Abstract
Transitional cell carcinoma (TCC) is the commonest cancer of the bladder. Although majority of TCC can be diagnosed at an early stage and removed easily by transurethral resection of tumor (TURT), the management of this carcinoma is complicated due to frequent recurrences usually within 6 months to one-year period. An imbalance between the Th1 and Th2 immune responses has been attributed to immune dysregulation in various malignancies. The present study aims to evaluate the Th1 and Th2 balance in Peripheral Blood Mononuclear Cells of 41 TCC patients (20 recurrent and 21 non-recurrent) using flow cytometry. It also further assesses immunological and cellular factors influencing the anti-neoplastic activity of the TCC patients and in 21 normal healthy subjects in terms of their cytokine expression and various cell surface markers. The findings of the study revealed that the cell surface markers CD3+, CD4+ and CD8+ along with NK cells were found to be significantly lower in patients than healthy controls (p<0.01). The mean percent expression of CD4+ was significantly lower in patients showing recurrence (23.9±9.84) as compared to patients with non-recurrence (31.1±12.27). The percentage of CD4+T-cells (mean±SD) producing IFN-γ, IL-2 and TNF-α were statistically significantly reduced in patients (19.1±4.94, 52.3±20.86 and 12.8±4.49) as compared to healthy controls (23.3±3.67, 67.5±12.0 and 17.6±5.96 respectively), (p<0.01, 0.018, 0.001). On the contrary, the mean levels of IL-4, IL-6 and IL-10 in patients (63.8±17.01, 60.4±14.79 and 65.7±14.84 respectively) were significantly higher as compared to healthy controls (24.4±8.77, 26.5±5.28 and 20.6±3.81 respectively), (p<0.001). No statistically significant difference was observed in the cytokine expression between patients showing recurrence and non-recurrence. Patients with bladder cancer seem to develop a Th2 dominant status with a deficient type1 immune response. The lymphocyte evaluation along with cytokine measurement can provide a sensitive and valuable tool for evaluating the function of cell-mediated immunity in these patients and can also find application in therapeutic monitoring of bladder cancer patients as new targets for immunotherapy.
Keywords: Flow cytometry, TCC, Cytokines
Introduction
Urinary bladder cancer represents a spectrum of neoplasms-superficial, invasive and metastatic, each requiring different primary management. Superficial transitional cell carcinoma (TCC) constitutes 90–95% of urothelial tumors [43] with recurrence rate of 30–90% [40, 46]. The treatment of such tumors aims to prevent recurrences and progression to an invasive stage.
Earlier report has shown that patients having bladder malignancies were immunodeficient [32]. It has been noted that depression of immune function in patients with advanced stage of bladder tumor worsens the prognosis and survival. This immunodeficiency manifests as impaired cell-mediated and humoral immunity as well as by impaired non-specific host defense mechanisms. Control of tumor growth presents a difficult problem for the host immune system. Increasing the ability of tumor reactive T-cells to mediate bladder tumor regression in vivo has been a major goal of tumor immunologists [24]. Even in view of results of previous studies, the role of immunological dysfunction has not been well elucidated for progression and recurrence of superficial bladder cancer.
According to the cytokine profile, the immune responses have been classified into 2 functionally distinct CD4+ T-cell subsets. The cytokines produced by the Th1 and Th2 cell subsets are important for the function and immune response of cytotoxic T-lymphocytes to regulate the differentiation of these cells. The characteristic cytokine products of Th1 and Th2 cells are mutually inhibitory for the differentiation and effector functions of the reciprocal phenotype. This cross regulation may partly explain the strong biases towards either Th1 or Th2 responses associated with many pathological conditions [14, 28].
Very few studies of the Th1 and Th2 balance have been performed in cancer patients [22, 35] moreover the role of two subsets in anti-tumor immunity in bladder cancer patients is also not clearly defined.
