Abstract
Human immunodeficiency virus type 1 (HIV-1) enters target cells by sequential binding to CD4 and specific seven-transmembrane-segment (7TMS) coreceptors. Viruses use the chemokine receptor CCR5 as a coreceptor in the early, asymptomatic stages of HIV-1 infection but can adapt to the use of other receptors such as CXCR4 and CCR3 as the infection proceeds. Here we identify one such coreceptor, Apj, which supported the efficient entry of several primary T-cell-line tropic (T-tropic) and dualtropic HIV-1 isolates and the simian immunodeficiency virus SIVmac316. Another 7TMS protein, CCR9, supported the less efficient entry of one primary T-tropic isolate. mRNAs for both receptors were present in phytohemagglutinin- and interleukin-2-activated peripheral blood mononuclear cells. Apj and CCR9 share with other coreceptors for HIV-1 and SIV an N-terminal region rich in aromatic and acidic residues. These results highlight properties common to 7TMS proteins that can function as HIV-1 coreceptors, and they may contribute to an understanding of viral evolution in infected individuals.
The human immunodeficiency viruses type 1 and type 2 (HIV-1 and HIV-2) are the etiologic agents of AIDS, which results from the depletion of CD4-positive T lymphocytes (3, 15, 17). HIV-1 infects T lymphocytes, monocytes/macrophages, dendritic cells, and microglial cells in the central nervous system. Efficient entry of HIV-1 into these cells is dependent on the binding of the viral envelope protein gp120 to the cellular receptor CD4 and subsequently to one of several seven-transmembrane-segment (7TMS) receptors (1, 5, 7, 8, 10, 11, 16, 22). Most of the known 7TMS receptors that serve as HIV-1 coreceptors have as their natural ligands members of the chemokine family. In addition, several orphan receptors, some of which are only distantly related to chemokine receptors, support the entry of various simian immunodeficiency viruses (SIV) and less efficient entry of some HIV-1 isolates (2, 9, 12, 21).
HIV-1 isolates are usually described in terms of their cellular tropism and passage history (5). Macrophage-tropic (M-tropic) viruses infect macrophages and primary T cells but not most immortalized T-cell lines. Dualtropic viruses can infect macrophages, primary T cells, and T-cell lines. T-cell line-tropic (T-tropic) viruses can infect primary T cells and T-cell lines but not primary macrophages. T-tropic viruses can be usefully divided into those that have been extensively passaged in laboratory cell lines (laboratory-adapted T-tropic viruses) and those that have been molecularly cloned after only limited in vitro passage (primary T-tropic viruses). HIV tropism is strongly correlated with coreceptor use. M-tropic viruses mainly use CCR5 as a coreceptor, although some M-tropic viruses can also use CCR3. The laboratory-adapted T-tropic viruses mainly use CXCR4 as a coreceptor. Most dualtropic and primary T-tropic viruses use CCR5, CXCR4, CCR3, and other receptors with different levels of efficiency (5, 10, 21).
Alanine-scanning mutagenesis of the exterior domains of CCR5 had shown that a small region in the N terminus of CCR5, rich in tyrosines and acidic residues, plays an important role in infection by HIV-1 and SIV isolates using this coreceptor (14). The presence of a similar array of residues in the N termini of two orphan 7TMS receptors helped to identify these receptors as efficient coreceptors for SIV (12). Here we show that a previously cloned 7TMS receptor, Apj (27), is expressed in the brain and in phytohemagglutinin (PHA)- and interleukin-2 (IL-2)-activated peripheral blood mononuclear cells (PBMC) and supports the efficient entry of recombinant HIV-1 pseudotyped with the envelope glycoproteins of several primary HIV-1 isolates. Apj also contains an array of aromatic and acidic residues in its N terminus that are similar to those of CXCR4, CCR3, and CCR5, although it has much higher overall sequence similarity to the angiotensin receptors I and II than to any known chemokine receptor (27). CCR9 (26), a newly identified 7TMS receptor with a similar array of tyrosines, less efficiently supported the entry of only 1 recombinant primary T-tropic virus of 10 isolates tested. These results underscore the importance of residues in the N terminus of coreceptor molecules for primary T-tropic as well as M-tropic viruses, and they may contribute to an understanding of HIV-1 evolution in the host.
