Abstract
During cold acclimation, winter rye (Secale cereale L. cv Musketeer) plants accumulate antifreeze proteins (AFPs) in the apoplast of leaves and crowns. The goal of this study was to determine whether these AFPs influence survival at subzero temperatures by modifying the freezing process or by acting as cryoprotectants. In order to inhibit the growth of ice, AFPs must be mobile so that they can bind to specific sites on the ice crystal lattice. Guttate obtained from cold-acclimated winter rye leaves exhibited antifreeze activity, indicating that the AFPs are free in solution. Infrared video thermography was used to observe freezing in winter rye leaves. In the absence of an ice nucleator, AFPs had no effect on the supercooling temperature of the leaves. However, in the presence of an ice nucleator, AFPs lowered the temperature at which the leaves froze by 0.3°C to 1.2°C. In vitro studies showed that apoplastic proteins extracted from cold-acclimated winter rye leaves inhibited the recrystallization of ice and also slowed the rate of migration of ice through solution-saturated filter paper. When we examined the possible role of winter rye AFPs in cryoprotection, we found that lactate dehydrogenase activity was higher after freezing in the presence of AFPs compared with buffer, but the same effect was obtained by adding bovine serum albumin. AFPs had no effect on unstacked thylakoid volume after freezing, but did inhibit stacking of the thylakoids, thus indicating a loss of thylakoid function. We conclude that rye AFPs have no specific cryoprotective activity; rather, they interact directly with ice in planta and reduce freezing injury by slowing the growth and recrystallization of ice.
Winter cereals are classified as freezing-tolerant plants because they survive the growth of extracellular ice in their tissues at subzero temperatures (Levitt, 1980; Pearce, 1988; Brush et al., 1994). Although the mechanism of freezing tolerance is complex, one component involves the secretion of antifreeze proteins (AFPs) into the apoplast of the leaves and crown (Griffith et al., 1992; Marentes et al., 1993; Antikainen et al., 1996; Griffith and Yaish, 2004). In winter rye (Secale cereale L. cv Musketeer), there are six different AFPs, ranging in mass from 16 to 35 kD, that all have the ability to bind onto the surface of ice and inhibit its growth in vitro (Hon et al., 1994). During cold acclimation, the accumulation of AFPs is correlated with increased freezing tolerance in winter and spring varieties of rye, wheat, and barley (Marentes et al., 1993; Antikainen and Griffith, 1997). Furthermore, a comparison of seven cold-acclimated rye, wheat, and barley cultivars showed that the level of freezing tolerance, determined by the survival of plants in the field, is highly correlated (R2 = 0.91) with the apoplastic protein content of the leaves (Chun and Griffith, 1998).
One limitation of these studies is that they do not demonstrate a cause-and-effect relationship between AFPs and freezing tolerance or winter survival. However, when apoplastic proteins were extracted from cold-acclimated winter rye leaves, the leaves exhibited greater injury after freezing and thawing, indicating that AFPs do have a protective function (Marentes et al., 1993). Besides binding to ice, it has also been shown that AFPs isolated from fish that inhabit the northern Atlantic Ocean can interact with membranes and proteins to reduce injury (Fletcher et al., 1999). When exposed to cold and/or freezing temperatures in the presence of AFPs from fish, mammalian cells, and tissues such as bovine oocytes, human blood platelets, mouse embryos, ram spermatozoa, and rat livers, all exhibit greater rates of survival and recovery of normal function (Fletcher et al., 1999). Therefore, the goal of this study was to determine whether AFPs enhance the ability of winter rye plants to survive freezing by interacting with ice and/or by acting as cryoprotectants.
When AFPs bind to the surface of ice, they adsorb irreversibly onto a specific plane of the crystal lattice, each of which is characterized by a different, regular spacing of the oxygen atoms (Knight et al., 1991, 1995; Jia and Davies, 2002). AFPs must be mobile in order to make the correct structural match with this crystal lattice (Knight et al., 1995). In winter rye, AFPs appear to be associated with the outer surface of cell walls and the middle lamellae in cold-acclimated winter rye leaves where ice propagates during freezing (Pihakaski-Maunsbach et al., 1996, 2003). This location may indicate that the AFPs are bound to the cell walls or it may be an artifact of fixation because the cell walls are the only structures within the apoplast to which the proteins can be cross-linked. Therefore, it was essential to first determine whether the apoplastic proteins are free in solution in planta so that they are able to interact with ice. We then observed the freezing process by infrared video thermography (IRVT) to determine the effect of AFPs on the propagation of ice in the tissues (Wisniewski et al., 1997; Wisniewski and Fuller, 1999; Pearce and Fuller, 2001). Once a plant is frozen, the ice may change its structure, and so we also examined the effects of winter rye AFPs on the recrystallization of ice. Finally, we subjected lactate dehydrogenase (LDH) and spinach (Spinacea oleracea) thylakoids to freezing and thawing in the presence and absence of winter rye AFPs to assay their cryoprotective activity. Together, these experiments provide insight into the functions of AFPs in cold-acclimated plants.
RESULTS
Antifreeze Activity Is Present in Guttation Fluid
To determine whether AFPs are mobile in the apoplast, we placed winter rye plants at high humidity to force guttation, then collected droplets of guttation fluid and assayed them for antifreeze activity. As shown in Figure 1, guttate from cold-acclimated plants modified the growth of ice so that hexagonal crystals formed, whereas only circular crystals (indicative of no antifreeze activity) were observed in guttate obtained from nonacclimated plants. One characteristic of AFPs is that they inhibit the growth of ice at concentrations well below those needed for freezing point depression. As a result, it is possible to quantify antifreeze activity by measuring thermal hysteresis, which is the difference between the freezing and melting point of an ice crystal in solution (DeVries, 1986). We observed no measurable thermal hysteresis of ice crystals in the guttate from nonacclimated winter rye leaves (n = 46), whereas guttation fluid collected from cold-acclimated leaves exhibited a low but statistically significant (P < 0.01) level of thermal hysteresis (mean ± se, 0.01°C ± 0.001°C; n = 17).
Figure 1.
Antifreeze activity of guttate from nonacclimated and cold-acclimated winter rye leaves. A, No antifreeze activity was observed in nonacclimated winter rye leaves. B and C, Low levels of antifreeze activity were observed in guttate from cold-acclimated leaves. D to F, Higher antifreeze activity was observed when guttate from cold-acclimated leaves was concentrated 30-fold. Magnification bar represents 20 μm.
AFPs Lower the Ice Nucleation Temperature of Winter Rye Leaves
We compared the freezing process in nonacclimated and cold-acclimated rye leaves that differ in their accumulation of AFPs and in median lethal temperature causing 50% death (LT50; Table I). In order to identify the specific effects of AFPs on the freezing process in planta, it was essential to obtain nonacclimated plant materials that were enriched in AFPs but lacked the other modifications that influence the freezing tolerance of the plants during cold acclimation. We were able to produce such plant materials by treating nonacclimated plants with either ethephon or abscisic acid (ABA). It was previously shown that plants treated with ethephon, which increases ethylene levels in the tissues, accumulate the same group of AFPs in the apoplast to the same level as cold-acclimated plants (Yu et al., 2001). Plants treated with ABA also accumulate apoplastic proteins to the same level as cold-acclimated and ethephon-treated plants, but these proteins are pathogenesis-related proteins that lack antifreeze activity (Yu and Griffith, 2001). In our experiments, treating the plants with ethephon or ABA had no effect on the LT50 of the plants (Table I). Thus, ethephon-treated plants were used to show the effects of accumulating AFPs in nonacclimated plants that lack freezing tolerance. ABA-treated plants were used to show the nonspecific effects of accumulating proteins with no antifreeze activity in the apoplast of nonacclimated plants.
Table I.
