Abstract
Objective
After lowering the Dutch threshold for active treatment from 25 to 24 completed weeks’ gestation, survival to discharge increased by 10% in extremely preterm live born infants. Now that this guideline has been implemented, an accurate description of neurodevelopmental outcome at school age is needed.
Design
Population-based cohort study.
Setting
All neonatal intensive care units in the Netherlands.
Patients
All infants born between 240/7 and 266/7 weeks’ gestation who were 5.5 years’ corrected age (CA) in 2018–2020 were included.
Main outcome measures
Main outcome measure was neurodevelopmental outcome at 5.5 years. Neurodevelopmental outcome was a composite outcome defined as none, mild or moderate-to-severe impairment (further defined as neurodevelopmental impairment (NDI)), using corrected cognitive score (Wechsler Preschool and Primary Scale of Intelligence Scale-III-NL), neurological examination and neurosensory function. Additionally, motor score (Movement Assessment Battery for Children-2-NL) was assessed. All assessments were done as part of the nationwide, standardised follow-up programme.
Results
In the 3-year period, a total of 632 infants survived to 5.5 years’ CA. Data were available for 484 infants (77%). At 5.5 years’ CA, most cognitive and motor (sub)scales were significantly lower compared with the normative mean. Overall, 46% had no impairment, 36% had mild impairment and 18% had NDI. NDI-free survival was 30%, 49% and 67% in live born children at 24, 25 and 26 weeks’ gestation, respectively (p<0.001).
Conclusions
After lowering the threshold for supporting active treatment from 25 to 24 completed weeks’ gestation, a considerable proportion of the surviving extremely preterm children did not have any impairment at 5.5 years’ CA.
Keywords: neonatology, neurology, epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Long-term outcomes in neonatal intensive care unit survivors are frequently classified as severe, moderate, mild or no impairment.
After lowering the threshold for supporting active treatment in the Netherlands from 25 completed weeks’ gestation to 24 completed weeks’ gestation in 2010, 62% of the extremely preterm survivors did not have any impairment at 2 years’ corrected age (CA).
WHAT THIS STUDY ADDS
In this population-based cohort study with nationwide agreed treatment practices at birth, which included 1003 live born extremely preterm infants, 46% of the survivors had no impairment, 36% had mild impairment and 18% had moderate-to-severe impairment at 5.5 years’ CA.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Major changes in neurodevelopmental performance between 2 and 5.5 years’ CA, with an increase in moderate-to-severely impaired children, emphasise the importance of continuing neonatal follow-up until at least school age.
The current study provides valuable information to clinicians who are counselling parents antenatally who are at risk of delivering before 27 weeks’ gestation.
Introduction
After lowering the threshold for active treatment of preterm infants from 25 to 24 completed weeks’ gestation in the Netherlands in 2010, survival to discharge increased by 10% in extremely preterm live born infants.1 Nevertheless, both higher risk for physical disabilities and cognitive problems later in life have been reported in preterm infants, and especially if born extremely preterm.2–6 Now that this guideline has been implemented, an accurate description of neurodevelopmental outcome at school age is needed, in order to provide national data for prenatal and neonatal counselling of parents and also for international comparison.7
The Extremely Preterm Infants-Dutch Analysis on Follow-up (EPI-DAF) study was designed to evaluate neurodevelopmental outcome at 2 and 5.5 years’ corrected age (CA), using outcome data collected at follow-up visits that are carried out in accordance with the national guidelines in the Netherlands. In a previous cohort of infants being 2 years’ CA, 62% of the extremely preterm survivors did not have any impairment at 2 years’ CA.8–10 However, several studies showed that the correlation between impairment classification at 2 years of age and at middle childhood is moderate.6 11–13 At later age, the degree of disability may be more clearly defined and may be more likely to be predictive of problems that will continue into later life.6 Therefore, this study evaluated the variation in overall impairment between 2 and 5.5 years of age.
The primary aim of the current report as part of the EPI-DAF study was to evaluate cognitive outcome, motor outcome and neurodevelopmental impairment (NDI) in extremely preterm born children at 5.5 years’ CA. As a secondary aim, change in neurodevelopmental outcome from 2 to 5.5 years’ CA was evaluated.