MATERIALS AND METHODS
Plasmids.
Plasmids pHXBH10ΔenvCAT and pSVIIIenv, used to produce recombinant HIV-1 virions containing the envelope glycoproteins from the HIV-1 isolates ADA, YU2, JR-FL, 89.6, ELI, UG21, MN, and HXB2 or the envelope glycoproteins from SIV isolates SIVmac239 and SIVmac316, have been described previously (5, 18, 20, 23, 25, 30). Plasmid pCD4, used to express full-length CD4 in Cf2Th cells, has been described elsewhere (4). CCR5, CCR3, CCR2b, CXCR4, and gpr15 in the expression vector pcDNA3 (Invitrogen) have been described previously (5, 12). CCR8 and HA-tagged CCR8 in the pcDNA3 plasmid were provided by Monica Napolitano. US28 was expressed in a pCEP4 vector (Invitrogen). Strl33 was isolated from a T-cell cDNA library described previously (12) and cloned into the pcDNA3 vector. An additional plasmid encoding Strl33 in a pCEP4 vector was provided by Joshua Farber (21). Apj and CCR9 were cloned from human brain QUICK-Clone cDNA (Clonetech) and QUICK-Clone MOLT-4 cDNA (Clontech), respectively, and subcloned into pcDNA3.
Cell lines.
Cf2Th canine thymocytes (ATCC CCRL 1430) were obtained from the American Type Culture Collection. Cells were maintained as described previously (5). Cf2Th-CD4 cells were generated by transfection with the expressor plasmid pcDNA3.1 (Invitrogen) encoding CD4, selection with 0.1 mg/ml hygromycin, and cloning by limiting dilution.
env complementation assay.
A single round of HIV-1 infection was assayed in a previously described env complementation assay (5). Briefly, recombinant HIV-1 with the nef gene replaced by a gene encoding chloramphenicol acetyltransferase (CAT) was pseudotyped with various HIV-1 and SIV envelope glycoproteins and used to infect Cf2Th or Cf2Th-CD4 cells. The target cells had been transfected, 48 h before infection, by the calcium phosphate method with either 10 μg of plasmid encoding CD4 and 20 μg of plasmid encoding a 7TMS receptor (Cf2Th), or 25 μg of plasmid encoding a 7TMS receptor (Cf2Th-CD4). For these assays, 25,000 cpm of reverse transcriptase activity of the recombinant viruses containing the envelope glycoproteins of YU2, ADA, JR-FL, 89.6, ELI, UG21, MN, HXB2, SIVmac239, or SIVmac316 was incubated at 37°C with Cf2Th target cells expressing various 7TMS molecules. Cells were lysed 72 h after infection, and CAT activity was measured, indicating the efficiency of infection.
Detection of mRNA encoding Apj and CCR9.
The mRNA from PBMC activated with 5 μg of PHA per ml, with or without 20 U of IL-2 per ml added 48 h later, was isolated and reverse transcribed as previously described (12). The cDNAs for Apj and CCR9 were detected by PCR. QUICK-clone brain, thymus, and spleen cDNAs (Clonetech) were also used to identify the presence of Apj and CCR9.
Quantitation of viral replication.
Replication-competent 89.6 or ELI virus was made by transfecting HeLa cells or 293T cells, respectively, by the calcium phosphate method with plasmids containing the proviral genome. Replication-competent virus was harvested 72 h after transfection and incubated with CF2Th cells 48 h after transfection of these cells with a plasmid expresing CD4 and either pcDNA3 or plasmids encoding Apj and CXCR4, in the presence or absence of 50 μM azidothymidine. Four days after infection, the p24gag protein was detected with an HIV-1 p24 enzyme-linked immunosorbent assay kit (Dupont Medical Products) as specified by the manufacturer.
