Abstract
Giant condyloma acuminata (GCA) is a rare, locally aggressive manifestation of human papillomavirus (HPV) infection, typically affecting the anorectal area. Patients with GCA often have a poor prognosis due to the high risk of malignant transformation. In this case report, we present a 39-year-old man with HIV who developed progressive and refractory anorectal GCA. Despite initially non-cancerous pathology results, there were concerns regarding a malignant component to the mass. Multidisciplinary discussions led to the decision to pursue definitive radiation therapy. This case report and review of the literature highlight the role of radiation in the management of GCA and the importance of a multidisciplinary approach in the treatment of complex cases.
Keywords: refractory treatment, non-cancerous pathology, hiv, anorectal area, multidisciplinary approach, malignant transformation, human papillomavirus, radiation therapy, rectal involvement, giant condyloma acuminata
Introduction
First described in 1924 by Abraham Buschke, giant condyloma acuminata (GCA), also known as Buschke-Lowenstein tumor, is a rare, locally aggressive variant of condyloma acuminata. While GCAs resemble both common condylomas and squamous cell carcinoma (SCC), these aggressive tumors are unique due to their benign histology, aggressive growth, malignant transformation, and high rates of recurrence after excision [1]. Typically arising in the anogenital region as persistent condyloma acuminatum, the progression to GCA may take three to seven years [2]. Over half of GCAs undergo malignant transformation, commonly followed by rapid infiltration of adjacent structures, with little histological distinction from benign condylomas [1,3]. These behavioral and histological features result in the high morbidity and mortality associated with GCAs, supporting the need for aggressive management strategies [2].
Traditionally, local resection is the standard of care. However, local invasion into perineal structures can complicate operative intervention and may necessitate the use of neoadjuvant therapies to shrink the tumor preoperatively. In locally advanced cases, surgery may not be a feasible option. In such cases, given the high malignant potential of GCA, it is reasonable to pursue a treatment paradigm similar to the management anal cancer. This would involve treating the mass definitively with radiation therapy, typically in combination with a radiosensitizing chemotherapy agent to achieve local control and improve patient outcomes. Although it is well known that concurrent chemotherapy improves local control and survival in patients with anal cancer, both acute and late toxicity are significantly higher with this treatment approach [4]. In patients with HIV, this may be of particular concern due to compromised immune function. Therefore, radiation therapy alone may be considered to be a balanced treatment approach in this patient population.
We report a case of a patient with HIV infection and a history of recurrent anal condylomas, who developed locally advanced GCA. Given the aggressive infiltration of the tumor into adjacent structures, this patient required a unique management strategy utilizing definitive radiotherapy alone.
Case presentation
A 39-year-old man with a longstanding history of HIV/AIDS on highly active antiretroviral therapy (HAART) of Biktarvy once a day, presented to his infectious disease specialist for routine follow-up. The patient's CD4 count was observed at 288, indicating an improvement from the previous year's count of 130. He had been experiencing two years of recurrent anal fistulas, requiring two fistulotomies eight and nine months prior to presentation.