Methods
Patient population
The EPI-DAF study included all live born infants with a gestational age (GA) of 24–26 completed weeks, who were 5.5 years’ CA in 2018–2020 (birth cohort 2012–2015).14
Data collection
Perinatal data collection and follow-up assessment has been reported previously in detail in a EPI-DAF cohort with follow-up at 2 years’ CA.8 Follow-up is part of standardised aftercare in children after very preterm birth and formalised in a national guideline for follow-up performed in all neonatal intensive care units (NICUs).15 This standardised protocol includes medical history taking, physical and neurological examination and assessment of cognitive and motor development by a trained team, consisting of a paediatrician/neonatologist, psychologist and physiotherapist and is usually taken place at the NICU site of birth of the child. Neurological examination was performed to identify cerebral palsy (CP), which was graded using the five levels defined in the Gross Motor Function Classification System (GMFCS).16 A combination of medical history and results of the assessment was used to rate hearing and vision status. Information on maternal education was collected during follow-up and classified as low, middle or high.17 Socioeconomic status (SES) was assessed using scores (mean 0, low SES <−1, high SES >1) defined by the Netherlands Institute for Social and Cultural Research (The Hague, The Netherlands) based on the postal code at birth.18
Outcome evaluation
Cognitive outcome was assessed using the Dutch version of the Wechsler Preschool and Primary Scale of Intelligence Scale-III (WPPSI-III-NL, normative mean 100 (SD 15) for all scores).19 This test provides an overall full-scale score (FSIQ) assessing global intelligence, a verbal composite score (VIQ) assessing verbal reasoning ability including vocabulary, information and word reasoning, a performance composite score (PIQ) measuring non-verbal reasoning ability and a processing speeds composite score (PSQ) evaluating visual scanning and motor response speed based on coding and symbol search. All scores were based on age corrected for prematurity.20
Motor outcome was tested using the Dutch version of the Movement Assessment Battery for Children (M-ABC-2-NL).21 The test yields a total standard score (normative mean 10 (SD 3)), and three component standard scores (normative mean 10 (SD 3)): manual skills, ball skills and balance skills scores. Developmental coordination disorders were defined as a total score less than or equal to the fifth centile.22
Neurodevelopmental outcome was a composite outcome measure classified as moderate-to-severe, mild or no impairment, using defined categories of cognitive development, neurological examination and neurosensory function.3 6 9 23
The entity NDI was restricted to children with a moderate-to-severe impairment and included an FSIQ <−2 SD, a CP with GMFCS level 2–5, functionally impaired vision or blindness or hearing loss requiring aids or severe sensorineural hearing loss despite aids.
Mild impairment included an FSIQ between −1 and −2 SD, non-CP-related neurology with abnormal neurological signs but with minimal functioning implications (GMFCS 1), mild visual problems (squints or refractive errors) or mild hearing loss (not sufficient to require aids). Outcome classification was based on the worst determinant in either one of the categories. If children did not visit the neonatal follow-up clinic, but a rehabilitation clinic instead due to severe neurodevelopmental problems, they were included in the study classified as moderate-to-severe impairment.
Of the children who were tested at 5.5 years’ CA, data were also available at 2 years’ CA. Neurodevelopmental outcome categorisation was similar at 2 compared with 5 years’ CA, except for cognitive functioning which was assessed using the Dutch version of the Bayley Scales of Infant and Toddler Development-III-NL (mean 100, SD 15).24
Statistical analysis
Statistical analysis was performed using R V.3.5.2. Baseline characteristics and outcome variables were compared between GA groups based on completed weeks of gestation using the one-way analysis of variance for continuous variables and using the χ2 test for categorical variables. A one-tailed one-sample T-test was used to test whether cognitive and motor test scores were lower than the general population using the normative mean scores. Impairment rates were compared between different GA groups using a χ2 test. Overall impairment status was imputed for 148 children who survived without follow-up data available, using an imputation model containing all baseline characteristics including SES using the R multivariate imputation by chained equation package. A p value <0.05 was considered statistically significant.
Results
Study population
Within the study period, 1003 infants were live born with a GA of 24–26 completed weeks, of whom 913 (91%) infants were admitted to a NICU (o nline supplemental appendix 1). Of these infants, 632 (69%) infants survived and were 5.5 years’ CA in 2018–2020. Of all survivors, 498 (79%) children were seen for follow-up at 5.5 years’ CA. Fourteen children were seen for follow-up, but parents refused to register data. Thus, in 484 (77%) children 5.5-year follow-up data were available for categorisation into neurodevelopmental outcome. Of these, 365 subjects were fully assessed using the WPPSI. In 90 subjects, estimate cognitive outcome was based on history (special education) or incomplete psychological tests. In another 29 children, cognitive outcome was estimated by the paediatrician/psychologist to classify children who were contacted by phone due to COVID-19.
fetalneonatal-2023-325732supp001.pdf (1.3MB, pdf)
In children without outcome data available, SES was lower compared with children with outcome data available (online supplemental appendix 2). With advancing GA, survivors were more often born as small-for-gestation or after caesarean section (table 1).
Table 1.
