Abstract
Polyamine (PA) titers and biosynthesis follow a basipetal decrease along the tobacco (Nicotiana tabacum) plant axis, and they also correlate negatively with cell size. On the contrary, the titers of arginine (Arg), ornithine (Orn), and arginase activity increase with age. The free (soluble)/total-PA ratios gradually increase basipetally, but the soluble conjugated decrease, with spermidine (Spd) mainly to determine these changes. The shoot apical meristems are the main site of Spd and spermine biosynthesis, and the hypogeous tissues synthesize mostly putrescine (Put). High and low Spd syntheses are correlated with cell division and expansion, respectively. Put biosynthetic pathways are differently regulated in hyper- and hypogeous tobacco tissues: Only Arg decarboxylase is responsible for Put synthesis in old hypergeous vascular tissues, whereas, in hypogeous tissues, arginase-catalyzed Orn produces Put via Orn decarboxylase. Furthermore, Orn decarboxylase expression coincides with early cell divisions in marginal sectors of the lamina, and Spd synthase strongly correlates with later cell divisions in the vascular regions. This detailed spatial and temporal profile of the free, soluble-conjugated, and insoluble-conjugated fractions of Put, Spd, and spermine in nearly all tobacco plant organs and the profile of enzymes of PA biosynthesis at the transcript, protein, and specific activity levels, along with the endogenous concentrations of the precursor amino acids Arg and Orn, offer new insight for further understanding the physiological role(s) of PAs. The results are discussed in the light of age dependence, cell division/expansion, differentiation, phytohormone gradients, senescence, and sink-source relationships.
Polyamines (PAs) are small polycations that represent important intrinsic developmental signals, linked to important developmental phenomena, including cell growth and division (Chattopadhyay et al., 2002; Theiss et al., 2002), morphogenesis (Paschalidis et al., 2001; Fos et al., 2003), stabilization of nucleic acids and membranes (Thomas and Thomas, 2001), protein synthesis and chromatin function (Mattheus, 1993), and biotic and abiotic stresses (Kurepa et al., 1998; Perez-Amador et al., 2002; Navakoudis et al., 2003; for review, see Bouchereau et al., 1999). Eukaryotic cells synthesize putrescine (Put) directly from Orn through the activity of Orn decarboxylase (ODC). Plants and some bacteria also synthesize Put indirectly from Arg, by the activity of Arg decarboxylase (ADC). An aminopropyl group derived from decarboxylated S-adenosylmethionine (dSAM) is transferred to Put by spermidine synthase (SPDS) to form spermidine (Spd), and another aminopropyl group is added to Spd by spermine synthase (SPMS) to form spermine (Spm). The dSAM is formed by the activity of S-adenosyl-l-Met decarboxylase (SAMDC; for review, see Wallace et al., 2003).
The positive charge of PAs enables them to interact electrostatically with polyanionic macromolecules within the cell. Spd and Spm can bridge the major and minor grooves of DNA, acting as a clamp holding together either two different molecules or two distant parts of the same molecule (Mattheus, 1993). Mutations in genes that control PA biosynthesis exert various effects on cell proliferation and viability, depending on the organisms (Wallace et al., 2003). For example, in Saccharomyces cerevisiae and Schizosaccharomyces pombe, null mutants of a gene encoding for SAMDC have an absolute requirement for Spd or Spm for growth (Chattopadhyay et al., 2002), whereas in Arabidopsis (Arabidopsis thaliana), SPDS genes are essential for survival, but not Spm (Imai et al., 2004). PAs in cells exist as free (soluble [S]), soluble-conjugated (soluble-hydrolyzed [SH]), or insoluble-conjugated (pellet-hydrolyzed [PH]) forms. The exact role of each of these fractions remains unknown.
The complexity in control of cell division, expansion, growth, and senescence in the tobacco (Nicotiana tabacum) plant is well documented (Chen et al., 2001). Growth-promoting or -limiting elements exist, and it is reasonable to presume that PA gradients within meristems, leaves, petioles, internodes, and/or roots could be a part of this network. Because these processes are associated with an enhanced carbon and nitrogen demand, a link to the regulation of sink-source relations is also suggested. During development, leaves undergo a transition from a sink (carbon-importing) to a source (carbon-exporting) organ (Oparka et al., 1999). On the other hand, nitrogen is supplied to sinks either via uptake from roots or retranslocation from old leaves (Hikosaka, 2005). PA contents, and mainly the S form, have been analyzed in various plant organs/tissues in in vitro systems (Tiburcio et al., 1997; Bouchereau et al., 1999). In addition, a number of enzymes of PA metabolism have been studied, mostly at the activity and protein level (Bouchereau et al., 1999). However, our in vivo detailed developmentally dictated gene expression analysis of the PA biosynthetic enzymes, in a truly tissue-specific manner, is essential to extend our knowledge about the molecular functions of PAs. In this work, we present the temporal and spatial distribution of the endogenous S-PA (Put, Spd, Spm), SH-PA (Put, Spd, Spm), and PH-PA (Put, Spd, Spm) contents throughout the entire tobacco plant, as well as the expression profiles of all biosynthetic genes at the transcriptional, translational, and posttranslational levels. The results suggest that PA synthesis and conjugation are likely to be an essential part of the homoeostatic mechanism that controls PA levels and their involvement in processes such as vascular differentiation. The genes of the PA biosynthesis network are down-regulated during development and are spatially related in a tissue/organ-specific manner. Put biosynthetic pathways work independently in tobacco, whereas Orn is mostly produced from Arg via arginase, as part of the urea cycle. Changes in PA metabolism during leaf development may be part of a mechanism controlling the transitions from cell division to expansion, depending on PA concentrations maintained within strict limits.
