Abstract
Syncytial feeding complexes induced by the cyst nematode Heterodera schachtii represent strong metabolic sinks for photoassimilates. These newly formed structures were described to be symplastically isolated from the surrounding root tissue and their mechanism of carbohydrate import has repeatedly been under investigation. Here, we present analyses of the symplastic connectivity between the root phloem and these syncytia in nematode-infected Arabidopsis (Arabidopsis thaliana) plants expressing the gene of the green fluorescent protein (GFP) or of different GFP fusions under the control of the companion cell (CC)-specific AtSUC2 promoter. In the same plants, phloem differentiation during syncytium formation was monitored using cell-specific antibodies for CCs or sieve elements (SEs). Our results demonstrate that free, CC-derived GFP moved freely from the phloem into the syncytial domain. No or only marginal cell-to-cell passage of GFP was observed into other root cells adjacent to these syncytia. In contrast, membrane-anchored GFP variants as well as soluble GFP fusions with increased molecular masses were restricted to the SE-CC complex. The presented data also show that nematode infection triggers the de novo formation of phloem containing an approximately 3-fold excess of SEs over CCs. This newly formed phloem exhibits typical properties of unloading phloem previously described in other sink tissues. Our results reveal the existence of a symplastic pathway between phloem CCs and nematode-induced syncytia. The plasmodesmata responsible for this symplastic connectivity allow the cell-to-cell movement of macromolecules up to 30 kD and are likely to represent the major or exclusive path for the supply of assimilates from the phloem into the syncytial complex.
The beet cyst nematode Heterodera schachtii resides in the soil, typically at a depth of 10 to 25 cm, where it infests the roots of sugar beet (Beta vulgaris). In addition to sugar beet, this parasite infects many other crop plants including radish, cabbage, cauliflower, and mustard. Sijmons and coworkers (Sijmons et al., 1991) established Arabidopsis (Arabidopsis thaliana) as a model system for plant-nematode interactions. Inoculation of Arabidopsis roots with freshly hatched beet cyst nematodes leads to the formation of a syncytial cell complex within the root vascular cylinder. The resulting syncytium serves as feeding site for the developing nematode. Cellular changes during the establishment of this syncytium from the root cambium or pericycle have been well studied (Golinowski et al., 1996; Sobczak et al., 1997). The most obvious structural rearrangements include cell wall dissolution, the formation of numerous small vacuoles, and the formation of additional phloem and/or cambial cells surrounding the syncytium.
Numerous changes in the expression of Arabidopsis genes were shown to accompany these structural modifications. Gene expression profiling using differential display or DNA microarray techniques on H. schachtii-infected versus noninfected roots revealed 24 genes (Hermsmeier et al., 2000) or 128 genes (Puthoff et al., 2003) with altered transcript levels. These changes in gene expression could not, however, clearly be assigned to the syncytium but rather to the root zone containing this nematode-induced structure. In contrast, analyses of transgenic Arabidopsis plants harboring promoter-reporter gene constructs revealed changes in the activities of various genes within the syncytia (Goddijn et al., 1993; Niebel et al., 1996; Barthels et al., 1997; de Almeida Engler et al., 1999; Puzio et al., 2000; Jürgensen et al., 2003; Thurau et al., 2003). Among these genes are a Gln-rich domain protein, cyclin-dependent kinases, mitotic cyclins, and a disaccharide transporter (Niebel et al., 1996; de Almeida Engler et al., 1999; Puzio et al., 2000; Jürgensen et al., 2003). In the Lemmi9 promoter of tomato (Lycopersicon esculentum), a cis-regulatory element involved in nematode-induced gene expression has been identified (Escobar et al., 1999). This 12-bp element resembles a CANNTG motif also found in stress-regulated genes and is recognized by a nuclear protein identified in root galls of tomato. Besides this, it is still unknown how the differential gene expression after nematode infestation and during formation of syncytia is regulated.
During the development of a cyst nematode, the parasite continuously withdraws nutrients from the metabolically active syncytium (Wyss, 1992). Therefore, syncytia represent major sinks for solutes, such as sugars or amino acids, which are imported into the root via the vascular system. In early studies, it has been suggested that these nutrients might be supplied by the xylem because of pronounced alterations of the cell wall at the syncytium-xylem interface (Jones, 1981). Investigations on phloem transport, however, revealed that both the fluorescent dye carboxyfluorescein (CF) and 14C-labeled solutes were able to enter cyst nematode-induced syncytia or root knot nematode-induced giant cells (Dorhout et al., 1993; Böckenhoff et al., 1996). These observations suggested that these probes might be symplastically unloaded from the phloem into these structures via connecting plasmodesmata. In fact, plasmodesmata connecting the syncytium with neighboring cells were identified, but after the detection of cell wall deposits it has been concluded that these plasmodesmata are nonfunctional (Grundler et al., 1998). The idea that syncytia are symplastically isolated from the surrounding host cells seemed to be supported by the observation that fluorescent dyes injected into syncytia were unable to move into neighboring cells (Böckenhoff and Grundler, 1994; Böckenhoff et al., 1996). Together, these data led to the widely accepted concept that solute transport from the host phloem into H. schachtii-induced syncytia is apoplastic, which involves transmembrane transport steps for the different solutes. In this context, the participation of putative nonspecific solute pores for CF has been discussed (Böckenhoff et al., 1996).
Another result supporting this apoplastic concept was the identification of AtSUC2 mRNA in cytoplasmic fractions extracted from syncytia that had been induced upon infection with female nematodes (Jürgensen et al., 2003). AtSUC2 is a plasma membrane-localized Suc-H+ symporter (Sauer and Stolz, 1994), and the identification of its mRNA in syncytia suggested that AtSUC2 might catalyze the uptake of Suc from the apoplast into the syncytium (Jürgensen et al., 2003).
In uninfected plants of Arabidopsis and common plantain (Plantago major), the SUC2 genes are expressed solely in companion cells (CCs) of the phloem (Stadler et al., 1995; Truernit and Sauer, 1995; Stadler and Sauer, 1996). Unexpectedly, analyses of Arabidopsis and other plants expressing the green fluorescent protein (GFP) gene under the control of the AtSUC2 promoter observed trafficking of this CC-synthesized GFP out of the CCs into the adjacent sieve elements (SEs), as well as unloading of GFP into sink tissues and postphloem cell-to-cell movement of unloaded GFP within these sinks (Imlau et al., 1999; Oparka et al., 1999; Crawford and Zambryski, 2001; Ayre et al., 2003; Stadler et al., 2005). In nematode-infected AtSUC2 promoter∷GFP Arabidopsis plants, strong GFP fluorescence was observed in syncytia that had formed after infection of roots with female nematodes (Jürgensen et al., 2003). Based on the concept of locked plasmodesmata and of apoplastic loading of syncytial, this result has been interpreted as a nematode-induced and syncytium-localized expression of AtSUC2.