Baseline characteristics of all infants with follow-up data available born <27 weeks’ GA who reached 5.5 years’ CA in 2018–2020, separately for infants born at 24, 25 and 26 weeks’ gestation
| Total | 24 weeks’ GA | 25 weeks’ GA | 26 weeks’ GA | P value | |
| Live born | N=1003 | N=286 | N=278 | N=439 | |
| Admitted | N=913 (91.0) | N=228 (79.7) | N=255 (91.7) | N=430 (97.9) | |
| Survived to 5.5 years’ CA | N=632 (69.2) | N=115 (50.7) | N=179 (70.2) | N=338 (78.6) | |
| Follow-up data available | N=484 (76.6) | N=95 (82.6) | N=145 (81.0) | N=244 (72.2) | |
| Birth weight (g) | 818 (159) | 687 (97) | 798 (127) | 880 (162) | <0.001* |
| Sex (male) | 266 (55.0) | 45 (47.4) | 87 (60.0) | 134 (54.9) | 0.157 |
| SGA (<10th percentile) | 95 (19.6) | 10 (10.5) | 25 (17.2) | 60 (24.6) | 0.009* |
| Caesarean section | 201 (41.5) | 14 (14.7) | 58 (40.0) | 129 (52.9) | <0.001* |
| Multiple birth | 161 (33.3) | 29 (30.5) | 50 (34.5) | 82 (33.6) | 0.806 |
| 5 min Apgar | 7 (6, 8) | 7 (6, 8) | 7 (6, 8) | 7 (6, 8) | 0.022* |
| Severe NEC or SIP | 46 (9.5) | 13 (13.7) | 16 (11.0) | 17 (7.0) | 0.126 |
| Severe brain injury | 81 (16.7) | 15 (15.8) | 28 (19.3) | 38 (15.6) | 0.610 |
| Socioeconomic status | 0.537 | ||||
| Low | 128 (26.9) | 30 (32.3) | 36 (25.4) | 62 (25.7) | |
| Intermediate | 286 (60.1) | 51 (54.8) | 91 (64.1) | 144 (59.8) | |
| High | 62 (13.0) | 12 (12.9) | 15 (10.6) | 35 (14.5) | |
| Maternal education | 0.054 | ||||
| Low | 59 (12.2) | 10 (10.5) | 12 (8.3) | 37 (15.2) | |
| Intermediate | 191 (39.5) | 40 (42.1) | 67 (46.2) | 84 (34.4) | |
| High | 177 (36.6) | 29 (30.5) | 49 (33.8) | 99 (40.6) | |
| Missing | 57 (11.8) | 16 (16.8) | 17 (11.7) | 24 (9.8) |
Birth weight is presented as mean (SD), Apgar score is presented as median (Q1, Q3), other variables are presented as N (%). SGA was defined as birth weight <10th percentile. The p value reflects differences between 24, 25 and 26 weeks’ gestation.
*P<0.05.
CA, corrected age; GA, gestational age; NEC, necrotising enterocolitis; SGA, small for gestational age.
Outcome at 5.5 years’ CA
The mean FSIQ score was 92.4 (SD 16.5), 95.4 (SD 14.1) and 98.0 (SD 14.2) for 24-week, 25-week and 26-week children, respectively (p=0.020, table 2). For the total cohort, (sub)scales (FSIQ, PIQ, PSQ) were significantly lower compared with the normative mean of 100, except for the VIQ.
Table 2.
Follow-up results at 5.5 years, born <27 weeks’ GA who reached 5.5 years’ CA in 2018–2020, separately for infants born at 24, 25 and 26 weeks’ gestation
| Total | 24 weeks | 25 weeks | 26 weeks | P value | |
| WPPSI-III-NL, N evaluated | 365 | 70 | 106 | 189 | |
| FSIQ, mean (SD) | 96.2 (14.8)* | 92.4 (16.5) | 95.4 (14.1) | 98.0 (14.2) | 0.020† |
| ≥85 (≥−1 SD) | 79% (75–83) | 71% (59–82) | 80% (71–87) | 82% (75–87) | 0.343‡ |
| 70–84 (−2 to −1 SD) | 17% (13–21) | 21% (13–33) | 15% (9–23) | 16% (11–22) | |
| <70 (<−2 SD) | 4% (2–7) | 7% (2–16) | 5% (2–11) | 3% (1–6) | |
| VIQ, mean (SD) | 99.0 (16.4) | 95.4 (18.1) | 98.5 (15.6) | 100.7 (15.9) | 0.065 |
| ≥85 (≥−1 SD) | 81% (77–85) | 77% (66–86) | 82% (74–89) | 83% (76–88) | 0.095‡ |
| 70–84 (−2 to −1 SD) | 14% (11–18) | 13% (6–23) | 12% (7–20) | 15% (11–21) | |
| <70 (<−2 SD) | 5% (3–7) | 10% (4–20) | 6% (2–12) | 2% (1–5) | |
| PIQ, mean (SD) | 96.4 (13.4)* | 93.1 (13.2) | 96.2 (13.5) | 97.8 (13.2) | 0.036† |
| ≥85 (≥−1 SD) | 83% (79–87) | 76% (65–86) | 82% (74–89) | 86% (81–91) | 0.290‡ |
| 70–84 (−2 to −1 SD) | 15% (12–19) | 21% (12–32) | 16% (10–23) | 13% (9–19) | |
| <70 (<−2 SD) | 1% (0–3) | 3% (0–10%) | 2% (0–7) | 1% (0–3) | |
| PSQ, mean (SD) | 92.0 (15.0)* | 89.3 (17.2) | 91.8 (14.5) | 93.1 (14.4) | 0.186 |
| ≥85 (≥−1 SD) | 70% (65–74) | 63% (51–75) | 74% (64–82) | 70% (63–77) | 0.063‡ |
