Abstract
In many flowering plants, such as petunia (Petunia × hybrida), ethylene produced in floral organs after pollination elicits a series of physiological and biochemical events, ultimately leading to senescence of petals and successful fertilization. Here, we demonstrate, using transgenic ethylene insensitive (44568) and Mitchell Diploid petunias, that multiple components of emission of volatile organic compounds (VOCs) are regulated by ethylene. Expression of benzoic acid/salicylic acid carboxyl methyltransferase (PhBSMT1 and 2) mRNA is temporally and spatially down-regulated in floral organs in a manner consistent with current models for postpollination ethylene synthesis in petunia corollas. Emission of methylbenzoate and other VOCs after pollination and exogenous ethylene treatment parallels a reduction in PhBSMT1 and 2 mRNA levels. Under cyclic light conditions (day/night), PhBSMT mRNA levels are rhythmic and precede emission of methylbenzoate by approximately 6 h. When shifted into constant dark or light conditions, PhBSMT mRNA levels and subsequent methylbenzoate emission correspondingly decrease or increase to minimum or maximum levels observed during normal conditions, thus suggesting that light may be a more critical influence on cyclic emission of methylbenzoate than a circadian clock. Transgenic PhBSMT RNAi flowers with reduced PhBSMT mRNA levels show a 75% to 99% decrease in methylbenzoate emission, with minimal changes in other petunia VOCs. These results implicate PhBSMT1 and 2 as genes responsible for synthesis of methylbenzoate in petunia.
Many flowers exhibit a colorful display of petals and emit a complex mixture of floral volatile organic compounds (VOCs) that are together attractive to both pollinators and humans. Regulation of floral volatiles corresponds to pollinator activity times and receptivity of the flower to a pollination event (Dudareva et al., 2000; Schiestl and Ayasse, 2001). In many flowers, physiological changes take place following pollination and fertilization including petal wilting and abscission, color changes, flower closure, fruit development, and seed development (for review, see O'Neill, 1997). The plant hormone ethylene has been shown to coordinate several of these postpollination processes in many different plant species (van Doorn, 1997).
In many plants, ethylene is synthesized and perceived in a localized, specific, and reproducible manner after pollination, underscoring the importance of understanding the progression of events and role of ethylene during pollination and fertilization. Petunia (Petunia × hybrida) is an excellent model system for studying postpollination responses because ethylene synthesis has been well characterized (Hoekstra and Weges, 1986; Tang and Woodson, 1996; Jones et al., 2003) and components of the ethylene-signaling pathway have been investigated (Wilkinson et al., 1997; Shibuya et al., 2004). In petunia, ethylene synchronizes pollen tube growth (Holden et al., 2003) and petal wilting (Hoekstra and Weges, 1986; Gubrium et al., 2000). An initial burst of ethylene is produced from the stigma/style within 2 to 4 h after pollination (Hoekstra and Weges, 1986), when pollen tubes have just started to germinate and grow into the stigma (Tang and Woodson, 1996). This is followed by sustained, autocatalytic ethylene production from the stigma/style and ovary, beginning approximately 12 h after pollination and peaking at 24 h after pollination. Ethylene production from the corolla is induced during this second phase between 24 and 36 h after pollination (Jones et al., 2003). The second phase of ethylene synthesis, which corresponds with the timing of fertilization, is responsible for corolla senescence (Hoekstra and Weges, 1986).
Floral fragrance is composed of low Mr VOCs that, together with other floral cues, are thought to stimulate pollinator activity. Floral VOCs are derived from multiple biosynthetic pathways in plant cells and include benzenoids, fatty acid derivatives, isoprenoids, and others (Knudsen et al., 1993). In flowers of some plant species, the availability of pollinator rewards and quality and intensity of the fragrance has been reported to reflect the pollination status (Burquez and Corbet, 1991; Schiestl and Ayasse, 2001). Postpollination changes in fragrance have been described for orchid (Platanthera bifolia: Tollsten, 1993; Ophyrys sphegodes: Schiestl and Ayasse, 2001) and recently for petunia and snapdragon (Antirrhinum majus) flowers (Negre et al., 2003). The changes in VOC emission presumably direct pollinators to unpollinated flowers. In petunia, the floral VOC methylbenzoate (MeBA) decreases after pollination (Negre et al., 2003). Genes characterized in vitro to catalyze MeBA synthesis, S-adenosyl-l-methionine:benzoic acid/salicylic acid carboxyl methyltransferases (PhBSMTs), also show decreased expression after pollination and exposure to ethylene in whole flowers, implicating a role for ethylene in regulating this process.
In this study, we investigated the physiological importance of ethylene in the regulation of floral volatile synthesis after pollination in petunia cv Mitchell Diploid (MD) and ethylene-insensitive CaMV35S∷etr1-1 (44568). PhBSMT1 and PhBSMT2 expression were examined in detail in individual floral organs after ethylene treatments and pollination. Our results indicated not only that PhBSMT expression and MeBA emission were ethylene regulated in a temporal and spatial manner, but also that there was additional rhythmic regulation that appeared to be controlled by both light and circadian factors. Changes in floral VOC emission after pollination provided strong evidence for ethylene regulation and rhythmic emission of overall floral VOC synthesis. The biochemical function of PhBSMT1 and 2 in vivo was determined using an RNAi approach to complement in vitro enzyme assays (Negre et al., 2003) and to support expression studies shown here. The physiological implications of this complex floral volatile regulation are discussed.
RESULTS
Floral VOC Emission Is Spatially Regulated
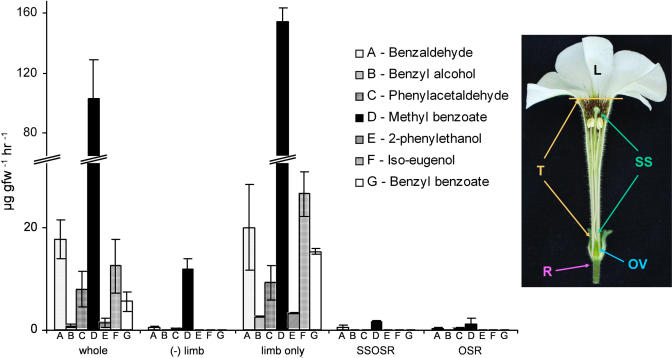
To determine how each part of a flower contributes to the overall aroma of petunia flowers, emission of VOCs was measured from individual floral organs (Fig. 1). Volatiles were collected between the hours of 7 pm and 8 pm at night from whole flowers, flowers with the limb removed, flowers with no corolla, and the gynoecium only. While there were measurable amounts of VOCs emitted from all floral organs tested, VOC production was primarily localized to the corolla and more specifically the petal limb (Fig. 1; Verdonk et al., 2003). These data show that VOC emission is spatially regulated in petunia floral organs.