Freezing characteristics of winter rye leaves from plants grown at 20°C (NA) or 5°C (CA), or grown at 20°C and treated with ABA or ethephon as described in “Materials and Methods”
Freezing and supercooling temperatures were determined by IRVT in the presence and absence, respectively, of ice+ bacteria. LT50 was calculated as the temperature at which the leaf lost 50% of the total leaf conductivity following freezing and thawing. Data are presented as means ± se (number of replicates). Within a row, means followed by different letters are statistically different from each other at P ≤ 0.05. Antifreeze activity was determined by observing the shape of ice crystals grown in solution. NA, Nonacclimated; CA, cold acclimated; FW, fresh weight.
| Characteristic
|
Treatment of Winter Rye Plants
|
|||
|---|---|---|---|---|
| NA | ABA | Ethephon | CA | |
| Apoplastic protein content (mg g−1 FW) | 0.1 ± 0.03 (3) aab | 0.4 ± 0.01 (3) ba | 0.3 ± 0.02 (3) bc | 0.3 ± 0.01 (3) bb |
| Freezing temperature (°C) | −2.4 ± 0.1 (16) a | −2.1 ± 0.1 (17) a | −2.7 ± 0.2 (19) b | −3.3 ± 0.2 (16) b |
| Supercooling temperature (°C) | −13.4 ± 0.3 (3) a | −13.8 ± 1.0 (3) a | −13.6 ± 0.5 (3) a | −12.2 ± 2.8 (3) a |
| LT50 (°C) | −7.0 ± 0.1 (3) a | −7.3 ± 0.1 (3) a | −6.3 ± 0.3 (3) a | −25.3 ± 0.7 (3) b |
| Antifreeze activity | No | No | Yes | Yes |
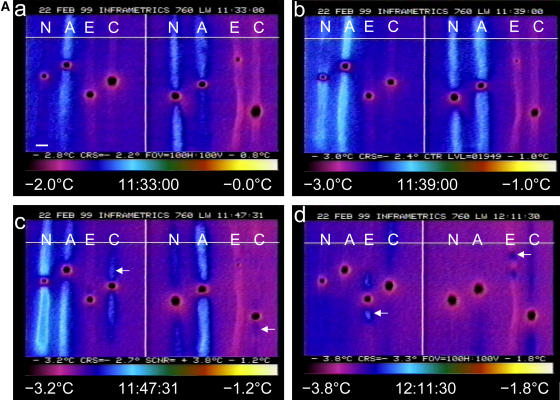
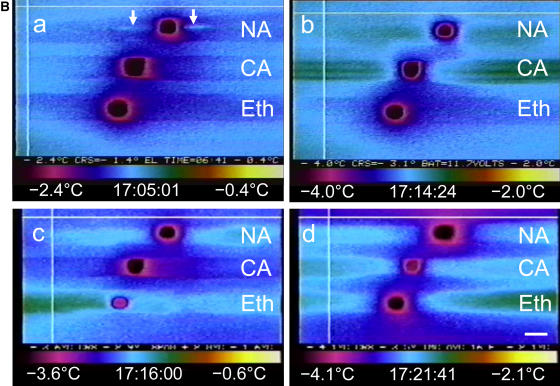
Because freezing is an exothermic process, we were able to observe freezing directly in the leaves of nonacclimated, cold-acclimated, ABA-treated, and ethephon-treated plants by IRVT. With IRVT, a freezing event is observed as a warming of the tissue relative to unfrozen tissue and ambient background temperature. Temperature is calculated from the level of infrared emission detected from an object. The temperature is then assigned a false color using a color pattern selected in the software, so that freezing events are seen as a change in color of the image (Figs. 2 and 3).
Figure 2.
A, Freezing in nonacclimated (N), ABA-treated (A), ethephon-treated (E), and cold-acclimated (C) winter rye leaves observed by IRVT as leaves were cooled 0.05°C min−1 over a period of 38 min 30 s. Each image shows eight winter rye leaves oriented vertically with a droplet of ice+ bacteria placed midleaf. After freezing, the droplet was colder than the ambient temperature due to sublimation of water. The temperature scale and range are shown below each image. a and b, ABA-treated leaves froze first, followed quickly by nonacclimated leaves. c, Initiation of freezing (arrows) in cold-acclimated leaves. d, Initiation of freezing (arrows) in ethephon-treated leaves. Magnification bar in a represents 0.5 cm. B, Freezing in nonacclimated (NA), cold-acclimated (CA), and ethephon-treated (Eth) winter rye leaves. Leaves were cooled at 0.10°C min−1 over a period of 16 min 40 s, and freezing was observed by IRVT. A droplet containing ice+ bacteria was placed midleaf. After freezing, the droplet was colder than the ambient temperature due to sublimation of water. The temperature range and time that the image was taken are shown below each image. a, Arrows indicate two freezing exotherms in nonacclimated leaves that were not observed in either cold-acclimated or ethephon-treated leaves. b, Nonacclimated, cold-acclimated, and ethephon-treated leaves froze within a 1-min interval. c, The exotherm dissipated in the cold-acclimated leaf as the temperature rose by 0.4°C. d, The cold-acclimated leaf refroze upon cooling. Magnification bar in d represents 0.5 cm.
Figure 3.
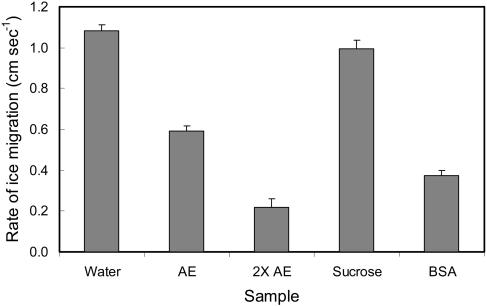
Effect of apoplastic proteins from cold-acclimated winter rye leaves on the rate of migration of ice through solution-saturated filter paper at −2.5°C. The samples were AE (apoplastic extract; 0.2 mg protein mL−1), and 2× AE (apoplastic extract concentrated 2-fold by ultrafiltration). Controls included water; Suc at a concentration of 40 mOs, which was the same osmotic concentration as AE and 2× AE; and BSA at a concentration of 5 mg protein mL−1. Data are presented as mean ice migration rates ± sem; n = 4.
When winter rye leaves were cooled in the absence of an extrinsic ice nucleator, all of the leaves in all four treatments froze below −12°C (Table I). The presence of apoplastic proteins had no effect on the supercooling temperature of the leaves, regardless of whether the proteins exhibited antifreeze activity (Table I). In contrast, when an ice nucleator was present, freezing patterns differed among the four treatments. Leaves were inoculated on their adaxial surfaces with a droplet of water containing ice+ bacteria (Pseudomonas syringae Cit7), cooled to −1°C, and held until the droplets froze. The frozen droplets were at least 0.5°C cooler than unfrozen leaf tissue whose temperature was near or at ambient temperature (Figs. 2 and 3). This was most likely due to the sublimation of ice to the vapor phase. Freezing was usually initiated in the tissues immediately subtending the droplet (Figs. 2 and 3). While this could occur because the tissues were coldest under the droplets, Wisniewski and Fuller (1999) and Wisniewski et al. (2002) have shown that ice must penetrate through the stomates or other openings in the cuticle in order to induce freezing of the leaf tissue. Their studies demonstrated that nucleation can be prevented by coating the leaf with a thin hydrophobic film. As shown in the series of infrared images in Figure 2A, the leaves of nonacclimated and ABA-treated plants that lacked AFPs froze before cold-acclimated and ethephon-treated leaves. The freezing temperature of inoculated leaves from both ethephon-treated and cold-acclimated plants that were enriched in AFPs was 0.3°C to 1.2°C lower than the leaves of nonacclimated and ABA-treated plants (Table I).