Here we present studies performed with nematode-infected Arabidopsis plants expressing free GFP, soluble GFP-fusions (GFP-Ubiquitin [GFP-UBI] and GFP-Sporamin [GFP-SPOR]), or two different membrane-anchored GFPs (transmembrane-GFP2 [tmGFP2] and tmGFP9). These open reading frames (ORFs) are all expressed under the control of the AtSUC2 promoter and were described only recently by Stadler and coworkers (Stadler et al., 2005). The soluble GFP and GFP fusions show cell-to-cell movement and were used to determine plasmodesmata size exclusion limits (SELs). In contrast, the membrane-attached GFPs (tmGFP9 is fused to the C terminus of the first 6 transmembrane helices of the AtSTP9 monosaccharide transporter [Sauer and Stolz, 1994]; tmGFP2 is fused to the C terminus of the AtSUC2 Suc transporter [Schneidereit et al., 2003]) were shown to be nonmobile. The obtained results in this paper demonstrate that AtSUC2 expression occurs exclusively and with high specificity in the CCs surrounding these syncytia. GFP synthesized within these CCs is able to move into the SEs and eventually into the syncytial complex. In conclusion, our data suggest that syncytial feeding with organic solutes occurs via large plasmodesmata formed between de novo-synthesized phloem cells and the syncytia.
RESULTS
GFP Moves Symplastically from the SE-CC Complex into Syncytia
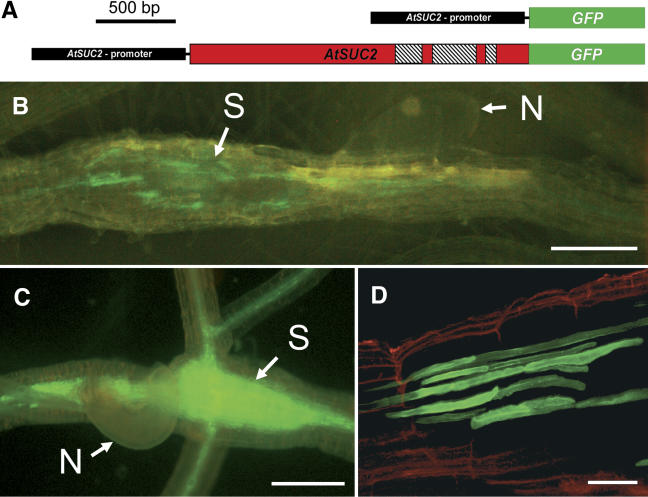
tmGFP2 Arabidopsis plants (Stadler et al., 2005; Fig. 1A) were inoculated with freshly hatched beet cyst nematodes and screened for fluorescent syncytia. In contrast to the even distribution of GFP fluorescence within the syncytia of AtSUC2 promoter∷GFP plants (Fig. 1C; Jürgensen et al., 2003), tmGFP2-dependent fluorescence was confined to distinct cells (Fig. 1B) that could be characterized as CCs (Fig. 1D). This suggests that in agreement with the data provided by Stadler and coworkers (Stadler et al., 2005) the C-terminal fusion of GFP to the AtSUC2 protein (tmGFP2) is trapped inside the CCs, where the AtSUC2 promoter is active.
Figure 1.
Fluorescence of GFP and tmGFP2 in nematode-infected roots of transgenic Arabidopsis plants. A, Schematic representation of the AtSUC2 promoter∷GFP (mobile) and the AtSUC2 promoter∷tmGFP2 (membrane-targeted) fusions present in the transgenic lines analyzed (Stadler et al., 2005). The AtSUC2 promoter is shown in black, the ORF of GFP frame is green, and the genomic-coding sequence of AtSUC2 is red. The 3 introns in the genomic AtSUC2 sequence are hatched. B, tmGFP2 fluorescence in AtSUC2 promoter∷tmGFP2 plants 13 d after infection (dai). tmGFP2 fluorescence is seen in distinct structures in the syncytial zone (N, Nematode; S, syncytium). C, Fluorescence of free GFP in AtSUC2 promoter∷GFP plants 11 dai. The GFP signal was observed in the root vasculature and in the syncytium. D, Optical section of the tmGFP2 signal in the syncytial area of an infected AtSUC2-promoter∷tmGFP2 root (14 dai). tmGFP2 fluorescence is seen only in elongated cells around the syncytium. Red fluorescence represents endogenous fluorescence of the cell walls. Scale bars represent 200 μm in B and C, and 40 μm in D.
Identical results were obtained with AtSUC2 promoter∷tmGFP9 plants, where GFP is membrane targeted as a C-terminal fusion to the 6 N-terminal transmembrane helices of AtSTP9 (Stadler et al., 2005; tmGFP9/AtSTP9; data not shown), a plasma membrane-localized Arabidopsis monosaccharide transporter (Schneidereit et al., 2003; Stadler et al., 2005). This complete absence of GFP fluorescence in the syncytia of plants expressing 2 different membrane-anchored, nondiffusible GFP fusions demonstrates that fluorescence observed in syncytia of AtSUC2 promoter∷GFP plants (Fig. 1C) cannot result from GFP synthesized within these syncytia (Jürgensen et al., 2003). It rather suggests that the GFP fluorescence in syncytia results from movement of GFP from the CCs into the SEs and finally into the syncytia.
The SEL for Macromolecular Trafficking into SyncytiaIs about 30 kD
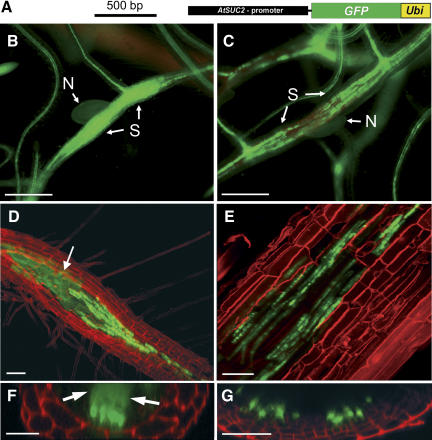
Only recently, the capacity of GFP to move cell to cell within and out of the Arabidopsis phloem was investigated in detail (Stadler et al., 2005). In these analyses, transgenic Arabidopsis lines were used that harbored constructs for several phloem-mobile GFP variants with different molecular masses, all expressed under the control of the AtSUC2 promoter. For our analyses of the SEL between the root phloem and the syncytia, we analyzed 2 Arabidopsis lines that had been used in the paper of Stadler and coworkers (Stadler et al., 2005): one expressing an AtSUC2 promoter∷GFP-UBI construct (Fig. 2A) and one harboring an AtSUC2 promoter∷GFP-SPOR insertion. Figure 2, B and C, represents epifluorescence analyses of syncytia from GFP (27 kD) plants and from GFP-UBI (36 kD) plants recorded under identical conditions. While the syncytia of AtSUC2 promoter∷GFP plants appeared homogenously fluorescent (Fig. 2B), the fluorescence in syncytia of AtSUC2 promoter∷GFP-UBI plants (Fig. 2C) was confined to the vascular tissue and looked as in tmGFPs plants (Fig. 1B).