Figure 1.
Volatile emission patterns (mean ± se; n = 3) from MD floral organs. VOCs were collected from whole flowers, flowers with petal limb excised (−limb), limbs only, flowers with excised corollas (SSOSR − stigma/style, ovary, sepals, and receptacle), and ovary + sepals + receptacle (OSR). Floral organs are explained in the picture to the right: L, petal limb; T, petal tube; SS, stigma/style; OV, ovary; R, receptacle.
Spatial and Temporal PhBSMT1 and PhBSMT2 Regulation in Petunia Flowers
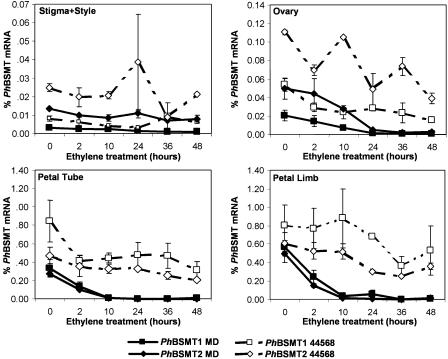
Petunia flowers spatially and temporally produce ethylene in response to pollination in order to coordinate postpollination changes in the individual floral organs (Hoekstra and Weges, 1986; Holden et al., 2003; Jones et al., 2003). PhBSMT expression levels were measured in individual floral organs of MD and transgenic ethylene-insensitive 35S∷etr1-1 (44568) to examine the spatial and temporal pattern of mRNA regulation after pollination and exogenous ethylene treatments. A real-time reverse transcription (RT-PCR) assay (TaqMan) was developed to allow us to measure the PhBSMT1 and PhBSMT2 mRNAs separately. The TaqMan primers and probes were designed to the 3′-untranslated region of each PhBSMT since this is the most divergent region between the 2 genes (65% identity). Expression of both PhBSMT1 and PhBSMT2 was highest in the petal limbs compared to the other floral organs (expression highest in petal limb > petal tube > ovary > stigma/style). PhBSMT1 and PhBSMT2 mRNA comprised between 0.3% to 0.6% and 0.6% to 0.8% of total RNA in petal limbs, respectively (Figs. 2, 3, and 4). Beginning 2 h after ethylene treatment and through the subsequent time course, the greatest decrease in both PhBSMT mRNAs was observed in the ovaries, petal limbs, and petal tubes in MD (Fig. 2). By 10 h, expression of both genes was reduced and maintained at low levels through the remainder of the experiment. There was a small decrease in mRNA levels in 44568 petal tube and ovary, but the magnitude of decrease was not as great as observed in MD. This decrease could possibly be due to some other type of regulation or effect, such as a small amount of residual ethylene sensitivity in these tissues, which has been reported in previous experiments with these plants (Clark et al., 1999). These results indicate that ethylene down-regulates expression of PhBSMT1 and PhBSMT2 in all organs of petunia flowers. Down-regulation in response to ethylene is rapid and is maintained in the corolla limb, the primary site of VOC emission.
Figure 2.
PhBSMT1 and PhBSMT2 % mRNA levels (mean ± se) after treatment with exogenous ethylene (2–3 μL L−1). Note differences in scale.
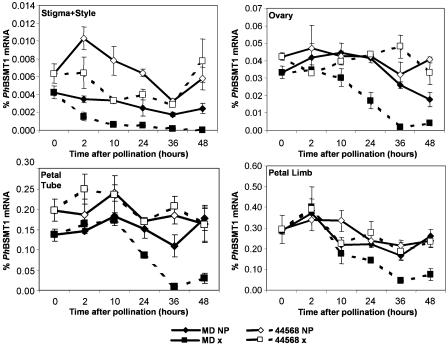
Figure 3.
PhBSMT1 mRNA expression (mean ± se) in wild-type MD petunia and ethylene-insensitive 44568 floral organs after pollination. NP, Nonpollinated; x, pollinated. Note differences in scale.
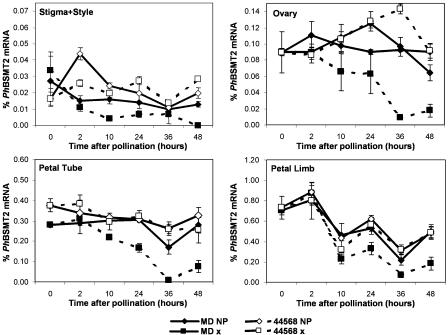
Figure 4.
PhBSMT2 mRNA expression (mean ± se) in wild-type MD petunia and ethylene-insensitive 44568 floral organs after pollination. NP, Nonpollinated; x, pollinated. Note differences in scale.
Since ethylene production is spatially and temporally regulated after pollination (Jones et al., 2003), spatial expression of PhBSMT1 (Fig. 3) and PhBSMT2 (Fig. 4) in individual floral organs was examined over a time course after pollination. Down-regulation of both PhBSMTs was observed in MD stigma/styles beginning approximately 2 h postpollination. At 10 h postpollination, expression of both PhBSMT1 and PhBSMT2 was reduced in the ovary, compared with corresponding expression in nonpollinated flowers. This decrease occurred as the first burst of ethylene was produced from the stigma/style during pollen tube growth. Subsequently, PhBSMT down-regulation was observed 24 h postpollination in the ovary, petal tube, and limb. This corresponded to the time of fertilization when the stigma/style, ovary, and corolla are all producing ethylene, which induces subsequent corolla senescence (Hoekstra and Weges, 1986; Jones et al., 2003). Overall, temporal and spatial ethylene evolution described for petunia (Hoekstra and Weges, 1986; Tang and Woodson, 1996; Jones et al., 2003) corresponded with decreases in PhBSMT levels in the organs producing ethylene and in organs in close proximity. Expression of both PhBSMTs in the ovary, petal tube, and petal limb of the ethylene-insensitive 44568 line was ultimately not different between pollinated and nonpollinated flowers. These data show that ethylene is the primary signal for terminating PhBSMT accumulation in a sequential manner in floral organs after pollination in petunia.