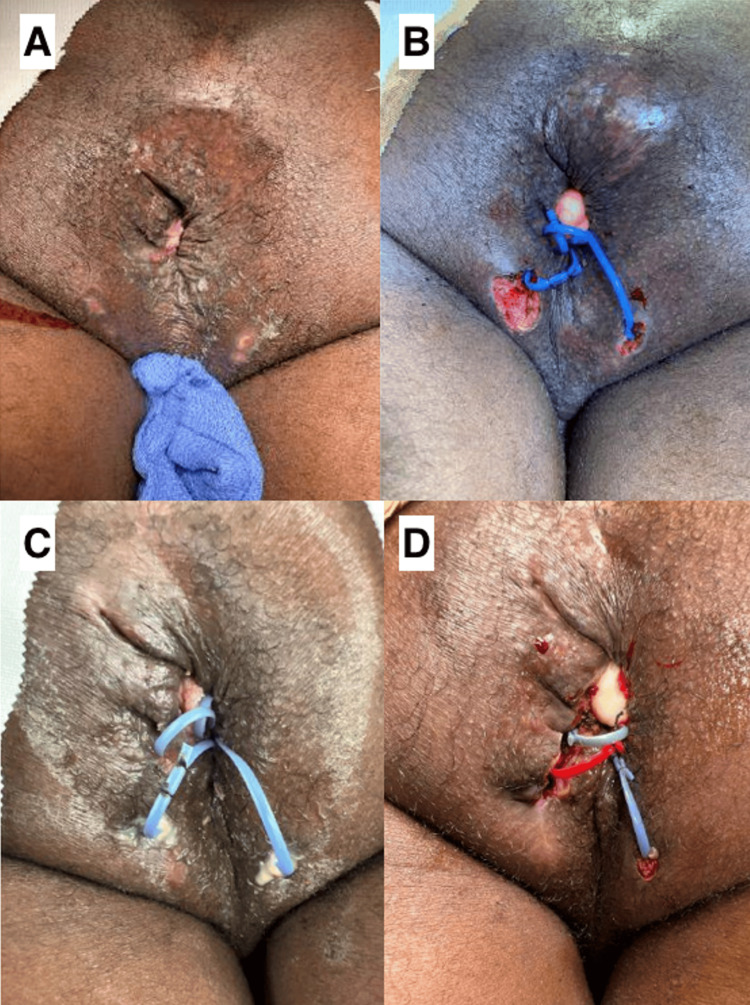
The patient expressed frustration with persistent rectal pain and anogenital warts and was referred to a general surgeon, who performed a colonoscopy. Advancement of the colonoscope was impeded by a large, pedunculated, partially obstructing anorectal mass. The mass was biopsied and the pathology was consistent with a low-grade squamous intraepithelial lesion (anal intraepithelial neoplasia grade I (AIN I)). A rectal examination under anesthesia (EUA) identified multiple abscesses and a recurrent fistula and scant stool in the rectal vault. The patient underwent fulguration of anal lesions, drainage of multiple abscesses, and with a seton placement (Figure 1). The patient was prescribed topical Imiquimod to be applied every other day prior to bedtime for the post-surgical management of the resected condylomas.
Figure 1. Appearance of anus following first and second fistulotomies with condyloma fulguration.
(A) The pre-operative appearance of the anal region prior to the first anal examination under anesthesia (EUA). (B) The post-operative appearance of the anal region following condyloma excision and seton placement during EUA. (C) The pre-operative appearance of the anal region prior to the second anal EUA (six months following the first condyloma excision). Pus originating from anus was noted; consistent with re-opening/drainage of prior abscess from first EUA. (D) The post-operative appearance of the anal region following the second EUA. The patient required a second fistulotomy with further condyloma fulguration and new seton placement.
The patient was initially scheduled for a three-month follow-up visit with the general surgeon; however, this appointment was postponed because the patient required emergency surgery for a stab wound in their left flank.
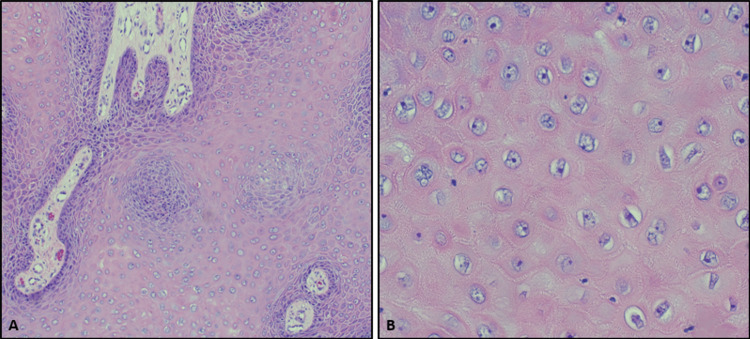
A follow-up visit at six months revealed several recurrent and unresolved condylomas. The patient underwent a second colonoscopy and EUA for condyloma re-excision. The biopsy specimens obtained during the colonoscopy were insufficient, so repeat biopsies were conducted during the EUA. Six fragments of varicoid skin were fixed with formalin and sent to pathology, along with samples of the left anterior fistula tract and an anterior skin tag. Histological examination of these fragments was remarkable for acanthosis and koilocytosis (irregular nuclei, multinucleation, and perinuclear vacuolization; Figure 2). Immunohistochemistry of this tissue was negative for p16 block-positive staining (not shown) and negative for definitive high-grade dysplasia, supporting a diagnosis of GCA. Immunohistochemistry of this tissue found that the tumor was negative for block-positive staining in A2-2 and negative for definitive high-grade dysplasia in C2-2 antibodies (Figure 2). Following the procedure, he reported difficulty urinating and significant perianal pain that radiated to the left groin.
Figure 2. H&E of tissue fragments from patient's anus.
Acanthosis, or thickening of the stratum spinosum, is present in the left-hand image (A, 10x), while koilocytosis (irregular nuclei, multinucleation, and perinuclear vacuolization) is noted in the right-hand image (B, 40x). These histologic findings in the patient's clinical context, along with negative p16 block-positive IHC staining (not shown) and absence of definitive high-grade dysplasia, support a diagnosis of GCA.