RESULTS
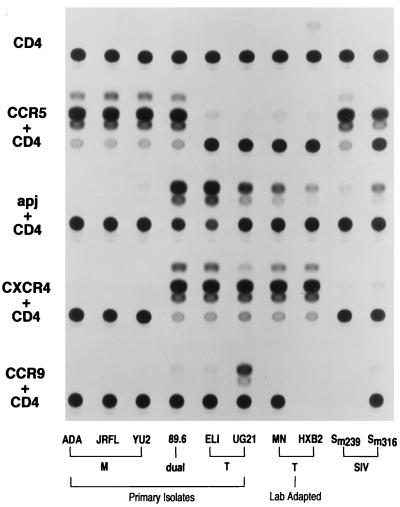
We investigated the properties of two human 7TMS receptors whose N termini exhibited sequence similarity to the N termini of CCR5 and/or CXCR4. Both the orphan receptor Apj and CCR9, whose murine counterpart binds murine MIP-1α and MIP-1β (26), possess N-terminal tyrosines that could be aligned with the tyrosines shown to be important for the ability of CCR5 to support HIV-1 and SIV entry (14). Figure 1 demonstrates that both Apj and CCR9 can serve as coreceptors for the entry of some HIV-1 or SIV isolates. As previously reported (5), virus pseudotyped with the envelope glycoproteins from M-tropic HIV-1 isolates (ADA, JR-FL, and YU2), a dualtropic HIV-1 isolate (89.6), or SIV isolates (SIVmac239 and SIVmac316) could efficiently enter Cf2Th-CD4 cells expressing CCR5. Also as expected, Cf2Th-CD4 cells expressing CXCR4 supported the entry of a dualtropic HIV-1 isolate (89.6), primary T-tropic HIV-1 isolates (ELI and UG21), laboratory-adapted T-tropic HIV-1 isolates (HXBc2 and MN), and, very inefficiently, SIVmac316. The same viruses were used to infect Cf2Th cells expressing CD4 and either Apj or CCR9. Cells expressing CD4 and Apj supported the entry of a primary dualtropic HIV-1 isolate (89.6), primary T-tropic HIV-1 isolates (ELI and UG21), and, to a lesser extent, laboratory-adapted T-tropic HIV-1 isolates (MN and HXB2). Interestingly, SIVmac316, which is derived from a rhesus macaque infected with SIVmac239 (25), can also infect cells expressing CD4 and Apj, although SIVmac239 does so only weakly. CCR9 was able to support the entry of virus pseudotyped with the envelope glycoproteins of the primary T-tropic HIV-1 isolate, UG21, but also demonstrated signals above those seen for the control cells expressing only CD4 when viruses containing the 89.6, ELI, or SIVmac316 envelope glycoproteins were tested.
FIG. 1.
Dualtropic and T-tropic viruses use Apj or CCR9 as coreceptors. Cf2Th-CD4 cells were transfected with either the pcDNA3 vector only or pcDNA3 plasmids encoding CCR5, CXCR4, Apj, or CCR9. The transfected cells were incubated with recombinant CAT-expressing viruses containing the envelope glycoproteins of the M-tropic HIV-1 ADA, JR-FL, or YU2; the dualtropic HIV-1 89.6; the primary T-tropic HIV-1 ELI, or UG21; the laboratory-adapted T-tropic HIV-1 MN or HXBc2; or the SIV isolates SIVmac239 or SIVmac316. Entry was quantitated as the ratio of the acetylated forms of chloramphenicol (upper spots) to the unacetylated form (bottom spot). Samples in which conversion is greater than 60% were diluted 1:10 and reassayed for quantitative comparisons. Entry of viruses pseudotyped with 89.6, ELI, UG21, MN, or HXBc2 envelope glycoproteins into cells expressing Apj was 5.6 ± 0.2, 10.2 ± 0.1, 7.7 ± 0.4, 1.5, and 0.3% ± 0.1%, respectively, of that of the same viruses entering cells expressing CXCR4. The entry of the virus pseudotyped with the envelope glycoproteins of SIVmac316 into cells expressing Apj was 21.9% ± 4.7% of that observed for cells expressing CCR5. Entry of virus pseudotyped with UG21 envelope glycoproteins into cells expressing CCR9 was 6.2% ± 1.9% of that observed for cells expressing CXCR4.