RESULTS
PA Levels Follow a Basipetal Decrease along Tobacco Plant Axis
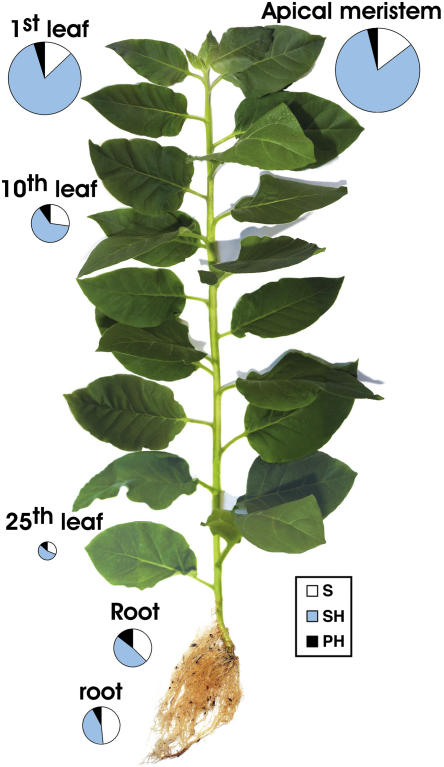
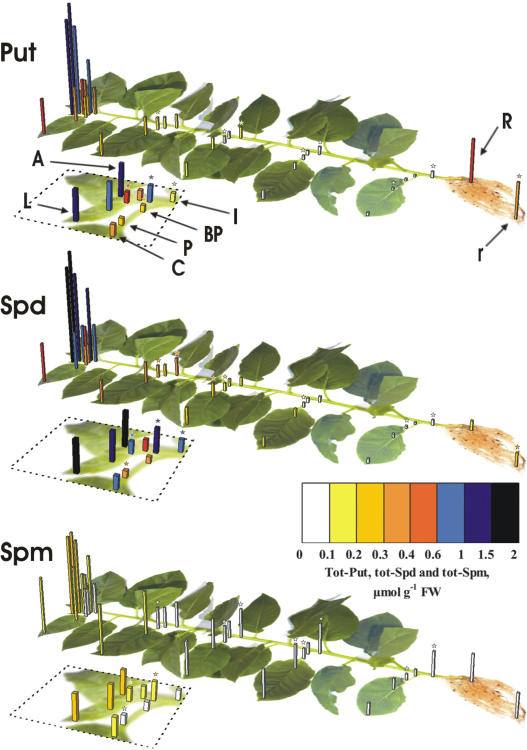
An inverse correlation is generally observed between leaf ontogenic stage and the titers of all fractions of total PA (Put + Spd + Spm; Fig. 1) independently of growth conditions (data not shown). PA concentrations are extremely high in sink leaves of less than 10 mg in weight and decrease drastically when cell division ceases and cell elongation is the sole process of leaf growth. The trend is same regardless the mode of expression of PA levels (nmol g−1 fresh weight [FW] or nmol mg−1 protein; data not shown). However, when PA levels are expressed per milligram of protein, differences were lower. This could be explained by the fact that the size of the vacuole and water content increase with cell age. The sums of S, SH, and PH fractions of total PA from the apical meristem to the 10th leaf from apex were 3,180 ± 142 and 684 ± 46 nmol g−1 FW, respectively (4.6-fold decrease), or 157 ± 12 and 49 ± 4 nmol mg−1 protein, respectively (3.2-fold decrease). The sum of S, SH, and PH fractions of total PA in leaves (lamina and petiole) also decreases from 2,577 ± 136 in the youngest (circle area of the first leaf) to 124 ± 3 nmol g−1 FW in the oldest (circle area of the 25th) leaf (Fig. 1). The sums of total Put (S + SH + PH), total Spd, and total Spm are high in sink tissues and gradually decrease 34-fold, 22-fold, and 7-fold, respectively, from the first developing (first) to the senescing (25th) leaf (Fig. 2). In petioles and internodes, total-Put, total-Spd, and total-Spm titers remain relatively constant compared to the respective values in leaf margins and centers from the first to the fifth developmental stage (Fig. 2), suggesting that the young vascular tissues mediate basipetal transport of PAs. Furthermore, the internodes contain significantly higher levels of total Put, total Spd, and total Spm than the acropetal and basipetal parts of the petioles (Fig. 2), indicating that PAs are transported and accumulate in shoots. Endogenous PAs were also analyzed using in vitro-grown plants, and the results reinforce the correlation reported herein between PA titers and developmental stages (data not shown).
Figure 1.
Total PA titers in tobacco plants. The size of the circles designates the PA values in different organs of the plant. The white portions of circles correspond to the amount of S-PAs, the blue portions to the SH-PAs, and the black portions to the PH-PAs. The size of the circle in the apical meristem corresponds to 3,180 nmol g−1 FW and includes the white, blue, and black portions corresponding, in turn, to 466, 2,590, and 124 nmol g−1 FW, respectively. The sizes of circles (and the respective portions) in the other plant organs are proportionally depicted.
Figure 2.
Total Put, total Spd, and total Spm content in different parts of tobacco plants. Dotted lines contain scale-ups of the figures, containing the shoot apex (A), the first leaf, and part of the fifth leaf. L, Apical and marginal leaf lamina; C, central lamina; P, acropetal petiole; BP, basipetal petiole; I, internode; R, primary root; r, secondary root. Data for L, C, P, BP, and I are depicted in the first, fifth, 10th, 15th, 20th, and 25th developmental stages (numbered from apex). Asterisk-marked data regions indicate statistically significant differences (P = 0.01) when compared with the adjacent acropetal ones of the same developmental stage. In the first two developmental stages, asterisks are depicted in the scale-up parts, whereas asterisks on secondary roots indicate statistically significant differences from the primary roots.
The Free/Total-PA Ratios Increase Basipetally from Apex to Base of the Plant, whereas the Conjugated Decrease
SH-Spd is the most abundant form in the hypergeous (aerial) plant organs (data not shown). The ratios of S-PAs/total PAs (white parts of circles/total circles, Fig. 1) increase 2.4-fold basipetally from shoot apex to the base of the plant, whereas the ratios of SH-PAs/total PAs decrease 1.5-fold (Fig. 1). The ratio S-Spd/total S-PAs increases 1.7-fold with increasing age in hypergeous tissues, whereas the ratios S-Put/total S-PAs and S-Spm/total S-PAs decrease 1.9- and 2.6-fold, respectively (Table I). Furthermore, the ratio S-Spd/total Spd also increases 4-fold, whereas S-Put/total Put increases only 2-fold and S-Spm/total Spm decreases 4-fold. Thus, taking into account also the respective conjugated ratios, it can be concluded that the basipetal increase in the S-PAs/total-PAs ratio is presumably due to decreased conjugation of Put and particularly Spd.
Table I.
Free/total-PA ratios in the hypergeous organs of the tobacco plant
S-Put/total S-PAs, S-Spd/total S-PAs, and S-Spm/total S-PAs ratios were determined in shoot apex and in the first, 10th, and 25th (numbered from shoot apex) leaves. Data are the means ± se.
| S-Put/Total S-PAs | S-Spd/Total S-PAs | S-Spm/Total S-PAs | |
|---|---|---|---|
| Shoot apex | 0.39 ± 0.02 | 0.42 ± 0.03 | 0.18 ± 0.01 |
| First leaf | 0.40 ± 0.02 | 0.45 ± 0.02 | 0.15 ± 0.01 |
| 10th leaf | 0.26 ± 0.01 | 0.61 ± 0.04 | 0.13 ± 0.00 |
| 25th leaf | 0.21 ± 0.01 | 0.72 ± 0.05 | 0.07 ± 0.00 |
Total-PA levels in primary and secondary root tissues do not differ significantly (Fig. 1), although total Spd is greater and total Put is lower in the secondary roots (Fig. 2), whereas all S fractions and SH-Spd are higher in the secondary roots (data not shown).
PAs Correlate with the Cell Division/Expansion Profile in the Youngest Leaves
Cell expansion in tobacco leaves increases, whereas nuclear DNA synthesis (K. Paschalidis and K. Roubelakis-Angelakis, unpublished data) and cell division (Chen et al., 2001) decrease, progressively with leaf age. All PA fractions as well as activities, transcripts, and proteins of the PA biosynthetic enzymes also decrease with age (Figs. 2 and 3). In the 1-cm-long tobacco leaf (first), cell division occurs throughout the leaf and petiole, but mostly at the peripheral regions (Chen et al., 2001). However, when the leaf reaches a length of more than 1.5 cm, cell divisions cease first at the apex and progressively further down the leaf, and gradually they are restricted to the basal part of the leaf blade, before ceasing completely (Poethig and Sussex, 1985; Chen et al., 2001). To investigate if the basipetal cessation of cell divisions and the developmental acquisition of cell size increases are mirrored by a similar reduction in PA content, all fractions of PAs were determined separately in the following sections of the leaves: the apical and marginal region, the central leaf region, the acropetal (apical) petiole, the basipetal (basal) petiole, and the internodes. Cells on the apical and marginal regions of the youngest tobacco leaf, where cells have the highest capacity for auxin-induced growth, maximal division rates, and minimal cell area (Jones et al., 1998), also have the highest titers of all PA fractions (Fig. 2), the highest biosynthetic activities (ADC, ODC, SAMDC, and SPDS; Fig. 3), and the highest odc transcript levels (Fig. 3), but lower Orn levels (Fig. 4), as compared to the acropetal petiole regions. Therefore, PA synthesis, abundance, and distribution seem to correlate positively with cell division, but negatively with the extent and distribution of cell expansion and cell size.
Figure 3.
Expression patterns of ADC, ODC, SAMDC, SPDS, and SPMS in the soluble fractions from tobacco plant tissues. A, Shoot apex; L, apical and marginal leaf lamina (⋄); P, acropetal petiole (▵); I, internode (dotted line); R, primary root; r, secondary root. A, Specific activities in L, P, and I at different developmental stages. B, Specific activities of ADC, ODC, and SAMDC (nmol CO2 h−1 mg−1 protein), SPDS (nmol Spd h−1 mg−1 protein), and SPMS (nmol Spm h−1 mg−1 protein) in shoot apex, primary, and secondary roots. C, RNA gel-blot analysis of the expression of adc, odc, samdc, and spds genes. D, Quantification of mRNA levels (mRNAs/actin mRNAs). E, Western-blot analysis of ADC protein. Numbering started from the apical organ being designated as first. Error bars represent ±se.