Figure 2.
GFP trafficking from the SE-CC complex into the syncytium. A, Schematic representation of the AtSUC2 promoter∷GFP-UBI construct (Stadler et al., 2005) used in the plants analyzed in C, E, and G. The AtSUC2 promoter is shown in black, the GFP ORF is green, and the coding sequence for UBI (Ubi) is shown in yellow. Plants analyzed in B, D, and F were transformed with the AtSUC2 promoter∷GFP construct shown in Figure 1. B, Epifluorescence microscopic detection of GFP fluorescence in the phloem and in the syncytium of an AtSUC2 promoter∷GFP root 14 d after infection (dai; N, nematode; S, syncytium). The GFP signal is evenly distributed within the syncytium. C, Epifluorescence microscopic detection of GFP-UBI fluorescence in the phloem and in a syncytium of an AtSUC2 promoter∷GFP-UBI root 14 dai. The GFP-UBI signal is detected only in distinct cells. Note: Approximately the same fluorescence intensity is seen in the vascular tissue of the AtSUC2 promoter∷GFP plant shown in A and of the AtSUC2 promoter∷GFP-UBI plants shown in B. D and E, Confocal images of fluorescent cells and of fluorescent syncytia in the root of an AtSUC2 promoter∷GFP plant (D) or of an AtSUC2 promoter∷GFP-UBI plant (E) both recorded 14 dai. The white arrow in D marks the diffuse fluorescence of free GFP in the syncytium that is absent in E. F and G, Optical z-sections of fluorescent cells and of fluorescent syncytia in the root of an AtSUC2 promoter∷GFP plant (F) or of an AtSUC2 promoter∷GFP-UBI plant (G). Both z-sections show distinctly labeled cells at the surface of the syncytia. Diffusion of GFP into the syncytium is only seen in F (white arrows). Roots shown in D to G were treated with propidium iodide to visualize the cell walls. Scale bars represent 500 μm in B and C, 80 μm in D, 40 μm in E, 25 μm in F, and 20 μm in G.
Strong labeling of CCs was also seen in confocal sections of syncytia from AtSUC2 promoter∷GFP plants (Fig. 2D); however, in contrast to the AtSUC2 promoter∷GFP-UBI plants (Fig. 2E), these plants showed additional, evenly distributed fluorescence within their syncytia (Fig. 2, D and E). This was even more obvious in confocal z-sections (Fig. 2, F and G), where the fluorescent CCs are located on the surface of the syncytia, which showed internal GFP fluorescence in AtSUC2 promoter∷GFP plants (arrows in Fig. 2F) but no internal fluorescence in AtSUC2 promoter∷GFP-UBI plants (Fig. 2G). This complete absence of fluorescence in syncytia of GFP-UBI plants (Fig. 2, E and G) suggested that the size of the GFP-UBI fusion (36 kD) exceeded the SEL of the connecting plasmodesmata. Identical results were obtained in analyses of syncytia of GFP-SPOR plants expressing an even larger GFP-fusion (47 kD; data not shown).
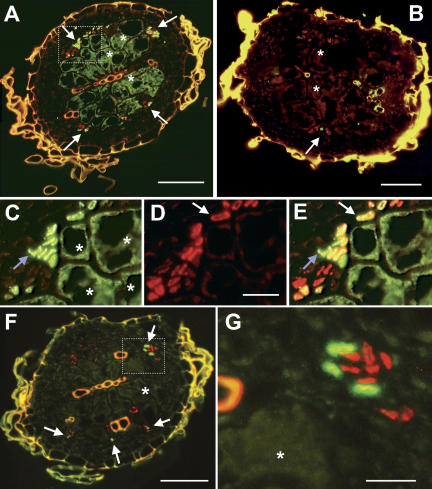
Immunohistochemical analyses of cross sections from AtSUC2 promoter∷GFP syncytia using an anti-GFP antiserum confirmed the presence of GFP in the syncytium and in the CCs at its surface (Fig. 3, A and C). In contrast and in agreement with the z-sections shown in Figure 2G, membrane-anchored tmGFP9 was immunolocalized only in these CCs adjacent to the syncytia of AtSUC2 promoter∷tmGFP9 plants (Fig. 3B). No tmGFP9 protein was immunolocalized inside the syncytia of these plants.
Figure 3.
Immunolocalization of GFP and tmGFP9 in cross sections from syncytia. A, Immunohistochemical staining of the cross section through the syncytium of an AtSUC2 promoter∷GFP plant decorated with anti-GFP antiserum (green fluorescence resulting from FITC-conjugated second antibody) and with the SE-specific monoclonal RS6 antiserum (red fluorescence results from TRITC-conjugated second antibody). Free GFP was detected in CCs and in some SEs (white arrows) as well as in the syncytium (asterisks). B, Identical staining as in A but in an AtSUC2 promoter∷tmGFP9 plant. The tmGFP9 protein was detected only in CCs (white arrows). No fluorescence was seen in the syncytium (asterisks). C, Higher magnification of the boxed area from A showing only the green fluorescence resulting from the immunolocalization of GFP. The blue arrow marks a cluster of cells from the vascular bundle. Large syncytial cells are marked with asterisks. D, Same section as in C showing only the red fluorescence resulting from the SE-specific RS6 antiserum. The white arrow marks a single SE. A faint red background reaction in all other cells is typical for this antibody. E, Merging images C and D identifies CCs (only green GFP fluorescence; blue arrow), SEs that do not contain GFP (only red RS6-derived fluorescence) and SEs that do contain GFP (the yellow staining results from GFP immunolocalization plus RS6-derived fluorescence; white arrow). F, Immunohistochemical staining of a cross section through the syncytium of an AtSUC2 promoter∷GFP plant (same syncytium as in A) decorated with anti-AtSUC2 antiserum (green fluorescence from FITC-conjugated second antibody) and with the SE-specific monoclonal RS6 antiserum (red fluorescence as in A). The AtSUC2 protein was detected only in CCs (white arrows) and not in the SEs or in the syncytium (asterisk). G, Higher magnification of the boxed area from F showing a section of a yellow xylem vessel on the left side, three CCs (green) labeled with anti-AtSUC2 antiserum, and eight SEs (red) labeled with the monoclonal RS6 antiserum. No green fluorescence is seen in the syncytial part shown in this section (asterisk). Yellow fluorescence in A, B and F results from the autofluorescence of cell wall phenolics. Scale bars are 50 μm in A, B and F, and 10 μm in C, D, E and G.
