Abstract
Kaposi’s sarcoma (KS) spindle cell growth and spread have been reported to be modulated by various cytokines as well as the human immunodeficiency virus (HIV) gene product Tat. Recently, HIV-1 Tat has been shown to act like a cytokine and bind to the Flk-1/KDR receptor for the vascular endothelial growth factor A (VEGF-A), which is expressed by KS cells. We have characterized signal transduction pathways stimulated by HIV-1 Tat upon its binding to surface receptors on KS cells. We observed that stimulation in KS 38 spindle cells resulted in tyrosine phosphorylation and activation of the Flk-1/KDR receptor. We also report that HIV-1 Tat treatment enhanced the phosphorylation and association of proteins found in focal adhesions, such as the related adhesion focal tyrosine kinase RAFTK, paxillin, and p130cas. Further characterization revealed the activation of mitogen-activated protein kinase, c-Jun amino-terminal kinase (JNK), and Src kinase. HIV-1 Tat contains a basic domain which can interact with growth factor tyrosine kinase receptors and a classical RGD sequence which may bind to and activate the surface integrin receptors for fibronectin and vitronectin. We observed that stimulation of KS cells with basic as well as RGD sequence-containing Tat peptides resulted in enhanced phosphorylation of RAFTK and activation of MAP kinase. These studies reveal that Tat stimulation activates a number of signal transduction pathways that are associated with cell growth and migration.
Kaposi’s sarcoma (KS) is the major neoplastic manifestation of AIDS (30, 37, 42, 55). Its pathogenesis has been the focus of considerable study. Several different cytokines including vascular endothelial growth factor A (VEGF-A), platelet-derived growth factor, interleukin-6, the KS virus-encoded homolog of the chemokine MIP1, and tumor necrosis factor alpha have been reported to modulate KS cell growth (17, 20, 24, 25, 38, 43, 47, 52, 56, 62). These cytokines have been postulated to be released in a paracrine fashion (by neighboring leukocytes and endothelial cells) or in an autocrine-paracrine fashion (by spindle cells themselves). Recent results suggest that the Tat protein of human immunodeficiency virus type 1 (HIV), known to be a transcriptional regulator of virus, potentiates KS cell growth in vitro and in in vivo animal models (2, 7, 11, 18). HIV Tat has been reported to act synergistically with inflammatory cytokines and basic fibroblast growth factor in promoting KS proliferation and migration (19).
HIV Tat is a protein of 86 to 104 amino acids encoded by two exons and is essential for viral replication. Tat can be divided into five distinct domains termed N terminal, cysteine rich, core, basic, and C terminal. The C-terminal domain contains an Arg-Gly-Asp (RGD) sequence, which represents the major cell attachment moiety recognized by integrin receptors. This Tat domain can bind with high affinity to the integrins α5β1, and α5β3, receptors for fibronectin and vitronectin, respectively (8, 60). The basic sequence of Tat (amino acids 42 to 64) is similar to the basic sequence of several growth factors (fibroblast growth factor, VEGF-A, hepatocyte growth factor, and heparin-binding epidermal growth factor) (12, 46, 57). The basic sequence of Tat has also been shown to bind to a novel integrin, αvβ5 (60). Because Tat can be released by infected cells during HIV infection and can act extracellularly in the microenvironment, it may function in a paracrine fashion as a protocytokine in KS pathogenesis (14, 21). Recently, another link between Tat and a cytokine-based model of KS pathogenesis was made by demonstrating that HIV Tat specifically binds with high affinity to the mitogenic Flk-1 (but not the Flt-1) receptor, also known as VEGFR-2, for VEGF-A (3). Moreover, VEGF-A was recently shown to act as a potent growth stimulator in KS cells (38).
Despite the increasingly prominent role of Tat in the induction of cell proliferation and migration of KS cells, relatively little is known about the signal transduction pathways which mediate these effects (41). In the present study, we observed that Tat treatment activates the Flk-1 receptor (VEGFR-2). Further characterization of downstream molecules revealed increased phosphorylation of various components of focal adhesions, structures which mediate adherent contacts with the extracellular matrix. These components included the related adhesion focal tyrosine kinase (RAFTK), paxillin, and p130cas. Tat treatment also activated various members of the mitogen-activated protein (MAP) kinase family and Src kinases. Our studies identify activation of specific signaling molecules that may participate in the Tat-induced growth and migration of KS cells.
MATERIALS AND METHODS
Cells and cell culture.
The KS cell line, KS 38, was derived from a biopsy specimen of a cutaneous lesion from an AIDS patient (13, 28, 39, 40). The cell line possesses many characteristics of primary KS cells, including endothelial markers and smooth muscle markers (13, 49), and has been used as a model for cytokine-mediated signaling studies (34). The cells were grown in flasks coated with 1.5% gelatin as described previously (34) and were suspended in RPMI 1640 containing 15% fetal calf serum, 2 mM glutamine, 1 mM MEM sodium pyruvate, 0.05 mM minimal essential medium (MEM) nonessential amino acids, 1× MEM amino acids, 1% Nutridoma-HU (Boehringer Mannheim Biochemicals, Indianapolis, Ind.), 50 μg of penicillin per ml, and 50 μg of streptomycin per ml. Cells were grown to confluence before being used in the signaling studies described below.
Reagents and antibodies.
