Abstract
Introduction
Chronic pain patients may experience impairments in multiple health-related domains. The design and interpretation of clinical trials of chronic pain interventions, however, remains primarily focused on treatment effects on pain intensity. This study investigates a novel, multidimensional holistic treatment response to evoked compound action potential-controlled closed-loop versus open-loop spinal cord stimulation as well as the degree of neural activation that produced that treatment response.
Methods
Outcome data for pain intensity, physical function, health-related quality of life, sleep quality and emotional function were derived from individual patient level data from the EVOKE multicenter, participant, investigator, and outcome assessor-blinded, parallel-arm randomized controlled trial with 24 month follow-up. Evaluation of holistic treatment response considered whether the baseline score was worse than normative values and whether minimal clinical important differences were reached in each of the domains that were impaired at baseline. A cumulative responder score was calculated to reflect the total minimal clinical important differences accumulated across all domains. Objective neurophysiological data, including spinal cord activation were measured.
Results
Patients were randomized to closed-loop (n=67) or open-loop (n=67). A greater proportion of patients with closed-loop spinal cord stimulation (49.3% vs 26.9%) were holistic responders at 24-month follow-up, with at least one minimal clinical important difference in all impaired domains (absolute risk difference: 22.4%, 95% CI 6.4% to 38.4%, p=0.012). The cumulative responder score was significantly greater for closed-loop patients at all time points and resulted in the achievement of more than three additional minimal clinical important differences at 24-month follow-up (mean difference 3.4, 95% CI 1.3 to 5.5, p=0.002). Neural activation was three times more accurate in closed-loop spinal cord stimulation (p<0.001 at all time points).
Conclusion
The results of this study suggest that closed-loop spinal cord stimulation can provide sustained clinically meaningful improvements in multiple domains and provide holistic improvement in the long-term for patients with chronic refractory pain.
Trial registration number
Keywords: spinal cord stimulation, chronic pain, treatment outcome
WHAT IS ALREADY KNOWN ON THIS TOPIC
It is well known and widely accepted that pain intensity alone does not fully capture the chronic pain experience and as such may be a poor metric to assess treatment response.
An expert panel previously proposed the concept of holistic treatment response as a framework to assess wider health impacts of treatments for chronic pain.
WHAT THIS STUDY ADDS
Using validated normative values and minimal clinical important differences for outcomes of interest for healthcare providers and patients, we evaluated the holistic treatment response to closed-loop spinal cord stimulation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The composite outcome of holistic treatment response used in this study provides a comprehensive approach to the assessment of treatment impact on chronic pain patients’ overall health and well-being.
The methods and concepts presented in this study have the potential to lead to a paradigm change in the assessment of response to spinal cord stimulation and other treatments for chronic pain.
Introduction
Chronic pain is a complex, multifaceted condition that is largely defined by the patient’s subjective experience. Since all pain is mediated by processing in the nervous system and may affect psychological features such as hypervigilance, emotional reactions, and avoidant behavior, it can be considered that pain is fundamentally a psychophysiological phenomenon.1 Many chronic pain patients experience deterioration in multiple domains including mental health, sleep quality, physical function, and overall health-related quality of life (HRQoL).2 Although many of these domains are now assessed in clinical trials of chronic pain interventions, the design and interpretation of such trials remain primarily focused on the magnitude of treatment effects on pain intensity alone. Consideration of several health-related domains in addition to pain intensity provides a more holistic view of the patient’s health state and treatment needs.2 A holistic assessment considers the critical biopsychosocial components of the pain experience for each individual patient by evaluating their impairment in each domain. Evaluation of a more holistic treatment response (ie, improvement in multiple impaired domains) aligns with the biopsychosocial complexity of the chronic pain experience, providing a more comprehensive synthesis of the overall patient outcomes, resulting in greater utility for patients and clinicians.3 Pain intensity, as a standalone measurement, is a poor assessment strategy for treatment of chronic pain.
Spinal cord stimulation (SCS) is a recognized and approved non-pharmacological intervention (sometimes referred to as electroceutical) for the management of several chronic intractable pain syndromes. As SCS technology advances and substantial reductions in pain intensity are more commonly observed in clinical trials with long-term follow-up,4–6 the pursuit of a more comprehensive, holistic treatment response measure beyond pain intensity alone has garnered increased interest.7–11 SCS involves the application of electrical stimuli to activate target spinal cord cells and/or fibers that contribute to the inhibition of pain transmission in the dorsal horn of the spinal cord.12 Historically, this activation has been established by patient report of paresthesia alone or assumed by the anatomical positioning of the SCS leads in paresthesia-free stimulation.11
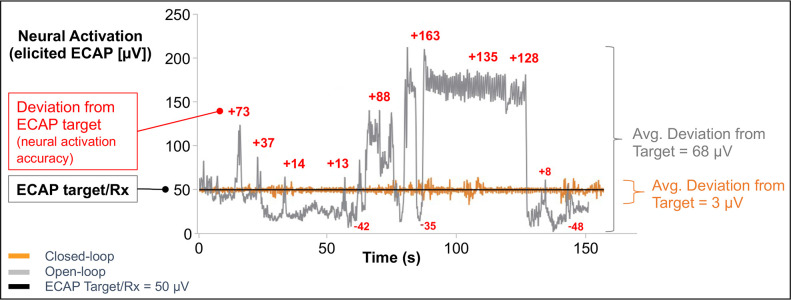
A novel SCS system uses evoked compound action potentials (ECAPs), an objective physiological biomarker for therapeutic activation of the spinal cord, to guide programming and optimize the neural activation and its accuracy. Closed-loop SCS (CL-SCS) automatically adjusts the output of the pulse generator with each pulse using real-time measured ECAPs and thus responds to this dynamic environment to deliver and maintain precise spinal cord activation at the targeted ECAP level. In contrast, the inherently dynamic relationship between the electrodes and spinal cord in all SCS paradigms means that open-loop SCS (OL-SCS) may not always produce the optimal neural response (figure 1).13
Figure 1.
