Abstract
The aluminum (Al)-induced secretion of citrate has been regarded as an important mechanism for Al resistance in soybean (Glycine max). However, the mechanism of how Al induces citrate secretion remains unclear. In this study, we investigated the regulatory role of plasma membrane H+-ATPase on the Al-induced secretion of citrate from soybean roots. Experiments performed with plants grown in full nutrient solution showed that Al-induced activity of plasma membrane H+-ATPase paralleled secretion of citrate. Vanadate and fusicoccin, an inhibitor and an activator, respectively, of plasma membrane H+-ATPase, exerted inhibitory and stimulatory effects on the Al-induced secretion of citrate. Higher activity of plasma membrane H+-ATPase coincided with more citrate secretion in Al-resistant than Al-sensitive soybean cultivars. These results suggested that the effects of Al stress on citrate secretion were mediated via modulation of the activity of plasma membrane H+-ATPase. The relationship between the Al-induced secretion of citrate and the activity of plasma membrane H+-ATPase was further demonstrated by analysis of plasma membrane H+-ATPase transgenic Arabidopsis (Arabidopsis thaliana). When plants were grown on Murashige and Skoog medium containing 30 μm Al (9.1 μm Al3+ activity), transgenic plants exuded more citrate compared with wild-type Arabidopsis. Results from real-time reverse transcription-PCR and immunodetection analysis indicated that the increase of plasma membrane H+-ATPase activity by Al is caused by transcriptional and translational regulation. Furthermore, plasma membrane H+-ATPase activity and expression were higher in an Al-resistant cultivar than in an Al-sensitive cultivar. Al activated the threonine-oriented phosphorylation of plasma membrane H+-ATPase in a dose- and time-dependent manner. Taken together, our results demonstrated that up-regulation of plasma membrane H+-ATPase activity was associated with the secretion of citrate from soybean roots.
Aluminum (Al) toxicity in plants is one of the major limitations to crops grown on acid soils (Horst, 1995; Kochian, 1995; Taylor, 1995; Matsumoto, 2000; Ryan et al., 2001). Exudation of organic acids is an important mechanism for plants to resist Al toxicity. Many plants were found to secrete organic acids in response to Al stress. For instance, citrate is released from the roots of soybean (Glycine max; Yang et al., 2001) and maize (Zea mays; Piñeros et al., 2002), oxalate from buckwheat (Fagopyrum esculentum; Ma et al., 1997), and malate is released from the roots of Al-tolerant genotypes of wheat (Triticum aestivum; Delhaize et al., 1993; Ryan et al., 1995). However, it remains unclear how Al activates organic acid secretion and how this process is regulated (Ryan et al., 2001). Recent studies indicated that the Al-dependent secretion of organic acids in soybean, wheat, and rye is poorly associated with changes in internal organic acid concentrations or with the activities of enzymes that synthesize these acids in the root cells (Ryan et al., 1995; Yang et al., 2001; Hayes and Ma, 2003). The Al-dependent secretion of malate from wheat roots and citrate from maize roots occurs via anion channels on the plasma membrane of the root cells (Ryan et al., 1997; Zhang et al., 2001; Piñeros et al., 2002; Sasaki et al., 2004). The activity of these channels depends on some direct or indirect interaction between Al and the channel protein, although the nature of these is unclear. Indirect interactions with Al might include kinase or phosphatase enzymes, or the activation of secondary messenger pathways that involve changes in cytoplasmic calcium or hormones (Haug et al., 1994; Ryan et al., 1995; Osawa and Matsumoto, 2001; Shen et al., 2004).
The plasma membrane H+-ATPase is the most abundant protein on the plasma membrane and is involved in multiple stress responses. This protein activates a series of secondary transporters since the transmembrane movement of many solutes, assimilates, or metabolites is dependent on proton motive force (Serrano, 1989; Sussman, 1994). Recent studies indicated that plasma membrane H+-ATPase is involved in the regulation of responses to a variety of environmental stimuli, such as Al stress (Ahn et al., 2004), NaCl stress (Niu et al., 1993; 1996), phosphorus deficiency (Yan et al., 2002), and ammonium stress (Jernejc and Legisa, 2001). Plasma membrane H+-ATPase is also involved in auxin-mediated cell elongation during wheat embryo development (Rober-Kleber et al., 2003). Transcript levels of plasma membrane H+-ATPase increase more in the roots of halophytes treated with NaCl than glycophytes (Niu et al., 1993), suggesting that the capacity of the plasma membrane H+-ATPase gene to respond to NaCl may contribute to the greater salt tolerance of halophytes. Furthermore, in tomato, modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses (Schaller and Oecking, 1999).
Since proton exudation contributes to charge balance during the secretion of organic acid anions from lupin roots (Yan et al., 2002), we hypothesized that plasma membrane H+-ATPase would also be involved in the Al-dependent secretion of citrate from soybean roots. In this study, we investigated whether plasma membrane H+-ATPase is involved in the Al-induced secretion of citrate from soybean roots and, if so, how it modulates this response.
RESULTS
Activity of Plasma Membrane H+-ATPase and Secretion of Citrate
To examine whether the plasma membrane H+-ATPase is involved in the Al-induced secretion of citrate, we compared the activity of this enzyme with the secretion of citrate from soybean roots. In contrast to a number of previous studies where organic acid secretion from roots was measured in a simple salt solution containing CaCl2 only (Li et al., 2000; Yang et al., 2001; Piñeros et al., 2002), we used a complete nutrient solution for soybean seedlings and Murashige and Skoog medium for Arabidopsis seedlings. To avoid contaminating the plasma membrane isolates with endoplasmic membranes, we examined the activity of various inhibitor-sensitive ATPases in both the microsomal and plasma membrane fractions (Table I). In the microsomal fraction, molybdate-sensitive ATPase was the major enzyme that showed the presence of unspecific acid phosphatases (Widell and Larsson, 1990). Vanadate (VA)-sensitive ATPase occupied 91.3% of the total activity in the plasma membrane fraction, and other inhibitor-sensitive enzyme activity was negligible (Table I).
Table I.
Specific activities of membrane-bound proteins in microsomes and plasma membrane vesicles isolated from soybean roots
Membranes were isolated from the root apices of 8-d-old Suzunari seedlings. Assays were conducted at 30°C The inhibitor-sensitive activity was calculated by subtracting the ATP-hydrolytic activity in the presence of inhibitor from the activity of the control. The values are means of three replicates of two independent experiments.
| Type | Microsome Fraction | Plasma Membrane Fraction |
|---|---|---|
| μmol min−1 mg−1 protein | ||
| VA-sensitive ATPase (1 mm) | 0.11 ca (7.15) | 1.16 a (91.3) |
| Nitrate-sensitive ATPase (50 mm) | 0.26 b (17.1) | 0.02 b (1.58) |
| Azide-sensitive ATPase (1 mm) | 0.13 c (8.55) | 0.03 b (2.37) |
| Molybdate-sensitive ATPase (1 mm) | 0.74 a (48.7) | 0.05 b (3.95) |
| NADH cyt-c reductase | 0.31 b (20.5) | 0.01 b (0.80) |
Different letters in the same column indicate a significant difference at the 0.05 level, according to Duncan's Multiple Range Test. Data in parentheses show the percentage of each enzyme to the total.
