Abstract
The ripening of a fleshy fruit represents the summation of an array of biochemical processes that are regulated by interactions between developmental programs and environmental inputs. Analysis of tomato (Solanum lycopersicum) mutants and inhibitor studies indicate that ethylene is necessary for full development of the ripening program of climacteric fruit such as tomato, yet ethylene alone is not sufficient. This suggests that an interaction between ethylene and nonethylene (or developmental) pathways mediates ripening. In this study, we have examined the physiological basis for ripening inhibition of the dominant Green-ripe (Gr) and Never-ripe 2 (Nr-2) mutants of tomato. Our data suggest that this inhibition is due to ethylene insensitivity in mutant fruit. Further investigation of ethylene responses in Gr and Nr-2 plants also revealed weak ethylene insensitivity during floral senescence and abscission and, during inhibition of root elongation, a phenotype associated with the triple response. However, ethylene-induced inhibition of hypocotyl elongation and petiole epinasty are normal in Gr and Nr-2, suggesting that these loci regulate a subset of ethylene responses. We have mapped both dominant mutations to a 2-cM overlapping region of the long arm of chromosome 1 of tomato, a region not previously linked to any known ethylene signaling loci. The phenotypic similarity and overlapping map location of these mutations suggest Gr and Nr-2 may be allelic and may possibly encode a novel component of the ethylene response pathway.
The ripening of a fruit represents the culmination of a series of biochemical processes that have evolved as a mechanism of seed dispersal. In the case of fleshy fruits, the changes that occur during ripening impart desirable characteristics to the fruit such as bright colors, softening, and sugar and volatile accumulation that attract animals and birds to aid dispersal. Fruits also form an essential component of the human diet providing sources of sugars, fiber, vitamins, minerals, and antioxidants. Therefore, the study of fruit ripening is of importance to both basic plant biology and agriculture.
Although there is great diversity in fruit anatomy and phenotypes, the biochemical changes that occur during ripening are conserved in many plant species. For example, research carried out on diverse species indicates that at the onset of ripening there is often a coordinated increase in gene expression and enzyme activity of many proteins involved in cell wall metabolism, pigment synthesis, and sugar metabolism (Seymour et al., 1993). These data suggest that the genetic mechanisms that regulate fruit ripening are likely conserved. Little is known of the identities of these genetic pathways, although experiments, largely performed using tomato (Solanum lycopersicum), have led to the hypothesis that ripening in climacteric fruits such as tomato, banana, apple, and stone fruits is regulated by ethylene dependent and independent pathways (Adams-Phillips et al., 2004a; Giovannoni, 2004).
The involvement of ethylene in regulating ripening has been defined by inhibitor studies, transgenic analysis through altered expression of genes involved in ethylene biosynthesis and signaling, and by characterization of mutants (Hobson et al., 1984; Oeller et al., 1991; Picton et al., 1993a; Lanahan et al., 1994; Wilkinson et al., 1995; Hackett et al., 2000; Tieman et al., 2000, 2001). Furthermore, the expression of many genes encoding enzymes that give rise to the ripe phenotype is known to be regulated by ethylene (Lincoln et al., 1987; Maunders et al., 1987; Zegzouti et al., 1999).
Evidence of the involvement of an ethylene-independent or developmental pathway that regulates ripening has come from the characterization of monogenic tomato mutants including ripening-inhibitor (rin), non-ripening (nor), and Colorless non-ripening (Cnr) in which ripening is severely impaired. These mutants fail to undergo an increase in ripening-related ethylene production and show inhibition of ripening-related gene expression, although gene expression but not ripening can be partially restored by ethylene treatment, indicating that they remain ethylene responsive (Tigchelaar et al., 1978; Lincoln and Fischer, 1988; Della-Penna et al., 1989; Picton et al., 1993b; Yen et al., 1995; Thompson et al., 1999). Although a complete molecular ripening pathway remains to be identified, an important first step toward this goal came from the discovery that the rin locus encoded a MADS-box transcription factor necessary for regulating ripening (Vrebalov et al., 2002).
In this study, we have examined the physiological basis of ripening inhibition in two dominant mutants of tomato, Green-ripe (Gr) and Never-ripe 2 (Nr-2), and conclude that their reduced ripening results from decreased ethylene sensitivity. Weak ethylene insensitivity is also apparent during petal senescence, ethylene-induced floral abscission, and ethylene inhibition of root elongation. However, ethylene-induced inhibition of hypocotyl growth and petiole epinasty are normal in Gr and Nr-2, suggesting that these loci regulate a subset of ethylene responses. Genetic mapping positioned Gr and Nr-2 to an overlapping region of the long arm of chromosome 1. The close physical proximity of these mutations in the genome coupled with their phenotypic similarities suggests that they may be allelic.