RAFTK antibodies were generated as described previously (33). Antibodies to the VEGF receptor Flk-1 (VEGFR-2), c-Src, JNK, ERK-1, ERK-2, and p38 MAP kinase were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). The monoclonal antibodies against p130cas and paxillin were obtained from Transduction Laboratories, Inc. (Lexington, Ky.). Antiphosphotyrosine antibody (4G10) was a generous gift from Brian Druker (Oregon Health Sciences University). Electrophoresis reagents were obtained from Bio-Rad Laboratories (Hercules, Calif.). The protease inhibitors leupeptin and aprotinin and all other reagents were obtained from Sigma Chemical Co. (St. Louis, Mo.). The nitrocellulose membrane was obtained from Bio-Rad Laboratories. HIV Tat (1 to 86 amino acids) was expressed in Escherichia coli and purified by heparin affinity chromatography. Further purification was done by high-performance liquid chromatography (data not shown). The protein was found to be homogenous by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie blue stain. The purified Tat preparation (500 ng/ml) was found to be endotoxin free by the timed gel formation method with the Limulus amoebocyte lysate reagent as recommended by the manufacturer (Sigma). It was found to be biologically active as assessed by the HIV rescue assay with a cell line (HLM-1) containing an integrated nonreversible Tat-defective provirus. The Tat protein was lyophilized and reconstituted in Tat buffer (phosphate-buffered saline containing 1 mg of bovine serum albumin and 0.1 mM per ml dithiothreitol and was used for further studies.
Stimulation of cells.
KS 38 cells, grown to confluence, were serum starved for 16 to 18 h and washed twice with Hanks’ balanced salt solution (Gibco BRL, Gaithersburg, Md.) before Tat treatment. The cells were treated with 100 ng of Tat per ml, and 10 IU of heparin per ml was added in each case. The addition of heparin enhanced the Tat-mediated effects. Tat protein was added to cell cultures singly for different periods in vitro. Controls included media with 10 IU of heparin per ml in the absence of Tat. The cell lysates were prepared directly within the culture dish by lysis in 500 μl of modified RIPA buffer (50 mM Tris-HCl [pH 7.4], 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 10 μg of pepstatin per ml, 10 mM sodium vanadate, 10 mM sodium fluoride, 10 mM sodium pyrophosphate) per dish at various time points. Total-cell lysates were clarified by centrifugation at 10,000 × g for 10 min. Protein concentrations were determined by the protein assay (Bio-Rad Laboratories).
Immunoprecipitation and Western blot analysis.
For the immunoprecipitation studies, identical amounts of protein from each sample were clarified by incubation with protein A-Sepharose or Gamma Bind Sepharose (Pharmacia Biotech, Piscataway, N.J.) for 1 h at 4°C followed by a brief centrifugation. The solution was incubated for 4 h with different primary antibodies for each experiment or clarified overnight at 4°C. The antibody-antigen complexes were immunoprecipitated by incubation for 2 h at 4°C with 50 μl of the protein A-Sepharose or Gamma Bind sepharose (10% suspension). Nonspecific proteins were removed by washing the Sepharose beads three times with the modified RIPA buffer and once with phosphate-buffered saline. Bound proteins were solubilized in 40 μl of 2× Laemmli buffer and further analyzed by immunoblotting. Samples were separated by SDS-PAGE (8% polyacrylamide) and then transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk protein and probed with primary antibody for 2 h at room temperature (RT) or overnight at 4°C. Immunoreactive bands were visualized by using horseradish peroxidase-conjugated secondary antibody and the enhanced chemiluminescence system (Amersham Corp., Arlington Heights, Ill.).
MAP and JNK kinase assays.
Cell lysates were immunoprecipitated with ERK-1, ERK-2 (1:1) (for MAP kinase), or JNK antibodies (for JNK kinase) (Santa Cruz Biotechnology). The immune complexes were washed twice with RIPA buffer and twice with kinase buffer (50 mM HEPES [pH 7.4], 10 mM MgCl2, 20 μM ATP). The complex was then incubated for 30 min at RT with the substrates myelin basic protein (MBP) (7 μg) or glutathione S-transferase (GST)–c-Jun (4 μg) for MAP and JNK kinase, respectively, and 5 μCi [γ-32P]ATP. The reaction was stopped by adding 2× SDS sample buffer and boiling the sample for 5 min at 100°C. Proteins were separated by SDS-PAGE (12 or 15% polyacrylamide) and detected by autoradiography.
c-Src kinase assay.
The c-Src kinase assay was carried out as described previously (23). Briefly, the immunoprecipitated complexes obtained by immunoprecipitating cell lysates with the c-Src antiserum were washed twice with RIPA buffer and once with kinase buffer (10 mM HEPES [pH 7.4], 5 mM MnCl2, 10 μM Na3VO4). For in vitro kinase assays, the immune complex was incubated for 30 min at RT in kinase buffer containing acid-denatured rabbit muscle enolase (Sigma Chemical Co.) and 5 μCi of [γ-32P]ATP. The reaction was stopped by adding 2× SDS sample buffer and boiling the samples for 5 min. The samples were subjected to SDS-PAGE (10% polyacrylamide) and detected by autoradiography.
Autophosphorylation assay.
The autophosphorylation assay was done as described by Albini et al. (3). The Flk-1/KDR immunoprecipitates were incubated in kinase buffer (50 mM HEPES [pH 7.4], 10 mM MnCl2, 10 mM MgCl2, 1 mM dithiothreitol, 20 μM ATP) plus 5 μCi [γ-32P]ATP for 30 min at 25°C. The reaction was stopped by adding 2× SDS sample buffer. The samples were then subjected to SDS-PAGE (8% polyacrylamide), and the proteins were detected by autoradiography.
RESULTS
HIV Tat induces tyrosine phosphorylation of cellular proteins in KS cells.