GCA: giant condyloma acuminatum; H&E: hematoxylin & eosin; IHC: immunohistochemistry
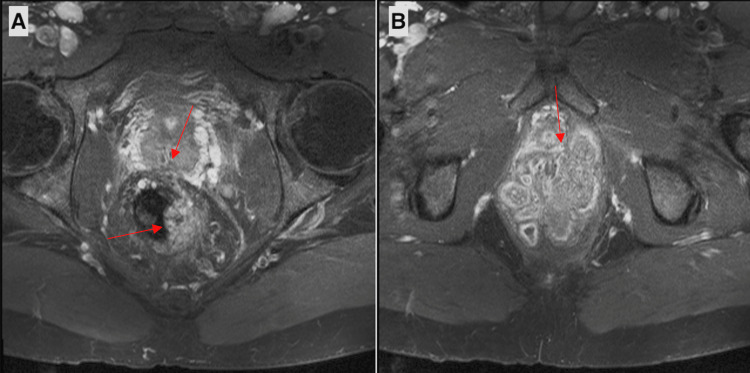
A two-month post-operative gadolinium-enhanced MRI revealed a 7.5 cm lobulated cauliflower-like mass extending into the mesorectal and periprostatic space, with invasion of the corpus spongiosum of the penis, and mild enlargement of several pelvic lymph nodes (Figure 3). Visual features of the mass and the degree of intraluminal obstruction raised concern for an underlying SCC.
Figure 3. Axial T2-weighted MRI images of the pelvis before radiation therapy showing rectal involvement of the mass at inferior arrow and invasion into the periprostatic space at superior arrow (A) and infiltrating the base of the penis (B).
Treatment
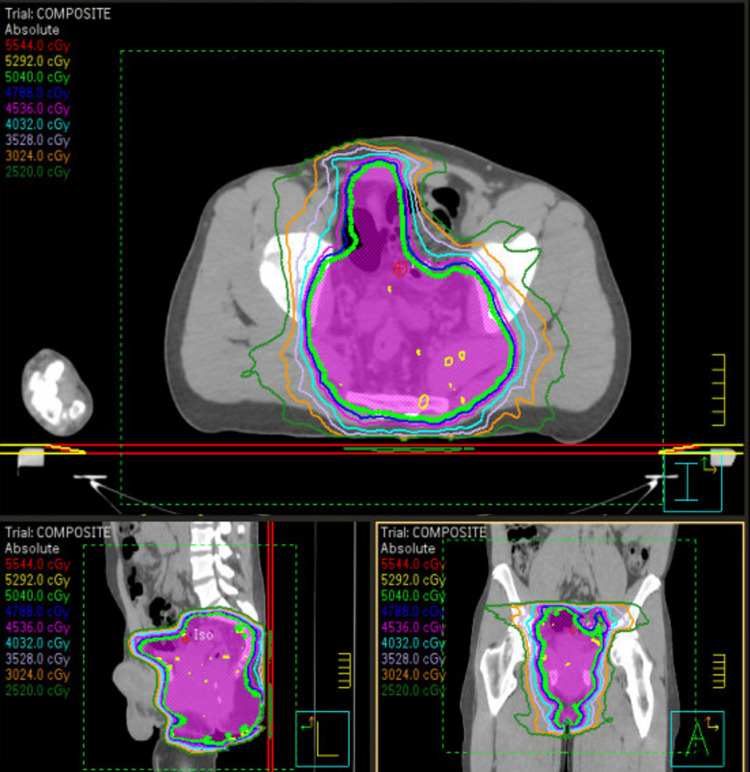
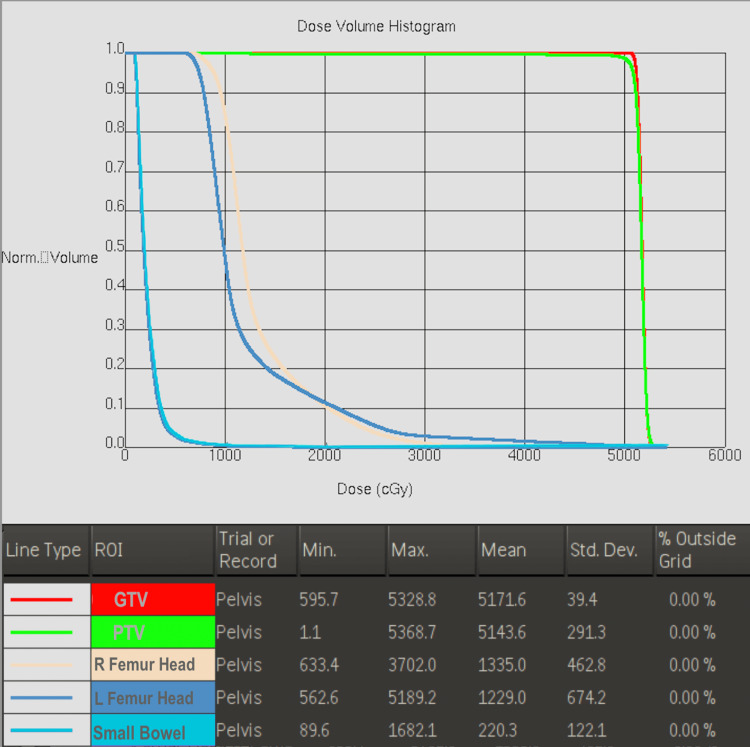
This case was presented to a multi-disciplinary colorectal tumor board, and a consensus was made to refer the patient to the radiation oncology department for consideration of definitive radiation therapy. Given the patient’s poor response to condyloma excision and adjuvant imiquimod and the alternative of a total exenteration, definitive radiotherapy was considered the most conservative treatment option [5]. The risks and benefits of radiotherapy were discussed with the patient, including the risk of infertility. Given the patient’s desire to preserve his fertility, a clamshell was used throughout the treatment. A total of 50.4 Gy was delivered to the pelvic region in 28 fractions. The contours, field design, and dose constraints were in concordance with the Radiation Therapy Oncology Group (RTOG) 0529 standard for the treatment of anal cancer (Figure 4) [5]. All dose constraints were met (Figure 5).
Figure 4. Patient radiation therapy treatment plan.
Legend: The isodose lines correlating with the respective colors are displayed in the top right-hand corner of the figure. The planning target volume (PTV) is displayed in a magenta color.
Figure 5. Dose-volume histogram of organs at risk in patient’s radiation treatment plan as detailed in Figure 4, compiled using MIM software (MIM Software Inc., Cleveland, OH, USA).
Outcomes and follow-up