RESULTS
Gr and Nr-2 Mutants Fail to Fully Ripen
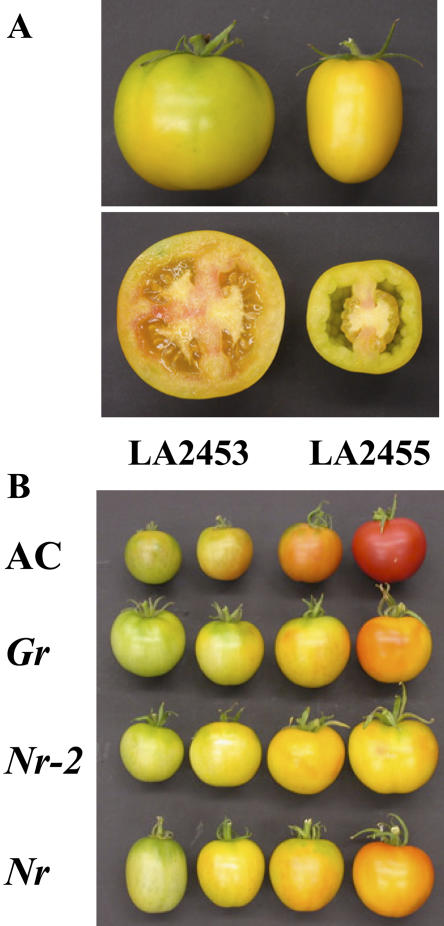
The Gr mutant was originally identified as a spontaneous mutant that retained chlorophyll in mature fruit (Kerr, 1958) and was subsequently described as a fruit ripening mutant (Jarret et al., 1984). The Nr-2 mutant was isolated as a spontaneous mutation in a breeding program and linkage to the long arm of chromosome 1 was demonstrated (Kerr, 1982a, 1982b). The C.M. Rick Tomato Genetics Resource Center (University of California at Davis) holds monogenic stocks of both mutants with the stock numbers LA2453 and LA2455 for Gr and Nr-2, respectively. The fruit phenotype at the ripe stage of development of these mutations is shown in Figure 1A. Fruit softening and lycopene accumulation are greatly inhibited compared to normal ripening fruits. However, some lycopene accumulation is apparent in both mutations in the inner pericarp surrounding the columella. No isogenic backgrounds are available for the LA2453 and LA2455 accessions, making detailed physiological and molecular comparisons difficult to interpret. We therefore backcrossed these mutations into the Ailsa Craig (AC) cultivar to create near isogenic lines (NILs) of each mutation. AC was chosen as the recurrent parent for this backcross analysis as many ripening studies have utilized this variety and the ripening mutants rin, nor, and Nr have also been introduced in to this background, facilitating meaningful comparisons (Darby et al., 1977). Gr was backcrossed in to AC five times and Nr-2 was backcrossed six times to generate the NILs used in this study. Comparison of plant growth and development revealed no obvious differences between AC and the Gr and Nr-2 NILs. Furthermore, fruit set and development were identical until the fruits started to ripen (“breaker” stage of fruit development). Following the initiation of ripening, fruits of AC ripened normally and showed substantial lycopene accumulation by 7 d post breaker. In contrast, like LA2453 and LA2455, the Gr and Nr-2 NILs failed to fully ripen, eventually turning yellow-orange (Fig. 1B). Furthermore, these color changes were substantially delayed in mutant fruits. On average, wild-type fruit reached the breaker stage of development at approximately 5 weeks postanthesis, whereas mutant fruits showed approximately a two and one-half week delay to the onset of color change (data not shown).
Figure 1.
Inhibition of fruit ripening in Gr and Nr-2 mutants. A, Fruit phenotype of the accessions LA2453 (Gr/Gr) and LA2455 (Nr-2/Nr-2) at the ripe stage of development. Fruit were photographed at 60 DPA. B, Ripening phenotype of AC, Gr, Nr-2, and Nr NILs. Photograph shows the normal pattern of ripening in AC fruits from the onset of ripening until the red ripe stage in comparison to mutant NILs. Fruits were harvested based on the accumulation of carotenoid pigments. Note that ripening inhibition is most severe in the Nr-2 NIL. Fruits are not comparable ages. The red ripe AC fruit is at 42 DPA, whereas mutant fruits with the most developed ripening are at 60 DPA.
Ethylene-Regulated Gene Expression Is Altered in Gr and Nr-2 Fruit
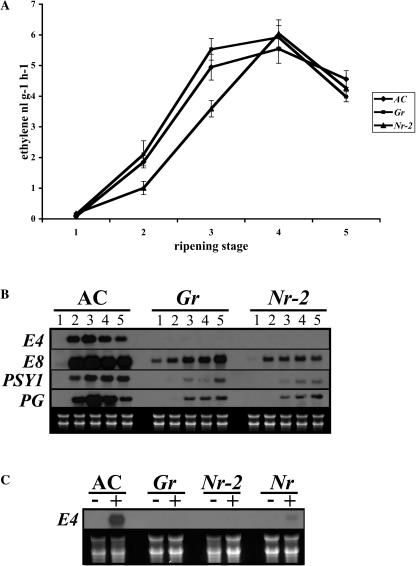
The ripening phenotype of Gr and Nr-2 is similar to that of the dominant Nr mutant (Fig. 1B). Nr carries a mutation in a member of the tomato ethylene receptor gene family rendering plants ethylene insensitive in all tissues examined (Lanahan et al., 1994; Wilkinson et al., 1995). Fruit phenotypic similarity to Nr prompted us to examine ethylene synthesis and responses in Gr and Nr-2. Ethylene synthesis was low in mature green fruits (stage 1) for all three genotypes examined and increased with the onset of color change and followed a similar pattern in the Gr and Nr-2 NILs as in the AC control (stages 2–5), indicating that ethylene synthesis is not qualitatively impaired in mutant fruits (Fig. 2A).