Neural activation and activation accuracy during open-loop and closed-loop SCS. Neural activation accuracy is the ability of the SCS device to adhere to the target neural response (ie, ECAP, prescription (Rx)) (black line at 50 µV in this example). In open-loop (gray line), with a fixed amplitude of 1.1 µC/pulse, over the course of 150 s, there was an average deviation from the target of 68 µV, which produced an erratic neural activation (elicited ECAP). In closed-loop (orange line), performing the same posture assessment, with the stimulation amplitude continuously modified with each pulse for a range of 0.5–1.4 µC, there was an average deviation of 3 µV, which produced a neural activation of 50 µV (aligned with the target ECAP). The neural activation (elicited ECAP) is more accurate in closed-loop (orange line), on average only deviating 3 µV from the ECAP target of 50 µV. In contrast, the neural activation is less accurate in open-loop, as can be observed by the more erratic gray line, on average deviating 68 µV from the ECAP target. Data are from two postural assessments (open-loop and closed-loop) for a single patient. ECAP, evoked compound action potential; SCS, spinal cord stimulation.
Using individual patient-level data from the EVOKE randomized controlled trial (RCT),14 the aim of this study is to investigate a novel holistic treatment response methodology in patients receiving ECAP-controlled CL-SCS compared with those receiving OL-SCS for the management of chronic intractable back and/or leg pain. Outcomes were evaluated through 24-month follow-up as was the degree of neural activation producing that treatment response.
Methods
Study design and participants
The EVOKE study (registered on ClinicalTrials.gov, October 5, 2016; NCT02924129) is a multicenter, participant, investigator, and outcome assessor-blinded, parallel-arm RCT conducted in 13 centers in the USA with patients randomized 1:1 to CL-SCS or OL-SCS. The study was conducted from January 27, 2017 (first patient enrolled) to September 9, 2022 (last patient complete). Full details of study design, patient demographics and random allocation sequence are presented elsewhere.4 14 In brief, the study eligibility criteria used was pragmatic and reflects clinical practice. The final patient selection was overseen by an independent medical monitor prior to randomization. The study was conducted in compliance with ethical and regulatory guidelines and was approved by local ethics committees prior to subject enrollment. Outcomes were assessed at 3-month, 12-month and 24-month follow-up with blinding of both the participants and the investigator team. The baseline patient demographics and characteristics were well balanced between the CL-SCS and OL-SCS groups. This study uses outcome data collected through 24-month follow-up.
Intervention and comparator
The Evoke SmartSCS System (Saluda Medical, Sydney, Australia) was used to deliver both CL-SCS (intervention) and OL-SCS (comparator). Measurement of the neural response elicited by SCS (ie, ECAPs) on every stimulation pulse was performed in both CL-SCS and OL-SCS modes to confirm activation of the intended target. In CL-SCS mode, the ECAP neural response was measured, compared with the target ECAP, and the stimulation current automatically adjusted in real time to maintain optimal neural activation at the prescribed level. In OL-SCS mode (feedback mechanism off), the system delivered fixed-output stimulation as with other existing SCS systems. When operating in OL mode, however, the system used ECAPs to confirm activation of the intended target and to inform program settings.
The type of stimulation (CL or OL) was enabled during the screening trial phase. Trial participants and investigators were both blinded to the settings and could not change the device settings from CL to OL and vice versa. The trial and implant procedures for both CL-SCS and OL-SCS were performed according to usual practice. Two percutaneous leads were placed in the dorsal epidural space over the dorsal column and connected to the external trial pulse generator during the trial phase or the pulse generator was implanted in a subcutaneous pocket for the permanent implant.
Outcome measures
The outcome measures were collected according to IMMPACT recommendations15 and assessed biopsychosocial components of chronic pain such as pain intensity, physical function, HRQoL, sleep quality and emotional function. Pain intensity was assessed using a 100 mm Visual Analog Scale (VAS) ranging from 0 (no pain) to 100 (worst possible pain).16 The VAS is considered a reliable and valid measure of subjective phenomena including chronic pain.16 17
Physical function was measured using the Oswestry Disability Index (ODI) to assess the level of pain interference with various activities of daily living. The ODI is a validated measure of condition-specific disability.18 It consists of 10 items/activities each with six scoring levels (range 0–5). The ODI is a core outcome measure to assess physical function in people with nonspecific low back pain and a commonly used secondary outcome of RCTs of SCS.19
HRQoL was assessed using the EQ-5D-5L. The EQ-5D-5L descriptive system comprises five dimensions (mobility, self-care, usual activities, pain/discomfort and depression/anxiety), where each dimension has five response levels: no problems, slight problems, moderate problems, severe problems, unable to/extreme problems.20 Responses to the EQ-5D-5L were converted into single (utility) indices using the US value set for EQ-5D-5L crosswalk to EQ-5D-3L.21
The Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality. The PSQI is a validated tool that comprises 19 individual items that generate seven component scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime impairment); the sum of the component scores produces a single global score.22
The Profile of Mood States-Brief (POMS-B) enables assessment of emotional function in response to therapeutic interventions. The POMS-B is a validated tool that consists of 30 adjectives that describe feelings or moods that an individual may have experienced during the prior week.23 A total score (total mood disturbance) is derived from six mood states (tension, depression, anger, vigor, fatigue, and confusion).