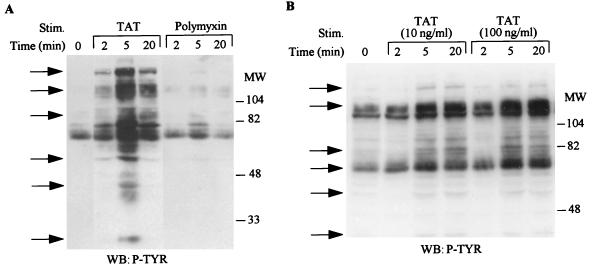
Signal transduction by the binding of ligands to cognate surface receptors involves the tyrosine phosphorylation of an array of targets. Since HIV Tat has been shown to bind to the Flk-1/KDR receptor (3) and to integrin receptors (8, 60), we analyzed the spectrum of substrates phosphorylated after its stimulation of KS cells. As shown in Fig. 1A, Tat stimulation resulted in the increased tyrosine phosphorylation of several different proteins with approximate molecular masses of 180, 120, 110, 100, 85, 70, 55, and 40 kDa. However, polymixin B treatment did not induce any increase in tyrosine phosphorylation of the various proteins. Furthermore, we found that Tat at 100 ng/ml had a slightly greater effect than Tat at 10 ng/ml (Fig. 1B). These results indicate that HIV-Tat can transduce signals by binding to surface receptors that result in induction of the tyrosine phosphorylation of a number of proteins.
FIG. 1.
Tyrosine phosphorylation of cellular proteins in KS cells after HIV Tat stimulation. Serum-starved KS cells were stimulated with either Tat at 100 ng/ml or polymixin at 100 ng/ml (A) or Tat at 10 ng/ml or 100 ng/ml (B) for the indicated times. Total-cell lysates (100 μg) obtained after cell lysis were fractionated on 10% polyacrylamide gels and subjected to Western blot (WB) analysis with the antiphosphotyrosine antibody 4G10. The arrows indicate the protein bands which show increased tyrosine phosphorylation after Tat treatment. MW, molecular weight in thousands. P-TYR, phosphotyrosine.
HIV Tat induces tyrosine phosphorylation and activation of the Flk-1/KDR receptor in KS cells.
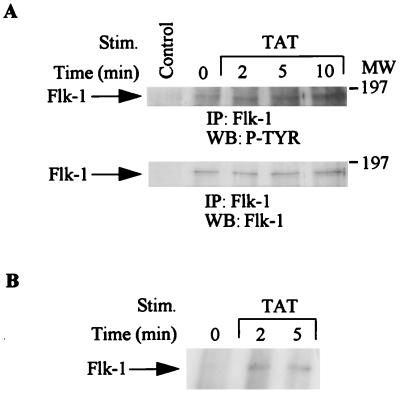
HIV Tat binds and activates the Flk-1/KDR receptor (VEGFR-2) in vascular endothelial cells (3). We have previously shown that the Flk-1/KDR receptor is expressed in KS cells (34). We therefore investigated whether HIV Tat activates the Flk-1/KDR receptor (VEGFR-2) in these cells. As shown in Fig. 2, Tat stimulation resulted in an increase in tyrosine phosphorylation of this receptor (Fig. 2A, top). Equivalent amounts of receptor protein were present in each lane, as confirmed by stripping and reprobing the membrane with anti-Flk-1/KDR antibody (Fig. 2A, bottom). The autokinase activity of the Flk-1/KDR receptor was also activated upon Tat stimulation (Fig. 2B).
FIG. 2.
Activation of the Flk-1/KDR receptor after HIV Tat treatments. KS cells were serum starved and treated with HIV Tat (100 ng/ml) for 2, 5, or 10 min. Total-cell lysates (1 mg) from treated or untreated cells were immunoprecipitated (IP) with anti-Flk-1/KDR receptor antibody. (A) Immunoprecipitates were separated by SDS-PAGE (8% polyacrylamide) and subjected to serial Western blotting (WB) with antiphosphotyrosine antibody (top) and anti-Flk-1/KDR receptor antibody (bottom). (B) Immunoprecipitates were subjected to autokinase assay, and 32P-incorporated proteins were resolved by SDS-PAGE (7.5% polyacrylamide) followed by autoradiography. Control lane represents immunoprecipitates of antibody alone. MW, molecular weight in thousands. P-TYR, phosphotyrosine.
RAFTK is tyrosine phosphorylated upon HIV Tat stimulation.
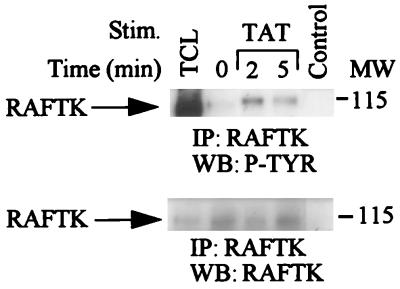
RAFTK is a novel signaling molecule of the focal adhesion kinase family that has been shown to link surface signals from integrins, cytokines, and T-cell receptors to the cytoskeleton and downstream to the MAP kinase pathway in certain cell types (4, 6, 26, 32, 33, 53). RAFTK was recently shown to be phosphorylated upon VEGF stimulation in KS cells (34). We therefore investigated whether HIV Tat phosphorylates RAFTK. HIV Tat treatment of KS cells resulted in rapid tyrosine phosphorylation of RAFTK (Fig. 3, top). Equivalent amounts of RAFTK protein were present in these experiments (Fig. 3, bottom).
FIG. 3.