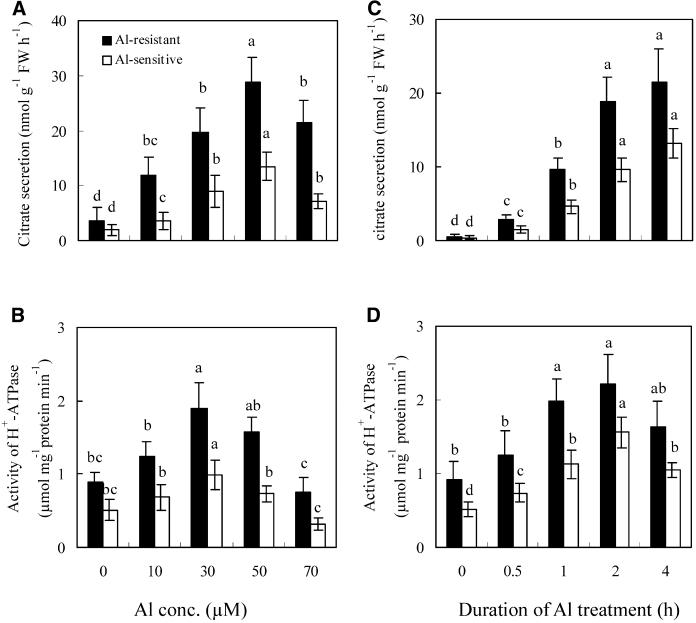
As reported previously (Shen et al., 2004), Al treatment induced the secretion of citrate in a dose- and time-dependent manner (Fig. 1, A and C). The activity of plasma membrane H+-ATPase in root cells was similarly stimulated by Al treatment (Fig. 1, B and D). Citrate secretion and activity of plasma membrane H+-ATPase increased with increasing Al concentrations up to 50 and 30 μm, respectively, but higher concentrations caused no extra stimulation and even began to inhibit these responses. Citrate secretion and plasma membrane H+-ATPase activity also increased with the time of Al treatment up to about 2 h (Fig. 1, C and D). The maximal activity of plasma membrane H+-ATPase was obtained by exposure to 30 μm Al for 2 h, and the maximal citrate secretion by exposure to 50 μm Al for 4 h. The covariance of the Al-induced activity of plasma membrane H+-ATPase and citrate secretion was analyzed at different Al levels and duration times. Results indicated that Al treatment had a significant effect on the relationship between the Al-induced activity of plasma membrane H+-ATPase and citrate secretion (F1, 96 = 38.9; P < 0.001). Under Al treatment, the activity of plasma membrane H+-ATPase had a linear correlation with citrate secretion. Greater activity of plasma membrane H+-ATPase coincided with more citrate secretion in Al-resistant than Al-sensitive cultivars (Fig. 1).
Figure 1.
Effect of Al concentration (A and B) and duration of Al treatment (C and D) on citrate secretion (A and C) and the activity of plasma membrane H+-ATPase (B and D). Soybean seedlings were incubated in nutrient solution for 8 d, then treated with Al in the nutrient solution at pH 4.5. In A and B, the duration of Al treatment was 4 h, and in C and D, the concentration of Al was 30 μm. After treatment, root exudates were analyzed for citrate by HPLC, and the root apices (1 cm) were used for analysis of the activity of plasma membrane H+-ATPase. See details in “Materials and Methods.” Each value is the mean ± se (n = 5). Different letters in the same column or cultivar indicate that the values are significantly different at the 0.05 level, according to Duncan's Multiple Range Test.
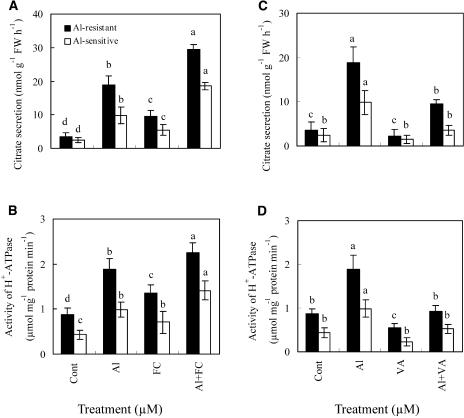
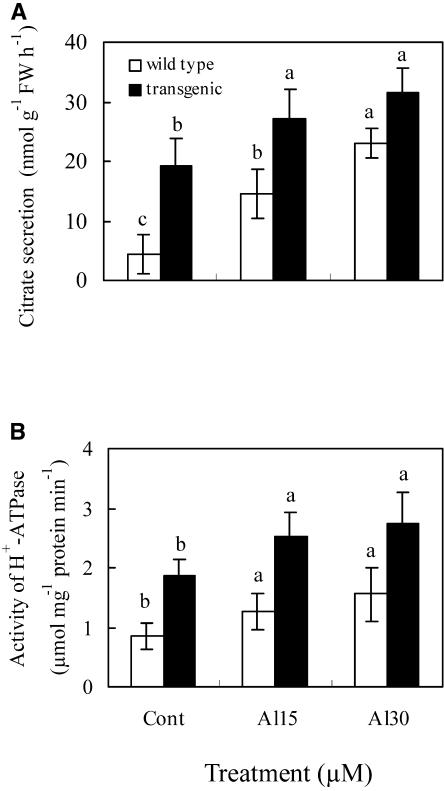
Addition of fusicoccin (FC), an activator of plasma membrane H+-ATPase, stimulated both citrate secretion and activity of plasma membrane H+-ATPase in an Al-resistant cultivar in the presence or absence of Al (Fig. 2, A and B). In an Al-sensitive cultivar, Al plus FC treatment also increased both citrate secretion and activity of plasma membrane H+-ATPase significantly in comparison to Al or FC treatment. Exposure to VA, an inhibitor of plasma membrane H+-ATPase, suppressed the Al-induced secretion of citrate and the activity of plasma membrane H+-ATPase (Fig. 2, C and D). To investigate the correlation between the Al-induced secretion of citrate and the activity of plasma membrane H+-ATPase, transgenic Arabidopsis was generated by overexpressing plasma membrane H+-ATPase using the 35S promoter. When plants were grown on Murashige and Skoog medium containing 30 μm Al (9.1 μm Al3+ activity), transgenic plants exuded more citrate compared with wild-type Arabidopsis. Al-induced secretion of citrate and activity of plasma membrane H+-ATPase in both wild-type and transgenic Arabidopsis showed similar changing patterns (Fig. 3, A and B). By contrast, Hoekenga et al. (2003) indicated that Al suppressed the secretion of citrate, but enhanced the secretion of malate from Arabidopsis roots. These contrasting results were probably due to differences in Al-treated levels and other experimental conditions.