| 70–84 (−2 to −1 SD) | 25% (20–27) | 27% (17–39) | 19% (12–27) | 27% (21–34) | |
| <70 (<−2 SD) | 5% (3–8) | 10% (4–19) | 8% (3–14) | 3% (1–6) | |
| M-ABC-2-NL, N evaluated | 383 | 70 | 114 | 199 | |
| Total standard score, mean (SD) | 6.7 (3.3)* | 5.8 (3.0) | 6.6 (3.4) | 7.1 (3.3) | 0.024† |
| ≤5 | 41% (36–46) | 55% (43–67) | 41% (32–51) | 36% (30–43) | 0.001‡ |
| Manual skills, mean (SD) | 7.4 (3.1)* | 6.9 (3.1) | 7.4 (3.4) | 7.6 (3.0) | 0.209 |
| ≤5 | 29% (25–34) | 32% (22–44) | 32% (23–41) | 26% (20–33) | 0.506‡ |
| Ball skills, mean (SD) | 8.1 (3.3)* | 7.2 (3.3) | 8.0 (3.3) | 8.4 (3.4) | 0.028† |
| ≤5 | 26% (21–31) | 32% (21–44) | 25% (17–34) | 24% (18–31) | 0.405‡ |
| Balance skills score, mean (SD) | 7.8 (3.5)* | 7.2 (3.5) | 7.2 (3.4) | 7.8 (3.5) | 0.240 |
| ≤5 | 32% (27–37) | 36% (25–48) | 32% (24–42) | 30% (23–37) | 0.588‡ |
| <7 (<−1 SD) | 41% (36–46) | 47% (35–59) | 44% (35–54) | 37% (30–44) | 0.206 |
| Neurological exam | |||||
| Normal | 79% (75–83) | 79% (69–87) | 75% (67–82) | 82% (76–86) | 0.394‡ |
| Mildly abnormal (eg, posture, coordination or tone dysregulation disorders) | 15% (12–19) | 11% (5–19) | 19% (13–26) | 14% (10–19) | |
| CP, GMFCS 1 | 2% (1–4) | 5% (2–12) | 2% (0–6) | 2% (0–4) | |
| CP, GMFCS 2–5 | 3% (2–5) | 5% (2–12) | 3% (1–8) | 2% (1–5) | |
| Vision | |||||
| Normal | 79% (75–82) | 71% (60–79) | 81% (73–87) | 81% (75–85) | 0.101‡ |
| Mild visual problems including squints or refractive errors | 18% (15–22) | 23% (15–33) | 16% (10–23) | 18% (13–23) | |
| Functionally impaired vision or blindness | 3% (2–5) | 6% (2–13) | 3% (1–8) | 2% (0–4) | |
| Hearing | |||||
| Normal | 94% (91–96) | 96% (90–99) | 94% (89–98) | 92% (88–95) | 0.262‡ |
| Mild hearing loss, not sufficient to require aids | 5% (4–8) | 2% (0–7) | 6%(2–11) | 7% (4–10) | |
| Hearing loss requiring aids or severe sensorineural hearing loss despite aids | 1% (0–2) | 2% (0–7) | 0% (0–3) | 1% (0–4) |
Results are presented as mean (SD) or as proportion (95% CI). Dutch version of the WPPSI-III-NL was available for 365 subjects. Dutch version of the M-ABC-2-NL was available for 383 subjects. Information on neurological exam, vision and hearing was available for 484 subjects (24 weeks, n=95, 25 weeks n=145 and 26 weeks n=244). The p value reflects differences between 24, 25 and 26 weeks’ gestation.
*Significantly lower compared with the normative mean, all p<0.001.
†P value is the result of a one-way analysis of variance applied on all three GA groups.
‡P value is the result of a χ2 test applied on all three categorical rows of the score. P<0.05 is considered significant.
CA, corrected age; CP, cerebral palsy; FSIQ, full-scale IQ; GA, gestational age; GMFCS, Gross Motor Function Classification System; M-ABC-2-NL, Movement Assessment Battery for Children; PIQ, performance composite score; PSQ, processing speeds composite score; VIQ, verbal composite score; WPPSI-III-NL, Wechsler Preschool and Primary Scale of Intelligence Scale-III.
The mean total motor score was 5.8 (SD 3.0), 6.6 (SD 3.4) and 7.1 (SD 3.3) for 24-week, 25-week and 26-week children, respectively (p=0.024, table 2). For the total cohort, all (sub)scales were significantly lower compared with the normative mean of 10.
Among all children with follow-up data available, 46% (95% CI 41 to 50) had no impairment, 36% (95% CI 32 to 41) had a mild impairment and 18% (95% CI 15 to 22) were children with NDI. The number of NDI decreased significantly from 23% at 24 weeks to 13% at 26 weeks’ GA (p=0.019, table 3). Including developmental coordination disorders in the definition of mild impairment increased proportions by 11%, 10% and 12% among 24-week, 25-week and 26-week children, respectively.