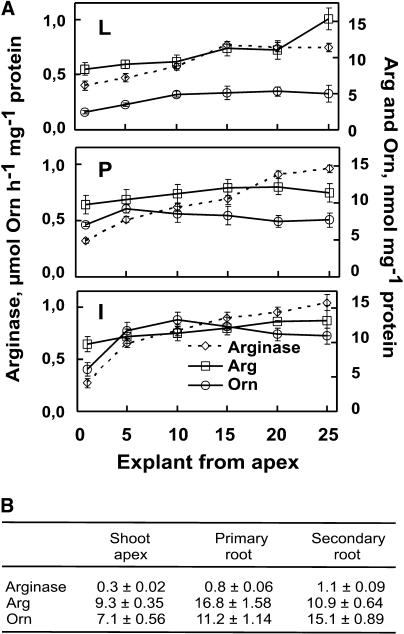
Figure 4.
Arginase-specific activities, and Arg and Orn levels in tobacco plants. A, Specific activities in L, P, and I. L, Apical and marginal leaf lamina; P, acropetal petiole; I, internode; arginase, ⋄ with dotted line; Arg, □; Orn, ○. B, Arginase activities (μmol Orn h−1 mg−1 protein) and endogenous levels of Arg and Orn (nmol mg−1 protein) in shoot apex, primary root, and secondary root. Error bars represent ±se.
The adc, samdc, and spds Transcripts and ADC Protein Do Not Coincide with Activity Levels in the Youngest Leaves
The transcripts of odc are consistent with the activity levels in the first leaf; they are high in leaf margin and decrease basipetally to the petiole (Fig. 3, C and D). However, the transcripts of adc, samdc, and spds and the protein level of ADC do not coincide with specific activities, being lower in leaf margin and gradually increasing in leaf center and petiolar parts (Fig. 3, C–E, and data not shown). The basipetally increasing gradients of transcripts continue also to the internode; the spds transcript in the first internode is almost 10-fold higher than in the marginal region of the first leaf (Fig. 3D). The opposite trend of adc transcript on one hand and ADC protein and activity levels on the other could suggest that regulation of ADC in tobacco plants is at the posttranslational level, as has been the case with ADC in oat (Avena sativa; Malmberg and Cellino, 1994), Arabidopsis (Watson and Malmberg, 1996), and grapevine (Vitis vinifera; Primikirios and Roubelakis-Angelakis, 2001). On the contrary, ODC activity is regulated at the transcriptional and the translational levels (Marton and Pegg, 1995). Lack of correlation between samdc and spds transcript levels and SAMDC and SPDS enzyme activities could also suggest a posttranscriptional, translational, and/or posttranslational regulation. SAMDC in mammals and plants is produced as a proenzyme maturing by autocatalytic proteolysis between two Ser residues (Heby and Persson, 1990; Schroder and Schroder, 1995), whereas SPDS is suggested to exist as a stable multienzyme complex (Panicot et al., 2002) or may be posttranscriptionally (Zhang et al., 2003) or posttranslationally (Yoon et al., 2000) modified. Because of the large volume of data, only those of the marginal leaf regions, the acropetal petiolar ones and the internodes, at each developmental stage and parameter, are shown.
Low SPDS Coincides with Cell Expansion in Tobacco
In expanding leaves, the activities of ADC and ODC (Fig. 3A) follow the basipetally decreasing gradient, from leaf margin to petiole, which is also found at the first developmental stage. On the contrary, in leaves of the fifth and 10th developmental stages, SPDS- and SPMS-specific activities increase from leaf margins to petioles following a reverse, to the first developmental stage, gradient (Fig. 3A). Both enzyme and transcript levels of SPDS are almost 2-fold higher in petioles than in leaves at the 10th developmental stage (Fig. 3, A, C, and D). In mammals (Marton and Pegg, 1995) and Chlamydomonas reinhardtii (Theiss et al., 2002), cell divisions are abolished by the addition of 4-methyl-cyclohexylamine, an inhibitor of SPDS, whereas addition of the ODC inhibitor dl-α-difluoromethylornithine does not abolish C. reinhardtii cell divisions but causes an early accumulation of dividing cells (Theiss et al., 2002). Furthermore, significantly higher ODC activities are measured after addition of the SPDS inhibitor 4-methyl-cyclohexylamine (Theiss et al., 2002), whereas inhibition of ODC by DFMO causes an increase in SAMDC activity, resulting in increased synthesis of Spd (Marton and Pegg, 1995).
As ODC activity has been correlated with cell divisions in many mammalian and higher plant systems (Marton and Pegg, 1995; Bouchereau et al., 1999), our results, together with the above, could suggest a tissue- or age-dependent expression of either ODC or SPDS in dividing tobacco cells, competing with each other for the progression of cell divisions. In the youngest tobacco leaf margin, ODC activity and transcript levels are almost 3- and 1.5-fold higher than in the youngest petiole, respectively (Fig. 3, A and C). SPDS activity, however, is only 1.9-fold higher in the youngest leaf margin than in the corresponding petiole (Fig. 3A). On the contrary, spds transcript levels are in the youngest leaf margin almost 3-fold and almost 10-fold lower than in the petiole and the corresponding internode, respectively (Fig. 3, C and D). In the expanding leaves (fifth and 10th), SPDS activity and transcript levels in leaf margins are almost 2-fold lower than in petioles, whereas ODC activities are higher in leaf margins (Fig. 3A). Thus, early in development (in the youngest leaf), ODC may correlate with the higher cell division in the peripheral leaf regions, whereas later (in the expanding leaves) high expression levels of SPDS in tobacco are strictly correlated with the onset and the intensity of cell division in the transport system. The extremely high mRNA levels of spds in the vascular youngest tissues could suggest that transcription of the spds in these tissues precedes their transition to high cell division phase later in development. In synchronized C. reinhardtii cultures (Theiss et al., 2002), PA synthesis also precedes the transition to the cell division phase. On the other hand, spatial changes in adc gene and enzyme activation in the youngest tissues and adc gene expression in the 10th leaf are in contrast to the cell division phases (Fig. 3, C, D, and E), confirming that adc gene is mostly related to stress responses in plants and not to cell division (Bouchereau et al., 1999; Paschalidis et al., 2001; Primikirios and Roubelakis-Angelakis, 2001).
Put Is Synthesized via ODC in Root Tissues and via ADC in Old Hypergeous Organs; Biosynthetic Enzymes of Spd and Spm Are Absent in the Particulate Fractions
The specific activities of all biosynthetic enzymes in the soluble fraction generally decrease with age in tobacco, with the exception of SPMS (Fig. 3, A and B), and are in agreement with the endogenous PA levels. ODC soluble-specific activity is significantly higher in the hypogeous than in the hypergeous organs, including shoot apical meristems, which also exhibit high ODC activity (Fig. 3, A and B). SAMDC activity is also high in roots, whereas ADC is lower in roots than in old and fully developed tissues (Fig. 3, A and B).
ADC and ODC activities in the particulate fractions of aerial tobacco tissues (leaves, petioles, and internodes) follow the same trend as the soluble ones (Table II). ODC particulate activity is approximately half of the soluble one, while in young roots it is extremely low as compared to the activity in the soluble fraction (Table II; Fig. 3), which is almost 30-fold higher than the respective ADC (Fig. 3, A and B). In contrary, higher ADC than ODC specific activities are present in the old hypergeous organs. Root apical tissues contain higher soluble but lower particulate ADC and ODC activities than the older root tissues. Furthermore, SAMDC, SPDS, and SPMS activities are absent from the particulate fractions.
