Abstract
Previous work has shown that 13-hydroxylated gibberellins (GAs) are predominant in the long-day (LD) plant spinach (Spinacia oleracea; GA53, GA44, GA19, GA20, GA1, GA8, and GA29). Also present in spinach are 2β-hydroxylated C20-GAs: GA97, GA98, GA99, and GA110. Levels of the most abundant GA, GA97, decreased when plants were transferred from short photoperiods (SD) to LD. When [14C]GA53 was fed to spinach plants, more GA53 was converted to GA97 in SD than in LD, and more radioactive GA20 was formed in LD than in SD. SoGA2ox3, encoding a GA 2-oxidase, was isolated from spinach. The recombinant protein converted only two C20-GA precursors, GA12 and GA53, to their respective products, GA110 and GA97. GA2ox3 competes with GA20ox1 for their common substrate, GA53. In SD, deactivation to GA97 prevails, whereas in LD conversion to GA20 is favored. Transcript levels of SoGA2ox3 were higher in shoot tips than in blades, petioles, and young leaves. Ectopic expression of SoGA2ox3 in the long-day plant Nicotiana sylvestris showed a range of dwarf phenotypes, such as reduced germination, short hypocotyl and stem, dark-green leaves, and late flowering, but normal flowers and seed production. The levels of GA53 and GA1 were 3- to 5-fold lower in transgenic plants than in wild type, whereas the levels of GA97 and GA110 increased 3- to 6-fold in transgenic plants. It is concluded that genetic manipulation of plant stature by increasing deactivation of precursors of active GA is more advantageous than increased deactivation of bioactive GA1 itself.
Long-day (LD) rosette plants, such as spinach (Spinacia oleracea), grow vegetatively and do not produce a stem when grown under short photoperiods (SD). Upon transfer to LD, stems elongate and flowering is initiated. In spinach, LD-induced stem elongation is dependent on GA-regulated processes. Although 136 different GAs (http://www.plant-hormones.info/gibberellins.htm) have been identified from natural sources, most of them are precursors or deactivated catabolites of a few biologically active GAs (Hedden and Phillips, 2000; Yamaguchi and Kamiya, 2000; Olszewski et al., 2002). The major endogenous GAs of spinach belong to the early-13-hydroxylation pathway (GA53, GA44, GA19, GA20, GA1, GA8, and GA29; Fig. 1A; Talon et al., 1991). Of these GAs, only GA1 is active per se (Zeevaart et al., 1993). A small gene family of 2-oxidases catalyzes deactivation of GA1 by 2β-hydroxylation to GA8. Genes encoding 2-oxidases have been isolated from several species: Phaseolus coccineus and Arabidopsis (Arabidopsis thaliana; Thomas et al., 1999), pea (Pisum sativum; Lester et al., 1999; Martin et al., 1999), rice (Oryza sativa; Sakamoto et al., 2001), spinach (Lee and Zeevaart, 2002), and poplar (Populus spp.; Busov et al., 2003). These GA 2-oxidases introduce a 2β-hydroxyl group to the bioactive GAs GA1 and GA4 and their respective precursors, GA9 and GA20. The two recombinant GA 2-oxidases (SoGA2ox1 and SoGA2ox2) isolated from spinach 2β-hydroxylated the C19-GAs GA1 and GA20 to GA8 and GA29, respectively. In addition, SoGA2ox1 also converted the C20-GA GA53 to GA97 (Lee and Zeevaart, 2002). In Arabidopsis, two GA 2-oxidases that 2β-hydroxylated GA12 and GA53 were identified by activation tagging. Increased expression of these genes resulted in decreased levels of active GAs and corresponding dwarf phenotypes in Arabidopsis and tobacco (Nicotiana tabacum; Schomburg et al., 2003).
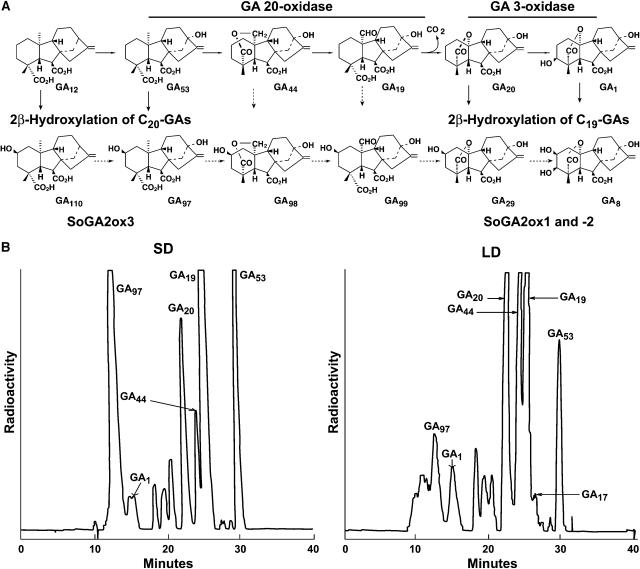
Figure 1.
A, The early-13-hydroxylation pathway from GA12 to GA8 of GA biosynthesis and deactivation in spinach. Each 2β-hydroxylated GA can be derived from the corresponding 13-hydroxylated GA, but only the conversions indicated by solid arrows have been experimentally demonstrated. B, Reverse-phase HPLC profiles of radioactive products after feeding of [14C]GA53 to spinach grown in SD or LD. Deactivation of radiolabeled GA53 by 2β-hydroxylation to GA97 was greater in SD than in LD. Full-scale radioactivity is 104 dpm.
In spinach, GA53 is at a branch point and can be converted to bioactive GA1 via the early-13-hydroxylation pathway, or it can be 2β-hydroxylated to GA97 (Fig. 1A). In addition to GA97, several other 2β-hydroxylated GAs are found in spinach: GA98 (2β-hydroxy-GA44), GA99 (2β-hydroxy-GA19), and GA110 (2β-hydroxy-GA12; Mander et al., 1996; Owen et al., 1998). Gilmour et al. (1986) found that cell-free extracts from spinach leaves are capable of converting [14C]GA12 to [14C]2β-hydroxy-GA12. These results led us to postulate that an early-2β-hydroxylation pathway operates in spinach and that a 2-oxidase in spinach specifically 2β-hydroxylates C20-GAs (Fig. 1A).
Here, we report the molecular cloning and characterization of SoGA2ox3 from spinach that 2β-hydroxylates two C20-GAs, GA12 and GA53. Ectopic expression of SoGA2ox3 in Nicotiana sylvestris produced a high level of GA97 with a concomitant decrease in the levels of active GA1. Transgenic plants overexpressing this 2-oxidase exhibited GA-deficient phenotypes, such as delayed germination, short hypocotyl, reduced stature, and late flowering.
RESULTS
Metabolism of [14C]GA53 in Spinach in SD and LD
[14C]GA53 was fed to spinach plants in SD and LD to determine differences in GA metabolism in plants under various photoperiodic conditions. As shown in Figure 1B, there were striking differences in the metabolism of GA53 between plants in SD and LD. First, much more GA53 remained unmetabolized in SD than in LD. Second, much more GA53 was converted to GA20 in LD than in SD. This is expected because GA 20-oxidase activity is much higher in LD than in SD (Lee and Zeevaart, 2002). Third, more radioactive GA1 was produced in LD than in SD. This is presumably due to the increased level of GA20, the substrate for GA 3-oxidase, which is not up-regulated by LD (Lee and Zeevaart, 2002). Fourth, and most striking, is the massive accumulation of GA97 in plants in SD as compared to those in LD. These data indicate that inactivation of GA53 by 2β-hydroxylation is predominant in SD, whereas in LD GA53 is preferentially converted to GA20 and further to bioactive GA1. On the basis of these results, it was of interest to investigate this GA 2-oxidase and its control at the molecular-genetic level.