When a lens that provided an additional 3× magnification was used in conjunction with the standard IRVT lens, we observed more detail in the freezing pattern. Prior to the major freezing exotherm, nonacclimated leaves exhibited many minor freezing events that originated beneath the frozen droplet of ice+ bacteria and traveled parallel to the veins (Fig. 2B, a). For example, within an 8-cm section of one nonacclimated leaf, 34 independent, minor freezing events were observed as the ambient temperature decreased from −1.2°C to −2.6°C over 22 min. In a second nonacclimated leaf, 32 independent, minor freezing events occurred between −1.4°C and −2.5°C over a period of 9 min. As the leaves were cooled further, these small exotherms were followed by the major exotherm (Fig. 2B, b) that was observed at the lower magnification (Fig. 2A, a–c) and represented freezing of the majority of water in the tissue. No freezing events were observed in cold-acclimated or ethephon-treated leaves in the temperature range of 0°C to −2.6°C (Fig. 2B, a), thus demonstrating that the presence of AFPs inhibited the propagation of ice from the frozen droplet on the leaf surface.
Once they reached their freezing temperature, ice propagated rapidly throughout cold-acclimated and ethephon-treated leaves in a pattern that paralleled the veins (Fig. 2B, b). Ethephon-treated leaves froze in one long exothermic event, like nonacclimated leaves. By contrast, cold-acclimated leaves froze with two distinct exotherms (Fig. 2B, b–d) that have been previously interpreted as the freezing of apoplastic water, followed by the freezing of water drawn from the symplast (Pearce and Fuller, 2001). However, it is important to note that freezing resulted in significant warming of the leaf tissue (Fig. 2B, c), which may have caused ice to melt in the cold-acclimated leaf and then refreeze as the ambient temperature fell again (Fig. 2B, d). An alternative explanation is that the initial freezing event within a leaf may decrease its freezing temperature sufficiently to bring a stop to the freezing process, so that a lower temperature is required for additional freezing to occur. Whether the two exothermic events represent a freeze-thaw-refreeze or a freeze-stop-freeze pattern, the cold-acclimated leaves must be freezing at a temperature that is closer to their equilibrium freezing point (Levitt, 1980) than nonacclimated leaves.
AFPs Decrease the Rate of Ice Migration in Vitro
Although AFPs lower the freezing temperature of cold-acclimated winter rye leaves, it is also important to know whether they can influence the rate at which ice propagates through the tissues. To mimic the migration of ice through the apoplast, we compared the rate of ice migration at −2.5°C along strips of filter paper saturated with (1) apoplastic extracts that contained all the AFPs at a concentration of 0.2 mg protein mL−1 or (2) a Suc solution of equal osmolality (40 mOs). As shown in Figure 3, there were no statistically significant differences in the rate of ice migration along saturated filter paper between distilled water and the 40 mOs Suc solutions. However, the rate of ice migration along the filter paper was significantly inhibited in the presence of apoplastic extracts (P < 0.05). Apoplastic fluids also contain low concentrations of many other components, including sugars (Antikainen and Griffith, 1997; Livingston and Premakumar, 2002). To distinguish between the effects of AFPs and small molecules, we used ultrafiltration to enrich the extracts 2-fold in proteins without affecting the small molecule content and tested the extracts again. The freezing rate decreased 4-fold in strips containing a higher concentration of apoplastic proteins (0.4 mg mL−1) compared with the control solution containing Suc at the same osmolality (Fig. 3). The osmolality of the extracts did not increase after ultrafiltration, thus demonstrating that proteins inhibited the migration of ice rather than small molecules with colligative properties as observed by Gusta et al. (2004). We also compared the nonspecific effects of a solution of 5 mg mL−1 bovine serum albumin (BSA) on the migration of ice. As shown in Figure 3, a 25-fold higher concentration of BSA was required to inhibit ice migration on a scale comparable to the apoplastic proteins from winter rye.
AFPs Inhibit the Recrystallization of Ice
Recrystallization is a spontaneous process in which water molecules migrate from small ice crystals to larger ones to minimize the surface area of ice (Knight et al., 1995). Once an organism has frozen, the population of ice crystals within the tissues can recrystallize into larger masses of ice that may cause physical injury to the cells (Knight and Duman, 1986). Ice recrystallization takes place most quickly just below the melting temperature (Tm) of a solution, which means that injury caused by the recrystallization of ice can occur at temperatures well above an organism's LT50. The technique used to study the rate of recrystallization of ice involves holding a population of ice crystals isothermally below the Tm of the solution and measuring changes in crystal size over time. Because the rate of recrystallization can be affected by solutes other than AFPs, recrystallization experiments are generally conducted at high solute concentrations to minimize nonspecific effects (Knight et al., 1995; Smallwood et al., 1999).
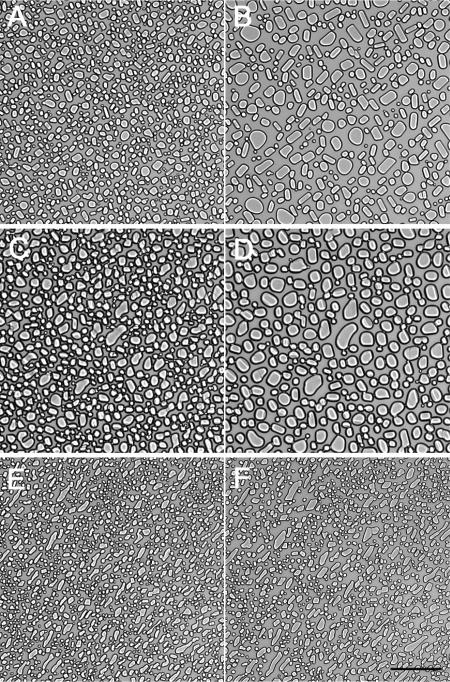
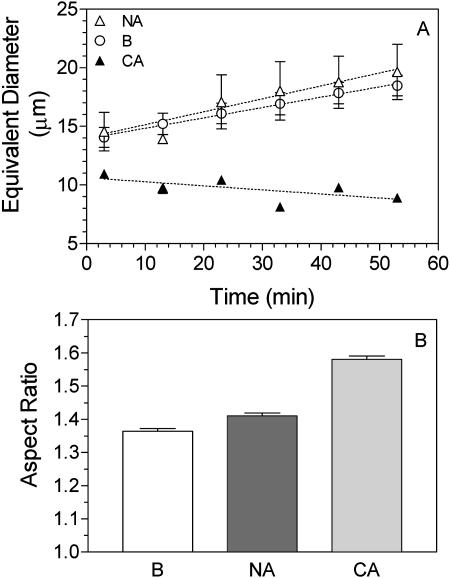
In order to determine the effect of winter rye AFPs on recrystallization, we dialyzed the apoplastic extracts to remove most small solutes, added a high concentration of Suc, froze the solution, and monitored ice crystal number and size for 50 min at −7°C. In the representative experiment shown in Figure 4, the number of ice crystals in the nonacclimated extract decreased from 3,990 to 2,139 and the mean area per crystal increased from 240 to 412 μm2. By contrast, the number of ice crystals in extracts from cold-acclimated plants increased from 6,012 to 6,617 and their mean area decreased from 118 to 89 μm2 per crystal. As shown in Figure 5A, the equivalent diameter for ice crystals grown in the presence of apoplastic proteins from cold-acclimated winter rye was significantly smaller (P < 0.05) at time 0 (y intercept from the linear regression) than the diameter of crystals grown in the presence of apoplastic proteins from nonacclimated rye or in the blank containing only 26% Suc. Over time, the equivalent diameter increased significantly (P < 0.05) for nonacclimated extracts and the blank, but not for solutions of cold-acclimated apoplastic proteins (P > 0.05). The aspect ratio, which is the maximum diameter divided by the minimum diameter, was 1.31 ± 0.02 (mean ± se; n = 12) for the nonacclimated sample and was significantly different (P < 0.05) for the cold-acclimated sample (1.58 ± 0.01; n = 6). The aspect ratio did not change significantly (P > 0.05) over time (Fig. 5B). The higher aspect ratio observed in frozen extracts from cold-acclimated plants demonstrated that the ice crystals were significantly elongated as well as being smaller.
