 | 0.036 |
| Middle cerebral artery M2 segment | 1.005 | 0.888 to 1.138 | 0.933 |
| Posterior cerebral artery | 0.984 | 0.723 to 1.338 | 0.918 |
| Vertebrobasilar | 0.866 | 0.713 to 1.052 | 0.148 |
| Stroke etiology | |||
| Large artery atherosclerosis | 1.521 | 1.359 to 1.702 | <0.001 |
| Cardioembolism | 0.961 | 0.865 to 1.067 | 0.454 |
| Dissection | 0.655 | 0.417 to 1.028 | 0.006 |
| Other | 0.804 | 0.615 to 1.051 | 0.110 |
| Undetermined | 0.601 | 0.511 to 0.706 | <0.001 |
| Treatment characteristics | |||
| Bridging intravenous thrombolysis | 0.786 | 0.707 to 0.873 | <0.001 |
| Primary admission at MT site | 1.091 | 0.978 to 1.218 | 0.119 |
| Symptom onset/time to recognition-to-groin puncture (per 10 min) | 1.001 | 1.000 to 1.003 | 0.038 |
| Door-to-groin puncture (per 10 min) | 1.000 | 0.997 to 1.003 | 0.969 |
| Outcome parameters | |||
| Successful recanalization (TICI 2b-3) | 0.929 | 0.792 to 1.089 | 0.361 |
| Good outcome at discharge (mRS 0–2) | 0.821 | 0.734 to 0.918 | <0.001 |
| Disabled at discharge (mRS 3–5) | 1.218 | 1.089 to 1.362 | <0.001 |
| Longer hospital stay per day | 0.992 | 0.985 to 0.998 | 0.010 |
| Discharge to home or neurorehabilitation vs hospital or institutional care | 1.754 | 1.558 to 1.976 | <0.001 |
ORs, 95% CI, and corresponding P values resulting from univariate logistic regression determining association with missing clinical outcome (mRS at 90 day follow-up) following discharge from the treating hospital alive despite complete baseline documentation (age, sex, NIHSS on admission, intravenous thrombolysis, post-mechanical thrombectomy TICI, and mRS at discharge from treating hospital).
mRS, modified Rankin Scale; MT, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; TICI, thrombolysis in cerebral infarction.
Figure 2.
Factors associated with missing 90 day clinical outcome in the total German Stroke Registry-Endovascular Treatment (GSR-ET) cohort and the subgroup of patients discharged from hospital alive with missing clinical outcome data (MOD) despite well documented baseline characteristics. ORs with 95% CI and P values of factors associated with MOD resulting from univariate logistic regression analyses. Circles=point estimates of factors associated with MOD in the total GSR-ET cohort; squares=subgroup analysis with point estimates of factors associated with MOD in the subgroup of patients who were discharged from hospital alive and had MOD despite well documented baseline characteristics (age, sex, admission National Institutes of Health Stroke Scale (NIHSS) score, intravenous thrombolysis (IVT), TICI, and mRS at discharge). P values indicating significance of predictors with a threshold of <0.05. MT, mechanical thrombectomy; mRS, modified Rankin scale score.
Discussion
Registry data from routine care MT is increasingly used to investigate MT under real world conditions and to assess efficacy and safety in distinct patient populations. Missing data is a common problem, not only in stroke registries, but among registry data from various disciplines.17–19 Analyses of registry data often apply complete case analysis as a strategy to handle missing data.20 Therefore, acknowledgment of systematic MOD is necessary when drawing conclusions from these data.
In this study, we found aspects for which analyses of registry MT data may not fully depict real world MT care when based on the availability of functional outcome at the 90 day follow-up. We found significant differences in basic patient, stroke, and treatment characteristics, especially with regard to pre-stroke disability, stroke severity, treatment characteristics, and post-stroke dependence on care that were disproportionately represented in patients with MOD versus those with 90 day clinical outcome data available. In a subgroup analysis of patients who were discharged from the treating hospital alive with complete follow-up until discharge, we identified patient and stroke characteristics that were significantly associated with MOD in order to prevent MOD by adjusting follow-up strategies. Among these characteristics were higher pre-stroke and post-stroke disability, shorter hospital stay, and discharge to hospital or institutional care. Increased efforts to perform clinical follow-up are needed in these patients and may help to increase follow-up rates and the representativeness of routine care MT datasets, which will benefit the validity and generalizability of conclusions drawn from these data.