Cloning of SoGA2ox3 cDNA
First-strand cDNA was synthesized with random primers and poly(A+) RNA isolated from spinach grown under SD conditions as a template. All combinations of degenerate primers were used in the PCR reactions with first-strand cDNA. The combination of degenerate primers JZ598 and JZ600 (Table I) yielded a 611-bp fragment of a putative GA 2-oxidase (data not shown). The predicted partial amino acid sequence of this reverse transcription (RT)-PCR product shares more identity with the amino acid sequences of AtGA2ox7 and AtGA2ox8 (Schomburg et al., 2003) than with those of the SoGA2ox1, SoGA2ox2, AtGA2ox1, AtGA2ox2, and AtGA2ox3 proteins (Thomas et al., 1999; Lee and Zeevaart, 2002). 5′- and 3′-RACE were performed with sequence-specific primers (JZ616, JZ617, JZ618, and JZ627).
Table I.
Primers used for amplification of the GA 2-oxidase3 gene from spinach
Single-letter codes for nucleotides were assigned as follows: Y (C/T), N (A/T/G/C), R (A/G), D (A/G/T), and W (A/T).
| Purpose | Primer | Primer Sequence (5′ to 3′) | Orientation |
|---|---|---|---|
| RT-PCR | JZ598 | GARTGGGGNTTYTTYCARRT | S |
| RT-PCR | JZ600 | GCYTGRAATAWRTCNCCDATRTT | AS |
| 3′-RACE | JZ617 | GCATGATGAGCTGACCTTTATTG | S |
| 3′-RACE | JZ618 | AGATACCCACCATGCCCTAAAT | S |
| 5′-RACE | JZ616 | TTGAATGGCTCCCTAAACACTT | AS |
| 5′-RACE | JZ627 | ACCGTTGAGCATAGATTGCTGA | AS |
| Coding region | JZ651 | TCCGGATCCAAATGGCTTCTACCAAGGTAG | S |
| Coding region | JZ656 | CTGAGAATTCCTAGTTCGAAATGAGGAAG | AS |
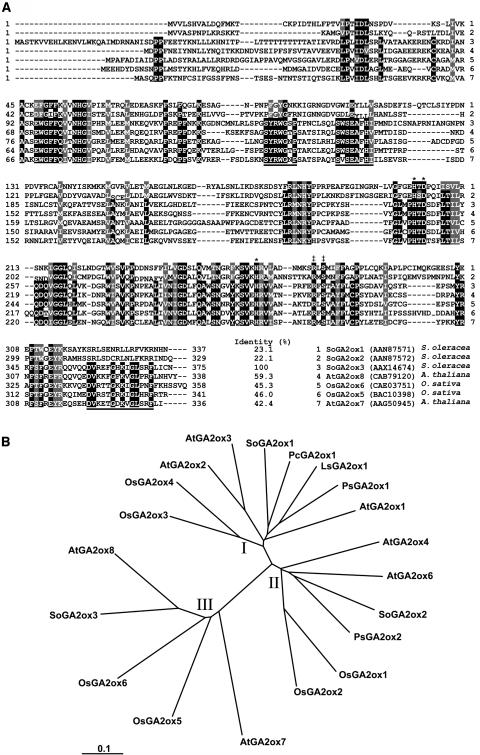
The coding region of SoGA2ox3 was obtained with primers JZ651 and JZ656 (Table I). The full-length cDNA clone of SoGA2ox3 (1,242 bp, GenBank accession no. AY935713) has an open reading frame of 1,128 bp, encoding a putative protein of 375 amino acids with a 79-bp 5′-untranslated sequence and a 35-bp 3′-untranslated sequence. The predicted molecular mass of the protein is 43 kD, with a pI of 8.42. Southern-blot analysis using the SoGA2ox3 cDNA as a probe indicated that there is at least one more related gene in the spinach genome (data not shown). Figure 2A shows an alignment of amino acid sequences of GA 2-oxidases from spinach, Arabidopsis, and rice. When compared with other 2-oxoglutarate-dependent dioxygenases, the deduced amino acid sequence of SoGA2ox3 fits best in the family of GA 2-oxidases (Fig. 2B). The predicted amino acid sequence shares 59.3%, 42.4%, 46%, and 45.3% identity with AtGA2ox8, AtGA2ox7, OsGA2ox5, and OsGA2ox6, respectively. However, it shares only 23.1% and 22.1% identity with the SoGA2ox1 and SoGA2ox2 proteins (Lee and Zeevaart, 2002). The amino acid sequence of the SoGA2ox3 protein contains gene-specific motifs (located at positions 143–149, 158–164, and 358–371) that are different from other GA dioxygenases, and a Thr homopolymer (located at positions 49–58). Three underlined regions show unique amino acid sequences that are highly conserved in SoGA2ox3, AtGA2ox7, AtGA2ox8, OsGA2ox5, and OsGA2ox6 but not in other GA dioxygenases, including SoGA2ox1 and SoGA2ox2 (Fig. 2, A and B). The amino acid sequence of SoGA2ox3 shares several conserved regions with other GA dioxygenases, such as putative 2-oxoglutarate-binding sites (Arg-319 and Ser-321) and iron-binding sites (His-253, Asp-255, and His-309; Thomas et al., 1999). However, the amino acid sequence (L-S-W-S-E-A, positions 157–162) shares only 50% identity with the L-P-W-K-E-T sequence, which is conserved in all GA 20-oxidases and which has been proposed to be involved in binding the substrates GA12 and/or GA53 (Wu et al., 1996; Kang et al., 1999; Sakamoto et al., 2004). SoGA2ox3 contains one intron of 781 bp (AY935714), which is located at the same position as the second intron of the AtGA2ox7 and AtGA2ox8 genes (Schomburg et al., 2003).
Figure 2.
Comparison of the deduced amino acid sequences of SoGA2ox3 with other GA 2-oxidases. A, Sequence alignment of the amino acid sequence of SoGA2ox3 cDNA with related GA 2-oxidases. The amino acid sequences were aligned using BCM Search Launcher. The three underlined regions show the unique amino acid sequences that are highly conserved in the SoGA2ox3, AtGA2ox7, AtGA2ox8, OsGA2ox5, and OsGA2ox6 proteins. Asterisks indicate putative amino acid residues that are supposed to bind iron. The symbol ‡ indicates the putative 2-oxoglutarate-binding sites. B, Phylogenetic tree based on the comparison of GA 2-oxidase genes. The amino acid sequences form three classes (I, II, and III). This unrooted phylogenetic tree was created using ClustalW (http://www.ebi.ac.uk/clustalw/). GenBank accession numbers of proteins are (in parentheses): AtGA2ox1 (CAB41007), AtGA2ox2 (CAB41008), AtGA2ox3 (CAB41009), AtGA2ox4 (AAG51528), AtGA2ox6 (AAG00891), AtGA2ox7 (AAG50945), AtGA2ox8 (CAB79120), LsGA2ox1 (BAB12442), OsGA2ox1 (BAB40934), OsGA2ox2 (BAC16751), OsGA2ox3 (BAC16752), OsGA2ox4 (AAU03107), OsGA2ox5 (BAC10398), OsGA2ox6 (CAE03751), PcGA2ox1 (CAB41036), PsGA2ox1 (AAF08609), PsGA2ox2 (AAD45424), SoGA2ox1 (AAN87571), SoGA2ox2 (AAN87572), and SoGA2ox3 (AAX14674). The scale value of 0.1 indicates 0.1 amino acid substitutions per site.
