Abstract
Oxalic acid secretion from roots is considered to be an important mechanism for aluminum (Al) resistance in buckwheat (Fygopyrum esculentum Moench). Nonetheless, only a single Al-resistant buckwheat cultivar was used to investigate the significance of oxalic acid in detoxifying Al. In this study, we investigated two buckwheat cultivars, Jiangxi (Al resistant) and Shanxi (Al sensitive), which showed significant variation in their resistance to Al stress. In the presence of 0 to 100 μm Al, the inhibition of root elongation was greater in Shanxi than that in Jiangxi, and the Al content of root apices (0–10 mm) was much lower in Jiangxi. However, the dependence of oxalic acid secretion on external Al concentration and the time course for secretion were similar in both cultivars. Furthermore, the variation in Al-induced oxalic acid efflux along the root was similar, showing a 10-fold greater efflux from the apical 0- to 5-mm region than from the 5- to 10-mm region. These results suggest that both Shanxi and Jiangxi possess an equal capacity for Al-dependent oxalic acid secretion. Another two potential Al resistance mechanisms, i.e. Al-induced alkalinization of rhizosphere pH and root inorganic phosphate release, were also not involved in their differential Al resistance. However, after longer treatments in Al (10 d), the concentrations of phosphorus and Al in the roots of the Al-resistant cultivar Jiangxi were significantly higher than those in Shanxi. Furthermore, more Al was localized in the cell walls of the resistant cultivar. All these results suggest that while Al-dependent oxalic acid secretion might contribute to the overall high resistance to Al stress of buckwheat, this response cannot explain the variation in tolerance between these two cultivars. We present evidence suggesting the greater Al resistance in buckwheat is further related to the immobilization and detoxification of Al by phosphorus in the root tissues.
Ionic aluminum (Al) is highly toxic to plant growth and appears to interfere with a number of physiological and biochemical processes (Rengel, 1992; Kochian, 1995). However, species vary widely in their ability to resist the harmful effect of Al, and significant differences in Al resistance have even been reported between genotypes of the same species (Yang et al., 2005). Over the past few decades, concerted efforts have been made to understand the genetic and physiological basis of Al resistance in many different species. As proposed by Taylor (1991), Al resistance mechanisms can be grouped into two categories. One is based on excluding Al from the root cells, and the other relies on improving the resistance of plants to the Al ions once they enter the cytosol. Among the likely exclusion mechanisms, a role for organic acid efflux has been well documented in several species (Ma, 2000; Ryan et al., 2001; Kochian et al., 2004). Other potential exclusion mechanisms include increases in rhizospheric pH (Degenhardt et al., 1998), phosphate efflux (Pellet et al., 1996), the secretion of proteins to bind Al ions (Basu et al., 1999), and the selective permeability of the plasma membrane to reduce Al uptake into the cytosol (Archambault et al., 1997). The possible mechanisms of Al resistance have been less well studied but include sequestration of the toxic ions to the vacuole or the adoption of metabolic processes that can better cope with Al in the cytosol (Kochian, 1995).
Buckwheat (Fygopyrum esculentum Moench) is considered a highly Al-resistant species and previous studies have suggested that two main mechanisms are responsible. One involves the secretion of oxalic acid from the roots, which is thought to bind and detoxify the Al ions in the rhizosphere (Zheng et al., 1998a), and the other relies on the internal complexation of Al by oxalate and storage in the vacuole (Ma et al., 1998; Shen et al., 2002). Thus, high Al resistance in buckwheat is the combination of both an exclusion process and internal detoxification (Ma et al., 1997). However, those earlier studies could not determine whether one of these mechanisms was relatively more important since only a single genotype was examined.
Recently, we described a large genotypic variation in Al resistance among buckwheat cultivars collected from different regions of China (Yang et al., 2005). In this study, we examined two of these genotypes representing differential Al-resistant hierarchies in detail and demonstrated that Al-induced organic acid secretion was not responsible for the genotypic differences in Al resistance. As an alternative, we hypothesize that Al resistance could be related to the precipitation of Al by phosphorus (P) and the accumulation of this compound in the root tissue. We discuss the possible ecological benefits of this mechanism in acid soils where P deficiency can also be a factor limiting crop production.