Table 3.
Neurodevelopmental results at 5.5 years of all 484 infants, born <27 weeks’ GA who reached 5.5 years’ CA in 2018–2020, separately for infants born at 24, 25 and 26 weeks’ gestation
| Total | 24 weeks | 25 weeks | 26 weeks | P value | |
| Impairment categorisation at 5.5 years’ CA, N evaluated | 484 | 95 | 145 | 244 | |
| None | 46% (41–50) | 40% (28–48) | 42% (34–51) | 51% (44–57) | 0.044* |
| Mild | 36% (32–41) | 39% (29–50) | 35% (27–44) | 36% (30–42) | |
| Moderate-to-severe | 18% (15–22) | 23% (15–33) | 23% (16–31) | 13% (9–18) | |
| Impairment categorisation at 2 years’ CA, N evaluated | 472 | 88 | 143 | 241 | |
| None | 61% (57–66) | 53% (43–64) | 60% (52–68) | 65% (59–71) | 0.116* |
| Mild | 31% (27–35) | 35% (25–46) | 29% (22–38) | 30% (24–36) | |
| Moderate-to-severe | 8% (6–11) | 11% (6–20) | 11% (6–17) | 5% (3–9) |
Of 484 children seen at 5.5 years’ CA, data of 472 children were also available at 2 years’ CA. Results are presented as % (95% CI).
*P value is the result of a χ2 test applied on all three categorical rows of the score. A p value <0.05 is considered significant.
CA, corrected age; GA, gestational age.
The number of children that survived without NDI relative to all live born infants increased from 30% at 24 weeks’ GA to 67% at 26 weeks’ GA (p<0.001, table 4, online supplemental appendix 3).
Table 4.
Summary of outcomes with imputed ‘survival without moderate-to-severe impairment’ among extremely preterm born children at 5.5 years of age
| 24 weeks’ GA | 25 weeks’ GA | 26 weeks’ GA | |
| Outcome | N=286 | N=278 | N=439 |
| Died in delivery room | 58 (20.3) | 23 (8.3) | 9 (2.1) |
| Admitted to NICU | 228 (79.7) | 255 (91.7) | 430 (97.9) |
| Died | 113 (49.6) | 76 (29.8) | 92 (21.4) |
| Survived to 5.5 years | 115 (50.7) | 179 (70.2) | 338 (78.6) |
| Lost to follow-up | 20 (17.4) | 34 (19.0) | 94 (27.8) |
| Follow-up data available | 95 (82.6) | 145 (81.0) | 244 (72.2) |
| Moderate-to-severe impairment | 23% (15–33) | 23% (16–31) | 13% (9–18) |
| Mild impairment | 39% (29–50) | 35% (27–44) | 36% (30–42) |
| Survived without moderate-to-severe impairment, after imputation of infants lost to follow-up | |||
| As a percentage of live births | 30% (25–36) | 49% (43–55) | 67% (63–72) |
| As a percentage of NICU admissions | 38% (32–45) | 54% (47–60) | 69% (64–73) |
Results are presented as n (%) or as % (95% CI). Overall impairment status was imputed for 148 children who survived without follow-up data available, using an imputation model containing all baseline characteristics including SES.
GA, gestational age; NICU, neonatal intensive care unit; SES, socioeconomic status.
Changes in categorisation of neurodevelopmental outcome between 2 and 5.5 years’ CA
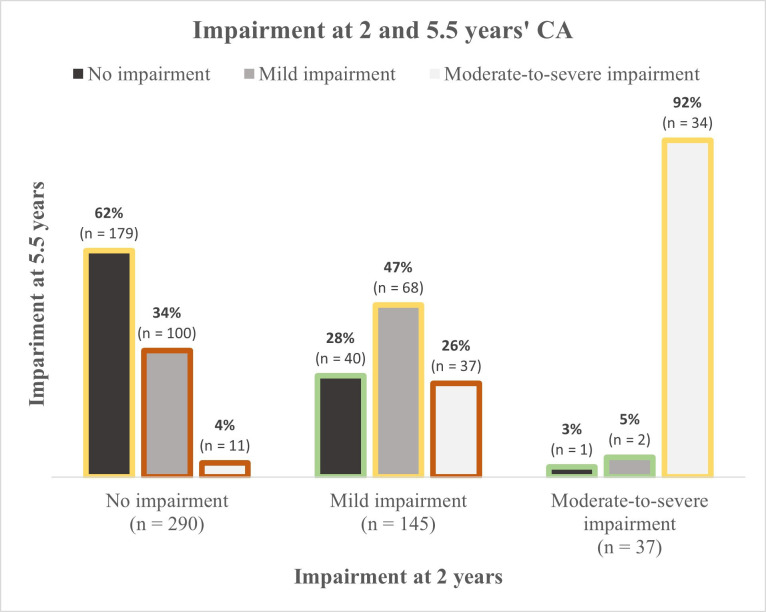
The overall number of children with moderate-to-severe impairment increased from 8% at 2 years to 18% at 5.5 years’ CA (table 3). From 2 to 5.5 years’ CA, 60% (281/472) remained in the same impairment category at 5.5 years of age, while 9% (43/472) were categorised better and 31% (148/472) were categorised worse (figure 1). The change to a lower category from age 2 to 5 years was not related to perinatal characteristics, but only to low maternal education.