To confirm that the highly fluorescent, tiny cells around these syncytia represent exclusively CCs additional immunohistochemical analyses were performed using the SE-specific monoclonal antibody RS6 (Meyer et al., 2004). Double labeling revealed green anti-GFP antiserum-derived fluorescence (Fig. 3C) and red anti-RS6 antiserum-derived fluorescence (Fig. 3D) in the same cross section. Merging both pictures resulted in green staining of CCs, in red staining of SEs with no GFP, and in yellow staining of SEs containing GFP (Fig. 3E). Merging similar pictures of double-labeled syncytial sections from AtSUC2-promoter∷tmGFP2 or from AtSUC2-promoter∷tmGFP9 plants yielded no colocalization of both antibodies, indicating that the membrane-anchored GFP was present exclusively in CCs and could not traffic into the SEs (Fig. 4D). Diffusible GFP, however, can move symplastically from CCs into SEs and eventually into the syncytia (Fig. 3, A and E).
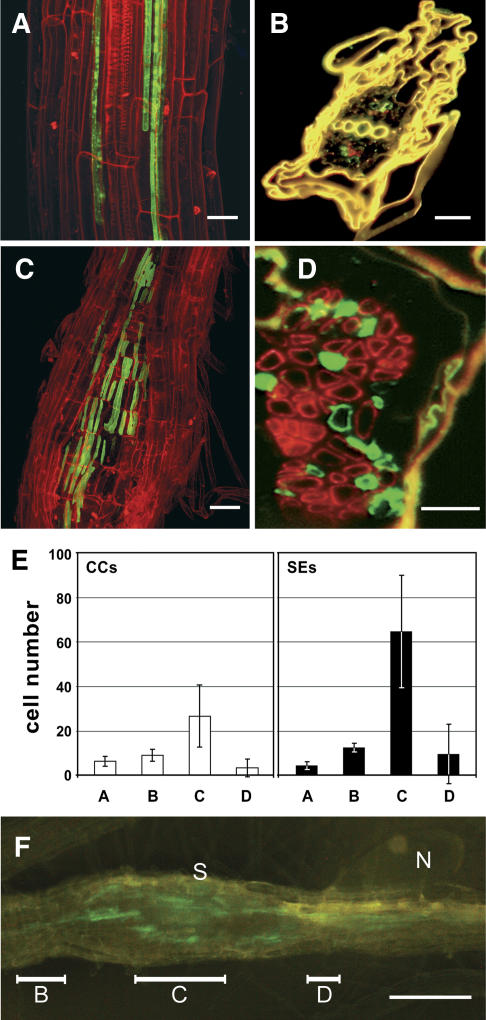
Figure 4.
The formation of syncytia triggers an increase in the numbers of SEs and CCs. A, Confocal image of tmGFP2 fluorescence (green) and in the 2 vascular strands typically seen in uninfected roots of AtSUC2-promoter∷tmGFP2 plants. B, Immunohistochemical staining of GFP and of SEs in an uninfected AtSUC2-promoter∷tmGFP2 root. The cross section was double labeled with anti-GFP antiserum (yielding green fluorescence in CCs) and with the SE-specific monoclonal RS6 antiserum (yielding red fluorescence). As shown in A, only very few cells of each type are present in the vasculature of uninfected Arabidopsis roots. The yellow color results from the autofluorescence of cell wall phenolics. C, Confocal image of tmGFP2 fluorescence in the phloem next to the syncytium of an AtSUC2-promoter∷tmGFP2 plant. D, Immunohistochemical staining of a cross section through the syncytium of a nematode-infected AtSUC2-promoter∷tmGFP2 root. The figure shows a section with numerous phloem cells that had been double labeled the using the same antibodies as in B. E, Numbers of CCs (white bars) and SEs (black bars) counted in cross sections from uninfected roots (A) or in cross sections from different syncytial regions: start of the syncytium (B), center of the syncytium (C), and region next to the nematode feeding site (D). F, Same syncytium as in Figure 1B with bars indicating the three regions used to determine CC and SE numbers in E (N, Nematode; S, syncytium). Roots shown in the confocal images A and C were treated with propidium iodide to visualize the cell walls. Scale bars represent 40 μm in A, 20 μm in B and C,10 μm in D and 200 μm in F.
AtSUC2 Protein Can Be Immunolocalized in CCs But Not in the Syncytium
In the paper of Jürgensen et al. (2003), a 650-bp AtSUC2 mRNA fragment was PCR amplified from a syncytial-mRNA preparation. It has, however, not been shown if full-length AtSUC2 mRNA is also present within the syncytia, and it cannot really be excluded that the amplified mRNA is a contamination from nonsyncytial cells or tissue. Nevertheless, this observation (Jürgensen et al., 2003) made it necessary to confirm the conclusions drawn from Figure 3, A and B, by an independent approach. To this end, we used sections from the same syncytium of an AtSUC2 promoter∷GFP plant that had been used for the immunodetection of GFP (Fig. 3A) and performed a double labeling with anti-AtSUC2 antiserum and the SE-specific RS6 antibody. Figure 3, F and G, shows that in contrast to the soluble GFP, the AtSUC2 protein is detected only in the CCs and not in the syncytium.
This immunolocalization of AtSUC2 suggests that there is no activity of the AtSUC2 promoter within the syncytia and that syncytial GFP has moved into these syncytia from the phloem. The syncytial AtSUC2 mRNA detected by Jürgensen et al. (2003) may also represent the result of mRNA movement from SEs into syncytia, which may occur via the large connecting plasmodesmata during the removal of syncytial content. In fact, plasmodesmal movement of Suc transporter mRNAs from CCs into SEs has been shown before (Kühn et al., 1997).
Analyses of de Novo Formation of SEs and CCs after Nematode Infection
Transgenic plants expressing in their CCs GFP or tmGFPs, which encode proteins that are either mobile or trapped within these CCs, do not only allow studies of the symplastic connectivity between the phloem and certain sink tissues. They also provide an elegant tool to investigate phloem formation and differentiation in nematode-infested roots.