The patient tolerated treatment well, without interruptions. Aside from acute Common Terminology Criteria for Adverse Events (CTCAE) grade 1 diarrhea, he also developed acute CTCAE grade 2 moist desquamation towards the end of treatment, which required aluminum sulfate tetradecahydrate (Domeboro® (Bayer HealthCare LLC, Whippany, USA)) soaks four times a day. Despite detailed follow-up instructions, the patient failed to present for a three-month follow-up CT of the abdomen and pelvis, and has been unreachable by phone due to phone service termination since the completion of treatment. This may be explained by the patient’s current homelessness, as relayed to the colorectal surgeon. The patient has followed up with general surgery six months following radiotherapy and reported that the patient is “feeling the best he has felt in a very long time since completion of RT." He denied any treatment-related toxicity at this visit. Perianal exam revealed the presence of chronic CTCAE grade 1 radiation-induced induration. Anoscopy showed a complete clinical response, noting no condylomas on the exam.
Discussion
The odds of developing GCA in the perianal region are increased in cases of immunosuppression, tobacco use, and early sexual intercourse [6]. The patient’s diagnosis of HIV and known history of tobacco use placed them at a higher risk of development. Within a seven-year time frame, the patient's recurrent anogenital warts developed into an aggressively invasive form of GCA. Given the locally aggressive nature of the GCA in this patient, paired with the proximity to vital structures, non-surgical treatment was necessary to preserve the quality of life.
Wide local excision or abdominal perineal resections are broadly considered the treatments of choice for patients presenting with GCA. According to a review of 42 GCA cases, up to 56% of those such cases malignantly transformed into SCC [1].
Several alternative treatment options have been explored in the literature. Generally, these conservative options have shown higher rates of local recurrence, and no singular treatment has been proven to achieve satisfactory disease control [7].
GCAs are often initially managed with topical therapies including imiquimod, podophyllotoxin, and sinecatechins. More commonly prescribed for the treatment of HIV-induced anogenital warts, these topical treatments are utilized as adjuvant therapies to more invasive therapies in the management of GCA [8,9]. As observed in our patient, who was prescribed topical imiquimod every other night, topical therapies alone have not proven to be effective in achieving total regression of condylomas. There has been a singular case of complete regression of vulvar GCA with no recurrence after three years [10].
