Abstract
Sulfate is the fourth most abundant anion in circulation. Despite being an essential nutrient for healthy growth and development, sulfate is not routinely measured in clinical settings. In research settings, animal studies have shown that hyposulfatemia and hypersulfaturia are associated with adverse developmental outcomes. Those findings have increased interest in measuring plasma and urine sulfate levels. In this study, we describe a modified assay to measure sulfate in low volumes of plasma and urine.
-
•
A streamlined microassay to measure sulfate levels using a microtiter plate format was developed.
-
•
To determine the robustness of the assay, this method assessed reagent stability and concentrations, as well as absorbance at different wavelengths and following a range of incubation times.
-
•
The optimized microassay was used to measure sulfate level in pig plasma and urine samples, which were compared to a validated ion chromatography method.
Keywords: Sulfate assay, Barium chloride, Sulfatemia
Method name: Turbidometric microassay for measurement of inorganic sulfate
Graphical abstract
Specifications table
| Subject area: | Chemistry |
| More specific subject area: | Biochemical pathology |
| Name of your method: | Turbidometric microassay for measurement of inorganic sulfate |
| Name and reference of original method: | Simple, rapid, turbidometric determination of inorganic sulfate and/or protein. S G Jackson, E L McCandless. Anal. Biochem. 1978 Oct 15;90(2):802–8. doi: 10.1016/0003–2697(78)90,171–9 |
| Resource availability: | Equipment: (1) Flat-bottom 96-well microtiter plates (Corning) (2) Microcentrifuge (3) Microplate spectrophotometer (Thermo Scientific™ Multiskan™ GO) Reagents: (1) Barium Chloride (Sigma-Aldrich Cat.# 202,738) (2) Agarose (Sigma-Aldrich Cat.# 05,066) (3) Trichloroacetic acid (Sigma-Aldrich Cat.# T6399) (4) Potassium sulfate (Sigma-Aldrich Cat.# 223,492) (5) Deionized H2O (Millipore) |
Background
Sulfate is an important nutrient in many cellular and physiological processes [[1], [2]]. Despite being the fourth most abundant anion in circulation, sulfate is not routinely measured in clinical settings. Animal models of hyposulfatemia display atypical phenotypes, including growth retardation, reduced fertility, seizures, behavioral abnormalities and osteochondrodysplasias [3], [4], [5]. Circulating sulfate level can be altered by genetic, environmental and physiological conditions [6], [7], [8], [9], [10]: (A) decreased by (i) loss-of-function mutations in the renal SLC13A1 gene, (ii) certain drugs such as acetaminophen and (iii) low sulfate diet; and (B) increased (i) in maternal circulation during pregnancy; (ii) in patients with chronic kidney disease; and (iii) by high sulfate diet. These findings have led to increased interest in methodologies for measuring sulfate, particularly in plasma and urine.
Several methods for measuring sulfate in biological samples have been described, but many of those methods use toxic reagents [11] or require high-cost specialized equipment such as ion chromatography [12]. A relatively low-cost and low-hazard methodology is the turbidometric assay that uses BaCl2 agarose solution to precipitate sulfate [13]. However, the previously published turbidometric assay requires milliliter volumes of sample, which can exceed the volume of blood available from small laboratory animals in research settings or from infants in clinical settings.
In this study, we report a modified turbidometric microassay for measuring sulfate in low sample volumes. In addition, we tested a range of assay parameters, including reagent stability and reagent incubation times, as well as comparison between the turbidometric microassay and a validated ion chromatography method [12].
Method details
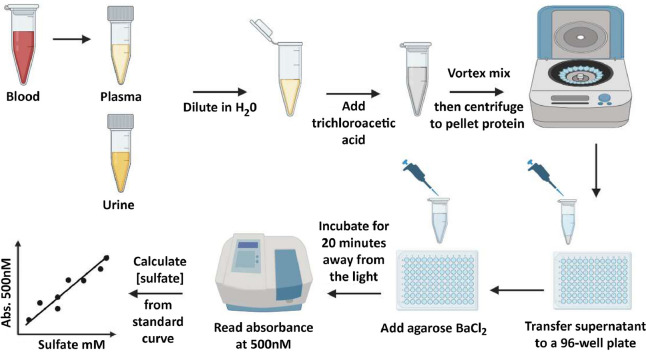
Plasma and urine samples
This study used samples from pigs up to 2 weeks of age. Whole blood was collected into a pediatric plasma separator tube, then gently inverted 5 times before centrifugal separation of the plasma at 9,000xg for 1.5 min in a microcentrifuge. The isolated plasma and a random urine sample were stored at −20 °C until assayed.
