Abstract
Approved therapies for tendon diseases have not yet changed the clinical practice of symptomatic pain treatment and physiotherapy. This review article summarizes advances in the development of novel drugs, biologic products, and biomaterial therapies for tendon diseases with perspectives for translation of integrated therapies. Shifting from targeting symptom relief toward disease modification and prevention of disease progression may open new avenues for therapies. Deep evidence-based clinical, cellular, and molecular characterization of the underlying pathology of tendon diseases, as well as therapeutic delivery optimization and establishment of multidiscipline interorganizational collaboration platforms, may accelerate the discovery and translation of transformative therapies for tendon diseases.
TENDON IN HEALTH AND DISEASE
Tendons connect muscle to bone and are critical for joint and body part movements. The hallmark of tendon function to enable joint movement is force transmission through its dense and extensive network of extracellular matrix (1). The tendon extracellular matrix consists of highly organized hierarchical and heterogeneous fibrils and fibers of collagens, proteoglycans, elastin, and fibronectins, deposited and organized by tenocytes, which are strategically well positioned within the extracellular matrix (2). Tendon extracellular matrix is one of the strongest and most fatigue-resistant structures of the human body. Although many tendons operate within the toe region of the stress-strain curve during routine function and experience a wide range of in vivo strains and strain rates, with different benchmarks in tension and compression (2), peak forces may exceed 3500 N in humans (70% of its maximum load) (3).
Tendon extracellular matrix undergoes constant regeneration and remodeling. A disturbed state of homeostasis in response to tendon chronic overuse or concomitant risk factors is considered a common driver of tendon disease and is described in several pathogenesis theories focusing on mechanical (4), inflammatory (5), apoptotic (6), or vascular or neurogenic (7) aspects or an integrated pathogenetic continuum (8). Tendon disease is characterized by persistent pain and impaired joint function, which without resolution will lead to restricted mobility, disease progression, tendon rupture, and long-term disability of patients (9). Tendon disease and its long-term consequence on patient mobility are not restricted to tendon, however, and could progress into generalized musculoskeletal disease because the morphology and function of connected muscle and bone tissues are affected by tendon disease, such as fatty infiltration, degeneration of muscle, and reduction in mineral density of bone (10).
Chronic overuse and the consequences of a disturbed state of equilibrium are proposed to result in gradual accumulation of extracellular matrix damage and disorganization over time (11), including altered collagen content, glycosaminoglycan deposition, lipid accumulation, and heterotopic ossification (12). Associated nonresolving inflammatory responses are considered to further drive the progression of tendon disease ultimately toward tendon rupture (13). In contrast to native uninjured tendon, healing tendon exhibits hypercellularity, matrix disorganization, cell rounding, and several other phenotypic differences (Fig. 1A).
Fig. 1. Tendon structure and function in health and disease.
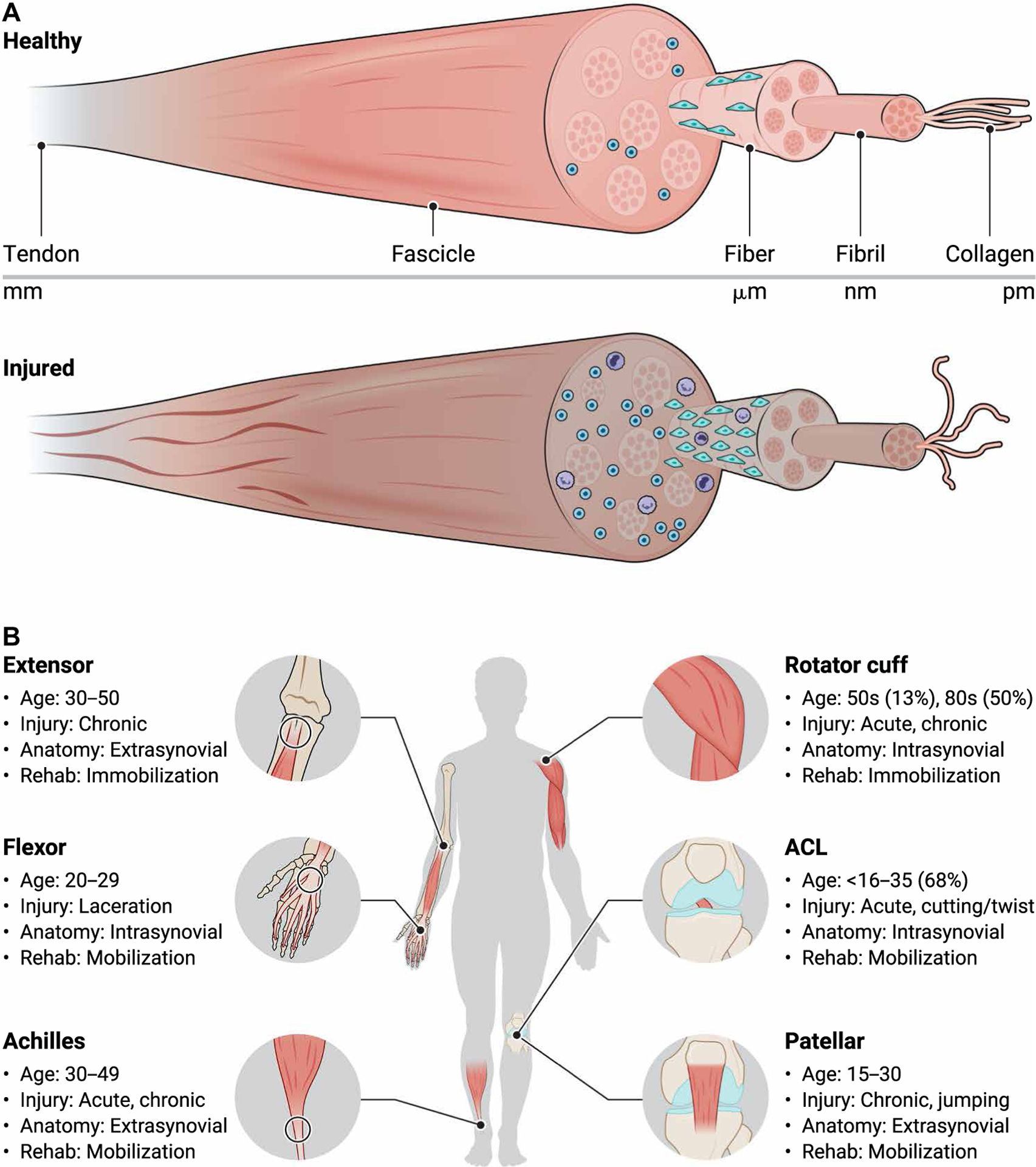
(A) Tendons have a highly organized hierarchical structure that includes the tendon, fascicle, fiber, fibril, and collagen levels. Tendon cells, termed tenocytes, exist in highly organized spindle-like arrays between tendon fibers. Compared to healthy tendon, diseased tendon has elevated collagen disorganization, smaller fibers, hypercellularity, increased cell rounding, elevated presence of immune cells, and increased denatured collagen. (B) Common tendon and ligament injuries including the lateral epicondyle, flexor tendon, Achilles tendon, rotator cuff tendons, cruciate ligaments, and patellar tendon. Injury type, severity, and physical therapy vary across tendon anatomical location and age. ACL, anterior cruciate ligament.
Tendon homeostasis is orchestrated by inflammatory mediators such as cytokines [e.g., interleukin-1β (IL-1β), tumor necrosis factor–α (TNF-α), IL-6, IL-17, and IL-33] and their effector biology on immune and tendon cells (e.g., regeneration and remodeling of extracellular matrix) (14, 15). Inadequate and nonresolving inflammatory responses to tendon trauma have been suggested to drive the molecular and cellular pathology of tendon disease via cytokine signaling pathways [e.g., mitogen-activated protein kinases (MAPKs), extracellular signal–regulated kinase (ERK), nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB), and Janus kinase and signal transducer and activator of transcription (JAK-STAT)] (16). Anatomical compartment specialization, such as the presence or absence of a tendon sheath and the presence or absence of a synovial environment, provides additional physiological and pathophysiological cues for immune modulation of tendon homeostasis (Fig. 1B).
Concomitant to chronic overuse, various risk factors are proposed to disturb tendon homeostasis and to tip the scale from tendon regeneration toward pathogenesis and disease progression, such as aging (17), metabolic disease (18), compression stress (19), and genetics (17). Although there is no direct genetic link associated with tendon disease, a substantial number of genes and gene signatures are differentially regulated and are associated with immune modulation (e.g., STAT3, JAK3, IL4R, IL13RA2, and S100A10), extracellular matrix integrity [e.g., collagen-1A1 (COL1A1), COL1A2, COL3A1, tenacin-C (TNC), and matrix metalloproteinase-2 (MMP2)], and tissue regeneration [e.g., fibroblast growth factor–1 (FGFR1), Wnt1-inducible signaling pathway protein 1 (WISP1), Dickkopf-related protein-3 (DKK3), WNT3, and lymphoid enhancer-binding factor 1 (LEF1)] (20).
CURRENT CLINICAL PRACTICE TO TREAT TENDON DISEASE
Physical therapy
Physical therapy is widely applied to support rehabilitation of tendon disease due to the proregenerative responsiveness of tendons to mechanical loading (10). Advanced rehabilitation protocols specific to tendon anatomy and injury (21, 22) are being defined in animal and human studies (23, 24). Although certain tendons are known to benefit from early rehabilitation to recover a range of motion (e.g., flexor tendon), other tendons (e.g., rotator cuff) might require immobilization. Treatment of chronic Achilles tendinopathy consists of early rehabilitation with eccentric strengthening protocols; however, intractable cases can result in continued symptoms and need for surgical intervention (25). When treated conservatively, eccentric strengthening protocols are superior to concentric strengthening protocols (1).
Surgical interventions
Repair surgery of tendon full rupture injury is the primary therapeutic intervention with >300,000 rotator cuff repair surgeries performed per year (26). The therapeutic outcome of rotator cuff repair surgery has several limitations as many patients (20 to 94%) suffer from postoperative complications such as rerupture (27), elongation (28), muscle atrophy (29), sustained impaired shoulder function (27), and poor reconstitution of the tendon-bone interface (30). Surgical repair of Achilles tendon rupture has recently also become more controversial as studies demonstrated that repair surgery is not superior to nonsurgical therapy with respect to reduction of rerupture rates (31) or improved tendon tissue properties (32). In addition to surgical repair, other interventional approaches include ligament reconstruction for anterior cruciate ligament (ACL) injuries (33), tissue debridement (34) for elderly patients where the primary focus is pain alleviation, and reverse total shoulder arthroplasty (34), tendon transfers (34), Mihata’s superior capsular reconstruction (35), or implantation of a subacromial spacer (36) for large-to-massive irreparable rotator cuff tears.
Molecular and cellular therapeutic modalities
Therapeutic modalities are commonly classified by their molecular size, their synthetic or biological origin, and their level of specification (i.e., low–molecular weight chemicals, biologics, biological products, and gene and cell therapy, not excluding functionalized biomaterials). Classical pharmacological therapeutics are based on chemicals and biologics typically of specific molecular structure and mechanism of action. Chemicals are typically orally available, whereas biologics such as peptides, growth factors, or antibodies typically require parenteral administration (i.e., subcutaneous, intravenous, or intramuscular injection) (37). Similarly, biological products and cell and gene therapies require parenteral administration.
Because the tendon proper is poorly vascularized, therapeutics for tendon diseases are preferentially applied locally by peritendon administration. Local administration of therapeutics offers advantages and opportunities such as increases in systemic safety window, staggered and extended duration of activity, and combination of therapeutics with additional functionalities of biomaterials. However, locally restricted therapy also introduces considerable complexity to type, timing, dosage, and delivery method for therapeutic treatment (38).
In the following section, we describe the molecular and cellular therapies in clinical use for tendinopathy and tendon injury. We detail the mechanism of action, indications, dosing, route of administration, and available safety/efficacy data.
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently used to treat tendon pain (Table 1). The classical NSAIDs are nonselective inhibitors of all cyclooxygenase (COX) subtypes and block the enzymatic conversion of arachidonic acid into prostaglandins, prostacyclin, and thromboxane (39), thereby reducing inflammation and causing antipyretic, antithrombotic, and analgesic effects. NSAIDs are administered orally (ibuprofen, aspirin, and naproxen), topically (diclofenac), and parenterally (ibuprofen) (40). Evidence demonstrates that nonselective NSAIDs provide short-term pain relief without a negative effect on tendon regeneration (41). In contrast, there is emerging evidence that the newer class of NSAIDs selectively inhibiting COX-2 (e.g., Celebrex, Vioxx, and Bextra) can negatively affect tendon regeneration (41).
Table 1. Current therapies in clinical use for tendon diseases.