Figure 2.
Effects of FC and VA on the Al-induced citrate secretion (A and C) and the activity of plasma membrane H+-ATPase (B and D) in soybean cultivars. Soybean seedlings were incubated in nutrient solution for 8 d, then treated with 30 μm Al together with 5 μm FC or 100 μm VA. After a 4-h treatment, exudates from soybean roots were analyzed for citrate by HPLC. The root apices (1 cm) of soybean were analyzed for the activity of plasma membrane H+-ATPase. See details in “Materials and Methods.” Each value is the mean ± se (n = 5). Different letters in the same column or cultivar indicate that the values are significantly different at the 0.05 level, according to Duncan's Multiple Range Test.
Figure 3.
Effect of Al concentration on the secretion (A) of citrate and the activity (B) of plasma membrane H+-ATPase in Arabidopsis roots. Arabidopsis seedlings were incubated in one-sixth Murashige and Skoog solution for 14 d, then exposed to 15 or 30 μm Al (9.1 μm Al3+ activity) in one-sixth Murashige and Skoog solution (pH 4.5) for 2 h. After treatment, the incubation solution was analyzed for citrate by an enzymatic method. The whole roots of Arabidopsis were analyzed for the activity of plasma membrane H+-ATPase. See details in “Materials and Methods.” Each value is the mean ± se (n = 5). Different letters in the same column or plant indicate that the values are significantly different at the 0.05 level, according to Duncan's Multiple Range Test.
Plasma Membrane H+-ATPase Gene Expression
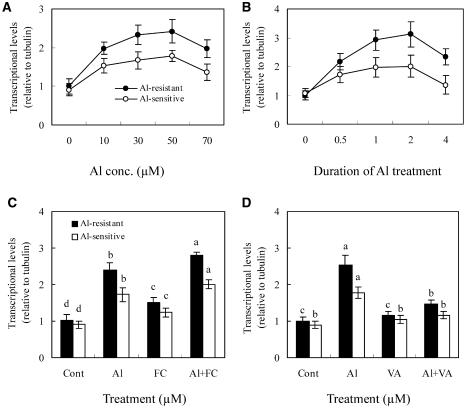
To examine whether transcriptional regulation is involved in the induction of the plasma membrane H+-ATPase gene during Al treatment, we performed real-time reverse transcription (RT)-PCR experiments using primers designed from AF091303 and mRNA isolated from the 1-cm root apices of soybean seedlings. Al increased the expression of the plasma membrane H+-ATPase gene in the root apices of soybean in a dose-dependent manner up to 30 μm Al, while higher concentrations up to 70 μm did not significantly affect the level of plasma membrane H+-ATPase transcript (Fig. 4A). Exposure to 30 μm of Al-induced accumulation of plasma membrane H+-ATPase mRNA in a time-dependent manner up to 2 h, but longer exposures decreased mRNA accumulation (Fig. 4B). Application of FC, which was previously shown to increase citrate secretion (Fig. 2A), significantly enhanced the expression of the plasma membrane H+-ATPase gene in the presence or absence of Al (Fig. 4C). By contrast, VA, which decreased citrate secretion, suppressed the transcriptional expression of the plasma membrane H+-ATPase gene greatly in the presence of Al (Fig. 4D). Furthermore, the Al-induced expression of plasma membrane H+-ATPase was consistently greater in the roots of the Al-resistant genotype than in the Al-sensitive soybean (Fig. 4, A and B). The β-tubulin gene is a constitutively expressed gene that was used as an internal standard for these measurements, and separate RT-PCR analysis showed that expression of the β-tubulin gene did not change significantly in response to these different treatments (data not shown).
Figure 4.
Quantitative real-time RT-PCR analysis of the transcriptional level of the plasma membrane H+-ATPase gene. After incubation in nutrient solution for 8 d, the seedling roots were treated in the nutrient solution (pH 4.5) as follows. A, Duration of Al treatment was 2 h. B, Concentration of Al was 30 μm. C, Concentration of Al and FC was 30 and 5 μm, respectively, and the duration of treatment was 2 h. D, Concentration of Al and VA was 30 and 100 μm, respectively, and the duration of treatment was 2 h. After the treatment, the root apices (1 cm) were excised and mRNA was isolated. See details in “Materials and Methods.” Each value is the mean ± se (n = 5). Different letters in the same column or cultivar indicate that the values are significantly different at the 0.05 level, according to Duncan's Multiple Range Test.
Western-Blot Analysis and Immunolocalization of Plasma Membrane H+-ATPase
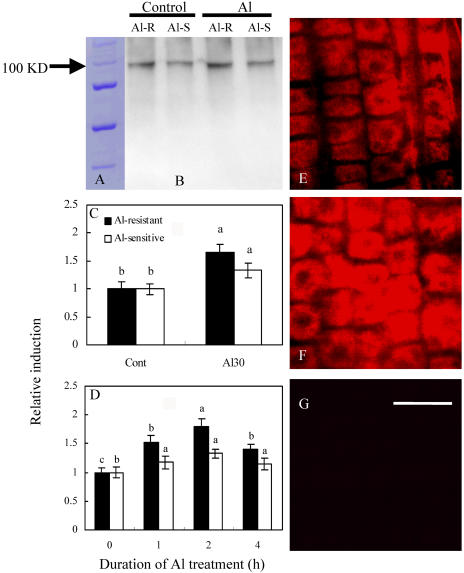
To investigate whether translational regulation is involved in the response to Al treatment, we conducted immunodetection of plasma membrane H+-ATPase, and relative induction was calculated using a fluorescence spectrophotometer (Fig. 5). Using a polyclonal antibody against maize plasma membrane H+-ATPase, we observed a signal band at 100 kD corresponding to plasma membrane H+-ATPase (Fig. 5A). Al increased the protein amount of plasma membrane H+-ATPase by 65% and 33% in the roots of Al-resistant and Al-sensitive cultivars, respectively (Fig. 5B). Furthermore, Al increased the accumulation of plasma membrane H+-ATPase in a dose- and time-dependent manner (Fig. 5, C and D). An Al-induced increase of plasma membrane H+-ATPase protein occurred within 2 h. The polyclonal antibody also decorated the plasma membrane of all cells along the root apices of Al-resistant soybean roots (Fig. 5, E and F). Al increased the fluorescence intensity of plasma membrane H+-ATPase by 48.5% in the root apices of Al-resistant seedlings. Figure 5G was the negative control of roots incubated without antibody.
Figure 5.