Figure 2.
Characterization of ethylene synthesis and response in Gr and Nr-2. A, Ethylene production in control AC, Gr, and Nr-2 NILs during fruit ripening. Fruit of different developmental stages (1–5) as defined in the methods were sealed in airtight jars and a 1-mL air sample was taken from the jars after 2 h and sampled for ethylene. Values represent means of at least eight individual fruit. Vertical bars represent se. B, Ripening-related gene expression in AC, Gr, and Nr-2 NILs during fruit ripening. Total RNA was extracted from fruit of stages 1 to 5 as defined above and 10 μg was subjected to RNA gel-blot analysis and hybridized to DNA probes for the ripening-related genes E4, E8, polygalacturonase, and phytoene synthase (PSY1). C, Ethylene responsiveness of AC, Gr, Nr-2, and Nr mature green fruits. Total RNA was extracted from mature green fruits of each genotype treated with 20 μL L−1 of ethylene (+) or held in air (−) for 16 h. A total of 15 μg was subjected to RNA gel-blot analysis and hybridized to a DNA probe for the ethylene-regulated gene E4.
The onset of fruit ripening in tomato is characterized by rapid changes in gene expression that ultimately result in the fully ripened fruit. The regulation of ripening-related changes in gene expression is not fully understood, but in tomato, several genes are coregulated by ethylene-independent and ethylene-dependent signaling pathways (for review, see Giovannoni, 2001, 2004). We examined the accumulation of transcripts for the ripening-related, ethylene-regulated genes encoding polygalacturonase, phytoene synthase, E4, and E8 in normal and mutant fruits (Fig. 2B). The transcripts of all of these genes increased at the onset of ripening in AC fruits (stage 2) as expected but were greatly reduced in both Gr and Nr-2 fruits throughout ripening. E4 expression during ripening has been shown to be entirely due to increased ethylene biosynthesis (Lincoln et al., 1987) and corresponding transcripts were absent in Gr and Nr-2 fruits. Furthermore, treatment of mature green fruits with ethylene failed to induce E4 expression in mutant fruit but transcripts were induced in AC (Fig. 2C). These data indicate that inhibition of ripening in Gr and Nr-2 is caused by reduced ethylene sensitivity in mutant fruit.
Gr and Nr-2 Effects in Nonfruit Tissues
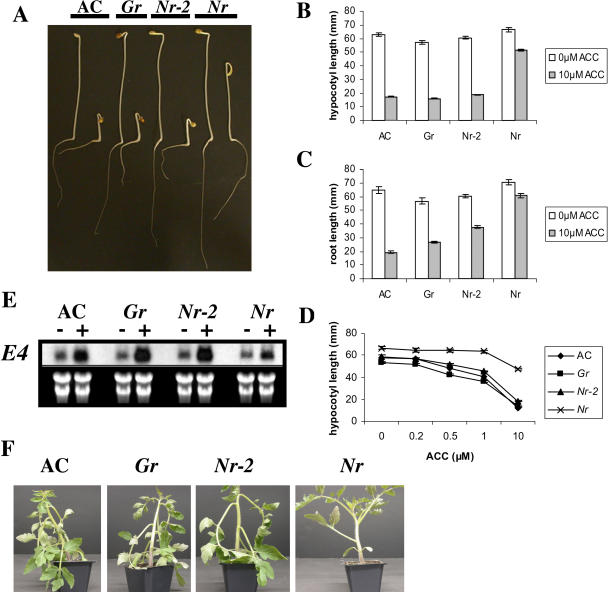
Given that Gr and Nr-2 appear to exhibit ethylene insensitivity in fruit tissues, we examined ethylene responses in nonfruit tissues and compared phenotypes to those of the Nr mutant. Treatment of dark grown seedlings with the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) results in development of the triple response phenotype characterized by a shortening and thickening of the hypocotyl and root in addition to enhanced curvature of the apical hook. The hypocotyls of dark grown AC control, Gr, and Nr-2 seedlings all showed a similar response to growth on 10 μm ACC, and as expected (Lanahan et al., 1994), Nr hypocotyls in the AC background showed partial insensitivity (Fig. 3, A and B). However, the roots of Gr and Nr-2 seedlings grown on ACC were intermediate in length compared to AC and Nr (Fig. 3, A and B), indicative of weak ethylene insensitivity. To assess whether hypocotyls of mutant seedlings also possessed weak ethylene insensitivity that was masked by treatment with 10 μm ACC, we performed a dose response experiment using concentrations of ACC ranging from 0.2 to 10 μm. The shortening of hypocotyl length in response to increasing concentrations of ACC was essentially the same in AC, Gr, and Nr-2 genotypes, indicating no alteration in ethylene responsiveness in this tissue (Fig. 3D). In contrast, Nr seedlings followed a typical ethylene-insensitive growth pattern displaying a greatly reduced inhibition of hypocotyl growth. The ethylene responsiveness of Gr and Nr-2 seedlings was also examined at the molecular level. Treatment of seedlings with 10 μm ACC caused a similar increase in E4 expression in AC, Gr, and Nr-2 genotypes, but this induction was reduced in Nr seedlings (Fig. 3E).