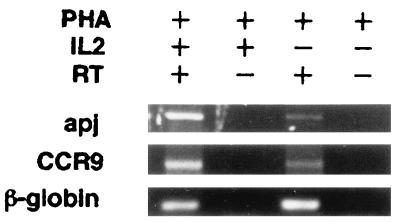
To assess the potential physiological relevance of these observations, we sought to determine whether Apj and CCR9 were present in PBMC stimulated with PHA or with PHA plus IL-2. Figure 2 shows that cDNAs for both CCR9 and Apj were detected in PBMC that had been activated with PHA plus IL-2 but only weak signals were seen for PBMC that had been stimulated with PHA alone. No signal was detected for either receptor if reverse transcriptase was excluded from the mRNA preparations used to generate cDNA, indicating that these signals were not derived from contaminating genomic DNA. We also detected Apj in preparations of cDNA from spleen and from brain (data not shown); the latter observation is consistent with previous reports (24, 27) and our own ability to clone Apj from brain cDNA. CCR9 was detectable in cDNA prepared from the thymus (data not shown).
FIG. 2.
Expression of Apj and CCR9 in PBMC. mRNA was isolated from PBMC activated with PHA (right-hand lanes) or with PHA plus IL-2 (left-hand lanes) and reverse transcribed to cDNA. PCR was performed with primers bounding an approximately 1,100-bp fragment of the Apj or CCR9 gene, or a 200-bp fragment of the β-globin gene control. In the lanes labeled RT−, the PCR was performed with the same reaction mixes used in the other two lanes, but prepared without reverse transcriptase.
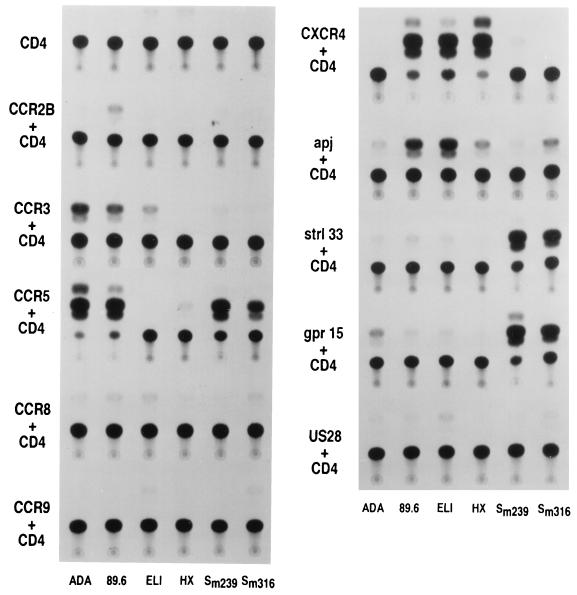
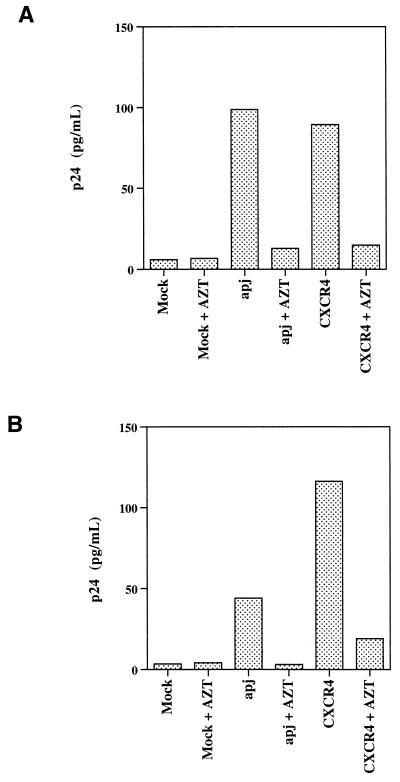
The ability of Apj and CCR9 to support virus entry was compared with that of a number of receptors previously reported to exhibit HIV and/or SIV coreceptor activity, by using a smaller panel of viruses. Figure 3 shows that entry of some HIV-1 isolates into Cf2Th cells expressing CD4 and CXCR4 or CD4 and CCR5 was efficient, as was SIV entry into Cf2Th cells expressing CD4 and Strl33, CCR5, or gpr15. These levels of entry were nearly 10-fold higher than those observed for the next most efficient receptors, Apj and CCR3. Although specific reagents were not available to normalize for the cell surface expression of receptors, some conclusions can be drawn. CCR2b only weakly supports the entry of one virus, 89.6, a result quantitatively consistent with the first report of CCR2b use (10). Signaling and binding experiments by MCP-1 on 293T cells have previously demonstrated that the CCR2b construct used here can be expressed efficiently in these cells (13). Consistent with previous reports, CCR3 can support the entry of M-tropic (ADA), dualtropic (89.6), and primary T-tropic (ELI) HIV-1 at efficiencies ranging from 5 to 10% of those of the entry of the same viruses when either CCR5 (ADA and 89.6) or CXCR4 (ELI) is used. Slightly higher levels of entry into cells expressing Apj were detected for 89.6 and ELI viruses. Values only slightly above background were detected for the entry of HIV-1 isolates into