RESULTS
Effect of Al on Root Growth and Al Content
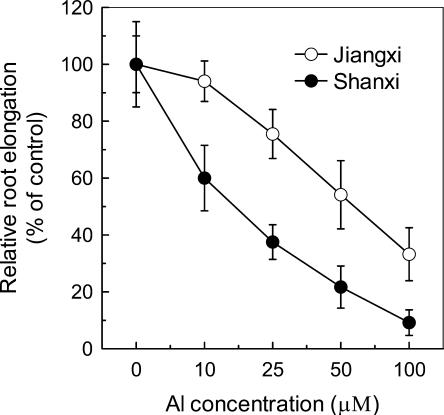
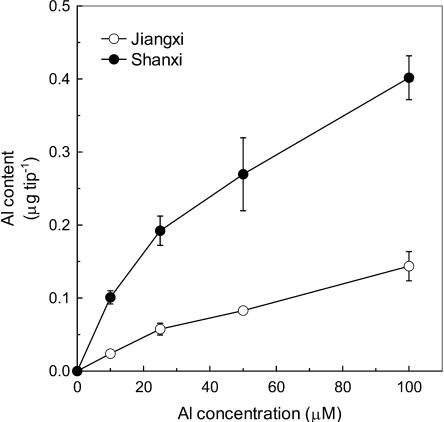
The trend of Al-induced root elongation inhibition in cv Shanxi was the same as that in cv Jiangxi, i.e. the inhibition of root elongation was increased with increasing Al concentration (Fig. 1). However, the relative root elongation (RRE) of Jiangxi at 100 μm Al concentration was comparable with that of Shanxi at 25 μm Al. The Al content of root apex (0–1 cm) was 3- to 4-fold higher in the Al-sensitive cultivar than in the Al-resistant plant irrespective of Al concentration in the culture solution (Fig. 2). In addition, over the whole range of Al concentration, Al content is negatively correlated with the RRE in both cultivars (data not shown).
Figure 1.
Effect of increasing Al concentrations on root elongation of buckwheat cv Jiangxi and cv Shanxi. RRE was calculated from root elongation of 3-d-old seedlings during a 24-h exposure to 0.5 mm CaCl2 solution, pH 4.5, containing 0, 10, 25, 50, or 100 μm Al. Data are means ± sd (n = 14).
Figure 2.
Al content in the root apices of buckwheat cv Jiangxi and cv Shanxi. Three-day-old seedlings were exposed for 24 h to a 0.5 mm CaCl2 solution, pH 4.5, containing 0, 10, 25, 50, or 100 μm Al. Root apices (0–1 cm) were excised and the Al concentration was determined by inductively coupled plasma atomic emission spectrometry. Data are means ± sd (n = 3).
The Pattern of Al-Induced Oxalic Acid Secretion
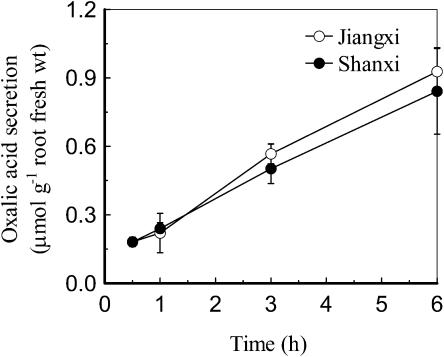
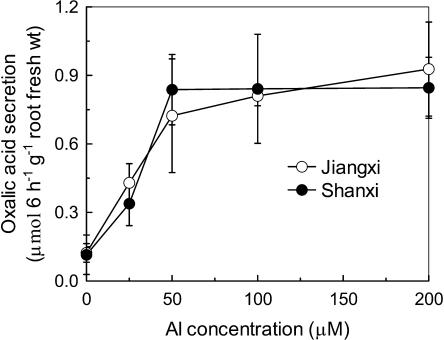
A 30-min exposure to 100 μm Al was sufficient to elicit oxalic acid secretion from the roots of both the resistant (Jiangxi) and sensitive (Shanxi) cultivars. Although the amount secreted from Shanxi was slightly lower than from Jiangxi during the first 3 h, the difference was negligible after a 6-h exposure (Fig. 3). Oxalic acid secretion showed a similar saturating dependence on external Al concentration in both cultivars with maximum levels occurring at approximately 50 μm Al (Fig. 4).
Figure 3.
Time course of oxalic acid secretion by Jiangxi and Shanxi exposed to 0.5 mm CaCl2 solution, pH 4.5, containing 100 μm AlCl3. Root exudates were collected after 0.5-, 1-, 3-, and 6-h exposure. Organic acids were determined by HPLC. Data are means ± sd (n = 3).
Figure 4.
Dependence of oxalic acid secretion on Al concentrations in Jiangxi and Shanxi. External solutions contained 0.5 mm CaCl2, pH 4.5, and a range of AlCl3 concentrations. Root exudates were collected after 6-h treatments. Organic acids were analyzed by HPLC. Data are means ± sd (n = 3).