Figure 4.
Recrystallization of ice in the presence of apoplastic proteins from nonacclimated (C and D) and cold-acclimated (E and F) winter rye. Apoplastic extracts dialyzed against 5 mm EDTA were adjusted to a final concentration of 0.14 mg protein mL−1 and Suc was added to a concentration of 26% (w/w). The samples were frozen on a cold stage as follows: cooled at 30°C min−1 to −50°C, warmed at 10°C min−1 to −10°C, held 10 min, warmed at 1°C min−1 to −7°C, cooled at 1°C min−1 to −8°C, warmed to −7°C, and held isothermally. Samples were photographed at 3 min (A, C, and E) and 53 min (B, D, and F). A solution of 26% Suc was used as a control (A and B). Magnification bar = 100 μm.
Figure 5.
Quantification of ice recrystallization in the presence of apoplastic proteins from nonacclimated (NA) and cold-acclimated (CA) winter rye. Ice crystals that formed in nonacclimated and cold-acclimated apoplastic extracts containing (26%, w/w) Suc were measured after holding at −7°C for 3 to 53 min to allow recrystallization. The blank (B) was a solution of 26% Suc. Data were calculated as equivalent diameter and as aspect ratio (maximum diameter/minimum diameter) and are presented as means ± se; n = 12 for the blank; n = 12 for nonacclimated samples; and n = 6 for cold-acclimated samples.
Cryoprotection of LDH Activity
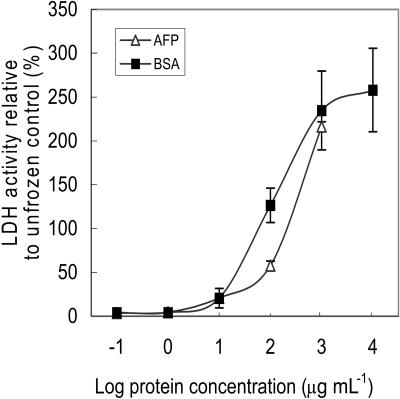
The ability of winter rye apoplastic proteins to preserve LDH activity following freeze-thaw cycles was compared with the level of cryoprotection provided by BSA. BSA is commonly used in cryoprotection studies to quantify nonspecific effects of high protein concentrations in samples that are frozen and thawed (Santarius and Franks, 1998). We chose to use apoplastic extracts in these experiments in order to test all of the AFPs for cryoprotective activity in their native, oligomeric state. In our experiments, LDH lost 85% to 98% of its original activity after eight freeze-thaw cycles in phosphate buffer. When LDH was frozen and thawed in the presence of 1 mg mL−1 apoplastic protein extracted from cold-acclimated winter rye leaves and compared with control LDH samples to which no extra protein was added, the enzyme exhibited a 2.2-fold increase in activity compared with the unfrozen control (Fig. 6) and a 72-fold increase in activity compared with the frozen control. When 1 mg mL−1 BSA was added before freezing, a 2.4-fold increase in LDH activity was obtained following freezing relative to the unfrozen control (Fig. 6) and an 80-fold increase relative to the frozen control. The recovery of greater than 100% of the unfrozen control occurs because the unfrozen LDH sample loses activity over time when stored in dilute solution at 4°C (Santarius and Franks, 1998). Although LDH activity was protected by both rye apoplastic proteins and BSA at concentrations of 1 mg mL−1 or higher (P < 0.05), there was no difference (P > 0.05) between the winter rye apoplastic proteins and BSA in their cryoprotective activities.
Figure 6.
Cryoprotection of LDH by rye apoplastic proteins and BSA. LDH was frozen and thawed eight times in the presence of either BSA or apoplastic proteins isolated from cold-acclimated winter rye leaves. LDH activity was measured spectrophotometrically as the oxidation of NADH and calculated as a percentage of the unfrozen control. The data are presented as means ± se; n = 4.
Cryoprotection of Spinach Thylakoids
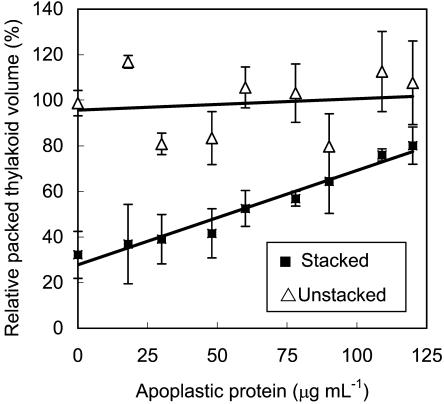
Isolated spinach thylakoids have been used as a model membrane system to determine whether additives can protect membranes against freezing injury (Hincha et al., 1989; Hincha and Schmitt, 1992). When thylakoid membranes are damaged by freezing, the vesicles lose their contents and collapse, which can be measured as a decrease in packed thylakoid volume (Hincha and Schmitt, 1992). In our experiments, NaCl-washed, unstacked spinach thylakoids were resuspended in solutions containing 0 to 120 μg mL−1 apoplastic protein from cold-acclimated winter rye leaves. They were then frozen to −20°C, thawed, and the packed thylakoid volume was measured. As shown in Figure 7, the thylakoid volume after freezing was similar for all treatments. Regression analysis showed that there was no relationship between the concentration of apoplastic protein and the packed volume of the thylakoids (r2 = 0.02).
Figure 7.
Cryoprotection of spinach thylakoids by rye apoplastic proteins. Unstacked spinach thylakoids were frozen and thawed in the presence of apoplastic proteins isolated from cold-acclimated winter rye leaves, then MgCl2 was added to one-half of the samples to stack the thylakoids. Relative packed thylakoid volume was calculated as a percentage of the packed thylakoid volume of the corresponding unfrozen controls × 100. Data are presented as means ± se; n = 3.
In the experiments described by Hincha and Schmitt (1992), MgCl2 was added after freezing and thawing the thylakoids to obtain a correlation between freeze-thaw injury (previously measured by release of plastocyanin from the thylakoids) and thylakoid volume. However, upon adding MgCl2, thylakoids stack together to form grana that pack more compactly than the NaCl-washed, unstacked thylakoids so that packed thylakoid volume is related to the proportion of thylakoids that are stacked rather than directly to the effects of a freeze-thaw cycle. As shown in Figure 7, when MgCl2 was added after the freeze-thaw cycle, the stacking of the thylakoids increased as indicated by the dramatic decrease in packed thylakoid volume in the presence of MgCl2 with no added apoplastic protein (Fig. 7). The packed volume of the thylakoids after adding MgCl2 was positively correlated with the concentration of apoplastic protein added before freezing (r2 = 0.96), thus showing that the apoplastic proteins inhibited thylakoid stacking. Ca2+ can also promote stacking, so there was a low level of stacking during the freeze-thaw cycle in our experiments because the apoplastic protein extract contained 13 mm CaCl2. However, the thylakoids remained responsive to MgCl2. Because the apoplastic proteins had no effect on thylakoid volume after a freeze-thaw cycle and because the proteins inhibited the ability of frozen-thawed thylakoids to stack upon adding MgCl2, we concluded that the winter rye proteins were not cryoprotective.