Floral VOC Emission in Response to Exposure to Ethylene and Pollination
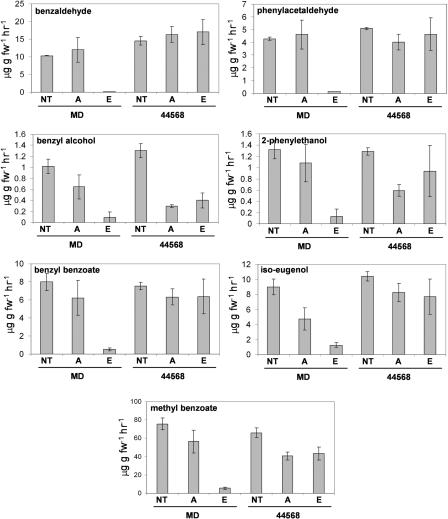
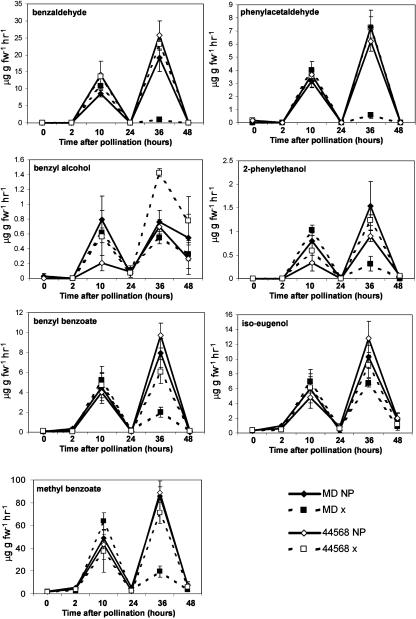
We characterized the production of seven major floral VOCs in response to exogenous ethylene and pollination. Most of the floral VOCs were reduced to almost negligible levels within 10 h of ethylene treatment in MD, but not in 44568 (Fig. 5). After pollination, emission of overall floral VOCs was virtually identical for nonpollinated and pollinated MD and 44568 flowers for the first 24 h (Fig. 6). At 36 h after pollination, emission of most VOCs was significantly reduced in MD-pollinated flowers but not in flowers of any other treatments. This corresponded to a time after the second burst of ethylene was produced in the ovaries and corolla after fertilization. Floral volatiles that decreased in response to both of these treatments included MeBA benzaldehyde, phenylacetaldehyde, benzyl alcohol, 2-phenylethanol, iso-eugenol, and benzyl benzoate. Iso-eugenol and benzyl alcohol were the least affected of all of the volatiles measured. These results show there is a coordinated down-regulation of overall floral volatile emission that is dependent upon ethylene signaling.
Figure 5.
Regulation of major volatiles from petunia in MD and ethylene-insensitive 44568 in response to ethylene (mean ± se; n = 3). Flowers were treated with ethylene (E), air (A), or nontreated (NT). Air-treated control flowers were treated the same as ethylene-treated flowers, except no ethylene was added to the chambers and KMnO4 was included in the chamber.
Figure 6.
Mean (±se; n = 3) emission of major volatiles from pollinated (x) and nonpollinated (NP) flowers of petunia MD and ethylene-insensitive 44568.
Substrate Regulation in Response to Pollination and Ethylene Treatments
While regulation of VOC emission by exogenous ethylene and pollination via mRNA levels may be a key point of regulation in petunia, it is likely that other factors such as substrate availability may also regulate this process. To address the possibility of substrate level regulation of MeBA emission, benzoic acid (BA), salicylic acid (SA), and cinnamic acid (CA) levels were measured in MD and 44568 corollas after pollination (Table I). BA and CA levels increased from 0 h to the 36 h time point in corollas from all treatments except those collected from pollinated MD flowers (Table I), similar to rhythmicity in BA levels observed by Kolosova et al. (2001). However, at 36 h after pollination, BA and CA were approximately 4- and 7-fold lower, respectively, in pollinated MD corollas compared with all other samples. SA levels were affected to a lesser degree, with a less than a 2-fold decrease after pollination in any sample. These results indicate that, in addition to its control over PhBSMT expression, ethylene exerts control of MeBA emission at the substrate level.
Table I.
Measurements of BA, SA, and CA (mean ± se) in MD petunia corollas 36 h after pollination
Amounts for each are in nanograms per gram fresh weight. NP, Nonpollinated; Pol, pollinated.
| Genotype
|
Time
|
|||
|---|---|---|---|---|
| 0 h | 36 h NP | 36 h Pol | ||
| MD | BA | 3,965 ± 442 | 24,214 ± 2,479 | 5,895 ± 2,104 |
| SA | 122 ± 13.7 | 191 ± 6.0 | 105 ± 17.2 | |
| CA | 43 ± 1.9 | 417 ± 27.9 | 56 ± 16.8 | |
| 44568 | BA | 5,311 ± 323 | 25,313 ± 222 | 20,541 ± 1,351 |
| SA | 191 ± 29.9 | 233 ± 14.9 | 265 ± 47.0 | |
| CA | 27 ± 4.3 | 491 ± 10.5 | 483 ± 46.9 | |
Rhythmic PhBSMT mRNA and MeBA Emission
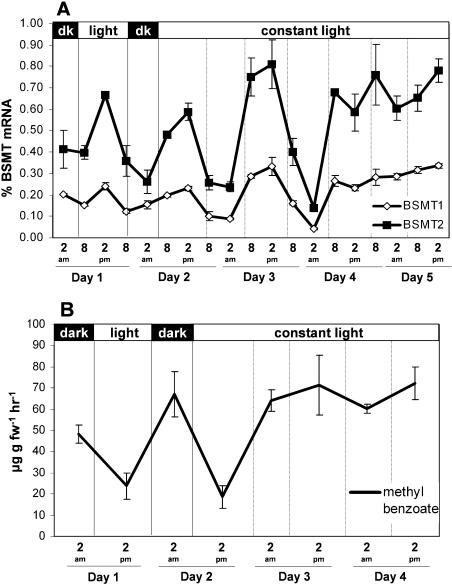
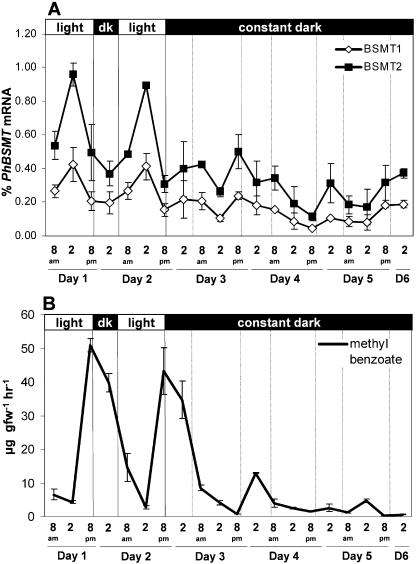
The pollination time course experiments also indicated that MeBA emission and mRNA expression is rhythmic over time in petunia. This is in agreement with observations of MeBA emission by Kolosova et al. (2001) and Verdonk et al. (2003). In many cases, rhythmic biological patterns indicate the possibility of a circadian-regulated process. To further examine the multiple factors regulating MeBA synthesis, we collected floral volatiles and measured PhBSMT mRNA levels through normal day/night cycles and then in continuous dark or light for an extended period. Both experiments demonstrated that PhBSMT expression and MeBA emission are rhythmic with expression peaking approximately 6 h prior to peak emission for the measured time points (Fig. 8), thus further indicating that emission of MeBA is tightly controlled by PhBSMT transcript level. When the plants were placed in complete darkness for an extended period, emission of MeBA was greatly reduced, but emission did continue through one oscillation 6 h after a relatively small increase in PhBSMT expression (Fig. 7). When the plants were placed in continuous light, PhBSMT expression and MeBA emission remained high after oscillating through one cycle (Fig. 8). The dampening of robust cycling the first day the plants were placed in the dark while cycling was maintained the first day in continuous light indicates that PhBSMT1 and PhBSMT2 expression and MeBA emission are influenced primarily by light and to a lesser degree by circadian factors.