The amino acid sequences of all GA 2-oxidases were aligned using the ClustalW 1.8 Multiple Sequence Alignment program, and a phylogenetic tree was generated (Fig. 2B). This analysis divides the GA 2-oxidase family into three different clades. Members of classes I and II catabolize C19-GAs. However, as far as tested, members of class III can only 2β-hydroxylate C20-GAs (SoGA2ox3, AtGA2ox7, and AtGA2ox8). The two GA 2-oxidases of rice in class III, OsGA2ox5 and OsGA2ox6, have not been characterized, but their amino acid sequences predict that they are able to 2β-hydroxylate C20-GAs.
Heterologous Expression of SoGA2ox3 in Escherichia coli
To demonstrate that the SoGA2ox3 cDNA clone encodes a GA 2-oxidase, we heterologously expressed the coding region as a fusion protein of the glutathione S-transferase fusion vector in E. coli strain BL21pLysS. The size of the fusion protein is about 69 kD. Soluble protein extracts were used for assays of GA 2-oxidase activity with several radioactive GAs as substrates. The reaction products were separated by reverse-phase HPLC with an on-line radioactivity detector (Lee and Zeevaart, 2002). Retention times (Rts) of the products were compared with those of standard 14C-labeled GAs for tentative identification. The recombinant SoGA2ox3 protein converted the radioactive C20-GAs GA12 (Rt = 37.6 min) and GA53 (Rt = 31.8 min) to products with Rts of 28.8 and 14.9 min, corresponding to those of GA110 and GA97, respectively. In some experiments, in addition to GA97, a minor unidentified product of GA53 was found at Rt = 19.6 min. We used 17,17-[2H2]GAs as substrates to identify the products of GA12 and GA53 catabolism by gas chromatography-mass spectrometry (GC-MS; Table II). Recombinant SoGA2ox3 did not convert [14C]GA44 (closed lactone ring) and [14C]GA19 to 2β-hydroxy-[14C]GA44 (=GA98) and 2β-hydroxy-[14C]GA19 (=GA99), respectively. Also, SoGA2ox3 was not able to convert C19-GAs, such as GA20 and GA1. Thus, SoGA2ox3 belongs to the class of 2-oxidases that specifically deactivates the C20-GA precursors GA12 and GA53 (Schomburg et al., 2003), which are also the substrates for GA 20-oxidases.
Table II.
Identification of products formed after incubation of recombinant GA 2-oxidase (SoGA2ox3) from spinach with GA12 or GA53
| Substrate | Product | Mass Spectra of Productsa |
|---|---|---|
| m/z (% relative abundance) | ||
| 17,17-[2H2]GA12 | 17,17-[2H2]GA110 | M+ 450 (5), 435 (6), 418 (25), 390 (44), 375 (4), 360 (2), 328 (6), 318 (7), 300 (100), 285 (66), 274 (40), 260 (26), 259 (22), 241 (98), 225 (24), 201 (17), 197 (10), 145 (34) |
| 17,17-[2H2]GA53 | 17,17-[2H2]GA97 | M+ 538 (35), 523 (9), 506 (6), 479 (9), 448 (3), 389 (10), 373 (5), 329 (11), 299 (2), 239 (44), 210 (65), 209 (100), 195 (11), 179 (16), 147 (3), 119 (14) |
As the methyl ester trimethylsilyl ethers.
Effects of Daylength on Gibberellin Levels in Spinach
The GA contents of spinach plants growing in SD or LD were determined by GC-MS-selected ion monitoring (Table III). As reported previously (Wu et al., 1996), transfer of spinach from SD to LD caused an increase in all GAs of the early-13-hydroxylation pathway, except for GA53 in mature leaves and petioles. By contrast, the levels of the 2β-hydroxylated C20-GAs GA97 and GA110 showed a decrease after transfer from SD to LD, which corroborates the results obtained with feeding [14C]GA53 (Fig. 1B). GA97 is by far the most abundant GA in spinach, whereas GA110 is present in levels comparable to those of the other GAs. This striking difference in contents of GA97 and GA110 raises the question whether SoGA2ox3 has a higher affinity for GA53 than for GA12. To answer this question, we incubated mixtures of the two substrates at equimolar concentrations with recombinant SoGA2ox3 for 1 h. GA12 and GA53 were equally well catabolized over a range of substrates from 30 to 300 pmol (data not shown), indicating that the much higher content of GA97 than of GA110 is probably not due to different affinities of the enzyme for the two substrates.
Table III.
Comparison of GA levels in various organs of spinach in SD and after 8 LD
Spinach plants were grown in SD for 6 weeks, then transferred to LD for 8 d. GA content was determined by GC-MS-selected ion monitoring, using deuterated GAs as internal standards.
| Plant Part | GA53 | GA19 | GA20 | GA1 | GA29+81 | GA8 | GA110 | GA97 |
|---|---|---|---|---|---|---|---|---|
| Mature blades | ||||||||
| SD | 4.6a | 31.8 | 2.7 | 1.7 | 13.5 | 1.3 | 8.5 | 191.1 |
| 8 LD | 2.1 | 23.5 | 23.6 | 2.7 | 36.4 | 3.2 | 1.3 | 73.0 |
| Petioles | ||||||||
| SD | 24.6 | 51.3 | 1.7 | 1.6 | 2.7 | 2.9 | 18.9 | 477.5 |
| 8 LD | 21.7 | 145.3 | 36.7 | 4.1 | 42.3 | 32.3 | 4.7 | 446.0 |
| Young leaves | ||||||||
| SD | 7.2 | 54.4 | 3.0 | 4.5 | 10.1 | 1.3 | 16.2 | 286.3 |
| 8 LD | 31.1 | 138.4 | 43.4 | 19.4 | 84.7 | 9.9 | 2.4 | 178.7 |
| Shoot tips | ||||||||
| SD | 24.7 | 61.3 | 2.2 | 3.8 | 16.0 | 3.8 | 18.3 | 880.0 |
| 8 LD | 66.6 | 173.5 | 31.4 | 16.4 | 66.7 | 37.1 | 13.4 | 696.2 |
Values are in ng g−1 dry weight.
Expression of SoGA2ox3 in Spinach in SD and LD
Two sizes of transcripts of SoGA2ox3 were observed around 1.3 kb and 0.7 kb in northern-blot analysis (data not shown). The larger size was assumed to be the mature message because it is the same size as the full-length cDNA (see above).
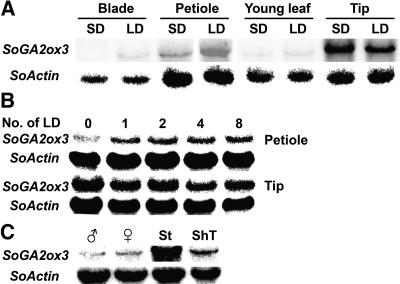
To investigate how expression of the SoGA2ox3 gene is regulated by the photoperiod, we isolated total RNA from spinach plants grown in SD and after 8 LD. Figure 3A shows that the levels of SoGA2ox3 transcripts were relatively high in petioles and shoot tips but low in blades and young leaves in both SD and LD. These expression patterns of SoGA2ox3 are correlated with the levels of GA97 in various organs of spinach (Fig. 3A; Table III).
Figure 3.
Northern analysis of SoGA2ox3 in spinach. A, Levels of SoGA2ox3 transcripts in spinach grown in SD and after 8 LD. B, Levels of SoGA2ox3 transcripts in petioles and tips with increasing numbers of LD. C, Levels of SoGA2ox3 transcripts in male (♂) and female (♀) flowers, stems (St), and shoot tips (ShT). Spinach plants were harvested 4 weeks after transfer to LD. The shoot tips included the upper 1 cm of shoots; stems were the next 1 cm of shoots. Total RNA (30 μg) was loaded for each sample. The blots were probed with SoGA2ox3 or SoActin.