cells expressing CD4 and CCR8, Strl33, or the cytomegalovirus-encoded 7TMS receptor, US28. These results are not quantitatively consistent with other reports of HIV-1 entry on these receptors (21, 28, 29), a discrepancy that may be accounted for by differences in the level of coreceptor expression achieved. However, expression of the identical US28 construct was verified on transfected 293T cells by using MIP-1β binding (data not shown). Some of the reported discrepancies may arise from the use of assay systems in which entry into cells expressing CCR5 or CXCR4 is saturated, thus exaggerating the relative efficiency of other receptors. Although care must be taken when comparing virus entry into cells expressing different receptors, we conclude that Apj is a relatively efficient receptor for dualtropic and primary T-tropic HIV-1 isolates. Figure 4 demonstrates that Apj can serve as an efficient coreceptor for primary dualtropic viruses in an assay system involving replication-competent viruses.
FIG. 3.
Comparison of virus entry by using Apj or other reported coreceptors. Cf2Th cells were transfected with a plasmid expressing CD4 and the pcDNA3 vector or plasmid expressing the indicated 7TMS receptor and incubated with recombinant viruses containing the ADA, 89.6, ELI, HXBc2, SIVmac239, or SIVmac316 envelope glycoproteins. CAT expression was analyzed in the target cells as described in Materials and Methods.
FIG. 4.
Apj supports the entry of replication-competent viruses. Replication-competent ELI (A) and 89.6 (B) HIV-1 isolates were incubated with Cf2Th cells transfected with CD4 and either a control plasmid or plasmids encoding Apj or CXCR4, in the presence or absence of 50 μM azidothymidine (AZT). The p24 values from 4 days after infection are given in picograms per milliliter, with a 1:5 dilution of cell supernatant.
DISCUSSION
During the course of HIV-1 infection, viruses emerge that use a broader range of coreceptors (6), including CXCR4, CCR3, CCR2b, and, as shown here, Apj. These dualtropic and primary T-tropic viruses probably infect a broader range of target cells, and their emergence may coincide with an accelerated disease progression. The ability of SIVmac316, but not SIVmac239, to use Apj as a coreceptor demonstrates that this expansion of receptor use may occur within SIV-infected rhesus macaques as well. SIVmac316 is derived from a macaque infected with SIVmac239 that had developed AIDS-like symptoms (25). The relative efficiency with which Apj is used as a coreceptor by primary viruses and its expression on activated PBMC suggest that it may play a role in viral dissemination in HIV-infected individuals. Moreover, the lower efficiency of Apj use by laboratory-adapted viruses, compared with primary T-tropic viruses, may suggest that Apj-using viruses are selected in vivo but not in vitro in humans as well as in macaques.