Holistic treatment assessment
‘Holistic’ indicates that a system and its properties are analyzed as a whole, in a global and integrated way. More detailed definition of holistic treatment response and its assessment have been described elsewhere.24 Such a holistic treatment response is plausible with SCS given the multiple physical and affective components of chronic pain.25 26 The holistic treatment response is a composite outcome that combines validated information from the five separate outcome domains outlined above.24 The holistic response also takes account of whether baseline scores for each outcome domain were indicative of impairment based on validated normative values.
Response across domains: breadth of response
The breadth of treatment response reflects having reached a validated minimal clinical important difference (MCIDs) in multiple domains. Multimodal treatment response was defined as improving by at least one MCID in at least two impaired domains. Holistic treatment response was defined as improving by at least one MCID in each of the domains that a patient reported as impaired at the time of baseline assessment. An additional concept of percent whole (proximity to attainment of holistic treatment response) and results are presented in online supplemental material 1.
rapm-2023-104639supp001.pdf (390.6KB, pdf)
Cumulative response: depth of response
Depth of treatment response is defined by the number of MCIDs attained for each impaired domain at baseline. Cumulative responder score reflects the total number of MCIDs obtained across all domains. MCIDs considered and population normative values are presented in table 1. A holistic MCID considers the cumulative responder score adjusted for the number of impaired domains at baseline for each patient.
Table 1.
Minimal clinical important differences and population normative values
| Domain | Normative value | MCID responder thresholds | Cumulative MCIDs (examples) |
| VAS | <60 mm (EVOKE RCT eligibility criterion)14 |
≥30% decrease=1 MCID35 | 50% decrease=1.67 MCID 80% decrease=2.67 MCID |
| ODI | <10.19 (normative value)14 18 |
≥10 point decrease=1 MCID36 | 15-point decrease=1.5 MCID 20-point decrease=2 MCID |
| EQ-5D | 0.830 (US normative value for 55–64 years)37 |
≥0.074 point increase=1 MCID38 | 0.148-point increase=2 MCID 0.1665-point increase=2.25 MCID |
| PSQI | 6.3 (US community sample)39 |
≥3 point decrease=1 MCID40 | 4-point decrease=1.33 MCID 6-point decrease=2 MCID |
| POMS | 17.7 (US adult normative value)23 |
≥10 point decrease=1 MCID35 | 15-point decrease=1.5 MCID 20-point decrease=2 MCID |
MCID, minimal clinical important difference; ODI, Oswestry Disability Index; POMS, Profile of Mood States; PSQI, Pittsburgh Sleep Quality Index; RCT, randomized controlled trial; VAS, Visual Analog Scale.
Neural activation in SCS therapy
Objective neurophysiological and device measurements were automatically recorded and collected on the device. Data were collected both in-clinic and outside the clinic (during home use) to provide an assessment of the degree of spinal cord activation that underpins the likely impact on patient-reported outcomes. The measurements analyzed included: an in-clinic metric of device performance using deviation from the prescribed target neural activation (ie, neural activation accuracy) and out-of-clinic neural activation (ie, mode ECAP amplitude elicited) and system utilization; in combination these metrics provide a quantitative method to evaluate a subject’s overall physiological response to SCS. Neural activation accuracy is calculated using root mean square error (RMSE, µV) to determine the deviation of the observed ECAP response from the prescribed ECAP. The mode ECAP (µV) was defined as the most frequent spinal cord activation level for the week up to the scheduled visit. System utilization was defined as the proportion of time the system was on for the week prior to the scheduled visit.
Data analysis
The sample size calculation, 3-month, 12-month and 24-month analysis of the primary and secondary outcomes have been previously described.4 14 The analysis included all randomized patients and followed the intention-to-treat principle with patients analyzed by group according to random allocation with missing follow-up outcomes imputed using last value carried forward.4 27 The primary analysis assessed patients with impairment at baseline for each of the domains. Sensitivity analysis assessed both patients with impairment at baseline for all the domains and all patients randomized regardless of impairment at baseline (online supplemental material 2, tables S1–S4).
The proportion of patients with CL-SCS or OL-SCS that reached MCIDs for the outcomes evaluated were calculated (absolute risk difference and 95% CI) at 3-month, 12-month and 24-month follow-up. Descriptive statistics are provided as mean±SD, median (IQR), or number of observations (percentage), as appropriate. Differences in categorical variables between treatment groups were evaluated using Fisher’s exact test and continuous measures with two-sample t-tests. Statistical significance was judged at the 5% level. All statistical analysis were conducted using SAS statistical software V.9.4 (SAS Institute).
Results
One-hundred and thirty-four patients were enrolled in the EVOKE RCT and randomized to CL-SCS (n=67) or OL-SCS (n=67) (Consolidated Standards of Reporting Trials (CONSORT) flow diagram available in online supplemental material 3, figure S1). Following a screening trial phase, 113 patients underwent implantation of the device (59 in the CL-SCS and 54 in the OL-SCS group). Follow-up assessments were completed by 58, 55, and 50 patients randomized to CL-SCS patients and by 53, 48, and 42 patients randomized to OL-SCS at 3 months, 12 months, and 24 months, respectively. There were no differences between groups in prescribed stimulation parameters for frequency, pulse duration, stimulation amplitude or ECAP neural activation for perception threshold, comfort or maximum level the patient could tolerate.4
At baseline, 100% of patients (134/134) presented scores worse than normative population values for pain intensity (VAS), physical function (ODI) and HRQoL (EQ-5D-5L), 97% (130/134) were also impaired for sleep quality (PSQI) and 61% (82/134) for emotional function (POMS). Ninety-nine per cent (132/134) of patients presented impaired scores for 4 domains and 60% (80/134) for all the five outcome domains (online supplemental material 4, figure S2). There were no statistically significant differences between CL-SCS and OL-SCS groups in baseline impairment.