Figure 3.
Seedling triple response and petiole epinasty in AC, Gr, Nr-2, and Nr. A, Triple response phenotype of AC, Gr, Nr-2, and Nr seedlings. Seeds were surface sterilized and sown on 1% water agar in the absence (−) or presence (+) of 10 μm ACC and incubated at 25°C in the dark for 7 d. Quantification of ethylene-induced inhibition of hypocotyl (B) and root (C) lengths. Wild-type and mutant seedlings were sterilized and grown as in A. Data is presented as the mean of at least 21 individual seedlings. Vertical bars represent se. D, Dose response curve of hypocotyl growth on ACC. Seeds were treated and grown as in A, except that five different ACC concentrations (0, 0.2, 0.5, 1, and 10 μm) were used. Following growth in the dark for 7 d, hypocotyl lengths were determined. Each data point is the result of measurements of at least 24 seedlings. Vertical bars represent se. E, Ethylene responsiveness of AC, Gr, Nr-2, and Nr seedlings. Total RNA was extracted from 7-d-old dark grown seedlings grown in the presence (+) or absence (−) of 10 μm ACC. A total of 20 μg was subjected to RNA gel-blot analysis and hybridized to a DNA probe for the ethylene-regulated gene E4. F, Petiole epinasty in AC, Gr, Nr-2, and Nr plants. Four-week-old plants were sealed in airtight chambers for 16 h in the presence of 20 μL L−1 of ethylene.
Tomato plants exposed to ethylene or water logging display petiole epinasty. We treated young plants with 20 μL L−1 of ethylene for 16 h (Fig. 3F). Both Gr and Nr-2 displayed phenotypes identical to that of the AC control lines, suggesting normal petiole ethylene responsiveness.
Gr and Nr-2 Have Altered Patterns of Floral Senescence and Abscission
In tomato, the senescence and abscission of floral organs is regulated in part by ethylene (Lanahan et al., 1994; Llop-Tous et al., 2000). We examined the floral phenotypes of Gr and Nr-2 and compared them to AC and Nr flowers. As expected, petal senescence was rapid in AC flowers both in the presence and absence of pollination and was slightly delayed in Nr with petals remaining attached to young developing fruit following successful pollination. In many instances, we observed petal retention in flowers of the Gr NIL and also in LA2455 flowers, the parental homozygous for Nr-2. These phenotypes are indicative of reduced ethylene responsiveness but were variable and difficult to quantify. They were also weaker than the phenotypes typically associated with Nr flowers (data not shown).
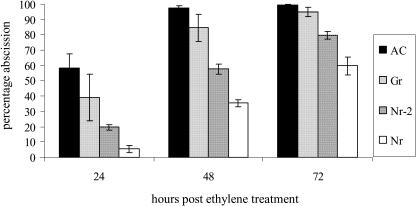
Treatment of tomato flowers with exogenous ethylene results in abscission at the pedicel abscission zone. This phenomenon is rapid in wild-type flowers and substantially delayed in Nr (Lanahan et al., 1994). We compared the ethylene responsiveness of pedicel abscission zones of the Gr and Nr-2 NILs to that of AC and Nr flowers (Fig. 4). As expected, almost 100% abscission occurred in AC flowers exposed to ethylene for 48 h. In contrast, abscission in Nr flowers was substantially delayed with only 60% abscission after 72 h. The phenotypes of the Gr and Nr-2 NILs were consistently intermediate between AC and Nr although the ethylene response shows greater inhibition in Nr-2 with approximately 80% abscission occurring after 72 h.
Figure 4.
Ethylene responsiveness of the pedicel abscission zone in AC, Gr, Nr-2, and Nr. Flower trusses were removed from plants and the cut ends immersed in water in conical flasks. Flasks were sealed in airtight chambers in 20 μL L−1 of ethylene and the percentage abscission was monitored every 24 h for 3 d. Data shown is derived from the average of three independent experiments. Total number of flowers examined for each genotype ranged from 134 to 163. Vertical bars represent se.
Genetic Mapping of Gr and Nr-2 Loci
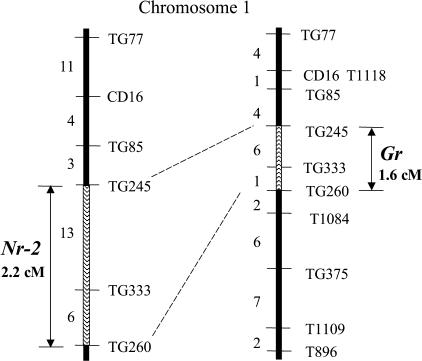
As a first step toward isolation of the Gr and Nr-2 loci using positional cloning strategies, we generated F2 populations segregating for normal and nonripening fruit between S. lycopersicum (Gr/Gr) × Solanum cheesmaniae (gr/gr) and S. lycopersicum (Nr-2/Nr-2) × S. cheesmaniae (nr-2/nr-2). Pooled-sample mapping (Giovannoni et al., 1991) was utilized to define linkage of normal ripening individuals to RFLP loci previously selected for polymorphism of parental alleles. This analysis revealed that both loci were linked to the long arm of chromosome 1 (data not shown). Maps were subsequently refined using DNA from individual F2 plants and additional chromosome 1 RFLP markers. Both loci were found to reside on overlapping regions of chromosome 1 between the RFLP markers TG260 and TG245 (Fig. 5).