Table II.
ADC and ODC (nmol CO2 h−1 mg−1 protein) specific activities of the particulate fractions in protein extracts of tobacco
Activities were measured in the first, fifth, 10th, 15th, 20th, and 25th developmental stages (numbered from shoot apex) and in the shoot apex, primary root, and secondary root. Data are the means ± se. ND, Not detected.
| First | Fifth | 10th | 15th | 20th | 25th | ||
|---|---|---|---|---|---|---|---|
| ADC | La | 1.2 ± 0.11 | 0.6 ± 0.04 | 0.4 ± 0.02 | 0.2 ± 0.00 | ND | ND |
| Pb | 0.6 ± 0.06 | 0.3 ± 0.02 | 0.2 ± 0.02 | 0.1 ± 0.00 | ND | ND | |
| Ic | 0.5 ± 0.04 | 0.7 ± 0.05 | 0.4 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.00 | 0.2 ± 0 | |
| ODC | L | 2.6 ± 0.24 | 2.2 ± 0.12 | 0.8 ± 0.09 | ND | ND | ND |
| P | 0.9 ± 0.09 | 0.7 ± 0.09 | 0.4 ± 0.03 | 0.2 ± 0.00 | ND | ND | |
| I | 0.5 ± 0.04 | 0.4 ± 0.03 | 0.5 ± 0.03 | 0.4 ± 0.01 | 0.3 ± 0.01 | 0.2 ± 0 | |
|
|
|
Shoot Apex
|
Primary Root
|
Secondary Root
|
|||
| ADC | 0.9 ± 0.07 | 0.2 ± 0.01 | 0.1 ± 0.00 | ||||
| ODC | 1.3 ± 0.09 | 0.7 ± 0.06 | 0.3 ± 0.01 | ||||
L, Tip and leaf margin.
P, Acropetal petiole.
I, Internode.
Arginase Activities and Arg and Orn Levels Increase with Age; Put Synthesis in Hypogeous Tissues Is Due to Successive Actions of Arginase and ODC
In tobacco plant, arginase-specific activities, as well as Arg and Orn levels, increase with increasing organ age (Fig. 4). Differences are more pronounced in internodes, where arginase, Arg, and Orn increase from the youngest to the oldest one, 3.9-, 1.3-, and 1.8-fold, respectively. The above three parameters generally increase from marginal leaf regions to petioles and internodes. The newly formed roots exhibit higher arginase and Orn but lower Arg levels than the primary roots (Fig. 4B). Furthermore, Put biosynthesis in roots (Fig. 2) uses Orn as a substrate and is due to successive actions of arginase (Fig. 4B) and ODC (Fig. 3B).
The Shoot Apical Meristems Are the Highest Biosynthetic Sites of Spd and Spm
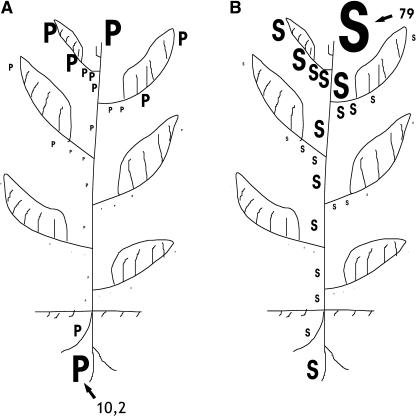
It is evident that all the studied organs of the tobacco plant are capable of synthesizing PAs (Fig. 5). The highest relative Put biosynthetic capacity is localized in the hypogeous apical meristems followed by the shoot apex and young developing leaves. The highest relative biosynthetic capacity of the higher PAs (Spd and Spm) is localized in the shoot apex and in the youngest aerial tissues, but roots also synthesize Spd and Spm at high rates (Fig. 5). These trends apply to plants of different ages and are independent of photoperiod (data not shown). Spd and Spm synthesis is more uniformly distributed (with lower temporal differences) than Put synthesis throughout tobacco development. Lower but still significant rates of PA biosynthesis are exhibited in expanding leaves and in old vascular tissues.
Figure 5.
PAs as signal-integrating developmental processes throughout the tobacco plant. PA synthesis is estimated from the enzyme-specific activities in the soluble fractions. Activity levels are represented by magnitude levels depicted by the sizes of letters P for Put synthesis and S for synthesis of higher PAs (Spd and Spm). A, Sites of Put synthesis. Synthesis of Put is depicted as a sum of ADC and ODC activities. B, Sites of synthesis of higher PAs. Synthesis of higher PAs is depicted as a sum of SPDS and SPMS activities. Numbers 10.2 and 79 show (with arrows) the activity levels (in nmol [of the product measured] h−1 mg−1 protein) depicted by the sizes of the biggest P and S, respectively. All the other sizes of P and S are depicted proportionally to the respective biggest ones.
DISCUSSION
Tobacco has been used as a model system to study the various events related to primary assimilation or remobilization of carbon and nitrogen during transition of sink to source leaves and senescence (Oparka et al., 1999; Masclaux et al., 2000). In addition, PAs are proposed to participate in the plasticity of carbon and nitrogen metabolism in higher plants (Bouchereau et al., 1999). Turano and Kramer (1993) determined the interaction of carbon and nitrogen availability on PA accumulation in soybean (Glycine max) by using a detached leaf system. Aziz (2003) suggests that Spd could regulate fruitlet abscission in grapevine by modulating the levels of sugars and amino acids in inflorescences, whereas, in tobacco, PAs and aromatic amines could be a limiting factor in sexual development (Martin-Tanguy, 1997) or play a role in apical dominance (Geuns et al., 2001). There is a lack of information on the relationship between the PA biosynthetic pathways and the metabolism of carbon and nitrogen or partitioning between source and sink organs in the vegetative plant. In tobacco, PA distribution follows a reduction from sink to source leaves, and the strongest reduction coincides with senescence. In this context, PAs could act either as carbon/nitrogen reserves or as signal molecule(s) regulating senescence and probably sink-source relationships. Also, PAs are present in thylakoid membranes, PSII, and the light-harvesting complex (Kotzabasis et al., 1993b; Sfichi et al., 2004), suggesting a functional role in the photosynthetic activity during senescence or in the regulation of photoassimilate synthesis and/or partitioning between organs. PAs also inhibit ethylene biosynthesis and other degrative processes associated with senescence, including chlorophyll loss, increase in membrane leakage, and rise in RNAse and protease activities (Pandey et al., 2000). PA involvement in delay of senescence was confirmed by elegant transgenic approaches (Mehta et al., 2002; Tucker and Seymour, 2002). However, the underlying molecular basis for this PA implication is still not understood. Other phytohormones may also affect PA metabolism. A transient increase in ADC and ODC activities as well as in the amount of free PAs and a decrease of conjugated PAs during early parthenocarpic fruit development induced by auxins and gibberellins (Alabadí et al., 1996) has been observed; expression of ODC and SPDS genes is also up-regulated (Alabadí and Carbonell, 1998, 1999). Fos et al. (2003) have suggested that parthenocarpy induced by the recessive mutation pat-2 in tomato (Lycopersicon esculentum) activates PA biosynthesis through the ODC pathway leading to elevated free-Spm content in unpollinated ovaries, probably as a result of a higher concentration of active GAs in pat-2 ovaries. Furthermore, jasmonate-induced up-regulation of PA biosynthetic genes (Biondi et al., 2001; Goossens et al., 2003) is differently modified by the presence/absence of exogenously supplied auxins and cytokinins (Biondi et al., 2003).