Western-blot analysis (A–D) and immunolocalization (E–G) of plasma membrane H+-ATPase. After an 8-d incubation in nutrient solution, soybean roots of Al-resistant (Al-R) and Al-sensitive (Al-S) cultivars were treated in the nutrient solution (pH 4.5) as follows. A, Marker. B, Control and 30 μm Al for 2 h. C and D, Relative induction of protein expression of plasma membrane H+-ATPase after setting the expression level of each cultivar at control or 0 h as 1. Treatment time was 2 h in C, and concentration of Al was 30 μm Al in D; E, Al-R, control. F, Al-R, 30 μm Al. G, Al-R, negative control. Bar in G = 250 μm. Treatment time in E to G was 2 h. After treatment, root apices were used for preparation of plasma membrane H+-ATPase proteins or for immunolocalization. See details in “Materials and Methods.” The results shown in Figure 5, C and D, are representative of three independent experiments. Different letters in the same column or cultivar indicate that the values are significantly different at the 0.05 level, according to Duncan's Multiple Range Test.
Phosphorylation of Plasma Membrane H+-ATPase
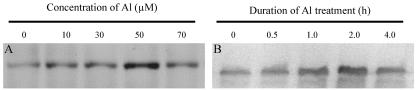
Since calcium-dependent phosphorylation has been shown to regulate the activity of plasma membrane H+-ATPase in maize (Nisi et al., 1999), we examined whether phosphorylation of plasma membrane H+-ATPase was involved in the Al-dependent increase in plasma membrane H+-ATPase activity. To verify the specificity of the antibody against phosphoamino acids, a positive control was always conducted with the samples in the experiments. By comparing the position of bands in the identical position on the membrane, the specificity of antibody would be confirmed. The Thr-, Tyr-, and Ser-oriented phosphorylation of plasma membrane H+-ATPase was measured in response to Al stress. Al did not induce the Tyr- and Ser-oriented phosphorylation of plasma membrane H+-ATPase (data not shown), but Al treatment did enhance the Thr-oriented phosphorylation of plasma membrane H+-ATPase in a dose- and time-dependent manner (Fig. 6). Phosphorylation of plasma membrane H+-ATPase increased with increasing Al concentration up to 50 μm, but further increase of Al concentration decreased the phosphorylation (Fig. 6A). Al at 30 μm activated the phosphorylation of plasma membrane H+-ATPase up to 2 h, followed by a decrease at 4 h after Al addition (Fig. 6B). A weak, but clear, band of phosphorylated plasma membrane H+-ATPase in the control suggested that a part of plasma membrane H+-ATPase was phosphorylated to maintain the basic activity in the absence of Al.
Figure 6.
Phosphorylated H+-ATPase on plasma membrane. After an 8-d incubation in nutrient solution, Al-resistant soybean roots were treated in nutrient solution (pH 4.5) as follows. A, Duration of Al treatment was 2 h. B, Concentration of Al was 30 μm. After the treatment, the root apices (1 cm) were excised and used for preparation of plasma membrane H+-ATPase proteins. See details in “Materials and Methods.”
DISCUSSION
Activity of Plasma Membrane H+-ATPase and Citrate Secretion
Plants showed a series of physiological changes in response to Al stress, and the secretion of organic acids is regarded as a protective measure in some species because many organic anions can chelate the harmful Al cations and detoxify them (Kochian, 1995; Matsumoto, 2000; Ryan et al., 2001). Recent progress in this area has shown that the conductance of anion transporters plays an important role in regulating this process (Ryan et al., 1997; Kollmeier et al., 2001; Zhang et al., 2001). Results presented here indicate that plasma membrane H+-ATPase is involved in the Al-induced secretion of citrate from soybean roots (Figs. 1–3) and this conclusion is consistent with several previous reports (Yan et al., 2002; Ohno et al., 2003; Ahn et al., 2004; Ligaba et al., 2004). For instance, an elevated level of plasma membrane H+-ATPase activity was measured from a mutant carrot (Daucus carota) cell line that showed greater citrate excretion than wild-type cells (Ohno et al., 2003). This could be related to the generation of an electrochemical gradient for protons across the plasma membrane by H+-ATPase, which can energize an array of secondary transporters (Arango et al., 2003). In this study, Al caused both stimulatory and inhibitory effects on plasma membrane H+-ATPase activity depending on the concentration of Al used and the exposure time (Fig. 1), and this has been reported previously (Ahn et al., 2001; Facanha and Okorokova-Facanha, 2002). Matsumoto (1988) observed a 50% decrease in plasma membrane H+-ATPase activity in barley roots treated with 100 μm Al for 48 h in 0.5 mm CaCl2 solution. In wheat, the activity of plasma membrane H+-ATPase declined by 13% to 19% after treatment with 50 μm AlCl3 for 24 h (Ahn et al., 2004). In nutrient solution (pH 4.5), 100 μm Al promoted ATP hydrolysis and stimulated the establishment of an ATP-dependent proton gradient in cells of corn roots, and 300 μm Al inhibited the root growth of seedlings (Facanha and Okorokova-Facanha, 2002). Kinraide (1988, 1993) showed that 100 μm Al increased the cell membrane electrical polarity and stimulated H+ extrusion (i.e. ATP hydrolytic activity). It is possible that Al stimulates the activity of plasma membrane H+-ATPase at concentrations below those that are toxic (Mullette, 1975; Clune and Copeland, 1999). We previously showed that Al strongly inhibited the activity of plasma membrane H+-ATPase in an Al-sensitive genotype of squash by altering the plasma membrane surface potentials (Ahn et al., 2001). These contrasting responses of the plasma membrane H+-ATPase to Al treatment might be ascribed to differences in the experimental conditions as well as plant species.
Gene Expression and Protein Phosphorylation
Plasma membrane H+-ATPase is encoded by a family of genes that show signs of both transcriptional and posttranscriptional regulation (Michelet et al., 1994). In soybean, plasma membrane H+-ATPase was encoded by gene accession number AF091303. To clone the plasma membrane H+-ATPase gene, we designed the primers based on gene accession number AF091303, and thus follow the same gene by RT-PCR in soybean. The expression of the plasma membrane H+-ATPase gene was influenced by various environmental stimuli, such as symbiotic status, and the phosphate and Suc levels (Requena et al., 2003). As an elicitor, FC activated pathogen-responsive gene expression and induced a programmed cell death (Malerba et al., 2003; Singh and Roberts, 2004). In an Al-tolerant genotype of soybean, Al stress enhanced the activity of phosphoenolpyruvate carboxylase and inosine-5′-monophosphate dehydrogenase, both of which are constitutive genes (Ermolayev et al., 2003). In this study, we showed that Al induced the expression of another constitutive gene, plasma membrane H+-ATPase, in a dose- and time-dependent manner (Fig. 4). Al initiated the gene expression of plasma membrane H+-ATPase very quickly (30 min after Al addition), with a maximal expression occurring 2 h after the addition of Al (Fig. 4B). Furthermore, FC and VA could modulate Al-induced gene expression of plasma membrane H+-ATPase. These results suggest that the plasma membrane H+-ATPase gene is among those that are rapidly induced by Al stress (Richards et al., 1998; Ezaki et al., 2000). Over 20 Al-induced genes have been isolated from wheat, soybean, tobacco (Nicotiana tabacum), and yeast (Saccharomyces cerevisiae) cells so far. Up-regulation of ATPase activity may also be an adaptive response related to Al resistance in wheat as well (Hamilton et al., 2001).