Tyrosine phosphorylation of RAFTK by HIV Tat stimulation. Unstimulated KS cells or KS cells stimulated with HIV Tat (100 ng/ml) were lysed in RIPA buffer. Total-cell lysates (1 mg) were immunoprecipitated (IP) with RAFTK polyclonal antibody. Immunoprecipitates were size-fractionated on 7.5% polyacrylamide gels, transferred to a nitrocellulose membrane, and then subjected to serial Western blotting (WB) with antiphosphotyrosine antibody (4G10; top) and anti-RAFTK antibody (bottom). Control lane represents immunoprecipitates of antibody alone, and TCL lane represents 50 μg of total-cell lysates. MW, molecular weight in thousands. P-TYR, phosphotyrosine.
HIV Tat stimulates tyrosine phosphorylation of paxillin and its association with RAFTK.
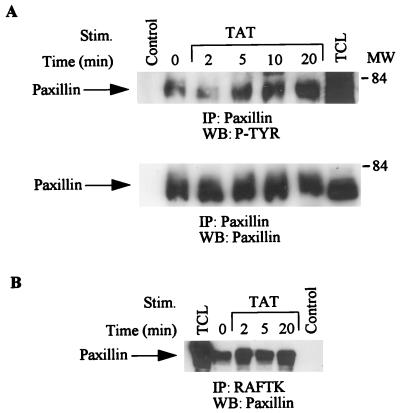
Cytoskeletal proteins such as paxillin have been shown to be modulated during cell functions related to migration and adhesion (35). Paxillin is a focal adhesion protein that serves as a binding site for a number of important signaling molecules including crk, Src, and RAFTK/Pyk2 (9, 10, 35, 50, 51, 54). Thus, we sought to investigate whether HIV Tat treatment of KS 38 cells resulted in changes in the phosphorylation state of paxillin and its association with other proteins. As shown in Fig. 4A (top), HIV Tat stimulation resulted in enhanced tyrosine phosphorylation of paxillin. Equivalent amounts of paxillin were present in each lane (Fig. 4A, bottom). Furthermore, we observed that paxillin was associated with RAFTK and that this association was enhanced upon HIV Tat treatment (Fig. 4B).
FIG. 4.
Phosphorylation of paxillin and its association with RAFTK. KS cells were stimulated with HIV Tat (100 ng/ml) for various times, and stimulated or unstimulated cell lysates were immunoprecipitated (IP) with anti-paxillin antibody. (A) The immunoprecipitates were then run on SDS-PAGE and subjected to Western blotting (WB) with antiphosphotyrosine antibody (top) and anti-paxillin antibody (bottom). (B) The cell lysates were immunoprecipitated with anti-RAFTK antibody run on SDS-PAGE (7.5% polyacrylamide) and blotted with paxillin antibody. Control lane represents immunoprecipitates of antibody alone, and TCL lane represents 50 μg of total-cell lysates. MW, molecular weight in thousands. P-TYR, phosphotyrosine.
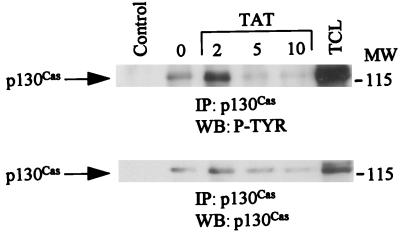
p130cas is tyrosine phosphorylated upon HIV Tat treatment.
p130cas, which is a component of focal adhesions, is essential for many functions including the regulation of cell shape and adhesive properties (36). We observed that HIV Tat treatment also resulted in enhanced tyrosine phosphorylation of p130cas (Fig. 5, top). The blots were stripped and blotted with anti-p130cas antibody (Fig. 5, bottom).
FIG. 5.
HIV Tat treatment of KS cells stimulates tyrosine phosphorylation of p130cas. Unstimulated or HIV Tat-stimulated KS cell lysates were immunoprecipitated (IP) with anti-p130cas. The immunoprecipitates were subjected to SDS-PAGE (7.5% polyacrylamide), transferred to a nitrocellulose membrane, and Western blotted (WB) with antiphosphotyrosine antibody 4G10 (top). The same blots were stripped and blotted with anti-p130cas antibody (bottom). Control lane represents immunoprecipitates of antibody alone, and TCL lane represents 50 μg of total-cell lysates. MW, molecular weight in thousands. P-TYR, phosphotyrosine.
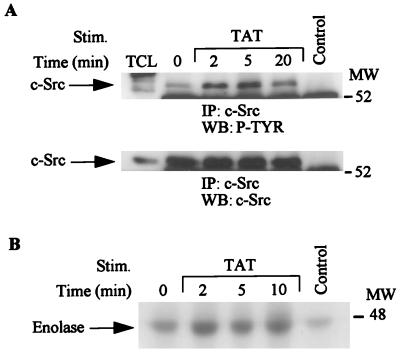
HIV Tat stimulates tyrosine phosphorylation and activation of c-Src kinase.
The Src family kinases have been shown to be activated by various growth factors and act to transmit growth signals downstream via adaptor molecules, RAFTK/Pyk2, and other substrates (15, 16, 61). KS 38 cells express c-Src kinase (data not shown). HIV Tat treatment of KS 38 cells resulted in rapid tyrosine phosphorylation of c-Src kinase (Fig. 6A, top). Equivalent amounts of c-Src were present in each lane (Fig. 6A, bottom). An in vitro kinase assay in which enolase was used as an exogenous substrate demonstrated that HIV Tat stimulation of KS cells resulted in the activation of the intrinsic kinase activity of c-Src (Fig. 6B).
FIG. 6.
Activation of Src kinases upon HIV Tat stimulation. Total-cell lysates (500 μg) from unstimulated or HIV Tat-stimulated cell lysates were immunoprecipitated (IP) with anti-Src antibody. The immune complexes were subjected to Western blotting (WB) with antiphosphotyrosine antibody (top) followed by c-Src antibody (bottom) (A) or an in vitro kinase assay with enolase as the substrate. The 32P-incorporated proteins were resolved by SDS-PAGE (7.5% polyacrylamide) followed by autoradiography. Control lane represents immunoprecipitates of antibody alone, and TCL lane represents 50 μg of total-cell lysates. MW, molecular weight in thousands. P-TYR, phosphotyrosine.