To determine the time course of changes in SoGA2ox3 expression, spinach plants were harvested at different times after transfer from SD to LD. The levels of SoGA2ox3 transcripts were slightly increased in blades and young leaves (data not shown) and petioles in a time-dependent manner, but the levels of SoGA2ox3 transcripts decreased slightly in shoot tips with increasing duration of LD (Fig. 3B).
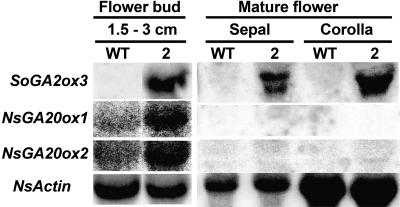
Expression patterns of SoGA2ox3 were also investigated in inflorescences. Male and female flowers, stems, and shoot tips were harvested from spinach plants that had been exposed to LD for 4 weeks (Fig. 3C). The SoGA2ox3 transcripts were more abundant in the upper 2 cm of stems than in shoot tips, and expression was relatively low in male and female flowers.
Ectopic Expression of the SoGA2ox3 Gene in N. sylvestris
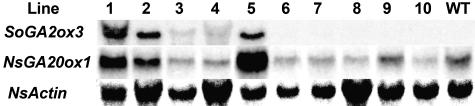
To determine whether ectopic expression of the SoGA2ox3 gene would produce GA97 and cause a GA-deficient phenotype in another species, we introduced the 35S∷SoGA2ox3 cDNA construct into N. sylvestris by Agrobacterium tumefaciens-mediated transformation. Ten homozygous lines expressing SoGA2ox3 were selected (Fig. 4), which exhibited a profound range of dwarfed phenotypes (data not shown). To ascertain the presence of the SoGA2ox3 gene in the transgenic plants, we performed genomic PCR amplification with a pair of primers, JZ651 and JZ656 (Table I). The PCR products (1.1 kb) were detected without any other PCR products in all transgenic lines (data not shown). Phenotypes of transgenic lines 6 to 10 were very mild, but transgenic lines 1 to 5 showed severe dwarfed phenotypes (data not shown). The severity of the phenotype was correlated with the accumulation of SoGA2ox3 transcripts (Fig. 4). The expression patterns of NsGA20ox1 of N. sylvestris were similar to those of SoGA2ox3 transcripts (Fig. 4), indicating that feedback regulation to maintain GA homeostasis operated in the transgenic plants.
Figure 4.
Northern analysis of SoGA2ox3 and NsGA20ox1 expression in transgenic N. sylvestris. Expression levels of the SoGA2ox3 transgene parallel those of the NsGA20ox1 gene. For each sample, 30 μg of total RNA was loaded. The blots were probed with SoGA2ox3, NsGA20ox1, or NsActin.
Transgenic line 2 was chosen for further characterization (Fig. 5). In SD, the leaves were slightly shorter and darker green than those of the wild type. The leaves of wild-type plants were smooth, whereas those of transgenic plants were somewhat wrinkled (Fig. 5A). The inset in Figure 5B shows that SoGA2ox3 was highly expressed in the transgenic plant. The time course of stem elongation (Fig. 5C) shows that the transgenic plants did not start to elongate until the wild-type plants had already reached their final height and were in full bloom. The final height of the transgenic plants was less than half of that of the wild-type plants, and the number of leaves was almost doubled (Table IV). Flower buds in the transgenic plants appeared 30 d later than those of the wild type (Fig. 5C; Table IV). However, the interval between appearance of flower buds and anthesis was the same in wild-type and transgenic plants, indicating that the rate of flower bud development was the same in both types of plants. Fruit and seed production were normal in the transgenic plants. This may indicate that these aspects of plant development require a lower GA1 level than stem growth. As in leaves (Fig. 4), SoGA2ox3 was highly expressed in flower buds and mature flowers (Fig. 6). Expression of native NsGA20ox1 and NsGA20ox2 in young flower buds was up-regulated as compared to their expressions in the wild type. However, expression of native 20-oxidases in mature flowers was below the limit of detection in northern blots (Fig. 6). Thus, at an early stage, GA 20-oxidase could channel sufficient GA53 through the early-13-hydroxylation pathway for normal flower development. It should be kept in mind, however, that flowers are heterotrophic, so that GAs, instead of being synthesized in situ, may be supplied by green tissue.
Figure 5.
Ectopic expression of SoGA2ox3 suppresses stem growth in N. sylvestris. A, Transgenic plant and wild-type plant (WT) show a similar phenotype in SD. B, WT and transgenic plants grown in LD following germination. Inset in B shows expression of SoGA2ox3 in the transgenic plant. C, Stem growth of WT and transgenic plants grown in LD following germination. Black arrows indicate the average number of days until appearance of flower buds. White arrows indicate the average number of days until anthesis. n = 12.
Table IV.
Characterization of transgenic plants overexpressing SoGA2ox3 in N. sylvestris (Ns)
| Observed Trait | Wild-Type Ns | 35S∷SoGA2ox3 |
|---|---|---|
| Days to flower buds | 67.3 ± 0.92a | 97.8 ± 0.86 |
| Days to anthesis | 83.5 ± 0.97 | 114.5 ± 0.96 |
| Number of leaves to inflorescence | 19.0 ± 0.35 | 35.8 ± 0.35 |
| Final plant height | 122.6 ± 2.8 | 56.1 ± 1.01 |
se; n = 12.
Figure 6.
Northern analysis of SoGA2ox3, NsGA20ox1 (GenBank accession no. AF494087), and NsGA20ox2 (accession no. AF494088) in young flower buds and mature flowers of transgenic N. sylvestris. For each sample, 30 μg of total RNA was loaded. The blots were probed with SoGA2ox3, NsGA20ox1, NsGA20ox2, or NsActin.
Effect of Ectopic Expression of SoGA2ox3 on Gibberellin Content
As expected, GA levels were affected by ectopic expression of SoGA2ox3 in transgenic plants (Table V). The level of GA53, which is the substrate for both GA 20-oxidase and GA 2-oxidase3, showed a marked decrease in transgenic plants in both SD and LD. The levels of GA19 and GA20, the products of GA 20-oxidase, also decreased in transgenic plants in LD with a concomitant 5-fold decrease in bioactive GA1. Overexpression of SoGA2ox3 did not affect the GA97 content in SD, but it caused a 3- to 6-fold increase in transgenic plants in LD. As in spinach, GA110 was present in much lower amounts than GA97; its levels also increased in LD. These data indicate that ectopic expression of SoGA2ox3 increased the levels of both GA97 and GA110 in transgenic plants and kept the GAs of the early-13-hydroxylation pathway at a low level. Thus, GA 2-oxidase3 in transgenic plants competes effectively with GA 20-oxidase for the common substrates GA12 and GA53, resulting in a reduced GA1 content and a dwarfed phenotype.
Table V.