Examples of products of a given therapeutic modality and mechanism of action are provided. GCR, Glucocorticoid Receptor.
| Therapeutic modality | Molecular/biological mechanism of action | Product | Company | Launch |
|---|---|---|---|---|
| Chemical drug | NSAID/anti-inflammatory, analgesic | Indocin (indomethacin) | GSK | 1965 |
| Mesulid (nimesulide) | Boehringer Ingelheim, GSK, Merck, Pfizer | 1985 | ||
| Aulin (nimesulide) | Sanofi | 1990 | ||
| Duraprox (oxaprozin) | Aventis | 2002 | ||
| Felbinac (felbinac) | Toko Pharmaceutical Industrial | 2018 | ||
| Chemical drug | Corticosteroid/anti-inflammatory, analgesic | Kenalog (triamcinolone acetonide) | Apothecon | 1978 |
| Chemical drug | Cox-2 inhibitor/anti-inflammatory, analgesic | Celebrex (celecoxib) | Astella Pharma | 2009 |
| Chemical drug | GCR agonist/anti-inflammatory, analgesic | Lederlon (triamcinolone hexacetonide) | Esteve | 2001 |
| Lederspan (triamcinolone hexacetonide) | Mylan | |||
| Biological product PRP | Not specified/tissue regeneration | Terumo BCT (PRP) | Terumo | 2010 |
| Cell therapy | Not specified/tissue regeneration | Ortho-ATI (autologous tendon cells) | Johnson & Johnson, Orthocell | 2010 |
| Biomaterial | Not specified/viscosupplement | CartiZol (atelocollagen) | Sewon Cellontech | 2007 |
| Ostenil (hyaluronan) | TRB Chemedica | 2012 | ||
| OrthoVisc (hyaluronan) | Anika Therapeutics | 2016 |
Corticosteroids and glucocorticoid receptor agonists
Synthetic corticosteroids (glucocorticoids) are in wide clinical use for anti-inflammatory pain management and are administered orally, parenterally, or by iontophoresis (Table 1). Glucocorticoids mediate their anti-inflammatory effects by down-regulation of the gene expression of proinflammatory proteins (transrepression); however, they also induce gene expression of certain other genes (transactivation) and exert nongenomic pathway modulation (42, 43).
In Achilles and rotator cuff tendinopathy, glucocorticoid treatment was shown to provide transient pain relief (44, 45), and in lateral elbow tendinopathy, glucocorticoid treatment improved pain and function (46). However, beyond transient pain relief, the overall therapeutic benefit of glucocorticoids in tendinopathy remains controversial, and glucocorticoids are under scrutiny as potentially supporting the progression of tendon disease. In vitro work suggests harmful disruption of the state of tendon equilibrium, evidenced by decreased viability and increased apoptosis of isolated human tenocytes (47), along with loss of fibroblastic appearance and decreased expression of the structural collagen type I (48).
Platelet-rich plasma
Platelet-rich plasma (PRP), an autologous blood product, is in clinical use to promote tendon regeneration in patients with tendinopathy and tendon injury (Table 1). After activation by exposure to thrombin and collagen, platelets release growth factors such as transforming growth factor–β (TGF-β), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) (49). Given that interaction of several different growth factors may be important in tendon healing, blood therapies intend to mimic these attributes by providing high concentrations of growth factors that promote tendon regeneration. Whole-blood injection (50), PRP (25), and leukocyte-rich PRP (51) are suggested to have clinical benefit; however, undisputed evidence for the therapeutic benefit of PRP on tendon regeneration in patients with tendon disease is pending (52).
Cell therapies
Autologous tendon cell therapies are in clinical use to promote tendon regeneration in patients with tendinopathy and tendon injury (Table 1). The interest in cell therapy is motivated by the low cellularity and regeneration capacity of tendon (10). The delivery of supplemental tendon-derived cells may promote tendon regeneration by tenocyte differentiation and proliferation, extracellular matrix deposition and remodeling along with stimulation of angiogenesis, and proregenerative immune modulation (53). In noncontrolled studies, injected autologous tendon–derived cells were shown to express CD44, STRO-1, and CD90, along with Col1a1 and Scx, indicating tenocyte differentiation and proliferation (54, 55).
Autologous tendon injection (ATI) (Ortho-ATI, Orthocell, Johnson & Johnson) is currently marketed in Australia. Ortho-ATI isolates cells from a biopsy of healthy superficial patellar tendon and expands them for 3 weeks before injection into diseased tendon. Results show that ATI increased grip strength, decreased pain, and decreased magnetic resonance (MR) T2 signal 1 and 4.5 years after injection in patients with severe refractory lateral epicondylitis (55).
Functional biomaterials
Biomaterials functionalized as viscosupplements or as mechanical grafts are in clinical use to augment tendon repair and to alleviate pain in patients with tendinopathy and tendon injury (Table 1). Viscosupplements provide local lubrication to tendon and reduce friction with adjacent tissues. Viscosupplements such as CartiZol (Sewon Cellontech) and OrthoVisc (Anika Therapeutics) are suggested to relieve pain and promote tissue gliding.
Naturally derived extracellular matrix such as dermis (GraftJacket) and small intestinal submucosa containing growth factors (CuffPatch) (56) as well as synthetic materials such as a bioabsorbable urethane urea (Artelon) are used as tendon grafts (57, 58). However, a Clinical Practice Guideline has concluded that “inconclusive,” “weak,” or “limited” evidence exists for grafts, PRP, or marrow stimulation to augment Achilles tendon (59) or rotator cuff tears (60), motivating the need for novel therapeutic paradigms for tendon diseases. Although viscosupplements, grafts, suture anchors (36), suture tape (61), and subacromial balloon spacers (36) are used clinically, they are not further addressed in this review article.
CURRENT REGULATORY PRACTICE AND GUIDELINES
The primary governing agencies ensuring the safety, efficacy, and security of drugs, biological products, and medical devices are the U.S. Food and Drug Administration (FDA; USA), European Medicines Agency (EMA; Europe), and Pharmaceuticals and Medical Devices Agency (PMDA; Japan). In addition, the Anatomical Therapeutic Chemical (ATC) Classification System is used to classify the active ingredients of drugs based on the organ system that they act. For the musculoskeletal system, ATC code “M” includes anti-inflammatory and antirheumatic products, topical products for joint and muscular pain, muscle relaxants, antigout preparation, and drugs for the treatment of bone diseases.
Several regulatory agencies provide published guidelines for important study considerations and endpoints. These documents are published by ASTM International (formerly the American Society for Testing and Materials), FDA, EMA, and the International Organization for Standardization (ISO) (62). The FDA approval process may take several years (63, 64) depending on the classification of the product (65) (Fig. 2). A cell therapy may be regulated as human cells, tissue, and cellular- and tissue-based products (HCT/Ps) if it meets certain criteria in 21 Code of Federal Regulations (CFR) 1271.10(a); otherwise, it is regulated as a biological product under Section 351. An example of an HCT/P regulated as a biological product is fascia lata allografts used for ACL defects. After preclinical evaluation for the safety of biological products, an investigational new drug (IND) is submitted to study the biological product in humans. If successful, a biologics license application (BLA) is submitted.
Fig. 2. Regulatory approval process and development timelines.
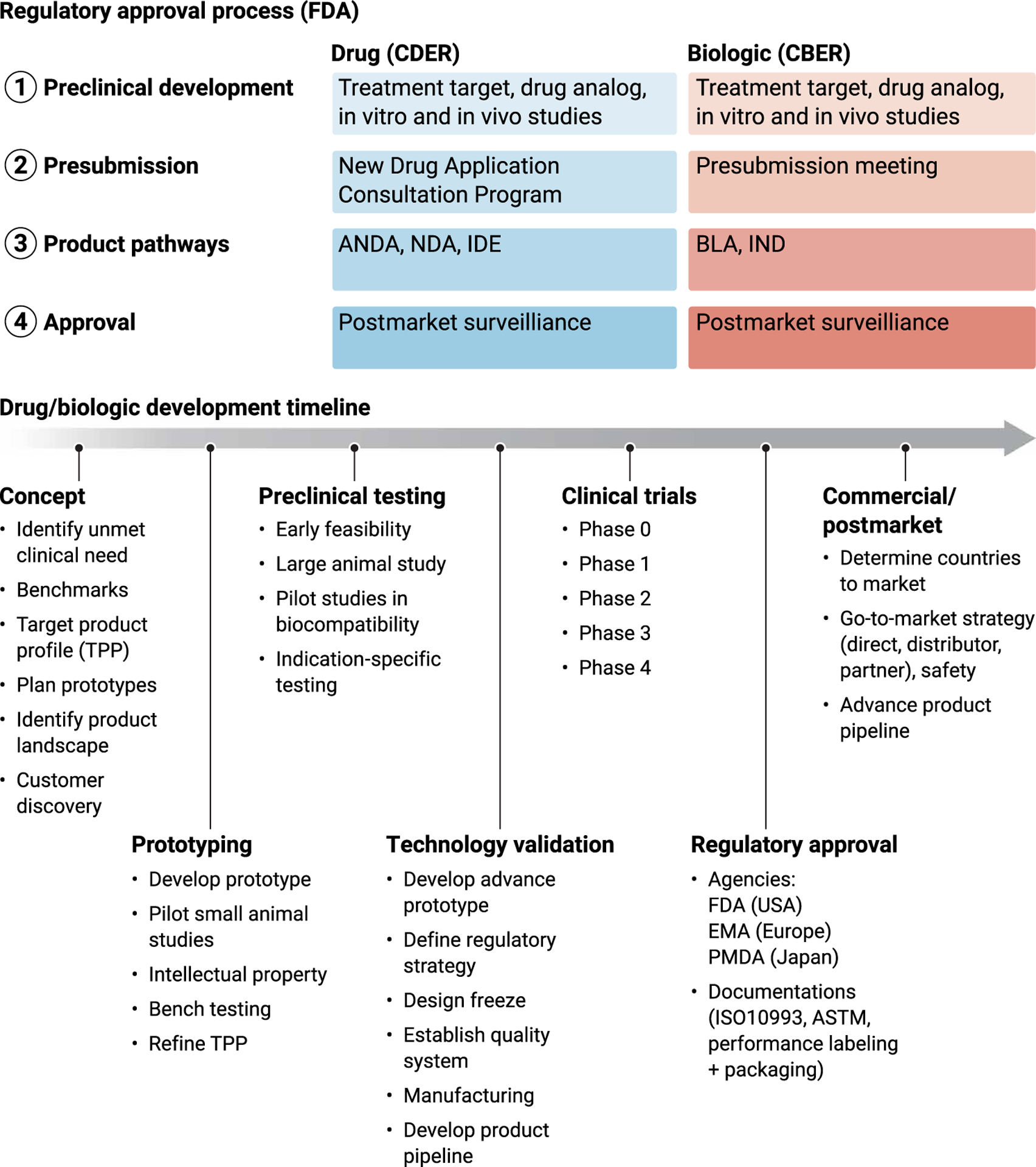
When developing new therapies to augment tendon, several steps are necessary for ultimate translation. For regulatory approval of new therapies, the agency and approval pathways at the FDA are dependent on whether the therapy is a drug or biologic. CDER, Center for Drug Evaluation and Research; CBER, Center for Biologics Evaluation and Research; ANDA, abbreviated new drug application; NDA, new drug application; IDE, investigational device exemption; BLA, biologics license application; IND, investigational new drug; FDA, Food and Drug Administration; EMA, European Medicines Agency; PMDA, Pharmaceuticals and Medical Devices Agency; ASTM, American Society for Testing and Materials; TPP, target product profile.
New biologic and drug therapies should seek guidance from the Center for Biologics Evaluation and Research (CBER), Center for Drug Evaluation and Research, or the Office of Combination Products (OCP) at the FDA for presubmission meetings before submission for approval. Novel cell therapies, human cell and tissue products, and combination products associated with a serious illness and unmet clinical need may qualify for Breakthrough or Regenerative Medicine Advanced Therapy (RMAT) designations. Both Breakthrough and RMAT designations have the benefits of fast track, including early interactions between the agency and sponsors. RMAT only requires preliminary clinical evidence of superiority over existing therapies.
Although intended to provide useful guidance, adherence to guidelines provided by these agencies varies greatly in preclinical studies (66). However, strict enforcement of biocompatibility and performance are required for overall approval per ISO 10993 and other performance standards set by the FDA and EMA. It may be possible to shorten the timeline to regulatory approval and to increase human translation by better incorporating themes from guidance documents published by the ISO, ASTM, FDA, and EMA that shift the focus of basic researchers toward considering translational feasibility earlier in the study planning process. For example, in cartilage repair and regeneration, most basic science studies being performed do not complete the full spectrum of recommended guidelines for bench/performance/preclinical testing (66), which limits their translational potential. However, these documents only highlight certain testing and performance standards and do not address the underlying limitations of models of disease states and functionality of a therapeutic within these different systems.
ADVANCES TOWARD TRANSFORMATIVE THERAPIES FOR TENDON DISEASES
Novel integrated therapies
Novel integrated therapies are beginning to target the promotion of tendon tissue regeneration and restoration of tendon function by providing extracellular matrix–inspired biophysical cues and by modulation of the immune response for adequate resolution of inflammation. These therapies are typically formulated as injectable or biomaterial-based delivery systems. The novel integrated therapy concept specifically includes the combination of drug and biological product therapies with biomaterials for additional synergistic therapeutic benefits. Their promise is supported by evidence that, similar to the extracellular matrix, biophysical cues present in biomaterials—such as strain stiffening (67), elasticity (68), viscoelasticity (69, 70), poroelasticity (71), porosity (72), cell adhesion ligands (53), mechanical loading (73, 74), and fiber orientation—are of critical importance for adequate cell infiltration, cell differentiation (75), cell alignment (76–78), matrix deposition (79, 80), and cell migration (81). Accordingly, biomaterials are also suggested as cell grafts for local administration of progenitor or stem cells to injured tendons, thus not only providing cues to promote differentiation and progression into appropriate tenogenic cell fate and cell state but also to shield grafted cells against immune defense responses (82).
Biomaterial-based delivery systems such as hydrogels can serve as sustained release depots (83, 84) and can also incorporate chemical, physical, and electrical charge interactions to control release, to restrict distribution, and to prevent degradation of drug and biologic product therapies. Local extended release and locally restricted effects using biomaterials may overcome the limitations of current therapies applied systemically or by repeated local injection. Local single administration of biomaterial-based delivery systems is aiming to minimize systemic exposure and/or distribution and thus reduce the risk of systemic safety liabilities. Furthermore, biomaterial-based single-injection delivery systems should reduce the incidence of infections and adverse local tissue reaction, which are inevitably increased with repeated injection regimens. In consequence, biomaterial-based delivery systems should also reduce the rate of therapy failure due to inappropriate patient adherence (85–88).