Agarose barium chloride, trichloroacetic acid and potassium sulfate solutions
Agarose, BaCl2 and trichloroacetic acid were purchased from Sigma-Aldrich. Glassware was rinsed several times in deionized H20 and then dried before preparing the reagents. A 0.5 % BaCl2 + 0.01 % agarose solution was prepared to a final volume of 50 ml using deionized H2O and a stock solution of 0.1 % agarose (dissolved by heating in deionized H2O) and then stored at room temperature from 1 to 11 weeks prior to use. An 8 % trichloroacetic acid solution was prepared in 50 ml deionized H2O. All reagents were stored away from light for up to 11 weeks. To prepare solutions with known sulfate levels, ranging from 0.05 to 2.0 mM, a stock 100 ml solution of 0.1 M K2SO4 (Sigma-Aldrich) was serially diluted in deionized H20. These standard solutions were used to generate a standard curve.
Sample preparation
Plasma and urine samples were diluted in deionized H2O at ratios of 1:1 and 1:49, respectively. In 1.5 ml tubes, 120 µl of 8 % trichloroacetic acid were added to 110 µl K2SO4 standards and the diluted plasma and urine samples. Samples were vortex mixed for 5 s and then centrifuged at 16,000xg for 3 min at room temperature. Supernatants were transferred to fresh 1.5 ml tubes and centrifuged at 16,000xg for 3 min at room temperature. Supernatants from this second round of centrifugation were used in the sulfate microassay.
Sulfate microassay
Duplicate 80 µl aliquots of supernatants containing plasma, urine, K2SO4 standards or deionized H2O (control no sulfate) were transferred to a 96-well flat bottom microtiter plate. To each well, 20.87µl agarose BaCl2 was added and mixed by stirring with the pipette tip, rather than pipetting up and down, to avoid bubbles. The microtiter plate, covered in foil to shield from light, was incubated at room temperature for 20 min. The absorbance of each well was then measured at 500 nM using a microplate spectrophotometer (Thermo Scientific™ Multiskan™ GO). Sulfate concentration in the plasma and urine samples was then calculated from the linear plot of absorbance values for each of the K2SO4 standards.
Method validation
Modified low volume sulfate assay
In this study, we modified a previously published turbidometric assay [13] for measuring sulfate by reducing the sample volume from 1.1 ml to 80 µl using a microtiter plate and a microplate spectrophotometer. Considering that plasma makes up approximately 55 % of blood [14], and accounting for the dilution of plasma (1:1) and urine (1:49) used in this assay, the minimum volumes of whole blood and urine required for this microassay are 100 µl and 2.2 µl, respectively.
Linearity of sulfate detection
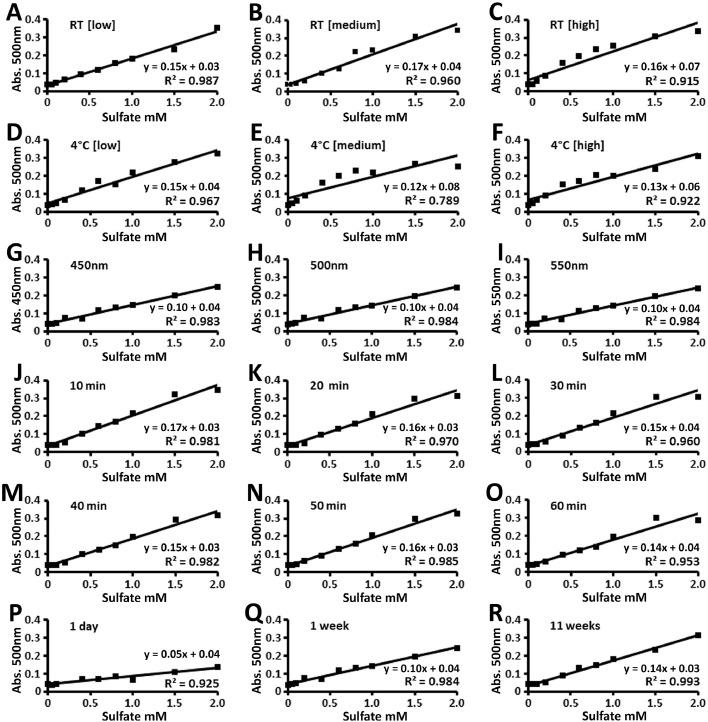
To determine whether the previously published assay [13] could be further improved, we tested a range of assay parameters (Fig. 1). We show that both low and high BaCl2 agarose concentrations stored at either room temperature or 4 °C gave similar linear responses to the previously published (medium) concentration stored at room temperature (Fig. 1A–F). In addition, the linearity of sulfate detection was similar when 450 nm, 500 nm (used in previously published method) or 550 nm wavelengths were applied (Fig. 1G–I). We next tested a range of incubation times post-mixing the sample and BaCl2 agarose reagent (Fig. 1J–O). Linearity of detection was similar between all incubation times, indicating the microassay can be completed at a minimum time of 10 min, which is less than the previous method that used an incubation time of 35–40 min. Stability of BaCl2 agarose was also tested, showing that this reagent is stable at room temperature up to at least 11 weeks post-preparation, with higher linear response and slope at 1 and 11 weeks when compared to 1 day (Fig. 1P–R).