Although all organs of a tobacco plant are competent to synthesize PAs (Fig. 5), the shoot apical tissues exhibit the highest Spd and Spm synthesis and high Put synthesis. Shoot apical meristems and actively dividing young tissues contain also the highest contents of PAs, which decrease drastically following the completion of their active development and probably the sink-source transition (Fig. 1). Spd titers are generally higher throughout development and Spm exhibits low variation (Fig. 2), suggesting a tighter regulation of cellular Spm metabolism, compared to Put or Spd, in agreement with results from camellia (Camellia sinensis) and grapevine cells (Pedroso et al., 1997; Primikirios and Roubelakis-Angelakis, 1999; Paschalidis et al., 2001). The limited size of the Spd pool may not be large enough to allow the conversion of excess Spd to Spm. Bassie et al. (2000) proposed a threshold model in terms of the size of the Put pool to explain why rice (Oryza sativa) tissues expressing the oat adc cDNA driven by a very strong constitutive promoter are able to accumulate higher levels of Spd and Spm compared to plants engineered with the same transgene driven by a weaker promoter (Capell et al., 1998) that do not show any changes in the levels of higher PAs.
At relatively low level of auxin, it is suggested that the auxin-binding protein 1 acts to mediate cell expansion, whereas high auxin levels stimulate cell division via an unidentified receptor (Chen et al., 2001). Cells at the tip of a young leaf have the capacity to respond to auxin, and this capacity moves basipetally as the leaf expands (Chen et al., 2001). In tobacco, there is a negative correlation between the abundance of PAs, the epidermal and mesophyll cell size, and leaf area. Before the leaf is fully expanded, it undergoes the sink-source transition progressing basipetally from the apical lamina to the base (Oparka et al., 1999). In this leaf, relatively low synthesis of higher PAs (Spd and Spm) occurs at the apical regions where cell expansion rate is maximum and cell division rate is minimum. On the other hand, at locations and times when cell division is maximum, SPDS expression is high. These correlations prompted us to propose that there are two separable Spd (and Spm) synthesis responses: a low Spd synthesis response leading to cell expansion and a high Spd synthesis response leading to cell division. Furthermore, ODC expression correlates with cell divisions in the youngest peripheral lamina regions, whereas SPDS correlates with cell division in the transport system of expanding tobacco leaves (Fig. 3, A and C). In eukaryotic cells, Spd is required for the activation of the initiation factor eIF-5A (Jakus et al., 1993) and, therefore, necessary for protein biosynthesis and cell growth, whereas in dividing and nondividing tobacco protoplasts (Siminis et al. 1994), PAs improve totipotency by preventing reactive oxygen species generation and induction of the programmed cell death syndrome (Papadakis and Roubelakis-Angelakis, 2005).
Our understanding of the specific PA pathways is still not precise enough to allow a safe correlation of total biosynthetic and catabolic fluxes in all pathways simultaneously. To our knowledge, this is the first exhaustive report on detailed temporal and spatial distribution of the PA anabolic (this work) and catabolic (K. Paschalidis and K. Roubelakis-Angelakis, unpublished data) pathways in plants. The tobacco secondary roots contain significantly higher free Put than other plant tissues (Fig. 6), except the shoot apical tissues, which contain similarly high free Put (data not shown). The root tissues use mainly as the substrate Orn and the ODC pathway for Put production (Fig. 6C). Therefore, the amount of Orn in the tissues could become limiting. The addition of either Orn or Arg to the medium results in increased accumulation of Put in poplar (Populus spp.) cells (Bhatnagar et al., 2001), showing that these precursors may be limiting also in tobacco root cells. ODC in mammals is known to be regulated by feedback mechanisms that operate at transcriptional and translational levels (Nilsson et al., 1997; Cohen, 1998), and the turnover is promoted by excess PAs via the induction of an antizyme protein (Hayashi and Murakami, 1995), whereas in grapevine we observed a feedback regulation of ADC protein by exogenous Put (Primikirios and Roubelakis-Angelakis, 1999). In tobacco root apical tissues, high free-Put contents reflect extremely high ODC, but although Arg levels are higher in roots than in the aerial tissues, no relevant increase in ADC activity, protein, and transcript levels are found (Figs. 3 and 4). In the old hypergeous tissues, ADC activities are higher than those of ODC, supporting that Put synthesis is accomplished by ADC in these tissues and by ODC in roots. Thus, Put biosynthetic pathways are regulated in an organ-specific manner in tobacco. A steady-state equilibrium may also be established between the rates of Put biosynthesis and its degradation (K. Paschalidis and K. Roubelakis-Angelakis, unpublished data), as both of them are responsible for the regulation of endogenous concentrations of Put in cells. Our previous results suggest that under conditions of abiotic stress a similar equilibrium would continuously recycle NH3, therefore minimizing its toxicity (Loulakakis et al., 2002).
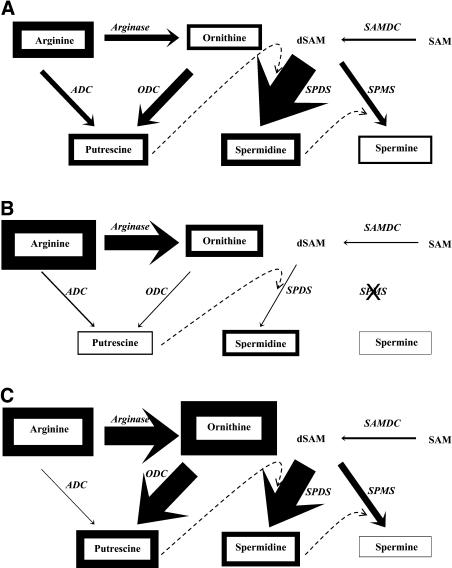
Figure 6.
The tobacco PA biosynthetic pathway in the youngest leaf (A), oldest leaf (B), and young roots (C). The endogenous contents of amino acids and free PAs are depicted by the thickness of their boxes, whereas the soluble-specific activities of the biosynthetic enzymes (in italics) are depicted by the thickness of the corresponding arrows. In the youngest leaf (A), the contents of Arg, Orn, Put, Spd, and Spm (nmol mg−1 protein), depicted by the corresponding thicknesses of boxes, are 8.6, 3.4, 6.8, 7.8, and 2.5 respectively, whereas the specific activities of ADC, ODC, SAMDC, SPDS, SPMS, and Arginase (nmol product h−1 mg−1 protein), depicted by the corresponding thicknesses of arrows, are 2.4, 4.3, 0.9, 28.1, 5.2, and 373, respectively. (For depicting reasons, thicknesses of ADC, ODC, and SAMDC are estimated 2-fold higher than thicknesses of SPDS and SPMS, whereas thicknesses of Arg are estimated 60-fold lower.) The thicknesses of boxes and arrows in B and C are depicted proportionally to the respective thicknesses in A.
A constitutive expression of the ODC enzyme in tobacco root cells could lead to a depletion of Orn pools. Since Orn is also the precursor of Arg, its depletion could reduce the availability of Arg and in turn limit the amount of Put synthesized via ADC (Fig. 6). Both low levels of Arg and ADC observed in newly formed roots, as compared to the primary root, together with high Orn, ODC, and arginase levels (Figs. 3 and 4) could support these hypotheses. Thus, most of the Orn in these cells is directly produced from Arg by arginase as part of the urea cycle and not from Glu. This production, however, is insufficient to saturate the available ODC enzyme in these cells. Although little is known about homeostatic regulation of Orn pools in plants (Bhatnagar et al., 2001), a homeostatic mechanism must exist to increase Orn production concomitant with its increased use.
The presence of alternative pathways for biosynthesis of PAs in plants (Bouchereau et al., 1999) has led to the hypothesis for differential regulation of the expression of each gene and compartmentalization of the respective proteins. SAMDC, SPDS, and SPMS are not present in the particulate fractions from tobacco organs at different developmental stages, whereas ADC and ODC exist in both the soluble and particulate fractions (Fig. 3; Table II). These results indicate that subcellular localization could be an additional means of regulation of PAs in individual cellular compartments during tobacco development.