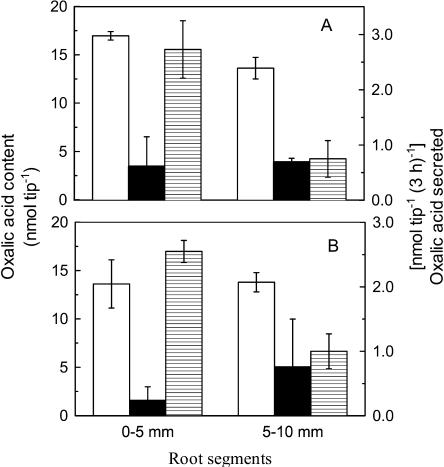
The spatial variation in oxalic acid secretion was investigated using excised root tissue (Fig. 5). Secretion of oxalic acid from the apical 0-to-5-mm region was up to 10-fold greater than from the next 5-mm region farther back, and this pattern was observed in Shanxi and Jiangxi. Although the oxalic acid content in apical 0-to-5-mm region of Jiangxi was statistically higher than in Shanxi, the oxalate content in both cultivars was very high (Fig. 5), indicating internal oxalate content contributes at best a minor role to the different Al resistance in buckwheat. Greater Al-dependent organic acid secretion from root apices has also been observed in wheat (Triticum aestivum efflux), and this pattern is consistent with root apices being the region most vulnerable to Al stress (Ryan et al., 1993, 1995a).
Figure 5.
Al-induced secretion of oxalic acid from different sections of roots from cv Jiangxi (A) or cv Shanxi (B). Root segments excised from the root apex (0–5 mm) and the next segment (5–10 mm) were washed in 0.5 mm CaCl2 solution, pH 4.5, and then transferred to a similar solution containing 0 or 100 μm AlCl3. After 3 h, the root exudates were collected. The oxalic acid content in root apices was analyzed as described in text. Values are means of three replicates ± sd. Shown are the oxalic acid content in roots (white bars), oxalic acid secreted by the roots not treated with Al (black bars), and oxalic acid secreted by roots treated during Al treatment (shaded bars).
Al-Induced Increase in Root Surface pH and Phosphate Release
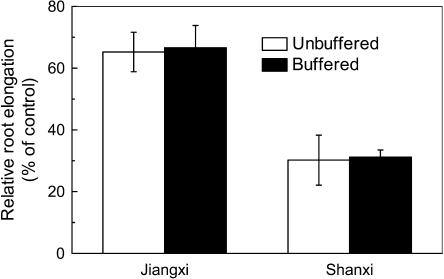
Al-induced increase in root surface pH and phosphate release have been suggested to be two additional Al-resistant mechanisms (Pellet et al., 1996; Degenhardt et al., 1998). In this study, to examine whether Al-induced pH change is involved in the different resistance of the two cultivars, we exposed both cultivars to a pH-buffered medium with or without Al according to Degenhardt et al. (1998). The difference in the sensitivity to Al between the resistant cultivar and the sensitive cultivar was evident after 24-h treatment, and there were no differences between buffered and unbuffered treatments (Fig. 6). Further, no phosphate was detected in the root exudates of both cultivars collected with 0.5 mm CaCl2 solution at pH 4.5 (data not shown).
Figure 6.
Effect of Al on root elongation in a pH-buffered solution. Three-day-old seedlings were exposed to Homo-PIPES buffer solution, pH 4.5, containing 0.5 mm CaCl2 and 0 or 50 μm Al. Root length was measured after 24-h treatment. Data are means ± sd (n = 10).
Mineral Concentrations
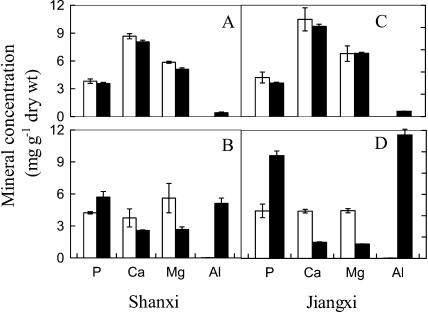
The concentrations of Al, P, calcium (Ca), and magnesium (Mg) in the leaves and roots of Shanxi and Jiangxi were measured following a 10-d intermittent treatment in 50 μm Al. Al, P, Ca, and Mg concentrations in the leaves were similar in each cultivar and unchanged by Al treatment (Fig. 7). Ca and Mg concentrations in the roots were reduced by Al treatment, whereas Al and P concentrations increased. Interestingly, the accumulation of P and Al was larger in Jiangxi, which resulted in 2-fold greater P and Al concentrations in Jiangxi compared to Shanxi. In control (−Al) treatments, the final ratio of P concentrations in the roots and leaves was similar for Shanxi and Jiangxi (1.11 and 1.27, respectively). However, after Al treatment, these ratios were slightly increased to 1.60 for Shanxi and greatly increased to 3.2 for Jiangxi. Similarly, the final ratio of Al concentrations in the roots and leaves was 12 for Shanxi and 24 for Jiangxi. All these suggest that more Al and P were accumulated in the roots of Jiangxi than in those of Shanxi.
