Abstract
A β-carbonic anhydrase (CA) in the marine diatom Phaeodactylum tricornutum (PtCA1) is encoded by the nuclear genome. This enzyme was previously found to be important for the operation of photosynthesis with a high affinity for dissolved inorganic carbon. A cDNA sequence that encodes PtCA1 (ptca1) was shown to possess a presequence of 138 bp (pre138), which encodes an N-terminal sequence of 46 amino acids (Pre46AA) that does not exist in the mature PtCA1. In this study, pre138 was ligated with the enhanced green fluorescent protein (GFP) gene (egfp), and introduced into P. tricornutum by microprojectile bombardment. Subsequently, the expressed Pre46AA-GFP fusion was shown to be localized in the chloroplast stroma, whereas the expressed GFP without Pre46AA was localized in the cytoplasm. Insertion of the DNA sequence, encoding a mature region of ptca1 (mptca1) between pre138 and egfp, resulted in the formation of particles with concentrated GFP fluorescence in the stroma of P. tricornutum. These particles, 0.3 to 3.0 μm in size, were shown to be distinct from the mitochondria and localized on the surface of the putative girdle lamella. The attachment of the initial one-half of the pre138 to the mptca1-egfp fusion caused the expressed GFP fusion to accumulate in areas surrounding the chloroplast, presumably due to the presence of the endoplasmic reticulum signal encoded by the initial half-sequence and to the absence of the chloroplast transit sequence. These results indicate that PtCA1 is targeted to the stroma by the bipartite sequences of Pre46AA and that the observed GFP particles are formed specifically in the stroma due to the function of the mptca1.
Diatoms (Bacillariophyceae) belong to Heterokontophyta, which, together with Haptophyta, are familiarly called Chromophyta. Chromophyta are a widespread and successful group of algae. These organisms are believed to have arisen by a secondary endocytobiotic event, which conferred on cells a unique and a relatively complicated plastid structure, namely, a double-layered chloroplast envelope surrounded by a double-layered chloroplast endoplasmic reticulum (ER), which is thought to have arisen by the engulfment of red algae by heterotrophic eukaryotes and the subsequent evolution of the chlorophyll a/c-based plastid (Saunders et al., 1997). These structural characteristics imply that nuclear-encoded chloroplastic proteins must be translocated across these four-layered membranes. In fact, it has been reported in the marine diatom Phaeodactylum tricornutum that the precursor of the γ-subunit of plastid ATP synthase contains a multipartite plastid-targeting sequence at its N terminus, which enables this protein to have a multistep traverse process across the chloroplast ER and the chloroplast envelope (Apt et al., 2002). Apparently, the initial translocation event of such a multistep targeting system seems to utilize an exocytosis-like mechanism that is mediated by the ER signals and the ER (Apt et al., 2002; Ishida and Green, 2002).
Marine diatoms are one of the major primary producers in the oceans and are thought to contribute more than 25% of the total global carbon fixation (Werner, 1977; Tréguer et al., 1995). The carbon-concentrating mechanism (CCM), a mechanism to facilitate an ample flux of CO2 to photosynthesis under CO2 limitation, is known to occur in the few marine diatoms studied so far (Colman and Rotatore, 1995; Johnston and Raven, 1996; Tortell et al., 1997; Badger et al., 1998; Matsuda et al., 2001a). Carbonic anhydrases (CA) in microalgae have been shown to be a crucial component for the operation of the CCM and for efficient CO2 fixation in a CO2-limited environment (Funke et al., 1997; Raven, 1997; Karlsson et al., 1998).
There are significant variations in the subcellular location of CAs, which have been reported in relation to their physiological functions in cyanobacteria and green algae. In the cyanobacterium, Synechococcus sp. PCC 7942, carboxysomal CAs were shown to be essential for the CCM (Price and Badger, 1989). In the green alga Chlamydomonas reinhardtii, on the other hand, CAs are expressed in the periplasmic space, the mitochondrion, and the thylakoid lumen. Recently, a new stromal form of CA (Cah6) was identified in C. reinhardtii and the function of this form as a putative efflux barrier of CO2 from the chloroplast was proposed (Mitra et al., 2004). Of these various forms of CAs in C. reinhardtii, only Cah3, the lumenal form, was demonstrated to be essential for growth under CO2 limitation (Funke et al., 1997; Hanson et al., 2003). These investigations using a model alga strongly suggest that the occurrences of internal CAs close to Rubisco and/or the interior of thylakoid are more crucial for growth under CO2 limitation than external CAs.
The pyrenoid, a structure with a protein matrix, which is contained within the stromal compartment, is another probable candidate for CA localization in algae (Badger et al., 1998). This organelle seems to occur in a large percentage of microalgal chloroplasts, either singly or in multiple numbers per chloroplast and usually contains all the chloroplast Rubisco protein within its matrix (Badger et al., 1998). Pyrenoid has been proposed to be an analogous structure to the cyanobacterial carboxysomes and hence to play a central role in the algal CCM (Badger and Price, 1992; Badger et al., 1998). In fact, it has been reported in several algae and bryophytes that the absence of pyrenoid structure might be correlated with the absence of a CCM (Palmqvist et al., 1995; Smith and Griffiths, 1996a, 1996b). These considerations have prompted the suggestion that pyrenoidal CA is required for the CCM. At present, however, there is no substantial body of evidence for the occurrence of pyrenoidal CA, and the function of pyrenoid in the CCM is still largely unexplained.
In addition to the organisms described above, the occurrences of internal and external forms of CA have been reported in other microalgae (Badger et al., 1998; Matsuda et al., 2002). Although these investigations have shown wide variations in the occurrence of CAs and in its intracellular location, few molecular mechanisms have been elucidated linking the localizations of CAs with their function in the CCM. Despite the significance of diatoms in marine primary productivity, CAs in marine diatoms have been studied to date in very few species. The CA from Thalassiosira weissflogii (TWCA1) was suggested to regulate cell growth, depending upon the concentrations of both extracellular dissolved inorganic carbon and trace metals (Tortell et al., 1997). It was suggested that TWCA1 seems to function by being buffered by the silica shell of the diatom (Milligan and Morel, 2002).