In conclusion, our data give a clearer picture of the homeostatic characteristics of PA biosynthesis and conjugation in tobacco plant organs during development. Biosynthetic capacity and PA pool size decrease basipetally in the aerial parts during growth and senescence, potentially implicating the sink-source transition. The ratios of soluble and conjugated PAs also change with development (Fig. 1). As the size of the total-PA pool and the relative ratio of SH-PAs/total PAs decrease, the relative ratio of S-PAs/total PAs increases, suggesting that, in addition to biosynthesis, conjugation exerts a significant role in the homeostatic mechanism of cellular PAs. The pool size of PAs in the hypogeous organs is comparative to their size in the mature and old hypergeous tissues (Fig. 1), even though the biosynthetic capacity of roots is severalfold higher (Fig. 5), suggesting different turnover rates between the organs. Moreover, the contribution of the ODC and ADC pathways to Put biosynthesis and the genes of Spd and Spm synthesis are developmentally regulated and exhibit different subcellular localization. Arginase-derived Orn is the substrate for Put production in roots via ODC, whereas Put in old hypergeous tissues is synthesized via the ADC pathway. Furthermore, ODC and SPDS depend on the spatial and temporal status of the plant for a role in the progression of cell division/expansion, whereas a high Spd synthesis response leading to cell division and a low response leading to cell expansion are proposed.
MATERIALS AND METHODS
Plant Material
Explants were removed from glasshouse-grown (25°C ± 2°C, 16/8 h photoperiod, and total energy of 55 μE m−2 s−1) 6- to 8-week-old tobacco (Nicotiana tabacum L. cv Xanthi) plants and numbered from shoot apex. They were divided in tip and leaf margin, leaf center, acropetal or apical petiole, and basipetal or basal petiole. Furthermore, shoot apex, primary or main root, and secondary or newly formed root explants were also removed.
PA, Arg, Orn, and Protein Determination
Free, soluble-conjugated, and insoluble-conjugated PAs were determined according to Kotzabasis et al. (1993a), with a HP 1100 HPLC (Hewlett-Packard, Waldbronn, Germany).
Arg and Orn were colorimetrically determined according to Ceriotti and Spandrio (1957) and to Roubelakis and Kliewer (1978), respectively.
Total proteins were extracted according to Papadakis et al. (2001) with 100 mm Tris-HCl, pH 8.0, 1 mm EDTA, 50 μm pyridoxal phosphate, 5 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 10 μm leupeptin, 10% (v/v) glycerol, and 0.2% Triton X-100. The homogenates were centrifuged and the supernatants (soluble fractions) were desalted in Bio-gel P-6 (Bio-Rad, Hercules, CA). The pellets (cell walls, nucleus, plastids, and mitochondria; de Marco and Roubelakis-Angelakis, 1996) were dissolved in half-strength buffer, supplemented with 1 m NaCl, centrifuged, and gave the particulate fractions. Protein concentration was determined according to Lowry et al. (1951) and by gel visualization.
Enzyme Assays
An isotopic method (Primikirios and Roubelakis-Angelakis, 1999) was used for the determination of ADC, ODC, and SAMDC activities by measuring the release of 14CO2. l-[1-14C]Arg, l-[1-14C]Orn, and adenosyl-l-Met S-[carboxyl-14C], respectively (ARC, St. Louis), were used as radioactive substrates. Usually, 100 μL of the protein extract were incubated with 25 μL (0.078 μCi) l-[1-14C]Arg (0.626 mCi/ mmol, 3.13 μCi/ mL, 1 mm final concentration of Arg), 25 μL (0.078 μCi) l-[1-14C]Orn (0.626 mCi/ mmol, 3.13 μCi/ mL, 1 mm final Orn concentration), and 25 μL (0.0156 μCi) l-[1-14C]adenosyl-l-Met (0.1252 mCi/ mmol, 0.626 μCi/ mL, 1 mm final adenosyl-l-Met concentration) in polypropylene tubes. For the ADC assay, 200 μm Nω-hydroxy-l-Arg (a competitive inhibitor of arginase; Iniesta et al., 2001) was also added in the reaction mixture. Labeled CO2 was counted in an LS 6000SE (Beckman, Fullerton, CA) scintillation counter.
Arginase activity was colorimetrically measured as the rate of Orn formation, as described by Roubelakis and Kliewer (1978), in a reaction mixture containing 100 mm Tris-HCl, pH 9.7, 25 mm l-Arg (adjusted to pH 9.7), 0.1 mL of total protein extract, and 1.5 mm MnCl2 (10 min of preincubation at room temperature) in a total volume of 1 mL. The reaction was terminated after 1 h by addition of 0.5 mL of 15% perchloric acid. The arginase activity was a linear function of incubation time, at least for 1 h, of substrate concentrations up to 20 mm and of the amount of protein extract under these conditions. Boiled enzyme preparations and reactions terminated with perchloric acid before incubation were used as controls, whereas 200 μm Nω-hydroxy-l-Arg inhibited arginase activity in tobacco tissues by 96%.
SPDS and SPMS were assayed by measuring the formation of Spd and Spm, respectively. The assay mixture for SPDS contained 3 mm Put, 0.2 mm dSAM, 100 mm Tris-HCI buffer, pH 9.0, and the enzyme in a total volume of 200 μL. The reaction was performed at 37°C for 1 h and stopped by the addition of 200 μL of 65 mm borate-KOH buffer, pH 10.5, followed with 1 mL of 2 n NaOH and 10 μL of benzoylchloride. The products were separated with an HP 1100 HPLC system. SPMS was assayed similarly to SPDS by replacing Put with Spd in the assay mixture.
Western Blotting, RNA Extraction, and Northern Analysis
Protein samples were electrophoretically resolved, transferred to nitrocellulose membranes, and incubated with an antiserum raised in rabbit against the hybrid protein MBP-ADC (Primikirios and Roubelakis-Angelakis, 2001). Total RNA was extracted as described by Logemann et al. (1987) and quantified by spectroscopy and ethidium bromide staining. RNA was denaturated, electrophoresed, and transferred to Gene Screen membranes (NEN Life Science Products, Boston) according to Sambrook et al. (1989) that were screened with the specific probes below.
A 1,047-bp tobacco adc cDNA clone obtained from suspension cell cultures (K. Primikirios and K. Roubelakis-Angelakis, unpublished data) was used as adc probe. Two degenerate oligonucleotide primers corresponding to two conserved regions in the aligned sequences of oat (Avena sativa; Bell and Malmberg, 1990) and tomato (Lycopersicon esculentum; Rastogi et al., 1993) ADC proteins (forward primer, CTNGARGCNGGNTCNAARCC; reverse primer, GGNCCNCCRAANAGRTTRTG) were used to obtain the 1,047-bp product, which was cloned and sequenced. A 2,112-kb grapevine (Vitis vinifera) adc cDNA fragment (Primikirios and Roubelakis-Angelakis, 1999) with 91% nucleotide identity to the tobacco adc was also used for hybridization revealing exactly the same RNA patterns, suggesting the high specificity of probes. A full-length tomato odc DNA (Alabadí and Carbonell, 1998), with deduced amino acid sequence 89.8% identical to that of tobacco, was used as odc probe. Furthermore, a grapevine 829-bp odc fragment (accession no. AY174164) revealed the same transcripts with the tomato probe. Finally, a tobacco samdc PCR-derived fragment (A.J. Michael, Institute of Food Research, Norwich, UK) and a 945-bp Nicotiana sylvestris spds reverse transcription-PCR fragment (Hashimoto et al., 1998) were also used as probes. The tobacco samdc was obtained from Agrobacterium rhizogenes-transformed hairy-root cultures. Two degenerate oligonucleotide primers, sense 5′-TT(T/C)GA(A/G)GGN(T/A/C)(T/C)NGA(A/G)AA-3′ and antisense 5′-TCNGGNGTNA(T/C)(A/G)TGNA(T/G/A)NG-3′ representing, respectively, the amino acid sequences FEG(F/T/P)EK and T(M/I/L)H(I/V)TPE, were used to obtain a 723-bp PCR product, which was cloned and sequenced (Taylor et al., 1992). PCR conditions, cloning, and sequencing are described by Michael et al. (1996).