Figure 8.
Mean (±se; n = 2) rhythmic mRNA levels of PhBSMT1 and PhBSMT2 (A) and mean (±se; n = 2) rhythmic emission of MeBA (B) through 2 regular day/night cycles and then transfer to continuous light.
Figure 7.
Mean (±se; n = 2) rhythmic mRNA levels of PhBSMT1 and PhBSMT2 (A) and mean (±se; n = 2) rhythmic emission of MeBA (B) through 2 regular day/night cycles and then transfer to continuous darkness.
Analysis of RNAi PhBSMT Transgenic Petunias
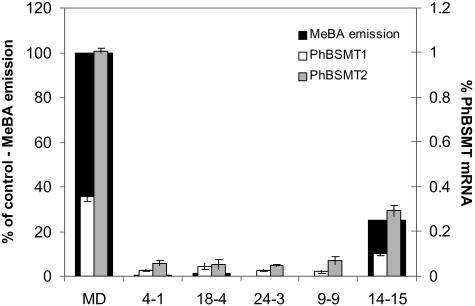
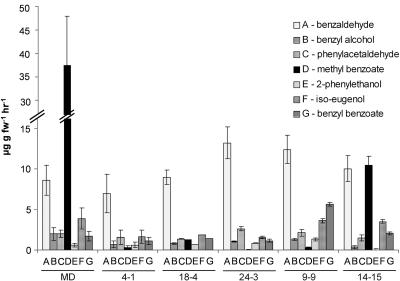
While there are multiple volatiles in petunia flowers whose synthesis is affected after pollination, we chose to focus on MeBA because we had isolated two PhBSMT cDNAs, characterized enzyme activity in vitro (Negre et al., 2003), and MeBA was the most prominent VOC in the volatile profile of MD petunia flowers. RNAi mediated posttranscriptional gene silencing of PhBSMT was employed to determine if the petunia PhBSMT1 and PhBSMT2 genes encode enzymes responsible for MeBA synthesis in vivo. Southern blots indicated that there are two BSMT genes present in the petunia genome. Since the coding regions of these 2 genes were so similar (99% identical), the RNAi construct targeted both PhBSMT1 and PhBSMT2. Eleven primary T0 transgenic lines were selected having significantly reduced MeBA levels and PhBSMT1 and PhBSMT2 expression. T1 progeny from five independent transgenic lines showed reduced MeBA levels (Fig. 9) based on the corresponding presence of NPTII, reduced accumulation of both PhBSMTs, and reduced MeBA emission. These lines emitted between 75% and 99% less MeBA and had a 30% to 99% reduction in PhBSMT1 and 70% to 99% reduction in PhBSMT2 compared to those of MD control flowers (Fig. 9). Additionally, PhBSMT levels directly corresponded with the amount of MeBA emitted; flowers from transgenic lines with the most reduced PhBSMT levels emitted the least MeBA. The petal limb was the primary site of VOC emission in these transgenic lines, similar to MD control flowers (Supplemental Fig. 1); thus, the spatial location of VOC synthesis was not significantly altered in these transgenic plants. We did observe some variation in the levels of other VOCs in a few of the transgenic lines (Fig. 10; Supplemental Fig. 1). There were no consistent trends for the variation in these other VOCs and the changes appeared to be minimal within the spring and summer seasons. However, we have observed some seasonal variation in VOC emission in petunia. It should be noted that the VOCs emission presented in the supplemental figure were collected in the winter season while all others were collected in spring and summer.
Figure 9.
RNA interference of petunia PhBSMT1 and PhBSMT2 reduces MeBA emission. Mean (±se; n = 3) MeBA emission (black bars, left axis) and % mRNA levels (right axis) of PhBSMT1 (white bars) and PhBSMT2 (gray bars) in flowers of MD control and T1 progeny from 5 independent transgenic lines 4-1, 18-4, 24-3, 9-9, and 14-15.
Figure 10.
Mean (±se) emission of benzaldehyde, benzyl alcohol, phenylacetaldehyde, methyl benzoate, 2-phenylethanol, iso-eugenol, and benzyl benzoate from MD flowers (n = 6) and T1 progeny from 5 independent transgenic lines 4-1, 18-4, 24-3, 9-9, and 14-15 (n = 3).
Since the RNAi PhBSMT petunia plants were lacking the major component of the volatile profile with minimal changes in other floral VOCs (Fig. 10), we tested to see if humans could perceive a difference between the fragrances of flowers from the RNAi PhBSMT (BSMT9-9, T1 plants) and MD control flowers. A human sensory panel was able to discriminate the differences in floral fragrance of the MeBA knockouts from MD fragrance. In this panel, 80% of the participants were able to correctly detect a significant difference between MD fragrance and BSMT9-9 fragrance at a probability of <0.1% (Supplemental Table I). Overall, the participants commented negatively on the floral fragrance of BSMT9-9 flowers, with a general consensus that they had less fragrance than MD flowers.