GA content of N. sylvestris (Ns) and transgenic plants overexpressing SoGA2ox3 grown under various photoperiodic conditions
Plants were grown in SD for 80 d and then transferred to LD for the number of days indicated prior to harvest. GA content was determined by GC-MS-selected ion monitoring, using deuterated GAs as internal standards. nd, Not detectable.
| Sample | Photoperiodic Treatment | GA53 | GA19 | GA20 | GA1 | GA97 | GA110 |
|---|---|---|---|---|---|---|---|
| Ns | SD | 0.7a | 3.8 | 1.2 | 0.9 | 11.7 | nd |
| Ns/35S∷SoGA2ox3 | SD | 0.1 | 2.1 | 0.3 | 0.8 | 11.6 | 0.5 |
| Ns | 14 LD | 13.2 | 32.4 | 16.7 | 4.9 | 16.9 | nd |
| Ns/35S∷SoGA2ox3 | 14 LD | 4.4 | 4.7 | 0.8 | 0.9 | 40.3 | 7.2 |
| Ns | 20 LD | 32.3 | 54.3 | 27.0 | 5.2 | 14.1 | 1.5 |
| Ns/35S∷SoGA2ox3 | 20 LD | 6.3 | 7.5 | 1.8 | 1.0 | 85.3 | 9.1 |
| Ns/35S∷SoGA2ox3 | 30 LD | 9.7 | 9.4 | 2.3 | 1.1 | 94.9 | 10.2 |
Values are in ng g−1 dry weight.
Applied Gibberellin Restores a Normal Phenotype in 35S∷SoGA2ox3 Plants
Treatment with GA1 reversed the phenotype of dwarf transgenic plants to that of wild type. In SD, leaves were elongated and the plants had started to bolt after 25 d. Although grown in SD, the GA-treated plants had the appearance of wild-type plants exposed to LD (Fig. 7A). In LD, the transgenic plants treated with GA1 showed essentially the same phenotype as wild-type plants (Fig. 7B). These results show that GA treatment effectively increased leaf and stem growth of transgenic plants both in SD and LD, indicating that application of GA1 could fully compensate for the decreased endogenous GA1 content of transgenic plants.
Figure 7.
Reversal of dwarf phenotype of transgenic plants by GA treatment. Plants were sprayed twice weekly with a solution of 10 mg L−1 GA1. GA treatment of transgenic plants in SD (A) and in LD (B). Photographs were taken 25 d after first GA application.
Effect of Ectopic Expression of SoGA2ox3 on Germination
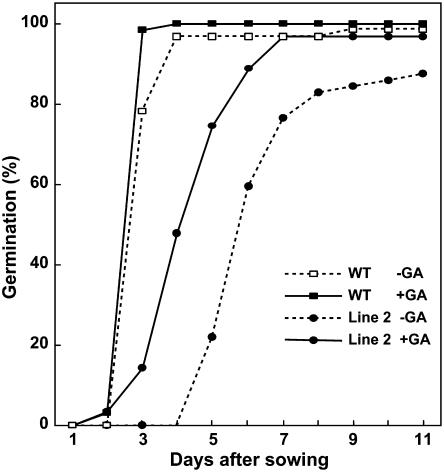
Because severely GA-deficient mutants do not germinate in the absence of exogenous GAs (Koornneef et al., 1983), we tested whether overexpression of SoGA2ox3 affects germination. As shown in Figure 8, a high percentage of wild-type seeds had germinated after 4 d, whereas germination of transgenic seeds did not begin until day 5. Treatment with GA3 accelerated germination and also caused a higher percentage of seeds to germinate than was observed for seeds without GA3 treatment (Fig. 8). Thus, although overexpression of SoGA2ox3 decreases the content of bioactive GA, transgenic seeds are still able to germinate, albeit with some delay.
Figure 8.
Effect of overexpression of SoGA2ox3 on germination in N. sylvestris. Seeds of wild-type (WT) or transgenic plants were spread on agar plates containing half-strength Hoagland solution. Treatment with GA partially restored germination of transgenic seeds. n = 60.
Effect of Ectopic Expression of SoGA2ox3 on Hypocotyl and Root Growth
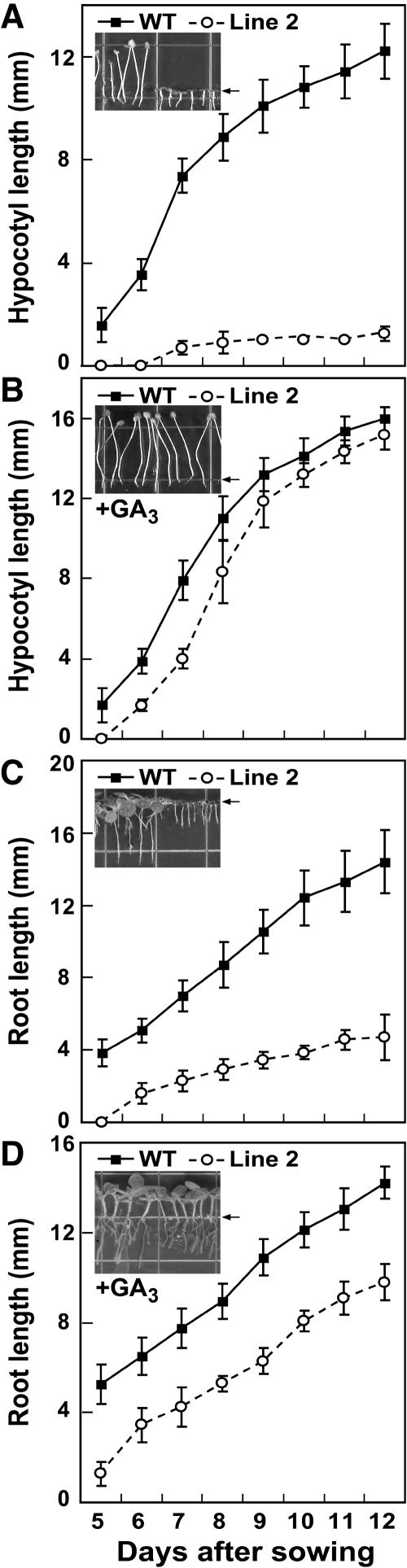
A role of GA in the regulation of root growth has been established by the use of GA-deficient mutants (Yaxley et al., 2001; Fu and Harberd, 2003). It was of interest, therefore, to investigate whether root growth is affected in seedlings with a low GA1 content due to overexpression of SoGA2ox3. As shown in Figure 9, ectopic expression of the SoGA2ox3 gene markedly affected both hypocotyl and root growth. The hypocotyls of wild-type seedlings were elongated in darkness, but those of transgenic seedlings remained very short (Fig. 9A). Treatment with 1 mg L−1 GA3 completely restored hypocotyl length of transgenic seedlings to that of wild type (Fig. 9B). Differences in root growth between wild-type and transgenic seedlings were most pronounced under continual light (Fig. 9C). Treatment with GA3 could only partially restore root growth in transgenic seedlings, but hypocotyl growth was promoted to the same extent in both types of seedlings (Fig. 9D).
Figure 9.
Effect of overexpression of SoGA2ox3 on hypocotyl and root growth in N. sylvestris seedlings. A, Hypocotyl length of seedlings grown in darkness. B, Treatment with GA restored to normal the hypocotyl growth of transgenic seedlings in darkness. C, Root growth of transgenic seedlings grown under continuous light. D, Treatment with GA partially restored the root growth of transgenic seedlings under continuous light. Arrows indicate transition from root to hypocotyl. Photographs taken 15 d after sowing. n = 15.