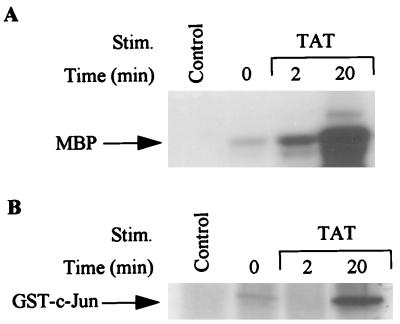
Activation of the MAP pathway in KS cells after HIV Tat treatment.
ERK and JNK kinases are important members of the MAP kinase family and have been shown to act as downstream mediators of the RAFTK/Pyk2 signaling pathway in PC12 neuronal cells (32, 59). We examined whether Tat treatment, which activated RAFTK, was also able to activate various members of the MAP kinase pathway. As shown in Fig. 7, HIV Tat stimulation of KS 38 cells resulted in activation of ERK and JNK kinases as determined by the phosphorylation of MBP and GST–c-Jun, respectively. p38 MAP kinase activity was not enhanced under these conditions (data not shown).
FIG. 7.
Activation of MAP kinase and JNK upon Tat stimulation. (A) KS cells were stimulated with Tat (100 ng/ml) and immunoprecipitated with ERK-1 or ERK-2 antibody and then subjected to an in vitro kinase assay with MBP (7 μg) as a substrate. (B) Stimulated KS cells were immunoprecipitated with JNK antibody and subjected to an in vitro kinase assay with GST–c-Jun (1 to 79 amino acids) as the substrate. The 32P-labeled proteins were subjected to SDS-PAGE (12% polyacrylamide) followed by autoradiography. Control lane represents immunoprecipitates of antibody alone.
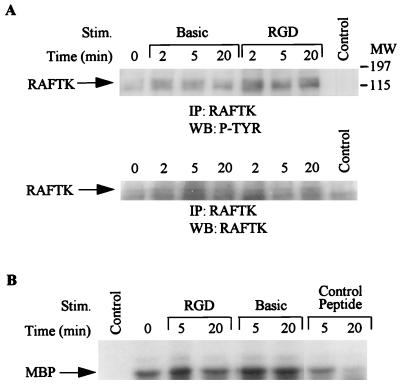
Tat basic and RGD peptides activate RAFTK and the MAP kinase pathway.
Tat contains several functional domains, including a basic domain spanning amino acids 46 to 60 and an RGD-containing domain spanning the carboxyl terminus of the molecule (amino acids 65 to 80). Having observed that full-length Tat stimulation resulted in phosphorylation of RAFTK and activation of the MAP kinase pathway, we studied whether treatment with basic or RGD-containing Tat peptides also resulted in activation of these kinases. As seen in Fig. 8, stimulation with both basic and RGD-containing peptides resulted in phosphorylation of RAFTK (Fig. 8A) and activation of MAP kinase (Fig. 8B). However, a control 15-amino-acid unrelated peptide did not activate MAP kinase under similar conditions (Fig. 8B).
FIG. 8.
Phosphorylation of RAFTK and activation of MAP kinase by basic and RGD domain-containing peptides of Tat. KS cells were stimulated with peptides containing either the basic or RGD domain of Tat or a control peptide. (A) The cell lysates were immunoprecipitated (IP) with RAFTK antibody, and immune complexes were subjected to SDS-PAGE (7.5% polyacrylamide) and subjected to Western blotting (WB) with antiphosphotyrosine antibody followed by anti-RAFTK antibody. (B) The cell lysates were immunoprecipitated with ERK-1 or ERK-2 antibody, and immune complexes were subjected to an in vitro kinase assay with MBP as the substrate. The samples were subjected to SDS-PAGE (12% polyacrylamide) followed by autoradiography. Control lane represents immunoprecipitates of antibody alone. MW, molecular weight in thousands. P-TYR, phosphotyrosine.
DISCUSSION
Various etiological factors implicated in KS include the recently discovered human herpesvirus 8 (HHV-8)/Kaposi’s sarcoma herpesvirus (KSHV) (45, 48), cytokines, and HIV Tat (2, 11, 17, 19, 25, 52). There is an extensive literature supporting a role for HIV Tat in regulating the growth and spread of KS cells and chemotaxis in monocytes (31, 44). However, relatively little is known about the signaling pathways used by HIV Tat that mediate these effects within KS cells or other monocytes (44). Tat can be released by HIV-infected cells (14, 21), and the protein might either be taken up by the target cells and trans-activate growth-related genes (11) or act as a paracrine growth factor by binding to cell surface receptors and leading to the generation of intracellular signals that modulate KS spindle cell growth and spread (3, 8, 19, 60). We observed that HIV Tat treatment of KS cells activated the Flk-1/KDR receptor (VEGFR-2) for VEGF-A. This effect may be mediated by direct binding of Tat to the Flk-1 receptor through its basic domain, since HIV Tat has recently been reported to bind to this receptor in endothelial cells (3). Moreover, VEGF-A has been shown to act as an autocrine growth factor for KS and therefore may play an important role in KS pathogenesis (38).