DISCUSSION
Apoplastic Proteins from Winter Rye Modify Freezing In Planta
AFPs inhibit the growth of ice by adsorbing onto its surface and lowering the freezing temperature by the Kelvin effect (Raymond and DeVries, 1977; Knight et al., 1991). The ice-binding domain has been characterized as a hydrophobic area on the surface of the protein that is complementary to the corrugated ice surface with hydrophilic groups spaced at regular intervals to stabilize the protein-ice interaction by a combination of van der Waals interactions and hydrogen bonds (Jia and Davies, 2002). As a result, adsorption of an AFP onto ice is driven by the increase in entropy that occurs as hydration water is pushed away from the ice surface by the approaching AFP. According to this model, an AFP must be able to rotate freely to make the match with the ice crystal lattice. If AFPs are tightly bound to the cell wall, they would be unable to reorient their ice-binding domains to match the ice surface. The presence of antifreeze activity in guttate obtained from cold-acclimated winter rye leaves (Fig. 1) shows that at least some of the AFPs are mobile in the apoplast and are available to bind to ice. Our results agree with those of Pihakaski-Maunsbach et al. (2003), who reported that winter rye suspension cells secrete AFPs into the culture medium, which also demonstrates that a population of AFPs is not bound to cell walls.
By comparing the freezing process in winter rye leaves that had accumulated apoplastic proteins with and without antifreeze activity, we were able to separate the effect of AFPs from the effects of other changes that occur during cold acclimation because the nonacclimated, ethephon-treated and ABA-treated leaves all had the same LT50 (Table I). Leaves that accumulated AFPs in response to cold or ethephon treatment froze at a lower temperature (Table I; Fig. 2). Because ice was present, these results show that AFPs are able to completely stall the growth of ice by as much as 1.2°C below the freezing temperature of the tissues. In an experiment conducted in vitro using saturated filter paper strips at −2.5°C, the AFPs also slowed the migration of ice to 0.6 cm s−1 (Fig. 3), which was slower than the rate of ice migration observed in intact barley leaves (2.36 cm s−1; Pearce and Fuller, 2001). The rate of ice migration is undoubtedly affected by both the concentration and specific activity of AFPs present in the apoplast, but it has proven difficult to measure these factors in situ. Nonetheless, it would be interesting to determine whether AFPs with different specific activities have a dramatic influence on winter survival.
The two-stage freezing process that has been observed by IRVT in cold-acclimated barley and canola leaves (Pearce and Fuller, 2001; Gusta et al., 2004) was also seen in cold-acclimated leaves in our experiments, but not in leaves treated with ethephon (Fig. 3). Therefore, this part of the freezing process is independent of AFPs. Each stage could represent freezing of different fractions of water in the leaf tissues, i.e. apoplastic water followed by water drawn from the cells (Pearce and Fuller, 2001). However, cold acclimation is the only one of the four treatments that results in greater sugar accumulation, a decrease in the osmotic potential, and lower relative water content (Huner et al., 1981; Gusta et al., 2004), which lowers the water potential and equilibrium freezing point of the cells. Therefore, an alternative explanation may be that only cold-acclimated winter rye leaves undergo equilibrium freezing (Fig. 3).
The range of freezing point depression (1.2°C) brought about by winter rye AFPs in planta is greater than the thermal hysteresis measured in vitro of 0.3°C for apoplastic extracts (Griffith et al., 1992) and 0.01°C for guttation fluid (Fig. 3), but none of these measurements can account for the level of freezing tolerance achieved by winter rye (LT50 is usually below −20°C). Therefore, the function of AFPs in winter rye is not to prevent freezing; rather, AFPs may reduce freezing injury at temperatures above LT50 by slowing the progression of ice through the tissues.
AFPs may have additional roles in modifying the freezing process that cannot be observed by IRVT. For example, after ice has formed, it can slowly recrystallize into larger crystals. Larger ice masses may be more damaging to tissues (Sakai and Larcher, 1987; Pearce, 2001). In fact, Knight and Duman (1986) consider inhibition of ice recrystallization to be essential to survival in overwintering organisms. Doucet et al. (2000) reported that total soluble extracts from all Antarctic plants and 25% of overwintering plants in the United Kingdom exhibit the ability to inhibit recrystallization. Many of the inhibitors detected by Doucet et al. (2000) were resistant to heat and protease treatment and may not have been AFPs. In fact, recrystallization can also be inhibited by molecules that either form networks to sterically block the movement of water to the surface of ice or else bind water and slow its diffusion to other crystals (Regand and Goff, 2002, 2003). In our experiments, the apoplastic proteins from cold-acclimated winter rye plants not only prevented the growth of ice crystals, but also modified their shape, thus showing that the inhibition of ice recrystallization was mediated by AFPs that adsorbed onto the ice surface.
Winter Rye Antifreeze Proteins Are Not Cryoprotective
Some solutes and proteins are more effective than others in stabilizing proteins and membranes subjected to freeze-thaw cycles. In solution, the most effective cryoprotectants are excluded from contact with the surface of the proteins so that they remain preferentially hydrated and their enzymatic activities are preserved (Carpenter and Crowe, 1988; Timasheff and Arakawa, 1988). Many overwintering plants accumulate proteins that are cryoprotective. For example, cold-induced dehydrins, such as COR85 purified from cold-acclimated spinach leaves, WCS120 isolated from cold-acclimated winter wheat leaves, and PCA60 isolated from the bark of cold-acclimated peach trees all provide cryoprotection of LDH activity at protein concentrations 10 to 100 times lower than BSA (Kazuoka and Oeda, 1994; Houde et al., 1995; Wisniewski et al., 1999). However, BSA and winter rye apoplastic proteins both produced similar levels of cryoprotection (Fig. 6); thus, the protective effects of the winter rye proteins appear to be nonspecific.
Hincha et al. (1997) reported that a tobacco (Nicotiana tabacum) β-1,3-glucanase is cryoprotective because its concentration was positively correlated with increased packed volume of stacked spinach thylakoids after a freeze-thaw cycle. Two of the winter rye AFPs are also β-1,3-glucanases (Hon et al., 1995), and we observed that the winter rye apoplastic proteins were effective in increasing packed thylakoid volume at a protein concentration 10-fold less than the tobacco β-1,3-glucanase (Fig. 7; Hincha et al., 1997). These results could be interpreted as showing that cold-induced winter rye β-1,3-glucanases are more cryoprotective than the tobacco β-1,3-glucanase. However, we have shown that the experiment actually measured a reduction in the ability of thylakoids to stack rather than a direct effect of freezing and thawing upon the integrity of the thylakoid membranes. Because the apoplastic proteins from cold-acclimated rye leaves inhibit thylakoid stacking after freezing and thawing, we conclude that the proteins are not cryoprotective of cellular membranes. Our results confirm those of Pihakaski-Maunsbach et al. (2003), who reported that apoplastic proteins isolated from cold-acclimated winter rye leaves have no effect on the survival of winter rye protoplasts exposed to a freeze-thaw cycle, and Hincha et al. (1993), who reported that AFPs and antifreeze glycoproteins isolated from fish are cryotoxic to spinach thylakoid membranes.
CONCLUSION
Our results demonstrate that AFPs from winter rye do not act as cryoprotectants; rather, they interact directly with ice and modify the freezing process in planta. In cold-acclimated leaves, AFPs inhibit the propagation of ice from the surface of the leaf so that no freezing occurs until the tissue reaches its equilibrium freezing point, and then ice propagates through the apoplast. After the leaves have frozen, AFPs inhibit the recrystallization of intercellular ice.
It is important to recognize that winter cereals and other overwintering plants are often killed in the field at temperatures above their LT50. Because AFPs modify the freezing process, they act to reduce freezing injury in cold-acclimated winter rye leaves at temperatures well above their LT50. AFPs may be particularly protective by slowing ice propagation in situations where fluctuating temperatures cause repeated freezing and thawing of tissues and also where an insulating snow cover may maintain ambient temperatures near the melting point of the intercellular ice and promote its recrystallization. Although ice recrystallizes more slowly at colder subzero temperatures, inhibition of ice recystallization may also be important in plants that are frozen for prolonged periods.