Statistical Analysis
Statistical analysis of data concerning endogenous PAs, activities, Arg and Orn levels, and the ratio mRNA/rRNAs was performed with one-way ANOVA to reveal any statistically significant differences (P < 0.01 or P < 0.05) that occurred. Data were logarithmically transformed to homogenize variances when necessary (Bartlett's test). If F values were significant, pairwise comparisons of means were made by Tukey's multiple comparisons test (P < 0.01). Data that contained two groups were compared using t tests. All experiments were carried out three times with similar results and included a minimum of three replicates per sample. Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
We thank Prof. J. Carbonnel (Universitad Politécnica de Valencia, Spain) for the odc DNA, Dr. A. Michael (Institute of Food Research, Norwich, UK) for the samdc PCR fragment, and Dr. T. Hashimoto (Nara Institute of Science and Technology, Japan) for the spds reverse transcription-PCR fragment. We are grateful to Prof. A. Shirahata and Dr. K. Samejima (University Yyo-Sai, Japan) for the generous gift of dSAM, to Dr. A. Papadakis (University of Crete, Greece) for helpful discussions, and to Dr. I. Corraliza (Department of Biochemistry and Molecular Biology, Cáceres, Spain) for comments on arginase inhibition.
This work was supported by the Interreg II and Pythagoras (Code No. 1945) programs.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.055483.
References
- Alabadí D, Agüero MS, Pérez-Amador MA, Carbonell J (1996) Arginase, arginine decarboxylase, ornithine decarboxylase, and polyamines in tomato ovaries: changes in unpollinated ovaries and parthenocarpic fruits induced by auxin or gibberellin. Plant Physiol 112: 1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabadí D, Carbonell J (1998) Expression of ornithine decarboxylase is transiently increased by pollination, 2,4-dichlorophenoxyacetic acid, and gibberellic acid in tomato ovaries. Plant Physiol 118: 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabadí D, Carbonell J (1999) Molecular cloning and characterization of a tomato (Lycopersicon esculentum Mill.) spermidine synthase cDNA (accession no. AJ006414). (PGR 99–103). Plant Physiol 120: 935 [Google Scholar]
- Aziz A (2003) Spermidine and related-metabolic inhibitors modulate sugar and amino acid levels in Vitis vinifera L.: possible relationships with initial fruitlet abscission. J Exp Bot 54: 355–363 [DOI] [PubMed] [Google Scholar]
- Bassie L, Noury M, Lepri O, Lahaye T, Christou P, Capell T (2000) Promoter strength influences polyamine metabolism and morphogenic capacity in transgenic rice tissues expressing the oat adc cDNA constitutively. Transgenic Res 9: 33–42 [DOI] [PubMed] [Google Scholar]
- Bell E, Malmberg RL (1990) Analysis of a cDNA encoding arginine decarboxylase from oat reveals similarity to the Escherichia coli arginine decarboxylase and evidence of protein processing. Mol Gen Genet 224: 431–436 [DOI] [PubMed] [Google Scholar]
- Bhatnagar P, Glasheen BM, Bains SK, Long SL, Minocha R, Walter C, Minocha SC (2001) Transgenic manipulation of the metabolism of polyamines in poplar cells. Plant Physiol 125: 2139–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi S, Scaramagli S, Capitani F, Altamura MM, Torrigiani P (2001) Methyl jasmonate up-regulates biosynthetic gene expression, oxidation and conjugation of polyamines, and inhibits shoot formation in tobacco thin layers. J Exp Bot 52: 231–242 [PubMed] [Google Scholar]
- Biondi S, Scoccianti V, Scaramagli S, Ziosi V, Torrigiani P (2003) Auxin and cytokinin modify methyl jasmonate effects on polyamine metabolism and ethylene biosynthesis in tobacco leaf discs. J Exp Bot 52: 231–242 [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tangui J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140: 103–125 [Google Scholar]
- Capell T, Escobar C, Liu H, Burtin D, Lepri O, Christou P (1998) Over-expression of the oat arginine decarboxylase cDNA in transgenic rice (Oryza sativa L.) affects normal development patterns in vitro and results in putrescine accumulation in transgenic plants. Theor Appl Genet 97: 246–254 [Google Scholar]
- Ceriotti G, Spandrio L (1957) An improved method for the microdetermination of arginine by use of 8-hydroxyquinoline. Biochem J 66: 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay MK, Tabor CW, Tabor H (2002) Absolute requirement of spermidine for growth and cell cycle progression of fission yeast (Schizosaccharomyces pombe). Proc Natl Acad Sci USA 99: 10330–10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Shimomura S, Sitbon F, Sandberg G, Jones AM (2001) The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J 28: 607–617 [DOI] [PubMed] [Google Scholar]
- Cohen SS (1998) A Guide to Polyamines. Oxford University Press, New York
- de Marco A, Roubelakis-Angelakis KA (1996) The complexity of enzymic control of hydrogen peroxide concentration may affect the regeneration potential of plant protoplasts. Plant Physiol 110: 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fos M, Proaño K, Alabadí D, Nuez F, Carbonell J, García-Martínez JL (2003) Polyamine metabolism is altered in unpollinated parthenocarpic pat-2 tomato ovaries. Plant Physiol 131: 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuns JMC, Smets R, Struyf T, Prinsen E, Valcke R, Onckelen HV (2001) Apical dominance in Pssu-ipt-transformed tobacco. Phytochemistry 58: 911–921 [DOI] [PubMed] [Google Scholar]
- Goossens A, Häkkinen ST, Laakso I, Seppänen-Laakso T, Biondi S, De Sutter V, Lammertyn F, Nuutila AM, Söderlund H, Zabeau M, et al (2003) A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proc Natl Acad Sci USA 100: 8595–8600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Tamaki K, Suzuki K, Yamada Y (1998) Molecular cloning of plant spermidine synthases. Plant Cell Physiol 39: 73–79 [DOI] [PubMed] [Google Scholar]
- Hayashi S, Murakami Y (1995) Rapid and regulated degradation of ornithine decarboxylase. Biochem J 306: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heby O, Persson L (1990) Molecular genetics of polyamine synthesis in eukaryotic cells. Trends Biochem Sci 15: 153–158 [DOI] [PubMed] [Google Scholar]
- Hikosaka K (2005) Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann Bot (Lond) 95: 521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A, Akiyama T, Kato T, Sato S, Tabata S, Yamamoto KT, Takahashi T (2004) Spermine is not essential for survival in Arabidopsis. FEBS Lett 556: 148–152 [DOI] [PubMed] [Google Scholar]
- Iniesta V, Gomez-Nieto LC, Corraliza I (2001) The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J Exp Med 193: 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus J, Wolff EC, Park MH, Folk EJ (1993) Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies: effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem 268: 13151–13159 [PubMed] [Google Scholar]
- Jones AM, Im KH, Savka MA, Wu MJ, DeWitt NG, Shillito R, Binns AN (1998) Auxin-dependent cell expansion mediated overexpressed auxin-binding protein 1. Science 282: 1114–1117 [DOI] [PubMed] [Google Scholar]
- Kotzabasis K, Christakis-Hampsas MD, Roubelakis-Angelakis KA (1993. a) A narrow-bore HPLC method for the identification and quantitation of free, conjugated and bound polyamines. Anal Biochem 214: 484–489 [DOI] [PubMed] [Google Scholar]
- Kotzabasis K, Fotinou C, Roubelakis-Angelakis KA, Ghanotakis D (1993. b) Polyamines in the photosynthetic apparatus: photosystem II highly resolved subcomplexes are enriched in spermine. Photosynth Res 38: 83–88 [DOI] [PubMed] [Google Scholar]