Complete case analysis is a strategy to handle missing data by deriving a study cohort for further analysis by excluding cases with missing variables of interest. This approach is both widely used and controversial.20 21 Especially in cases of missing data that do not occur completely at random but are associated with subject specific characteristics or certain degrees of the missing variable itself, complete case analysis may depict a distorted dataset compared with the actual population it was derived from. Consequently, estimated effects derived from subsequent analyses may be biased. Proper accounting for observed factors that are associated with MOD may reduce the bias of complete case analyses. However, if MOD is systematically related to the missing outcome itself, it cannot be fully addressed by adjusting and has to be acknowledged as a study limitation.14
In this study, we identified patient, stroke, and treatment characteristics that were associated with MOD and therefore indicated that MOD that does not occur completely at random, potentially resulting in selection bias by complete case analyses. Specifically, we observed that relevant pre-stroke disability (mRS>2) was strongly associated with MOD. This is especially important because most high level evidence studies for MT explicitly excluded patients with relevant pre-stroke disability. Therefore, evidence of MT efficacy in this subgroup of patients is generally limited.1 Data from observational studies suggest effective MT in patients with relevant pre-stroke disability by means of increasing odds of not accumulating more disability by MT.22 23 However, with complete case analysis strategies applied, it is likely that observational data may disproportionately represent this subgroup of patients, compared with their actual share of MT treated patients. Moreover, patients with relevant pre-stroke disability may have MOD not occurring at random, resulting in potentially biased study estimates, which are even more difficult to address by adjusting or imputation. Also, in our subgroup analysis of MOD despite complete follow-up until discharged from hospital, relevant pre-stroke disability was strongly associated with 3.4-fold increased odds of MOD, stressing the importance to increase follow-up in this subgroup of patients.
Furthermore, we found a strong association of MOD with functional stroke outcome following acute stroke treatment. Specifically, we suggest an inverted U shaped relationship between early functional stroke outcome and MOD at the 90 day follow-up. Based on our data, we suggest that there is less MOD in patients with fatal outcomes during hospital stay and in those with good functional outcomes (mRS ≤2) at discharge. In our data, this was depicted by an inverse association of discharge mRS score of 3–6 with MOD by a factor of 0.85, lower mean admission NIHSS in patients with MOD and, at the same time, increased odds (by a factor of 1.23) for MOD for patients with a discharge mRS score of 3–5 versus 0–2. In line with this, successful recanalization (TICI 2b–3) appeared to be associated with an increase in MOD by 24%, whereas futile recanalization was associated with available 90 day outcome data. This depicts thorough follow-up of inhouse patient outcome, which is not influenced by obstacles of follow-up after discharge, such as missing contact information or non-traceable relocation. Consequentially, fatal strokes with inhouse mortality may be overrepresented, compared with other categories of functional stroke outcome.
Consistent with this interpretation, in our subgroup analysis of MOD despite complete follow-up until discharged alive, futile recanalization was not significantly associated with MOD. But higher stroke severity (ie, higher NIHSS on admission) as well as higher resulting stroke disability at discharge (mRS 3–5 vs 0–2) were associated with MOD in this subgroup, calling for increased efforts to perform follow-up in this patient group. It is noteworthy that the described U shaped relationship is contrary to observational registry data of other disciplines (eg, chronic diseases such as heart failure and cancer). These report missing data to be associated with worse outcome and increased mortality.19 24 25 This emphasizes a disease specific distribution of missing data in observational registries. Hence strategies aiming to increase completeness of data need to take into account disease and population specific factors associated with MOD.
We found a strong association of MOD after discharge from hospital alive in patients with shorter duration of hospital stay and in patients discharged to further hospital or institutional care. This might potentially reflect missing contact information. Therefore, focusing on obtaining valid contact information for patients or relatives in a timely manner after admission for MT might be a promising strategy to increase follow-up rates in these patients.