The ability to assess cytokine profile in different immune cells is fundamental to understand both normal immunoregulation and its dysfunction in superficial TCC of bladder. Profiles of cytokine reactivity have been extremely important determinants of disease outcome for a large number of infections. It has been made clear by many investigators that production of most cytokines is not confined to one cell type. Thus, a method to detect cytokines at the single cell level would be helpful to study the contribution of different cell types to cytokine production in heterogeneous cell populations in bladder cancer patients.
It has been observed that cellular level cytokine assessment has been problematic, relying on in situ hybridization, limiting dilution, plaque/ELISPOT or T-cell cloning techniques [23]. All these approaches are laborious and complex, and have practical difficulties. Investigators have usually measured plasma or culture supernatant cytokine titres, which reflect the combinations of many cells and/or a physiological environment. Moreover, this type of analysis does not measure cytokine profile at single cell level. Cytokine expression in T-cells has also been analyzed by RT-PCR, but the limitation of the type of analysis is that it does not give information about the contribution of different cells to cytokine production in a heterogeneous cell population. With technical advancement it has now become possible to investigate cytokine profile on a cellular level and concurrently examine other cell characteristics. Several groups have reported the use of mutiparameter flow cytometry for assessment of single cell intracellular cytokine production in human PBMCs [7, 15] and in mouse T-cells [2]. This present communication aims to evaluate the role of Th1 and Th2 subsets and their functional balance in progression and recurrence of superficial bladder cancer by flow cytometry.
Materials and methods
Patients and Healthy Subjects
Forty-one newly diagnosed patients (39 men and 2 women) with histologically confirmed superficial transitional cell carcinoma treated by transurethral resection followed by intravesical adjuvant chemo/immunotherapy formed the study group. Age range was 30–85 yrs; with a mean and SD of 59.4 and 10 years. Patients with tumors at stage (Ta or T1) of any grade were eligible in this study. Patients presenting with clinical features such as haematuria and CT scan, ultrasound or urine cytology suggesting malignancy were confirmed by histopathologic examination as superficial papillary transitional cell carcinoma (TCC pT1-T3) or carcinoma in situ (CIS). Blood samples were collected prior to surgery in all the patients. Transurethral resection of tumor was followed by adjuvant chemo/immunotherapy. Patients with muscle invasive or metastatic disease, or those associated with upper urinary tract transitional cell carcinoma were excluded from this study. Patients were followed up every third month for duration of 6 months to 3 years (median follow–up of 22.5 months) with urine cytology, cystoscopy, haematological status, U/S by transrectal probe and cystoscopic biopsy in case of recurrence. Twenty patients amongst this group showed recurrence of tumor within 6–18 months of follow-up (Recurrent) and the remaining 21 patients remained recurrence-free throughout the follow–up period (Non-recurrent). All the patients were treated for any urinary tract infection before being included in the study. Controls constituted 32 normal healthy subjects (laboratory personnel) who volunteered to donate 10 ml of blood for this study. Of these 32 subjects, 11 individuals who had past history of major illness, recent viral infections, urinary tract infections, or lymphadenopathies and other metabolic disorders like diabetes and hypertension during the last 6 months of this study were strictly excluded. Thus a total of twenty-one healthy subjects, whose age ranged from 25 to 55 years were used as controls (Male 20, Female 1). Informed consent and ethical committee clearance was granted for the study.
Blood Collection
Heparinized peripheral venous blood (5–10 ml) was collected by venipuncture from bladder cancer patients and normal healthy volunteers and transported immediately to the laboratory, in a sterile tube at room temperature for isolation of the PBMCs.
Isolation of PBMC from Peripheral Blood
Heparinized (GIBCO-BRL, Grand Island, N.Y.) peripheral blood was obtained from all patients and healthy subjects. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (SIGMA-Aldrich, St. Louis, MO.) density gradient centrifugation [4]. The PBMCs were then washed twice with phosphate buffered saline (pH 7.4). The cells were counted in a haemocytometer and the viability was determined by the trypan blue dye exclusion test. Viability of the cells was 95% and above in all the samples.