MATERIALS AND METHODS
Plant Material and Hormonal Treatments
Seeds of winter rye (Secale cereale L. cv Musketeer) were surface sterilized with 6% (v/v) sodium hypochlorite with 0.01% (v/v) Tween 20 for 15 min, rinsed well with distilled water, then grown in potting soil (ProMix BX; Premier Horticulture, Riviere de Loup, Canada) at 20°C with a 16-h daylength and a photosynthetic photon flux density of 250 μmol photons m−2 s−1 for 4 weeks to produce nonacclimated plants. To produce cold-acclimated plants comparable in physiological age to the nonacclimated plants, some of the plants grown in nonacclimating conditions for 1 week were transferred to low temperature (5°C day and 2°C night) with a short daylength (8 h) for an additional 7 to 9 weeks (Krol et al., 1984; Griffith and McIntyre, 1993). Hormonal treatments were carried out as described previously (Yu and Griffith, 2001; Yu et al., 2001). Briefly, 3-week-old nonacclimated plants were sprayed daily to runoff at the beginning of the dark period with an aqueous solution of either 100 μm ABA or 10 μm ethephon (Sigma Chemical Company, St. Louis) in 0.005% (v/v) Tween 20 for 5 to 7 d (Yu and Griffith, 2001; Yu et al., 2001), and plants were harvested within 24 h of the last application.
Guttation
To promote guttation, pots of plants were placed in a tray filled with water to a depth of 4 cm, which was, in turn, placed in a large, clear plastic bag to maintain high humidity, and all plants were held overnight at 20°C. In the morning, the bags were slit and fluid was collected from the leaf tips. Guttate was assayed for antifreeze activity immediately and then stored at −20°C. When 200 μL of guttate were collected from both nonacclimated and cold-acclimated plants, the fluid was lyophilized, solubilized in 10 μL of distilled water, centrifuged at 10,000g for 5 min, and the supernatant was assayed again for antifreeze activity.
Apoplastic Protein Extraction
Proteins were extracted from the apoplast of cold-acclimated winter rye leaves by vacuum infiltrating leaves with 20 mm ascorbate and 20 mm CaCl2 (pH 3), followed by centrifugation to recover the infiltrate, as described by Hon et al. (1994). These extracts were used directly in thylakoid experiments. For LDH experiments, the extracts were concentrated by ammonium sulfate (75% of saturation) precipitation at 4°C overnight. After centrifugation, the precipitate was redissolved in a minimal volume of 10 mm K2HPO4, pH 7.5, and dialyzed against 10 mm K2HPO4 at 4°C overnight. Protein concentrations were measured by the Bradford (1976) method, as modified by Bio-Rad Laboratories (Mississauga, Canada), using BSA as the standard protein. Although the data are not shown, the presence of AFPs in the apoplastic extracts was confirmed by SDS-PAGE and assays of antifreeze activity as described by Hon et al. (1995). Antifreeze activity was determined by observing the morphology of ice crystals grown in solution using a nanoliter osmometer (Clifton Technical Physics, Hartford, NY) with the freezing stage mounted on an Olympus phase contrast light microscope (model BH-2; Carsen Medical and Scientific, Markham, Canada). Thermal hysteresis was measured as the difference in the Tm and the freezing temperature for an ice crystal in an apoplastic extract. Values of thermal hysteresis in extracts from nonacclimated and cold-acclimated plants were compared using a t test (Sokal and Rohlf, 1995).
Freezing of LDH
Cryoprotection of LDH (EC 1.1.1.27; rabbit muscle LDH-5 [M5] isozyme type V-S; Sigma; 0.134 mg protein mL−1) was assayed as previously described by Wisniewski et al. (1999). LDH, BSA (20 mg protein L−1), and the apoplastic extracts from cold-acclimated winter rye leaves were dialyzed against 1 L of 10 mm K2HPO4, pH 7.5, at 4°C overnight. Serial dilutions of BSA and apoplastic protein were added to 2 μL of LDH to achieve a final enzyme concentration of 2.7 μg protein mL−1 in 100 μL. Controls included samples lacking LDH (100 μL of 10 mm K2HPO4 or diluted BSA or apoplastic extract) and samples containing 2 μL of LDH that was stored at 4°C and 98 μL 10 mm K2HPO4. In each of the three independent freezing experiments, LDH samples were frozen in polypropylene microfuge tubes in liquid nitrogen for 30 s, and then thawed rapidly at 20°C, for a total of seven freeze-thaw cycles. For the eighth cycle, the samples were placed at −20°C for 3 h before thawing at 20°C. After the last thaw, 10 μL of the LDH-buffer-protein mixture was added to a reaction mixture containing 80 mm Tris-HCl, pH 7.5, 100 mm KCl, and 0.3 mm NADH. The reaction was initiated by adding pyruvate to a final concentration of 2 mm and final assay volume of 200 μL. Enzyme activity was quantified as the decrease in A340 as NADH was oxidized to NAD− by using a SpectraMax 190 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). Every sample was assayed twice and the average Vmax was calculated by SoftMax Pro software (Molecular Devices). The specific activity of LDH was corrected using the appropriate controls, then divided by the unfrozen control and multiplied by 100 to obtain the percent of cryoprotection. The experiment was conducted four times and the data were analyzed using a factorial ANOVA, with day of experiment, type of protein (BSA or AFP), and protein concentration (five levels) as fixed effects, followed by comparisons of means using t tests (Sokal and Rohlf, 1995).
Freezing of Spinach Thylakoids
Thylakoids were isolated from fresh spinach (Spinacea oleracea) leaves using the method of Hincha and Schmitt (1992). Spinach leaves from the market were washed, homogenized for 10 s in 100 mL of grinding buffer (160 mm NaCl, 240 mm Suc, 1 mm MnCl2, 1 mm MgCl2, 2 mm EDTA, 1 mm KH2PO4, 5 mm Tris, and 1.25 mm ascorbic acid, pH 7.8), filtered through Miracloth (Calbiochem, La Jolla, CA), and centrifuged at 2,000g for 5 min. The pellet was washed three times in 5 mm NaCl and resuspended to 0.5 mg chlorophyll mL−1 in 2.5 mm NaCl and 5 mm Suc (resuspension buffer). Chlorophyll was assayed in 90% acetone according to Arnon (1949).
All samples contained 400 μL of resuspended thylakoids plus 264 μL of a combination of 20 mm ascorbate and 20 mm CaCl2, pH 3, and apoplastic extract from cold-acclimated winter rye leaves at a final protein concentration of 0 to 120 μg apoplastic protein mL−1. The samples were frozen at −20°C for 3 h and thawed in a water bath at 20°C (Hincha and Schmitt, 1992). Aliquots (75 μL) of the frozen-thawed thylakoids were either used directly to fill capillary tubes (unstacked) or mixed with an equal volume of 10 mm MgCl2 and then placed in capillary tubes (stacked). The capillary tubes were centrifuged at 12,000g for 15 min, and the packed thylakoid volume was measured as height in millimeters and expressed as the percentage of the height of the total solution. The percent of packed thylakoid height of each sample was then divided by the corresponding stacked or unstacked controls and multiplied by 100 to obtain the relative packed thylakoid volume in percent (Hincha and Schmitt, 1992; Sieg et al., 1996). Thylakoids resuspended in only 2.5 mm NaCl and 5 mm Suc were used as controls: The unfrozen sample was the control for 100% packed thylakoid height and the sample frozen to −20°C represented 0% packed thylakoid height. The controls represented 100% and 0% cryoprotection, respectively, after freezing. However, they represented 0% and 100% stacking, respectively, after adding MgCl2. Data were collected in three independent experiments, and the relationship between apoplastic protein concentration and relative packed thylakoid volume was examined by linear regression (Sokal and Rohlf, 1995).