Figure 7.
Effect of 10-d intermittent Al treatment on the concentrations of P, Ca, Mg, and Al in cv Shanxi (A, leaves; B, roots) and cv Jiangxi (C, leaves; D, roots). The intermittent Al treatment was conducted as follows: The plants after 10-d culture in nutrient solution were exposed to Al treatment solution (50 μm AlCl3 in 0.5 mm CaCl2 at pH 4.5) for 1 d and nutrient solution on alternative days for 10 d (black bars). For the treatment lacking Al, they were transferred to 0.5 mm CaCl2 solution at pH 4.5 for 1 d and nutrient solution on alternative days for 10 d (white bars). Values shown are means ± sd (n = 3).
Localization of Al
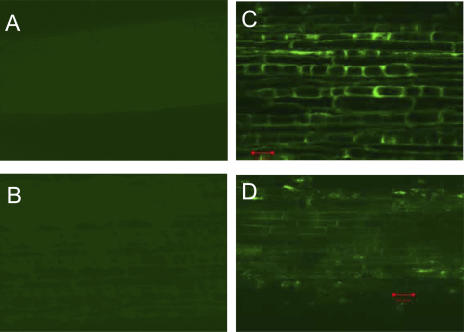
Localization of Al using an Al-specific stain, Morin, coupled with confocal laser scanning microscopy revealed that more Al was localized in the cell walls of root apex in Jiangxi than that in Shanxi after a 10-d intermittent Al treatment (Fig. 8, C and D). In the −Al treatment, no Al was detected in the cell walls of both cultivars (Fig. 8, A and B).
Figure 8.
Al location as detected by the Morin fluorescence using confocal laser scanning microscopy. Confocal images of buckwheat cultivar roots of Jiangxi (A and C) and Shanxi (B and D) subjected to a 10-d intermittent treatment with CaCl2 solution (A and B) or AlCl3 solution (C and D). Bar represents 20 μm for images.
DISCUSSION
The Al-dependent secretion of organic acid from roots is widely considered an important mechanism whereby some plants can minimize the toxic effects of Al present in acid soils (Ma et al., 2001; Ryan et al., 2001; Kochian et al., 2004). For instance, the relative Al resistance of 37 different genotypes of wheat was significantly correlated with the Al-dependent efflux of malate (Ryan et al., 1995b). The first gene for Al resistance was recently cloned from wheat (Sasaki et al., 2004), designated ALMT1. This gene encodes a membrane-bound protein that facilitates the Al-dependent malate efflux from several different transgenic cell types. The transgenic barley (Hordeum vulgare) expressing ALMT1 conferred an Al-activated malate efflux that was accompanied by an Al tolerance phenotype, indicating that ALMT1 is a major gene for Al tolerance in wheat (Delhaize et al., 2004). Experiments with divided-root chambers have demonstrated that organic acid release is confined to the root apex in wheat and maize (Zea mays; Ryan et al., 1993; Mariano and Keltjens, 2003) and similar results are now available for other species. The organic acids are believed to chelate and detoxify the harmful Al cations near the root apex, which is the most sensitive region for Al stress (Ryan et al., 1993). Correlations between organic acid secretion and Al resistance have now been described in a range of plant species (Zhao et al., 2003). By contrast, Parker and Pedler (1998) reported that the efflux of organic acids plays only a minor role in the differential resistance to Al in wheat, and they emphasized that a multifaceted, more integrative mode of resistance was probably occurring. Wenzl et al. (2001) demonstrated that organic acid secretion does not account for the high level of Al resistance in signalgrass (Brachiaria sp.), which indicates that organic acid secretion is not the only mechanism for Al resistance in plants. Recently, Piñeros et al. (2005) also reported that citrate efflux could not explain the difference in Al resistance in some maize cultivars.