Figure 5.
Genetic mapping of Gr and Nr-2 loci to tomato chromosome 1. Populations were constructed as described in the text. The Gr map is based upon a population of 220 F2 individuals. The Nr-2 map is based upon a population of 438 F2 individuals. The position of each locus between the flanking markers TG260 and TG245 is indicated by hatched bars and the distances between these markers for each population is given in centiMorgans. RFLP markers are depicted on the right of each map and the number of recombinant plants between each adjacent marker is shown on the left.
DISCUSSION
Inhibition of Ripening in Gr and Nr-2 Is Due to Ethylene Insensitivity
Previously described mutants that inhibit fruit ripening in tomato share two common phenotypic characteristics: an inability of ethylene to restore the ripening process and reduced expression of ripening-related genes (Tigchelaar et al., 1978; Lincoln and Fischer, 1988; Picton et al., 1993b; Yen et al., 1995; Thompson et al., 1999). However, these mutants can be grouped into two classes based on their ability to synthesize ethylene during ripening and for the ability of ethylene to restore ripening-related gene expression. The first class is characterized by the rin, nor, and Cnr loci. Ethylene synthesis does not increase during ripening in fruits of these mutants but remains at a baseline level. However, treatment of mutant fruits with ethylene will restore some ethylene-regulated gene expression but not fruit ripening. For these reasons, it has been proposed that these loci regulate an ethylene-independent component of fruit ripening and may govern the competency of the fruits to ripen (Adams-Phillips et al., 2004a; Giovannoni, 2004). The second class of ripening mutant is characterized by the ethylene receptor mutant Nr. Fruits of Nr produce ethylene during ripening, but the expression of ripening-related genes is greatly reduced and cannot be restored by ethylene treatment, indicating that the physiological basis of ripening inhibition is due to reduced ethylene sensitivity (Lanahan et al., 1994).
We have examined the physiological basis for inhibition of fruit ripening in the Gr and Nr-2 mutants of tomato using NILs generated in the AC genetic background. Our results indicate that the fruit phenotype of both Gr and Nr-2 is similar to that of Nr. For example, ethylene synthesis during ripening follows a similar pattern to that of AC control fruits; ethylene-regulated and ripening-related gene expression is reduced in mutant fruits and ethylene treatment fails to restore the expression of the ethylene-regulated gene E4 (Fig. 2) or ripening. These data strongly suggests that inhibition of ripening in Gr and Nr-2 is due to reduced ethylene sensitivity.
Ethylene Signaling Specificity
Our knowledge of the components of the ethylene signaling pathway and their mechanism of action in higher plants has come largely from studies on the model plant Arabidopsis (for review, see Guo and Ecker, 2004 and refs. therein). These components have been isolated based upon altered phenotypes of seedlings grown in the dark in the presence or absence of ethylene, the triple response phenotype. Subsequent analysis has revealed that the majority of these loci have pleiotropic effects on plant ethylene responses. However, a subset including hookless 1, ethylene-insensitive root 1 (eir1), enhanced ethylene response 1 (eer1), and weak ethylene insensitive 2 and 3 (wei2, wei3) have altered tissue-specific ethylene responses (Guzman and Ecker, 1990; Roman et al., 1995; Larsen and Chang, 2001; Alonso et al., 2003). In the examples where these mutant loci have been cloned, they have been shown to encode either downstream components of the ethylene signaling pathway or peripheral components that function in or integrate multiple hormone signaling pathways. For example, hookless 1 encodes a putative N-acetyltransferase that integrates ethylene, auxin, and light signals to control apical hook formation during seed germination (Lehman et al., 1996; Li et al., 2004). EIR1 functions as an auxin transporter and may be a target for ethylene regulation of auxin transport to mediate gravitropic responses in the root (Luschnig et al., 1998). The eer1 mutant results from a loss-of-function mutation in the protein phosphatase 2A A regulatory subunit, RCN1 (Larsen and Cancel, 2003). This gene has also been implicated in regulating auxin responses in roots and abscisic acid signaling in guard cells (Garbers et al., 1996; Kwak et al., 2002) Subsequent biochemical characterization indicated that the PP2A catalytic subunit, PP2A-1C, interacts with the kinase domain of CTR1 but is not a phosphorylation substrate of CTR1. These findings led the authors to postulate that PP2A activity may modulate ethylene responses by altering CTR1 activity (Larsen and Cancel, 2003).
In addition, in a separate study, we provided evidence that the Epinastic (Epi) mutant of tomato regulated a subset of ethylene responses controlling cell growth and expansion but did not influence fruit ripening and abscission (Barry et al., 2001). Similarly, in melon, two loci that regulate ethylene responsiveness during ripening and abscission but not the triple response have been reported (Périn et al., 2002).