- Kurepa J, Smalle J, Van Montagu M, Inzé D (1998) Polyamines and paraquat toxicity in Arabidopsis thaliana. Plant Cell Physiol 39: 987–992 [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 [DOI] [PubMed] [Google Scholar]
- Loulakakis KA, Primikirios NI, Nikolantonakis MA, Roubelakis-Angelakis KA (2002) Immunocharacterization of Vitis vinifera L. ferredoxin-dependent glutamate synthase, and its spatial and temporal changes during leaf development. Planta 215: 630–638 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Malmberg RL, Cellino ML (1994) Arginine decarboxylase of oats is activated by enzymatic cleavage into two polypeptides. J Biol Chem 269: 2703–2706 [PubMed] [Google Scholar]
- Martin-Tanguy J (1997) Conjugated polyamines and reproductive development: biochemical, molecular and physiological approaches. Physiol Plant 100: 675–688 [Google Scholar]
- Marton LJ, Pegg AE (1995) Polyamines as target for therapeutic intervention. Annu Rev Pharmacol Toxicol 35: 55–91 [DOI] [PubMed] [Google Scholar]
- Masclaux C, Valadier MH, Brugière N, Morot-Gaudry JF, Hirel B (2000) Characterization of the sink-source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211: 510–518 [DOI] [PubMed] [Google Scholar]
- Mattheus HR (1993) Polyamines, chromatin structure and transcription. Bioessays 15: 561–567 [DOI] [PubMed] [Google Scholar]
- Mehta RA, Cassol T, Li N, Ali N, Handa AK, Mattoo AK (2002) Engineered polyamine accumulation in tomato enhances phytonutrient comtent, juice quality, and vine life. Nat Biotechnol 20: 613–618 [DOI] [PubMed] [Google Scholar]
- Michael AJ, Furze JM, Rhodes MJ, Burtin D (1996) Molecular cloning and functional identification of a plant ornithine decarboxylase cDNA. Biochem J 314: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakoudis E, Lütz C, Langebartels C, Lütz-Meindl U, Kotzabasis K (2003) Ozone impact on the photosynthetic apparatus and the protective role of polyamines. Biochim Biophys Acta 1621: 160–169 [DOI] [PubMed] [Google Scholar]
- Nilsson J, Koskiniemi S, Persson K, Grahn B, Holm I (1997) Polyamines regulate both transcription and translation of the gene encoding ornithine decarboxylase antizyme in mouse. Eur J Biochem 250: 223–231 [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts IM, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel BL (1999) Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97: 743–754 [DOI] [PubMed] [Google Scholar]
- Pandey S, Ranade SA, Nagar PK, Kumar N (2000) Role of polyamines and ethylene as modulators of plant senescence. J Biosci 25: 291–299 [DOI] [PubMed] [Google Scholar]
- Panicot M, Minguet EG, Ferrando A, Alcázar R, Blázquez MA, Carbonell J, Altabella T, Koncz C, Tiburcio AF (2002) A polyamine metabolon involving aminopropyl transferase complexes in Arabidopsis. Plant Cell 14: 2539–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis AK, Roubelakis-Angelakis KA (2005) Polyamines inhibit NADPH oxidase-mediated superoxide generation and putrescine prevents programmed cell death syndrome induced by the polyamine oxidase-generated hydrogen peroxide. Planta 10.1007: s00425-004-1400-9. [DOI] [PubMed] [Google Scholar]
- Papadakis AK, Siminis CI, Roubelakis-Angelakis KA (2001) Reduced activity of antioxidant machinery is correlated with suppression of totipotency in plant protoplasts. Plant Physiol 126: 434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschalidis KA, Aziz A, Geny L, Primikirios NI, Roubelakis-Angelakis KA (2001) Polyamines in grapevine. In KA Roubelakis-Angelakis, ed, Molecular Biology and Biotechnology of the Grapevine. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 109–152
- Pedroso MC, Primikirios N, Roubelakis-Angelakis KA, Pais MS (1997) Free and conjugated polyamines in embryogenic and non-embryogenic leaf regions of camellia leaves before and during direct somatic embryogenesis. Physiol Plant 101: 213–219 [Google Scholar]
- Perez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol 130: 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS, Sussex IM (1985) The developmental morphology and growth dynamics of the tobacco leaf. Planta 165: 158–169 [DOI] [PubMed] [Google Scholar]
- Primikirios NI, Roubelakis-Angelakis KA (1999) Cloning and expression of an arginine decarboxylase cDNA from Vitis vinifera L. cell-suspension cultures. Planta 208: 574–582 [DOI] [PubMed] [Google Scholar]
- Primikirios NI, Roubelakis-Angelakis KA (2001) Indications for post-translational regulation of Vitis vinifera L. arginine decarboxylase. Plant Mol Biol 45: 669–678 [DOI] [PubMed] [Google Scholar]
- Rastogi R, Dulson J, Rothstein SJ (1993) Cloning of tomato (Lycopersicon esculentum Mill.) arginine decarboxylase gene and its expression during fruit ripening. Plant Physiol 103: 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubelakis KA, Kliewer WM (1978) Enzymes of Krebs-Henseleit cycle in Vitis vinifera L.: in vivo and in vitro studies of arginase. Plant Physiol 62: 344–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsh EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schroder G, Schroder J (1995) cDNAs for S-adenosyl-L-methionine decarboxylase from Catharanthus roseus, heterologous expression, identification of the proenzyme-processing site, evidence for the presence of both subunits in the active enzyme, and a conserved region in the 5′ mRNA leader. Eur J Biochem 228: 74–78 [PubMed] [Google Scholar]
- Sfichi L, Ioannidis N, Kotzabasis K (2004) Thylakoid-associated polyamines adjust the UV-B sensitivity of the photosynthetic apparatus by means of light-harvesting complex II changes. Photochem Photobiol 80: 499–506 [DOI] [PubMed] [Google Scholar]
- Siminis CI, Kanellis AK, Roubelakis-Angelakis KA (1994) Catalase is differentially expressed in dividing and nondividing protoplasts. Plant Physiol 105: 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MA, Mad Arif SA, Kumar A, Davis HV, Scobie LA, Pearce SR, Flavell AJ (1992) Expression and sequence analysis of cDNAs induced during early stages of tuberization in different organs of the potato plant (Solanum tuberosum L.). Plant Mol Biol 20: 641–651 [DOI] [PubMed] [Google Scholar]
- Theiss C, Bohley P, Voigt J (2002) Regulation by polyamines of ornithine decarboxylase activity and cell division in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol 128: 1470–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Thomas TJ (2001) Polyamine in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci 58: 244–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio AF, Altabella T, Borrell A, Masgrau C (1997) Polyamine metabolism and its regulation. Physiol Plant 100: 664–674 [Google Scholar]
- Tucker G, Seymour G (2002) Life on the vine. Nat Biotechnol 20: 558–560 [DOI] [PubMed] [Google Scholar]
- Turano FJ, Kramer GF (1993) Effect of metabolic intermediates on the accumulation of polyamines in detached soybean leaves. Phytochemistry 34: 959–968 [Google Scholar]
- Wallace HM, Fraser AV, Hughes A (2003) A perspective of polyamine metabolism. Biochem J 376: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Malmberg RL (1996) Regulation of Arabidopsis thaliana (L.) Heynh arginine decarboxylase by potassium deficiency stress. Plant Physiol 111: 1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Lee YS, Lee SH, Cho YD (2000) Polyamine synthesis in plants: isolation and characterization of spermidine synthase from soybean (Glycine max) axes. Biochim Biophys Acta 1475: 17–26 [DOI] [PubMed] [Google Scholar]
- Zhang J, Honda C, Kita M, Hu C, Nakayama M, Moriguchi T (2003) Structure and expression of spermidine synthase genes in apple: two cDNAs are spatially and developmentally regulated through alternative splicing. Mol Genet Genomics 268: 799–807 [DOI] [PubMed] [Google Scholar]