DISCUSSION
The petunia floral volatile synthesis profile is complex and regulated at many levels. There are factors that direct tissue specificity, ethylene sensitivity, and rhythmic regulation. Petunia VOCs are emitted primarily from the corolla limb with lower emission from other floral parts. Six of these volatiles exhibited temporal down-regulation after pollination and ethylene treatments. The down-regulation of VOC biosynthesis by ethylene is physiologically meaningful because the timing of regulation corresponds to the second major phase of pollination-induced ethylene production, when ethylene is simultaneously being produced from multiple floral organs as a result of a successful fertilization event. These results demonstrate that ethylene sensitivity regulates total floral fragrance output in addition to controlling petal senescence. Once the flowers are pollinated, they begin making ethylene, which leads to petal senescence and a reduction in fragrance VOC synthesis and emission. This occurs as the flowers transition into a new phase of development from an attractive phase to one of seed production.
Floral VOC emission is spatially regulated in petunia flowers, and the greatest amount of VOCs were collected from the corolla limb. Emission of many VOCs was reduced at least 20-fold when the limb was removed from the flower. Perhaps this is the primary site of emission since there is such a large exposed surface area for VOCs to disperse into the surrounding environment. It appears from these data that the corolla is specialized for VOC synthesis and emission in petunia, making it similar to snapdragon (Kolosova et al., 2001). While VOC emission was much lower from other floral parts (gynoecium and sepals), there were still measurable amounts of these chemicals being emitted. Lower PhBSMT expression levels in these tissues compared with the corolla are consistent with lower levels of MeBA emission. Perhaps emission from these parts contributes to overall fragrance, providing continued surface area for VOC emission. There are also reports that some VOCs have a signaling role in microbial defense (Chen et al., 2003) where lower levels would be biologically meaningful. It is possible that these volatiles not only attract pollinators, but also serve a role in defense against microbial invasion of floral parts until fertilization is achieved. A defensive type of role also may be important in an area where sugar-rich nectar is produced and accumulated and is thus more prone to microbial growth.
PhBSMT1 and PhBSMT2 were both down-regulated rapidly in response to exogenous ethylene and pollination in all MD floral organs, but not in ethylene-insensitive 44568 floral organs. Studies in petunia, Phalaenopsis spp., and carnation have shown that ethylene synthesis is temporally and spatially regulated in the flower and coordinates developmental changes such as pollen tube growth, petal senescence, and ovule development (O'Neill et al., 1993; Tang and Woodson, 1996; Bui and O'Neill, 1998; Jones and Woodson, 1999; Holden et al., 2003; Jones et al., 2003). Results presented here, combined with Negre et al. (2003), show that ethylene has direct control of VOC biosynthesis. Models for postpollination regulation of ethylene synthesis indicate that pollination induces ethylene synthesis first from the stigma/style, then from the ovary at fertilization, followed by the corolla. Here, we show that the temporal and spatial down-regulation of both PhBSMT1 and PhBSMT2 in the floral organs follows this sequential pattern of postpollination ethylene production observed in petunia (Tang and Woodson, 1996; Jones et al., 2003). These data also suggest that the ethylene gas produced from the stigma/style may regulate ethylene-sensitive processes in the corolla due to the close proximity of these organs in petunia (Fig. 1). Down-regulation of PhBSMT2 begins as early as 10 h in the MD petal tube, a time when ethylene is produced from the stigma/style (Jones et al., 2003). Kolosova et al. (2001) localized the snapdragon BA carboxyl methyltransferase to the conical cells of the inner epidermis of the petals. In petunia, these cells would be in the immediate vicinity to ethylene produced from the stigma/style. These data suggest that ethylene produced in the stigma/style likely regulates PhBSMT2 in the petal tube after pollination.
Another layer of regulation of MeBA emission in petunia occurs at the substrate level. Free BA and CA levels decreased in pollinated MD corollas compared with corollas from nonpollinated control flowers (Table I). These data indicate that the decrease in MeBA synthesis after pollination results from both decreased substrate levels and decreased PhBSMT expression. After pollination, these substrates may be used in other processes or remobilized to other organs, resulting in the measurable decrease observed in corollas. There was little difference in BA levels in 44568 after pollination, suggesting that ethylene has a central role in controlling BA levels in the corollas. These results, combined with the observation that ethylene down-regulates emission of most floral VOCs in petunia, suggest that genes encoding enzymes upstream of MeBA or enzymes involved in the biosynthetic pathways of the other floral VOCs may also be down-regulated by ethylene. If this is the case, it will be imperative to determine whether there are common transcription factors influenced by ethylene that recognize common promoter elements in these genes. Levels of SA were relatively low compared with BA and CA and were not influenced to the same degree by pollination or ethylene sensitivity. Since SA is a plant hormone and MeSA is virtually undetectable in petunia flowers, low SA levels would be expected. Additionally, since SA is involved in eliciting cell death and plant defense responses, maintenance of stable SA levels in petals during pollination may assist in promoting ethylene-independent cell death processes or in defensive mechanisms against pathogen infection as the flower senesces around the developing fruit.
The nature of rhythmic MeBA emission was investigated to examine additional relationships between mRNA and MeBA emission and the influence of a possible circadian rhythm on VOC synthesis. Rhythmic emission of floral VOCs has been demonstrated in multiple species including petunia (Verdonk et al., 2003; Simkin et al., 2004), rose (Helsper et al., 1998), Antirrinhum (Kolosova et al., 2001), and Stephanotis (Pott et al., 2002). Recently, emission of the floral VOC β-ionone was shown to be regulated by both circadian and light factors in petunia (Simkin et al., 2004). To demonstrate true circadian control of a process, a robust rhythm must continue under constant environmental conditions (Jones and Mansfield, 1975). Under normal light/dark conditions, peak PhBSMT expression precedes peak MeBA emission by approximately 6 h. Although PhBSMT expression levels in corollas continued to cycle when plants were placed into constant light or dark conditions, MeBA emission was reduced during constant darkness to minimal levels and was elevated in constant light to maximal levels 1 d after the onset of treatments. These data suggest a role for both light and circadian factors in control of MeBA synthesis. Since light generates energy in plants and methylation is a metabolically expensive process (Atkinson, 1977), it is possible that light regulation of the PhBSMT transcripts would help to control the amount of energy spent on MeBA synthesis. Substrate levels also likely have a role in the rhythmic regulation of MeBA, since BA was shown to be rhythmic in petunia (Kolosova et al., 2001). An understanding of genes involved in BA synthesis and their regulation will help to address the questions of how these multiple factors regulate MeBA synthesis.