DISCUSSION
In this study, we isolated a full-length cDNA clone encoding a GA 2-oxidase from spinach. Heterologous expression in E. coli showed that the product of SoGA2ox3 catalyzes 2β-hydroxylation of the C20-GAs GA12 and GA53 to GA110 and GA97, respectively. The same activities have previously been found for AtGA2ox7 and AtGA2ox8 (Schomburg et al., 2003). The recombinant proteins from other GA 2-oxidases characterized so far 2β-hydroxylate C19-GAs. The only exceptions reported are for PcGA2ox1 and AtGA2ox2, which 2β-hydroxylated GA15, a C20-GA (Thomas et al., 1999); and OsGA2ox1, which converted GA44 to GA98 (Sakamoto et al., 2001). Spinach produces GA98 and GA99, but recombinant SoGA2ox3 did not metabolize their possible precursors, GA44 and GA19. In transgenic N. sylvestris overexpressing SoGA2ox3, there was an increase in GA97 (Table V), but no GA98 and GA99 could be detected in these plants (J.A.D. Zeevaart, unpublished data). Considering these combined results, it is unlikely that SoGA2ox3 is involved in the synthesis of GA98 and GA99. Another possibility is that GA 20-oxidase converts GA97 to GA98 and GA99, as indicated in the hypothetical pathway in Figure 1A. But GA97 was not converted by recombinant SoGA20ox (J.A.D. Zeevaart, unpublished data). Perhaps recombinant SoGA2ox1 can 2β-hydroxylate GA44 and GA19, as it was able to convert GA53 to GA97 (Lee and Zeevaart, 2002). Finally, the possibility remains that there are additional GA 2-oxidases that catalyze the formation of GA98 and GA99.
GA97 is much more abundant than GA110 in spinach (Table III), and the same is true for N. sylvestris in both wild-type and transgenic plants (Table V). Given the results of the experiment in which the two precursors GA12 and GA53 were mixed, it is unlikely that GA 2-oxidase3 has a higher affinity for GA53 than for GA12. The cellular location of the GA 13-oxidase that converts GA12 to GA53 is not known, but there may be compartmentation of the substrates in such a manner that GA53 is more readily available to GA 2-oxidase3 than is GA12. Further metabolism of GA97 is not known, so that its very high level may not reflect a high rate of synthesis but rather accumulation of a stable inactive end product. A relatively low rate of synthesis of GA97 is also indicated by the low levels of SoGA2ox3 transcripts in spinach (Fig. 3). In addition, antiserum raised against recombinant SoGA2ox3 protein did not detect any SoGA2ox3 protein from spinach or transgenic N. sylvestris in western blots, indicating that the level of SoGA2ox3 protein in vivo is very low (D.J. Lee, unpublished data).
GA53 is at a branch point in GA metabolism in spinach (Fig. 1A). It can be converted to bioactive GA1 via the early-13-hydroxylation pathway, or it can be deactivated by 2β-hydroxylation to GA97. The latter step appears to prevail in SD, whereas in LD most GA53 will be channeled through the early-13-hydroxylation pathway. As shown in Figure 3, the levels of SoGA2ox3 transcripts undergo little change when spinach plants are shifted from SD to LD. Nevertheless, the levels of GA97 decreased, presumably because expression of GA 20-oxidase was strongly up-regulated in LD (Lee and Zeevaart, 2002). Thus, the fate of GA53 will be determined by the relative activities of the two enzymes GA 2-oxidase3 and GA 20-oxidase. In LD, 20-oxidase activity is high, so that GA53 is preferentially converted to GA20. By contrast, in SD the activity of 20-oxidase is low, and more GA53 will be deactivated via 2β-hydroxylation to GA97. Whereas SoGA2ox1 and SoGA2ox2 can directly regulate the level of active GA1, SoGA2ox3 can only indirectly regulate the level of active GA1 by reducing the amount of precursor that is channeled through the early-13-hydroxylation pathway. Thus, 2β-hydroxylation of GA precursors of bioactive GA1 adds an additional step at which the levels of active GAs can be regulated.
Overexpression of SoGA2ox3 in N. sylvestris had a profound effect on growth and development from germination to flowering (Figs. 5, 7, 8, and 9). Analysis of the endogenous GAs showed a reduced GA1 level in transgenic plants and an increase in GA97 and GA110. Wild-type N. sylvestris also produced GA97, although not at the high levels found in spinach. The reduction in active GA1 resulted in reduced germination, shorter radicles and hypocotyls of seedlings, reduced stature, and later flowering. The GA-deficient phenotype of older plants could be readily overcome by applied GA1 (Fig. 7).
Application of GA to the LD rosette plant N. sylvestris in SD induces stem elongation and, ultimately, flower formation (Lang, 1989). As shown in Table V, the level of GA1 increased 5- to 6-fold when plants were transferred from SD to LD. This increase indicates that a high level of bioactive GA is a prerequisite for stem elongation and subsequent flowering. Conversely, a reduced level of GA1 in transgenic plants delayed stem elongation and flower formation (Fig. 5; Table IV). This retardation in flowering is most vividly expressed by the number of leaves formed prior to the inflorescence in transgenic plants compared to wild-type plants (Table IV).
Semidwarfism is a desirable trait in agriculture (Busov et al., 2003; Hedden, 2003). Overexpression of GA 2-oxidase is an easy way to reduce GA levels in transgenic plants, but ectopic expression of SoGA2ox1 and SoGA2ox2 in N. sylvestris caused severely dwarfed plants that produced few seeds (D.J. Lee, unpublished results) because active GA was probably deactivated as soon as it was produced. Singh et al. (2002) also reported that ectopic expression of class II GA 2-oxidases (PsGA2ox2) caused defective pollen tube growth and seed abortion. Overexpression of AtGA2ox1 in tobacco reduced the number of capsules 40% to 70% (Biemelt et al., 2004). In rice, ectopic expression of OsGA2ox1 inhibited not only stem elongation but also development of reproductive organs. In a more direct approach, OsGA2ox1 was expressed under the control of the promoter of OsGA3ox2, which is specifically expressed in the shoot apex. This caused a semidwarf phenotype with normal flowering and grain development (Sakamoto et al., 2003). When SoGA2ox3 was overexpressed, GA precursors were deactivated, but GA 20-oxidase could still convert a small amount of the precursor to GA1 (Table V), which could act without being quickly deactivated. This finding demonstrates that we can manipulate plant stature by overexpressing a gene that deactivates precursors of GA1 rather than active GA1 itself. The result is plants with reduced GA1 levels, short stature, and delayed flowering but that otherwise retain desired traits, such as normal flowering and seed production.
MATERIALS AND METHODS
Plant Material and Growing Conditions
Spinach (Spinacia oleracea, Savoy Hybrid 612) plants were grown in a SD growth chamber for approximately 6 or 7 weeks as described earlier and then transferred to a LD growth chamber for experimental use (Lee and Zeevaart, 2002). Nicotiana sylvestris Spegaz. et Comes was grown under the same conditions. Plants used for GA extraction were grown in SD for approximately 3 months before being exposed to LD. All plants were watered with half-strength Hoagland solution daily. SD consisted of 8 h of light from fluorescent tubes and incandescent bulbs of approximately 300 μmol m−2 s−1 at 23°C, followed by 16 h darkness at 20°C. In the case of LD, the 8-h main light period was followed by 16 h of weak light from incandescent bulbs of 10 μmol m−2 s−1.
Feeding of [14C]GA53 to Spinach in SD and LD
[14C]GA53 (specific activity 127.5 mCi/mmol) was dissolved in a small amount of acetone, to which water containing 0.05% (v/v) Tween 20 was added to give a 75% (v/v) aqueous solution. This solution was injected into petioles and midribs of spinach leaves by means of a 100-μL Hamilton syringe. Each plant received 0.3 × 106 dpm of [14C]GA53 (=0.36 μg GA53). Spinach plants were either kept in SD or had been exposed to 8 LD. The plants, 4 per treatment, were harvested after 2 d, frozen in liquid N2, and lyophilized. The dried material was extracted with methanol, and an acidic fraction was prepared from each sample (Zeevaart et al., 1993; Schomburg et al., 2003). The acidic fractions were analyzed by reverse-phase HPLC with on-line radioactivity detection. Radioactive fractions were collected, the derivatized materials were identified, and their specific activities determined by GC-MS (Zeevaart et al., 1993).