Cell Culture
PBMCs (0.5×106 –1×106/ml) were cultured in 96 well flat bottom plates (NUNC, Wiesbaden, Germany) for 24 hrs at 37°C and 5% CO2 in RPMI-1640 medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 IU/ml Penicillin, 100 μg/ml Streptomycin, 2×10−5 M mercaptoethanol and 10% v/v autologous serum. Cells were stimulated with PMA (phorbol 12-myristate 13-acetate; 2 ng/ml) and ionomycin (1 μM), PHA 2.4 μg/ml in the presence of 1 μg/ml Brefeldin A (BFA), (Golgi PlugTM, Pharmingen, Becton Dickinson Mountain View, Calif.) a carboxylic ionophore that blocks the transport of cytokines in a pre-golgi compartment [18, 33, 36]. Brefeldin A was added for a duration of 6 hrs before the termination of the culture. It inhibits intracellular traffic pathways leading to the accumulation of protein and subsequently enhanced fluorescence signal.
Staining for Cell Surface Markers and Intracellular Cytokines
Following activation, 0.5×105 PBMC’s (approximately) were incubated in 50 μl of staining buffer (0.1 M PBS, pH 7.2–7.4) containing 0.1% Bovine serum albumin (BSA) and 0.01% NaN3 (Sigma Aldrich, USA.) in an optimal concentration of (0.5 μg/5 μl) FITC-conjugated anti-human CD69, CD3, CD4, CD8 and CD56 MAbs (Pharmingen, Becton Dickinson, Mountain View, Calif) for cell surface markers according to the manufacturers instructions. The cell activation marker CD69 was used to determine the activation of stimulated PBMCs, and it was observed that majority of cells >85% were activated. Cells were then fixed in 500 μl of 2% paraformaldehyde (PFA, Sigma) in 0.1 M PBS and were kept at room temperature in dark for 20 min. After incubation, cells were washed twice in staining buffer and pelleted by centrifugation.
Cells were then resuspended in 100 μl Cytofix/Cytoperm TM Solution (2090 KZ Cytofix/Cytoperm PlusTM kit, Pharmingen, BD Mountain View, calif.) and permeabilized for 20 min. at 4°C in dark. Fixed/Permeabilized cells were resuspended in 50 μl of 1X Perm/Wash solution (Cytofix/Cytoperm PlusTM kit, Pharmingen, BD Mountain View, Calif.) and incubated with an optimal concentration (0.5 μg/5 μl) of a PE-conjugated anti-cytokine antibodies (anti-Human IFN-γ, IL-2, IL-4, IL-6, IL-10 and TNF-α (Pharmingen, BD Mountain View, Calif). FITC-conjugated mouse IgG2a and PE-conjugated mouse IgG1 were used as controls. Stained cells were washed twice with Perm/Wash Solution and fixed in 2% PFA and acquired immediately on a FACS Calibur.TM (Becton Dickinson, Mountain View, U.S.A.)
Data Analysis
A FACS Calibur TM flow cytometer (Becton Dickinson, Mountain View, U.S.A.) equipped with a 15 mW argon ion laser and filter settings for FITC, PE were used in this study. The flow cytometer was calibrated and compensated using CaliBRITETM beads using FACSCOMP software according to the manufacturer’s recommendations. Total 10,000 events were acquired for each sample. The compensation standards, used were lymphocytes stained with strongly–positive single-color monoclonal antibodies, eg. CD3 or CD4 or CD8 in separate tubes for each of the fluorochromes FITC and PE. Initial standardization of the technique was done on samples from control subjects. As a result of the differences in the cell size and granularity, light scattering separates the blood cells into three major populations: lymphocytes, monocytes and granulocytes. For each analysis, dot plot graphs of forward scatter versus side scatter were drawn, and a lymphocyte region was defined, separate dot plots of cytokine fluorescence were then constructed for CD3+, CD4+, and CD8+ T-cells. The percentage positive cells were recorded by carefully excluding all cellular debris, using a quadrant and identifying the number of antibody positive cells. The data have been expressed as the values of the actual percentage of cytokine expressing cells, i.e. double positive for CD4+ and cytokine positive.