This study examines many aspects of regulation of floral VOC synthesis in petunia, with particular attention given to MeBA synthesis. These data show that PhBSMT expression and MeBA emission are ethylene regulated, pollination regulated, and rhythmically regulated by light and circadian factors. Down-regulation of MeBA synthesis after pollination is controlled by ethylene through decreased mRNA and substrate levels in the corolla and, as observed by Negre et al. (2003), at the posttranslational level. Reducing the levels of mRNA by RNAi shows that PhBSMT abundance determines how much MeBA is synthesized. While posttranslational regulation does appear to have a role in regulating MeBA (Negre et al., 2003), decreased mRNA levels are likely to have a key role since this would presumably limit enzyme abundance and disturb the substrate-to-enzyme ratio, thus resulting in decreased MeBA synthesis. Recent results suggest that a similar transcriptional down-regulation is occurring in response to ethylene with at least one or more of the other VOC synthesis genes in petunia (Dexter et al., 2004). Down-regulation of these genes and their respective volatiles suggest a broad role for ethylene in regulating floral VOC synthesis at the level of transcription. The promoters and regulatory elements of these genes will be of interest for studying factors involved with this complex regulation.
Transgenic plants with decreased PhBSMT mRNA levels emit greatly reduced levels of MeBA, thus demonstrating a role for these genes in MeBA synthesis in vivo. These results are consistent with in vitro studies showing that the BSMT enzymes methylate SA and BA substrates (Negre et al., 2003). We measured at least a 32-fold difference in the levels of these 2 substrates in petal tissue (Table I). Since SA is present at relatively low levels in the petal tissue, methyl salicylate emission is not consistently observed in MD. Consistent with this observation, no change was observed in the status of methyl salicylate in the RNAi lines, while the most notable change was a large decrease in MeBA levels. Many of the transgenic lines exhibited some differences in the levels of other VOCs compared with MD control flowers (Fig. 10). Many of these benzenoid-type compounds have been shown to be intermediates in benzenoid metabolism in petunia (Boatright et al., 2004). Therefore, it is likely that disruption of MeBA synthesis could result in a change in levels of these other compounds. Comprehensive studies of how floral VOCs are regulated and the genes responsible for their synthesis are important for understanding floral biology and for potential commercial applications.
MATERIALS AND METHODS
Plant Material
In all experiments, petunia (Petunia × hybrida) cv MD was used as the wild-type line and is also the genetic background of ethylene-insensitive 35S∷etr1-1 line 44568 (Wilkinson et al., 1997). Plants were grown in air-conditioned glass greenhouses at 25°C day/18°C night. Plants were potted in Fafard 2B potting medium (Fafard, Apopka, FL) in 1.2-L pots and fertilized at every irrigation with 150 mg L−1 Scott's Excel 15-5-15 (Scotts, Marysville, OH).
cDNA Isolation
Three cDNA libraries were constructed from petunia MD whole flowers collected at multiple developmental stages (from early bud to anthesis), ethylene-treated flowers (2.5 μL L−1 ethylene treatments for 30 min and 1, 3, 6, and 12 h), and pollinated flowers (1, 2, 5, 10, 24, and 34 h after pollination). Total RNA was extracted by a phenol:chloroform extraction method with lithium chloride precipitations as described in Ciardi et al. (2000). Messenger RNA was isolated using Oligotex mRNA purification (Qiagen, Valencia, CA). cDNA libraries were constructed using a λ-ZAPII cDNA synthesis kit from Stratagene (La Jolla, CA). Approximately 6,000 clones from these libraries were randomly sequenced. A minimally redundant subset of these clones was used for microarray experiments to find ethylene-regulated genes. From these microarray experiments, the S-adenosyl-l-methionine:salicylic acid carboxyl methyltransferase homologs were isolated as being putative ethylene down-regulated cDNAs (Underwood, 2003). Two full-length cDNAs encoding for putative SAMT orthologs, PhBSMT1 (AAO45012) and PhBSMT2 (AAO45013), were isolated from the cDNA libraries and were used in subsequent experiments. RNA gel-blot analysis was used to verify down-regulation by ethylene and corresponding activity was assayed in vitro with recombinant BSMT proteins (Negre et al., 2003).
Tissue Treatments and Collections
All ethylene treatments and pollinations were initiated the day after anthesis at 10 am under sunny weather conditions to help reduce developmental, temporal, and environmental variability. For ethylene and control air treatments, flowers were excised and placed into 1.5-mL centrifuge tubes containing 1.0 mL distilled water. Flowers were sealed in 37.85-L glass chambers and treated with 2 to 3 μL L−1 ethylene. For air treatments, flowers were placed in the same conditions, but no ethylene was added and potassium permanganate (Fisher Scientific, Hampton, NH) was placed in the chambers. Concentrations of exogenous ethylene were verified at the beginning and end of indicated treatment times using a gas chromatograph (Hewlett-Packard model 5890, Series II; Palo Alto, CA) equipped with a flame ionization detector and an alumina column. For pollinated flower collections, flowers were pollinated and remained on the plant until collection time. For all experiments, all treated (ethylene or pollination) and control (air or nonpollinated) flowers were collected at the following times (with treatment times in parenthesis): 10 am (0 h), 12 pm (2 h), 8 pm (10 h), 10 am (24 h), 10 pm (36 h), and 10 am (48 h) after ethylene treatment or pollination. For constant dark or light circadian studies, tissue was collected from plants placed into continuous darkness (0.06 μmol m−2 s−1) and plants in continuous light (380 μmol m−2 s−1) at a temperature of 25°C ± 3°C.