In the marine diatom P. tricornutum, external CA activities may or may not be present, depending upon strain (John-McKay and Colman, 1997). Most of the diatoms studied so far exhibit an efficient direct uptake of HCO3− from the bulk medium even in the presence of the weakly permeable CA inhibitor, acetazolamide (Badger et al., 1998). In P. tricornutum UTEX642, at least one major soluble CA (PtCA1) activity was detected and 2 mm acetazolamide were shown to be ineffective in inhibiting direct HCO3− uptake, whereas 0.5 mm ethoxyzolamide, a highly permeable CA inhibitor, dramatically suppressed high-affinity photosynthesis (Matsuda et al., 2001a; Satoh et al., 2001). Considering the fact that internal forms of CAs seem to be more crucial for cell growth under CO2 limitation than external forms in the green alga C. reinhardtii, the importance of internal CA for the primary production under CO2 limitation would also be of significant interest in marine diatoms.
PtCA1 is encoded by the nuclear genome and its expression is regulated at the transcriptional level by changes in ambient [CO2]; that is, the level of CA gene transcripts in air-grown cells is 5- to 10-fold higher than in 5% CO2-grown cells (Satoh et al., 2001). However, the intracellular location of PtCA1 is unknown and detailed mechanisms of temporal and spatial regulation of PtCA1 expression has yet to be elucidated. Both cDNA (ptca1) and PtCA1 protein have been isolated and PtCA1 was shown to be a β-type CA with a well-conserved zinc coordination domain (Satoh et al., 2001). The ptca1 cDNA was also shown to possess a presequence of 138 bp (pre138), which encodes 46 N-terminal amino acids (Pre46AA) that do not occur in the mature PtCA1 (corresponding gene sequence is termed hereafter mptca1; Satoh et al., 2001). Pre46AA is relatively rich in hydrophobic amino acids and it has been proposed that it might function as a sorting signal for PtCA1 (Satoh et al., 2001). In this study, the intracellular location of PtCA1 and the localization mechanisms of PtCA1 were investigated.
RESULTS
The Expression of Green Fluorescent Protein Fusions
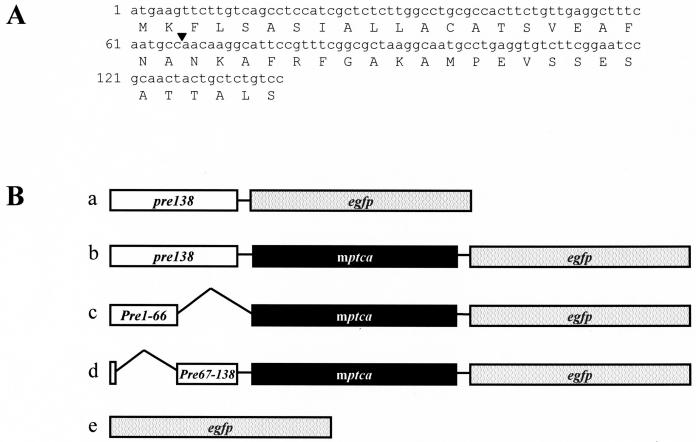
The cDNA sequence of pre138 (Fig. 1A) and two truncation forms of pre138 were ligated with either enhanced green fluorescent protein gene (egfp) or ptca1-egfp fusion (Fig. 1B, a–d) and were packaged into the transformation vector pPha-T1. A vector with egfp alone was used as a control (Fig. 1B, subsection e), and each vector was introduced into P. tricornutum. These genes are thought to be randomly integrated and stably maintained on the genome of P. tricornutum (Apt et al., 1996). As pPha-T1 possesses the Zeocin-resistant (Zeor) gene cassette, transformed cells were selected on solid agar palates containing 100 μg mL−1 of Zeocin for about 3 weeks. Thirty Zeor cells were obtained out of 108 cells transformed by the pre138-egfp fusion construct (Fig. 1B, subsection a). Twenty-three cells of these 30 Zeor cells exhibited emissions of GFP fluorescence. Similarly, for the transformation with pre138-mptca1-egfp, pre1-66-mptca1-egfp, and pre67-138-mptca1-egfp fusions (Fig. 1, subsections b–d), 48, 3, and 5 Zeor cells, respectively, were obtained. Of these, 24, 3, and 4 cells, respectively, were shown to be GFP positive. In these transformants, the inserted constructs were shown to be retained in a stable form on the genome by PCR amplification of genomic DNA (data not shown) and the production of GFP fusions was also confirmed by western blotting (Supplemental Fig. 1).
Figure 1.
Primary structures of pre138 and Pre46AA and fusion constructs inserted in the transformation vector. A, The nucleotide sequence is shown for the top line and the amino acid sequence for the bottom line. An arrowhead indicates the dividing point for the N-terminal and C-terminal halves of Pre46AA. B, pre138 was ligated directly with egfp (subsection a); the full length of ptca1 was ligated with egfp (subsection b); pre1-66, which encodes the N-terminal half of Pre46AA, was ligated with mptca1-egfp fusion (subsection c); pre67-138, which encodes the C-terminal half of Pre46AA with attached initiation codon, atg, was ligated with mptca1-egfp fusion (subsection d); egfp for the control (subsection e).