The greater steady-state activity of the plasma membrane H+-ATPase enzyme observed in the roots of the Al-resistant soybean cultivar compared to the Al-sensitive cultivar may result from a higher gene expression (transcriptional level), enhanced protein synthesis (translational level), or from a lower degradation rate of plasma membrane H+-ATPase protein (Sze et al., 1999). In the coleoptile of maize, auxin not only stimulated gene expression of plasma membrane H+-ATPase but also enhanced the membrane flow from endoplasmic reticulum to plasma membrane, resulting in a high steady-state H+-ATPase concentration in the plasma membrane (Hager et al., 1991). In this study, increased expression of plasma membrane H+-ATPase was observed by Al treatment in a dose- and time-dependent manner (Fig. 4, A and B). Furthermore, the rate of increase was higher in Al-resistant than in Al-sensitive cultivars. The amount of plasma membrane H+-ATPase protein was increased by Al treatment, suggesting that plasma membrane H+-ATPase synthesis was stimulated by this treatment (Fig. 5). Therefore, the increased activity of the plasma membrane H+-ATPase of soybean root tips under Al stress is caused by enhancement at the transcriptional and translational levels. The 14-3-3 proteins have been reported to be involved in the regulation of phosphorylation of plasma membrane H+-ATPase by forming a complex with the plasma membrane H+-ATPase at the C-terminal end of the protein (Olsson et al., 1998; Kinoshita and Shimazaki, 1999). Al also triggered the Thr-oriented phosphorylation of plasma membrane H+-ATPase in a dose- and time-dependent manner (Fig. 6). It is likely that Al stimulated the activity of endogenous 14-3-3 proteins and, together, they mediated the phosphorylation of plasma membrane H+-ATPase. Protein phosphorylation was reported to contribute to the high activity of plasma membrane H+-ATPase (Serrano, 1989; Chang and Slayman, 1991; Palmgren, 1998). We showed that the activation of plasma membrane H+-ATPase by Al is caused, in part, by the enhanced phosphorylation of plasma membrane H+-ATPase. Consistent with our results, the phosphorylation of plasma membrane H+-ATPase could trigger the ammonium-induced activation of proton extrusion in Aspergillus niger (Jernejc and Legisa, 2001). In wheat, protein phosphorylation was suggested to be involved in Al-responsive malate efflux from the root apices (Osawa and Matsumoto, 2001). The level of phosphorylation, the activity of plasma membrane H+-ATPase, and the secretion of citrate showed similar tendencies in response to Al stress (Figs. 1, 2, and 6), suggesting that the phosphorylation of plasma membrane H+-ATPase was involved in its activation, and that the increased plasma membrane H+-ATPase activity is related to the Al-induced secretion of citrate.
Ohno et al. (2003) reported that a mutant carrot cell line (insoluble phosphate grower), which grows normally in an Al-phosphate medium, displayed enhanced citrate secretion and greater plasma membrane H+-ATPase activity. They speculated that plasma membrane H+-ATPase is involved in the exudation process of citrate in the insoluble phosphate grower cells, perhaps even via a citrate-proton cotransport system. Recently, Ligaba et al. (2004) found that exudation of citrate in greater purple lupin roots under phosphate deficiency is inhibited by VA treatment in vivo. Furthermore, plasma membrane H+-ATPase activity from phosphate-deficient roots was much higher than that of phosphate-sufficient roots. In this study, we have provided further insight into the role of plasma membrane H+-ATPase on citrate secretion from soybean root tips under Al stress.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of soybean (Glycine max) were gently ground with sea sand (20–30 mesh) for 10 s to facilitate germination. Pretreated seeds were soaked in a solution containing 0.5 mm CaCl2 for 30 min and then germinated in peat moss mixed with sand quartz for 3 d at 25°C. After germination, the seedlings were transferred to nutrient solution (in micromolars) containing KNO3 (750), Ca(NO3)2 (250), MgSO4 (325), KH2PO4 (20), Fe-EDTA (20), H3BO3 (8), CuSO4 (0.2), ZnSO4 (0.2), MnCl2 (0.2), and (NH4)6Mo7O24 (0.2) in 2-L plastic pots. The seedlings were grown in a growth cabinet at 25°C/20°C, 14-h-day/10-h-night cycles, 40 μmol m−2 s−1 light intensity, and 70% relative humidity. After 8 d of growth, the seedling roots were treated with the test chemicals in the nutrient solution (pH 4.5). FC was dissolved in methanol at 1 mm as stock solution. Methanol in each treatment was less than 0.2%, which did not affect citrate secretion and activity of plasma membrane H+-ATPase. After the treatment, root exudates were collected for citrate analysis, and the seedling roots were collected for analysis of the activity of plasma membrane H+-ATPase. Arabidopsis (Arabidopsis thaliana) seedlings were cultured as follows. Sterilized seeds were incubated on a nylon mesh square cup (mesh size, 300 μm; cup size, 4 cm wide × 4 cm long × 1 cm high) under irradiation at 50 μE m−2 s−1, 16-h-light/8-h-dark cycles at 22°C. The square cup was kept floating with a sponge supporter on 140 mL of one-sixth Murashige and Skoog solution containing 3% (w/v) Suc. After a 14-d incubation, the seedlings were treated with Al in the one-sixth Murashige and Skoog solution adjusted to pH 4.5. After treatment, citrate in the incubation solution and activity of plasma membrane H+-ATPase in the roots were analyzed. Each experiment was performed at least two times independently. Transgenic lines of Arabidopsis (ecotype Landsberg erecta) were obtained by cloning plasma membrane H+-ATPase into a binary vector and expressing under the control of the cauliflower mosaic virus 35S promoter in sense or antisense orientation according to Harper et al. (1989). Binary vector constructions were transformed in planta Agrobacterium tumefaciens GV3101/Pmp90 by electroporation. Vacuum infiltration in plant transformation was performed. The transformed plants were allowed to continue to grow and set seeds. Positive transformants were identified as normal seedlings grown on Murashige and Skoog agar plates supplemented with 50 μg mL−1 kanamycin. Single-locus insertions were scored by observing a 3:1 ratio (kanamycin resistant versus kanamycin sensitive) in T2 progeny resulted from self-pollination of T1 parents. Homologous T3 progeny (nine lines) were used for subsequent assays.