Combination of MT with bridging IVT as well as shorter onset-to-groin puncture time were significantly associated with available 90 day clinical outcome in our dataset. This may also reflect subsequent better functional outcome in this subgroup of patients which, as described previously, was associated with availability of 90 day outcome. However, bridging IVT and patients treated in the early time window may be overrepresented in complete case analyses of registry data. In contrast, patients treated with MT within the extended time window may be disproportionately represented, which is noticeable, especially because evidence based decision making in acute stroke treatment in this patient group is just evolving. Similar to our observation, different treatment patterns in patients with missing data have also been described for observational data of heart failure, where patients with missing ejection fraction data depicted a group less likely to receive evidence based treatment.25
Despite broadly analyzing MOD in a multicenter, prospective registry study of routine care MT, our study approach had several limitations. We investigated an extensive set of parameters potentially associated with MOD. However, we did not capture additional patient characteristics that have previously been reported to be associated with missing data (eg, sociodemographic characteristics such as immigrant status,26 education, and language skills27). We cannot conclude on these aspects, although it is likely that MOD in acute stroke care registries also depends on sociodemographic factors. It might be of value to include these baseline variables in registry study datasets to conclude on such aspects in these patient groups, which may be even more relevant in geographic areas other than western Europe.
Furthermore, the generalizability and transferability of results to other stroke registries might be limited due to unique organizational structures, such as the strategies and technology used for data acquisition or follow-up and acquisition of contact information for patients or relatives. Although we believe that systematic MOD is likely to be driven by similar organizational obstacles, strategies to increase follow-up rates will have to be based on registry and site specific conditions. Our analysis was also limited with regard to analyzing the association between MOD and functional outcome. Because of the nature of our dataset, information on functional outcome at discharge from hospital of patients with MOD at the 90 day follow-up was not complete. Although information regarding discharge mRS was available in the majority (72%) of patients with MOD at the 90 day follow-up, 28% were also lacking information about discharge mRS. We therefore cannot quantify the extent to which inhouse mortality was overrepresented by complete case analysis. However, inhouse mortality would only not truly be overrepresented in the cohort of patients with 90 day mRS available if as many as 80.1% (582/722) of patients with MOD and missing discharge mRS actually died inhouse. We do not consider this to be likely, especially because inhouse mortality is comparatively easy to investigate and report.
By analyzing an up-to-date large and complex nationwide cohort of >13 000 MT procedures, our study benefited from a strong data foundation. Past analyses of MOD in acute stroke studies have focused on the accuracy of imputation methods and their consequence for study estimates in acute stroke studies, some of them assuming that MOD occurred completely at random.12 28 However, imputation methods will not fully be able to adjust biases resulting from systematic MOD because there are substantial theoretical reasons to assume that missing outcome data are occurring not completely randomly but depending on observed and unobserved variables and the missing outcome itself. Imputation cannot fully compensate for missing data depending on the outcome itself or unobserved variables.14 We therefore believe that our report of patient, treatment, and outcome characteristics associated with MOD in a large routine care MT registry is important in order to acknowledge aspects in which observational data may not fully represent routine MT care and may influence subsequent analyses.
Because 30 centers were involved in enrollment and data acquisition over more than 6 years, we were able to minimize the impact of site and time specific factors influencing MOD. Furthermore, the broad dataset of the GSR-ET, including functional outcome at hospital discharge, allowed us to augment our study by conducting a subgroup analysis, excluding patients with inhouse mortality. We agree with Yeatts and Martin,29 suggesting that developing an approach for handling missing data produces the best results when proactively preventing or minimizing the occurrence of missing data. In this sense, our findings have direct implications for data acquisition in registry studies within the field of acute stroke care and will enable study groups to increase efforts or implement additional strategies to succeed with clinical follow-up in patients that are at increased risk of MOD.