Laser-Scanning Confocal Microscopy of Wild-Type Cells and Transformants
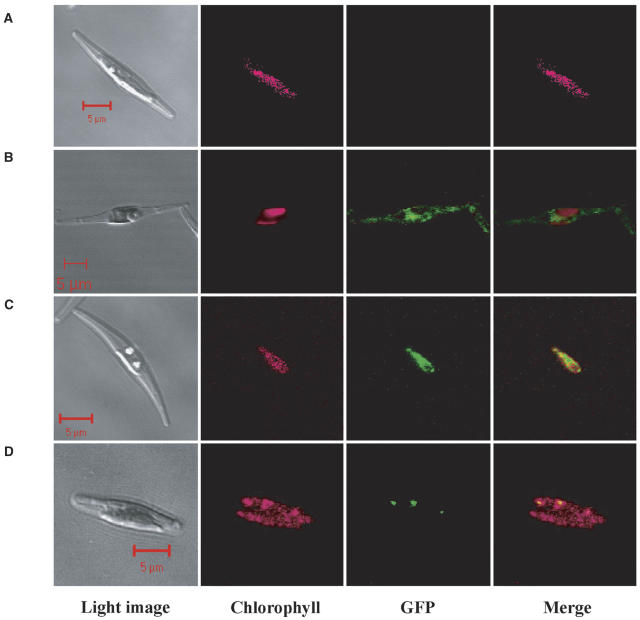
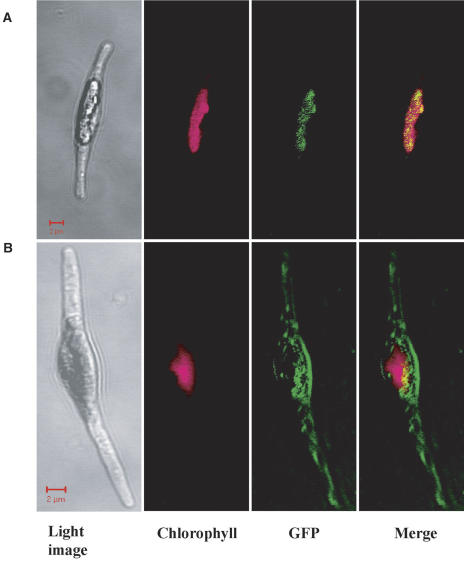
Wild-type cells showed no GFP fluorescence (Fig. 2A). In the transformants by egfp insertion with no presequence, GFP was expressed outside the chlorophyll fluorescence and the GFP signal was dispersed throughout the cytoplasm (Fig. 2B). The attachment of pre138 to egfp (pre138-egfp), in contrast, caused the GFP fusion to be expressed within the stroma (Fig. 2C); namely, GFP fluorescence was shown to be located at the same area as the chlorophyll fluorescence. Interestingly, when a cDNA fragment, encoding mptca1, was inserted between pre138 and egfp and this chimeric fusion (pre138-mptca1-egfp) was expressed in P. tricornutum, GFP fluorescence was observed as several small particles, which seemed to be located close to the thylakoid membranes (Fig. 2D). In sharp contrast, the expression of GFP alone in the chloroplast did not form particles (Fig. 2C). The numbers and sizes of these particles were analyzed for nine different clones of transformants, with the pre138-mptca1-egfp fusion, using 20 independent cells out of each clone. The number of PtCA1-GFP particles was shown to range from 1.5 ± 0.5 to 2.9 ± 1.1 per cell, depending on the clone (Table I), and these particles were 0.3 to 3 μm in size.
Figure 2.
Fluorescent microscope images of wild-type cells and transformants of P. tricornutum. Wild-type cell (A); transformants with construct e (B); with construct a (C); and with construct b (D). Light images, chlorophyll autofluorescence, GFP fluorescence, and merged images of chlorophyll and GFP fluorescence are presented, respectively, from the left column. Images were taken with a laser-scanning confocal microscope under conditions described in the text. Scale bars, 5 μm. Cells were grown in air-level (0.037%) CO2.
Table I.
Numbers of PtCA1-GFP-containing particles observed in nine clones of pre138-mptca1-egfp transformants
Transformation was carried out as described in the text. After the primary screening on 100 μg mL−1 Zeocin, nine clones out of 24 GFP-positive clones were selected, and the numbers of PtCA1-GFP-containing particles per cell were counted.
| Clone No. | No. of Particles per Cella |
|---|---|
| 1 | 1.9 ± 0.9 |
| 3 | 1.8 ± 0.8 |
| 4 | 1.5 ± 0.7 |
| 8 | 2.7 ± 1.4 |
| 9 | 2.2 ± 0.7 |
| 12 | 1.5 ± 0.5 |
| 16 | 2.9 ± 1.1 |
| 18 | 2.0 ± 0.9 |
| 19 | 2.2 ± 0.9 |
Values are the means ± sd counted with 20 independent cells in each clone.
Differentiation of PtCA1-GFP Particles from Mitochondria
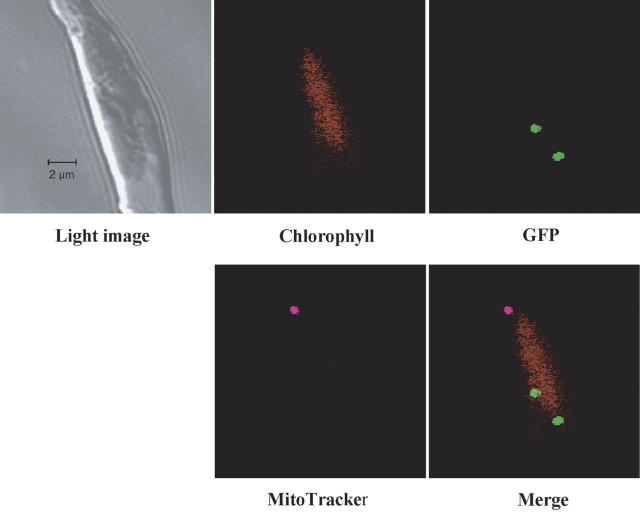
Mitochondria were stained with the mitochondria-specific fluorescent dye, MitoTracker Deep Red 633, and the location of the mitochondria in the pre138-mptca1-egfp transformants was observed by laser-scanning confocal microscopy. At the central part of the P. tricornutum cell (Fig. 3, light image), a single chloroplast that emits autofluorescence was detected (Fig. 3, chlorophyll). The GFP signal, converged into two particles, was also clearly observed (Figs. 3, GFP and merged). A single particle emitting MitoTracker-specific fluorescence, which was colored magenta, was observed (Fig. 3, MitoTracker). The location of the mitochondria was clearly differentiated from that of the GFP-containing particles (Fig. 3, merged). A mitochondrion was found to be located distinct from the chlorophyll fluorescence at an apparent distance of about 0.5 μm (Fig. 3, merged), whereas two PtCA-GFP particles observed in this transformant appeared to be located at the edge of the chlorophyll fluorescence (Fig. 3, merged).
Figure 3.
Staining of a pre138-mptca1-egfp transformant with MitoTracker Deep Red 633. Mitochondrion of a pre138-mptca1-egfp transformant was stained with a specific indicator dye, MitoTracker, as described in the text. A light image (top left) and fluorescent images of the corresponding portion are presented. Fluorescent images of chlorophyll (top middle), GFP (top right), and MitoTracker (bottom left) were merged (bottom right). Scale bars, 2 μm. Cells were grown in 5% CO2.