Treatment response
SCS with ECAP-guided programming resulted in treatment response (defined as attainment of at least one MCID, so-called ‘responder’ or ‘responded’) in multiple impaired domains with both CL and OL-SCS up to 24 month follow-up.
A greater proportion of patients improved by at least one MCID for each of the domains, at all time points with CL-SCS than with OL-SCS (online supplemental material 5, table S5). In addition, a greater proportion of patients with CL-SCS consistently obtained a wider breadth of response, reflected by response in more impaired domains than patients with OL-SCS (table 2). A significantly greater proportion of patients with CL-SCS were holistic treatment responders at 24-month follow-up, with at least one MCID in all impaired domains (absolute risk difference: 22.4%, 95% CI 6.4% to 38.4%, p=0.01) (figure 2).
Table 2.
Multimodal and holistic treatment response—breadth of response
| 3 months | 12 months | 24 months | ||||
| Closed-loop SCS | Open-loop SCS | Closed-loop SCS | Open-loop SCS | Closed-loop SCS | Open-loop SCS | |
| Multimodal treatment responders (MCID in at least two impaired domains out of VAS≥30%, ODI≥10, EQ-5D≥0.074, PSQI≥3, POMS≥10) | ||||||
| Responders in ≥1 impaired domain (%) | 63/67 (94.0) | 58/67 (86.6) | 63/67 (94.0) | 56/67 (83.6) | 63/67 (94.0) | 56/67 (83.6) |
| Responders in ≥2 impaired domains (%) | 56/67 (83.6) | 51/67 (76.1) | 55/67 (82.1) | 45/67 (67.2) | 53/67 (79.1) | 45/67 (67.2) |
| Responders in ≥3 impaired domains (%) | 54/67 (80.6) | 46/67 (68.7) | 49/67 (73.1) | 39/67 (58.2) | 47/67 (70.1) | 39/67 (58.2) |
| Responders in ≥4 impaired domains (%) | 41/66 (62.1) | 34/66 (51.5) | 41/66 (62.1) | 30/66 (45.5) | 38/66 (57.6) | 27/66 (40.9) |
| Responders in five impaired domains (%) | 25/44 (56.8) | 9/36 (25.0) | 28/44 (63.6) | 10/36 (27.8) | 23/44 (52.3) | 6/36 (16.7) |
| Holistic treatment responder (%) | 36/67 (53.7) | 26/67 (38.8) | 38/67 (56.7) | 26/67 (38.8) | 33/67 (49.3) | 18/67 (26.9) |
| Risk difference (%) and 95% CI | 14.9 (-1.8, 31.6), p=0.12 | 17.9 (1.3, 34.6), p=0.06 | 22.4 (6.4, 38.4), p=0.01 | |||
The denominator for the number of patients varies according to the number of domains impaired at baseline.
.MCID, minimal clinical important difference; ODI, Oswestry Disability Index; POMS, Profile of Mood States; PSQI, Pittsburgh Sleep Quality Index; SCS, spinal cord stimulation; VAS, Visual Analog Scale.
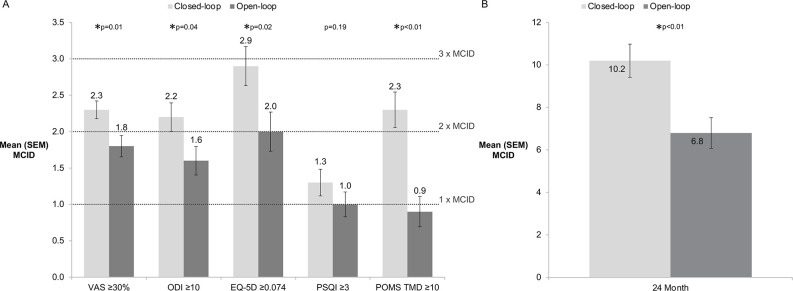
Figure 2.
Breadth and depth of treatment response 24 months after implantation of SCS device. (A) MCIDs obtained for each impaired domain (mean and SE of mean (SEM)). (B) Cumulative Responder Score (mean and SE of mean). MCID, minimal clinical important difference; ODI, Oswestry Disability Index; POMS, Profile of Mood States; PSQI, Pittsburgh Sleep Quality Index; SCS, spinal cord stimulation; TMD, total mood disturbance; VAS, Visual Analog Scale.
Similarly, patients with CL-SCS obtained a greater intensity of response than patients with OL-SCS with a greater number of MCIDs for each of the domains at all time points (figure 3). Significant differences in mean number of MCIDs were observed for at least one time point in each domain; pain intensity and emotional function (both 3, 12 and 24 months), sleep (12 months), physical function and HRQoL (24 months). The cumulative responder score, which reflects the total number of MCIDs obtained across all domains, was significantly greater for CL-SCS patients at all time points and resulted in more than three additional MCIDs at 12-month and 24-month follow-up (online supplemental material 6, table S6). The holistic MCID was significantly greater for CL-SCS patients when compared with OL-SCS patients at 12-month (mean difference (MD) 0.6, 95% CI 0.1 to 1.1, p=0.02) and 24-month follow-up (MD 0.6, 95% CI 0.2 to 1.1, p<0.01) (online supplemental material 6, table S6).