Figure 1.
Severity of impairment at 2 and 5.5 years age among 472 children with impairment status available at 2 and 5.5 years’ corrected age (CA).
Discussion
This national cohort study aimed to assess neurodevelopmental outcome at 5.5 years’ CA in extremely preterm infants during a 3-year period, after the threshold of active treatment was lowered from 25 to 24 weeks’ gestation in 2010. Due to that change in active treatment, 10% more extremely preterm live born infants survived to discharge. At 5.5 years’ CA, mild impairment was seen in 36% of the children and moderate-to-severe impairment (ie, NDI) was seen in 18% of the children. The percentage of children that survived without NDI relative to all live born infants increased from 30% at 24 weeks’ GA to 49% and 67% at 25 and 26 weeks’ GA.
At 5.5 years’ CA, 46% of the surviving infants had no neurodevelopmental impairment, 36% had mild neurodevelopmental impairment and 18% of the children showed NDI. In 2018, a meta-analysis was published including 15 cohorts with a broad geographical representation, presenting neurodevelopmental impairment in preterm survivors at age 4–10 years.23 Rates of moderate-to-severe neurodevelopmental impairment (ie, NDI) were 32% (95% CI 25 to 39) at 24 weeks and 23% (95% CI 18 to 29) at 25 weeks. For 24-week infants, the EPI-DAF outcome (23% (95% CI 15 to 33)) is slightly lower than the lower CI boundary of the meta-analysis. For 25-week infants, our results (23% (95% CI 16 to 31)) are within the range of the meta-analysis CI.
To put these rates in perspective, the denominator of the outcome parameter is relevant. While numbers in the previous paragraph are related to survivors, the rate of survival needs to be taken into account as well. In this study, calculating NDI relative to admitted infants, this study showed that 38%, 54% and 69% of the admitted infants born at 24, 25 and 26 weeks’ gestation, respectively had moderate-to-severe impairment-free survival up to 5.5 years’ CA. The EPICure study (birth cohort 1995) showed lower rates of survival without severe or moderate impairment at 6 years of age in only 12% and 24% of the 24-week and 25-week admitted infants, respectively.6 The EXPRESS (Extremely Preterm Infants in Sweden Study) study (birth cohort 2004–2007) related rates of NDI to live born infants, and showed survival without moderate-to-severe impairment at 6.5 years of age in 35%, 50% and 57% of 24-week, 25-week and 26-week live births, compared with 30%, 49% and 67% in this study.11 Although the moderate-to-severe impairment-free survival rates appear higher in the EPI-DAF study compared with the EPICure and EXPRESS studies, it should be acknowledged that the EPI-DAF cohort (birth cohort 2012–2015) is a substantially more contemporary cohort.
Considerable changes in neurodevelopmental outcome were seen between 2 and 5.5 years’ CA. Only 60% of the infants were classified in the same impairment category at both follow-up moments. Moreover, only 41% (34/82) of the children with NDI at 5.5 years’ CA was already identified at 2 years’ CA. The relationship between disability rates over time has been discussed previously, showing that, although severe disability at early age is highly predictive of outcome at 5–6 years, it is hard to identify later onset disabilities or less severe disabilities at early age.6 11 25 For upcoming trials, measuring outcomes after the age of 2 years is highly recommended, as these results are more likely to be predictive to problems that will continue through life.
Apart from CP, many high-risk children have motor problems during infancy.26 As shown in our study, the rate of children with mild impairment increased with 10% when including developmental coordination disorders (defined a motor score ≤5th percentile) in the definition of mild impairment. It confirms that non-CP motor impairment can remain a risk for apparently healthy preterm infants.27 International inclusion of developmental coordination disorders in the definition of mild impairment will help to improve the complex description of difficulties in extremely preterm born children.22
Children that were categorised in a worse category at age 5.5 years were less often born to mothers with high education, compared with children that remained in the same category or improved. Although the association between the level of maternal education and neurodevelopment has been frequently reported, less is known on the effect of maternal education on the trajectory of neurodevelopment. One study determining trajectories of cognitive test scores from infancy to adulthood did not find different slopes for children born to mothers with high education versus lower education.5 However, another study showed that higher parental education may predict stronger gain in cognitive score from 1.5 to 5 years of age.28 The results from the current study underline that increased attention is necessary for children born to mothers with low education, to ensure that they will complete the follow-up programme up to later age and to initiate interventions on time.