The data presented in Figure 3 demonstrate that the number of phloem cells increased after infection and during formation of syncytia, an observation that has already been described by Golinowski et al. (1996). In fact, sections through healthy, uninfected areas of the roots possess only very few SEs and CCs (Fig. 4A) and all of these SEs and CCs are located in two phloem files, representing the situation in uninfected Arabidopsis roots (Fig. 4B). In contrast, irregular clusters with different, but clearly increased, numbers of SEs and CCs were found in all parts of roots adjoining syncytial complexes (Fig. 4C). For a detailed description and quantification of these newly formed cell types, we used an immunohistochemical approach to identify and visualize SEs and CCs in different parts of roots from AtSUC2-promoter∷tmGFP2 plants infected with beet cyst nematodes. Double labeling with anti-GFP antiserum and the monoclonal RS6 antibody labels specifically either the CCs, where GFP is synthesized and targeted to the membrane system, or the SEs (RS6).
For quantification, the SEs and CCs were counted in several sections obtained from independent uninfected or syncytial regions and the results are summarized in Figure 4E. The low numbers of CCs (white bars) and SEs (black bars) in cross sections from uninfected roots (6.4 ± 2.3 CCs and 4.5 ± 1.8 SEs/cross section; n = 5) are strongly increased in those parts of the root vascular tissue surrounding the central part of the syncytia (region C in Fig. 4F). Interestingly, the increase in CCs (about 4-fold; 26.7 ± 13.9 CCs/cross section; n = 5) is less pronounced than the increase in SEs (about 15-fold; 64.7 ± 25.3 SEs/cross section; n = 5), which underlines the specific role of SEs in symplastic-phloem unloading. This massive increase in phloem cell types is restricted to the center of the syncytia. At the syncytial endings (region B in Fig. 4F) and close to the nematode infection site (region D in Fig. 4F), no or only a marginal increase in the numbers of CCs and SEs is observed.
DISCUSSION
In this paper, the activity of the promoter of the AtSUC2 Suc transporter gene was studied in Arabidopsis roots infected with the beet cyst nematode H. schachtii. In contrast to an earlier report (Jürgensen et al., 2003), promoter activity was found exclusively in the CCs and not inside the syncytia. Consequently, GFP fluorescence that has previously been observed inside these syncytia and that has been discussed to result from AtSUC2 promoter activity must be a consequence of GFP movement from the CCs into these syncytia. Therefore, the symplastic connectivity between the syncytia and the adjacent unloading phloem was analyzed, the SEL of the connecting plasmodesmata was determined, and the differentiation of new phloem SEs and CCs during syncytia formation was investigated.
Our data provide clear evidence that H. schachtii-induced syncytia and the surrounding phloem are connected via functional plasmodesmata with an SEL of about 30 kD. The presented data also show that the formation of the syncytial sink induces the de novo formation of a typical unloading phloem with more SEs than CCs. Our observations suggest that assimilate import into syncytia occurs via the symplastic pathway and that phloem SEs are responsible for assimilate unloading.
Arabidopsis Root Phloem and Syncytia Possess Functional Symplastic Connections
It was known from numerous analyses that in noninfected Arabidopsis plants, AtSUC2 is expressed most strongly in the CCs of the phloem (Truernit and Sauer, 1995; Stadler and Sauer, 1996; Imlau et al., 1999; Stadler et al., 2005). In Arabidopsis rosettes, this expression is confined to the vascular tissue of source leaves, whereas sink leaves show no AtSUC2 expression in their CCs. In contrast, the AtSUC2 promoter is active in the CCs of roots, a typical sink tissue (Truernit and Sauer, 1995; Stadler and Sauer, 1996), and it has been proposed that in roots AtSUC2 may play a role in Suc unloading (Truernit and Sauer, 1995). The high preference of the AtSUC2 promoter for CCs made it a useful and elegant tool for analyses of cell-to-cell movement of GFP within and out of the phloem. Imlau and coworkers (Imlau et al., 1999) showed that GFP expressed under the control of this promoter can move from the CCs into the SEs and migrate toward the different sinks, where it is eventually unloaded and distributed by cell-to-cell transport. In subsequent analyses, microinjection of GFP or of GFP mRNA as well as AtSUC2-driven expression of GFP or of different GFP-fusions were used to monitor the development of plasmodesmata and the modulation of their SEL, to correlate GFP movement with long-distance allocation and distribution of assimilates, or to determine the symplastic connectivity between specific cell types, tissues, or even between plants and the holoparasite Cuscuta reflexa (Oparka et al., 1999; Haupt et al., 2001; Ayre et al., 2003; Wright et al., 2003; Stadler et al., 2005). All of these analyses clearly demonstrated that microscopic and confocal monitoring of the cell-to-cell movement of GFP and of GFP fusions represents a reliable, noninvasive approach for the determination of SELs of plasmodesmata, and that GFP movement is a perfect indicator for assimilate and macromolecular trafficking through these structures.
In this paper, this approach was extended toward analyses of the symplastic connectivity between the root phloem and nematode-induced syncytia. Our data demonstrate (1) that there are symplastic connections between the root phloem and the syncytium and (2) that the SEL of the plasmodesmata forming these symplastic connections are large enough (almost 30 kD) to allow the cell-to-cell movement of GFP (27 kD), but not of larger fusions, such as GFP-UBI (36 kD) or GFP-SPOR (47 kD).
It has been discussed before (Stadler et al., 2005) that the molecular mass of GFP or of GFP fusions is not the ideal unit to measure the SEL of plasmodesmata. The fusion product of globular GFP (Stokes radius = 2.82 nm; Terry et al., 1995) with another globular protein, such as UBI (Stokes radius about 1.2 nm) or SPOR (Stokes radius about 2.2 nm), will not result in one larger globule but rather in a dimer of 2 globules. Therefore, it is not only the increased molecular mass but also the altered shape that influences the movement of GFP fusions through plasmodesmata. However, in the absence of structural information on these fusion proteins, their molecular masses (in kD) are a crude but generally accepted unit for describing the SELs of plasmodesmata.
Previous data showing movement of CF into H. schachtii-induced syncytia were in line with such a symplastic path between the host phloem and the syncytia (Böckenhoff et al., 1996). However, electron microscopic analyses of the cell walls in these tissues showed only very few plasmodesmata, and these seemed to be nonfunctional (Grundler et al., 1998). Finally, microinjection analyses of low-molecular-weight fluorescent dyes into syncytia were interpreted as additional proof for a symplastic isolation of this structure, because in no case could a spreading of fluorochromes from injected syncytia be observed (Böckenhoff and Grundler, 1994; Böckenhoff et al., 1996).