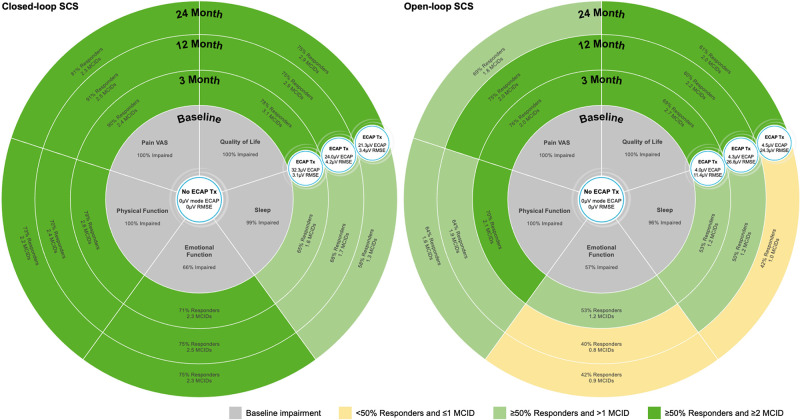
Figure 3.
Durability of breadth and depth of treatment response with closed-loop and open-loop SCS ECAP treatment (Tx) was defined by neural activation (mode ECAP) and accuracy (RMSE), as well as system utilization. CL-SCS provided statistically significant greater neural activation and accuracy at all time points (depicted in the figure). Both arms had high system utilization greater than 80% at all time points (3 months (closed-loop 88%, open-loop 92%); 12 months (closed-loop 82%, open-loop 83%); 24 months (closed-loop 86%, open-loop 82%)). CL-SCS, closed-loop SCS; ECAP, evoked compound action potentials; MCID, minimal clinical important difference; RMSE, root mean square error; SCS, spinal cord stimulation; VAS, Visual Analog Scale.
Sensitivity analysis based on patients with impairment in all domains at baseline showed a higher response rate for each of the domains, significantly more MCID improvements, per cent whole and greater proportions of patients with CL-SCS obtaining a holistic treatment response at all time points (online supplemental material 2, tables S1 and S2). Sensitivity analysis considering all patients regardless of impairment at baseline also shows superior results for those patients that received CL-SCS (online supplemental material 2, tables S3 and S4).
Neural activation and system utilization
Utilization of the SCS system was similar between treatment groups with patients having the system turned on for more than 80% of the time at all time points (online supplemental material 7, table S7). The degree of neural activation, however, was greater for the CL-SCS group as compared with the OL-SCS group at all time points. The most frequent neural activation (ECAP elicited) was at least four times greater in closed-loop than in OL patients (p<0.001 at all time points). Additionally, neural activation accuracy (the deviation of the observed ECAP response from the target ECAP response) was at least three times more accurate in the CL-SCS group compared with the OL-SCS group (p<0.001 at all time points).
Discussion
The field of neuromodulation for chronic pain has developed beyond using pain intensity as the single subjective measure of success to endeavor towards personalized medicine. It has been recognized that pain intensity alone does not adequately capture the entire chronic pain experience and by focusing primarily on pain reduction, clinical trials of chronic pain may potentially miss clinically important responses in other outcome domains (such as physical function, HRQoL, sleep and emotional function) that are valued by patients.27 In the current study, we observed that 81% of CL-SCS and 69% of OL-SCS patients improved by at least one MCID for pain intensity but a higher proportion of patients (CL-SCS=94%, OL-SCS=84%), were considered responders when other impaired domains are taken into account. In other words, 13% of CL-SCS and 15% of OL-SCS patients who did not improve by at least one MCID in pain intensity still obtained clinically important differences in another impaired outcome domain of physical function, HRQoL, sleep or emotional function. Although there were no significant differences in PSQI at 24 months, the improvement by at least one MCID in both groups was greater than that reported in a previous OL-SCS RCT.28 In parallel with the holistic response observed, 66.7% (18/27) of CL-SCS patients and 56.5% (13/23) of OL-SCS patients taking opioids at baseline voluntarily reduced their opioid use at a clinically meaningful level.4
Evaluation of a holistic treatment response is paramount in chronic pain populations given that impairment can be present in several domains other than just pain intensity. In the EVOKE study, all included patients presented impairment in three outcome domains and a majority (60%) were impaired in five distinct outcome domains. By implication, a substantial number of patients with chronic refractory pain and experiencing significant impairment across multiple health-related domains can obtain a holistic treatment response to SCS (ie, improving by at least 1 MCID for each impaired outcome domain prior to SCS). Importantly, responders in multiple domains were observed as early as 3 months following SCS implantation and sustained up to 24 months later. This was particularly evident in the CL-SCS group which showed responder rates above 50% across multiple domains over this period. The superior patient-reported outcomes observed with CL-SCS show that ECAP-controlled therapy was delivered with greater accuracy and that this can provide a greater breadth and depth of improvements in multiple domains. This is unique in the current SCS literature.