M-tropic HIV-1 isolates that have been isolated from the central nervous system have been shown to use CCR3, as well as CCR5, as a coreceptor (5, 19). Both CCR3 and CCR5 are present on the surface of brain microglia, and infection of fetal brain cultures by these isolates has been demonstrated (19). Apj is also expressed in brain tissue and has sequence similarity to CCR5 and CCR3 in an N-terminal region of these molecules that has been shown to be important for coreceptor function (14). However, neither of the tested central nervous system-derived M-tropic viruses, JR-FL or YU2, can use Apj as a coreceptor, although both use CCR3. It remains to be determined whether there is a specific role for Apj as a coreceptor in the central nervous system following the emergence of dualtropic or T-tropic viruses.
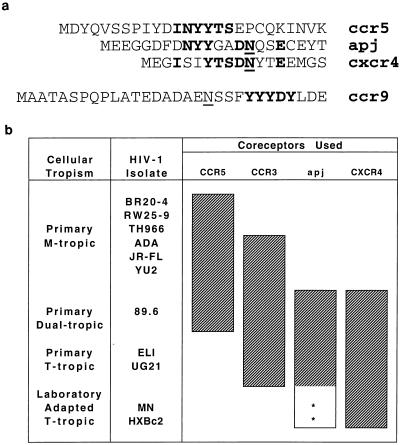
The identification of Apj and CCR9 as HIV-1 coreceptors supports earlier observations indicating that a specific array of tyrosines and acidic amino acids in the N termini of these molecules plays a critical role in infection by HIV-1 and SIV (14). Single-amino-acid changes in this region of CCR5 interfere with the ability of primary M-tropic and dualtropic HIV-1 and with SIVmac239 to infect cells expressing these mutants. The amino termini of three alternate SIV coreceptors, Strl33, gpr15, and gpr1, exhibit sequence similarity to this region of CCR5. The results described above suggest that primary T-tropic HIV-1 isolates may also require the presence of specific N-terminal tyrosines in their coreceptors. The tyrosine-rich, acidic motif is less well developed in CXCR4 than in CCR5, with the Apj N terminus sharing characteristics of both sequences (Fig. 5a). Apj and CCR3 could facilitate the adaptation of CCR5-using viruses to CXCR4 by providing an N terminus that diverges from that of CCR5 and possesses similarity to that of CXCR4. Further adaptation to CXCR4 could require increased dependence on other regions of the molecule, including the second extracellular loop (29). Figure 5b summarizes coreceptor usage data for CCR5, CCR3, Apj, and CXCR4.
FIG. 5.
7TMS receptor sequences and coreceptor function. (a) Alignment of CCR5, Apj, and CXCR4 N-terminal sequences. The sequences of the N termini, beginning with the initiator methiones, of CCR5, Apj, and CXCR4 are shown. Tyrosines 10, 14, and 15, aspartic acid 11, asparagine 13, and glutamic acid 18 of CCR5 have been previously demonstrated to be important for M-tropic and dualtropic viral entry by using CCR5 (14). Common residues are shown in boldface type, and possible N-linked glycosylation sites are underlined. Also shown is the N terminus of CCR9, with a tyrosine-rich region indicated in bold. (B) Summary of the coreceptor usage of various HIV-1 isolates. The ability of various HIV-1 isolates to enter Cf2Th cells expressing CD4 and each of the four coreceptors shown to be most efficient in this study is indicated by shaded bars. Stars indicate relatively inefficient entry of laboratory-adapted T-tropic viruses into cells expressing the Apj receptor.
CCR9 also possesses a prominent array of tyrosines in its N terminus (Fig. 5a) yet supports the entry of only a single virus of 10 tested. Whether subtleties in the arrangement of these tyrosines, or other properties of the molecule, limit its ability to function as an efficient coreceptor remains to be demonstrated.