Spatial and Temporal Analysis of mRNA Expression in Flowers
Spatial and temporal mRNA accumulation was analyzed after ethylene treatments and pollination in petunia MD and ethylene-insensitive 44568. Expression was examined from individual floral organs including petal limbs, petal tubes, stigma/styles, and ovaries dissected from ethylene-treated, air-treated, pollinated, and nonpollinated flowers. The day after anthesis, flowers were either collected for ethylene treatments or pollinated on the plant for the time courses described above. At each respective time point, tissue was harvested, dissected, and immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using an RNeasy Mini Plant RNA extraction kit with on-column DNase digestion performed during the extraction (Qiagen). RNA was quantified by spectrophotometry, and RNA quality was verified by gel electrophoresis. Real-time RT-PCR was performed for quantification of PhBSMT mRNA transcripts from 100 ng of total RNA using TaqMan One-Step RT-PCR reagents (Applied Biosystems, Foster City, CA). Reactions were conducted in 25-μL volumes in 96-well optical reaction plates on a Gene Amp 5700 Sequence Detection System (Applied Biosystems). Primers and TaqMan probes were designed using Primer Express software (Applied Biosystems). Specificity of each of the primer and probe sets was verified by performing PCR reactions with in vitro transcribed PhBSMT1 template with the primer and probe set specific to PhBSMT2 and vice versa. In vitro transcribed RNA was synthesized using a MAXIscript In vitro Transcription kit (Ambion, Austin, TX) according to manufacturer's instructions. PhBSMT1 and PhBSMT2 were used as templates for in vitro transcription and the tritiated transcripts were collected from separate gels to prevent possible contamination. Primer and probe sequences used for individual detection of each gene corresponded to the 3′-untranslated region of the cDNA and are as follows: PhBSMT1 forward primer, AAATGTCATCATCTCCTTGACCAA; PhBSMT1 reverse primer, CGGATCACTACTAAAATATTCGGGTTT; PhBSMT TaqMan probe, 6FAM-AAGGCACTCAATGTCTATTTTCGGTCGA-BHQ1; PhBSMT2 forward primer, TGTACCAATTCTCTATTGTTGTTTTGC; PhBSMT2 reverse primer, CTGAAAGGACCCCTAGTGTACAAGA; PhBSMT2 TaqMan probe, 6FAM-CTTCATAGGTGGTCGAGGTGCTAATTTATCTAGTC-BHQ1. TaqMan Real-time PCR reactions were run under the following conditions: 48°C 30 min, 95°C 10 min, followed by 40 cycles of 95°C 15 s and 60°C 1 min. Reactions were repeated twice with one set of RNAs and once with RNA collected from a duplicate set of tissues. PCR reactions of tritiated in vitro-transcribed PhBSMT1 or PhBSMT2 standards were run in duplicate and in tandem with the sample RNAs to generate a standard curve from which the level of each PhBSMT mRNA in the samples was quantified.
Generation of Transgenic PhBSMT RNAi Petunias
Petunia plants were transformed with and generated from one of two different BSMT RNAi constructs. Transgenic lines BSMT-9 and BSMT-14 were generated from transformations that utilized a pHANNIBAL-derived construct (Wesley et al., 2001). For this construct, the segment between 661 and 1,002 nucleotides of the PhBSMT1 open reading frame was cloned in the sense and antisense orientation to flank the intron in the pHANNIBAL vector. Lines BSMT-4, BSMT-18, and BSMT-24 were generated from the construct SAMJR. For this construct, the region spanning nucleotides 720 to 1,020 and 720 to 1,430 were cloned in the sense and antisense orientation without a flanking intron into the pFMV cloning vector. The RNAi chimeric genes were subsequently cloned into an Agrobacterium transformation vector containing the neomycin phosphotransferase II (NPTII) gene. This transformation vector was introduced into Agrobacterium tumefaciens by triparental mating and used for transforming leaf explants from 5-week-old MD seedlings according to the methods of Jorgensen et al. (1996). Primary transformants were grown under standard greenhouse conditions, and transgenic plants were selected by PCR for presence of NPTII, reduced BSMT mRNA levels, and reduced MeBA emission. Flowers were self pollinated to produce T1 progeny from phenotype positive lines that were analyzed for presence of transgene by PCR analysis. T1 progeny plants from each line were analyzed for phenotypes by measurement of MeBA emission and measurement of BSMT mRNA levels.
Volatile Collection and Analysis
For ethylene treatment volatile collections, flowers were excised and treated with ethylene as described in tissue collection methods. An additional untreated control was included for the ethylene experiments to control for flower excision, induced variability in air, and ethylene treatments. For this control, flowers at the same developmental stages were collected fresh from the plants to compare with air-treated control flowers. For pollinated flower samples, flowers were pollinated on the plant and not excised until the time of volatile collection. Flowers used for spatial volatile analysis were collected from freshly opened flowers in the evening between 8 and 9 pm. Flowers and floral organs were weighed prior to volatile collection. Three flowers were collected per treatment and each time point/treatment was repeated three times. Flowers from the BSMT RNAi screen were collected three times with 3 to 5 flowers/collection at 8 pm for the initial screen and once more with putative positive lines at 12 am to verify that reduced MeBA emission was reduced when MeBA emission is maximal in MD (Kolosova et al., 2001). Volatiles were collected for 1 h according to collection protocol described by Schmelz et al. (2001). Briefly, filtered air was flowed at a constant rate for 1 h over flowers in glass tubes (17 mm × 61 cm, 127-mL volume) with volatiles collecting on columns with Super-Q resin. Volatiles collected on the Super-Q resin were eluted with methylene chloride (Fisher Scientific). A total of 400 ng nonyl acetate was added to the columns as an internal standard before the volatiles were eluted. Identification of each of the floral volatiles was verified by gas chromatography-mass spectrometry (Schmelz, et al., 2001).
BA and SA Extraction and Quantification
BA and SA were extracted and quantified by gas chromatography-mass spectrometry (Schmelz et al., 2003). Basal control levels of BA may have been overestimated due to trace BA contaminants in analytical grade solvents; however, significant differences between treatment groups were unaffected and remain valid (Schmelz et al., 2004). Petal tissue was excised from MD and 44568 whole flowers after 10 h treatment with 2 to 3 μL L−1 ethylene or 36 h after pollination and stored at −80°C until extraction. Two replicate sets of tissues were used for substrate quantification.
Human Sensory Panels
Human sensory panels were used to determine if differences in fragrance of the reduced MeBA flowers and MD wild-type flowers could be discriminated by human olfaction. The flower samples were prepared from freshly excised flowers at anthesis from MD and knockout line BSMT-9. Excised flowers were placed immediately into 5-mL water agarose blocks, then placed into 210-mL glass jars and sealed with lids for approximately 120 min before testing. A triangle test (Lawless and Heymann, 1998) was performed with 60 human subjects, each randomly given a set of 3 unmarked flowers for sampling of the floral fragrances. Each set of flowers consisted of two flowers of the same genotype and one of the other genotype. The test was performed both with two controls and one transgenic or two transgenics and one control and panelists were asked to judge which flower had a different fragrance. Additional descriptive comments were also solicited from the test subjects to determine if there were preferences in floral fragrance. The statistical significance of the correct number of judgments was determined as described (Lawless and Heymann, 1998).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AAO45012 and AAO45013.