The high degree of Al resistance observed in buckwheat has been attributed, in part, to the Al-dependent release of oxalate from the roots (Ma et al., 1997, 1998; Zheng et al., 1998a). It is now clear that buckwheat shows genotypic variation in Al resistance, and in this study we investigated two contrasting cultivars collected from different regions of China. We found no relationship between the amount of oxalic acid secreted and RRE (Figs. 1, 3, and 4), although the Al exclusion mechanisms seem to be involved in the high resistance to Al in seedling stage (Fig. 2). Our results were similar to those found in maize lines (Piñeros et al., 2005). The Al-resistant cultivar tested, Jiangxi, was collected from the acidic soils of southern China. The sensitive cultivar, Shanxi, was collected from northwest China where the soils are mostly alkaline (Yang et al., 2005). Therefore, this difference appears to stem from the original genotypes adapted to those specific environments, perhaps explaining why greater Al resistance was present in the cultivars collected from the acidic soils of southern China (Yang et al., 2005).
Previous studies showed that the release of oxalic acid from the roots of the Al-resistant cultivar Jiangxi occurred soon after the Al treatment was applied (Zheng et al., 1998a), which classifies the response as Pattern I in the system proposed by Ma et al. (2001). In Pattern II, by contrast, a lag phase of several hours occurs between the addition of Al and the onset of organic acid secretion. We examined the time dependence of the oxalic acid response to see if this could explain the difference in Al resistance between Shanxi and Jiangxi. We confirmed that oxalic acid secretion from Jiangxi is activated rapidly by Al (detectable within 30 min) and that it continues at a constant rate for at least 6 h (Fig. 3). We also showed that the same pattern occurs in cv Shanxi. Therefore, the contrasting phenotypes of these two cultivars cannot be explained by the more rapid activation of the oxalic acid secretion in one cultivar compared to the other. Additionally, the dependence of oxalic acid secretion on external Al concentration is also identical in Shanxi and Jiangxi with each showing a saturating response at concentrations above 50 μm Al (Fig. 4). This differs from the earlier report by Ma et al. (1997) who found that the rate of secretion continued to increase above 50 μm. This disparity might result from experimental conditions, since Yang et al. (2001) demonstrated that even light intensity can affect the Al-induced secretion of citrate from soybean (Glycine max) roots. Finally, the variation in Al-induced oxalic acid efflux along the root was similar in both cultivars, showing a 10-fold greater efflux from the apical 0-to-5-mm region than from the 5-to-10-mm region (Fig. 5). Collectively, these results suggest that the mechanism for Al-induced secretion of oxalic acid is identical in Shanxi and Jiangxi and cannot explain the genotypic variation in Al resistance displayed by the two cultivars.
Taylor (1991) hypothesized that the increase in rhizosphere pH might be a potential Al resistance mechanism. Degenhardt et al. (1998) reported that, in an Al-resistant Arabidopsis (Arabidopsis thaliana) mutant (alr-104), exposure to Al induced a 2-fold increase in net H+ influx localized to the root apex. Piñeros and Kochian (2002) also found that H+ influx occurs at the root apical zone in maize, whereas the elongation zone exhibits H+ efflux. On the other hand, Pellet et al. (1996) reported that root apical phosphate release is another Al-resistant mechanism in an Al-resistant wheat cultivar Atlass 66. In this study, Al-induced changes of rhizosphere pH were not involved in high Al resistance in Jiangxi (Fig. 6), nor did phosphate release (data not shown).
The one significant difference that was observed between Shanxi and Jiangxi emerged after plants were exposed intermittently to Al for 10 d. Although the accumulation of Al and P in the leaves was similar in each cultivar, significantly higher concentrations of Al and P were detected in the roots of the Al-resistant Jiangxi than in the sensitive cultivar Shanxi. Insoluble Al-P precipitates can accumulate on the root surface, in the cell wall, or in the root cells (Taylor, 1991) and are generally considered nontoxic to plants. We suggest that the immobilization of Al in roots by precipitation with P might contribute to the genotypic differences in buckwheat. Previous reports on the accumulation of Al in the cv Jianxi indicated that 90% of the 400-mg kg−1 dry weight of Al accumulated in leaves was present in the leaf vacuoles in the form of Al oxalate at a molar ratio of 1:3 (Ma et al., 1997; Ma and Hiradate, 2000; Shen et al., 2002). The total Al content of the roots was much higher than the leaves but an estimated 40% of this was located in the apoplasm (Ma et al., 1998) adsorbed to the galacturonic acid polymers and phosphate molecules in the cell wall (Horst, 1995). The formation of Al-P complexes in the cell wall, and especially insoluble compounds like Al4(PO4)3, may be helpful by retarding the uptake of Al into the cytosol. Significant correlations between Al and phosphate concentrations have also been found in the cell wall of Avena sativa and maize (Marienfeld and Stelzer, 1993; Gaume et al., 2001). Gaume et al. (2001) proposed that the Al resistance of maize was associated with the immobilization of Al by P in the root tissues, and they also observed that the more Al-resistant cultivar had a higher capability to utilize P. Furthermore, Vázquez et al. (1999) reported that resistance in maize relied on the active transport of Al-P complex from the cell wall to vacuoles. Watanabe and Osaki (2002) proposed that species exposed to Al for extended periods might induce a combination of protective mechanisms themselves that rely on exclusion and internal detoxification. However, many of these processes are probably yet to be discovered (Wenzl et al., 2001). In this study, we found that the concentration of Al and P in the Al-resistant cv Jiangxi was significantly higher than that of the sensitive one, and that the increased Al absorbed by Jiangxi was localized in the cell walls (Figs. 7 and 8). As morin staining was reported to be not a good index of Al bound to cell wall pectin, but it could detect Al bound to phosphate (Eticha et al., 2005), therefore our results clearly indicated that immobilization of P with Al in cell wall was involved in high Al resistance in buckwheat.