Our data clearly contradict this apoplastic model for assimilate import into syncytia. We speculate that the observed lack of CF efflux (Böckenhoff et al., 1996) is the consequence of a symplastic mass flow from the phloem into the syncytia, which results from the permanent withdrawal of syncytial content by the parasite and prevents diffusion of CF in the opposite direction.
New Phloem Is Formed during Syncytia Formation
During the development of syncytial, both the anatomy and morphology of the root change extensively. In syncytia induced by male juveniles, an increase of SEs from 2 to a maximum of 15 has been published (Golinowski et al., 1996), and the same authors also described a general increase in the number of phloem and cambial cells next to the syncytia induced by female nematodes. However, these authors did not distinguish between individual cell types. Our immunohistochemical analyses (Fig. 4, B and D) allow a direct quantification of both CCs and SEs, and show (1) the differential de novo formation of these cells and (2) that the maximal number of these cells is formed around the center of the syncytia (Fig. 4E).
The Newly Formed Phloem Has Unloading Phloem Properties
The detected SEL of about 30 kD between the phloem and the syncytial complex is similar to that described for the plasmodesmata in the unloading phloem at the very tip of Arabidopsis roots (Stadler et al., 2005). In this tissue, where phloem unloading occurs from the protophloem, only the free form of GFP (27 kD) but none of the tested GFP fusions were able to exit the phloem (Stadler et al., 2005). Moreover, CCs are lacking in the protophloem of Arabidopsis root tips, indicating that unloading is mediated only by SEs (Esau, 1969; Stadler et al., 2005). Interestingly, the new phloem formed during the formation of syncytia contains much more SEs (70%–75% after a 15-fold increase in number) than CCs (25%–30% after a 4-fold increase in number). In contrast, no increased number of SEs (Fig. 4) and only little symplastic unloading of CF (Oparka et al., 1994) or of GFP (Stadler et al., 2005) was found in the transport phloem of uninfected roots. This suggests that symplastic unloading into the syncytia occurs mainly or even exclusively from the newly formed SEs and not or only to a limited extent from the CCs (Figs. 3E and 4, D and E). Moreover, these results demonstrate that the newly formed phloem differs from the transport phloem in uninfected roots both with respect to the SELs of its plasmodesmata and with respect to the number of SEs.
Symplastic unloading of solutes into pathogen-induced feeding structures has previously been proposed for other nematodes, such as Meloidogyne incognita and Criconemella xenoplax (Hussey et al., 1992; Dorhout et al., 1993). Moreover, a functional symplastic pathway has been demonstrated between several host plants and the holoparasite C. reflexa (Haupt et al., 2001).
In the paper of Jürgensen et al. (2003), β-glucuronidase (GUS) histochemical staining has been observed inside the syncytia of AtSUC2 promoter∷GUS plants, although the AtSUC2 promoter is not active in these cells (this paper). However, the observed GUS staining cannot result from diffusion of GUS into these syncytia for 2 reasons: (1) because GUS had been fused to a 159-bp fragment of the AtSUC2 sequence that encoded a membrane anchor; and (2) because functional GUS forms a homotetramer of 4 68-kD proteins. Even the monomers are unlikely to move through plasmodesmata with a SEL of 30 kD. We, therefore, suggest that the observed staining results from diffusion of hydrolyzed dye into the syncytia.
In this context, it should be mentioned that in independent analyses of other GUS reporter plants the promoters of several vascular tissue-expressed genes were also described to be active in cyst nematode-induced syncytia (Barthels et al., 1997; de Almeida Engler et al., 1999; Puzio et al., 2000). If our interpretation is right, there is a possibility that these localizations are also due the diffusion of dye and not to the activities of the analyzed promoters. For future analyses, membrane-anchored GFPs may represent a better tool to study promoter activities in or around tissues having plasmodesmata with large SELs.
Is the de Novo Formation of Phloem Induced by Increased Sink Strength?
During the early stages of cyst nematode infection, genes encoding cyclins and cyclin-dependent kinases show promoter induction in the syncytium but also in neighboring cells. In addition, an activation of these genes was observed in consecutively forming lateral roots close to the infection site (Niebel et al., 1996; de Almeida Engler et al., 1999). In contrast to the syncytia, where this gene induction is transient, expression of these genes persists much longer in the neighboring cells. In the syncytial complex, the activity of these cell cycle genes is believed to be necessary for the incorporation of cells into this complex (de Almeida Engler et al., 1999). In the vascular cylinder, these cyclins and cyclin-dependent kinases may modulate cell division in the phloem and contribute to the observed increase in SEs and CCs.
The formation of nematode feeding structures was also shown to increase the root levels of auxin and cytokinin that in turn may trigger cell differentiation (Kochba and Samish, 1972). The same two phytohormones promote vascularization at sites infected with Agrobacteria, and the resulting extended-vascular network provides growing tumors with nutrients (Ullrich and Aloni, 2000; Veselov et al., 2003). Transport studies in Agrobacterium-infected tissues with fluorescent dyes clearly demonstrated that assimilates were unloaded symplastically from this newly formed phloem into the parenchyma cells of the tumor indicating a functional connection via plasmodesmata (Pradel et al., 1996, 1999). Thus, our results demonstrate that, in addition to Agrobacterium-induced tumors, nematode-induced syncytia may represent a system to study the possible phytohormone signaling related to phloem differentiation. It will be interesting to see if the signals causing this differentiation process are delivered directly from the nematode, if nematode-derived factors induce hormone synthesis, or if the newly formed sink per se is sufficient to trigger its own supply with assimilates.
MATERIALS AND METHODS
Strains, Growth Conditions, and Nematode Infection
Five transgenic Arabidopsis (Arabidopsis thaliana) lines expressing GFP or GFP fusions under the control of the AtSUC2 promoter (Stadler et al., 2005) were used for infection with the beet cyst nematode Heterodera schachtii. Seeds of these lines were surface sterilized in 1.5% sodium hypochloride and 0.05% Tween 20 for 5 min and washed 3 times with sterile water. Sterilized seeds were resuspended in 0.1% agarose and sown in petri dishes containing Murashige and Skoog medium (Murashige and Skoog, 1962; pH 5.7, 1% Suc, 0.8% phyto agar). After 4 d of vernalization (4°C) plates were incubated in a growth chamber under long-day conditions (16-h light/8-h dark) at 22°C and 70% relative humidity. After 4 to 5 d, seedlings were transferred to petri dishes containing a modified Knop nutrient medium (Sijmons et al., 1991) and grown for an additional 4 to 5 d. For nematode infection of the roots of these seedlings, nematode cysts were harvested from sterile agar stock cultures, sterilized in 1.5% sodium hypochloride and 0.05% Tween 20 for 5 min, washed 3 times with sterile water, and kept in 3-mm ZnCl2 solution at 22°C to 26°C. After 5 d, freshly hatched beet cyst nematodes (second stage juveniles [J2]) were treated with 0.05% HgCl2 for 3 min, washed 3 times with sterile water, and used for inoculation of plant cultures.