Supplementary Material
This work was supported by the Fred Gloeckner Foundation (grant to D.G.C.), by the American Floral Endowment (grant to D.G.C.), by the U.S. Department of Agriculture Floral and Nursery Crops Initiative (grant to D.G.C.), by the Florida Agricultural Experiment Station, National Science Foundation (grant no. DBI–0211875 to H.J.K.), and by the University of Florida (alumni fellowship to B.A.U.). This paper is contribution R-10643 from the Florida Agricultural Experiment Station.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.051144.
References
- Atkinson DE (1977) Functional stoichiometric coupling and metabolic prices. In Cellular Energy Metabolism and Its Regulation. Academic Press, New York, pp 31–83
- Boatright J, Negre F, Chen X, Kish CM, Wood B, Peel G, Orlova I, Gang D, Rhodes D, Dudareva N (2004) Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol 135: 1993–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui AQ, O'Neill SD (1998) Three 1-aminocyclopropane-1-carboxylate synthase genes regulated by primary and secondary pollination signals in orchid flowers. Plant Physiol 116: 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burquez A, Corbet SA (1991) Do flowers resorb nectar? Funct Ecol 5: 369–379 [Google Scholar]
- Chen F, D'Auria JC, Tholl D, Ross JR, Gershenzon J, Noel JP, Pichersky E (2003) An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, as a role in defense. Plant J 36: 577–588 [DOI] [PubMed] [Google Scholar]
- Ciardi J, Tieman DM, Lund SD, Jones JB, Stall RE, Klee HJ (2000) Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol 123: 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Klee HJ, Barrett JE, Nell TA (1999) Root formation in ethylene insensitive plants. Plant Physiol 121: 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter RJ, Shibuya K, Loucas HM, Clark DG, Underwood BA, Tieman DM, Klee HJ (2004) The isolation of BEBT from Petunia hybrida: a flower specific gene involved in floral volatile synthesis (abstract no. 273). In Plant Biology 2004, American Society of Plant Biologists, July 24–28, 2004, Lake Buena Vista, FL
- Dudareva N, Murfitt LM, Mann CJ, Gorenstein N, Kolosova N, Kish CM, Bonham C, Wood K (2000) Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 12: 949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubrium EK, Clevenger DJ, Clark DG, Barrett JE, Nell TA (2000) Reproduction and horticultural performance of transgenic ethylene-insensitive petunias. J Am Soc Hortic Sci 125: 277–281 [Google Scholar]
- Helsper JPFG, Davies JA, Bouwmeester HJ, Krol AF, van Kampen MH (1998) Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L. cv. Honesty. Planta 207: 88–95 [Google Scholar]
- Hoekstra FA, Weges R (1986) Lack of control by early pistillate ethylene on the accelerated wilting of Petunia hybrida flowers. Plant Physiol 80: 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden MJ, Marty JA, Singh-Cundy A (2003) Pollination-induced ethylene promotes the early phase of pollen tube growth in Petunia inflata. J Plant Physiol 160: 261–269 [DOI] [PubMed] [Google Scholar]
- Jones MB, Mansfield TA (1975) Circadian rhythms in plants. Sci Prog Oxf 62: 103–125 [Google Scholar]
- Jones ML, Langston BJ, Johnson F (2003) Pollination-induced senescence of ethylene sensitive and insensitive petunias. In M Vendrell, F Romojaro, eds, Biology and Biotechnology of the Plant Hormone Ethylene III. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Jones ML, Woodson WR (1999) Interorgan signaling following pollination in carnations. J Am Soc Hortic Sci 124: 598–604 [Google Scholar]
- Jorgensen RA, Cluster PD, English J, Oue O, Napoli CA (1996) Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense vs antisense constructs and single-copy vs complex T-DNA sequences. Plant Mol Biol 31: 957–973 [DOI] [PubMed] [Google Scholar]
- Knudsen JT, Tollsten L, Bergstrom LG (1993) Review article number 76: Floral scents: a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33: 253–280 [Google Scholar]
- Kolosova N, Gorenstein N, Kish CM, Dudareva N (2001) Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 13: 2333–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless HT, Heymann H (1998) Sensory Evaluation of Food. Chapman and Hall Publishers, New York
- Negre F, Kish CM, Boatright J, Underwood BA, Shibuya K, Wagner C, Clark DG, Dudareva N (2003) Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. Plant Cell 15: 2992–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SD (1997) Pollination regulation of flower development. Annu Rev Plant Physiol Plant Mol Biol 48: 547–574 [DOI] [PubMed] [Google Scholar]
- O'Neill SD, Nadeau JA, Zhang XS, Bui AQ, Halevy AH (1993) Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell 5: 419–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott MB, Pichersky E, Piechulla B (2002) Transcriptional and post-translational regulation of S-adenosyl-L-methionine: salicylic acid carboxyl methyltransferase (SAMT) during Stephanotis floribunda flower development. J Plant Physiol 159: 925–934 [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M (2001) Post-pollination emission of a repellent compound in a sexually deceptive orchid: a new mechanism for maximising reproductive success? Oecologia 126: 531–534 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2001) The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214: 171–179 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Alborn HT, O'Donnell P, Sammons M, Toshima H, Tumlinson JH (2003) Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc Natl Acad Sci USA 100: 10522–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT (2004) The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J 39: 790–808 [DOI] [PubMed] [Google Scholar]
- Shibuya K, Barry KG, Ciardi JA, Loucas HM, Underwood BA, Nourizadeh S, Ecker JR, Klee HJ, Clark DG (2004) The central role of PhEIN2 in ethylene responses throughout plant development in petunia. Plant Physiol 136: 2900–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya E, Schmelz E, Clark DG, Klee HJ (2004) Circadian regulation of the PhCCD1 carotenoid dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol 136: 3504–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XY, Woodson WR (1996) Temporal and spatial expression of 1-aminocyclopropane-1-carboxylate oxidase mRNA following pollination of immature and mature petunia flowers. Plant Physiol 112: 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollsten L (1993) A multivariate approach to post-pollination changes in the floral scent of Platanthera bifolia (Orchidaceae). Nord J Bot 13: 495–499 [Google Scholar]
- Underwood BA (2003) Effects of ethylene on floral fragrance in Petunia x hybrida Mitchell Diploid. PhD dissertation. University of Florida, Gainesville, FL
- van Doorn WG (1997) Effects of pollination on floral attraction and longevity. J Exp Bot 48: 1615–1622 [Google Scholar]
- Verdonk JC, de Vos CHR, Verhoeven HA, Haring MA, van Tunen AJ, Schuurink RC (2003) Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62: 997–1008 [DOI] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27: 581–590 [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ (1997) A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol 15: 444–447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.