Statistical analysis
The data obtained were tabulated and analyzed. All the quantitative parameters were expressed as percent mean±standard deviations. The mean values between the two groups were statistically tested for significance by employing Mann-Whitney U test, or the Kruscal Wallis test as appropriate. The relationship between the various Th1 and Th2 cytokines was determined using Pearson’s correlation test. The level of significance was set at p<0.05.
Results
Phenotypic characterization of PBMCs
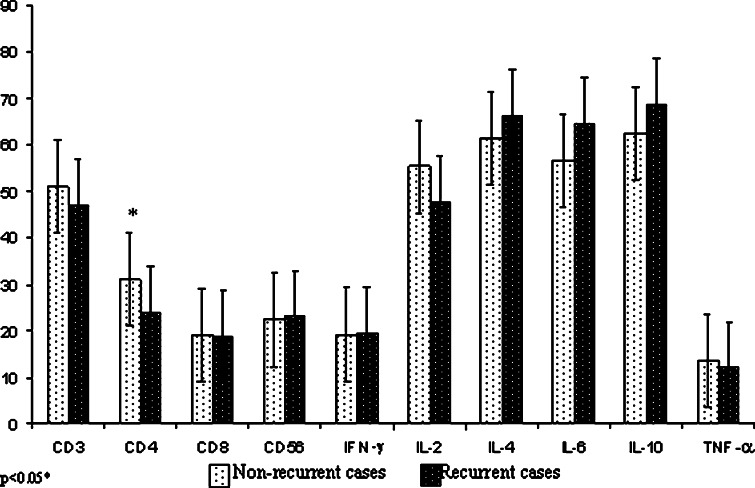
The percentages of CD3+T-cells and its CD4+ and CD8+ expressing subsets along with CD56 for NK cells were examined in both unstimulated (data not shown) and stimulated PBMCs of 41 patients with superficial TCC of bladder and 21 normal healthy subjects. The mean percentages of CD3+, CD4+ and CD8+ expressing T-cells were significantly reduced in patients (48.8±16.75, 27.6±11.91, 18.8±9.27 respectively) as compared to healthy subjects, (72.8±4.0, 42.4±2.61, 22.8±5.52 respectively) and the mean difference was statistically significant between the two groups (p<0.01, p<0.01& p<0.05). The mean percentage of CD56 expressing NK cells was also significantly lower in patients 22.5±13.11 as compared to normal healthy volunteers 26.2±6.95 (p<0.01) (Fig. 1). The mean CD3 values were lower in patients who had recurrence of the disease (47.0±16.01) as compared to the group of patients with non-recurrence of the disease (51.1±17.02) (Fig. 2). The mean CD3 value was highest in normals (72.8±4.00), than in non-recurrent (51.1±17.02) and recurrent group (47.0±16.01) in decreasing order. Similarly, the mean CD4 values also showed a decreasing trend between normal (42.4±2.61), non-recurrent (31.1±12.26) and recurrent groups (23.9±9.80) of patients (Table 1). An intercomparison of the differences in the mean CD3 and CD4 values amongst the normals and different patient groups showed similar statistical significance (p<0.001). No statistical significance were obtained for the difference in the means of CD3, CD8 and CD56 values between the recurrent and non-recurrent group of patients however, significant difference was observed in the mean CD4 values amongst these groups (Fig. 2). Patients showing recurrence of the disease showed significantly lower (p<0.04) mean CD4+ value as compared to the non-recurrent patients. The difference in the mean values of CD3+, CD4+, and CD56+ between normals and recurrent patients (p<0.001, p<0.001, p<0.05 respectively) as well as normals and non-recurrent patients were statistically significant. However, the difference in the mean value of CD8 was found statistically significant only between normal and recurrent patients (p<0.02) (Table 1).