Conclusions
MOD in stroke registries is not a rare event and does not occur completely at random. While registries depicting MT in routine care are valuable tools to analyze patterns of care and functional outcome after LVO in distinct populations, missing data may impact the accuracy and generalizability of these analyses. Bias may result from case selection on the basis of complete data and cannot be fully adjusted for by imputation of missing data, because MOD is significantly dependent on other observed (and unobserved) variables and the outcome itself. We have reported factors associated with missing data on 90 day functional outcome in a large multicenter registry of MT in acute stroke care. These include patients with higher pre-stroke disability, patients treated later after symptom onset, patients not receiving bridging IVT, and patients with relevant disability at discharge from the treating hospital, which are at increased risk of MOD. These patient groups may therefore be underrepresented in acute stroke care studies based on complete case analysis, compared with the actual cohort treated by MT. In contrast, patients with inhouse mortality after MT treatment are likely to have complete data on 90 day stroke outcome and might therefore be disproportionately present. In addition to patients with higher pre-stroke and post-stroke disability, increased efforts to perform 90 day follow-up should also be undertaken for patients with a shorter duration of hospital stay and those discharged to institutional care or hospital.
Acknowledgments
The authors thank Dr Cheryl Ernest for proofreading and editing the manuscript.
Footnotes
@TimoUphaus
Collaborators: The German Stroke Registry Endovascular Treatment–Steering Committee: Joachim Röther (Asklepios Klinik Altona, Hamburg), Bernd Eckert (Asklepios Klinik Altona, Hamburg), Michael Braun (Bezirkskrankenhaus Günzburg), Gerhard F Hamann (Bezirkskrankenhaus Günzburg), Eberhard Siebert (Charité-Campus Benjamin Franklin und Mitte, Berlin), Christian H Nolte (Charité-Campus Benjamin Franklin und Mitte, Berlin), Sarah Zweynert (Charité-Campus Virchow Klinikum, Berlin), Georg Bohner (Charité-Campus Virchow Klinikum, Berlin), Jörg Berrouschot (Klinikum Altenburger Land), Albrecht Bormann (Klinikum Altenburger Land), Christoffer Kraemer (Klinikum Lüneburg), Martina Petersen (Klinikum Osnabrück), Florian Stögbauer (Klinikum Osnabrück), Tobias Boeckh-Behrens (Klinikum rdIsar, München), Silke Wunderlich (Klinikum rdIsar, München), Alexander Ludolph (Sana Klinikum Offenbach), Karl-Heinz Henn (Sana Klinikum Offenbach), Christian Gerloff (UKE Hamburg-Eppendorf), Jens Fiehler (UKE Hamburg-Eppendorf), Götz Thomalla (UKE Hamburg-Eppendorf), Anna Alegiani (UKE Hamburg-Eppendorf), Franziska Dorn (Uniklinik Bonn), Gabor Petzold (Uniklinik Bonn), Waltraud Pfeilschifter (Uniklinik Frankfurt/Main), Fee Keil (Uniklinik Frankfurt/ Main), Martin Dichgans (Uniklinik München (LMU)), Steffen Tiedt (Uniklinik München (LMU)), Lars Kellert (Uniklinik München (LMU)), Christoph Trumm (Uniklinik München (LMU)), Ulrike Ernemann (Universitätsklinik Tübingen), and Sven Poli (Universitätsklinik Tübingen).
Contributors: MH and TU designed and conceptualized the study. TU, SG, and MH contributed to data acquisition. MH performed statistical analyses with support from TU. KG, SG, TU, and MH interpreted the data. MH drafted the manuscript for intellectual content. KG, AO, LB, MAB, AC, SG, and TU critically revised the manuscript. TU accepts full responsibility for the work and conduct of the study, had access to the data and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: AO reports speakers bureau from Cerenovus and Canon Medical. KG reports personal fees and/or non-financial support from Bayer, Boehringer Ingelheim, Bristol-Meyers Squibb, Daiichi Sankyo, and Pfizer. MH reports personal fees from Bristol-Meyers Squibb, outside of the submitted work. TU reports personal fees from Merck Serono and Pfizer, and grants from Else Kröner-Fresenius Stiftung.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: On behalf of German Stroke Registry Endovascular Treatment–Steering Committee, Joachim Röther, Bernd Eckert, Michael Braun, Gerhard F Hamann, Eberhard Siebert, Christian H Nolte, Sarah Zweynert, Georg Bohner, Jörg Berrouschot, Albrecht Bormann, Christoffer Kraemer, Martina Petersen, Florian Stögbauer, Tobias Boeckh-Behrens, Silke Wunderlich, Alexander Ludolph, Karl-Heinz Henn, Christian Gerloff, Jens Fiehler, Götz Thomalla, Anna Alegiani, Franziska Dorn, Gabor Petzold, Waltraud Pfeilschifter, Fee Keil, Martin Dichgans, Steffen Tiedt, Lars Kellert, Christoph Trumm, Ulrike Ernemann, and Sven Poli
Data availability statement
Data are available upon reasonable request. The data supporting the findings of this study are available from the corresponding author on reasonable request from any qualified investigator.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the ethics committee of the leading center (Ludwig-Maximilians University Munich, protocol No 689-15), and by the local ethics committees. Participants gave informed consent to participate in the study before taking part.