Laser-based therapies are a better treatment option for GCA. Photodynamic therapy (PDT), which engages a photodynamic reaction to target selective tissues, has been largely successful in the treatment of classic condyloma acuminata. When combined with oral acitretin and aminolevulinic acid (ALA), patients experienced a 94% cure rate [11]. When applied to GCA, PDT has yielded more ambiguous outcomes. While PDT has completely eradicated the underlying GCA in some patients [12], in others PDT resulted in a reduction of GCA symptoms without a significant reduction in lesion size [13].
Although CO2 lasers have been historically successful in managing verrucous carcinoma [8], CO2 laser vaporization is reserved for cases of surface level, non-penetrating GCAs, post-operative ablation, and recurrent GCA following surgical intervention [14].
Cryotherapy offers another minimally invasive treatment option for patients with GCA. Cryotherapy has been successfully utilized for decades to achieve complete local control in patients with GCA [15]. However, the requirement of combination therapy in patients with locally advanced disease serves as a limitation to broader use [16]. Furthermore, cryotherapy imposes a significant burden on the patient, as the frequency with which cryotherapy must be conducted exceeds that of all laser and topical therapies.
The most extensively studied alternative to surgical excision is chemotherapy. While there have been cases of anogenital verrucous carcinomas successfully treated with systemic chemotherapy, the existing data on GCAs only pertains to smaller, superficial lesions [17]. For local control of larger lesions, the use of chemotherapeutics is focused on reducing the tumor burden in the pre- and postoperative settings [18,19]. Oral chemotherapy with fluorouracil (5-FU) as neoadjuvant therapy is largely effective when combined with local surgical excision and radiation therapy [20,21]. However, while concurrent chemotherapy and radiotherapy regimens traditionally used to treat anal SCC may be effective in patients with GCA, there remains a need for a more thorough investigation to evaluate the potential efficacy and long-term treatment safety of this treatment approach [18]. For GCAs that remain refractory to chemoradiation, surgical intervention through an abdominoperineal resection (APR) is warranted.
Through a multidisciplinary tumor board for discussion of management options, surgical resection was confirmed to be limited due to the lack of clear planes between the mass and adjacent structures. Wide local excision would be near impossible without excision of portions of the patient's lower left penis, prostate, and perianal region. While flap reconstruction is possible for some patients [22], radiotherapy was deemed a more suitable treatment choice due to age and quality of life. The unique management outlined in this case report has been utilized in 11 other reported cases of locally advanced GCA in a literature review spanning over 30 years (Table 1).
Table 1. A literature review of published cases with GCA treated with radiation therapy spanning from 1987 to 2019.
GCA: giant condyloma acuminata, HPV: human papillomavirus, 5FU: fluorouracil, SCC: squamous cell carcinoma, APR: abdominoperineal resection
| First Author/Pub Year | Gender | Age | HPV/HIV | Therapy | Fractions | Follow-Up | Recurrence | Additional Therapy | Pathology |
| Butler, 1987 [18] | Male | 40 | N/A | Definitive Radiotherapy, 5FU, mitomycin | 45Gy (28 fractions over 44d) | 32w | No | Diverting colostomy, surgical excisions | GCA w/ marked acanthosis, hyperkeratosis, and papillomatosis with well-differentiated in situ and invasive SCC |
| Hyacinthe, 1998 [23] | Male | 60 | HPV+ (6/11/16/18/31/33/35) | Radiotherapy, 5FU, mitomycin-C, posterior pelvic exenteration | 46.8Gy (26 fractions over 46d) | 2y | No | No | GCA with SCC transformation |
| Sobrado, 2000 [24] | Male | 42 | N/A | Definitive Radiation therapy | 45 Gy (28 fractions) during additional radiation therapy | 20mo | No | PRIOR to Radiation Therapy: Sigmoidostomy, bleomycin, topical podophyllin (recurrence in <30d) | |
| Dolanc, 2002 [25] | Female | 56 | HPV+ (6/11) | Radiotherapy, APR (Abdominoperineal Resection) | 50Gy | No | SCC arising in GCA w/ clear resection margins | ||