Sectioning Fluorescent Microscopy of Chloroplast and GFP Particles
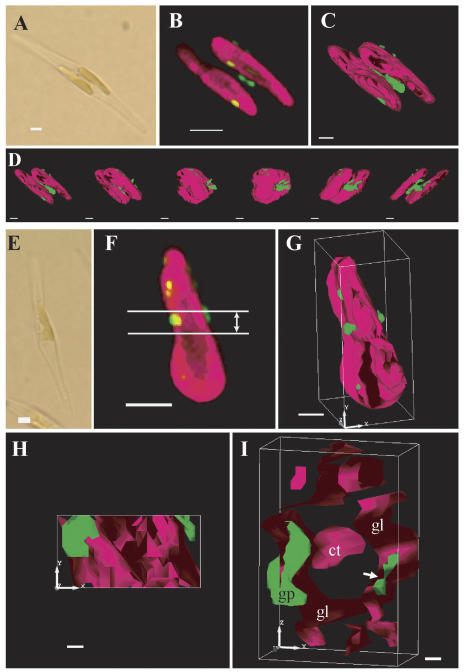
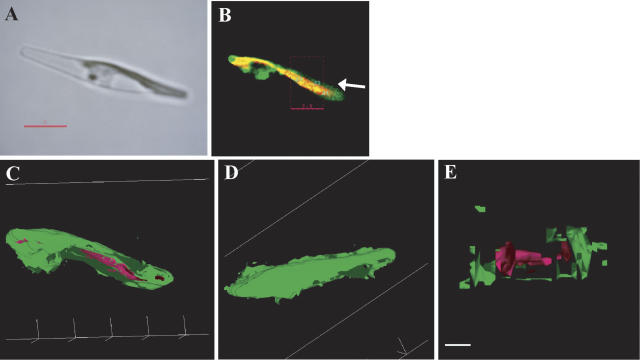
To determine the detailed topological relationship between the thylakoid membranes and the PtCA1-GFP-containing particles in transformants pre138-mptca1-egfp, data from microscopic sections were integrated to form three-dimensional images (Fig. 4). In the transformant shown in Figure 4A, two large parts of the pigment-containing portion within a chloroplast were observed. The GFP fluorescence clearly converged into four to five particles, which were probably attached at the surface of the outermost membranes of the thylakoid (Fig. 4B), and no GFP fluorescence was detected in other parts of the stroma (Fig. 4B). The shapes and sizes of these particles appeared to vary considerably (Fig. 4, B and C). Polygonization of integrated sectioning microscopic images was carried out to create three-dimensional models with enhanced surface information. The model clearly showed that clusters of GFP-containing substances were located at the surface of thylakoid membranes (Fig. 4C). A large particle of GFP fluorescence was shown to occur sandwiched between two large compartments of the thylakoid membranes (Fig. 4C). Views of the chloroplast from different directions, by rotating the three-dimensional model, clearly showed that the thylakoid was shaped as a flat disc and that the PtCA1-GFP-containing particles took indefinite shapes and sizes (Fig. 4D) and were attached at the surface of the outermost thylakoid membranes.
Figure 4.
Analyses of a three-dimensional model of inner chloroplast structures in two independent pre138-mptca1-egfp transformants of P. tricornutum by sectioning fluorescent microscopy. A light image (A); a merged image of chlorophyll and GFP fluorescence of the corresponding portion to A (B); a three-dimensional model of B (C); X-axial rotation of the three-dimensional model (D); a light image (E); a merged fluorescence image of chlorophyll and GFP fluorescence of the corresponding portion to E (F); a three-dimensional model of F (G); a surface image of the cross-sectioned portion indicated in F (H); and a cross-section image that is rotated on the x axis about 90° from H (I). Magenta parts indicate chlorophyll fluorescence and green parts indicate GFP fluorescence. Images in D were taken at every 30° rotation. The cross-sectioned portion of 1.0-μm thickness is indicated in F. x, y, and z axes indicate the topological relationship among G, H, and I. gl, Girdle lamellae; ct, central thylakoid stacks; gp, GFP particle. Scale bars, 2 μm in A, B, E, and F; 1 μm in C, D, and G; 0.2 μm in H and I. Cells were transformed with the construct b in Figure 1B and grown in air-level CO2.
Cross-sectional images of the middle part of the chloroplast were obtained by slicing the three-dimensional model of one of the pre138-mptca1-egfp transformants (Fig. 4, E–G). A sliced section of 1.0-μm thickness (Fig. 4, H and I) was shown to comprise central stacks of thylakoid membrane (Fig. 4I, ct), which was further surrounded by outer thylakoid membranes (Fig. 4I, gl). This outermost thylakoid membrane is probably the girdle lamella, which is the characteristic structure in Heterokontophyta and is known to be located immediately inside the chloroplast envelope. A PtCA1-GFP particle was located at the surface of the putative girdle lamella (Fig. 4I, gp) and also appeared in the interior of the putative girdle lamella (Fig. 4I, arrow). A part of the PtCA1-GFP particle was thus most likely within the girdle lamella rather than attached to the outside (Fig. 4I). However, no PtCA1-GFP particles related to central thylakoid membranes, or penetrating the girdle lamella toward the central thylakoid membranes, were detected in any of the pre138-mptca1-egfp transformants analyzed in this study.
The Expression of GFP Fusions Attached with Manipulated Presequences
Signal peptide analysis by the SignalP algorithm for the N terminus of the Pre46AA of the PtCA1 precursor showed a high probability of the occurrence of a signal peptide sequence of the N-terminal 19 amino acids (data not shown) within the Pre46AA. This result prompted us to follow up the mechanism by which PtCA1 traverses the chloroplast ER membranes and envelopes to form particles in the stroma. Laser-scanning confocal microscopy showed that attachment of the N-terminal 22 amino acids of the Pre46AA, which contains the putative ER signal segment as described above, to the PtCA1-GFP fusion resulted in the localization of GFP fluorescence at the same area as the chlorophyll autofluorescence (Fig. 5A). In sharp contrast, the expression of the PtCA1-GFP fusion with the attached C-terminal half of the Pre46AA resulted in a dispersed distribution of GFP fluorescence throughout the cytoplasm (Fig. 5B). Detailed analyses of GFP localization in the pre1-66-mptca1-egfp transformants were further carried out with the sectioning fluorescent microscope by polygonization modeling to produce integrated three-dimensional images (Fig. 6). GFP fluorescence was shown to occur covering almost all of the chlorophyll autofluorescence (Fig. 6, A–D). This GFP localization seemed to be strictly limited to the edges of the chlorophyll fluorescence (Fig. 6, C and D), but no GFP signal was detected at the cytoplasmic space (Fig. 6, A–D). Cross-sections of the polygonized model clearly showed that GFP fluorescence occurred surrounding the chlorophyll fluorescence of the putative girdle lamella (Fig. 6E). Unlike the pre138-mptca1-egfp transformant, PtCA1-GFP-containing particles attached to, or penetrating the thylakoid membrane of the girdle lamella, were not observed in the pre1-66-mptca1-egfp transformant (Fig. 6E).