Immunohistochemistry
Arabidopsis roots with or without syncytia were prepared, fixed in ethanol/acetic acid (3:1), embedded in methacrylate, sectioned into 3-μm slices (Leica Ultracut R, Leica Microsystems, Bensheim, Germany), and transferred to adhesion microscope slides (Linaris, Wertheim, Germany) as described (Stadler and Sauer, 1996). Embedding was performed in ethanol/methacrylate (1:1) for 30 min followed by a second incubation in pure methacrylate for 2 d. Polymerization was achieved by UV-light irradiation (310 nm) for 15 h. After removal of methacrylate by incubation in 100% (v/v) acetone for 3 min, sections were rehydrated in ethanol of decreasing concentration (100%, 70%, and 30% [w/v]). Blocking of the sections for 1 h in 50 mm Tris-HCl, pH 7.5, 150-mm NaCl, and 1% (w/v) skim milk powder was followed by an overnight incubation with affinity-purified monoclonal mouse RS6-antibody (diluted 1:3 in blocking solution; Meyer et al., 2004), with polyclonal rabbit anti-GFP antiserum (diluted 1:500 in blocking solution, Abcam, Cambridge, UK) or with affinity-purified, polyclonal anti-AtSUC2 antiserum (diluted 1:10 in blocking solution; Stadler and Sauer, 1996). After this incubation, sections were washed five times in blocking solution. Incubations with anti-mouse IgG-tetramethylrhodamine isothiocyanate (TRITC)-isomer conjugate and anti-rabbit IgG-fluorescein isothiocyanate (FITC)-isomer (both diluted 1:300; Sigma-Aldrich, Deisenhofen, Germany) were performed for 1 h. After incubation with the secondary antibody, sections were washed five times in blocking solution and mounted in anti-fading medium (ProLong Antifade Kit, Molecular Probes, Leiden, The Netherlands).
Epifluorescence and Confocal Laser-Scanning Microscopy
GFP fluorescence was monitored after excitation with light of 460 to 500 nm using an epifluorescence stereomicroscope (Leica MZFLIII, Leica Microsystems) and a Color View II camera controlled by the analySIS Doku 3.2 imaging software (Soft Imaging Systems, Münster, Germany). Emitted fluorescence was detected at wavelengths above 510 nm. High-resolution images of GFP fluorescence were made with a confocal laser-scanning microscope (Leica TCS SP II, Leica Microsystems) as described (Meyer et al., 2004; Stadler et al., 2005). Confocal images were processed using the Leica Confocal Software 2.5 (Leica Microsystems) and presented as maximum projection or optical z-sections. Immunohistochemical analyses were performed on an epifluorescence microscope (Zeiss Axioskop, Carl Zeiss, Jena, Germany) in combination with a Sony 3CCD color video camera (Sony, Tokyo) controlled by Imaging System KS200 software (Kontron Elektronik, Munich). FITC was excited with 460- to 500-nm light and fluorescence was detected at 510 to 560 nm. TRITC was excited with 510- to 560-nm light and fluorescence was detected at 573 to 648 nm. For cell wall staining, roots grown on the agar surface were covered with a drop of 0.5% propidium iodide and incubated for 10 min at room temperature. After two washes with water, roots were usually imaged directly on the petri dishes. Propidium iodide-stained cell walls were detected with the argon laser 488-nm line using a detection window of 595 to 640 nm.
Acknowledgments
We are grateful to Florian Grundler (Universität für Bodenkultur, Vienna) for providing sterile stock cultures of H. schachtii and to Ruth Stadler (Molecular Plant Physiology, University of Erlangen-Nürnberg, Germany) for helpful comments on the manuscript.
This work was supported by the Körber Foundation (Körber European Science Award 2001 to N.S.) and by the Deutsche Forschungsgemeinschaft (grant nos. SA 382–8/1 and 2 to N.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.058800.
References
- Ayre BG, Keller F, Turgeon R (2003) Symplastic continuity between companion cells and the translocation stream: Long-distance transport is controlled by retention and retrieval mechanisms in the phloem. Plant Physiol 131: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthels N, van der Lee FM, Klap J, Goddijn OJ, Karimi M, Puzio P, Grundler FM, Ohl SA, Lindsey K, Robertson L, et al (1997) Regulatory sequences of Arabidopsis drive reporter gene expression in nematode feeding structures. Plant Cell 9: 2119–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böckenhoff A, Grundler FMW (1994) Studies on the nutrient uptake by the beet cyst nematode H. schachtii by in situ microinjection of fluorescent probes into the feeding structures in Arabidopsis thaliana. Parasitology 109: 249–254 [Google Scholar]
- Böckenhoff A, Prior DA, Grundler FM, Oparka KJ (1996) Induction of phloem unloading in Arabidopsis thaliana roots by the parasitic nematode Heterodera schachtii. Plant Physiol 112: 1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford KM, Zambryski PC (2001) Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol 125: 1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL Jr, Inze D, Van Montagu M, Engler G, Gheysen G (1999) Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 11: 793–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhout R, Gommers FJ, Kollöffel C (1993) Phloem transport of carboxyfluorescein through tomato roots infected with Meloidogyne incognita. Physiol Mol Plant Pathol 43: 1–10 [Google Scholar]
- Esau K (1969) The phloem. In W Zimmermann, P Ozenda, HD Wulff, eds, Handbuch der Pflanzenanatomie. Gebrüder Borntraeger Verlag,Berlin
- Escobar C, De Meutter J, Aristizabal FA, Sanz-Alferez S, del Campo FF, Barthels N, Van der Eycken W, Seurinck J, van Montagu M, Gheysen G, et al (1999) Isolation of the LEMMI9 gene and promoter analysis during a compatible plant-nematode interaction. Mol Plant Microbe Interact 12: 440–449 [DOI] [PubMed] [Google Scholar]
- Goddijn OJ, Lindsey K, van der Lee FM, Klap JC, Sijmons PC (1993) Differential gene expression in nematode-induced feeding structures of transgenic plants harbouring promoter-gusA fusion constructs. Plant J 4: 863–873 [DOI] [PubMed] [Google Scholar]
- Golinowski W, Grundler FMW, Sobczak M (1996) Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma 194: 103–116 [Google Scholar]
- Grundler FMW, Sobczak M, Golinowski W (1998) Formation of wall openings in root cells of Arabidopsis thaliana following infection by the plant-parasitic nematode Heterodera schachtii. Eur J Plant Pathol 104: 545–551 [Google Scholar]