Analysis of Citrate
Citrate secretion from intact roots of soybean was measured using HPLC according to the method of Yang et al. (2001). Citrate secretion from Arabidopsis roots was determined by the method of Delhaize et al. (1993), with some modifications. After the treatments, the incubation solution was concentrated from 140 to 4 mL for citrate analysis. Two milliliters of the concentrated solution were incubated in the solution consisting of 95 μL of buffer (1 m Tris-HCl, pH 7.8), 12 μL of 10 mm NADH, and 4 μL of a lactate dehydrogenase/malate dehydrogenase mixture. After a stable A340 reading was obtained, 4 μL of citrate lyase were added and the decline in A340 was recorded. The decrease in NADH concentration was directly proportional to the amount of citric acid in the sample. Al and other elements did not interfere with the assay for citrate because the exact amount of citrate could be detected when standard citrate was added.
Plasma Membrane Isolation
Plasma membrane vesicles were prepared according to the method of Faraday and Spanswick (1992), with minor modifications. Briefly, collected samples were ground in ice-cold homogenization buffer containing 250 mm Suc, 2 mm EGTA, 10% (v/v) glycerol, 0.5% (w/v) bovine serum albumin, 2 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 5 mm 2-mercaptoethanol, and 50 mm 1,3-bis(tris[hydroxymethyl]methylamino) propane (BTP), adjusted to pH 7.8 with 2-(N-Morpholino) ethanesulfonic acid. The homogenate was filtered through two layers of Miracloth and centrifuged in a swinging bucket rotor at 10,000g for 10 min at 4°C. The supernatants were centrifuged at 100,000g for 55 min, and the microsomal pellets were resuspended in phase buffer (250 mm Suc, 3 mm KCl, and 5 mm KH2PO4, pH7.8). The microsomal membrane preparation was fractionated by two-phase partitioning in aqueous dextran T-500 (Sigma, St. Louis) and PEG-3350 (Sigma) according to the method of Larsson (1985). After centrifugation, the residue was suspended in microsome suspension buffer and centrifuged at 100,000g for 30 min. Then the pellet was suspended in microsome suspension solution to make 12-g aliquots. The phase component solution was prepared to a weight of 24 g by mixing different phase components. After phase partitioning, the upper phase containing the plasma membrane was mixed with microsome suspension buffer and then centrifuged. The plasma membrane was suspended in suspension buffer for analysis of plasma membrane H+-ATPase. The protein concentration was determined by the Bradford method (Bradford, 1976). Isolation of the plasma membrane should be performed carefully. To evaluate feasibility, a specific inhibitor of plasma membrane H+-ATPase was added. Results indicated the activity of VA-sensitive ATPase occupied 91.3% of the total activity in the plasma membrane fraction, and other inhibitor-sensitive enzyme activity was negligible. The results suggested that the techniques for isolation of the plasma membrane were correct and practicable.
Assay of Plasma Membrane H+-ATPase Activity
Plasma membrane H+-ATPase was measured in 0.5 mL of reaction solution that contained 30 mm BTP/N-Morpholino ethanesulfonic acid, 5 mm MgSO4, 50 mm KCL, and 4 mm Tris-ATP or BTP-ATP. Brij 58 (0.02% w/v) was added to the reaction solution and reactions were initiated by adding 1 to 10 μg of membrane protein. After 30 min at 30°C, the reactions were stopped by adding 1 mL of stopping solution 2% (v/v) H2SO4, 5% (w/v) SDS, 0.7% (w/v) sodium molybdate, and 50 μL of 10% (w/v) ascorbic acid. Color development of the phosphomolybdate complex proceeded for 30 min. A700 was measured with a spectrophotometer. Plasma membrane H+-ATPase activity was calculated as the phosphate liberated in excess of boiled-membrane controls. The difference of the samples with and without 0.1 mm VA is expressed as the activity of plasma membrane H+-ATPase. A standard curve of phosphate in the reaction mixture was included in each assay.
Real-Time RT-PCR Analysis of Plasma Membrane H+-ATPase Gene Expression
After treatment, soybean root apices (1 cm) were excised and used for isolation of total RNA using an extraction reagent (TRIZOL Reagent; Invitrogen, Carlsbad, CA), then total RNA was purified by RNase-free DNase. First-strand cDNA was synthesized in a 20-μL reaction solution containing approximately 2 μg of total RNA using the SuperScriptII reverse transcriptase amplification system for first-strand cDNA synthesis and oligo(dT)12 to 18 as a primer. Using the synthesized first-strand cDNA as a template and two gene-specific primers derived from the plasma membrane H+-ATPase gene, 5′-CTTGGGATAATCTTTTGGAGAAC-3′ as a sense primer and 5′-CTCGGCACGTCTCTTA-G-3′ as an antisense primer according to gene accession number AF091303, PCR with 30 cycles was performed. The β-tubulin gene was used to normalize the amount of first-strand cDNA. The β-tubulin sense primer (5′-CTCAGGTGATTTCATCTTTG-3′) and antisense primer (5′-GAATTCAGTCACATCCAC-3′) were designed according to gene accession number CA936138. Transcriptional levels were quantified using Light Cycler data analysis software.
Western Blot and Immunolocalization of Plasma Membrane H+-ATPase
Plasma membrane proteins were separated by SDS-PAGE using the system of Laemmli (1970). Membrane vesicles were solubilized in SDS-loading buffer containing 0.125 mm Tris-HCl, pH 7.4; 10% (w/v) SDS; 10% (v/v) glycerol; 0.2 m dithiothreitol, 0.002% (w/v) bromocresol blue, and 5 mm phenylmethylsulfonyl fluoride. The samples were loaded on a discontinuous SDS-PAGE (5% [w/v] acrylamide stacking gel and 8% [w/v] acrylamide separating gel). For western-blot analysis, after separation by SDS-PAGE, samples were transferred to polyvinylidene difluoride membrane filters (0.2 μm). To identify and quantitate the plasma membrane H+-ATPase, we incubated the blot with a polyclonal antibody against maize (Zea mays) plasma membrane H+-ATPase. The antiserum was diluted to 1:1,000 in Tris-buffered saline plus Tween (TBST) buffer (1 mm Tris-HCl, pH 8.0, mm NaCl, and 0.1% [v/v] Tween 20) and incubation was carried out for 1 h at room temperature. After rinsing in TBST, a polyvinylidene difluoride membrane was incubated at room temperature for 1 h with a 1:10,000 (v/v) diluted secondary antibody (enhanced chemiluminescence anti-rabbit IgG, peroxidase-linked species-specific whole antibody from donkey). After rinsing in TBST, the membrane was incubated for 5 min in enhanced chemiluminescence-based signal detection solution. Intensities of signals on the film were quantified by a densitometer (Bio-Rad Laboratories, Hercules, CA). Immunolocalization of plasma membrane H+-ATPase was examined as described by Ahn et al. (2001), with minor modifications. The images of plasma membrane H+-ATPase from roots were obtained using the 543-nm excitation line of an He-Ne laser fitted to a Zeiss (Oberkochen, Germany) confocal microscope using Ph3-Plan-Neofluar 100× oil immersion objective. The root surface images were the overlay of 7 to 11 optical sections (0.75 μm thick), and scan configurations were kept constant between treatments using the recycle option of the LSM 510 software to assess the intensity differences among treatments. A model F-4500 fluorescence spectrophotometer (Hitachi Instruments, San Jose, CA) was used to measure the fluorescence intensity, and relative induction was calculated.