Recently, Zhu et al. (2002) demonstrated that buckwheat utilizes sparingly of soluble P sources very efficiently. Since P deficiency is another factor that can limit plant production in acidic soils (Jones, 1998), we can hypothesize that buckwheat cultivars native to these acid soils have evolved mechanisms to utilize P efficiently and to detoxify Al simultaneously, thus helping it adapt to these hostile soils.
In conclusion, the genotypic difference between the two buckwheat cultivars was unrelated to oxalic acid secretion. The higher Al resistance of Jiangxi appears to be associated with a higher accumulation of Al and P in the roots. The capacity of buckwheat to acquire poorly soluble P, and the genetic links, if any, of this with Al resistance is an interesting area for future study.
MATERIALS AND METHODS
Plant Materials and Growth Environments
Buckwheat (Fygopyrum esculentum Moench) cv Jiangxi and cv Shanxi were collected from southern China and northwest China, respectively. Seeds were fully imbibed with deionized water and then germinated at 26°C in the dark. After germination, the seeds were transferred to a net tray floated on a container filled with 5 L of 0.5 mm CaCl2 solution at pH 4.5. The solution was renewed daily. On d 3, seedlings with similar size were selected to evaluate Al resistance. After another 2-d culture in CaCl2 solution, the remaining seedlings were transplanted into 1.1-L plastic pots (16 seedlings per pot) containing 1 L aerated nutrient solution. One-fifth strength Hoagland solution was used containing the following macronutrients in mm: KNO3, 1.0; Ca(NO3)2, 1.0; MgSO4, 0.4; NH4H2PO4, 0.2, and the following micronutrients in μm: NaFeEDTA, 20; H3BO3, 3; MnCl2, 0.5; CuSO4, 0.2; ZnSO4, 0.4; and (NH4)6Mo7O24, 1. The solution was adjusted to pH 4.5 with 1 m HCl and renewed every 3 d. All the experiments were conducted at an environmentally controlled growth room with a 14-h/26°C day and a 10-h/23°C night regime, a light intensity of 150 μmol photon m−2 s−1, and a relative humidity of 70%.
Assessment of Al Resistance in Buckwheat
Al resistance in buckwheat was examined by measuring root elongation of primary roots of 3-d-old seedlings grown in 0.5 mm CaCl2 solution, pH 4.5, containing 0, 10, 25, 50, or 100 μm AlCl3. For root elongation measurement, the seedlings were subjected to a compartmental hydroponic system (Yang et al., 2005). Root length was measured with a ruler before and after treatment (24 h). RRE was defined as the percentage of the root elongated of the Al treatment compared to the Al-free control.
Al Treatments
Prior to beginning the treatments, plants were transferred to 0.5 mm CaCl2 solution at pH 4.5 overnight. In the dose-response experiment, 20-d-old seedlings were exposed to 0.5 mm CaCl2 solution containing 0, 25, 50, 100, or 200 μm AlCl3. All treatment solution was adjusted to pH 4.5 with 0.1 m HCl. Root exudates were collected after 6-h exposure. In the time course experiment, the 20-d-old seedlings were exposed to 0.5 mm CaCl2 solution containing 100 μm AlCl3, pH 4.5. Root exudates were collected after 0.5-, 1-, 3-, and 6-h exposure. In the longer term experiments, an intermittent Al treatment was adopted to avoid interaction between Al and other nutrients such as P (see Zheng et al., 1998b). After a 10-d treatment with intermittent Al supply, plants were harvested, separated into roots and shoots, and dried in an oven at 70°C for 2 d for the mineral content analysis.