The contribution of HIV-1 coreceptors other than CCR5, CXCR4, and perhaps, CCR3 to natural infection and pathogenesis remains unclear. The efficiency of Apj coreceptor function relative to that of most of the other reported HIV-1 coreceptors, its use by a range of dualtropic and primary T-tropic viruses, and its presence in activated PBMC and in the central nervous system suggest that further examination of its role is warranted.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported by grants to Joseph Sodroski from the National Institutes of Health (AI 24755 and AI 41851) and by a Center for AIDS Research grant to the Dana-Farber Cancer Institute (AI 28691). Dana-Farber Cancer Institute is also the recipient of a Cancer Center grant from the National Institutes of Health (CA 06516). Craig Gerard was supported by NIH grants HL 51366 and AI 36162, as well as by the Rubenstein/Cable Fund at the Perlmutter Laboratory. Martin Dorf was supported by an NIH grant (NS 37284). Mark Cayabyab is a recipient of a Ford Foundation Fellowship. Michael Farzan was supported by a training grant (CA 09141). This work was made possible by gifts from the late William McCarty-Cooper, from the G. Harold and Leila Y. Mathers Charitable Foundation, and from the Friends 10.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Liao F, Berger E A, Farber J M, Peden K W. A new SIV co-receptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 3.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 4.Brand D, Srinivasan K, Sodroski J. Determinants of human immunodeficiency virus type 1 entry in the CDR2 loop of the CD4 glycoprotein. J Virol. 1995;69:166–171. doi: 10.1128/jvi.69.1.166-171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 6.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1–infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 10.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 11.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC- CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 12.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farzan M, Choe H, Martin K A, Sun Y, Sidelko M, Mackay C R, Gerard N P, Sodroski J, Gerard C. HIV-1 entry and macrophage inflammatory protein-1beta-mediated signaling are independent functions of the chemokine receptor CCR5. J Biol Chem. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 14.Farzan M, Choe H, Vaca L, Martin K, Sun Y, Desjardins E, Huffing N, Wu L, Wyatt R, Gerard N, Gerard C, Sodroski J. A tyrosine-rich region in the N terminus of CCR5 is important for human immunodeficiency virus type 1 entry and mediates an association between gp120 and CCR5. J Virol. 1998;72:1160–1164. doi: 10.1128/jvi.72.2.1160-1164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fauci A S, Macher A M, Longo D L, Lane H C, Rook A H, Masur H, Gelmann E P. NIH Conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 17.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 18.Gao F, Morrison S G, Robertson D L, Thornton C L, Craig S, Karlsson G, Sodroski J, Morgado M, Galvao-Castro B, von Briesen H, Beddows S, Weber J, Sharp P M, Shaw G M, Hahn B H. Molecular cloning and analysis of functional envelope genes from human immunodeficiency virus type 1 sequence subtypes A through G. The WHO and NIAID Networks for HIV Isolation and Characterization. J Virol. 1996;70:1651–1667. doi: 10.1128/jvi.70.3.1651-1667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 20.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 23.Marcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newman W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus, SIVmac239. J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto M, Hidaka K, Akiho H, Tada S, Okada M, Yamaguchi T. Low stringency hybridization study of the dopamine D4 receptor revealed D4-like mRNA distribution of the orphan seven-transmembrane receptor, APJ, in human brain. Neurosci Lett. 1996;219:119–122. doi: 10.1016/s0304-3940(96)13198-0. [DOI] [PubMed] [Google Scholar]
- 25.Mori K, Ringler D, Desrosiers R. Tenth Symposium on Nonhum Primate Models of AIDS. 1992. Restricted replication of SIVmac239 in macrophages is determined by env but is not due to restricted entry, abstr. 19. [Google Scholar]
- 26.Nibbs R J B, Wylie S M, Pragnell I B, Graham G J. Cloning and characterization of a novel murine beta chemokine receptor, D6. Comparison to three other related macrophage inflammatory protein-1alpha receptors, CCR-1, CCR-3, and CCR-5. J Biol Chem. 1997;272:12495–12504. doi: 10.1074/jbc.272.19.12495. [DOI] [PubMed] [Google Scholar]
- 27.O’Dowd B F, Heiber M, Chan A, Heng H H, Tsui L C, Kennedy J L, Shi X, Petronis A, George S R, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 28.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 29.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]