Fig. 1.
Mean and standard deviation of the phenotypic characterization and the frequency of CD4+T-cells producing Th1 and Th2 cytokines in healthy controls and bladder cancer patients
Fig. 2.
Mean and standard deviation of the phenotypic characterization and the frequency of CD4+T-cells producing Th1 and Th2 cytokines in recurrent and non-recurrent bladder cancer patients
Table 1.
Mean and SD (%) of phenotypic characterization and cytokines expression in patients and normals
| Mean±SD% | CD3 | CD4 | CD6 | CD66 | IFN-γ | IL-3 | IL-4 | IL-6 | IL-10 | TNF-α |
|---|---|---|---|---|---|---|---|---|---|---|
| Normals (n=31) | 72.8±4.00 | 42.4±2.61 | 22.8±5.52 | 26.2±6.95 | 23.3±3.67 | 67.5±12.0 | 24.4±8.77 | 26.5±5.28 | 20.6±3.81 | 17.6±5.96 |
| Patients (n=41) | 48.8±16.75 | 27.6±11.91 | 18.8±9.27 | 22.5±13.11 | 19.1±4.94 | 52.3±20.85 | 63.8±17.01 | 60.4±14.79 | 65.7±14.84 | 12.8±4.49 |
| Non-recurrent (n=31) | 51.1±17.02 | 31.1±12.26 | 19.1±7.14 | 22.4±13.26 | 19.2±5.10 | 55.4±18.80 | 61.6±19.42 | 56.7±18.07 | 62.6±16.60 | 13.6±4.39 |
| Recurrent (n=30) | 47.0±16.01 | 23.9±9.80 | 18.6±11.10 | 23.1±12.8 | 19.4±4.60 | 47.7±21.50 | 66.2±13.59 | 64.5±8.50 | 68.7±11.80 | 12.0±4.40 |
| Significance (p-value) | 0.001*** | 0.001*** | 0.05* | 0.05* | 0.05* | 0.03* | 0.001*** | 0.001*** | 0.001*** | 0.003** |
n number of subjects
Cytokine Expression in PBMCs
The expressions of intracellular cytokines were determined in unstimulated (data not shown) and stimulated cultured PBMCs of both normal healthy subjects and patients. The proportions of CD4+cytokine positive T-cells in patients and healthy controls are shown in (Fig. 3). The frequencies of CD4+ T-cells producing IFN-γ, IL-2 and TNF-α were significantly reduced in patients (19.1±4.94, 52.3±20.86, 12.8±4.49 respectively) as compared to normal healthy controls (23.3±3.67, 67.5±12.0, 17.6±5.96 respectively) (Fig. 1) (p<0.01, p<0.018, p<0.001 respectively). On the contrary, significantly enhanced values of IL-4, IL-6 and IL-10 producing CD4+T-cells were observed in patients (63.8±17.01, 60.4±14.7, 65.7±14.84 respectively) as compared to healthy volunteers (24.4±8.77, 26.5±5.28, 20.6±3.81 respectively) (p<0.001, 0.001, 0.001 respectively) (Table 1) (Fig. 1). The expression of IFN-γ was seen highest in normal healthy subjects (23.3±3.67) as compared to recurrent (19.4±4.60), and non-recurrent patients (19.2±5.10) (p<0.03). The expression of IL-2 was lowest in recurrent patients as compared to the non-recurrent (Fig. 2) and the expression was highest in normals (67.5±12.0) (Table 1). The difference in the mean IFN-γ and IL-2 values between the recurrent and non-recurrent group of patients were not found statistically significant (Fig. 2) however, significant difference in the expression of IL-2 was seen between the normals and recurrent patients (p<0.009). The mean percent expression of Th2 cytokines like IL-4, IL-6 and IL-10 was although higher in the patients who had recurrence, as compared to those with non-recurrence, but the difference obtained were not statistically significant (p=0.38, 0.08, 0.18 respectively). On the contrary, expression of IL-4, IL-6 and IL-10 was found significantly reduced in normal controls when compared with recurrent and non-recurrent patients (p<0.001 for both groups). Also, the difference in the mean TNF-α values between the recurrent and non-recurrent patients was not statistically significant (p=0.25) (Fig. 2) whereas the difference between normals and different patient groups was significant (p<0.003), highest expression of TNF-α was seen in normal healthy subjects (Table 1).