Since HIV Tat has been shown to affect cell migration, which involves alteration in cytoskeletal elements, we analyzed the phosphorylation of RAFTK, paxillin, and p130cas, components of focal adhesions. We observed that Tat stimulation resulted in the tyrosine phosphorylation of all three components of focal adhesions. RAFTK, which is also known as Pyk2 or Cak-β, is a novel member of the focal adhesion kinase family and has previously been shown to be activated upon VEGF-A stimulation in KS cells (34) and integrin stimulation in megakaryocytes and B cells (4, 33). RAFTK has been shown to act as a “platform kinase” and to link growth factor and stress signals (such as UV and osmotic shock) to the nucleus and cytoskeleton in neuronal and hematopoietic cells (4, 26, 32). Paxillin and p130cas have also been shown to participate in integrin- and VEGF-mediated signal transduction pathways (1, 4, 5, 36, 54). Our data revealed that Tat induces a rapid tyrosine phosphorylation of RAFTK, which reaches a maximum around 2 min and declines thereafter. However, paxillin tyrosine phosphorylation reaches a maximum after 5 min. This suggests that tyrosine phosphorylation of RAFTK leads to its activation and that activated RAFTK may phosphorylate paxillin. RAFTK has been shown to phosphorylate paxillin in hematopoietic cells (29, 50). We also observed that there was an enhanced association of paxillin with RAFTK upon Tat stimulation. Paxillin has previously been shown to be associated with RAFTK and FAK in various cell types through the proline-rich COOH terminus (9, 26, 27, 50). Phosphorylation of RAFTK, p130cas, and paxillin and association of RAFTK with paxillin may result in the formation of a cytoskeletal complex, which contributes to chemotaxis.
c-Src, MAP, and JNK kinases are also activated upon Tat treatment in KS cells. These kinases are thought to play important roles in regulating cellular proliferation (22, 58). Furthermore, c-Src also plays a role in regulating adhesion, since v-Src-transformed cells have morphologically abnormal focal adhesions and are defective in cell substrate adhesion (22, 58). Recent studies also indicate that RAFTK mediates G-protein-coupled activation of Src and MAP kinases in PC12 cells (16).
Both the basic and RGD Tat peptides resulted in the activation of KS cells, with phosphorylation of RAFTK and activation of MAP kinase. The basic-domain-containing peptide may mediate its effect by binding to the Flk-1/KDR receptor (VEGFR-2). Recently, Albini et al. (3) showed that Tat basic peptide can induce tyrosine phosphorylation of the Flk-1/KDR receptor (VEGFR-2) whereas the RGD-containing peptide does not activate the Flk-1/KDR receptor. However, the RGD-containing peptide may induce signaling by binding to integrin receptors. RAFTK has been shown to participate in both Flk-1- and integrin-mediated signaling pathways in hematopoietic and other cells (4, 34, 36).
Our data provide new information on HIV-Tat-induced signal transduction pathways in KS cells and demonstrate how Tat may act at a molecular level to modulate chemotaxis and cell proliferation in these cells. Tat has previously been shown to activate phosphatidylinositol 3-kinase in PC12 neuronal cells (41). We add to this report data on Tat stimulation and subsequent activation of the Flk-1 receptor and phosphorylation of the recently identified focal adhesion components RAFTK and p130cas. The characterization of the Tat-induced signal transduction pathway in KS may provide insights into developing targeted therapies to inhibit its growth and spread.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported in part by NIH grants HL 55187, HL 53745, HL 43510, and CA76950.
We thank Hava and Shalom Avraham for the generous gift of RAFTK antibody. We also thank our colleagues Stephanie Brubaker for technical assistance, Janet Delahanty for editing and preparation of figures, and Nancy DesRosiers for assistance with the figures. We are grateful to Delroy Heath for facilitating receipt of needed reagents and to Tee Trac for typing the manuscript.
Footnotes
Dedicated to Ronald Ansin for his continuing support of our research program.
REFERENCES
- 1.Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- 2.Albini A, Barillari G, Benelli R, Gallo R C, Ensoli B. Angiogenic properties of human immunodeficiency virus type 1 Tat protein. Proc Natl Acad Sci USA. 1995;92:4838–4842. doi: 10.1073/pnas.92.11.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 4.Astier A, Avraham H, Manie S N, Groopman J E, Canty T, Avraham S, Freedman A S. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after B1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 5.Astier A, Manie S N, Avraham H, Hirai H, Law S F, Zhang Y, Golemis E A, Fu Y, Druker B J, Haghayeghi N, Freedman A S, Avraham S. The related adhesion focal tyrosine kinase differentially phosphorylated p130cas and the cas-like protein, p105HEF1. J Biol Chem. 1997;272:19719–19724. doi: 10.1074/jbc.272.32.19719. [DOI] [PubMed] [Google Scholar]
- 6.Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor L M, White R A, Groopman J E, Avraham H. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 7.Barillari G, Buonaguro L, Fiorelli V, Hoffman J, Michaels F, Gallo R C, Ensoli B. Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS-Kaposi’s sarcoma pathogenesis. Immunology. 1992;149:3727–3734. [PubMed] [Google Scholar]