Children lost to follow-up were significantly more often born to parents of lower SES. It has been shown previously that drop-outs are more likely to occur in families with social disadvantages, while preterm children from socially disadvantaged families may have poorer neurodevelopment.6 11 For presentation of overall outcome relative to all live born and admitted infants, a multiple imputation model including a SES variable that was available from the registry was used to account for possible selective missing data.
Strengths and limitations
The strengths of this study include a nationwide standardised follow-up programme as part of NICU aftercare, yielding standardised data for scientific purpose. Collection thereof in the EPI-DAF study database was done over a 3-year period with a creditable follow-up rate. Given the examination of impairment rates over time, age correction for prematurity was used to account for bias. However, this study has limitations as well. First, a weakness is the lack of a term control group, which might overrate favourable outcome. Impairment rates reported in this paper may be lower than the true rate of impairment. Second, the disability criteria did not include behaviour, attention and learning disabilities that are all commonly found among extremely preterm born children. Nevertheless, NDI as defined in our study is often used internationally as an outcome measure for neurodevelopmental outcome in very preterm infants. Third, due to COVID-19, for 29 children developmental outcome was determined based on medical history, partial assessment or phone interviews, instead of validated assessments. Fourth, currently no information is available on impairment rates prior to the guideline change. Last, although reflecting standard care, data were collected by different people, which might have introduced some intra-observer bias. However, standardised tests were used as much as possible.
Conclusions
The EPI-DAF study presents recent nationwide data on neurodevelopmental outcome at 5.5 years’ CA for extremely preterm infants, a few years after the treatment threshold was lowered countrywide from 25 to 24 weeks’ gestation. A considerable proportion of the surviving children did not have any impairment at 5.5 years’ CA. The number of children that survived without NDI relative to all live born infants increased from 30% at 24 weeks’ GA to 67% at 26 weeks’ GA. A twofold increase in moderate-to-severe impairment rates was seen between 2 and 5.5 years’ CA, with children. This emphasises the importance of continuing neonatal follow-up until school age. The current study provides valuable information to clinicians who are counselling parents antenatally who are at risk of delivering before 27 weeks’ gestation, and it might influence the Dutch NICU guidelines on management of extremely preterm infants.
Acknowledgments
The Extremely Preterm Infants-Dutch Analysis on Follow-up (EPI-DAF) study group is indebted to all paediatricians, psychologists, physiotherapists and research nurses who have contributed to the follow-up of extremely preterm infants in one of the 10 perinatal centres in the Netherlands. We would also especially like to thank the parents and children who were involved in the study. Research included in this paper was part of the thesis of the first author (PvB (2022)). Extremely Preterm Infants: Dutch Analysis on Follow-up (PhD Thesis 1 (Research TU/e /Graduation TU/e), Electrical Engineering, Eindhoven University of Technology). As such, preliminary data on the EPI-DAF study have been published.
Footnotes
Contributors: PvB, MR, AGvW-L, PA and EPI-DAF study group had a substantial contribution to the methodological design of the study. MR, HJtH, CK-E, EdK, ARCL, SMM, KS, RMS, EvW-K and AGvW-L had a substantial contribution to data acquisition, being the principal investigators of the 10 Dutch perinatal centres. PvB, MR, LB, AGvW-L and PA had a substantial contribution to the analysis and interpretation of the data. PvB wrote the first draft of the manuscript. PA was responsible for the financial funding of the project and supervised the project. PvB and PA are guarantors.
Funding: This project has been funded by an unrestricted grant from Stichting Tiny & Anny van Doorne Fonds. As this was a private grant, no grant number is available.
Disclaimer: The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Other data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
In accordance with the Dutch law on medical research involving human subjects, a waiver (no. N17.166) was provided by the medical ethical committee of Máxima MC (Veldhoven, The Netherlands) and approved by all other perinatal centres. Parents signed informed consent for research use of the follow-up data and for transport of data to the Dutch Perinatal Registry (Perined).