Biomaterials are typically designed with added functional cues to the delivery system to target for synergistic therapeutic benefits in combination with other therapies. Biomaterials with additional mechanical and structural cues were shown preclinically to provide mechanical tendon augmentation for several weeks after surgery (28), to have slow degradation, and to allow endogenous cells to infiltrate (2). Previous pioneering work in the osteology field using decellularized matrices to promote bone formation (89) has inspired biomaterial science to discover silk (90), synthetic [e.g., polycaprolactone (PCL), poly-d, l-lactic-co-glycolic acid (PLGA), and poly-l-lactic acid)] (91), and collagen-based (92) biomaterials to promote tendon regeneration. These biomaterials recapitulate certain structural alignment (93), fiber diameter (94), and mechanics (95) of native tendon extracellular matrix, but they have not yet advanced to clinical development neither as therapeutic biomaterials alone nor in combination with other therapies (96).
Biomaterials with viscosupplementation are typically based on hyaluronic acid. Increasing clinical evidence suggests that peritendon injection of hyaluronic acid may reduce scar formation after tendon injury, reduce adhesions and gliding resistance, and improve tendon healing attributed to anti-inflammatory activity, enhanced cell proliferation, and collagen deposition, besides the lubricating action on the gliding surface of the tendon (97). The concept of synergistic therapeutic effects combining viscosupplementary biomaterials with drug therapies has yet only been implemented for osteoarthritis but not for tendon diseases. Cingal (Anika Therapeutics) is a marketed combination of cross-linked hyaluronic acid and the corticosteroid triamcinolone hexacetonide for knee osteoarthritic pain.
Several factors may affect local drug delivery including external stimulation (e.g., saline flushing of the joint/tissue during surgery, local cooling due to icing, and post-op mobilization) and local tissue anatomy. Drug delivery systems for tendon should avoid burst release due to uncontrolled stimuli during and after surgery that may accelerate release and exhibit tough mechanical properties that do not fracture during joint movement and/or tissue adhesion. Delivery systems should also consider the permeability of surrounding tissues (e.g., nerves, vessels, fat, skin, and sheath), which may affect drug targeting.
In the next sections, we first summarize the advances in preclinical research on novel concepts to integrate biomaterials in drug and biologic product therapies for tendon diseases. We then summarize the advances in clinical research and development (R&D) of therapeutic modalities, some of which may have the potential to transform the treatment of tendon diseases.
Preclinical advances toward novel integrated therapies
The long-term benefit and the appropriate timing for the delivery of anti-inflammatory drugs, such as corticosteroids and NSAIDs, for tendon diseases remain controversial. Nonetheless, anti-inflammatory drugs are the most common therapies used to alleviate symptomatic pain in tendon diseases (>50 approved products). Recent work suggests that delayed drug delivery may result in superior tendon healing (98). Exploratory research combining biomaterials with drug therapies using porous microspheres loaded with dexamethasone (Dex/PMS) showed extended drug release in vitro, and combined Dex/PMS suppressed the expression of inflammatory cytokines both in vitro and in vivo after intratendon injection (99). In a preclinical in vivo collagenase tendon injury model, combined Dex/PMS enhanced collagen content and biomechanics in rat Achilles tendons (99).
The combination of biomaterials with established drugs approved and marketed for other indications may offer novel avenues of integrated therapies for tendon diseases. Rapamycin, an mammalian target of rapamycin (mTOR) kinase inhibitor, is a macrolide immune modulator marketed primarily for the prevention of organ transplant rejection. In preclinical studies, the combination of rapamycin with PLGA nanoparticles modified with a collagen hybrid peptide was shown to have affinity to collagen, sustained release, and bioactivity and suppressed heterotopic ossification progression in mouse models of Achilles tendinopathy and Achilles tendon rupture, presumably via prevention of osteochondrogenic differentiation (100).
Drug discovery and optimization programs for novel targeted therapies of tendon diseases implement strategies to combine biomaterials and drugs more often in the early phase of programs for technical, pharmacokinetic, and pharmacodynamic reasons. For localized delivery and extended activity, the low–molecular weight drugs oxotremorine (Oxo-M) and 4-Phenyl-1-(4-phenylbutyl)piperidine (4-PPBP) were encapsulated in PLGA microspheres with beneficial effects on healing in a preclinical rat patellar tendon model (101).
Many growth factors are potential biologic drug targets to promote tendon tissue regeneration and restoration of tendon function [e.g., insulin-like growth factor 1 (IGF-1) (102), PDGF (103), VEGF (104–106), FGF (107), TGF-β, and bone morphogenetic proteins (BMPs) (108)]; however, they undergo succinct spatial and temporal biological regulation and are prone to rapid degradation and clearance from the application site. In a preclinical model of supraspinatus repair in rabbits, TGF-β was combined with an alginate-based biomaterial implant for sustained locally restricted activity. The TGF-β biomaterial implant improved the tendon biomechanical tensile strength; however, tendon material properties were not changed 12 weeks after implantation (109). The synergistic pharmacological and mechanical promotion of tendon-to-bone healing was demonstrated by integrating TGF-β, BMP-2, and connective tissue growth factor (CTGF) in a three-dimensional (3D) printed porous PCL biomatrix template. In a preclinical model of supraspinatus tendon rupture, the drug-loaded biomaterial template promoted the recruitment of tendon cells and suggested regeneration of the enthesis (101).
Electrospun biomaterials such as a bilayer tube (DegraPol) have been used as a drug delivery system for PDGF-BB, in addition to providing mechanical reinforcement and structural guidance for migrating cells after complete transection of rabbit Achilles tendons (110). This results in homogeneous cell distribution, elevated proteoglycan content, reduced α–smooth muscle actin (α-SMA), and reduced collagen I and III (110).
Porous sutures have been used in combination with heparin/fibrin delivery systems for sustained drug delivery of CTGF into canine flexor tendons. Repair surgery with porous sutures serving as matrix for a biomaterial drug delivery system was mechanically competent. There was no evidence of adhesion or other negative inflammatory reaction 14 days after repair, supporting the concept of a suture-based biomaterial drug depot to avoid additional traumatic and proinflammatory intervention for drug depot placement (111).
Many approaches have been investigated to also deliver cells within biomaterials to tendon. When implanted in an allogeneic fibrin glue construct, tendon cells promoted earlier and more complete repair (112). Cells may also be expanded and delivered using electrospun scaffolds (113). Adipose-derived stem cells (ADSCs) that expanded to form a cell sheet and transplanted on rotator cuffs in rats led to improvements in histologic scoring and bone volumes (114). ADSCs may also home to tendon injury sites after tail vein injection, although the functional benefits remain to be elucidated (115).
Recent studies suggest that cell-derived agents may be a key driver mediating tissue healing in certain settings, rather than the direct reparative capacity of the cells. Bone marrow mesenchymal stem cell–derived exosomes promoted proliferation, migration, and tenogenic differentiation of tendon stem/progenitor cells in vitro (116). These exosomes were encapsulated in fibrin and injected into defects in rat patellar tendons, with controlled release and internalization within tendon (116). The approach improved histologic scores and the expression of tendon markers compared to fibrin vehicle (116).
Clinical advances toward transformative therapies
Of novel therapies for tendon diseases, autologous cell therapies and therapies using allogenic biological products have advanced the furthest in clinical development. Adipose-derived regenerative cells (ADRCs; Transpose RT system, InGeneron) were assessed as autologous cell therapy for the functional improvement of symptomatic patients with partial-thickness rotator cuff tears in a clinical trial after intratendon injection (NCT03752827) (Fig. 3). For point-of-care isolation of autologous ADSCs, the Transpose RT system consists of a tissue processing unit with Matrase Reagent that enzymatically releases cells from collected tissue. Single injection of ADRCs into the partial-thickness supraspinatus tendon tear compared to the administration of a single corticosteroid injection into the associated subacromial space showed increased functional scores of patient-reported outcome measures (PROs) and supraspinatus tendon strength 24 and 52 weeks after injection (117).
Fig. 3. Therapeutic modalities in current clinical use and advances in clinical development of novel therapies for tendon disease.
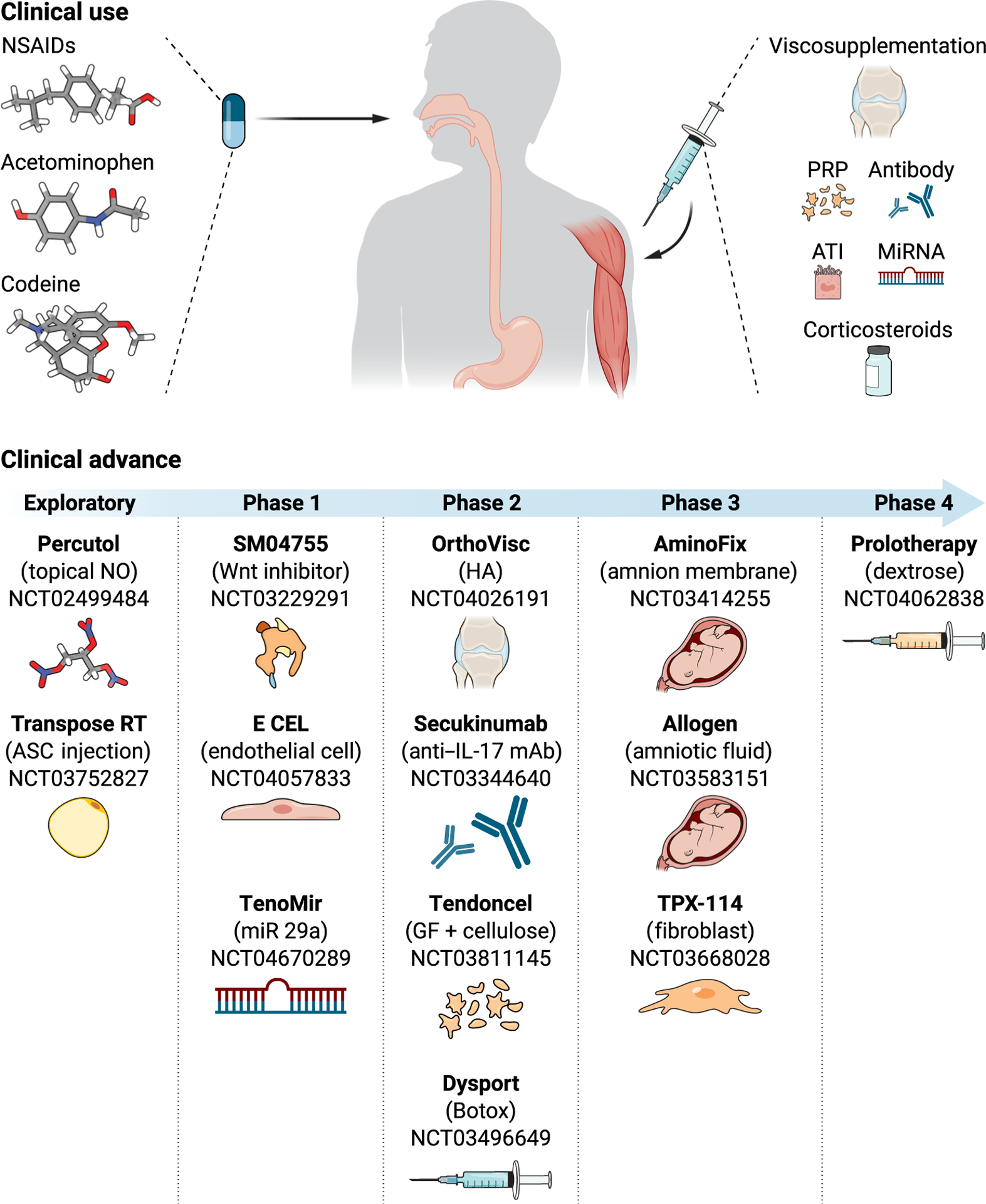
Phase 1: Safety and tolerability. Phase 2: Efficacy dose range. Phase 3: Pivotal therapeutic benefit. Phase 4: Postmarket surveillance.
A second autologous cell therapy in clinical development uses dermal fibroblast cells (TXP-114, Tego Science) to evaluate efficacy and safety on the improved structural outcome and reduction in retear rates using MR imaging (MRI) in patients with full-thickness rotator cuff tears (NCT03668028). Dermal fibroblasts are locally implanted during arthroscopic surgical repair of full-thickness rotator cuff tear. Preclinical work in a chronic rabbit rotator cuff tear model showed that trans-osseous repair surgery and injection of dermal fibroblasts with fibrin increased the tensile strength and promoted greater collagen fiber continuity compared to groups receiving only fibrin or saline treatments (118).
An allogenic cell therapy using umbilical vein–derived cells (E-CEL, AB-205 Gel Matrix, Angiocrine Bioscience) is being tested for safety and feasibility in a phase 1 clinical trial after local implantation during the arthroscopic surgical repair of full-thickness rotator cuff tears (NCT04057833). E-CEL are genetically modified umbilical vein–derived CD31+ endothelial cells that simulate tendon-resident stem/progenitor cells by inserting a prosurvival gene (Ad5 E4ORF1). Motivation for the work derives from preclinical in vivo studies demonstrating that endothelial cell transplantation could rejuvenate hematopoietic stem cell function in aged mice (119).