Analysis of Phosphorylation of Plasma Membrane H+-ATPase
All protein samples used for phosphorylation studies on SDS-PAGE and immunoblotting were obtained with immunoprecipitation by using an antibody raised against maize plasma membrane H+-ATPase. For immunoprecipitation of the plasma membrane H+-ATPase, 10 to 50 μg of isolated plasma membrane were boiled for 5 min in 2× immunoprecipitation buffer (20 mm sodium phosphate, pH 7.2, 300 mm NaCl, 2 mm EDTA, and 3% Triton X-100). After centrifugation, the buffer was added to the supernatant together with 5 μL of antibody, and after 10 min of gentle mixing, they were incubated overnight at 4°C. An equal volume of 10% prewashed protein A agarose was added. Protein A agarose was washed with 50 volumes of 1× immunoprecipitation buffer by mixing, then centrifugated and resuspended in immunoprecipitation buffer, giving a final concentration of 10%. The samples were incubated at room temperature for 2 h before centrifugation for 4 min at 15,000g in a microcentrifuger. The supernatant was discarded, and the pellet was washed three times with 1 mL of immunoprecipitation buffer by mixing and microcentrifugation. The pellet was finally resuspended in SDS-PAGE sample buffer and centrifuged at 15,000g, and the supernatant was used for SDS-PAGE. The antibody against phosphoamino acids (antiphosphothreonine) was used for western-blot analysis of phosphorylated plasma membrane H+-ATPase.
Statistical Analysis
The experiments were arranged in a randomized complete design and the data were analyzed by the SAS Institute (1988). ANOVA was performed for each variable with comparison of means by Duncan's Multiple Range Test. Data transformation before ANOVA was judged to be necessary if variance was found to be correlated with means.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AF091303 and CA936138.
Acknowledgments
We thank Michiyo Ariyoshi for culturing Arabidopsis seedlings, Sanae Rikiishi for experimental assistance in the laboratory (Research Institute for Bioresources, Okayama University, Japan), and Dr. Peter R. Ryan (Commonwealth Scientific and Industrial Research Organization Plant Industry, Australia) for his critical review of the manuscript.
This work was supported by the Program for the Promotion of Basic Research Activities in Innovative Biosciences and a Grant-in-Aid for General Research from the Ministry of Education, Sports, Culture, Science and Technology of Japan (grant no. 14206008 to H.M.), by the International Foundation for Science (grant no. C/3042–2), by the National Natural Scientific Foundation of China (grant no. 30471040/30230220), by the Guangdong Natural Scientific Foundation (grant no. 000642), and by the Japan Society for the Promotion of Science (postdoctoral fellowships to H. Shen).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.058065.
References
- Ahn SJ, Rengel Z, Matsumoto H (2004) Aluminum-induced plasma membrane surface potential and H+-ATPase activity in near-isogenic wheat lines differing in tolerance to aluminum. New Phytol 162: 71–79 [Google Scholar]
- Ahn SJ, Sivaguru M, Osawa H, Chung GC, Matsumoto H (2001) Aluminum inhibits the H+-ATPase activity by permanently altering the PM surface potentials in squash roots. Plant Physiol 126: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216: 355–365 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Chang A, Slayman CW (1991) Maturation of the yeast plasma membrane H+-ATPase involves phosphorylation during intracellular transport. J Cell Biol 115: 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clune TS, Copeland L (1999) Effects of aluminum on canola roots. Plant Soil 216: 27–33 [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolayev V, Weschki W, Manteuffel R (2003) Comparison of Al-induced gene expression in sensitive and tolerant soybean cultivars. J Exp Bot 54: 2745–2756 [DOI] [PubMed] [Google Scholar]
- Ezaki B, Gardner RC, Ezaki Y, Matsumoto H (2000) Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol 122: 657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facanha AR, Okorokova-Facanha AL (2002) Inhibition of phosphate uptake in corn roots by aluminum-fluoride complexes. Plant Physiol 129: 1763–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday CD, Spanswick RM (1992) Maize root plasma membranes isolated by aqueous polymer two-phase partitioning: assessment of residual tonoplast ATPase and prophosphatase activities. J Exp Bot 43: 1583–1590 [Google Scholar]
- Hager A, Debus G, Edel HG, Stransky H, Serrano R (1991) Auxin induces exocytosis and the rapid synthesis of a high turnover pool of plasma membrane H+-ATPase. Planta 185: 527–537 [DOI] [PubMed] [Google Scholar]
- Hamilton C, Good AG, Taylor GJ (2001) Induction of vacuolar ATPase and mitochondrial ATP synthase by aluminum in an aluminum-resistant cultivar of wheat. Plant Physiol 125: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Surowy TK, Sussman MR (1989) Molecular cloning and sequence of cDNA encoding the plasma membrane proton pump (H+-ATPase) of Arabidopsis thaliana. Proc Natl Acad Sci USA 86: 1234–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug A, Shi B, Vitrorello M (1994) Aluminum interaction with phosphoinositide-associated signal transduction. Arch Toxicol 68: 1–7 [DOI] [PubMed] [Google Scholar]
- Hayes JE, Ma J (2003) Al-induced efflux of organic acid anions is poorly associated with internal organic acid metabolism in triticale roots. J Exp Bot 54: 1753–1759 [DOI] [PubMed] [Google Scholar]
- Hoekenga QA, Vision TJ, Shaff JE, Monforte AJ, Lee GP, Howell SH, Kochian LA (2003) Identification and characterization of aluminum tolerance loci in Arabidopsis (Landsberg erecta × Columbia) by quantitative trait locus mapping. A physiologically simple but genetically complex trait. Plant Physiol 132: 936–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ (1995) The role of the apoplast in aluminum toxicity and resistance of higher plants: a review. Z Pflanzenernäbrung Bodenkunde 158: 419–428 [Google Scholar]
- Jernejc K, Legisa M (2001) Activation of plasma membrane H+-ATPase by ammonium ions in Aspergillus niger. Appl Microbiol Biotechnol 57: 368–373 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB (1988) Proton extrusion by wheat roots exhibiting severe aluminum toxicity symptoms. Plant Physiol 88: 418–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB (1993) Aluminum enhancement of plant growth in acid rooting media. A case of reciprocal alleviation of toxicity by two toxic cations. Physiol Plant 88: 619–625 [DOI] [PubMed] [Google Scholar]
- Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260 [Google Scholar]