Figure 5.
Fluorescent microscope images of cells transformed with egfp fusions comprised of truncated presequences. Images of a pre1-66-mptca1-egfp transformant (A) and a pre67-138-mptca1-egfp transformant (B). Light images, chlorophyll autofluorescence, GFP fluorescence, and merged images of chlorophyll and GFP fluorescence are presented, respectively, from the left column. Images were taken with the laser-scanning confocal microscope under conditions described in the text. Scale bars, 2 μm. Cells were transformed with construct c in Figure 1B (A), with construct d in Figure 1B (B), and grown in air-level CO2.
Figure 6.
Analyses of a three-dimensional model of the chloroplast in a pre1-66-mptca1-egfp transformant. A light image (A); a merged image of chlorophyll and GFP fluorescence of the corresponding portion to A (B); a three-dimensional model viewed from the bottom of B (C); viewed from the top of B (D); and a cross-section image of the model (E). Magenta parts indicate chlorophyll fluorescence and green parts indicate GFP fluorescence. The cross-sectioned portion of the chloroplast is indicated by dashed box in B. The thickness of the section was 3.5 μm. Scale bar in A, 5 μm; scale bar in (E), 2 μm. Cells were transformed with construct c in Figure 1B and grown in air-level CO2.
DISCUSSION
A direct attachment of pre138 on the 5′ terminus of egfp caused a translocation of the expressed GFP from the cytosol to the chloroplast that clearly demonstrates the requirement of Pre46AA in sorting PtCA1 to the chloroplast (Fig. 2, B and C). As shown by Apt et al. (2002), nuclear-encoded plastid proteins in P. tricornutum have a general requirement, in secondary endocytobiotes with plastidal structures surrounded by four-layered membranes, to possess a bipartite presequence of an ER signal followed by a plastid transit sequence. In fact, the N-terminal 19-amino acid sequence of Pre46AA of the PtCA1 precursor was shown to be a putative ER signal. A truncation of the N-terminal 22-amino acid sequence of Pre46AA resulted in the blocking of the entry of the truncated protein into the ER and/or the chloroplast ER (Fig. 5B), indicating that the truncated portion included an ER signal. This N-terminal half of the Pre46AA was, in turn, shown to be enough to let the PtCA1-GFP fusion traverse the chloroplast ER, but not the chloroplast envelope, and thus the product of pre1-66-mptca1-egfp accumulated in the space encircling the chloroplast, most probably the periplastidal compartment (Figs. 5A and 6). It is therefore probable that the following C-terminal half of Pre46AA includes a region that functions as a transit peptide to traverse the chloroplast envelope. It was shown by Apt et al. (2002) that three amino acids, Thr-Thr-Gln, immediately after the ER signal in the presequence of the γ-subunit of plastid ATP synthetase in one strain of P. tricornutum, exhibited a function as a chloroplast transit peptide. In contrast, although the N-terminal 22 amino acids of Pre46AA contains three extra amino acids, Asn-Lys-Ala, after the highly probable cleavage site of the ER signal next to Ala at position 19 (Fig. 1A), this truncated presequence failed to target the PtCA1-GFP fusion to the chloroplast (Figs. 5 and 6). This suggests that the critical motif for chloroplast membrane transit may reside nearer the C-terminal portion of Pre46AA after Ala-22. There are no notable similarities in amino acid sequence after the Ala-22 of Pre46AA as compared to the other known chloroplast transits, such as that of the γ-subunit of ATP synthetase AtpC; several photosystem components; triose phosphate translocator Tpt1; cytochrome c553 PetJ; Fru-1,6-bisP aldolase FbaC1 in P. tricornutum (Apt et al., 2002; Kilian and Kroth, 2004); and in the oxygen-evolving enhancer1 PsbO in dinoflagellates, Haptophyta, Heterokontophyta, Rhodophyta, and higher plants (Ishida and Green, 2002; comparison data not shown). Several prediction algorithms, such as TargetP, gave indecisive results on this C-terminal half of Pre46AA (data not shown). As a noteworthy characteristic in this region, the C-terminal half sequence of Pre46AA comprises hydrophobic residues between which several cationic residues, such as Lys and Arg, are located (Fig. 1A). This characteristic seems to be common to many stroma transit domains in plants, including the Chromophyta (Schnell, 1998; Apt et al., 2002; Ishida and Green, 2002; Kilian and Kroth, 2004), although it is questionable that the particular region in Pre46AA could form a typical amphipathic helix because the distances between positively charged residues are relatively short (two to three residues; Fig. 1A). In Pre46AA, the sequence required for localization to the thylakoid membrane or the lumen was not found (Fig. 1A).
Manipulation of the Pre46AA could alter the localization of PtCA1 selectively within the complicated membrane structures of the chloroplast in P. tricornutum. The four-layered chloroplast membrane also creates at least three intermembrane compartments between the cytosol and the stroma that must form a dense influx and efflux barrier for small molecules like CO2, as compared to that in primary symbiotes. As strongly suggested in the green alga Chlamydomonas and the cyanobacteria, the location of CAs might be closely related to the flux balance of CO2 across the plasmalemma and/or chloroplast envelope and maintain a close proximity of CO2 and its reserve form HCO3− to Rubisco (Raven, 1997; Badger et al., 1998; Hanson et al. 2003). This suggests the possibility that the CO2 fluxes through four-layered chloroplast membranes toward Rubisco in secondary symbiotes could be partially governed by specific CAs. Alterations of PtCA1 localization by manipulation of presequences could be a powerful tool to investigate these problems.