Two allogenic biological products derived from human amnion membrane matrix (AmnioFix, MiMedx) and human amnion fluid matrix (Allogen, Vivex Biologics) are being investigated in phase 3 clinical trials for the alleviation of pain in patients with Achilles tendonitis (NCT03414255) and in patients with stenosing tenosynovitis (NCT03583151), respectively, as assessed with PROs after local injection. AmnioFix is a micronized dehydrated human amnion/chorion membrane, whereas Allogen is a human amniotic fluid allograft. Both contain an undefined number of biological substrates including growth factors, cytokines, collagen substrates, amino acids, polyamines, lipids, carbohydrates, and extracellular matrix molecules like hyaluronic acid and fibronectin. Both are suggested to modulate inflammation, to protect and promote tendon, and to provide tendon lubrification (120). A more defined allogenic biological product in clinical development is based on a platelet lysate (Tendoncel, Celixir). Tendoncel combines isolated platelet growth factors and a cellulose derivative gel and is being evaluated for efficacy and safety in a phase 2 trial to alleviate pain and function assessed with PROs and grip strength in patients with lateral epicondylitis after daily topical administration (NCT03811145).
Biologics and small molecular weight chemicals are also advancing in clinical development. They represent therapeutic modalities targeting well-defined molecular targets and pathways for the resolution of inflammation and/or promotion of tissue regeneration in tendon diseases. The fully human, high-affinity, anti–IL-17A monoclonal antibody (secukinumab, Cosentyx, Novartis) is being evaluated on efficacy to alleviate pain and improve tendon function assessed with PROs and MRI in patients with symptomatic rotator cuff tendinopathy in a phase 2 clinical trial after systemic administration (NCT03344640). Cosentyx modulates immune responses in a variety of clinical conditions with demonstrated therapeutic potential in rheumatoid arthritis, moderate-to-severe plaque psoriasis, noninfectious uveitis, and ankylosing spondylitis. Preclinical studies demonstrated an elevated number of IL-17A–positive cells in human tendinopathy and the abundant expression of IL-17A receptor in tenocytes with increased expression correlated to increased disease severity. Moreover, in tenocytes, IL-17A induced proinflammatory cytokine signatures (e.g., IL-6, IL-8, CXCL1, and CCL20) and increased the expression of collagen III and apoptotic markers (14).
Inhibition of the Wnt/β-catenin signaling pathway with a topical low–molecular weight chemical (SM04755, Samumed LLC) is being assessed for safety and feasibility in a phase 1 clinical trial (NCT03229291). Preclinical work demonstrated the up-regulation of Wnt in tendinopathy that suggests a causal link to inflammation and tenocyte differentiation. In vitro, SM04755 decreases Wnt, promotes tenocyte differentiation, and inhibits catabolic enzymes and proinflammatory cytokines after induction with IL-1β (e.g., MMPs) (121). Further preclinical supportive evidence was generated in a rat model of collagenase-induced Achilles tendon injury. In this tendon disease paradigm, SM04755 reduced tendon inflammation, weight-bearing function, and pain and induced tendon regeneration (121).
MicroRNA29a (miR29a) mimetic (TenoMiR, Causeway Therapeutics) is a novel microRNA replacement therapy assessed in a phase 1 clinical trial for safety and tolerability in patients with lateral epicondylitis after intratendon injection (NCT04670289). TenoMiR directly targets the key changes in collagen production associated with tendinopathy. Loss of miR29a from human tendons results in increased expression of IL-33 and collagen type III, a key feature of tendon disease. Replacement of miR29a in damaged tendon cells restores collagen production to preinjury concentrations (122). In horse superficial digital flexor tendinopathy, injection of miR29a reduced collagen type III and lesion cross-sectional area and improved histological outcomes (123).
Botulinum toxin A and nitric oxide (NO) are less novel therapeutic modalities. Expanding on their mechanism of action and demonstrated potential in other indications, clinical R&D suggests that botulinum toxin A and NO may address the unmet therapeutic needs of tendon diseases. Botulinum toxin A (abobotulinumtoxinA, Dysport, Ipsen; onabotulinumtoxinA, Botox, Allergan) is being examined in phase 2 clinical trials for efficacy to alleviate symptomatic pain assessed with PRO in adductor tendinopathy in the groin area (NCT03496649) and to alleviate symptomatic pain assessed with PROs and grip strength in patients with lateral epicondylitis after intramuscular injection (NCT00930709). Botulinum toxin A is a nicotinic acetylcholine receptor antagonist and, as a profound muscle relaxant, has demonstrated therapeutic effect for cervical dystonia, cerebral palsy, hyperhidrosis, muscle spasticity, and blepharospasm.
Nitroglycerin (metabolized NO) is being tested in exploratory clinical trials for the promotion of tendon healing after topical administration. NO is an unstable, free radical small molecule that functions as a messenger of the immune system and vasodilator. In patients with lateral epicondylitis, transdermal application of nitroglycerin transiently reduced pain at 2 weeks but not at 6 months after treatment (124). The outcome of randomized controlled trials in Achilles tendinopathy with topical application of nitroglycerin is controversial, either reporting no effect (125) or reporting less pain episodes during night sleep and improved tendon function at 12 and 24 weeks after treatment (126). In patients with supraspinatus tendinopathy, nitroglycerin reduced shoulder pain with activity and improved joint function, with 46% of patients reporting no symptoms at 6 months after treatment (124). The role of NO in tendon injury is further supported by complementary translational preclinical research, showing elevated NO synthase (NOS) in human rotator cuff tendon samples and in rodent models of rotator cuff injury and overuse (124). In vitro and in vivo experiments suggest that NO increases collagen synthesis of tenocytes (124). Rodents receiving NOS inhibitors had inferior healing (reduced cross-sectional area and load to failure) (124). Clinical trials are continuing to examine whether the addition of topical glyceryl trinitrate over 24 weeks in addition to a 12-week exercise program improves clinical outcome in Achilles tendinopathy (NCT02499484).
PERSPECTIVES TO ACCELERATE TRANSLATION
Over the past decades, approved and marketed therapies for tendon diseases have not drastically changed clinical practice. Today, symptomatic pain treatment together with physical therapy remains the forefront of therapeutic intervention of tendon diseases that do otherwise not require surgical intervention. Many novel therapies for tendon diseases have failed in clinical development despite excellent scientific rationale and supportive preclinical evidence due to the lack of forward and reverse translational verification, lack of measures for patient stratification, shifting risk-benefit assessment, undefined regulatory paths, or changing market assessments and business cases (Table 2). For example, RCT-01 (RepliCel), an autologous cell therapy using nonbulbar dermal sheath–derived fibroblasts (127, 128) for chronic Achilles tendinosis, was terminated in phase 2 clinical development because of unsuccessful patient enrollment (NCT02330146). However, substantial progress in basic and translational science opens new avenues for novel therapies, supported by the paradigm shift from symptom relief toward disease modification and the prevention of recurrence of disease. In the following sections, we discuss challenges and perspectives for the discovery and development of novel therapies targeting the promotion of tendon tissue regeneration and restoration of tendon function by considering scientific, clinical, technical, regulatory, and commercial aspects (Fig. 4).
Table 2.
Abandoned drug and biologic product therapies for tendon diseases (terminated in clinical development).
| Phase 1 | Phase 2 | Phase 3/4 |
|---|---|---|
| PDGF (becaplermin; β-tricalcium phosphate, Augment LT, Wright Medical Group) (NCT01256242) | Adipose Cell Therapy (ALLO-ASC Injection, Bukwang Pharmaceuticals) (NCT03449082) | Corticosteroid (Ketorolac, Kenalog) (NCT04115644) |
| BMP-655/ACS (bone morphogenetic protein ligand, Pfizer/Wyeth) (NCT01122498) | Autologous human platelet lysate (Bharat Serums and Vaccines) (NCT01668862) | NSAID (Ketprofen Topical Patch 20%) (NCT00426985) |
| Hypoxia-inducible factor inhibitor (daprodustat, GSK) (NCT02231190) | Nitrate/nitrite (nitroglycerin) (OrthoDerm, Cure Therapeutics) (NCT00447928) | NSAID [etodolac (lidocaine), Etoreat, MEDRx] (NCT01506154) |
| Nonbulbar dermal sheath cells (RCT-01, RepliCel Life Sciences) (NCT02330146) | NSAID [ketoprofen (transdermal patch), Endo Health Solutions] (NCT00426985) |
Fig. 4. Translation of integrated science into innovative therapy.
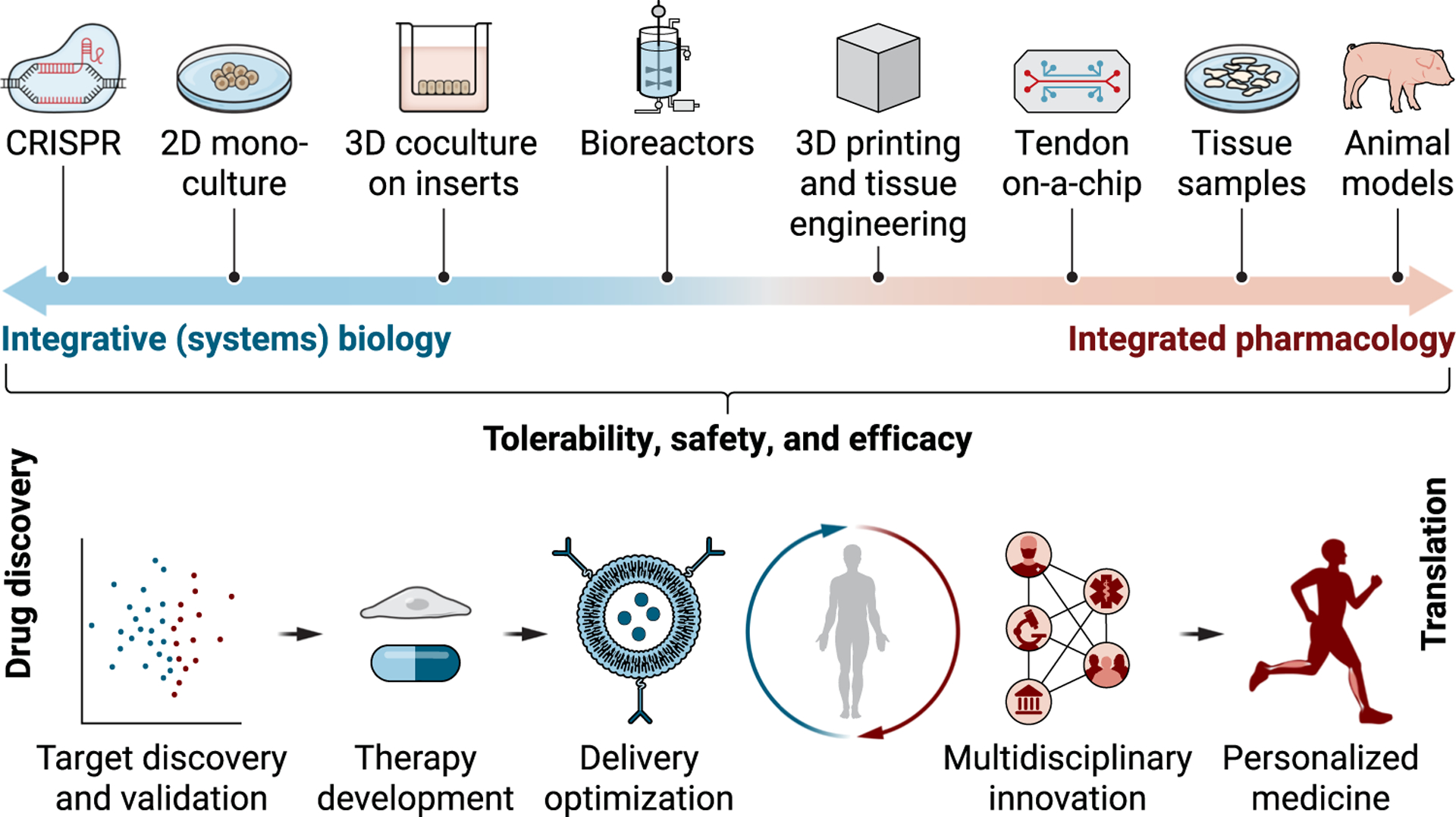
Future treatments for tendon disease require evidence-based clinical, cellular, and molecular characterization of the underlying pathology of tendon disease. Insights from omics, therapy development, delivery optimization, advances in clinical data science, and establishment of multidisciplinary research and technology platforms should accelerate the discovery of novel therapeutic cues, molecular diagnostic markers, and predictors for therapy outcome, which are interrelated and of critical importance for targeted disease-modifying therapy of tendon disease.
Understanding of pathogenesis and clinical molecular pathology of tendon disease
Fundamental understanding of the pathogenesis of tendon diseases is currently lacking in the tendon field, with limited validation between model systems and the human condition. The discovery and development of novel and targeted disease-modifying therapies critically rely on in-depth insights into the cellular and molecular alterations in human tendon disease (129, 130). Emerging evidence derived from human studies proposes that altered cross-talk between immune cells and stromal tendon cells may convert controllable normal inflammatory responses into chronic tendon disease (16). In addition, transcriptomic and proteomic research and related clinical data science are warranted to further elucidate the key cellular and molecular pathways implicated in tendon disease and to rationalize novel therapeutic targets. New integrated data science approaches such as The Tendon Seed Network are helping to map the transcriptome of tendon in health and disease across multiple anatomic and microanatomic sites and to develop clinical, laboratory, bioinformatic, and mathematical tools and technology platforms for the discovery and development of novel therapies (www.chanzuckerberg.com/science/programs-resources/single-cell-biology/seednetworks/the-tendon-seed-network).
The path to innovation in therapy discovery can be further advanced by data science, notably bioinformatics and machine learning, and such tools are just beginning to affect the tendon field. Continued deconvolution of biological cross-talk is likely to cause a paradigm shift from target and pathway monotherapies toward polymodal therapies (131). For example, novel bispecific monoclonal antibodies are in clinical development for inflammatory diseases (132), and anticancer therapies with rationalized combination therapies are improving objective response rates and prolonging disease control in comparison with monotherapies (133).