Location of Oxalic Acid Secretion Site
To determine the spatial variation in oxalate secretion along the root, excised root segments were used from 3-d-old seedlings. The apical 0-to-5-mm region and the next 5-mm segment farther back (5–10 mm from the root apex) were excised and collected in petri dishes containing 20 mL 0.5 mm CaCl2 solution at pH 4.5. After 1 h (washed three times, each for 20 min) the root apices were transferred to 10-mL centrifuge tubes containing 6 mL 0.5 mm CaCl2 solution, pH 4.5. Al treatment was initiated by replacing the solution with 6 mL 0.5 mm CaCl2 solution containing 100 μm AlCl3. The treatment was conducted at dark for 3 h, during which the tubes were gently shaken by hand at 10-min intervals. After the treatment period, the organic acid contents in the solution and in the root tissue were measured according to Zheng et al. (1998a). In this experiment, all solutions were prepared using Milli-Q water (Millipore S.A. 67120, Molshem, France).
Mineral Analysis
Root and shoot samples collected from intermittent Al treatment experiments were ground to fine powder. Then they were digested in an HNO3/HClO4 mixture (4:1, v/v). The mineral concentration was determined by inductively coupled plasma atomic emission spectrometry (IRIS/AP optical emission spectrometer, Thermo Jarrel Ash, San Jose, CA).
Quantifying Secreted Organic Acids by HPLC
Organic acids in the root exudates were determined according to Zheng et al. (1998a). Briefly, the above-collected exudates either from the intact roots or from the excised roots were passed through a cation exchange column (16 mm × 14 cm) filled with 5 g of Amberlite IR-120B resin (H+ form, Muromachi Chemical, Tokyo), followed by an anion exchange column (16 mm × 14 cm) filled with 2 g of Dowex 1 × 8 resin (100–200 mesh, formate form). The organic acids retained on anion exchange resin were eluted by 15 mL of 1 m HCl, and the eluate was concentrated to dryness by a rotary evaporator (40°C). After the residue was redissolved in 50 mm HClO4 solution, the concentration of organic acids was analyzed by HPLC. Aliquots (50 μL) were analyzed on a Shodex RSpak KC-811 cation exchange column (300 × 8 mm) using 50 mm HClO4 solution as mobile phase at 1.0 mL min−1 and 50°C.
Al Localization
After the Al treatments mentioned above, the roots were washed in deionized water for 5 min, stained with 10 mm MES buffer, pH 5.5, containing 100 μm Morin (Sigma, Tokyo) for 30 min. After a further wash in MES buffer, the images were obtained using a Zeiss confocal microscope (Axioplan 2 connected with LSM 510, Carl Zeiss, Oberkochen, Germany) at 488 nm (Argon laser) excitation wavelength.
Sensitivity to Al in a pH-Buffered Solution
The experiment was performed in a pH-buffered solution containing 0 or 50 μm AlCl3 at pH 4.5. The pH-buffered solution contained 10 mm Homo-PIPES (Degenhardt et al., 1998). Root length of 10 seedlings (3 d old) each was measured after 24 h.
Quantifying Secreted Phosphate by Ion Chromatography
To analyze the inorganic phosphate in the root exudates, an ion chromatography system (Dionex 300, Dionex, Sunnyvale, CA) was used according to Pellet et al. (1996).
Acknowledgments
Thanks are given to Dr. Peter R. Ryan (Commonwealth Scientific and Industrial Research Organization, Canberra, Australian Capital Territory, Australia) for providing comments on the manuscript.
This work was supported by the Natural Science Foundation of China (contract no. 30170548) and by the Fund for New Century Talent from the Education Ministry of China, Huoyingdong Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.059667.