- 8.Barillari G, Gendelman R, Gallo R C, Ensoli B. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc Natl Acad Sci USA. 1993;90:7941–7945. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellis S L, Miller J T, Turner C E. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem. 1995;270:17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- 10.Birge R B, Fajardo J E, Reichman C, Shoelson S E, Songyang Z, Cantley L C, Hanafusa H. Identification and characterization of a high-affinity interaction between v-Crk and tyrosine-phosphorylated paxillin in CT10-transformed fibroblasts. Mol Cell Biol. 1993;13:4648–4656. doi: 10.1128/mcb.13.8.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonaguro L, Barillari G, Chang H K, Bohan C A, Kao V, Morgan R, Gallo R C, Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992;66:7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bussolino F, Arese M, Montrucchio G, Barra L, Primo L, Benelli R, Sanavio F, Aglietta M, Ghigo D, Rola-Pleszczynski M, Bosia A, Albini A, Camussi G. Platelet activating factor produced in vitro by Kaposi’s sarcoma cells induces and sustains in vivo angiogenesis. J Clin Invest. 1995;96:940–952. doi: 10.1172/JCI118142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai J, Gill P S, Masood R, Chandrasoma P, Jung B, Law R E, Radka S F. Oncostatin-M is an autocrine growth factor in Kaposi’s sarcoma. Am J Pathol. 1994;145:74–79. [PMC free article] [PubMed] [Google Scholar]
- 14.Chang H C, Samaniego F, Nair B C, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparin sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Cooper J A, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 16.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 17.Ensoli B, Barillari G, Gallo R C. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Immunol Rev. 1992;127:147–155. doi: 10.1111/j.1600-065x.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 18.Ensoli B, Barillari G, Salahuddin S Z, Gallo R C, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 19.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang H K, Brady J N, Gallo R C. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature. 1994;371:674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 20.Ensoli B, Nakamura S, Salahuddin S Z, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo R C. AIDS-Kaposi’s sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989;243:223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- 21.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan R A, Wingfield P, Gallo R C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erpel T, Courtneidge S A. Src family protein tyrosine kinases and cellular signal transduction pathways. Curr Opin Cell Biol. 1995;7:176–182. doi: 10.1016/0955-0674(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 23.Feder D, Bishop J M. Purification and enzymatic characterization of pp60c-src from human platelets. J Biol Chem. 1990;265:8205–8211. [PubMed] [Google Scholar]
- 24.Fiorelli V, Gendelman R, Samaniego F, Markham P D, Ensoli B. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi’s sarcoma spindle cells. J Clin Invest. 1995;95:1723–1734. doi: 10.1172/JCI117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganem D. AIDS. Viruses, cytokines and Kaposi’s sarcoma. Curr Biol. 1995;5:469–471. doi: 10.1016/s0960-9822(95)00093-5. [DOI] [PubMed] [Google Scholar]
- 26.Ganju R K, Hatch W C, Avraham H, Ona M A, Druker B, Avraham S, Groopman J E. RAFTK, a novel member of the focal adhesion kinase family, is phosphorylated and associates with signaling molecules upon activation of mature T lymphocytes. J Exp Med. 1997;185:1–9. doi: 10.1084/jem.185.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganju R K, Dutt P, Wu L, Newman W, Avraham H, Avraham S, Groopman J E. β-Chemokine receptor CCR5 signals via the novel tyrosine kinase RAFTK. Blood. 1998;91:791–797. [PubMed] [Google Scholar]
- 28.Guo W, Antakly T, Cadotte M, Kachra Z, Kindel L, Masood R, Gill P. Expression and cytokine regulation of glucocorticoid receptors in Kaposi’s sarcoma. Am J Pathol. 1996;148:1999–2007. [PMC free article] [PubMed] [Google Scholar]
- 29.Hiregowdara D, Avraham H, Fu Y, London R, Avraham S. Tyrosine phosphorylation of the related adhesion focal tyrosine kinase in megakaryocytes upon stem cell factor and phorbol myristate acetate stimulation and its association with paxillin. J Biol Chem. 1997;272:10804–10810. doi: 10.1074/jbc.272.16.10804. [DOI] [PubMed] [Google Scholar]
- 30.Karp J E, Pluda J M, Yarchoan R. AIDS-related Kaposi’s sarcoma. A template for the translation of molecular pathogenesis into targeted therapeutic approaches. Hematol Oncol Clin North Am. 1996;10:1031–1049. doi: 10.1016/s0889-8588(05)70383-x. [DOI] [PubMed] [Google Scholar]
- 31.Lafrenie R M, Wahl L M, Epstein J S, Hewlett I K, Yamada K M, Dhawan S. HIV-1-tat protein promotes chemotaxis and invasive behavior by monocytes. J Immunol. 1996;157:974–977. [PubMed] [Google Scholar]
- 32.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Avraham H, Rogers R, Raja S, Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- 34.Liu Y, Ganju R, Ona M, Hatch W, Wang J, Zheng T, Gill P, Avraham S, Groopman J. Cytokine signaling through the novel tyrosine kinase RAFTK in Kaposi’s sarcoma cells. J Clin Invest. 1997;99:1798–1804. doi: 10.1172/JCI119344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo S H, Chen L B. Focal adhesion as a signal transduction organelle. Can Metas Rev. 1994;13:9–24. doi: 10.1007/BF00690415. [DOI] [PubMed] [Google Scholar]
- 36.Manie S N, Astier A, Haghayeghi N, Canty T, Druker B J, Hirai H, Freedman A S. Regulation of integrin-mediated p130cas tyrosine phosphorylation in human B cells. J Biol Chem. 1997;272:15636–15641. doi: 10.1074/jbc.272.25.15636. [DOI] [PubMed] [Google Scholar]