Emerging opportunities to enable innovative therapies
The implementation of focused academic and applied reverse translational preclinical research in tendon disease models mimicking clinical tendon injury enables a more predictive assessment of novel therapy concepts (134). Moreover, gain/loss-of-function experiments (135), inducible genetic mouse models, and gene editing techniques (e.g., CRISPR) (136) are accelerating the discovery of novel therapeutic pathways and molecular targets and the subsequent validation in preclinical models and optimization of drug candidate molecules. The recent advances in clinical tendon research, ranging from tendon-muscle-joint biophysical performance (137) to transcriptional profiling of tendon pathology (138), offer novel opportunities to enable the discovery and development of improved model systems (i.e., spanning 2D and 3D mono/coculture, bioreactors, organ on chip, and animal) to test innovative therapies for tendon diseases. In particular, the progression of complementary technologies provides novel clinical tools to allow monitoring and quantification of joint and muscle mechanical loading generated by tendon (139); to assess tendon composition, structure, and mechanics (140); and to analyze the molecular fate and state of tendon-derived cells associated with tendon disease and regeneration (141). Once validated, these tools will allow pathology staging, patient stratification, and monitoring of disease-modifying therapy outcome in patients after the onset of treatment (Fig. 4).
Inspired by innovation in biomedical material science, the combined design and optimization of integrated drug-biomaterial therapies represent another opportunity to enable the discovery and development of novel treatments for tendon diseases. The concept to use biomaterials as delivery systems for therapeutic modalities recently evolved. In addition to low–molecular weight drugs, delivery of larger–molecular weight therapeutic modalities such as peptides (~1 to 10 kDa) and proteins (>10 kDa) that previously required injection or parenteral administration for delivery (37) because of their restricted oral bioavailability (142, 143) is now possible using biomaterials (144). Although the use of biomaterials with excellent biocompatibility historically focused on basic drug release features, the importance of sustained local release, attachment to tissue surfaces using adhesives without the need for suturing, and recapitulation of native tendon properties has become more evident (2). Poor viability and homing after direct injection of cell-based therapies have further motivated the use of biomaterials to mechanically protect cells after injection and to provide a niche environment conducive for cell-based tissue regeneration. Recapitulating certain features of tendon (e.g., extracellular matrix composition and structure and mechanical strength) in biomaterials may itself enable mechanotherapeutic benefits (2).
Multidiscipline partnership models for innovative therapies
Novel multidiscipline interorganizational partnership models governed by operational, legal, financial, and regulatory excellence that connects distinct academic disciplines and applied science industries such as biomedical science, material science, data science, biophysical science, sensor science, kinematic science, and diagnostic science should become the key catalysts for the multidisciplinary discovery and development of innovative therapies for tendon disease. Indeed, partnership models are already applied by networks such as the recently founded Alliance for Advanced Therapies in Orthopedics (www.atioalliance.org) aiming to collaboratively advance and accelerate the development of transformative therapies for musculoskeletal diseases and The Tendon Seed Network (www.chanzuckerberg.com/science/programs-resources/single-cell-biology/seednetworks/the-tendon-seed-network) bringing together scientists, computational biologists, software engineers, and physicians to generate a unified and shared resource of data, tools, and open-source analysis methods of the human tendon transcriptome. However, partnership models are not only expected to generate novel opportunities to advance and accelerate early R&D but also to share and lower the cost and risk of R&D. In this context, the cell therapy company InGeneron (www.ingeneron.com) established a strategic partnership with the integrated health care provider Sanford Health for clinical trial cooperation and cosponsoring of the pivotal program on their Transpose RT system for rotator cuff tears (NCT03752827).
The chance of approval for a new therapeutic modality that enters phase 1 trials is currently about 10% (145). The costs of development of new therapies to reach market authorization typically amount to $314 million to $2.8 billion and are capitalized at a real cost of capital rate of ~10% per year (i.e., the required rate of return for an investor) (146). The models used to calculate the probability of success at estimated costs are based on data from biomedical industry big players including all failed resource-absorbing late-stage clinical programs. Accordingly, risks and costs are much lower at early-stage R&D biotech companies (145) and can be further reduced by applying virtual business model concepts to keep fixed costs to an absolute minimum and to be highly agile and responsive to fast-progressing science. The early clinical-stage biopharmaceutical company Causeway Therapeutics (www.causewaytherapeutics.com) exemplifies a virtual business model, applied to develop an academia spun-out microRNA technology (TenoMiR) for the treatment of tendinopathy (NCT04670289). The rise in virtual business models over the past decade was directly and indirectly supported by structural changes in the biomedical industry now contracting specialist activities to contract research organizations (CROs) and contract development/manufacturing organizations (CDMOs). Indeed, the CDMO markets for medical devices are experiencing compound annual growth rates exceeding 11% across many specialties including drug delivery, orthopedics, and surgery devices (147).
Multidiscipline interorganizational partnership models are a strategic opportunity either for direct funding such as collaboration, licensing, and co-development or for attracting public and private funding such as scientific grants, governmental subsidies, and venture capital. The high pressure on intellectual diversity is apparent at all phases and disciplines of R&D. In this respect, biomedical industry big players have established specific collaborations with academic centers of excellence and biotech such as the research collaboration of Johnson & Johnson with the regenerative medicine company Orthocell (www.orthocell.com.au) for its Ortho-ATI stem cell approach to treat tendinopathy (ACTRN12617000684325). Moreover, they have built innovation centers, made joint ventures with academic institutions, established precompetitive consortia, or experimented with crowdsourcing and virtual business model concepts. Currently, many companies have put greater focus on leveraging external knowledge, licensing, or acquiring and changing their R&D models from primarily inside-driven concepts to plans that more closely follow the open innovation paradigm (148). Indeed, biomedical clusters have emerged around clinical and academic centers of excellence such as Boston, Massachusetts, where all major biomedical industry big players have established a physical presence to access and share resources of top-tier universities, health care systems, and other biomedical technology companies. For example, close collaborations between a big pharma and a local university have led to the development of new biomaterial-based tendon therapies (84). The overall catalytic implication on innovation is evidenced by the region being top ranked in patents (7565), National Institutes of Health (NIH) funding (4735 awards totaling $2.5 billion), venture capital funding ($6.2 billion in 156 deals), and laboratory space (26.8 million square feet) in 2017.
For academic partners, partnership models might offer additional and facilitated access to funding compared to most other grants. For instance, multidiscipline interorganizational collaboration is warranted to participate in the European Union–funded Horizon research and innovation programs such as Perspectives For Future Innovation in Tendon repair (P4 FIT) aiming to enable the discovery and development of predictive, preventive, personalized, and participatory novel therapies for tendon disease (www.cordis.europa.eu/project/id/955685).
Moreover, partnership models may also provide new opportunities for academic partners to participate in the commercial success of basic research prospectively translating into applied science and eventually to marketed therapy (149). Most recently, the unparalleled potential of partnership models was demonstrated by the NIH and the Foundation for the NIH bringing together more than a dozen leading biopharmaceutical companies, the Health and Human Services Office of the Assistant Secretary for Preparedness and Response, the Centers for Disease Control and Prevention, the FDA, and the EMA to develop an international strategy for a coordinated research response to the coronavirus disease 2019 (COVID-19) pandemic. Applying the partnership model to develop mRNA vaccines for the prevention of COVID-19 was critical to implement fast and wide-spread vaccination programs to successfully fight off the pandemic. However, it is apparent that the applied partnership model also boosted innovation around RNA-based therapeutic modality and delivery technology in general, and it is envisaged that RNA-based therapies will rapidly advance for cancer immunotherapy and other indications in the near future (150). Innovation around RNA-based therapies might inspire the use of inhibitory antisense oligonucleotides and small interfering RNAs (siRNAs) as well as mRNAs encoding for therapeutic proteins as a new class of therapeutic modality also for the treatment of tendon diseases (151).
Multidiscipline interorganizational partnership models may offer an unprecedented opportunity to promote innovation; however, in practice, they require acceptance to relinquish control and to change organizational culture from competitive to collaborative behaviors. Accordingly, the establishment of trust and faith into the partnership is key to interorganizational collaboration performance (152). Leverage on multidiscipline interorganizational partnership requires awareness and skilled management of cultural diversity of partner organizations and the establishment and maintenance in transparency and clarity on resources and accountabilities, legal and administrative agreements, coordination of information exchange, and interpersonal communication (153).
Innovative regulatory and clinical concepts
Advances to transform the therapy of tendinopathy from symptom based toward disease modification will inevitably stimulate the evolution of tools and technologies for diagnostics and for monitoring of therapeutic effect size and will concomitantly transform current regulatory and clinical concepts. The International Scientific Tendinopathy Symposium Consensus recommends that tendinopathy is the preferred terminology for tendon diseases with persistent tendon pain and loss of function related to mechanical loading. Diagnosis of tendinopathy should use condition-specific PRO measures that capture tendon function testing, participation in life activities, psychological factors, physical function capacity, and disability (154).
Tendinopathy-specific PRO measures are conceptually viewed as tools to assess improvement in tendinopathy and represent the current state of art to monitor the effect size of a therapy for tendinopathy. Accordingly, PRO measures are accepted clinical endpoints and are also used for the therapy benefit-risk assessment by regulatory authorities (155). However, validity assessment applying Consensus Standards for the Selection of Health Measurement Instruments (COSMIN) revealed the insufficient or inconsistent quality of PROs in content and structural validity of symptom severity and disability in tendinopathy [e.g., Victorian Institute of Sport Assessment (VISA) questionnaires]. The use of PRO measures is only recommended because of lacking alternatives, and cautious data interpretation is warranted (156).
Future innovative regulatory and clinical concepts are thus envisaged to be built on evolving technologies providing adequate disease-relevant outcome measures such as validated digital ePROs with integrated biosensor real-time recordings (157), molecular imaging [e.g., positron emission tomography (PET)/single-photon emission computed tomography (SPECT)] (158), cellular and molecular biomarkers of disease (e.g., transcriptomics) (159), and prognostic indicators (e.g., genetics and risk models) (160) (Fig. 4). In addition, innovative regulatory and clinical concepts are envisaged to evolve by consolidation and integration of evidence obtained from prospective observational clinical studies without therapeutic intervention (161) and by consolidation and integration of hypotheses generated retrospectively from interventional clinical trials (i.e., virtual evidence) (162).
In accordance, the FDA published a novel strategic Real-World Evidence (RWE) Framework (163) to encourage innovative clinical concepts to further explore the use of Real-World Data (RWD) for regulatory decision-making. Rather than testing hypotheses in traditional randomized clinical trials, hybrid or pragmatic trial designs and observational studies are supported to generate hypotheses (e.g., identification of drug development tools including biomarker and composite signatures, examining the impact of planned inclusion/exclusion criteria in the relevant population, informing prior probability distributions, identifying prognostic indicators or patient baseline characteristics for enrichment or stratification, and assembling geographically distributed research cohorts). The scope of the FDA RWE framework offers opportunities to explore novel clinical trial concepts with new therapy outcome measures for tendinopathy (e.g., composite signatures). A recent observational study assessed PRO (Achilles Total Rupture Score) together with recovery of tendon biomechanics (sonoelastography) and gait patterns (wearable pressure-sensitive insoles) over 12 weeks after Achilles tendon rupture (164). The association between symptoms, tendon biomechanics, and the dynamics of weight loading on the foot suggests that an innovative composite signature was identified, potentially suitable for clinical diagnostics and monitoring of therapeutic effect size (164).
The advances in sensors technology and data science toward digital health care are already beginning to transform regulatory and clinical concepts as to capture patient experience beyond the current therapy outcome assessments and to establish more integrated insights of how a therapy functions, how to measure disease modification, and how to predict long-term therapy outcome (165). Moreover, the learnings on the impact of the COVID-19 pandemic on traditional clinical trials—with nearly all clinical studies disrupted, many paused enrollment, and new trials were left on hold (166)—will further accelerate the transformation of regulatory and clinical concepts toward decentralized patient-centric remote trials (167). Patient-centric strategies are further reinforced by patient organizations to become equal partners in clinical trial design and to participate in health care decision-making along with the movement toward personalized therapies for tendinopathy.
The Clinical Trials Transformation Initiative (www.ctti-clinicaltrials.org), a public-private partnership cofounded by Duke University and the FDA, has outlined a common vision for how future controlled prospective clinical trials should be completed. The initiative envisages future prospective clinical trials to be patient-centric and easily accessible, with patients and patient organizations fully integrated in trial design and governance. Patients are enrolled regardless of geography and mobility, reflecting the diversity of the patient population expected to benefit from the therapy. Future prospective clinical trials should also be fully integrated within health care systems. Health care systems and health plans should be involved in trial planning and should accelerate the integration of insights into clinical practice. Clinical trials should maximally leverage available clinical, nonclinical, digital, and consumer data to minimize the collection of necessary trial-specific data. Eventually, the initiative envisages that controlled prospective clinical trials should contribute knowledge about how to prevent, diagnose, and treat disease as one of many other sources of information. Future prospective clinical trials should therefore fall within the broader context of evidence generation for regulatory decision-making.
Cost-effective commercialization of innovative therapies
Tendon disease is considered self-limiting by managed care organizations, with low to moderate direct costs for standard of care (e.g., <$300/year symptomatic pain treatment, ~$100/visit physical therapy, $12,000 Achilles tendon repair surgery, and $22,000 rotator cuff repair surgery). However, the societal costs of tendon diseases are immense considering the indirect costs such as lost income due to inability to work or lower wages, missed workdays, and disability payments (168), and are similar to rheumatoid arthritis, osteoarthritis, and lower back pain (169). For example, up to 5% of patients with lateral epicondylitis claim sickness absence with an average duration of 29 days in a year (170) and rotator cuff tendinopathy may take about 10 months to heal, resulting in considerable sick leave (171). Novel disease-modifying therapies for tendon diseases are thus considered of high socioeconomic value, evidenced by the fact that for repair surgery of rotator cuff full-thickness tears alone, long-term societal savings of $3.4 billion per year have been estimated with the model prediction of a high 75% long-term success rate of the surgical treatment (172).