Freezing Tolerance
Freezing tolerance was measured by ion leakage of leaves subjected to a freeze-thaw cycle (Dexter et al., 1932). Fully expanded winter rye leaves were cut into 2-cm segments, rinsed, rolled in damp cheesecloth, placed in test tubes in a polyethylene glycol bath held at −1°C, nucleated with ice, and held at −1°C for 12 h, then cooled at a rate of 2°C h−1. Sample temperatures were monitored using copper-constantan thermocouples (0.25-mm diameter) and a microcomputer thermometer (model HH-72T; Omega Engineering, Stamford, CT). Samples withdrawn at 2°C intervals were thawed at 5°C, placed in 6 mL of double-distilled water, shaken at 5°C overnight, and the conductivity at 20°C was measured using a YSI model 31 conductivity bridge (Yellow Springs Instruments, Yellow Springs, OH) before and after boiling. The experiment was repeated three times, and the data were analyzed to obtain LT50 values as described by Griffith and McIntyre (1993).
IRVT
Freezing of winter rye leaves was observed by IRVT as described previously (Wisniewski et al., 1997). For these experiments, mature leaf blades were cut at the junction with the leaf sheath from nonacclimated, ethephon-treated, ABA-treated, and cold-acclimated plants. The cut ends were sealed with petroleum jelly, and the leaves were taped with their adaxial surface facing upward onto a horizontal cardboard surface. One 10-μL droplet of ice+ bacteria (Pseudomonas syringae Cit7) was placed midleaf and the leaves were placed in a dark, Tenney T20S (Lunaire, Williamsport, PA), programmable, environmental chamber at a set temperature of 0°C, cooled to −1.5°C, and held until all droplets had frozen. Then, the temperature was lowered by 0.05°C to 0.10°C min−1 and freezing of winter rye leaves was observed in real time using an imaging radiometer (model 760; Inframetrics, North Billerica, MA) with an HgCdTe long-wave (8–12 μm) detector as described in detail by Wisniewski et al. (1997). The false color images were chosen to reflect a 2°C temperature interval and were recorded on videotape using frame averaging (16 frames s−1) to smooth the image. The midpoint of the temperature scale was lowered as the temperature decreased to capture exothermic freezing events. The experiment was repeated three times. Freezing processes were quantified by the number of exothermic freezing events observed and the temperatures at which they were initiated. To determine supercooling temperatures, the experiment was repeated without application of ice+ bacteria and the leaves were cooled at 0.15°C min−1.
Rate of Ice Migration
The effect of AFPs on the rate of ice migration was measured according to the technique of Gusta et al. (2004). Apoplastic extracts from 7-week-old cold-acclimated winter rye plants were adjusted to a protein concentration of 0.2 mg mL−1. Aliquots of these extracts were concentrated 2-fold by ultrafiltration using Microcon YM-3 spin filters with a 3-kD molecular mass cutoff (Millipore, Bedford, MA) at 3,200g. The osmolality of the apoplastic extracts before and after concentration by ultrafiltration was determined to be 40 mOs using a nanoliter osmometer (Clifton Technical Physics). Control samples were prepared with Suc to obtain an equivalent osmolality (Hall and Sherrill, 1933) and checked using the nanoliter osmometer. In addition, a solution of 5 mg BSA mL−1 was prepared as a control for the nonspecific effects of protein on ice migration. Each sample was absorbed onto an 8 × 0.5-cm strip of Whatman number 2 filter paper, and one end of this strip was placed perpendicular to a second fuse strip that was wetted with double-distilled water in a shallow plastic container. A 10-μL droplet of water containing ice+ bacteria was placed on the end of the fuse strip to initiate freezing. The strips were covered with a sheet of plastic film, cooled to −2.5°C, and monitored by IRVT. The length of time required to freeze the entire strip was determined for four strips per sample.
Recrystallization of Ice
Apoplastic extracts (5 mL) from nonacclimated and cold-acclimated winter rye were dialyzed against two 500-mL changes of 5 mm EDTA, pH 5.6, using dialysis tubing with a 12- to 14-kD molecular mass cutoff (Spectrum Laboratories, Rancho Dominguez, CA), concentrated by centrifugal ultrafiltration (Microcon YM-10; Millipore), and adjusted to a final protein concentration of 0.14 mg mL−1. After adding 26% (w/w) Suc to each extract, they were frozen on a cold stage (Linkham Instruments, Surrey, UK) of a light microscope (Olympus BH, Tokyo) as follows: cool at 30°C min−1 to −50°C, warm at 10°C min−1 to −10°C, hold 10 min, warm at 1°C min−1 to −7°C, cool at 1°C min−1 to −8°C, warm at 1°C min−1 to −7°C, and hold for 60 min. A blank solution used as the control contained the same amount of water as the nonacclimated and cold-acclimated samples plus 26% (w/w) Suc. Images were processed by the image-processing tool kit (version 3.0; ISBN 1–928808–00–X) created by Chris Russ and John Russ that was implemented within Adobe Photoshop 7.0, and the data were analyzed as described by Russ (2002). Statistical analyses were carried out by linear regression and one-way ANOVA in Prism 3.03 (Graphpad, San Diego).
Acknowledgments
We thank Lisa Kraemer and Dr. Patricia M. Schulte for their assistance with LDH assays, Nadine Saikaly and Nicholas Stavrinou for collecting guttation fluid, Anne Tutte, Shauna A. McCabe, and Halina Chik for preparing samples for measuring ice recrystallization and migration, Alejandra Regand for assistance in recrystallization assays, and Dr. G. Carr for help with statistical analyses. Musketeer winter rye seeds were a gift from Dr. G. McLeod, Agriculture Canada, Swift Current, Saskatchewan, Canada.
This work was supported by a research grant from the Natural Science and Engineering Research Council of Canada to M.G.
In memory of Prof. Marilyn Griffith, her endless energy, her openness, and her dedication to the pursuit of scientific excellence.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.058628.