| Chao, 2005 [26] | Male | 57 | HIV- | Radiotherapy, 5FU, mitomycin | 50.4Gy to primary tumor, 45Gy to perirectal LN, 36Gy to inguinal LN | 1y | No | No | |
| Tytherleigh, 2006 [27] | Male | 40 | HIV+ | chemoradiation, APR (Abdominoperineal Resection) | 12mo | Yes, patient passed | N/A | GCA | |
| Male | 51 | HIV+ | chemoradiation, APR (Abdominoperineal Resection) | 5y | No | N/A | GCA | ||
| Armstrong, 2009 [28] | Male | 46 | HIV-, HPV+ (6/11) | Unsuccessful APR, chemoradiotherapy | 34mo | No | No | GCA | |
| Handisurya, 2009 [29] | Male | 45 | HIV+, HPV+ (6/11) | Surgical intervention | 60Gy (additional radiation therapy) | 6mo | Yes | Palliative surgery and chemoradiotherapy | GCA differentiating into invasive SCC |
| Haque, 2009 [30] | Male | 38 | HIV- | Definitive Radiotherapy, 5FU, mitomycin | 54Gy (30 fractions) | 6mo | No | PRIOR to Radiation Therapy: Two Surgical excisions (recurrence in <5mo) | GCA (Buschke-Lewenstein tumor-type) w/ focal transformation to verrucous carcinoma |
| Indinnimeo, 2013 [21] | Male | 43 | HIV+, HPV+ (6) | Radiotherapy, 5FU, mitomycin-C | 45Gy (28 fractions) to the pelvis plus a boost with 14.40Gy (1.8Gy/fr) to the primary tumor | 3y | No | EUS and high-resolution anoscopy (HRA) | GCA with SCC transformation |
| Shenoy, 2019 [31] | Male | 70 | HIV-, HPV+ | Radiotherapy, 5FU, mitomycin | 46Gy (30 fractions) | 3y | No | No | GCA, SCC of the anus |
| Male | 58 | HIV+, HPV+ | Radiotherapy, 5FU, mitomycin, diverting sigmoid colostomy | 46Gy (30 fractions) | 3mo | Yes | No | GCA, SCC of the anus |
The patient underwent a course of external beam radiation therapy delivering 50.4 Gy in 28 fractions. While concurrent chemotherapy was also considered to enhance treatment efficacy, the decision was made to treat with radiation alone. The patient was informed about the potential acute and long-term side effects of radiation therapy and presented with fertility preservation options.
While wide surgical excision would be the standard of care and result in the greatest chance of survival, the patient would be required to have an ostomy for the remainder of their life which would necessitate total reconstruction of the penis. Given the patient's standing as a relatively healthy 39-year-old male, excluding HIV and GCA diagnoses, preserving the patient’s quality of life was deemed a priority.
At six months follow-up with general surgery, anoscopy reflected complete regression of condylomas in this patient. Given the nature of GCA to undergo malignant transformation [1], a complete clinical response is vital to preventing further recurrence or progression of the cancer. Based on the literature review conducted on those cases utilizing radiation therapy as a definitive treatment option, primarily after the failure of more common treatment options, only 15% experienced recurrence. The patient's positive clinical outcome could be correlated to a decreased probability of recurrence and/or progression of disease to a malignant state.
GCA with rectal involvement poses significant challenges in terms of treatment planning and management decisions. The case report emphasizes the role of radiation therapy in the multimodal management of this condition, especially when surgical options are limited. A comprehensive and multidisciplinary approach is crucial in determining the optimal treatment strategy and addressing patient concerns. While further studies are warranted to evaluate the efficacy and long-term outcomes of radiation therapy in this rare and aggressive disease, for this patient, the adequacy of radiation therapy in the treatment of GCA is evident through the patient’s total regression of disease and most notably through the patient’s improved quality of life.
Conclusions
In summary, this case report details a progressive and treatment-resistant GCA, emphasizing the challenges associated with its management. The patient's anorectal GCA, refractory to traditional surgical and topical treatments, raised concerns for possible malignant transformation. After a thorough multidisciplinary evaluation, definitive radiation therapy was pursued, resulting in a complete clinical response and significant improvement in the patient's quality of life. This case supports radiation therapy as a viable treatment option for GCA, particularly in complex cases where surgical interventions fail and malignancy is a concern.