Cost-effectiveness analysis for value proposition and commercial pricing of a novel therapy is based on estimates of therapy costs and overall benefits to patients, employers, and payers (172). The increasing costs necessary to develop novel therapies (medical devices, $50 million to $800 million; drug, $314 million to $2.8 billion) (173, 174) are factored into the cost estimation of a therapy. Accordingly, with low to moderate costs of current standard of care and concomitant high development costs, a novel therapy for tendon disease must transform current clinical practice by offering superior therapeutic outcome that results in superior cost-effectiveness compared to current standard of care. There is evidence for acute Achilles tendon rupture that an excellent therapeutic outcome, rather than lower costs, results in a superior cost-effectiveness assessment (175). Surgical repair of acute Achilles tendon rupture is associated with higher direct costs than nonsurgical conservative standard of care; however, it is similarly cost-effective because of superior therapy outcome on function and quality of life associated with earlier return to work (175). Obviously, the evaluation of therapy outcome and the associated cost-effectiveness analysis depends on the etiology, duration, and severity of tendon disease and on patient population (e.g., elderly, general population, workers, and athletes). Cost-effectiveness will be a critical-to-meet criterion, and concomitant prediction of therapeutic value and cost-effectiveness is proposed to be incorporated early and to be continued along the entire development path of a novel therapy for tendon disease.
CONCLUDING REMARKS TO ADVANCE THE FIELD
This review has highlighted current clinical practices to treat tendon disease and outlined perspectives to advance to novel disease-modifying therapies, considering scientific, clinical, technical, regulatory, and commercial aspects. The tendon disease therapy field is moving toward a more evidence-based clinical, cellular, and molecular characterization of the underlying pathology of tendon disease. Novel insights from omics, advances in clinical data science, and establishment of multidisciplinary research platforms should further inspire, motivate, and accelerate the discovery of novel therapeutic cues, molecular diagnostic markers, and predictors for therapy outcome, which are interrelated and of critical importance for the targeted disease-modifying therapy of tendon disease.
Recent advances in preclinical, clinical, material, and technology sciences offer unprecedented opportunities to enable the discovery and development of novel disease-modifying therapies for tendon disease. However, novel R&D partnership models are warranted to integrate and leverage multidiscipline interorganizational innovation. Virtual business model concepts might serve to explore and stress-test the operational, legal, financial, and regulatory excellence required for partnering resources, intellectual properties, incentives, and accountabilities.
Pathology-based integrated assessments of etiological, diagnostic, and therapy outcome measures and prognostic indicators are considered the cornerstone to implement innovative regulatory and clinical concepts toward disease modification of tendinopathy. Meanwhile, current concepts of patient-reported outcomes may be advanced and complemented with digital health technologies and data science, providing objective therapy outcome measures (e.g., duration to return to predisease activity, quality of activity, and quality of life) and predictors for the prevention of recurrence of disease, relevant for both regulatory decision-making and therapy cost-effectiveness assessment. For superior cost-effectiveness, novel therapies are required to transform current clinical practice by offering superior therapeutic outcome compared to current standard of care. Novel therapies should be disease modifying, restore predisease tendon functionality, and prevent the recurrence of disease. For ultimate success and adoption, novel therapies must lower socioeconomic disease burden by reducing productivity loss and disease compensation associated with tendon disease.
Funding:
This work was supported by the National Institute on Aging of the NIH (K99 AG065495) and Novartis.
Competing interests:
B.R.F. has been a paid consultant for Amend Surgical and has the following interests: Limax, equity. D.J.M. has been a paid consultant for Ethicon and Boston Scientific and has the following interests: Lyell, equity; Attivare, equity; IVIVA Medical, consulting and equity; Limax, equity; Epoulosis, equity; Revela, equity; Amend Surgical and Sirenex, licensed intellectual property. B.R.F. and D.J.M. are inventors on patent/patent application PCT/US62/903,315 held/submitted by Harvard University that covers tough adhesive hydrogels for drug delivery. The authors receive grant support through Novartis. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the position of the Wyss Institute for Biologically Inspired Engineering at Harvard University or Novartis.
REFERENCES AND NOTES
- 1.Freedman BR, Gordon JA, Soslowsky LJ, The Achilles tendon: Fundamental properties and mechanisms governing healing. Muscles Ligaments Tendons J. 4, 245–255 (2014). [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman BR, Mooney DJ, Biomaterials to mimic and heal connective tissues. Adv. Mater 31, e1806695 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukashiro S, Komi PV, Järvinen M, Miyashita M, In vivo Achilles tendon loading during jumping in humans. Eur. J. Appl. Physiol. Occup. Physiol 71, 453–458 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Burry HC, Pathogenesis of some traumatic and degenerative disorders of soft tissue. Aust. N. Z. J. Med 8 Suppl. 1, 163–167 (1978). [DOI] [PubMed] [Google Scholar]
- 5.Abate M, Silbernagel KG, Siljeholm C, Di Iorio A, De Amicis D, Salini V, Werner S, Paganelli R, Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther 11, 235 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan J, Murrell GAC, Wei A-Q, Wang M-X, Apoptosis in rotator cuff tendonopathy. J. Orthop. Res 20, 1372–1379 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Fredberg U, Stengaard-Pedersen K, Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand. J. Med. Sci. Sports 18, 3–15 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Cook JL, Rio E, Purdam CR, Docking SI, Revisiting the continuum model of tendon pathology: What is its merit in clinical practice and research? Br. J. Sports Med 50, 1187–1191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, Murrell GAC, McInnes IB, Rodeo SA, Tendinopathy. Nat. Rev. Dis. Primers 7, 1 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Freedman BR, Bade ND, Riggin CN, Zhang S, Haines PG, Ong KL, Janmey PA, The (dys)functional extracellular matrix. Biochim. Biophys. Acta 1853, 3153–3164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran PHT, Malmgaard-Clausen NM, Puggaard RS, Svensson RB, Nybing JD, Hansen P, Schjerling P, Zinglersen AH, Couppe C, Boesen M, Magnusson SP, Kjaer M, Early development of tendinopathy in humans: Sequence of pathological changes in structure and tissue turnover signaling. FASEB J. 34, 776–788 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Kannus P, Józsa L, Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Joint Surg. Am 73, 1507–1525 (1991). [PubMed] [Google Scholar]
- 13.Steinmann S, Pfeifer CG, Brochhausen C, Docheva D, Spectrum of tendon pathologies: Triggers, trails and end-state. Int. J. Mol. Sci 21, 844 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millar NL, Akbar M, Campbell AL, Reilly JH, Kerr SC, McLean M, Frleta-Gilchrist M, Fazzi UG, Leach WJ, Rooney BP, Crowe LAN, Murrell GAC, McInnes IB, IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep 6, 27149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dakin SG, Newton J, Martinez FO, Hedley R, Gwilym S, Jones N, Reid HAB, Wood S, Wells G, Appleton L, Wheway K, Watkins B, Carr AJ, Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med 52, 359–367 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millar NL, Murrell GAC, McInnes IB, Inflammatory mechanisms in tendinopathy—Towards translation. Nat. Rev. Rheumatol 13, 110–122 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Abat F, Alfredson H, Cucchiarini M, Madry H, Marmotti A, Mouton C, Oliveira JM, Pereira H, Peretti GM, Spang C, Stephen J, van Bergen CJA, de Girolamo L, Current trends in tendinopathy: Consensus of the ESSKA basic science committee. Part II: Treatment options. J. Exp. Orthop 5, 38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliva F, Barisani D, Grasso A, Maffulli N, Gene expression analysis in calcific tendinopathy of the rotator cuff. Eur. Cell. Mater 21, 548–557 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Cook JL, Purdam C, Is compressive load a factor in the development of tendinopathy? Br. J. Sports Med 46, 163–168 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Jelinsky SA, Rodeo SA, Li J, Gulotta LV, Archambault JM, Seeherman HJ, Regulation of gene expression in human tendinopathy. BMC Musculoskelet. Disord 12, 86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyer R, Kongsgaard M, Hougs Kjaer B, Ohlenschlaeger T, Kjaer M, Magnusson SP, Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: A randomized controlled trial. Am. J. Sports Med 43, 1704–1711 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT, Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J. Gerontol. A Biol. Sci. Med. Sci 51, B54–B59 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrick JG, Does accelerated functional rehabilitation after surgery improve outcomes in patients with acute Achilles tendon ruptures? Clin. J. Sport Med 22, 379–380 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Mortensen HM, Skov O, Jensen PE, Early motion of the ankle after operative treatment of a rupture of the Achilles tendon. A prospective, randomized clinical and radiographic study. J. Bone Joint Surg. Am 81, 983–990 (1999). [DOI] [PubMed] [Google Scholar]
- 25.de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SMA, Verhaar JAN, Weinans H, Tol JL, Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA 303, 144–149 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL, National trends in rotator cuff repair. J. Bone Joint Surg. Am 94, 227–233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iannotti JP, Full-thickness rotator cuff tears: Factors affecting surgical outcome. J. Am. Acad. Orthop. Surg 2, 87–95 (1994). [DOI] [PubMed] [Google Scholar]
- 28.McCarron JA, Derwin KA, Bey MJ, Polster JM, Schils JP, Ricchetti ET, Iannotti JP, Failure with continuity in rotator cuff repair “healing”. Am. J. Sports Med 41, 134–141 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S, Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J. Shoulder Elbow Surg 12, 550–554 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Rodeo SA, Biologic augmentation of rotator cuff tendon repair. J. Shoulder Elbow Surg 16, S191–S197 (2007). [DOI] [PubMed] [Google Scholar]
- 31.van der Eng DM, Schepers T, Goslings JC, Schep NWL, Rerupture rate after early weightbearing in operative versus conservative treatment of Achilles tendon ruptures: A meta-analysis. J. Foot Ankle Surg 52, 622–628 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Freedman BR, Salka NS, Morris TR, Bhatt PR, Pardes AM, Gordon JA, Nuss CA, Riggin CN, Fryhofer GW, Farber DC, Soslowsky L, Temporal healing of Achilles tendons after injury in rodents depends on surgical treatment and activity. J. Am. Acad. Orthop. Surg 25, 635–647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton KK, Hamilton T, Irrgang JJ, Karlsson J, Harner CD, Fu FH, Anatomic anterior cruciate ligament (ACL) reconstruction: A global perspective. Part 1. Knee Surg. Sports Traumatol. Arthrosc 22, 1467–1482 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Merolla G, Chillemi C, Franceschini V, Cerciello S, Ippolito G, Paladini P, Porcellini G, Tendon transfer for irreparable rotator cuff tears: Indications and surgical rationale. Muscles Ligaments Tendons J. 4, 425–432 (2014). [PMC free article] [PubMed] [Google Scholar]
- 35.Mihata T, Lee TQ, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, Kinoshita M, Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Art Ther. 29, 459–470 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Holschen M, Brand F, Agneskirchner JD, Subacromial spacer implantation for massive rotator cuff tears: Clinical outcome of arthroscopically treated patients. Obere Extrem. 12, 38–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelikin AN, Ehrhardt C, Healy AM, Materials and methods for delivery of biological drugs. Nat. Chem 8, 997–1007 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Walden G, Liao X, Donell S, Raxworthy MJ, Riley GP, Saeed A, A clinical, biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng. Part B Rev 23, 44–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons DL, Botting RM, Hla T, Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev 56, 387–437 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Ghlichloo I, Gerriets V, Nonsteroidal anti-inflammatory drugs (NSAIDs), in StatPearls (Treasure Island (FL): StatPearls Publishing, 2022). [PubMed] [Google Scholar]