Fig. 3.
Dot plots of Flow cytometric analysis in a bladder cancer patient and a healthy control subject showing the a phenotypic characterization and the b frequency of CD4+ cytokine-positive T-lymphocytes by flow cytometry. 10,000 lymphocytes were analyzed with cell-size gating. The frequencies of CD4+IFN-γ+, IL-2+, and TNF-α+ T-cells were reduced whereas; CD4+IL-4+, IL-6+ and IL-10+ were higher in patients with TCCs than in healthy controls
Correlation among the Th1 and Th2 Cytokines
Normal healthy subjects
No statistically significant correlation was observed between IFN-γ and IL-2, and IFN-γ with Th2 cytokines in normal healthy subjects (Table 2). Relationship of IL-2 with Th2 cytokines in normals showed a significant inverse correlation between the pairs (IL-2 and IL-4, p<0.003 and IL-2 and IL-10, p<0.004). Expression of IL-2 was associated with a concomitant suppression of Th2 cytokines like IL-4 and IL-10. None of the other pairs of cytokines revealed any statistical significance.
Table 2.
Correlation between Th1 and Th2 cytokines in patients and normals
| Groups | Th1/Th2 cytokines | IFN-γ | IL-2 | IL-4 | IL-6 | IL-10 | TNF-α |
|---|---|---|---|---|---|---|---|
| Normals (n=21) | IFN-γ | −0.101 | 0.345 | −0.448 | −0.032 | −0.03 | |
| IL-2 | −0.101 | −0.709** | −0.427 | −0.695** | 0.082 | ||
| Patients (n=41) | IFN-γ | 0.395 | −0.224 | −0.252 | −0.392* | 0.333 | |
| IL-2 | 0.395 | −0.479** | −0.321 | −0.467** | 0.37 | ||
| Recurrent cases (n=20) | IFN-γ | 0.297 | −0.164 | 0.076 | −0.291 | 0.183 | |
| IL-2 | 0.297 | −0.317 | 0.023 | −0.231 | 0.1 | ||
| Non-recurrent cases (n=21) | IFN-γ | 0.517* | −0.27 | −0.42 | −0.477 | 0.478 | |
| IL-2 | 0.517* | −0.603* | −0.483 | −0.632** | 0.619* |
Bladder Cancer Patients
A significant positive (p<0.02) correlation was found between the pair (IFN-γ and IL-2) whereas a significant negative correlation (p<0.02) existed between IFN-γ and IL-10 (Table 2). Similarly, significant positive correlation (p<0.04) of IL-2 was seen with TNF-α, whereas significant negative correlations were observed between the pairs (IL-2 and IL-4, p<0.006; IL-2 and IL-10, p<0.008). This indicated that enhanced production of IL-4 and IL-10 in patients was associated with a concomitant suppression of IFN-γ IL-2 and TNF-α. These data indicated that increased levels of Th2 cytokines were associated with decreased levels of Th1 cytokines in bladder cancer patients. Also, no significant correlations were observed between other pairs of cytokines.