References
- 1. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2. Jovin TG, Nogueira RG, Lansberg MG, et al. Thrombectomy for anterior circulation stroke beyond 6 H from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet 2022;399:249–58. 10.1016/S0140-6736(21)01341-6 [DOI] [PubMed] [Google Scholar]
- 3. Deb-Chatterji M, Pinnschmidt H, Flottmann F, et al. Stroke patients treated by thrombectomy in real life differ from cohorts of the clinical trials: a prospective observational study. BMC Neurol 2020;20:81. 10.1186/s12883-020-01653-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dittrich TD, Sporns PB, Kriemler LF, et al. Mechanical thrombectomy for large vessel occlusion between 6 and 24 hours: outcome comparison of DEFUSE-3/DAWN eligible versus non-eligible patients. Int J Stroke 2022;2022:17474930221140792. 10.1177/17474930221140793 [DOI] [PubMed] [Google Scholar]
- 5. Forlivesi S, Cappellari M, Bovi P. Missing data on 3-month modified Rankin scale may influence results of functional outcome after intravenous thrombolysis in observational studies. J Thromb Thrombolysis 2016;42:585. 10.1007/s11239-016-1392-x [DOI] [PubMed] [Google Scholar]
- 6. Akbik F, Alawieh A, Dimisko L, et al. Bridging thrombolysis in atrial fibrillation stroke is associated with increased hemorrhagic complications without improved outcomes. J Neurointerv Surg 2022;14:979–84. 10.1136/neurintsurg-2021-017954 [DOI] [PubMed] [Google Scholar]
- 7. Matusevicius M, Cooray C, Rand V-M, et al. Stroke etiology and outcomes after endovascular thrombectomy: results from the SITS Registry and a meta-analysis. J Stroke 2021;23:388–400. 10.5853/jos.2021.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jumaa MA, Castonguay AC, Salahuddin H, et al. Middle cerebral artery M2 thrombectomy in the STRATIS Registry. Stroke 2021;52:3490–6. 10.1161/STROKEAHA.120.033951 [DOI] [PubMed] [Google Scholar]
- 9. Ali M, van der Meij A, van Os HJA, et al. Sex differences in onset to hospital arrival time, prestroke disability, and clinical symptoms in patients with a large vessel occlusion: a MR CLEAN Registry substudy. J Neurointerv Surg 2022:jnis-2022-019670. 10.1136/jnis-2022-019670 [DOI] [PubMed] [Google Scholar]
- 10. Dellborg M, Eriksson P. Randomized trials of closure of persistent foramen ovale (PFO) vs medical therapy for patients with cryptogenic stroke - effect of lost-to-follow-up and withdrawal of consent. Int J Cardiol 2016;207:308–9. 10.1016/j.ijcard.2016.01.185 [DOI] [PubMed] [Google Scholar]
- 11. Engels JM, Diehr P. Imputation of missing longitudinal data: a comparison of methods. J Clin Epidemiol 2003;56:968–76. 10.1016/s0895-4356(03)00170-7 [DOI] [PubMed] [Google Scholar]
- 12. Fernandez-Ferro J, Schwamm LH, Descalzo MA, et al. Missing outcome data management in acute stroke trials testing IV thrombolytics Eur Stroke J 2020;5:148–54. 10.1177/2396987320905457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garg R. Methodological survey of missing outcome data in an alteplase for ischemic stroke meta‐analysis. Acta Neurol Scand 2022;146:252–7. 10.1111/ane.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mack C, Su Z, Westreich D. Managing missing data in patient registries: Addendum to registries for evaluating patient outcomes: A user’s guide. 3rd edn. Rockville (MD), 2019. [PubMed] [Google Scholar]