- Haupt S, Oparka KJ, Sauer N, Neumann S (2001) Macromolecular trafficking between Nicotiana tabacum and the holoparasite Cuscuta reflexa. J Exp Bot 52: 173–177 [PubMed] [Google Scholar]
- Hermsmeier D, Hart JK, Byzova M, Rodermel SR, Baum TJ (2000) Changes in mRNA abundance within Heterodera schachtii-infected roots of Arabidopsis thaliana. Mol Plant Microbe Interact 13: 309–315 [DOI] [PubMed] [Google Scholar]
- Hussey RS, Mims CW, Sobczak M (1992) Changes in the structure of Arabidopsis thaliana during female development of the plant parasitic nematode Heterodera schachtii. Protoplasma 167: 55–65 [Google Scholar]
- Imlau A, Truernit E, Sauer N (1999) Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 11: 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MGK (1981) The development and function of plant cells modified by endoparasitic nematodes. In BM Zuckerman, RA Rohde, eds, Plant Parasitic Nematodes. Academic Press, New York, 255–279
- Jürgensen K, Scholz-Starke J, Sauer N, Hess P, van Bel AJ, Grundler FM (2003) The companion cell-specific Arabidopsis disaccharide carrier AtSUC2 is expressed in nematode-induced syncytia. Plant Physiol 131: 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochba J, Samish RM (1972) Levels of endogenous cytokinins and auxin in roots of nematode resistant and susceptible peach root-stocks. J Am Soc Hortic Sci 97: 115–119 [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298–1300 [DOI] [PubMed] [Google Scholar]
- Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N (2004) Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiol 134: 684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Niebel A, de Almeida Engler J, Hemerly A, Ferreira P, Inze D, Van Montagu M, Gheysen G (1996) Induction of cdc2a and cyc1At expression in Arabidopsis thaliana during early phases of nematode-induced feeding cell formation. Plant J 10: 1037–1043 [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Duckett CM, Prior DAM, Fisher DB (1994) Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J 6: 759–766 [Google Scholar]
- Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts I, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel B (1999) Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97: 743–754 [DOI] [PubMed] [Google Scholar]
- Pradel KS, Rezmer C, Krausgrill S, Rausch T, Ullrich CI (1996) Evidence for phloem unloading with a concomitant high level of acid cell-wall invertase in Agrobacterium tumefaciens-induced plant tumours. Bot Acta 109: 397–404 [Google Scholar]
- Pradel KS, Ullrich CI, Santa Cruz S, Oparka KJ (1999) Symplastic continuity in Agrobacterium tumefaciens-induced tumours. J Exp Bot 50: 183–192 [Google Scholar]
- Puthoff DP, Nettleton D, Rodermel SR, Baum TJ (2003) Arabidopsis gene expression changes during cyst nematode parasitism revealed by statistical analyses of microarray expression profiles. Plant J 33: 911–921 [DOI] [PubMed] [Google Scholar]
- Puzio PS, Lausen J, Heinen P, Grundler FM (2000) Promoter analysis of pyk20, a gene from Arabidopsis thaliana. Plant Sci 157: 245–255 [DOI] [PubMed] [Google Scholar]
- Sauer N, Stolz J (1994) SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker's yeast and identification of the histidine tagged protein. Plant J 6: 67–77 [DOI] [PubMed] [Google Scholar]
- Schneidereit A, Scholz-Starke J, Büttner M (2003) Functional characterization and expression analyses of the glucose-specific AtSTP9 monosaccharide transporter in pollen of Arabidopsis. Plant Physiol 133: 182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P, Grundler FMW, von Mende N, Burrows P, Wyss U (1991) Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J 1: 245–254 [Google Scholar]
- Sobczak M, Golinowski W, Grundler FMW (1997) Changes in the structure of Arabidopsis thaliana roots induced during development of males of the plant parasitic nematode Heterodera schachtii. Eur J Plant Pathol 103: 113–124 [Google Scholar]
- Stadler R, Brandner J, Schulz A, Gahrtz M, Sauer N (1995) Phloem loading by the PmSUC2 sucrose carrier from Plantago major occurs into companion cells. Plant Cell 7: 1545–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Sauer N (1996) The Arabidopsis thaliana AtSUC2 gene is specifically expressed in companion cells. Bot Acta 109: 299–306 [Google Scholar]
- Stadler R, Wright MW, Lauterbach C, Ammon G, Gahrtz M, Feuerstein A, Oparka KJ, Sauer N (2005) Expression of GFP-fusions in Arabidopsis companion cells reveals extensive non-specific protein trafficking into sieve elements, and identifies a novel post-phloem domain in roots. Plant J 41: 319–331 [DOI] [PubMed] [Google Scholar]
- Terry BR, Matthews EK, Haseloff J (1995) Molecular characterisation of recombinant green fluorescent protein by fluorescence correlation microscopy. Biochem Biophys Res Commun 217: 21–27 [DOI] [PubMed] [Google Scholar]
- Thurau T, Kifle S, Jung C, Cai D (2003) The promoter of the nematode resistance gene Hs1pro-1 activates a nematode-responsive and feeding site-specific gene expression in sugar beet (Beta vulgaris L.) and Arabidopsis thaliana. Plant Mol Biol 52: 643–660 [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N (1995) The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of beta-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta 196: 564–570 [DOI] [PubMed] [Google Scholar]
- Ullrich CI, Aloni R (2000) Vascularization is a general requirement for growth of plant and animal tumours. J Exp Bot 51: 1951–1960 [DOI] [PubMed] [Google Scholar]
- Veselov D, Langhans M, Hartung W, Aloni R, Feussner I, Gotz C, Veselova S, Schlomski S, Dickler C, Bachmann K, et al (2003) Development of Agrobacterium tumefaciens C58-induced plant tumors and impact on host shoots are controlled by a cascade of jasmonic acid, auxin, cytokinin, ethylene and abscisic acid. Planta 216: 512–522 [DOI] [PubMed] [Google Scholar]
- Wright KM, Roberts AG, Martens HJ, Sauer N, Oparka KJ (2003) Structural and functional vein maturation in developing tobacco leaves in relation to AtSUC2 promoter activity. Plant Physiol 131: 1555–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss U (1992) Observation on the feeding behaviour of Heterodera schachtii throughout development, including events during molting. Fundam Appl Nematol 15: 75–89 [Google Scholar]