- 41.Ghosh N, Kolade OO, Shontz E, Rosenthal Y, Zuckerman JD, Bosco III JA, Virk MS, Nonsteroidal anti-inflammatory drugs (NSAIDs) and their effect on musculoskeletal soft-tissue healing: A scoping review. JBJS Rev. 7, e4 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Sundahl N, Bridelance J, Libert C, De Bosscher K, Beck IM, Selective glucocorticoid receptor modulation: New directions with non-steroidal scaffolds. Pharmacol. Ther 152, 28–41 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Newton R, Holden NS, Separating transrepression and transactivation: A distressing divorce for the glucocorticoid receptor? Mol. Pharmacol 72, 799–809 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Kearney RS, Parsons N, Metcalfe D, Costa ML, Injection therapies for Achilles tendinopathy. Cochrane Database Syst. Rev 5, CD010960 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamadi A, Chan JJ, Claessen FMAP, Ring D, Chen NC, Corticosteroid injections give small and transient pain relief in rotator cuff tendinosis: A meta-analysis. Clin. Orthop. Relat. Res 475, 232–243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.da Luz DC, de Borba Y, Ravanello EM, Daitx RB, Dohnert MB, Iontophoresis in lateral epicondylitis: A randomized, double-blind clinical trial. J. Shoulder Elbow Surg 28, 1743–1749 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Harada Y, Kokubu T, Mifune Y, Inui A, Sakata R, Muto T, Takase F, Kurosaka M, Dose- and time-dependent effects of triamcinolone acetonide on human rotator cuff-derived cells. Bone Joint Res. 3, 328–334 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tempfer H, Gehwolf R, Lehner C, Wagner A, Mtsariashvili M, Bauer HC, Resch H, Tauber M, Effects of crystalline glucocorticoid triamcinolone acetonide on cultered human supraspinatus tendon cells. Acta Orthop. 80, 357–362 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eppley BL, Woodell JE, Higgins J, Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast. Reconstr. Surg 114, 1502–1508 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Bell KJ, Fulcher ML, Rowlands DS, Kerse N, Impact of autologous blood injections in treatment of mid-portion Achilles tendinopathy: Double blind randomised controlled trial. BMJ 346, f2310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitzpatrick J, Bulsara M, Zheng MH, The effectiveness of platelet-rich plasma in the treatment of tendinopathy: A meta-analysis of randomized controlled clinical trials. Am. J. Sports Med 45, 226–233 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Docheva D, Muller SA, Majewski M, Evans CH, Biologics for tendon repair. Adv. Drug Deliv. Rev 84, 222–239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bogdanowicz DR, Lu HH, Designing the stem cell microenvironment for guided connective tissue regeneration. Ann. N. Y. Acad. Sci 1410, 3–25 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Wang A, Breidahl W, Mackie KE, Lin Z, Qin A, Chen J, Zheng MH, Autologous tenocyte injection for the treatment of severe, chronic resistant lateral epicondylitis: A pilot study. Am. J. Sports Med 41, 2925–2932 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Wang A, Mackie K, Breidahl W, Wang T, Zheng MH, Evidence for the durability of autologous tenocyte injection for treatment of chronic resistant lateral epicondylitis: Mean 4.5-year clinical follow-up. Am. J. Sports Med 43, 1775–1783 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP, Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J. Bone Joint Surg. Am 88, 2665–2672 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Shoaib A, Mishra V, Surgical repair of symptomatic chronic achilles tendon rupture using synthetic graft augmentation. Foot Ankle Surg. 23, 179–182 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Dempsey DK, Robinson JL, Iyer AV, Parakka JP, Bezwada RS, Cosgriff-Hernandez EM, Characterization of a resorbable poly(ester urethane) with biodegradable hard segments. J. Biomater. Sci. Polym. Ed 25, 535–554 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Pedowitz RA, Yamaguchi K, Ahmad CS, Burks RT, Flatow EL, Green A, Wies JL, Andre JS, Boyer K, Iannotti JP, Miller BS, Tashjian R, Watters III WC, Weber K, Turkelson CM, Raymond L, Sluka P, Gowan RM, American Academy of Orthopaedic Surgeons clinical practice guideline on: Optimizing the management of rotator cuff problems. J. Bone Joint Surg. Am 94, 163–167 (2012). [PubMed] [Google Scholar]
- 60.American Academy of Orthopaedic Surgeons, in Evidence-Based Clinical Practice Guideline (American Academy of Orthopaedic Surgeons, 2019), p. 79. [Google Scholar]
- 61.Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM, Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Art Ther. 33, 190–198 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Sah RL, Ratcliffe A, Translational models for musculoskeletal tissue engineering and regenerative medicine. Tissue Eng. Part B Rev 16, 1–3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proffen BL, Perrone GS, Roberts G, Murray MM, Bridge-enhanced ACL repair: A review of the science and the pathway through FDA investigational device approval. Ann. Biomed. Eng 43, 805–818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Norman GA, Drugs, devices, and the FDA: Part 2: An overview of approval processes: FDA approval of medical devices. JACC Basic Transl. Sci 1, 277–287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, Mooney DJ, Designing hydrogels for controlled drug delivery. Nat. Rev. Mater 1, 16071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfeifer CG, Fisher MB, Carey JL, Mauck RL, Impact of guidance documents on translational large animal studies of cartilage repair. Sci. Transl. Med 7, 310re319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller KS, Connizzo BK, Feeney E, Soslowsky LJ, Characterizing local collagen fiber re-alignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. J. Biomech 45, 2061–2065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA, Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater 12, 458–465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ, Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater 15, 326–334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freedman BR, Knecht RS, Tinguely Y, Eskibozkurt GE, Wang CS, Mooney DJ, Aging and matrix viscoelasticity affect multiscale tendon properties and tendon derived cell behavior. Acta Biomater. 143, 63–71 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin L, Elliott DM, A biphasic and transversely isotropic mechanical model for tendon: Application to mouse tail fascicles in uniaxial tension. J. Biomech 37, 907–916 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ, Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater 14, 1269–1277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nirmalanandhan VS, Dressler MR, Shearn JT, Juncosa-Melvin N, Rao M, Gooch C, Bradica G, Butler DL, Mechanical stimulation of tissue engineered tendon constructs: Effect of scaffold materials. J. Biomech. Eng 129, 919–923 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Goncalves AI, Rodrigues MT, Gomes ME, Tissue-engineered magnetic cell sheet patches for advanced strategies in tendon regeneration. Acta Biomater. 63, 110–122 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, Lu HH, The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 34, 1942–1953 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keller R, Shook D, Skoglund P, The forces that shape embryos: Physical aspects of convergent extension by cell intercalation. Phys. Biol 5, 015007 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Horne-Badovinac S, The Drosophila egg chamber—A new spin on how tissues elongate. Integr. Comp. Biol 54, 667–676 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barocas VH, Tranquillo RT, An anisotropic biphasic theory of tissue-equivalent mechanics: The interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J. Biomech. Eng 119, 137–145 (1997). [DOI] [PubMed] [Google Scholar]
- 79.Peach MS, Ramos DM, James R, Morozowich NL, Mazzocca AD, Doty SB, Allcock HR, Kumbar SG, Laurencin CT, Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLOS ONE 12, e0174789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orr SB, Chainani A, Hippensteel KJ, Kishan A, Gilchrist C, Garrigues NW, Ruch DS, Guilak F, Little D, Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 24, 117–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perris R, Perissinotto D, Role of the extracellular matrix during neural crest cell migration. Mech. Dev 95, 3–21 (2000). [DOI] [PubMed] [Google Scholar]
- 82.Marquardt LM, Heilshorn SC, Design of injectable materials to improve stem cell transplantation. Curr. Stem Cell Rep 2, 207–220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langer R, Folkman J, Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976). [DOI] [PubMed] [Google Scholar]
- 84.Freedman BR, Kuttler A, Beckmann N, Nam S, Kent D, Schuleit M, Ramazani F, Accart N, Rock A, Li J, Kurz M, Fisch A, Ullrich T, Hast MW, Tinguely Y, Weber E, Mooney DJ, Enhanced tendon healing by a tough hydrogel with an adhesive side and high drug-loading capacity. Nat. Biomed. Eng, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ashley GW, Henise J, Reid R, Santi DV, Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. U.S.A 110, 2318–2323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Florence AT, Jani PU, Novel oral drug formulations. Their potential in modulating adverse effects. Drug Saf. 10, 233–266 (1994). [DOI] [PubMed] [Google Scholar]
- 87.Cohen J, IL-12 deaths: Explanation and a puzzle. Science 270, 908 (1995). [DOI] [PubMed] [Google Scholar]
- 88.Langer R, Drug delivery and targeting. Nature 392, 5–10 (1998). [PubMed] [Google Scholar]
- 89.Urist MR, Strates BS, Bone formation in implants of partially and wholly demineralized bone matrix. Including observations on acetone-fixed intra and extracellular proteins. Clin. Orthop. Relat. Res 71, 271–278 (1970). [PubMed] [Google Scholar]
- 90.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL, Silk-based biomaterials. Biomaterials 24, 401–416 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Zhu C, Pongkitwitoon S, Qiu J, Thomopoulos S, Xia Y, Design and fabrication of a hierarchically structured scaffold for tendon-to-bone repair. Adv. Mater 30, e1707306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith LJ, Deymier AC, Boyle JJ, Li Z, Linderman SW, Pasteris JD, Xia Y, Genin GM, Thomopoulos S, Tunability of collagen matrix mechanical properties via multiple modes of mineralization. Interface Focus 6, 20150070 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murugan R, Ramakrishna S, Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 13, 1845–1866 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Pham QP, Sharma U, Mikos AG, Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 7, 2796–2805 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Czaplewski SK, Tsai TL, Duenwald-Kuehl SE, Vanderby R Jr., Li WJ, Tenogenic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells dictated by properties of braided submicron fibrous scaffolds. Biomaterials 35, 6907–6917 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Prabhath A, Vernekar VN, Vasu V, Badon M, Avochinou JE, Asandei AD, Kumbar SG, Weber E, Laurencin CT, Kinetic degradation and biocompatibility evaluation of polycaprolactone-based biologics delivery matrices for regenerative engineering of the rotator cuff. J. Biomed. Mater. Res. A 109, 2137–2153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osti L, Buda M, Buono AD, Osti R, Massari L, Clinical evidence in the treatment of rotator cuff tears with hyaluronic acid. Muscles Ligaments Tendons J. 5, 270–275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blomgran P, Hammerman M, Aspenberg P, Systemic corticosteroids improve tendon healing when given after the early inflammatory phase. Sci. Rep 7, 12468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choi S, Song MH, Shim K-S, Kim H-J, Lim Y-M, Song H-R, Park K, Kim SE, Therapeutic efficacy of intratendinous delivery of dexamethasone using porous microspheres for amelioration of inflammation and tendon degeneration on Achilles tendinitis in rats. Biomed. Res. Int 2020, 5052028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y, Shen W, Tang C, Huang J, Fan C, Yin Z, Hu Y, Chen W, Ouyang H, Zhou Y, Mao Z, Chen X, Targeted pathological collagen delivery of sustained-release rapamycin to prevent heterotopic ossification. Sci. Adv 6, eaay9526 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tarafder S, Brito JA, Minhas S, Effiong L, Thomopoulos S, Lee CH, In situ tissue engineering of the tendon-to-bone interface by endogenous stem/progenitor cells. Biofabrication 12, 015008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP, From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports 19, 500–510 (2009). [DOI] [PubMed] [Google Scholar]
- 103.Gao HGL, Fisher PW, Lambi AG, Wade CK, Barr-Gillespie AE, Popoff SN, Barbe MF, Increased serum and musculotendinous fibrogenic proteins following persistent low-grade inflammation in a rat model of long-term upper extremity overuse. PLOS ONE 8, e71875 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marqueti RC, Durigan JLQ, Oliveira AJS, Mekaro MS, Guzzoni V, Aro AA, Pimentel ER, Selistre-de-Araujo HS, Effects of aging and resistance training in rat tendon remodeling. FASEB J. 32, 353–368 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ, Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J. Shoulder Elbow Surg 14, S79–S83 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Rooney SI, Tobias JW, Bhatt PR, Kuntz AF, Soslowsky LJ, Genetic response of rat supraspinatus tendon and muscle to exercise. PLOS ONE 10, e0139880 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Molloy T, Wang Y, Murrell G, The roles of growth factors in tendon and ligament healing. Sports Med. 33, 381–394 (2003). [DOI] [PubMed] [Google Scholar]
- 108.Kisiel M, Martino MM, Ventura M, Hubbell JA, Hilborn J, Ossipov DA, Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials 34, 704–712 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Yoon JP, Lee CH, Jung JW, Lee HJ, Lee YS, Kim JY, Park GY, Choi JH, Chung SW, Sustained delivery of transforming growth factor β1 by use of absorbable alginate scaffold enhances rotator cuff healing in a rabbit model. Am. J. Sports Med 46, 1441–1450 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Meier Burgisser G, Evrova O, Calcagni M, Scalera C, Giovanoli P, Buschmann J, Impact of PDGF-BB on cellular distribution and extracellular matrix in the healing rabbit Achilles tendon three weeks post-operation. FEBS Open Bio 10, 327–337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Linderman SW, Shen H, Yoneda S, Jayaram R, Tanes ML, Sakiyama-Elbert SE, Xia Y, Thomopoulos S, Gelberman RH, Effect of connective tissue growth factor delivered via porous sutures on the proliferative stage of intrasynovial tendon repair. J. Orthop. Res 36, 2052–2063 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mienaltowski MJ, Adams SM, Birk DE, Regional differences in stem cell/progenitor cell populations from the mouse Achilles tendon. Tissue Eng. Part A 19, 199–210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huegel J, Kim DH, Cirone JM, Pardes AM, Morris TR, Nuss CA, Mauck RL, Soslowsky LJ, Kuntz AF, Autologous tendon-derived cell-seeded nanofibrous scaffolds improve rotator cuff repair in an age-dependent fashion. J. Orthop. Res 35, 1250–1257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shin MJ, Shim IK, Kim DM, Choi JH, Lee YN, Jeon IH, Kim H, Park D, Kholinne E, Yang HS, Koh KH, Engineered cell sheets for the effective delivery of adipose-derived stem cells for tendon-to-bone healing. Am. J. Sports Med 48, 3347–3358 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Franklin A, Gi Min J, Oda H, Kaizawa Y, Leyden J, Wang Z, Chang J, Fox PM, Homing of adipose-derived stem cells to a tendon-derived hydrogel: A potential mechanism for improved tendon-bone interface and tendon healing. J. Hand Surg. Am 45, 1180. e1–1180.e12 (2020). [DOI] [PubMed] [Google Scholar]