RT-PCR
RT-PCR was used for the cloning of GA 2-oxidase. First-strand cDNA from 1 μg poly(A+) RNA was synthesized by SuperScript II RNase H− reverse transcriptase (Invitrogen, Carlsbad, CA), using oligo(dT) primers at 42°C for 1 h. The cDNA product was then used in PCR reactions containing Taq polymerase and several pairs of degenerate primers (Table I). For GA 2-oxidase, the degenerate primers were synthesized based on conserved regions of AtGA2ox7 and AtGA2ox8 (Schomburg et al., 2003). These primers were used in all combinations in PCR reactions with first-strand cDNA as a template. The PCR amplifications were performed with a DNA thermal cycler (RTC-200; MJ Research, Waltham, MA) in a 50-μL reaction mixture containing 1× PCR buffer, 1.5 mm MgCl2, 200 μm dNTP, 2.5 pmol of each primer, and 1 unit of Taq DNA polymerase (Invitrogen). The reaction mixtures were heated to 94°C for 2 min and then subjected to 30 cycles of 94°C for 1 min, 48°C for 1 min, and 72°C for 45 s. A final extension was performed at 72°C for 10 min. A 5-μL volume of the PCR reaction products was analyzed by 1.2% agarose gel electrophoresis, purified with a Wizard PCR purification system (Promega, Madison, WI), and then cloned into the pGEM-T Easy vector (Promega).
RACE and Cloning
5′- and 3′-RACE of spinach GA 2-oxidase3 cDNA were performed using the SMART RACE cDNA amplification kit from CLONTECH (Palo Alto, CA). Poly(A+) RNA was isolated from spinach using the PolyATtract mRNA Isolation System IV (Promega) according to the manufacturer's instructions. Following RT with primers supplied by CLONTECH, the first-strand cDNA was used directly in 5′- and 3′-RACE PCR reactions. Primary PCR amplification reactions were achieved using a high-fidelity enzyme (Pfu Turbo polymerase; Stratagene, La Jolla, CA) and gene-specific primers JZ616 and JZ627 or JZ617 and JZ618 for GA2ox3 to generate the 5′- or 3′-cDNA RACE fragments, respectively. The PCR reaction consisted of the first denaturation for 3 min at 94°C, a series of 30 cycles (1 min at 94°C, 1 min at 54°C or 55°C, 1 min at 72°C), and a final extension for 5 min at 72°C using a thermal cycler (MJ Research). A 5-μL aliquot of the RT-PCR and RACE reaction solution was analyzed by 1.2% agarose gel electrophoresis. PCR products were purified and cloned into the pCR-Script Cam SK(+) cloning vector from Stratagene. These constructs were sequenced.
The National Center for Biotechnology Information Blast program was used to search genes homologous with the SoGA2ox3 gene. Analysis of the DNA sequences was carried out using the DNASTAR program (DNASTAR, Madison, WI). Multiple sequence alignment of the amino acid sequences was performed using BCM Search Launcher (http://searchlauncher.bcm.tmc.edu/multi-align/multi-align.html) and printed with BOXSHADE 3.21 (http://www.ch.embnet.org/software/BOX_form.html). The phylogenetic tree was created by means of the ClustalW program (http://www.ebi.ac.uk/clustalw/).
Northern-Blot Analysis
Leaf blades, petioles, young leaves, shoot tips, and other organs were harvested, frozen immediately in liquid N2, and stored at −80°C (Lee and Zeevaart, 2002). Northern blots were prepared by electrophoresis of 30 μg of total RNA in the presence of formaldehyde (Sambrook et al., 1989), and RNA was transferred to nitrocellulose. Full-length cDNAs were labeled with [32P]dCTP by the Random Primers DNA labeling system (Invitrogen) and used to probe northern and Southern blots. Hybridization was carried out at 42°C using a 50% formamide system (Sambrook et al., 1989). Membranes were washed twice in 2× SSC at room temperature for 10 min and then twice in 0.2× SSC with 0.1% SDS at 55°C for 10 min. At low stringency, membranes were washed once in 2× SSC at room temperature for 10 min and then in 2× SSC at 55°C for 10 min. Each blot was first probed with SoGA2ox3 cDNA, stripped, and reprobed with SoActin. Each experiment was repeated at least three times with similar results.
Expression of Recombinant GA 2-Oxidase3 Protein
The coding region of GA 2-oxidase3 was produced by PCR with the following primer set designed from the RACE product: JZ651 and JZ656 for the mature GA2ox3 cDNA (BamHI and EcoRI sites inserted to facilitate cloning). The resulting PCR fragments were cloned into the pGEM-T Easy cloning vector, digested with BamHI/EcoRI, and subcloned into the corresponding restriction sites of the pGEX-5X-2 vector (Amersham Biosciences, Piscataway, NJ). This full-length cDNA clone (pGEXGA2ox3) was transformed into Escherichia coli strain BL21pLysS. Fifty mL of freshly cultured cells were added to 1 L of Luria-Bertani medium with 100 mg L−1 ampicillin and incubated at 37°C with vigorous shaking. When the optical density at 600 nm reached 0.6, isopropyl-β-d-thiogalactopyranoside was added to give a final concentration of 3 mm, and the culture was incubated for another 2 h. The cells were harvested, suspended, and incubated in lysis buffer (100 mm Tris-HCl, pH 7.5, and 10 mg L−1 lysozyme) at room temperature for 10 min. Crude extracts were briefly sonicated by using a Sonifier Cell Disruptor 200 (Branson Ultrasonics, Danbury, CT), carried out for 10 cycles (10 × 10 s) on ice. The lysates were submerged in liquid N2 for 2 min and then thawed in an ice bath for 15 min (Johnson and Hecht, 1994). The lysates were centrifuged at 13,000 rpm for 30 min, and the supernatant was stored at −80°C until needed for enzyme assays.
Enzyme Assays and Product Identification
Enzyme assays with recombinant GA 2-oxidase3 were performed with approximately 30,000 dpm of [14C]-labeled GAs. The assays and methods for product identification have been described (Lee and Zeevaart, 2002; Schomburg et al., 2003).
Gibberellin Extraction and Quantification
The procedures for extraction, purification, and quantification of endogenous GAs with deuterated GAs as internal standards were the same as described (Talon and Zeevaart, 1990; Zeevaart et al., 1993). All GA measurements were repeated at least twice with similar results. The four prominent ions monitored for GA97 were mass-to-charge ratio (m/z) 477/479 and 536/538, and for GA110 m/z 298/300 and 388/390. Deuterated GA97 and GA110 were prepared by means of incubating 50 μg of 17,17-[2H2]GA53 and 17,17-[2H2]GA12, respectively, with 2.5 mL of recombinant SoGA2ox3 protein. The GC-MS conditions were as described by Schomburg et al. (2003).
Construction of 35S∷SoGA2ox3 Plasmid and Transformation
The full-length SoGA2ox3 cDNA was introduced into the pGEM-T Easy vector, and then reintroduced into the BamHI and SacI sites of the pBI121 vector after removing the GUS gene. This vector, pBISoGA2ox3, was introduced into Agrobacterium tumefaciens strain LBA4404, for transformation of N. sylvestris according to Horsch et al. (1988). Leaf discs of N. sylvestris were incubated with A. tumefaciens for 10 min and then transferred to Murashige and Skoog medium containing 1 mg L−1 6-benzylaminopurine and 0.1 mg L−1 1-naphthaleneacetic acid (NAA) in darkness. After coculture for 3 d, leaf discs were transferred to selection medium (Murashige and Skoog, 1 mg L−1 6-benzylaminopurine, 0.1 mg L−1 NAA, 100 mg L−1 kanamycin, and 500 mg L−1 carbenicillin). When shoots appeared, individual shoots were cut and transferred to root inducing medium (Murashige and Skoog, 0.1 mg L−1 NAA, 100 mg L−1 kanamycin, and 500 mg L−1 carbenicillin). After rooting, plantlets (T0) were planted in soil and grown in a greenhouse. Independent transgenic lines were selected on half-strength Hoagland medium containing 50 mg L−1 kanamycin. The T1 generations were self-pollinated to get T2 generations. Each line showing a 3:1 segregation (resistant:sensitive) was selected to obtain homozygous T3 plants; these homozygous lines were used in further experiments.