The most intriguing aspect of the intracellular state of PtCA1 is its formation of particles in the chloroplast. Distributions of soluble proteins in the stroma are not necessarily uniform but could constitute reaction complexes in the stroma presumably to close proximities of linked reactions. In many microalgae and in some bryophyta, Rubisco is known to be localized in a protein matrix termed the pyrenoid. In dinoflagellates, Rubisco has been shown to exhibit circadian changes of localization between the stroma area and the pyrenoid during dark and light periods, respectively (Nassoury et al., 2001), supporting the idea that assembled soluble protein complexes form an integrated unit with a specific function. As the pyrenoid seems not to be surrounded by any membrane or carboxysome-like protein shell, this structure is hypothesized to be formed by self-assemblage, driven by the intermolecule interaction among pyrenoid-composing proteins. The mechanisms of the formation of pyrenoids, however, are not known, and proteins composing the pyrenoid have not been identified except for Rubisco. The existence of a pyrenoid in P. tricornutum has not been reported consistently. In one strain of P. tricornutum UTEX646, a pyrenoid-like structure has been reported at the central part of the chloroplast within girdle lamellae (Apt et al., 2002). The presence of the pyrenoid in P. tricornutum could be variable even within the single genus (Badger et al., 1998), and the absence of a starch sheath around the pyrenoid in these microalgae could also account for the apparent lack of a clearly observable pyrenoid structure. CAs are thought to be necessary in the pyrenoid if its function is to create CO2-saturating conditions for Rubisco (Badger et al., 1998), but the presence of CAs in the pyrenoid has not been shown and the proposed role of pyrenoids in the CCM is largely speculation. Considering that pyrenoids are sometimes not present in P. tricornutum or, if present, are localized as a relatively large single compartment in the central part of the chloroplast within the girdle lamella (Apt et al., 2002), the PtCA1-GFP-containing particles observed in this study are probably not conventionally defined pyrenoids, since these particles seem to be localized on the surface of the girdle lamella or in the girdle lamella, but were not observed at the central part of the chloroplast (Fig. 4). Despite the apparent lack of homology of these particles with pyrenoids, the occurrence of CA particles at the surface of the girdle lamella is still noteworthy when assuming any functional assembly with which a tight proximity of reactions is constituted to create an ample flow of inorganic carbon toward the site of CO2 fixation. Some supporting data for this idea are that the inhibition of intracellular CAs by a specific inhibitor, ethoxyzolamide, severely suppressed high-affinity photosynthesis in P. tricornutum (Satoh et al., 2001). It is also possible that these particles might merely be storage forms of PtCA1 in the stroma. Other components of the PtCA1-containing particles have not yet been identified and the implications of such particle formation are not known.
The expression of GFP alone, either in the cytosol or the stroma, did not result in the formation of the converged GFP signal (Fig. 2, B and C). This is also the case in the expression of PtCA1-GFP fusions when they were localized in the cytoplasm or the periplastidal compartment (Figs. 5 and 6). It is therefore clear that this particle formation occurs strictly in the stroma only when mptca1 was present and that the attached GFP to the C terminus of PtCA1 does not cause formation of particles. Unknown effects of the overexpression of the PtCA1/PtCA1-GFP group also should be considered. The activity of the fcpA promoter is independent of ambient CO2 concentration and is about one-third that of the ptca1 promoter under limited CO2 (data not shown). It is expected, therefore, that the total amount of the PtCA1 population in the pre138-mptca1-egfp transformant could exceed up to 30% under limited CO2 conditions as compared with wild-type cells. In fact, western-blot analysis of this transformant agreed reasonably well with the above assumption (Supplemental Fig. 1). However, such slight overexpression of the PtCA1 population is not likely to cause the formation of particles since PtCA1-GFP expression, driven constitutively by the fcpA promoter, formed PtCA1-GFP-containing particles even in 5% CO2-grown cells of P. tricornutum in which the endogenous PtCA1 is almost completely repressed (Fig. 3) and, hence, the concentration of PtCA1 is much lower than that in air-grown cells.
The fact that only the GFP fused with mptca1 at the N terminus, specifically when expressed in the stroma, resulted in the convergence of the GFP signal (Fig. 2D) indicates that a part of mptca1 would play a key role in constituting the protein complexes observed in this study. A previous study showed that purified PtCA1 did not show a clear single molecular mass in the native state in vitro on gel filtration assay (Matsuda et al., 2001b), whereas that determined on SDS-PAGE was clearly about 28 kD (Satoh et al., 2001); that is, PtCA1 eluted from gel filtration columns at a molecular mass of about 30 to 800 kD throughout and did not give any clear sharp peak in the elution profile (Matsuda et al., 2001b). This suggests that PtCA1 possesses some physicochemical characteristics that interact weakly with each other in vitro and hence may cause particle formation on the girdle lamella in vivo. The nature of PtCA1, however, is not sufficient to account for particle formation, since particles were not observed when the PtCA1-GFP fusion was expressed in the cytosol (Fig. 5B) or the periplastidal compartment (Figs. 5A and 6). It is probable, therefore, that some additional stromal factors may be needed to cooperate with a structural motif in the mptca1 for the assemblage and growth of the protein complex.
Although the physiological role of PtCA1 is not known, it is of particular interest that clustering CA is located on the outermost thylakoid membrane, probably being faced to the chloroplast envelope. Considering that the environment surrounding the PtCA1 particles would be alkaline when cells photosynthesize actively, PtCA1 catalyzes a hydration of CO2 to form HCO3−. Since the girdle lamella is located immediately inside the chloroplast envelope, the hydration of CO2 at the layer between the chloroplast envelope and the girdle lamella may create both a strong resistance for CO2 efflux from the chloroplast and a proton driving force to secondary transport systems on the chloroplast envelope. The nature of components of PtCA1-containing particles and the metabolic relations between these particles and CO2 fixation remain to be studied.
MATERIALS AND METHODS
Algal Culture
The marine diatom Phaeodactylum tricornutum Bohlin UTEX642 was obtained from the University of Texas Culture Collection (Austin, TX) and was cultured axenically in artificial seawater containing one-half-strength Gullard's F solution (F/2ASW; Guillard and Ryther, 1962; Harrison et al., 1980), which was additionally buffered with 10 mm Tris-HCl, pH 8.2, under continuous illumination at photon flux density of 50 μmol m−2 s−1 at 20°C. CO2 concentrations in the in-flow air were 5% (v/v) or 0.037% (v/v), depending on experiments as described in the each figure legend.
Signal Analysis of Pre46AA
Probabilities of occurrence of a signal peptide and a possible cleavage site in Pre46AA were analyzed by the SignalP 3.0 algorithm (http://www.cbs.dtu.dk/services/SignalP; Nielsen et al., 1997).