Our data suggest that Gr and Nr-2 also affect a subset of ethylene responses regulating fruit ripening, floral senescence, and abscission and root elongation (Figs. 2, 3C, and 4) but not ethylene responses controlling inhibition of hypocotyl elongation or petiole epinasty (Fig. 3, B and F). However, the extent of ethylene insensitivity varies between tissues. For example, ethylene induction of E4 expression is absent in Gr and Nr-2 fruit but can be partially induced in Nr fruit, suggesting that Gr and Nr-2 fruit display a strong inhibition of ethylene responses (Fig. 2C). In contrast, ethylene insensitivity during floral senescence, abscission, and root growth inhibition is weak in Gr and Nr-2 when compared to that displayed in the Nr mutant (Figs. 3 and 4). The cause of this differential responsiveness is currently unknown but is suggestive of varying degrees of penetrance of these dominant mutations in different tissues.
Gr and Nr-2 May Be Allelic
Using RFLP mapping, we have positioned both Gr and Nr-2 onto overlapping regions of the long arm of chromosome 1 to a window of approximately 2 cM (Fig. 5). The overlapping map positions of Gr and Nr-2 coupled with their similar dominant phenotypes raises the possibility that the mutations may be allelic. A number of loci encoding proteins involved in the ethylene response pathway have been mapped onto the tomato genome (Yen et al., 1995; Giovannoni et al., 1999; Adams-Phillips et al., 2004b). One gene corresponding to EIN3-LIKE 3 (EIL3) maps to the long arm of chromosome 1. We mapped this gene in our mapping populations and found that it was not linked to either Gr or Nr-2. Based upon the overlapping map positions and phenotypic similarities of Gr and Nr-2 and the fact that these features are distinct from previously characterized ethylene response loci, it is possible that these mutations encode a novel ethylene-signaling component. The dominance and tight genetic linkage of these loci would confuse efforts to perform a standard genetic test of allelism. Current efforts are focused toward isolation of the GR and NR-2 loci using positional cloning strategies.
MATERIALS AND METHODS
Plant Material and Treatments
Homozygous lines carrying the Gr/Gr and Nr-2/Nr-2 mutations and the wild tomato species Solanum cheesmaniae (accession nos. LA2453, LA2455, and LA483, respectively) were obtained from the Tomato Genetics Resource Center, UC Davis. Homozygous Nr/Nr seed and the parental cultivar AC (nr/nr) were originally obtained from the Glasshouse Crops Research Institute (Littlehampton, Sussex, UK). Plants were grown in peat-based compost supplemented with fertilizer in greenhouses equipped with heating and cooling systems and supplemental lighting at Cornell University. Fruit were harvested at five developmental stages (termed 1–5 in this study). For the AC cultivar, these were mature green (stage1), breaker (stage 2), breaker + 3 d (stage 3), breaker + 7 d (stage 4), and breaker + 10 d (stage 5). To account for the more protracted ripening observed for the Gr and Nr2 NILs fruit were harvested as follows based upon changes in color: mature green (stage 1), early breaker, defined as changes in internal color only (stage 2), breaker (stage 3), yellow (stage 4), and orange (stage 5). Stage 5 fruits were taken at around 60 d postanthesis (DPA). For comparison, stage 5 AC control fruit are approximately 42 DPA.
Experiments on dark grown seedlings were performed as follows. Surface sterilized seeds were sown on 1% water agar supplemented with ACC at 0, 0.2, 0.5, 1, and 10 μm and incubated in the dark for 7 d at 25°C. Ethylene treatment of light grown plants and mature green fruits was performed by sealing the aforementioned in airtight chambers and injecting ethylene to a final concentration of 20 μL L−1 for 16 h. Experiments on floral abscission were performed in the same way except that responses were monitored for up to 72 h following ethylene exposure.
Ethylene Measurements
Ethylene was measured from fruits of different developmental stages by sealing whole fruits in airtight jars for 2 h at 22°C after which a 1-mL sample of the headspace was taken and injected on to a Hewlett-Packard 5890 series II gas chromatograph equipped with a flame ionization detector. Samples were compared to a standard of known concentration and normalized for fruit mass.
RNA Isolation and Gel-Blot Analysis
Total RNA was extracted from the pooled pericarp of three individual fruits of different developmental stages and fractionated through 1% denaturing agarose gels as described by Griffiths et al. (1999). Gels were blotted for 20 h in 10 mm sodium phosphate buffer onto Hybond N membranes (Amersham Biosciences, Piscataway, NJ). Following transfer, membranes were baked at 80°C for 2 h to fix the RNA to the membrane.