Analysis of Transgenic Plants by PCR
Isolation of genomic DNA from transgenic plants was performed as described (Lee and Kim, 1998). The PCR amplification reaction was for 25 cycles of 30 s at 94°C, 60 s at 50°C, and 60 s at 72°C with two primers (JZ651 and JZ656; Table I) after an initial denaturation step for 2 min at 94°C. The 5 μL of reaction products was analyzed by 1.2% agarose gel electrophoresis.
Germination Assays
Sixty seeds were sown in petri dishes containing half-strength Hoagland medium in the presence or absence of 1 mg L−1 GA3 and incubated in a growth room at 23°C under continual light from fluorescent lamps (50–80 μmol m−2 s−1). Germination was defined as radicle emergence and was scored daily.
Hypocotyl and Root Growth
Growth of hypocotyls and roots was determined by sowing seeds on half-strength Hoagland medium containing 0.8% phytoagar with or without 1 mg L−1 GA3. The petri dishes were vertically oriented so that the seedlings could grow vertically along the agar surface. Wild-type and transgenic lines were sown in the same petri dish at 23°C. Seedlings were kept in darkness to determine hypocotyl length, or kept under continuous light (same source as used for germination assays) to determine root growth. Both hypocotyl length and root length were measured until 12 d after sowing.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY935713 (SoGA2ox3, cDNA) and AY935714 (intron of SoGA2ox3).
Acknowledgments
We thank Dr. Lew Mander of the Australian National University for providing [2H2]GAs, and Bev Chamberlin (Michigan State University Mass Spectrometry Facility) for assistance with GC-MS.
This work was supported by the U.S. Department of Agriculture (grant no. 2002–35304–1269) and by the U.S. Department of Energy (grant no. DE–FG02–91ER20021).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.056499.
References
- Biemelt S, Tschiersch H, Sonnewald U (2004) Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants. Plant Physiol 135: 254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busov VB, Meilan R, Pearce DW, Ma C, Rood SB, Strauss SH (2003) Activation tagging of a dominant gibberellin catabolism gene (GA 2-oxidase) from poplar regulates tree stature. Plant Physiol 132: 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421: 740–743 [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Zeevaart JAD, Schwenen L, Graebe JE (1986) Gibberellin metabolism in cell-free extracts from spinach leaves in relation to photoperiod. Plant Physiol 82: 190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P (2003) The genes of the green revolution. Trends Genet 19: 5–9 [DOI] [PubMed] [Google Scholar]
- Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5: 523–530 [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry J, Hoffmann N, Neidermeyer J, Rogers SG, Fraley RT (1988) Leaf disc transformation. In SB Gelvin, RA Schilperoort, eds, Plant Molecular Biology Manual. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp A5: 1–9
- Johnson BH, Hecht MH (1994) Recombinant proteins can be isolated from Escherichia coli cells by repeated cycles of freezing and thawing. Biotechnology (N Y) 12: 1357–1360 [DOI] [PubMed] [Google Scholar]
- Kang HG, Jun SH, Kim J, Kawaide H, Kamiya Y, An G (1999) Cloning and molecular analyses of a gibberellin 20-oxidase gene expressed specifically in developing seeds of watermelon. Plant Physiol 121: 373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, van Eden J, Hanhart CJ, de Jongh AMM (1983) Genetic fine-structure of the GA-1 locus in the higher plant Arabidopsis thaliana (L.) Heynh. Genet Res Cambridge 41: 57–68 [Google Scholar]
- Lang A (1989) Nicotiana. In AH Halevy, ed, Handbook of Flowering, Vol VI. CRC Press, Boca Raton, FL, pp 427–483
- Lee DJ, Kim SS (1998) The regulation of 5′ upstream regions of a Korean radish cationic peroxidase gene by gibberellic acid and abscisic acid. Plant Sci 139: 105–115 [Google Scholar]
- Lee DJ, Zeevaart JAD (2002) Differential regulation of RNA levels of gibberellin dioxygenases by photoperiod in spinach. Plant Physiol 130: 2085–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester DR, Ross JJ, Smith JJ, Elliott RC, Reid JB (1999) Gibberellin 2-oxidation and the SLN gene of Pisum sativum. Plant J 19: 65–73 [DOI] [PubMed] [Google Scholar]
- Mander LN, Owen DJ, Croker SJ, Gaskin P, Hedden P, Lewis MJ, Talon M, Gage DA, Zeevaart JAD, Brenner ML, et al (1996) Identification of three C20-gibberellins: GA97 (2β-hydroxy-GA53), GA98 (2β-hydroxy-GA44) and GA99 (2β-hydroxy-GA19). Phytochemistry 43: 23–28 [DOI] [PubMed] [Google Scholar]
- Martin DN, Proebsting WM, Hedden P (1999) The SLENDER gene of pea encodes a gibberellin 20-oxidase. Plant Physiol 121: 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N, Sun T-p, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell (Suppl) 14: S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Mander LN, Storey JMD, Huntley RP, Gaskin P, Lenton JR, Gage DA, Zeevaart JAD (1998) Synthesis and confirmation of structure for a new gibberellin, 2β-hydroxy-GA12 (GA110), from spinach and oil palm. Phytochemistry 47: 331–337 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kobayashi M, Itoh H, Tagiri A, Kayano T, Tanaka H, Iwahori S, Matsuoka M (2001) Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol 125: 1508–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Morinaka Y, Ishiyama K, Kobayashi M, Itoh H, Kayano T, Iwahori S, Matsuoka M, Tanaka H (2003) Genetic manipulation of gibberellin metabolism in transgenic rice. Nat Biotechnol 21: 909–913 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, et al (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134: 1642–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Plainview, NY
- Schomburg FM, Bizzell CM, Lee DJ, Zeevaart JAD, Amasino RM (2003) Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 15: 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DP, Jermakow AM, Swain SM (2002) Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 14: 3133–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M, Zeevaart JAD (1990) Gibberellins and stem growth as related to photoperiod in Silene armeria L. Plant Physiol 92: 1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M, Zeevaart JAD, Gage DA (1991) Identification of gibberellins in spinach and effects of light and darkness on their levels. Plant Physiol 97: 1521–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SG, Philips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA 96: 4698–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Li L, Gage DA, Zeevaart JAD (1996) Molecular cloning and photoperiod-regulated expression of gibberellin 20-oxidase from the long-day plant spinach. Plant Physiol 110: 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kamiya Y (2000) Gibberellin biosynthesis: its regulation by endogenous and environmental signals. Plant Cell Physiol 41: 251–257 [DOI] [PubMed] [Google Scholar]
- Yaxley JR, Ross JJ, Sherriff LJ, Reid JB (2001) Gibberellin biosynthesis mutations and root development in pea. Plant Physiol 125: 627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Gage DA, Talon M (1993) Gibberellin A1 is required for stem elongation in spinach. Proc Natl Acad Sci USA 90: 7401–7405 [DOI] [PMC free article] [PubMed] [Google Scholar]