- 116.Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, Yang P, Duan X, Zhang J, Fu X, Hu X, Ao Y, Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 106, 328–341 (2020). [DOI] [PubMed] [Google Scholar]
- 117.Hurd JL, Facile TR, Weiss J, Hayes M, Hayes M, Furia JP, Maffulli N, Winnier GE, Alt C, Schmitz C, Alt EU, Lundeen M, Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: A prospective, randomized, controlled first-in-human pilot study. J. Orthop. Surg. Res 15, 122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kwon J, Kim YH, Rhee SM, Kim TI, Lee J, Jeon S, Oh JH, Effects of allogenic dermal fibroblasts on rotator cuff healing in a rabbit model of chronic tear. Am. J. Sports Med 46, 1901–1908 (2018). [DOI] [PubMed] [Google Scholar]
- 119.Poulos MG, Ramalingam P, Gutkin MC, Llanos P, Gilleran K, Rabbany SY, Butler JM, Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J. Clin. Invest 127, 4163–4178 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koob TJ, Lim JJ, Massee M, Zabek N, Denoziere G, Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater 102, 1353–1362 (2014). [DOI] [PubMed] [Google Scholar]
- 121.Deshmukh V, Seo T, O’Green AL, Ibanez M, Hofilena B, Kc S, Stewart J, Dellamary L, Chiu K, Ghias A, Barroga C, Kennedy S, Tambiah J, Hood J, Yazici Y, SM04755, a small-molecule inhibitor of the Wnt pathway, as a potential topical treatment for tendinopathy. J. Orthop. Res 39, 2048–2061 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, Murrell GAC, Liew FY, Kurowska-Stolarska M, McInnes IB, MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat. Commun 6, 6774 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Watts AE, Millar NL, Platt J, Kitson SM, Akbar M, Rech R, Griffin J, Pool R, Hughes T, McInnes IB, Gilchrist DS, MicroRNA29a treatment improves early tendon injury. Mol. Ther 25, 2415–2426 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bokhari AR, Murrell GAC, The role of nitric oxide in tendon healing. J. Shoulder Elbow Surg 21, 238–244 (2012). [DOI] [PubMed] [Google Scholar]
- 125.Kane TPC, Ismail M, Calder JDF, Topical glyceryl trinitrate and noninsertional Achilles tendinopathy: A clinical and cellular investigation. Am. J. Sports Med 36, 1160–1163 (2008). [DOI] [PubMed] [Google Scholar]
- 126.Hunte G, Lloyd-Smith R, Topical glyceryl trinitrate for chronic Achilles tendinopathy. Clin. J. Sport Med 15, 116–117 (2005). [DOI] [PubMed] [Google Scholar]
- 127.Clarke AW, Alyas F, Morris T, Robertson CJ, Bell J, Connell DA, Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. Am. J. Sports Med 39, 614–623 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Obaid H, Clarke A, Rosenfeld P, Leach C, Connell D, Skin-derived fibroblasts for the treatment of refractory Achilles tendinosis: Preliminary short-term results. J. Bone Joint Surg. Am 94, 193–200 (2012). [DOI] [PubMed] [Google Scholar]
- 129.Riley G, Tendinopathy—From basic science to treatment. Nat. Clin. Pract. Rheumatol 4, 82–89 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GAC, McInnes IB, Inflammation is present in early human tendinopathy. Am. J. Sports Med 38, 2085–2091 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Sawant MS, Streu CN, Wu L, Tessier PM, Toward drug-like multispecific antibodies by design. Int. J. Mol. Sci 21, 7496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao Q, Bispecific antibodies for autoimmune and inflammatory diseases: Clinical progress to date. BioDrugs 34, 111–119 (2020). [DOI] [PubMed] [Google Scholar]
- 133.Meric-Bernstam F, Larkin J, Tabernero J, Bonini C, Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet 397, 1010–1022 (2021). [DOI] [PubMed] [Google Scholar]
- 134.Hagiwara Y, Dyrna F, Kuntz AF, Adams DJ, Dyment NA, Cells from a GDF5 origin produce zonal tendon-to-bone attachments following anterior cruciate ligament reconstruction. Ann. N. Y. Acad. Sci 1460, 57–67 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Otabe K, Nakahara H, Hasegawa A, Matsukawa T, Ayabe F, Onizuka N, Inui M, Takada S, Ito Y, Sekiya I, Muneta T, Lotz M, Asahara H, Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J. Orthop. Res 33, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Suzuki H, Ito Y, Shinohara M, Yamashita S, Ichinose S, Kishida A, Oyaizu T, Kayama T, Nakamichi R, Koda N, Yagishita K, Lotz MK, Okawa A, Asahara H, Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc. Natl. Acad. Sci. U.S.A 113, 7840–7845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hullfish TJ, O’Connor KM, Baxter JR, Medial gastrocnemius muscle remodeling correlates with reduced plantarflexor kinetics 14 weeks following Achilles tendon rupture. J. Appl. Physiol. (1985) 127, 1005–1011 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Abraham AC, Shah SA, Golman M, Song L, Li X, Kurtaliaj I, Akbar M, Millar NL, Abu-Amer Y, Galatz LM, Thomopoulos S, Targeting the NF-κB signaling pathway in chronic tendon disease. Sci. Transl. Med 11, eaav4319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hullfish TJ, O’Connor KM, Baxter JR, Instrumented immobilizing boot paradigm quantifies reduced Achilles tendon loading during gait. J. Biomech 109, 109925 (2020). [DOI] [PubMed] [Google Scholar]
- 140.Huang AH, Lu HH, Schweitzer R, Molecular regulation of tendon cell fate during development. J. Orthop. Res 33, 800–812 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Freedman BR, Rodriguez AB, Hillin CD, Weiss SN, Han B, Han L, Soslowsky LJ, Tendon healing affects the multiscale mechanical, structural and compositional response of tendon to quasi-static tensile loading. J. R. Soc. Interface 15, 20170880 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roger E, Lagarce F, Garcion E, Benoit JP, Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine (Lond.) 5, 287–306 (2010). [DOI] [PubMed] [Google Scholar]
- 143.Antosova Z, Mackova M, Kral V, Macek T, Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 27, 628–635 (2009). [DOI] [PubMed] [Google Scholar]
- 144.Li J, Weber E, Guth-Gundel S, Schuleit M, Kuttler A, Halleux C, Accart N, Doelemeyer A, Basler A, Tigani B, Wuersch K, Fornaro M, Kneissel M, Stafford A, Freedman BR, Mooney DJ, Tough composite hydrogels with high loading and local release of biological drugs. Adv. Healthc. Mater 7, e1701393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.DiMasi JA, Grabowski HG, Hansen RW, Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ 47, 20–33 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Wouters OJ, McKee M, Luyten J, Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 323, 844–853 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.AliraHealth, “Global trends & opportunities: Medical device contract engineering and manufacturing” (2021).
- 148.Holmes D, A new chapter in innovation. Nature 533, S54–S55 (2016). [DOI] [PubMed] [Google Scholar]
- 149.Duda GN, Grainger DW, Frisk ML, Bruckner-Tuderman L, Carr A, Dirnagl U, Einhäupl KM, Gottschalk S, Gruskin E, Huber C, June CH, Mooney DJ, Rietschel ET, Schütte G, Seeger W, Stevens MM, Urban R, Veldman A, Wess G, Volk H-D, Changing the mindset in life sciences toward translation: A consensus. Sci. Transl. Med 6, 264cm12 (2014). [DOI] [PubMed] [Google Scholar]
- 150.Miao L, Zhang Y, Huang L, mRNA vaccine for cancer immunotherapy. Mol. Cancer 20, 41 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Groth K, Berezhanskyy T, Aneja MK, Geiger J, Schweizer M, Maucksch L, Pasewald T, Brill T, Tigani B, Weber E, Rudolph C, Hasenpusch G, Tendon healing induced by chemically modified mRNAs. Eur. Cell. Mater 33, 294–307 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Aunger JA, Millar R, Greenhalgh J, Mannion R, Rafferty A-M, McLeod H, Why do some inter-organisational collaborations in healthcare work when others do not? A realist review. Syst. Rev 10, 82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gersdorf T, He VF, Schlesinger A, Koch G, Ehrismann D, Widmer H, von Krogh G, Demystifying industry-academia collaboration. Nat. Rev. Drug Discov 18, 743–744 (2019). [DOI] [PubMed] [Google Scholar]
- 154.Scott A, Squier K, Alfredson H, Bahr R, Cook JL, Coombes B, de Vos RJ, Fu SN, Grimaldi A, Lewis JS, Maffulli N, Magnusson SP, Malliaras P, Mc Auliffe S, Oei EHG, Purdam CR, Rees JD, Rio EK, Gravare Silbernagel K, Speed C, Weir A, Wolf JM, Akker-Scheek IVD, Vicenzino BT, Zwerver J, ICON 2019: International scientific tendinopathy symposium consensus: Clinical terminology. Br. J. Sports Med 54, 260–262 (2020). [DOI] [PubMed] [Google Scholar]
- 155.Macdermid JC, Silbernagel KG, Outcome evaluation in tendinopathy: Foundations of assessment and a summary of selected measures. J. Orthop. Sports Phys. Ther 45, 950–964 (2015). [DOI] [PubMed] [Google Scholar]
- 156.Korakakis V, Kotsifaki A, Stefanakis M, Sotiralis Y, Whiteley R, Thorborg K, Evaluating lower limb tendinopathy with victorian institute of sport assessment (VISA) questionnaires: A systematic review shows very-low-quality evidence for their content and structural validity-part I. Knee Surg. Sports Traumatol. Arthrosc 29, 2749–2764 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nowell WB, Curtis JR, Nolot SK, Curtis D, Venkatachalam S, Owensby JK, Poon JL, Calvin AB, Kannowski CL, Faries DE, Gavigan K, Haynes VS, Digital tracking of rheumatoid arthritis longitudinally (DIGITAL) using biosensor and patient-reported outcome data: Protocol for a real-world study. JMIR Res. Protoc 8, e14665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Docking SI, Ooi CC, Connell D, Tendinopathy: Is imaging telling us the entire story? J. Orthop. Sports Phys. Ther 45, 842–852 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Klatte-Schulz F, Minkwitz S, Schmock A, Bormann N, Kurtoglu A, Tsitsilonis S, Manegold S, Wildemann B, Different Achilles tendon pathologies show distinct histological and molecular characteristics. Int. J. Mol. Sci 19, 404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.September A, Rahim M, Collins M, Towards an understanding of the genetics of tendinopathy. Adv. Exp. Med. Biol 920, 109–116 (2016). [DOI] [PubMed] [Google Scholar]
- 161.Zheng F, Wang H, Gong H, Fan H, Zhang K, Du L, Role of ultrasound in the detection of rotator-cuff syndrome: An observational study. Med. Sci. Monit 25, 5856–5863 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schieker M, Conaghan PG, Mindeholm L, Praestgaard J, Solomon DH, Scotti C, Gram H, Thuren T, Roubenoff R, Ridker PM, Effects of interleukin-1β inhibition on incident hip and knee replacement: Exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med 173, 509–515 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Framework for FDA’s Real-World Evidence Program, (2018) https://www.fda.gov/media/120060/download.
- 164.Laurent D, Walsh L, Muaremi A, Beckmann N, Weber E, Chaperon F, Haber H, Goldhahn J, Klauser AS, Blauth M, Schieker M, Relationship between tendon structure, stiffness, gait patterns and patient reported outcomes during the early stages of recovery after an Achilles tendon rupture. Sci. Rep 10, 20757 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Corrigan-Curay J, Sacks L, Woodcock J, Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA 320, 867–868 (2018). [DOI] [PubMed] [Google Scholar]
- 166.Broderick JP, Elm JJ, Janis LS, Zhao W, Moy CS, Dillon CR, Chimowitz MI, Sacco RL, Cramer SC, Wolf SL, Johnston KC, Saver JL, Marshall RS, Brown D, Wintermark M, Elkind MSV, Kamel H, Tirschwell DL, Longstreth WT, Chervin RD, Adeoye OM, Barreto AD, Grotta JC, Ramey SL, Lo WD, Feng W, Schlaug G, Sheth KN, Selim M, Naidech AM, Lansberg MG, Lazar RM, Albers GW, Griffin JS, Sirline LP, Frasure J, Wright CB, Khatri P; NIHS StrokeNet Investigators, National Institutes of Health strokenet during the time of COVID-19 and beyond. Stroke 51, 2580–2586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dockendorf MF, Hansen BJ, Bateman KP, Moyer M, Shah JK, Shipley LA, Digitally enabled, patient-centric clinical trials: Shifting the drug development paradigm. Clin. Transl. Sci 14, 445–459 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hopkins C, Fu SC, Chua E, Hu X, Rolf C, Mattila VM, Qin L, Yung PS, Chan KM, Critical review on the socio-economic impact of tendinopathy. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol 4, 9–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kramer JS, Yelin EH, Epstein WV, Social and economic impacts of four musculoskeletal conditions. A study using national community-based data. Arthritis Rheum. 26, 901–907 (1983). [DOI] [PubMed] [Google Scholar]
- 170.Walker-Bone K, Palmer KT, Reading I, Coggon D, Cooper C, Occupation and epicondylitis: A population-based study. Rheumatology (Oxford) 51, 305–310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bonde JP, Mikkelsen S, Andersen JH, Fallentin N, Baelum J, Svendsen SW, Thomsen JF, Frost P, Thomsen G, Overgaard E, Kaergaard A; PRIM Health Study Group, Prognosis of shoulder tendonitis in repetitive work: A follow up study in a cohort of Danish industrial and service workers. Occup. Environ. Med 60, E8 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mather RC III, Koenig L, Acevedo D, Dall TM, Gallo P, Romeo A, Tongue J, Williams G Jr., The societal and economic value of rotator cuff repair. J. Bone Joint Surg. Am 95, 1993–2000 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hunziker E, Spector M, Libera J, Gertzman A, Woo SL-Y, Ratcliffe A, Lysaght M, Coury A, Kaplan D, Vunjak-Novakovic G, Translation from research to applications. Tissue Eng. 12, 3341–3364 (2006). [DOI] [PubMed] [Google Scholar]
- 174.Van Norman GA, Drugs, devices, and the FDA: Part 1: An overview of approval processes for drugs. JACC Basic Transl. Sci 1, 170–179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Koltsov JCB, Gribbin C, Ellis SJ, Nwachukwu BU, Cost-effectiveness of operative versus non-operative management of acute Achilles tendon ruptures. HSS J 16, 39–45 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