Previous studies have defined a holistic treatment response across multiple outcome domains in a variety of different ways, for example, a clinical improvement in two outcomes with or without a response in pain intensity,9 reduction in VAS and/or ODI without an increase in either,29 any of four outcomes and score within normal population values at follow-up,7 development of a new outcome based on three different domains,8 and models considering overall pain intensity and walking time.10 Response in pain intensity should still be considered since it was often ranked higher as a treatment goal in a survey of patients with chronic pain,30 and remains the key interventional intent when treating a population with chronic pain. However, a holistic view that takes into account the level of response across a range of outcome domains provides a more comprehensive understanding of the treatment effects in a complex condition such as chronic pain. In comparison to pain response alone, a composite holistic response provides a better summary of the totality of treatment effectiveness that is important for patients and their families, physicians, and payers alike.
CL-SCS offers a more transparent measure of therapy efficacy based on physiologic markers that provides direct evidence of the therapy effect than OL-SCS systems that deliver a set stimulation intensity without regard for how the spinal cord neurons respond. We have shown that CL-SCS can result in true relief of the complex, multifactorial personal experience that patients describe as chronic pain. In the current study, both CL-SCS and OL-SCS arms underwent ECAP-guided programming to optimize the stimulation parameters based on objective individual patient neurophysiology (vs assumed and subjective patient report). OL-SCS therapy, as with all other commercially available SCS systems, however, is not able to maintain consistent levels of neural activation at the prescribed ECAP target, resulting in partial adherence or nonadherence to therapy and potentially poorer treatment response. ECAP-guided programming delivered via OL-SCS and CL-SCS appear to both provide clinical benefit. As CL-SCS therapy maintains a consistent level of neural activation at the prescribed level in real-time, however, the improvements observed with CL-SCS were greater and durable over the study period, while some of the initial improvements reported with OL-SCS lessened overtime (figure 3).
All patients included in this analysis were blinded to their therapy and the effectiveness of the blinding was tested.14 As reported, there were no differences in programmed device settings (Hz, pulse width), or device utilization between groups. Given that patients were unaware of their treatment allocation and the parity in stimulation settings, the only therapy difference between groups was the closed-loop function. This resulted in significant differences in the therapy delivered, specifically the absolute level of spinal cord activation (ECAP size), and the accuracy of spinal cord activation (RMSE of ECAP variability vs target).
Strengths and weaknesses
We believe this to be the first study report to quantify a comprehensive holistic treatment response to SCS based on a double-blind, parallel arm RCT up to 24-month follow-up. Use of validated MCIDs to evaluate treatment response is a well-accepted approach in the clinical trial literature that reflects changes after a clinical intervention that are clinically meaningful to healthcare providers and important for patients.31 Furthermore, we used validated cut-offs both for the evaluation of MCIDs and for population normative values. However, while the definition of treatment responders is based on validated cut-offs, dichotomization of continuous data can lead to a loss of information and reduced power for statistical comparisons. We recognize the limitations of post hoc analysis and as such, further prospective research is warranted and ongoing to confirm the value of the holistic treatment response outcome described. In addition, as a post hoc analysis, a limitation of the current work was that the sample size was not powered to identify differences in holistic treatment response between groups.
The five outcome domains considered for evaluation of a holistic treatment response are those recommended by IMMPACT as core outcome measures,15 considered as crucial goals by patients,30 and healthcare providers.32 In addition, the analysis was based on a recently developed framework for evaluation of holistic treatment response.24 The development of the holistic treatment response composite aligns with recent Food and Drug Administration recommendations for composite outcomes.33 We acknowledge, however, that we may not have captured all outcomes of importance to all patients.
Last value carried forward was performed for imputation of missing data to make full use of data with careful attention to the assumptions about the nature of the missing data. This was performed as a conservative measure to minimize potential bias of an enriched population (ie, only patients benefiting from treatment remained in the study and those not benefiting withdrew early).34
Conclusion
The composite outcome of holistic response used in this study provides a comprehensive approach to the assessment of treatment impact on chronic pain patients’ overall health and well-being. Using the EVOKE trial data set, we demonstrate that SCS can provide sustained clinically meaningful improvements in multiple domains and a holistic response for patients with chronic refractory back and/or leg pain. In addition, with consistent neural activation at the prescribed level, CL-SCS provided superior and durable outcomes in all domains and at all time points when compared with OL-SCS. The reporting of objective physiologic evidence that underpins the mechanistic basis of improvement in patient-reported outcomes is paramount to increase our understanding of SCS as well as the transparency and replication in future SCS studies. Furthermore, we propose that in addition to traditional pain intensity scores, future studies should report the impact of SCS using composite measures of holistic response.
Footnotes
Twitter: @CAGPain, @drsimonthomson, @doctdeer
Contributors: All authors made substantial contributions to the study design, data analysis, and data interpretation, actively participated in drafting and critically revising the manuscript, provided final approval of the submitted version, and agree to be held accountable for the accuracy and integrity of the finished publication. RD is a guarantor who accepts full responsibility for the finished work and the conduct of the study as well as having access to the data and controlled the decision to publish.
Funding: This study was supported by Saluda Medical.