Fig. 1.
Linearity of sulfate detection using K2SO4 standards to test reagent concentrations and stability, as well as absorbance at different wavelengths and incubation times in the modified turbidometric microassay. Data are representative of at least 10 independent experiments and the within run imprecision coefficient of variation (CVa) was <1.0 %. A–F) Assays used BaCl2 agarose solution at low (0.25 % BaCl2 + 0.005 % agarose), medium (0.5 % BaCl2 + 0.01 % agarose) or high (1.0 % BaCl2 + 0.02 % agarose) concentrations that were stored at room temperature (RT) or 4 °C for 1 week. G–I) Using the medium concentration of BaCl2 agarose solution stored at RT for 24hr, the absorbance values were measured at 450 nm, 500 nm or 550 nm. J–O) Using the conditions from panel H, absorbance values were measured at timed intervals, from 10 to 60 min, after mixing the BaCl2 agarose solution with K2SO4 standard solutions. P–R) Samples were assayed with the parameters used in panel K, following storage of the BaCl2 agarose solution at RT for up to 11 weeks.
Recovery of sulfate
Using assay parameters shown in Fig. 1, the limit of detection (LOD=3.3σ/S, 0.097 mmol/L) and quantification (LOQ=10σ/S, 0.294 mmol/L) were calculated from 3.3 to 10 times, respectively, the SDs of responses, where the slope of the calibration curve (S)=0.17 and the SD of y intercepts of regression lines (σ)=0.005. These LOD and LOQ values are low enough for the detection and quantification of sulfate in diluted plasma and urine samples from laboratory animals, including pigs and mice which have plasma and urine sulfate levels in the millimolar range [3,15]. To calculate the recovery of sulfate in this turbidometric microassay, we added known amounts of sulfate to pig plasma and urine followed by dilution and processing as described above. The average sulfate recovery ranged from 45 to 66 % for plasma and 58–66 % for urine spiked with 0.8–1.5 mmol/L K2SO4 and 25–100 mmol/L K2SO4, respectively (Table 1). This finding suggests that the turbidometric microassay underestimates the sulfate level in plasma and urine by at least 34 %. We found that to be the case when we compared sulfate levels measured with the turbidometric microassay and an ion chromatography method [12]. The turbidometric microassay consistently yielded sulfate levels that were 32 % and 33 % lower in pig plasma (n = 33) and urine (n = 5) samples, respectively, when compared to the ion chromatography method. Taken together, these findings indicate that a 1.3-fold factor should be used when calculating plasma and urine sulfate levels with the turbidometric microassay.
Table 1.
Recovery of sulfate using the turbidometric microassay.
| *Sample | K2SO4 spike (mmol/L) | Recovery sulfate% (Mean±SEM) |
|---|---|---|
| Plasma | 0.8 | 66±18 |
| 1.0 | 52±11 | |
| 1.5 | 45±9 | |
| Urine | 25 | 66±15 |
| 50 | 58±14 | |
| 100 | 63±22 |
*Plasma and urine samples were spiked with known concentrations of K2SO4 and then diluted with H20 1:1 and 1:50, respectively, before being processed and assayed.
In this study, we report a modified assay for measuring sulfate level in low sample volumes. This is particularly relevant to research studies that require sulfate testing in multiple blood and urine samples from laboratory animals. In addition, we reduced the assay incubation time and showed that the assay reagents can be stored for at least 11 weeks at room temperature, avoiding the need for freshly prepared solutions and cold storage. We also show that the microassay underestimates sulfate concentration by approximately 33 %. Accordingly, we recommend including a 1.3x factor when calculating plasma and urinary sulfate level.
Ethics statements
All procedures were approved (2022/AE000846) by the University of Queensland Animal Ethics Committee. This study used plasma and urine samples from both male and female pigs to compare methodology. Data were not compared among pigs.
CRediT authorship contribution statement
Prasidhee Vijayakumar: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Visualization. Avis McWhinney: Investigation, Resources, Writing – review & editing. Yvonne A. Eiby: Conceptualization, Methodology, Investigation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition. Paul A. Dawson: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by an Ideas grant (2020999) from the Australian National Health and Medical Research Council. We also acknowledge funding support from Mater Research and the Mater Foundation for this research at the Translational Research Institute, which is supported by a grant from the Australian Government. PV is supported by a Research Training Program PhD scholarship from the University of Queensland, as well as a Frank Clair Scholarship from Mater Research Ltd. PD is supported by a Mater Foundation Principal Research Fellowship.