- 37.Masood R, Cai J, Law R, Gill P. AIDS-associated Kaposi’s sarcoma pathogenesis, clinical features, and treatment. J Opin Oncol. 1993;5:831–834. doi: 10.1097/00001622-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Masood R, Cai J, Zheng T, Smith D L, Naidu Y, Gill P S. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc Natl Acad Sci USA. 1997;94:974–984. doi: 10.1073/pnas.94.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masood R, Husain S R, Rahman A, Gill P. Potentiation of cytotoxicity of Kaposi’s sarcoma related to immunodeficiency syndrome (AIDS) by liposome-encapsulated doxorubicin. AIDS Res Hum Retroviruses. 1993;9:741–746. doi: 10.1089/aid.1993.9.741. [DOI] [PubMed] [Google Scholar]
- 40.Masood R, Lunardi-Iskandar Y, Jean L, Murphy J, Waters C, Gallo R, Gill P. Inhibition of AIDS-associated Kaposi’s sarcoma cell growth by DAB389-interleukin 6. AIDS Res Hum Retroviruses. 1994;10:969–975. doi: 10.1089/aid.1994.10.969. [DOI] [PubMed] [Google Scholar]
- 41.Milani D, Mazzoni M, Borgatti P, Zauli G, Cantley L, Capitani S. Extracellular human immunodeficiency virus type-1 tat protein activates phosphatidylinositol 3-kinase in PC12 neuronal cells. J Biol Chem. 1996;271:22961–22964. doi: 10.1074/jbc.271.38.22961. [DOI] [PubMed] [Google Scholar]
- 42.Miles S A. Pathogenesis of HIV-related Kaposi’s sarcoma. J Opin Oncol. 1994;6:497–502. doi: 10.1097/00001622-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Miles S A, Rezai A R, Salazar-Gonzalez J F, Vander Meyden M, Stevens R H, Logan D M, Mitsuyasu R T, Taga T, Hirano T, Kishimoto T, Martinez-Maza O. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci USA. 1990;87:4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitola S, Sozzani S, Luini W, Primo L, Borsatti A, Weich H, Bussolino F. Tat-human immunodeficiency virus-1 induces human monocyte chemotaxis by activation of vascular endothelial growth factor receptor-1. Blood. 1997;90:1365–1372. [PubMed] [Google Scholar]
- 45.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 46.Naidu Y M, Rosen E M, Zitnick R, Goldberg I, Park M, Naujokas M, Polverini P J, Nickoloff B J. Role of scatter factor in the pathogenesis of AIDS-related Kaposi sarcoma. Proc Natl Acad Sci USA. 1994;91:5281–5285. doi: 10.1073/pnas.91.12.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nair B C, DeVico A L, Nakamura S, Copeland T D, Chen Y, Patel A, O’Neil T, Oroszlan S, Gallo R C, Sarngadharan M G. Identification of a major growth factor for AIDS-Kaposi’s sarcoma cells as oncostatin M. Science. 1992;255:1430–1432. doi: 10.1126/science.1542792. [DOI] [PubMed] [Google Scholar]
- 48.Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H-G, Hayward G S, Reitz M S. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 49.Rabkin C S, Janz S, Lash A, Coleman A E, Musaba E, Liotta L, Biggar R, Zhuang Z. Monoclonal origin of multicentric Kaposi’s sarcoma lesions. N Engl J Med. 1997;336:988–993. doi: 10.1056/NEJM199704033361403. [DOI] [PubMed] [Google Scholar]
- 50.Salgia R, Avraham H, Pisick E, Li J-L, Raja S, Greenfield E, Sattler M, Avraham H, Griffin J. The related adhesion focal tyrosine kinase forms a complex with paxillin in hematopoietic cells. J Biol Chem. 1996;271:31222–31226. doi: 10.1074/jbc.271.49.31222. [DOI] [PubMed] [Google Scholar]
- 51.Salgia R, Uemura N, Okuda K, Li J L, Pisick E, Sattler M, de Jong R, Druker B, Heisterkamp N, Chen L B, Groffen J, Griffin J D. CRKL links p210BCR/ABL with paxillin in chronic myelogenous leukemia cells. J Biol Chem. 1995;270:29145–29150. doi: 10.1074/jbc.270.49.29145. [DOI] [PubMed] [Google Scholar]
- 52.Samaniego F, Markham P D, Gallo R C, Ensoli B. Inflammatory cytokines induce AIDS-Kaposi’s sarcoma-derived spindle cells to produce and release basic fibroblast growth factor and enhance Kaposi’s sarcoma-like lesion formation in nude mice. J Immunol. 1995;154:3582–3592. [PubMed] [Google Scholar]
- 53.Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 54.Schaller M D, Parsons J T. pp125fak-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz R A. Kaposi’s sarcoma: advances and perspectives. J Am Acad Dermatol. 1996;34:804–814. doi: 10.1016/s0190-9622(96)90018-3. [DOI] [PubMed] [Google Scholar]
- 56.Sturzl M, Roth W K, Brockmeyer N H, Zietz C, Speiser B, Hofschneider P H. Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci USA. 1992;89:7046–7050. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sturzl M, Brandstetter H, Zietz C, Eisenburg B, Raivich G, Gearing D P, Brockmeyer N H, Hofschneider P H. Identification of interleukin-1 and platelet-derived growth factor-B as major mitogens for the spindle cells of Kaposi’s sarcoma: a combined in vitro and in vivo analysis. Oncogene. 1995;10:2007–2016. [PubMed] [Google Scholar]
- 58.Superti-Furga G, Courtneidge S A. Structure-function relationships in Src family and related protein tyrosine kinases. Bioessays. 1995;17:321–330. doi: 10.1002/bies.950170408. [DOI] [PubMed] [Google Scholar]
- 59.Tokiwa G, Dikic I, Lev S, Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- 60.Vogel B E, Lee S J, Hildebrand A, Craig W, Pierschbacher M D, Wong-Staal F, Ruoslahti E. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol. 1993;121:461–468. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu W, Harrison S C, Eck M J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Xu Y, Zhu C, Hagan M K, Lawley T, Offermann M K. Regulation of adhesion molecule expression in Kaposi’s sarcoma cells. J Immunol. 1994;152:361–373. [PubMed] [Google Scholar]