Competing interests: LK reports receiving grants from Nevro, Neuros, Avanos, Medtronic, Neuralace, and Xalud Therapeutics and financial support from Nevro, Avanos, and Saluda Medical. NM reports receiving grants from Neuros and Mesoblast, as well as consulting as a medical monitor for Saluda Medical, Nevro, Vivex, Mainstay, and Vertos. SC reports receiving grants from Saluda Medical, Vertos, Mainstay, and Vivex. CG reports personal fees and other from SPR, and personal fees from Nevro, Nalu, Biotronik, Boston Scientific, Saluda Medical, and Shiratronics. JP serves as a consultant for Abbott, Medtronic, Saluda Medical, Flowonix, SpineThera, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, and Thermaquil; has received grant and research support from: Abbott, Flowonix, Saluda Medical, Aurora, Painteq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, and Thermaquil; and is a shareholder of Vertos, SPR Therapeutics, Painteq, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil, and Anesthetic Gas Reclamation. SL reports receiving grants from Avanos, Boston Scientific, Nalu Medical, SPR Therapeutics, Saluda Medical, Averitas Pharma, Biotronik, SGX Therapeutics, and PainTeq, as well as serving as a consultant for Abbott, Avanos, Boston Scientific, Nevro, SPR Therapeutics, Averitas Pharma, Biotronik, Nalu Medical, PainTeq, and Saluda Medical, as well as holding stock options for Nalu Medical. CH has received consultancy fees from Saluda Medical and Genecentrix. PSS has received consultancy fees from Medtronic, Saluda Medical, Nalu, and Biotronic and has stock options from Saluda Medical and Nalu. RST reports consultancy fees from Medtronic, Nevro and Saluda Medical. SE reports consultancy fees from Medtronic, Mainstay Medical, Boston Scientific, and Abbott. He has received department research funding from the National Institute of Health Research, Medtronic and Nevro. JWK is an advisory board member for Boston Scientific, Medtronic, Abbott, and Saluda Medical. ST has received consultancy fees from Boston Scientific, Mainstay Medical, and Saluda Medical. He has received department research funding from the National Institute for Health Research, Boston Scientific, Saluda Medical, and Mainstay Medical. EP has received research support from Mainstay, Medtronic, Neuros Medical, Nevro Corp, ReNeuron, SPR, and Saluda Medical, as well as personal fees from Abbott Neuromodulation, Biotronik, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, Presidio Medical, Saluda Medical, and Vertos. She holds stock options from SynerFuse and neuro42. DS is a consultant for Abbott, Medtronic, Nevro, SPR, Vertos, PainTEQ, and Saluda Medical and has received research funding from and holds minority equity in Vertos. TD is a consultant for Abbott, Vertos, SpineThera, Saluda Medical, Cornerloc, SPR Therapeutics, PainTeq, Spinal Simplicity, and Biotronik. He has minor equity in form of stock options for Vertos, Saluda Medical, Cornerloc, Nalu, PainTeq, and Spinal Simplicity, and minor equity in common stock for SpineThera, and Ethos. He is an advisory board member or participates on a data safety monitoring board for Abbott, Vertos, SPR Therapeutics, and Biotronik. AA reports consultancy fees from Boston Scientific. AL, NS and RD are employees of Saluda Medical. RD has previously received consultancy fees from Mainstay Medical, Medtronic Ltd, and Saluda Medical. RL is an uncompensated consultant for Nalu, Saluda Medical, and Mainstay Medical and has stock options from Nalu and Saluda Medical obtained before 2019, not exercisable through the duration of his term as International Neuromodulation Society President and editor-in-chief of the journal Neuromodulation: Technology at the Neural Interface.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Saluda Medical is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit https://www.saludamedical.com/us/contact-us/.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the trial was conducted at 13 investigation sites throughout the USA. The study protocol was approved by each participating site’s institutional review board: Western Institutional Review Board (IRB IDs 1168219, 1168118, 1168713, 1174388, 1171961, 1172489, 1169008, 1173993, 1178269, 1180823), Forsyth Medical Center Institutional Review Board (IRB ID 16-518) St. Luke’s University Health Network IRB (IRB ID SLUHN 2016-92), Cleveland Clinic Foundation IRB (IRB ID 16-1465). Participants gave informed consent to participate in the study before taking part.
References
- 1. Garland EL. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care 2012;39:561–71. 10.1016/j.pop.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nugraha B, Gutenbrunner C, Barke A, et al. The IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain 2019;160:88–94. 10.1097/j.pain.0000000000001433 [DOI] [PubMed] [Google Scholar]
- 3. Evans SR, Follmann D. Using outcomes to analyze patients rather than patients to analyze outcomes: a step toward pragmatism in benefit:risk evaluation. Stat Biopharm Res 2016;8:386–93. 10.1080/19466315.2016.1207561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mekhail N, Levy RM, Deer TR, et al. Durability of clinical and quality-of-life outcomes of closed-loop spinal cord stimulation for chronic back and leg pain: a secondary analysis of the evoke randomized clinical trial. JAMA Neurol 2022;79:251–60. 10.1001/jamaneurol.2021.4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russo M, Brooker C, Cousins MJ, et al. Sustained long-term outcomes with closed-loop spinal cord stimulation: 12-month results of the prospective, multicenter, open-label Avalon study. Neurosurgery 2020;87:E485–95. 10.1093/neuros/nyaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized. Neurosurgery 2016;79:667–77. 10.1227/NEU.0000000000001418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilitsis JG, Fahey M, Custozzo A, et al. Composite score is a better reflection of patient response to chronic pain therapy compared with pain intensity alone. Neuromodulation 2021;24:68–75. 10.1111/ner.13212 [DOI] [PubMed] [Google Scholar]
- 8. Goudman L, Billot M, Duarte RV, et al. Gradation of clinical holistic response as new composite outcome to evaluate success in spinal cord stimulation studies for pain. Neuromodulation 2023;26:139–46. 10.1016/j.neurom.2021.10.020 [DOI] [PubMed] [Google Scholar]