- 15. Sheffet AJ, Voeks JH, Mackey A, et al. Characteristics of participants consenting versus declining follow-up for up to 10 years in a randomized clinical trial. Clin Trials 2015;12:657–63. 10.1177/1740774515590807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alegiani AC, Dorn F, Herzberg M, et al. Systematic evaluation of stroke Thrombectomy in clinical practice: the German stroke Registry Endovascular treatment. Int J Stroke 2019;14:372–80. 10.1177/1747493018806199 [DOI] [PubMed] [Google Scholar]
- 17. Ueland TE, Carreira DS, Martin RL. Substantial loss to follow-up and missing data in national Arthroscopy registries: A systematic review. Arthroscopy 2021;37:761–70. 10.1016/j.arthro.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 18. Mendelsohn AB, Dreyer NA, Mattox PW, et al. Characterization of missing data in clinical Registry studies. Ther Innov Regul Sci 2015;49:146–54. 10.1177/2168479014532259 [DOI] [PubMed] [Google Scholar]
- 19. Yang DX, Khera R, Miccio JA, et al. Prevalence of missing data in the National cancer database and association with overall survival. JAMA Netw Open 2021;4:e211793. 10.1001/jamanetworkopen.2021.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shivasabesan G, Mitra B, O’Reilly GM. Missing data in trauma registries: A systematic review. Injury 2018;49:1641–7. 10.1016/j.injury.2018.03.035 [DOI] [PubMed] [Google Scholar]
- 21. Vollenweider D, Boyd CM, Puhan MA. High prevalence of potential biases threatens the interpretation of trials in patients with chronic disease. BMC Med 2011;9:73. 10.1186/1741-7015-9-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Havenon A, Castonguay A, Nogueira R, et al. Prestroke disability and outcome after thrombectomy for emergent anterior circulation large vessel occlusion stroke. Neurology 2021;97:e1914–9. 10.1212/WNL.0000000000012827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sykora M, Michel P, Strambo D, et al. Mechanical thrombectomy in acute stroke patients with moderate to severe pre-stroke disability. J Stroke 2022;24:396–403. 10.5853/jos.2022.00906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plichta JK, Rushing CN, Lewis HC, et al. Implications of missing data on reported breast cancer mortality. Breast Cancer Res Treat 2023;197:177–87. 10.1007/s10549-022-06764-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poppe KK, Squire IB, Whalley GA, et al. Known and missing left ventricular ejection fraction and survival in patients with heart failure: a MAGGIC meta-analysis report. Eur J Heart Fail 2013;15:1220–7. 10.1093/eurjhf/hft101 [DOI] [PubMed] [Google Scholar]
- 26. Vyas MV, Fang J, Austin PC, et al. Importance of accounting for loss to follow-up when comparing mortality between immigrants and long-term residents: a population-based retrospective cohort. BMJ Open 2021;11:e046377. 10.1136/bmjopen-2020-046377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Razzaghi H, Tinker SC, Herring AH, et al. Impact of missing data for body mass index in an epidemiologic study. Matern Child Health J 2016;20:1497–505. 10.1007/s10995-016-1948-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young-Saver DF, Gornbein J, Starkman S, et al. Handling of missing outcome data in acute stroke trials: advantages of multiple imputation using baseline and postbaseline variables. J Stroke Cerebrovasc Dis 2018;27:3662–9. 10.1016/j.jstrokecerebrovasdis.2018.08.040 [DOI] [PubMed] [Google Scholar]
- 29. Yeatts SD, Martin RH. What is missing from my missing data plan Stroke 2015;46:e130–2. 10.1161/STROKEAHA.115.007984 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnis-2023-020435supp001.pdf (185.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data supporting the findings of this study are available from the corresponding author on reasonable request from any qualified investigator.