Data availability
Data will be made available on request.
References
- 1.Cole D.E., Evrovski J. The clinical chemistry of inorganic sulfate. Crit. Rev. Clin. Lab. Sci. 2000;37(4):299–344. doi: 10.1080/10408360091174231. [DOI] [PubMed] [Google Scholar]
- 2.Dawson P.A. Role of sulphate in development. Reproduction. 2013;146(3):R81–R89. doi: 10.1530/REP-13-0056. [DOI] [PubMed] [Google Scholar]
- 3.Dawson P.A., Beck L., Markovich D. Hyposulfatemia, growth retardation, reduced fertility and seizures in mice lacking a functional NaSi-1 gene. Proc. Natl. Acad. Sci. USA. 2003;100(23):13704–13709. doi: 10.1073/pnas.2231298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neff M.W., Beck J.S., Koeman J.M., Boguslawski E., Kefene L., Borgman A., Ruhe A.L. Partial Deletion of the Sulfate Transporter SLC13A1 Is Associated with an Osteochondrodysplasia in the Miniature Poodle Breed. PLoS ONE. 2012;7(12):e51917. doi: 10.1371/journal.pone.0051917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X., Onteru S.K., Piripi S., Thompson K.G., Blair H.T., Garrick D.J., Rothschild M.F. In a shake of a lamb's tail: using genomics to unravel a cause of chondrodysplasia in Texel sheep. Anim. Genet. 2012;43(1):9–18. doi: 10.1111/j.1365-2052.2011.02304.x. [DOI] [PubMed] [Google Scholar]
- 6.Dawson P.A., Elliott A., Bowling F.G. Sulphate in pregnancy. Nutrients. 2015;7(3):1594–1606. doi: 10.3390/nu7031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson P.A., Petersen S., Rodwell R., Johnson P., Gibbons K., McWhinney A., Bowling F.G., McIntyre H.D. Reference intervals for plasma sulfate and urinary sulfate excretion in pregnancy. BMC Pregnancy Childbirth. 2015;15:96. doi: 10.1186/s12884-015-0526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S., Dawson P.A., Hewavitharana A.K., Shaw P.N., Markovich D. Disruption of NaS1 sulfate transport function in mice leads to enhanced acetaminophen-induced hepatotoxicity. Hepatology. 2006;43(6):1241–1247. doi: 10.1002/hep.21207. [DOI] [PubMed] [Google Scholar]
- 9.Tise C.G., Perry J.A., Anforth L.E., Pavlovich M.A., Backman J.D., Ryan K.A., Lewis J.P., O'Connell J.R., Yerges-Armstrong L.M., Shuldiner A.R. From genotype to phenotype: nonsense variants in SLC13A1 are associated with decreased serum sulfate and increased serum aminotransferases. G3 (Bethesda) 2016;6(9):2909–2918. doi: 10.1534/g3.116.032979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yildirim I., Hur E., Magden K., İlikhan S., Engin H., Can M., Yıldız G., Özer İ. Serum sulphate levels in hemodialysis patients. Int. J. Nephrol. 2019;2019 doi: 10.1155/2019/1063514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleeman C.R., Taborsky E., Epstein F.H. Improved method for determination of inorganic sulfate in biologic fluids. Proc. Soc. Exp. Biol. Med. 1956;91(3):480–483. doi: 10.3181/00379727-91-22299. [DOI] [PubMed] [Google Scholar]
- 12.Dawson P.A., McWhinney A., Reimer M., Bowling F.G. Evaluation of an ion chromatography method for quantitating sulfate in plasma, serum and urine. J. Chromatogr. Sep. Tech. 2018;9(411) doi: 10.4172/2157-7064.1000411. [DOI] [Google Scholar]
- 13.Jackson S.G., McCandless E.L. Simple, rapid, turbidometric determination of inorganic sulfate and/or protein. Anal. Biochem. 1978;90(2):802–808. doi: 10.1016/0003-2697(78)90171-9. [DOI] [PubMed] [Google Scholar]
- 14.Mathew J., Sankar P., Varacallo M. StatPearls Publishing; 2024. Physiology, Blood Plasma.https://www.ncbi.nlm.nih.gov/books/NBK531504/ [PubMed] [Google Scholar]
- 15.Dawson P., Lingwood B.E., Eiby Y.A., Barnes S.K., Colditz P., Boyd R., Badawi N., Koorts P., Kumar S., Flenady V., Hurrion E. Plasma sulphate levels decline in the neonatal preterm piglet. J. Paediatr. Child Health. 2017;53(S2):25–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.