- 9. Deer T, Wilson D, Schultz D, et al. Ultra-low energy cycled burst spinal cord stimulation yields robust outcomes in pain, function, and affective domains: a subanalysis from two prospective, multicenter, international clinical trials. Neuromodulation 2022;25:137–44. 10.1111/ner.13507 [DOI] [PubMed] [Google Scholar]
- 10. Russo M, Verrills P, Santarelli D, et al. A novel composite metric for predicting patient satisfaction with spinal cord stimulation. Neuromodulation 2020;23:687–97. 10.1111/ner.13072 [DOI] [PubMed] [Google Scholar]
- 11. Katz N, Dworkin RH, North R, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: initiative on methods, measurement, and pain assessment in clinical trials/Institute of Neuromodulation/International Neuromodulation Society recommendations. Pain 2021;162:1935–56. 10.1097/j.pain.0000000000002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation 2017;20:525–33. 10.1111/ner.12624 [DOI] [PubMed] [Google Scholar]
- 13. Chakravarthy K, Reddy R, Al-Kaisy A, et al. A call to action toward optimizing the electrical dose received by neural targets in spinal cord stimulation therapy for neuropathic pain. JPR 2021;Volume 14:2767–76. 10.2147/JPR.S323372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (evoke): a double-blind, randomised, controlled trial. Lancet Neurol 2020;19:123–34. 10.1016/S1474-4422(19)30414-4 [DOI] [PubMed] [Google Scholar]
- 15. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 16. Price DD, McGrath PA, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17:45–56. 10.1016/0304-3959(83)90126-4 [DOI] [PubMed] [Google Scholar]
- 17. McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med 1988;18:1007–19. 10.1017/s0033291700009934 [DOI] [PubMed] [Google Scholar]
- 18. Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976) 2000;25:2940–52. 10.1097/00007632-200011150-00017 [DOI] [PubMed] [Google Scholar]
- 19. Chiarotto A, Boers M, Deyo RA, et al. Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain 2018;159:481–95. 10.1097/j.pain.0000000000001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Hout B, Janssen MF, Feng Y-S, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value SETS. Value Health 2012;15:708–15. 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 22. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 23. McNair D, Heuchert J. Profile of mood states—technical update. North Tonawanda, NY: Multi-Health Systems, 2003. [Google Scholar]
- 24. Levy RM, Mekhail N, Abd-Elsayed A, et al. Holistic treatment response: an international expert panel definition and criteria for a new paradigm in the assessment of clinical outcomes of spinal cord stimulation. Neuromodulation 2023;26:1015–22. 10.1016/j.neurom.2022.11.011 [DOI] [PubMed] [Google Scholar]
- 25. Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci 2012;13:421–34. 10.1038/nrn3231 [DOI] [PubMed] [Google Scholar]
- 26. Staats PS, Hekmat H, Staats AW. The psychological behaviorism theory of pain: a basis for unity. Pain Forum 1996;5:194–207. 10.1016/S1082-3174(96)80031-6 [DOI] [Google Scholar]
- 27. Patel KV, Allen R, Burke L, et al. Evaluation of composite responder outcomes of pain intensity and physical function in neuropathic pain clinical trials: an ACTTION individual patient data analysis. Pain 2018;159:2245–54. 10.1097/j.pain.0000000000001324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Food and Drug Administration . Summary of safety and effeciveness data (SSED): Senza spinal cord stimulation (SCS) system. 2015. Available: https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130022B.pdf
- 29. Gilligan C, Volschenk W, Russo M, et al. Long-term outcomes of restorative neurostimulation in patients with refractory chronic low back pain secondary to multifidus dysfunction: two-year results of the Reactiv8-B pivotal trial. Neuromodulation 2023;26:87–97. 10.1016/j.neurom.2021.10.011 [DOI] [PubMed] [Google Scholar]
- 30. Goudman L, De Smedt A, Linderoth B, et al. Identifying goals in patients with chronic pain: a European survey. Eur J Pain 2021;25:1959–70. 10.1002/ejp.1814 [DOI] [PubMed] [Google Scholar]
- 31. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–15. 10.1016/0197-2456(89)90005-6 [DOI] [PubMed] [Google Scholar]
- 32. Goudman L, De Smedt A, Billot M, et al. Opinions of health care providers about Neuromodulation for pain: results of an online survey at the 2nd joint congress of the International Neuromodulation Society European chapters. Neuromodulation 2022. 10.1016/j.neurom.2022.04.038 [Epub ahead of print 9 May 2022]. [DOI] [PubMed] [Google Scholar]
- 33. Food and Drug Administration . Multiple endpoints in clinical trials: guidance for industry. 2022. Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/multiple-endpoints-clinical-trials-guidance-industry
- 34. National Research Council Panel on Handling Missing Data in Clinical T . The prevention and treatment of missing data in clinical trials. Washington (DC): National Academies Press (US), 2010. [PubMed] [Google Scholar]
- 35. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–21. 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 36. Ostelo RWJG, de Vet HCW. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 2005;19:593–607. 10.1016/j.berh.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 37. Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer, 2014. 10.1007/978-94-007-7596-1 [DOI] [PubMed] [Google Scholar]
- 38. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523–32. 10.1007/s11136-004-7713-0 [DOI] [PubMed] [Google Scholar]
- 39. Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh sleep quality index (PSQI), Epworth sleepiness scale (ESS), and clinical/Polysomnographic measures in a community sample. J Clin Sleep Med 2008;4:563–71. [PMC free article] [PubMed] [Google Scholar]
- 40. Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med 2011;171:887–95. 10.1001/archinternmed.2010.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rapm-2023-104639supp001.pdf (390.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. Saluda Medical is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit https://www.saludamedical.com/us/contact-us/.