Genomic DNA Extraction and Genetic Mapping
Genomic DNA was extracted from fresh meristematic leaves using a microprep isolation protocol modified from Fulton et al. (1995). Approximately six meristematic leaves were placed into a 2-mL screw-cap tube and kept on ice. Samples were homogenized in 290 μL of extraction buffer (0.35 m sorbitol, 0.1 m Tris-base, 5 mm EDTA, pH 7.5, containing 3.8 mg/mL sodium bisulfite) in a Savant FP120 Fast Prep machine. A total of 290 μL nuclear lysis buffer (0.2 m Tris-HCl, pH 8, 0.05 m EDTA, pH 8, 2 m NaCl, 2% (w/v) hexadecyl-trimethyl-ammonium bromide) and 140 μL 5% sodium lauryl sarcosine were added and the samples vortexed and incubated for 40 min at 65°C. A total of 700 μL of chloroform/octanol (24:1) was added and the samples were vortexed and centrifuged at 8,000 rpm for 15 min. The supernatant was transferred to a 1.5-mL microfuge tube and the DNA precipitated using 540 μL of cold isopropanol. DNA was pelleted by microcentrifugation for 10 min at 13,000 rpm and pellets were washed in 70% ethanol and airdried. DNA was resuspended by incubating pellets in 50 μL of sterile distilled water for 10 min at 65°C. Twenty microliters of DNA was digested in a total volume of 30 μL using restriction enzymes supplied by New England Biolabs (Beverly, MA) as per manufacturer's instructions. Digested DNA was fractionated through 1% agarose gels and “nicked” by UV light for 60 s. Gels were blotted in 0.4 n NaOH onto Hybond N+ membranes (Amersham Biosciences). Following transfer, membranes were baked at 80°C for 2 h to fix the DNA to the membrane.
Details of tomato genetic maps and DNA markers can be accessed through the Solanaceous Genomics Network (http://www.sgn.cornell.edu). Restriction enzymes yielding RFLPs between S. lycopersicum and S. cheesmaniae for given DNA probes are as follows: TG77, EcoRV; T896, EcoRI; TG85, TG333, and T1109, HaeIII; TG260, DraI; TG38, AccI; T1118, HinCII; TG375 and T1084, HinFI; CD16 and TG245, αTaqI.
Synthesis of Radiolabeled Probes and Hybridization Conditions
Membranes were hybridized at 65°C to 32P-labeled random primed DNA probes, synthesized as described by Feinberg and Vogelstein (1983), in a buffer containing 5× SSC, 0.5% SDS, 50 mm potassium phosphate buffer, pH 6.5, and 5× Denhardt's solution. Hybridizations were performed for at least 16 h following which the filters were washed in 2× SSC, 0.1% SDS, and then 1× SSC, 0.1% SDS at 65°C. Signal intensity was visualized by autoradiography using Kodak X-OMAT-AR film with two intensifying screens at −80°C.
Acknowledgments
We thank Dr. Chris Watkins and Jackie Nock (Cornell University) for use of their gas chromatograph and Dr. Steve Tanksley (Cornell University) for providing RFLP markers.
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (award no. 2002–35304–12530 to C.S.B. and J.J.G.) and by the U.K. Biotechnology and Biological Sciences Research Council (to G.B.S. and A.J.T.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.057745.
References
- Adams-Phillips L, Barry C, Giovannoni J (2004. a) Signal transduction systems regulating fruit ripening. Trends Plant Sci 9: 331–338 [DOI] [PubMed] [Google Scholar]
- Adams-Phillips L, Barry C, Kannan P, Leclercq J, Bouzayen M, Giovannoni J (2004. b) Evidence that CTR1-mediated ethylene signal transduction in tomato is encoded by a multigene family whose members display distinct regulatory features. Plant Mol Biol 54: 387–404 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solana R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Fox EA, Yen H-C, Lee S, Ying T-J, Grierson D, Giovannoni JJ (2001) Analysis of the ethylene response in the epinastic (epi) mutant of tomato. Plant Physiol 127: 58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby LA, Ritchie DB, Taylor IB (1977) Isogenic lines of the tomato “Ailsa Craig”. Annual Report Glasshouse Crops Research Institute, Littlehampton, UK, pp 168–184
- DellaPenna D, Lincoln JE, Fischer RL, Bennett AB (1989) Transcriptional analysis of polygalacturonase and other ripening associated genes in Rutgers, rin, nor and Nr tomato fruit. Plant Physiol 90: 1372–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B (1983) A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 [DOI] [PubMed] [Google Scholar]
- Fulton T, Julapark C, Tanksley S (1995) Miniprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol Biol Rep 13: 207–209 [Google Scholar]
- Garbers C, DeLong A, Deruere J, Bernasconi P, Soll D (1996) A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J 15: 2115–2124 [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ (2001) Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 52: 725–749 [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell (Suppl) 16: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ, Wing RA, Ganal MW, Tanksley SD (1991) Isolation of molecular markers from specific chromosomal intervals using DNA from existing mapping populations. Nucleic Acids Res 19: 6552–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni J, Yen H, Shelton B, Miller S, Vrebalov J, Kannan P, Tieman D, Hackett R, Grierson D, Klee H (1999) Genetic mapping of ripening and ethylene-related loci in tomato. Theor Appl Genet 98: 1005–1013 [Google Scholar]
- Griffiths A, Barry CS, Alpuche-Solis A, Grierson D (1999) Ethylene and developmental signals regulate expression of lipoxygenase genes during tomato fruit ripening. J Exp Bot 50: 793–798 [Google Scholar]