Acknowledgments
We would like to acknowledge Dr. Grace Y. Wang, faculty dermatopathologist at Corewell Health William Beaumont University Hospital in Royal Oak, MI, for access to relevant histology slides.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Bailey A. Loving, John M. Robertson
Acquisition, analysis, or interpretation of data: Bailey A. Loving, Shaveena Sivapalan, Siddharth Ramanathan, Casey P. Schukow
Critical review of the manuscript for important intellectual content: Bailey A. Loving, John M. Robertson, Siddharth Ramanathan
Supervision: Bailey A. Loving, John M. Robertson
Drafting of the manuscript: Shaveena Sivapalan, Casey P. Schukow
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.The pathogenesis of giant condyloma acuminatum (Buschke-Lowenstein tumor): an overview. Purzycka-Bohdan D, Nowicki RJ, Herms F, Casanova JL, Fouéré S, Béziat V. Int J Mol Sci. 2022;23:4547. doi: 10.3390/ijms23094547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giant condyloma acuminatum (Buschke-Lowenstein tumor) of the anorectal and perianal regions. Analysis of 42 cases. Chu QD, Vezeridis MP, Libbey NP, Wanebo HJ. Dis Colon Rectum. 1994;37:950–957. doi: 10.1007/BF02052606. [DOI] [PubMed] [Google Scholar]
- 3.An armamentarium of wart treatments. Lipke MM. Clin Med Res. 2006;4:273–293. doi: 10.3121/cmr.4.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Br J Cancer. 2010;102:1123–1128. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Kachnic LA, Winter K, Myerson RJ, et al. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staged resection in the management of HIV-related anogenital giant condyloma acuminatum. A case report. Loo GH, Lim LY, Zainuddin ZM, Fam XI. Ann Med Surg (Lond) 2019;48:73–76. doi: 10.1016/j.amsu.2019.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giant condyloma acuminatum or buschke-Lowenstein tumor: review of the literature and report of three cases treated by CO2 laser surgery. A long-term follow-up. Frega A, P Stentella, Tinari A, Vecchione A, Marchionni M. https://europepmc.org/article/med/12168925. Anticancer Res. 2002;10:1201–1204. [PubMed] [Google Scholar]
- 8.Treatment of verrucous carcinoma with imiquimod and CO2 laser ablation. Heinzerling LM, Kempf W, Kamarashev J, Hafner J, Nestle FO. Dermatology. 2003;207:119–122. doi: 10.1159/000070963. [DOI] [PubMed] [Google Scholar]
- 9.Giant condyloma acuminata of Buschke-Löwenstein: successful treatment with a combination of surgical excision, oral acitretin and topical imiquimod. Erkek E, Basar H, Bozdogan O, Emeksiz MC. Clin Exp Dermatol. 2009;34:366–368. doi: 10.1111/j.1365-2230.2008.02938.x. [DOI] [PubMed] [Google Scholar]
- 10.Giant condyloma acuminatum of the vulva: successful management with imiquimod. Combaud V, Verhaeghe C, El Hachem H, Legendre G, Descamps P, Martin L, Bouet PE. JAAD Case Rep. 2018;4:692–694. doi: 10.1016/j.jdcr.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ALA-PDT combined with oral acitretin in the treatment of refractory condyloma acuminatum in anal canal. Zhang L, Zeng Q, Li J, et al. Photodiagnosis Photodyn Ther. 2022;40:103048. doi: 10.1016/j.pdpdt.2022.103048. [DOI] [PubMed] [Google Scholar]
- 12.Buschke-Löwenstein tumor (giant condyloma acuminatum) successfully treated by topical photodynamic therapy: a case report. Chu GY, Chang TCC, Chang CH. Dermatologica Sinica. 2013;31:94–97. [Google Scholar]
- 13.Modified photodynamic therapy of vulvar giant condyloma acuminatum complicated by systemic lupus erythematosus: A case report. Photodiagnosis and Photodynamic Therapy. Zhang Y, Zhang L, Zhang H, et al. Photodiagnosis Photodyn Ther. 2022;1:103089. doi: 10.1016/j.pdpdt.2022.103089. [DOI] [PubMed] [Google Scholar]
- 14.Recurrent Buschke-Löwenstein tumor treated using CO(2) laser vaporization. Perniola G, d'Itri F, Di Donato V, Achilli C, Lo Prete E, Panici PB. J Minim Invasive Gynecol. 2010;17:662–664. doi: 10.1016/j.jmig.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Cryosurgery of verrucous carcinoma of the penis (Buschke-Lowenstein tumor) Hughes PS. https://europepmc.org/article/med/477377. Cutis. 1979;24:43–45. [PubMed] [Google Scholar]