References
- 1. van Beek PE, Groenendaal F, Broeders L, et al. Survival and causes of death in extremely Preterm infants in the Netherlands. Arch Dis Child Fetal Neonatal Ed 2021;106:251–7. 10.1136/archdischild-2020-318978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linsell L, Johnson S, Wolke D, et al. Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely Preterm birth: a prospective cohort study. Eur Child Adolesc Psychiatry 2019;28:531–42. 10.1007/s00787-018-1219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torchin H, Morgan AS, Ancel P-Y. International comparisons of neurodevelopmental outcomes in infants born very Preterm. Semin Fetal Neonatal Med 2020;25:101109. 10.1016/j.siny.2020.101109 [DOI] [PubMed] [Google Scholar]
- 4. Anderson P, Doyle LW, Victorian Infant Collaborative Study Group . Neurobehavioral outcomes of school-age children born extremely low birth weight or very Preterm in the 1990s. JAMA 2003;289:3264–72. 10.1001/jama.289.24.3264 [DOI] [PubMed] [Google Scholar]
- 5. Linsell L, Johnson S, Wolke D, et al. Cognitive Trajectories from infancy to early adulthood following birth before 26 weeks of gestation: a prospective, population-based cohort study. Arch Dis Child 2018;103:363–70. 10.1136/archdischild-2017-313414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marlow N, Wolke D, Bracewell MA, et al. Neurologic and developmental disability at six years of age after extremely Preterm birth. N Engl J Med 2005;352:9–19. 10.1056/NEJMoa041367 [DOI] [PubMed] [Google Scholar]
- 7. Rysavy MA, Li L, Bell EF, et al. Between-hospital variation in treatment and outcomes in extremely Preterm infants. N Engl J Med 2015;372:1801–11. 10.1056/NEJMoa1410689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Beek PE, Rijken M, Broeders L, et al. Two-year neurodevelopmental outcome in children born extremely Preterm: the EPI-DAF study. Arch Dis Child Fetal Neonatal Ed 2022;107:467–74. 10.1136/archdischild-2021-323124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serenius F, Källén K, Blennow M, et al. Neurodevelopmental outcome in extremely Preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013;309:1810–20. 10.1001/jama.2013.3786 [DOI] [PubMed] [Google Scholar]
- 10. Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely Preterm children born in England in 1995 and 2006: the epicure studies. BMJ 2012;345. 10.1136/bmj.e7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serenius F, Ewald U, Farooqi A, et al. Neurodevelopmental outcomes among extremely Preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr 2016;170:954–63. 10.1001/jamapediatrics.2016.1210 [DOI] [PubMed] [Google Scholar]
- 12. Knijnenburg PJC, Spruijt MS, Jansen L, et al. Neurodevelopmental trajectories of Preterm born survivors of twin-twin transfusion syndrome: from birth to 5 years of age. J Pediatr 2022;240:51–7. 10.1016/j.jpeds.2021.09.002 [DOI] [PubMed] [Google Scholar]
- 13. van Beek PE, van der Horst IE, Wetzer J, et al. Developmental trajectories in very preterm born children up to 8 years: a longitudinal cohort study. Front Pediatr 2021;9. 10.3389/fped.2021.672214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Netherlands perinatal Registry (Perined); [DOI] [PubMed]
- 15. Aanbeveling Landelijke Neonatale Follow-up- NICU follow-up, Available: https://www.nvk.nl
- 16. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23. 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 17. Statistics Netherlands, Education level, Available: https://www.cbs.nl/en-gb/news/2018/20/well-being-not-distributed-equally/education-level [Accessed 30 Nov 2021].
- 18. Netherlands Institute for Social Research (SCP), SCP Statusscores 2017, Available: http://www.scp.nl/Formulieren/Statusscores_opvragen
- 19. Hendriksen J, Hurks P. WPPSI-III-NL | Wechsler preschool and primary scale of intelligence. Manual 2009. [Google Scholar]
- 20. Doyle LW, Anderson PJ. Do we need to correct age for Prematurity when assessing children? J Pediatr 2016;173:11–2. 10.1016/j.jpeds.2016.03.038 [DOI] [PubMed] [Google Scholar]
- 21. Smits-Engelsman BC. Movement ABC-2 NL | movement assessment battery for children. Manual 2010. [Google Scholar]
- 22. Pierrat V, Marchand-Martin L, Marret S, et al. Neurodevelopmental outcomes at age 5 among children born Preterm: EPIPAGE-2 cohort study. BMJ 2021;373:741. 10.1136/bmj.n741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding S, Lemyre B, Daboval T, et al. A meta-analysis of neurodevelopmental outcomes at 4-10 years in children born at 22-25 weeks gestation. Acta Paediatr 2019;108:1237–44. 10.1111/apa.14693 [DOI] [PubMed] [Google Scholar]
- 24. van Baar AL, van Wassenaer AG, Briët JM, et al. Very Preterm birth is associated with disabilities in multiple developmental domains. J Pediatr Psychol 2005;30:247–55. 10.1093/jpepsy/jsi035 [DOI] [PubMed] [Google Scholar]
- 25. Roberts G, Anderson PJ, Doyle LW, et al. The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very Preterm children born in 1997. Archives of Disease in Childhood 2010;95:786–90. 10.1136/adc.2009.160283 [DOI] [PubMed] [Google Scholar]
- 26. Brown RN, Burnett AC, Thompson DK, et al. Motor performance and attention outcomes in children born very Preterm. Dev Med Child Neurol 2023;65:1501–10. 10.1111/dmcn.15620 [DOI] [PubMed] [Google Scholar]
- 27. Hee Chung E, Chou J, Brown KA. Neurodevelopmental outcomes of Preterm infants: a recent literature review. Transl Pediatr 2020;9(Suppl 1):3–8. 10.21037/tp.2019.09.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manley BJ, Roberts RS, Doyle LW, et al. Social variables predict gains in cognitive scores across the preschool years in children with birth weights 500 to 1250 grams. J Pediatr 2015;166:870–6. 10.1016/j.jpeds.2014.12.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fetalneonatal-2023-325732supp001.pdf (1.3MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Other data may be obtained from a third party and are not publicly available.