References
- Archambault DJ, Zhang GC, Taylor GJ (1997) Spatial variation in the kinetics of aluminium (Al) uptake in roots of wheat (Triticum aestivum L.) exhibiting differential resistance to Al: evidence for metabolism-dependent exclusion of Al. J Plant Physiol 151: 668–674 [Google Scholar]
- Basu U, Good AG, Aung T, Slaski J, Basu A, Briggs KG, Taylor GJ (1999) A 23-kDa root exudates polypeptide co-segregates with aluminum resistance in Triticum aestivum. Physiol Plant 106: 53–61 [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV (1998) Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum increase in rhizosphere pH. Plant Physiol 117: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci USA 101: 15249–15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst WJ (2005) Localization of aluminium in the maize root apex: Can morin detect cell wall-bound aluminium? J Exp Bot 10.1093/jxb/eri136 [DOI] [PubMed]
- Gaume A, Mächler F, Frossard E (2001) Aluminum resistance in two cultivars of Zea may L.: root exudation of organic acids and influence of phosphorous nutrition. Plant Soil 234: 73–81 [Google Scholar]
- Horst WJ (1995) The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernaehr Bodenkd 158: 419–428 [Google Scholar]
- Jones DL (1998) Organic acids in the rhizosphere: a critical review. Plant Soil 205: 25–44 [Google Scholar]
- Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260 [Google Scholar]
- Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55: 459–493 [DOI] [PubMed] [Google Scholar]
- Ma JF (2000) Role of organic acids in detoxification of Al in higher plant. Plant Cell Physiol 44: 482–488 [DOI] [PubMed] [Google Scholar]
- Ma JF, Hiradate S (2000) Form of aluminium for uptake and translocation in buckwheat (Fygopyrum esculentum Moench). Planta 211: 355–360 [DOI] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Matsumoto H (1998) High aluminum resistance in buckwheat: Oxalic acid detoxifies aluminum internally. Plant Physiol 117: 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6: 273–278 [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Hiradate S, Matsumoto H (1997) Detoxifying aluminium with buckwheat. Nature 390: 569–5709403684 [Google Scholar]
- Mariano ED, Keltjens WG (2003) Evaluating the role of root citrate exudation as a mechanism of aluminium resistance in maize genotypes. Plant Soil 256: 469–479 [Google Scholar]
- Marienfeld S, Stelzer R (1993) X-ray microanalyses in roots of Al-treated Avena sativa plants. J Plant Physiol 141: 569–573 [Google Scholar]
- Parker DR, Pedler JF (1998) Probing the “malate hypothesis” of differential aluminum tolerance in wheat by using other rhizotoxic ions as proxies for Al. Planta 205: 389–396 [Google Scholar]
- Pellet DM, Papernik LA, Kochian LV (1996) Multiple aluminum-resistance mechanisms in wheat: roles of root apical phosphate and malate exudation. Plant Physiol 112: 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Kochian LV (2002) H+ currents around plant roots. In Z Rengel, ed, Handbook of Plant Growth: pH as the Master Variable. Marcel Dekker, New York, pp 299–322
- Piñeros MA, Shaff JE, Manslank HS, Carvalho VM, Kochian LV (2005) Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiology study. Plant Physiol 137: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z (1992) Role of calcium in aluminum toxicity. New Phytol 121: 499–513 [Google Scholar]
- Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52: 527–560 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ (1995. a) Characterisation of Al-stimulated efflux of malate from the apices of Al wheat roots. Planta 196: 103–110 [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ (1995. b) Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Aust J Plant Physiol 22: 531–536 [Google Scholar]
- Ryan PR, DiTomaso J, Kochian LV (1993) Spatial aspects of aluminium toxicity and the role of the root cap. J Exp Bot 44: 437–446 [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37: 645–653 [DOI] [PubMed] [Google Scholar]
- Shen R, Ma JF, Kyo M, Iwashita T (2002) Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta 215: 394–398 [DOI] [PubMed] [Google Scholar]
- Taylor GJ (1991) Current views of the aluminum stress response: the physiological basis of tolerance. Curr Top Plant Biochem Physiol 10: 57–93 [Google Scholar]
- Vázquez M, Poschenrieder C, Corrales I, Barceló J (1999) Changes in apoplastic aluminum during the initial growth response to aluminum by roots of tolerant maize variety. Plant Physiol 119: 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Osaki M (2002) Mechanisms of adaptation to high aluminum condition in native plant species growing in acid soils: a review. Commun Soil Sci Plant Anal 33: 1247–1260 [Google Scholar]
- Wenzl P, Patiño GM, Chaves AL, Mayer JE, Rao IM (2001) The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiol 125: 1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zheng SJ, Lin XY, Tang CX, Zhou GD (2005) Genotypic differences among plant species in response to aluminum stress. J Plant Nutr (in press)
- Yang ZM, Nian H, Sivaguru M, Tanakamaru S, Matsumoto H (2001) Characterization of aluminium-induced citrate secretion in aluminium-tolerant soybean (Glycine max) plants. Physiol Plant 113: 64–71 [Google Scholar]
- Zhao Z, Ma JF, Sato K, Takeda T (2003) Differential Al resistance and citrate secretion in barley (Hordeum vulgare L.). Planta 217: 794–800 [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H (1998. a) High aluminum resistance in buckwheat. Al-induced special secretion of oxalic acid from root tips. Plant Physiol 117: 745–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H (1998. b) Continuous secretion of organic acids is related to aluminium resistance during relatively long-term exposure to aluminium stress. Physiol Plant 103: 209–214 [Google Scholar]
- Zhu YG, He YQ, Smith SE, Smith FA (2002) Buckwheat (Fygopyrum esculentum Moench) has high capacity to take up phosphorous (P) from calcium (Ca)-bound source. Plant Soil 239: 1–8 [Google Scholar]