- 16.Recalcitrant extragenital giant condyloma acuminatum: a need for combination therapy. Lee CN, Hsu CK, Lee JY. Dermatol Ther. 2019;32:0. doi: 10.1111/dth.12867. [DOI] [PubMed] [Google Scholar]
- 17.Buschke-Lowenstein tumor: therapeutic options including systemic chemotherapy. Ilkay AK, Chodak GW, Vogelzang NJ, Gerber GS. Urology. 1993;42:599–602. doi: 10.1016/0090-4295(93)90288-l. [DOI] [PubMed] [Google Scholar]
- 18.Squamous-cell carcinoma of the anus in condyloma acuminatum. Successful treatment with preoperative chemotherapy and radiation. Butler TW, Gefter J, Kleto D, Shuck EH 3rd, Ruffner BW. Dis Colon Rectum. 1987;30:293–295. doi: 10.1007/BF02556178. [DOI] [PubMed] [Google Scholar]
- 19.Buschke-Löwenstein tumor in childhood: a case report. Ambriz-González G, Escobedo-Zavala LC, Carrillo de la Mora F, et al. J Pediatr Surg. 2005;40:0–7. doi: 10.1016/j.jpedsurg.2005.05.070. [DOI] [PubMed] [Google Scholar]
- 20.Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. WOMAN Trial Collaborators. Lancet. 2017;389:2105–2116. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buschke-Löwenstein tumor with squamous cell carcinoma treated with chemo-radiation therapy and local surgical excision: report of three cases. Indinnimeo M, Impagnatiello A, D'Ettorre G, et al. World J Surg Oncol. 2013;11:231. doi: 10.1186/1477-7819-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reconstructive surgery in anal giant condyloma: report of two cases. Mingolla GP, Potì O, Carbotta G, Marra C, Borgia G, De Giorgi D. Int J Surg Case Rep. 2013;4:1088–1090. doi: 10.1016/j.ijscr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Squamous-cell carcinoma of the pelvis in a giant condyloma acuminatum: use of neoadjuvant chemoradiation and surgical resection: report of a case. Hyacinthe M, Karl R, Coppola D, et al. Dis Colon Rectum. 1998;41:1450–1453. doi: 10.1007/BF02237065. [DOI] [PubMed] [Google Scholar]
- 24.Radiation-induced total regression of a highly recurrent giant perianal condyloma: report of case. Sobrado CW, Mester M, Nadalin W, Nahas SC, Bocchini SF, Habr-Gama A. Dis Colon Rectum. 2000;43:257–260. doi: 10.1007/BF02236991. [DOI] [PubMed] [Google Scholar]
- 25.Malignant transformation of perianal Buschke-Löwenstein tumor. Extensive abdominoperineal rectum excision and reconstruction with transpelvic myocutaneous rectus abdominis muscle flap [Article in German] Dolanc R, Kocher T, Langer I, Marti WR, Pierer G, Harder F. Chirurg. 2002;73:370–374. doi: 10.1007/s00104-001-0402-4. [DOI] [PubMed] [Google Scholar]
- 26.Squamous cell carcinoma arising in a giant condyloma acuminatum (Buschke-Lowenstein tumour) Chao MWT, Gibbs P. Asian J Surg. 2005;28:238–240. doi: 10.1016/S1015-9584(09)60352-3. [DOI] [PubMed] [Google Scholar]
- 27.Combined surgery and chemoradiation as a treatment for the Buschke-Löwenstein tumour. Tytherleigh MG, Birtle AJ, Cohen CE, Glynne-Jones R, Livingstone J, Gilbert J. Surgeon. 2006;4:378–383. doi: 10.1016/s1479-666x(06)80114-9. [DOI] [PubMed] [Google Scholar]
- 28.Successful treatment of a large Buschke-Lowenstein tumour with chemo-radiotherapy. Armstrong N, Foley G, Wilson J, Finan P, Sebag-Montefiore D. Int J STD AIDS. 2009;20:732–734. doi: 10.1258/ijsa.2009.009012. [DOI] [PubMed] [Google Scholar]
- 29.Rapid progression of an anal Buschke-Lowenstein tumour into a metastasising squamous cell carcinoma in an HIV-infected patient. Handisurya A, Rieger A, Bago-Horvath Z, et al. Sex Transm Infect. 2009;85:261–263. doi: 10.1136/sti.2008.034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Successful treatment of recurrent Buschke-Lowenstein tumor by radiation therapy and chemotherapy. Haque W, Kelly E, Dhingra S, Carpenter LS. Int J Colorectal Dis. 2010;25:539–540. doi: 10.1007/s00384-009-0826-8. [DOI] [PubMed] [Google Scholar]
- 31.Anal carcinoma in giant anal condyloma, multidisciplinary approach necessary for optimal outcome: two case reports and review of literature. Shenoy S, Nittala M, Assaf Y. World J Gastrointest Oncol. 2019;11:172–180. doi: 10.4251/wjgo.v11.i2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]