References
- Antikainen M, Griffith M (1997) Antifreeze accumulation in freezing-tolerant cereals. Physiol Plant 99: 423–432 [Google Scholar]
- Antikainen M, Griffith M, Zhang J, Hon WC, Yang DSC, Pihakaski-Maunsbach K (1996) Immunolocalization of antifreeze proteins in winter rye leaves, crown and roots by tissue printing. Plant Physiol 110: 845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DJ (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brush RA, Griffith M, Mlynarz A (1994) Characterization and quantification of intrinsic ice nucleators in winter rye (Secale cereale) leaves. Plant Physiol 104: 725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JF, Crowe JH (1988) The mechanism of cryoprotection of proteins by solutes. Cryobiology 25: 244–255 [DOI] [PubMed] [Google Scholar]
- Chun JU, Griffith M (1998) Variation of antifreeze proteins during cold acclimation among winter cereals and their relationship with freezing resistance. Korean J Crop Sci 43: 172–178 [Google Scholar]
- DeVries AL (1986) Antifreeze glycoproteins and peptides: interactions with ice and water. Methods Enzymol 127: 293–303 [DOI] [PubMed] [Google Scholar]
- Dexter ST, Tottingham WE, Graber LF (1932) Investigations of the hardiness of plants by measurement of electrical conductivity. Plant Physiol 7: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet CJ, Byass L, Elias L, Worrall D, Smallwood M, Bowles DJ (2000) Distribution and characterization of recrystallization inhibitor activity in plant and lichen species from the UK and maritime Antarctic. Cryobiology 40: 218–227 [DOI] [PubMed] [Google Scholar]
- Fletcher GL, Goddard SV, Wu Y (1999) Antifreeze proteins and their genes: from basic research to business opportunity. Chemtech 30: 17–28 [Google Scholar]
- Griffith M, Ala P, Yang DSC, Hon WC, Moffat B (1992) Antifreeze protein produced endogenously in winter rye leaves. Plant Physiol 100: 593–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M, McIntyre HCH (1993) The interrelationship of growth and frost tolerance in winter rye. Physiol Plant 87: 335–344 [Google Scholar]
- Griffith M, Yaish MWF (2004) Antifreeze proteins in overwintering plants: a tale of two activities. Trends Plant Sci 9: 399–405 [DOI] [PubMed] [Google Scholar]
- Gusta LV, Wisniewski M, Nesbitt NT, Gusta ML (2004) The effect of water, sugars, and proteins on the pattern of ice nucleation and propagation in acclimated and nonacclimated canola leaves. Plant Physiol 135: 1642–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RE, Sherrill MS (1933) International Critical Tables of Numerical Data, Physics, Chemistry and Technology, Vol 2. National Research Center (USA), New York, pp 254–255
- Hincha DK, DeVries AL, Schmitt JM (1993) Cryotoxicity of antifreeze proteins and glycoproteins to spinach thylakoid membranes—comparison with cryotoxic sugar acids. Biochim Biophys Acta 1146: 258–264 [DOI] [PubMed] [Google Scholar]
- Hincha DK, Heber U, Schmitt JM (1989) Freezing ruptures thylakoid membranes in leaves, and rupture can be prevented in vitro by cryoprotective proteins. Plant Physiol Biochem 27: 795–801 [Google Scholar]
- Hincha DK, Meins F Jr, Schmitt JM (1997) β-1,3-Glucanase is cryoprotective in vitro and is accumulated in leaves during cold acclimation. Plant Physiol 114: 1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hincha DK, Schmitt JM (1992) Cryoprotective leaf proteins: assay methods and heat stability. J Plant Physiol 140: 236–240 [Google Scholar]
- Hon W-C, Griffith M, Chong P, Yang DSC (1994) Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves. Plant Physiol 104: 971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon W-C, Griffith M, Mlynarz A, Kwok YC, Yang DSC (1995) Antifreeze proteins in winter rye are similar to pathogenesis-related proteins. Plant Physiol 109: 879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Daniel C, Lachapelle M, Allard F, Laliberte JF, Sarhan F (1995) Immunolocalization of freezing tolerance-associated proteins in the cytoplasm and nucleoplasm of wheat crown tissues. Plant J 8: 583–593 [DOI] [PubMed] [Google Scholar]
- Huner NPA, Palta JP, Li PH, Carter JV (1981) Anatomical changes of Puma rye in response to growth at cold hardening temperatures. Bot Gaz 142: 55–62 [Google Scholar]
- Jia Z, Davies PL (2002) Antifreeze proteins: an unusual receptor-ligand interaction. Trends Biochem Sci 27: 101–106 [DOI] [PubMed] [Google Scholar]
- Kazuoka T, Oeda K (1994) Purification and characterization of COR95-oligomeric complex from cold-acclimated spinach. Plant Cell Physiol 35: 601–611 [Google Scholar]
- Knight CA, Cheng CC, DeVries AL (1991) Adsorption of α-helical antifreeze polypeptides on specific ice crystal surface planes. Biophys J 59: 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CA, Duman JG (1986) Inhibition of recrystallization of ice by insect thermal hysteresis proteins: a possible cryoprotective role. Cryobiology 23: 256–262 [Google Scholar]
- Knight CA, Wen DY, Laursen RA (1995) Nonequilibrium antifreeze peptides and the recrystallization of ice. Cryobiology 32: 23–34 [DOI] [PubMed] [Google Scholar]
- Krol M, Griffith M, Huner NPA (1984) An appropriate physiological control for low temperature studies: growth kinetics of winter rye. Can J Bot 62: 1062–1068 [Google Scholar]
- Levitt J (1980) Responses of Plants to Environmental Stresses: Chilling, Freezing and High Temperature Stresses, Ed 2, Vol 1. Academic Press, New York
- Livingston DP, Premakumar R (2002) Apoplastic carbohydrates do not account for differences in freezing tolerance of two winter-oat cultivars that have been second phase cold-hardened. Cereal Res Commun 30: 375–381 [Google Scholar]
- Marentes E, Griffith M, Mlynarz A, Brush RE (1993) Proteins accumulate in the apoplast of winter rye leaves during cold acclimation. Physiol Plant 87: 499–507 [Google Scholar]
- Pearce RS (1988) Extracellular ice and cell shape in frost-stressed cereal leaves: a low-temperature scanning-electron-microscopy study. Planta 175: 313–324 [DOI] [PubMed] [Google Scholar]
- Pearce RS (2001) Plant freezing and damage. Ann Bot (Lond) 87: 417–424 [Google Scholar]
- Pearce RS, Fuller MP (2001) Freezing of barley studied by infrared video thermography. Plant Physiol 125: 227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihakaski-Maunsbach K, Griffith M, Antikainen M, Maunsbach AB (1996) Immunogold localization of glucanase-like antifreeze proteins in cold-acclimated winter rye. Protoplasma 191: 115–125 [Google Scholar]
- Pihakaski-Maunsbach K, Tamminen I, Pietiäinen M, Griffith M (2003) Antifreeze proteins are secreted by winter rye cells in suspension culture. Physiol Plant 118: 390–398 [Google Scholar]
- Raymond JA, DeVries AL (1977) Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci USA 74: 2589–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regand A, Goff HD (2002) Effect of biopolymers on structure and ice recrystallization in dynamically frozen ice cream model systems. J Dairy Sci 85: 2722–2732 [DOI] [PubMed] [Google Scholar]
- Regand A, Goff HD (2003) Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocoll 17: 95–102 [Google Scholar]
- Russ JC (2002) The Image Processing Handbook, Ed 4. CRC Press, Boca Raton, FL, pp 512–514
- Sakai A, Larcher W (1987) Frost Survival of Plants. Springer-Verlag, New York, pp 31–33
- Santarius KA, Franks F (1998) Cryopreservation of lactate dehydrogenase: interactions among various cryoprotectants. Cryo Letters 19: 37–48 [Google Scholar]
- Sieg F, Schröder W, Schmitt JM, Hincha DK (1996) Purification and characterization of a cryoprotective protein (cryoprotectin) from leaves of cold-acclimated cabbage. Plant Physiol 111: 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M, Worrall D, Byass L, Elias L, Ashford D, Doucet CJ, Holt C, Telford J, Lillford P, Bowles DJ (1999) Isolation and characterization of a novel antifreeze protein from carrot (Daucus carota). Biochem J 340: 385–391 [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ (1995) Biometry. The Principles and Practice of Statistics in Biological Research, Ed 3. W.H. Freeman, San Francisco
- Timasheff SN, Arakawa T (1988) Mechanism of protein precipitation and stabilization by co-solvents. J Cryst Growth 90: 39–46 [Google Scholar]
- Wisniewski M, Fuller M (1999) Ice nucleation and deep supercooling in plants: new insights using infrared thermography. In R Margesin, F Schinner, eds, Cold-Adapted Organisms: Ecology, Physiology, Enzymology and Molecular Biology. Springer-Verlag, Berlin, pp 105–118
- Wisniewski M, Fuller M, Glenn DM, Gusta L, Duman J, Griffith M (2002) Extrinsic ice nucleation in plants: What are the factors involved and can they be manipulated? In PH Li, ET Palva, eds, Plant Cold Hardiness: Gene Regulation and Genetic Engineering. Kluwer Academic/Plenum Publishers, New York
- Wisniewski M, Lindow SE, Ashworth EN (1997) Observations of ice nucleation and propagation in plants using infrared video thermography. Plant Physiol 113: 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski M, Webb R, Balsamo R, Close TJ, Yu XM, Griffith M (1999) Purification, immunolocalization, cryoprotective and antifreeze activity of PCA60: a dehydrin from peach (Prunus persica). Physiol Plant 105: 600–608 [Google Scholar]
- Yu XM, Griffith M (2001) Winter rye antifreeze activity increases in response to cold and drought, but not abscisic acid. Physiol Plant 112: 78–86 [DOI] [PubMed] [Google Scholar]
- Yu XM, Griffith M, Wiseman SB (2001) Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol 126: 1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]