- Kollmeier M, Dietrich P, Bauer CS, Horst WJ, Hedrich R (2001) Aluminum activates a citrate-permeable anion channel in the aluminum-sensitive zone of the maize root apex. A comparison between an aluminum-sensitive and an aluminum-resistant cultivar. Plant Physiol 126: 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Larsson C (1985) Plasma membrane. In HF Linskens, JF Jackson, eds, Modern Methods of Plant Analysis, New Series Vol 1: Cell Components. Springer-Verlag, Berlin, pp 85–104
- Li XF, Ma JF, Matsumoto H (2000) Pattern of Al-induced secretion of organic acids differs between rye and wheat. Plant Physiol 123: 1537–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligaba A, Yamaguch M, Shen H, Sasaki T, Yamamoto Y, Matsumoto H (2004) Phosphorus deficiency enhances plasma membrane H+-ATPase activity and citrate exudation in greater purple lupin (Lupinus pilosus). Funct Plant Biol 31: 1075–1083 [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H, Hiradate S (1997) Detoxifying aluminum with buckwheat. Nature 390: 569–5709403684 [Google Scholar]
- Malerba M, Ceran AR, Crosti P (2003) Fusicoccin induces in plant cells a programmed cell death showing apoptotic features. Protoplasma 222: 113–116 [DOI] [PubMed] [Google Scholar]
- Matsumoto H (1988) Inhibition of proton transport activity of microsomal membrane vesicles of barley roots by aluminum. Soil Sci Plant Nutr 34: 499–506 [Google Scholar]
- Matsumoto H (2000) Cell biology of Al tolerance and toxicity in higher plants. Int Rev Cytol 200: 1–46 [DOI] [PubMed] [Google Scholar]
- Michelet B, Lukaszewicz M, Dupriez V, Boutry M (1994) A plant plasma membrane proton-ATPase gene is regulated by development and environment and shows signs of a translation regulation. Plant Cell 6: 1375–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullette KJ (1975) Stimulation of growth in eucalyptus due to aluminum. Plant Soil 42: 495–499 [Google Scholar]
- Nisi PD, Dellorto M, Pirovano L, Zocchi G (1999) Calcium-dependent phosphorylation regulates the plasma membrane H+-ATPase activity of maize (Zea mays L.) roots. Planta 209: 187–194 [DOI] [PubMed] [Google Scholar]
- Niu X, Damsz B, Kononowicz AK, Bressan RA, Hasegawa PM (1996) NaCl-induced alterations in both cell structure and tissue-specific plasma membrane H+-ATPase gene expression. Plant Physiol 111: 679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Narasimhan ML, Salzman RA, Bressan RA, Hasegawa PM (1993) NaCl regulation of plasma membrane H+-ATPase gene expression in a glycophyte and a halophyte. Plant Physiol 103: 713–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T, Koyama H, Hara T (2003) Characterization of citrate transport through the plasma membrane in a carrot mutant cell line with enhanced citrate excretion. Plant Cell Physiol 44: 156–162 [DOI] [PubMed] [Google Scholar]
- Olsson A, Svennelid F, Ek B, Sommarin M, Larsson C (1998) A phosphothreonine residue at the C-terminal end of the plasma membrane H+-ATPase is protected by fusicoccin-induced 14-3-3 binding. Plant Physiol 118: 551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa H, Matsumoto H (2001) Possible involvement of protein phosphorylation in aluminum-responsive malate efflux from wheat root apex. Plant Physiol 126: 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG (1998) Proton gradient and plant growth: role of plasma membrane H+-ATPase. Adv Bot Res 28: 2–70 [Google Scholar]
- Piñeros MA, Magalhaes JV, Alves VMC, Kochian LV (2002) The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize. Plant Physiol 129: 1194–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena N, Breuninger M, Franken P, Ocon A (2003) Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus. Plant Physiol 132: 1540–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KD, Schott EJ, Sharma YK, Davis KR, Gardner RC (1998) Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol 116: 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rober-Kleber N, Albrechtova TP, Fleig S, Fischer-Iglesias C (2003) Plasma membrane H+-ATPase is involved in auxin-mediated cell elongation during wheat embryo development. Plant Physiol 131: 1302–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52: 527–560 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ (1995) Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta 196: 103–110 [Google Scholar]
- Ryan PR, Skerrett M, Findlay GP, Delhaize E, Tyermann SD (1997) Aluminum activates an anion channel in the apical cells of wheat roots. Proc Natl Acad Sci USA 94: 6547–6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute (1988) SAS/SAST User's Guide. Release Edition 6.03. SAS Institute, Inc, Cary, NC
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37: 645–653 [DOI] [PubMed] [Google Scholar]
- Schaller A, Oecking C (1999) Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 11: 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R (1989) Structure and function of plasma membrane ATPase. Annu Rev Plant Physiol Plant Mol Biol 40: 61–94 [Google Scholar]
- Shen H, Ligaba A, Yamaguchi M, Osawa H, Shibata K, Yan XL, Matsumoto H (2004) Effect of K-252a and abscisic acid on the efflux of citrate from soybean roots. J Exp Bot 55: 663–671 [DOI] [PubMed] [Google Scholar]
- Singh J, Roberts MR (2004) Fusicoccin activates pathogen-responsive gene expression independently of common resistance signaling pathways, but increases disease symptoms in Pseudomonas syringae-infected tomato plants. Planta 219: 261–269 [DOI] [PubMed] [Google Scholar]
- Sussman MR (1994) Molecular analysis of proteins in the plant plasma membrane. Annu Rev Plant Physiol Plant Mol Biol 45: 211–234 [Google Scholar]
- Sze H, Li X, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11: 677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ (1995) Overcoming barriers to understanding the cellular basis of aluminum resistance. Plant Soil 171: 89–103 [Google Scholar]
- Widell S, Larsson C (1990) A critical evaluation of markers used in plasma membrane purification. In C Larsson, IM Moller, eds, The Plant Plasma Membrane. Springer-Verlag, Berlin, pp 16–43
- Yan F, Zhu Y, Müller C, Zörb C, Schubert S (2002) Adaptation of H+-pumping and PM H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129: 50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZM, Nian H, Sivaguru M, Tanakamaru S, Matsumoto H (2001) Characterization of aluminum-induced citrate secretion in aluminum-tolerant soybean (Glycine max L.) plants. Physiol Plant 113: 64–71 [Google Scholar]
- Zhang WH, Ryan PR, Tyerman SD (2001) Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiol 125: 1459–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]