Vector Constructs
Fusion constructs were designed as shown in Figure 1B. The gene for the presequence of PtCA1 precursor, pre138 (Fig. 1A; Satoh et al., 2001; accession no. AF414191), was ligated directly with egfp (Fig. 1B, subsection a) or the coding region of mptca1 (accession no. AF414191; Satoh et al., 2001) with a deleted termination codon inserted between pre138 and egfp (Fig. 1B, b). egfp fusions with two manipulated presequences were also prepared; namely, the initial 66 bp of pre138 (pre1-66) or the next 67 to 138 bp of pre138 with an attached initiation codon at the 5′ terminus (pre67-138) were ligated with the mptca1-egfp fusion (Fig. 1B, subsection c or d, respectively). To prepare these constructs, forward and reverse primer sets for PCR amplifications of each part sequence were used as follows: ptcasigE1Fw (5′-GGGAATTCCCATGAAGTTCTTGTCAGC-3′) and ptcasigRv (5′-GGACAGAGCAGTAGTTGCGGATTCCG-3′) for pre138; egfpFw (5′-ATGGTGAGCAAGGGCGAGGA-3′) and egfpH3Rv (5′-CCCAAGCTTGGGTTACTTGTACAGCT-3′) for egfp; ptcasigE1Fw and ptcanoTAARv (5′-GGCAGGGATCTTGGCGTTTTC-3′) for the whole sequence of ptca1 cDNA (pre138-mptca1); ptcasigE1Fw and ptcasig1-66Rv (5′-GGCATTGAAAGCCTCAACAGAAGTGG-3′) for pre1-66; and ptcasig67-138ATGE1Fw (5′-GGGAATTCCCATGAACAAGGCATTCCGTTTCGGCG-3′) and ptcasigRv for pre67-138. As a control, egfp was amplified (Fig. 1B, subsection e) using egfpE1Fw (5′-GGGAATTCCCATGGTGAGCAAG-3′) and an egfpH3Rv primer pair. The DNA polymerase, KOD-plus (Toyobo, Osaka), was used for all PCR amplifications and EcoRI and HindIII restriction sites were attached to the 5′ terminus of presequences and the 3′ terminus of egfp upon PCR amplifications of these terminal fragments, respectively. These egfp fusions were treated by EcoRI and HindIII and were inserted into the EcoRI and HindIII sites of the P. tricornutum transformation vector, pPha-T1, (Zaslavskaia et al., 2000). The inserted egfp fusions were flanked by the fcpA promoter and the fcpA terminator. pPha-T1 was equipped with the Zeor cassette as a primary selection marker system that is driven by the fcpB promoter and the fcpA terminator (Zaslavskaia et al., 2000).
Transformation of P. tricornutum
Each vector containing an egfp fusion was introduced into wild-type cells of P. tricornutum by microprojectile bombardment using the Bio-Rad Biolistic PDS-1000/He particle delivery system (Bio-Rad, Hercules, CA) as shown by Apt et al. (2002). The employed pressure range of the rupture disc was 1,550 psi and the tungsten M17 particle of 1.1-μm median diameter was used as a micro carrier. Three milligrams of micro carrier were coated with 5 μL of 0.1% (w/v) plasmid DNA in the presence of 1.0 m CaCl2 and 16 mm of spermidine as instructed by the manufacturer. Wild-type cells of P. tricornutum at midlogarithmic phase (OD730 nm = 0.2–0.3) were harvested and 5 × 107 cells were plated on a 1.2% (w/v F2ASW) agar plate to form a plaque of 30-mm diameter. This plate was placed at the second level of the biolistic chamber and was bombarded under the negative pressure of 27 inches of mercury. The bombarded cells were heated in the dark for a day at 20°C and resuspended in 5 mL of F/2ASW. The cell suspension was centrifuged at 3,000g for 5 min at 20°C, resuspended in 0.5 mL of F/2ASW, and plated on an F/2ASW agar plate containing 100 μg mL−1 of Zeocin.
Laser-Scanning Confocal Microscopy
Fluorescent microscopy of chlorophyll a/c autofluorescence, GFP, and MitoTracker Deep Red 633 (Molecular Probes, Eugene, OR) was carried out with a laser-scanning confocal microscope, LSM 510 META, version 3.0 (Carl Zeiss, Oberkochen, Germany), at the lambda mode. The high-frequency transduction UV/488/543/633 filter was selected as the main dichroic splitter and three laser lines (488, 543, and 633 nm) were activated for the excitation of the specimen. The lambda mode scanning data were taken in a range from 488 to 700 nm. Each specific fluoroimage was extracted by subtracting prescanned fluorescent spectrum information of each material (chlorophyll a/c, GFP, or MitoTracker) from the total fluorescent information.
Sectioning Fluorescent Microscopy
A three-dimensional microscopic fluorescent image of chlorophyll a/c autofluorescence and GFP was modeled by combination of a sectioning fluorescent microscopy system, DeltaVision (Applied Precision, Issaquah, WA), and softWoRx version 2.5″, an image analysis and model-building software (Applied Precision). To acquire the chlorophyll a/c autofluorescence images, a CY-5 (640/20) excitation filter and a CY-5 (685/40) emission filter were selected for excitation and emission, respectively. The GFP fluorescent images were also taken by setting the GFP (470/40) excitation filter and the GFP (525/50) emission filter for excitation and emission, respectively.
Supplementary Material
Acknowledgments
We are grateful to Dr. Kirk E. Apt (Martek Biosciences Corporation, Columbia, MD) for sharing the transformation vector, pPha-T1; Dr. Brian Colman (York University, Toronto) for critical reading and comments on this manuscript; Ms. Nobuko Higashiuchi for her technical assistance; and Ms. Miyabi Inoue for her skillful secretarial aid.
This work was supported in part by the Showa-Shell-Sekiyu Environmental Research Foundation (to Y.M.), by the Kato Memorial Bioscience Foundation (to Y.M.), and by the University-Industry Joint Research Project of the Ministry of Education, Culture, Sports, Science and Technology.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.058982.