- Guo H, Ecker JR (2004) The ethylene signalling pathway: new insights. Curr Opin Plant Biol 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett RM, Ho C-W, Lin Z, Foote HCC, Fray RG, Grierson D (2000) Antisense inhibition of the Nr gene restores normal ripening to the tomato Never-ripe mutant, consistent with the ethylene receptor-inhibition model. Plant Physiol 124: 1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson GE, Nichols R, Davies JN, Atkey PT (1984) The inhibition of tomato fruit ripening by silver. J Plant Physiol 116: 21–29 [DOI] [PubMed] [Google Scholar]
- Jarret RL, Tigchelaar EC, Handa AK (1984) Ripening behavior of the Green ripe tomato mutant. J Am Soc Hortic Sci 109: 712–717 [Google Scholar]
- Kerr EA (1958) Mutations of chlorophyll retention in ripe fruit. Rep Tomato Genet Coop Tomato Genet Coop 8: 22 [Google Scholar]
- Kerr EA (1982. a) Never ripe-2 (Nr-2) a slow ripening mutant resembling Nr and Gr. Rep Tomato Genet Coop Tomato Genet Coop 32: 33 [Google Scholar]
- Kerr EA (1982. b) New residents on chromosome 1. Rep Tomato Genet Coop Tomato Genet Coop 32: 16–17 [Google Scholar]
- Kwak JM, Moon JH, Murata Y, Kuchitsu K, Leonhardt N, DeLong A, Schroeder JI (2002) Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in Arabidopsis. Plant Cell 14: 2849–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ (1994) The Never ripe mutation blocks ethylene perception in tomato. Plant Cell 6: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Cancel JD (2003) Enhanced ethylene responsiveness in the Arabidopsis eer1 mutant results from a loss-of-function mutation in the protein phosphatase 2A A regulatory subunit, RCN1. Plant J 34: 709–718 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Chang C (2001) The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiol 125: 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyls. Cell 85: 183–194 [DOI] [PubMed] [Google Scholar]
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR (2004) Convergence of signalling pathways in the control of differential cell growth in Arabidopsis. Dev Cell 7: 193–204 [DOI] [PubMed] [Google Scholar]
- Lincoln JE, Cordes S, Read E, Fischer RL (1987) Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci USA 84: 2793–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln JE, Fischer RL (1988) Regulation of gene expression by ethylene in wild-type and rin tomato (Lycopersicon esculentum) fruit. Plant Physiol 88: 370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop-Tous I, Barry CS, Grierson D (2000) Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol 123: 971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunders M, Holdsworth M, Slater A, Knapp J, Bird C, Schuch W, Grierson D (1987) Ethylene stimulates the accumulation of ripening-related mRNAs in tomatoes. Plant Cell Environ 10: 177–184 [Google Scholar]
- Oeller PW, Wong LM, Taylor LP, Pike DA, Theologis A (1991) Reversible inhibition of tomato fruit senescence by antisense 1-aminocyclopropane-1-carboxylate synthase. Science 254: 437–439 [DOI] [PubMed] [Google Scholar]
- Périn C, Gomez-Jimenez MC, Hagen L, Dogimont C, Pech J-C, Latché A, Pitrat M, Lelièvre J-M (2002) Molecular and genetic characterization of a non-climacteric phenotype in melon reveals two loci conferring altered ethylene response in fruit. Plant Physiol 129: 300–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D (1993. a) Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. Plant J 3: 469–481 [Google Scholar]
- Picton S, Gray J, Barton S, AbuBakar U, Lowe A, Grierson D (1993. b) cDNA cloning and characterization of novel ripening-related mRNAs with altered patterns of accumulation in the ripening inhibitor (rin) tomato ripening mutant. Plant Mol Biol 23: 193–207 [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber J, Rothenberg M, Ecker J (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour G, Taylor J, Tucker G, eds (1993) Biochemistry of Fruit Ripening. Chapman and Hall, London
- Thompson AJ, Tor M, Barry CS, Vrebalov J, Orfila C, Jarvis MC, Giovannoni JJ, Grierson D, Seymour GB (1999) Molecular and genetic characterisation of a novel pleiotropic tomato-ripening mutant. Plant Physiol 120: 383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ (2001) Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J 26: 47–58 [DOI] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ (2000) The tomato ethylene receptors NR and LeETR4 are negative regulators of the ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA 97: 5663–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigchelaar EC, McGlasson WB, Buescher RW (1978) Genetic regulation of tomato fruit ripening. HortScience 13: 508–513 [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296: 343–346 [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ (1995) An ethylene-inducible component of signal transduction encoded by Never-ripe. Science 270: 1807–1809 [DOI] [PubMed] [Google Scholar]
- Yen H, Lee S, Tanksley S, Lanahan M, Klee HJ, Giovannoni JJ (1995) The tomato Never-ripe locus regulates ethylene-inducible gene expression and is linked to a homologue of the Arabidopsis ETR1 gene. Plant Physiol 107: 1343–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegzouti H, Jones B, Frasse P, Marty C, Maitre B, Latché A, Pech J-C, Bouzayen M (1999) Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J 18: 589–600 [DOI] [PubMed] [Google Scholar]