References
- Apt KE, Kroth-Pancic PG, Grossman AR (1996) Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol Gen Genet 252: 572–579 [DOI] [PubMed] [Google Scholar]
- Apt KE, Zaslavskaia L, Lippmeier JC, Lang M, Kilian O, Wetherbee R, Grossman AR, Kroth PG (2002) In vivo characterization of diatom multipartite plastid targeting signals. J Cell Sci 115: 4061–4069 [DOI] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD (1998) The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot 76: 1052–1071 [Google Scholar]
- Badger MR, Price GD (1992) The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiol Plant 84: 606–615 [Google Scholar]
- Colman B, Rotatore C (1995) Photosynthetic inorganic carbon uptake and accumulation in two marine diatoms. Plant Cell Environ 18: 919–924 [Google Scholar]
- Funke RP, Kovar JL, Weeks DP (1997) Intracellular carbonic anhydrase is essential to photosynthesis in Chlamydomonas reinhardtii at atmospheric levels of CO2. Plant Physiol 114: 237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8: 229–239 [DOI] [PubMed] [Google Scholar]
- Hanson DT, Franklin LA, Samuelsson G, Badger MR (2003) The Chlamydomonas reinhardtii cia3 mutant lacking a thylakoid-lumen localized carbonic anhydrase is limited by CO2 supply to Rubisco and not photosystem II function in vivo. Plant Physiol 132: 2267–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Waters FJR, Taylor FJR (1980) A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J Phycol 16: 28–35 [Google Scholar]
- Ishida K, Green BR (2002) Second- and third-hand chloroplasts in dinoflagellates: phylogeny of oxygen-evolving enhancer 1 (PsbO) protein reveals replacement of a nuclear-encoded plastid gene by that of a haptophyte tertiary endosymbiont. Proc Natl Acad Sci USA 99: 9294–9299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John-McKay ME, Colman B (1997) Variation in the occurrence of external carbonic anhydrase among strains of the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 33: 988–990 [Google Scholar]
- Johnston AM, Raven JA (1996) Inorganic carbon accumulation by the marine diatom Phaeodactylum tricornutum. Eur J Phycol 31: 285–290 [Google Scholar]
- Karlsson J, Clarke AK, Chen ZY, Hugghins SY, Park YI, Husic HD, Moroney JV, Samuelsson G (1998) A novel α-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J 17: 1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian O, Kroth PG (2004) Presequence acquisition during secondary endocytobiosis and the possible role of introns. J Mol Evol 58: 712–721 [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Hara T, Colman B (2001. a) Regulation of the induction of bicarbonate uptake by dissolved CO2 in the marine diatom, Phaeodactylum tricornutum. Plant Cell Environ 24: 611–620 [Google Scholar]
- Matsuda Y, Satoh K, Harada H, Satoh D, Hiraoka Y, Hara T (2002) Regulation of the expressions of HCO3− uptake and intracellular carbonic anhydrase in response to CO2 concentration in the marine diatom Phaeodactylum sp. Funct Plant Biol 29: 279–287 [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Satoh D, Hiraoka Y, Harada H (2001. b) β-Type carbonic anhydrase, one of major soluble proteins in the marine diatom Phaeodactylum tricornutum grown in air-level CO2. In Proceedings of the XIIth International Congress on Photosynthesis, August 18–23, 2001, Brisbane, Australia, S19-004
- Milligan AJ, Morel FMM (2002) A proton buffering role for silica in diatoms. Science 297: 1848–1850 [DOI] [PubMed] [Google Scholar]
- Mitra M, Lato SM, Ynalvez RA, Xiao Y, Moroney JV (2004) Identification of a new chloroplast carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 135: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassoury N, Fritz L, Morse D (2001) Circadian changes in ribulose-1,5-bisphosphate carboxylase/oxygenase distribution inside individual chloroplasts can account for the rhythm in dinoflagellate carbon fixation. Plant Cell 13: 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10: 1–6 [DOI] [PubMed] [Google Scholar]
- Palmqvist K, Sültemeyer D, Baldet P, Andrews TJ, Badger MR (1995) Characterization of inorganic carbon fluxes, carbonic anhydrase(s) and ribulose-1,5-bisphosphate carboxylase-oxygenase in the green unicellular alga Coccomyxa: comparisons with low CO2 cells of Chlamydomonas reinhardtii. Planta 197: 352–361 [Google Scholar]
- Price GD, Badger MR (1989) Expression of human carbonic anhydrase in the cyanobacterium Synechococcus PCC7942 creates a high CO2-requiring phenotype. Plant Physiol 91: 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA (1997) CO2-concentrating mechanisms: a direct role for thylakoid lumen acidification? Plant Cell Environ 20: 147–154 [Google Scholar]
- Satoh D, Hiraoka Y, Colman B, Matsuda Y (2001) Physiological and molecular biological characterization of intracellular carbonic anhydrase from the marine diatom Phaeodactylum tricornutum. Plant Physiol 126: 1459–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders GW, Potter D, Anderson RA (1997) Phylogenic affinities of the Sarcinochrysidales and Chrysomeridales (Heterokonta) based on analyses of molecular and combined data. J Phycol 33: 310–318 [Google Scholar]
- Schnell JD (1998) Protein targeting to the thylakoid membrane. Annu Rev Plant Physiol Plant Mol Biol 49: 97–126 [DOI] [PubMed] [Google Scholar]
- Smith EC, Griffiths H (1996. a) The occurrence of the chloroplast pyrenoid is correlated with the activity of a CO2-concentrating mechanism and carbon isotope discrimination in lichens and bryophytes. Planta 198: 6–16 [Google Scholar]
- Smith EC, Griffiths H (1996. b) A pyrenoid based carbon-concentrating mechanism is present in terrestrial bryophytes of the class Anthocerotae. Planta 200: 203–212 [Google Scholar]
- Tortell PD, Reinfaelder JR, Morel FMM (1997) Active uptake of bicarbonate by diatoms. Nature 390: 243–2449384376 [Google Scholar]
- Tréguer P, Nelson DM, Bennekom AJ, DeMaster DJ, Leynaert A, Quéquiner B (1995) The silica balance in the world ocean: a reestimate. Science 268: 375–379 [DOI] [PubMed] [Google Scholar]
- Werner D (1977) Introduction with a note on taxonomy. In D Werner, ed, The Diatoms. Blackwell Scientific Publications, Oxford, pp 1–17
- Zaslavskaia LA, Lippmeier JC, Kroth PG, Grossman AR, Apt KE (2000) Transformation of the marine diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J Phycol 36: 379–386 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.