Relationship between different pairs of cytokines associated with IFN-γ and IL-2 did not reveal any significant correlations in recurrent patients (Table 2). On the contrary, analysis of the correlations in non-recurrent patients showed significant correlations between various pairs of cytokines. Significant positive correlations (p<0.04, p<0.01) were seen between the pairs (IFN-γ and IL-2, IL-2 and TNF-α) respectively, whereas significant negative correlations were observed between IL-2 and Th2 cytokines (IL-4 and IL-10) in non-recurrent patients (p<0.01 and p<0.009 respectively). Thus, non-recurrent patients expressed Th1 cytokines more frequently, as compared to Th2 cytokines. Cross-regulation of cytokine gene expression was also seen in these patients. Enhanced expression of IFN-γ, IL-2 and TNF-α were associated with a concomitant suppression of IL-4, IL-6 and IL-10 in non-recurrent patients.
Discussion
The present study was performed to evaluate the functional activity of T-cell subsets on the basis of their cytokine profile in PMA and ionomycin stimulated PBMCs of patients of superficial transitional cell carcinoma of the human bladder along with normals using dual-color flow cytometry since this technique could reveal the Th1/Th2 balance in patients in vivo.
The cytokines produced by Th1 and Th2 cell subsets of CD4+T-cells play a vital role in determining the functionality and the immune response of cytotoxic T-lymphocytes (CTL), because they can regulate the differentiation of these cells. Th1 cells produce IFN-γ, IL-2, lymphotoxin and TGF-beta [6, 29] and they mediate cellular immunity, macrophage activation, ADCC and DTH responses while the Th2 cells produce cytokines like IL-4, IL-6, IL-5, IL-10 and IL-13 which direct anti-inflammatory responses as well as provide help for some B-cell responses [44, 45]. TNF-α is known to be an initiator cytokine [31] and it interacts with Th1 and Th2 cytokines as part of the interregulatory cytokine network. Cross regulation of Th1 and Th2 subsets of CD4+ T-cells through cytokine network has also been observed, IFN-γ inhibits Th2 cell functions [9] whereas, IL-4 and IL-10 are reported to inhibit Th1 response [37].
The findings of our study showed that the percentages of cells bearing T-lineage markers (CD3+, CD4+, CD8+) along with CD56 were markedly reduced in patients as compared to the normal healthy controls (Table 1). The results of our study are in accordance with the findings of other studies that have also shown systemic immunosupression in cancer patients [10, 17] but no suppression of humoral immune response [27]. Reduced percentages of CD8+ and NK cell populations in bladder cancer patients (recurrent/non-recurrent) indicated profound depression of cell-mediated immune function along with inappropriate lysis of tumor cells in these patients as compared to normal controls, since both cell types are required to generate potent anti-tumor responses (Table 1) [42].
Measurement of cytokines produced from peripheral blood mononuclear cells in vitro in bladder cancer patients has been done to examine the systemic changes of Th1 and Th2 function in immune response. In our study, following activation of PBMCs with PMA and ionomycin, the proportions of CD4+T-cells producing Th1 cytokines IFN-γ and IL-2 along with TNF-α were significantly reduced, whereas proportions of CD4+T-cells producing Th2 cytokines IL-4, IL-6 and IL-10 were significantly enhanced in patients as compared to normal controls (Fig. 1). It therefore seems that a novel increase in the Th2 cytokines in these patients is responsible for the pre-dominance of type II immune response. Non-T-cells, such as basophils and mast cells can also provide IL-4, but after PBMC separation, the CD4+T-cells of patients are the main source of higher levels of IL-4. These data suggest that in patients of bladder cancer, the presence of IL-4 along with IL-10 favors the expansion of Th2 cells and leads to the suppression of Th1 response which is required for the generation of effector CTLs that can cause lysis of tumor cells.
The correlation among the proportions of CD4+T-cells producing Th1/Th2 cytokines showed no significant correlations between IFN-γ and other cytokines in normal controls (Table 2). IL-2 in particular, had significant negative correlation with Th2 cytokines like IL-4 and IL-10. These findings suggest that the local presence of IL-2 along with concomitant suppression of IL-4 and IL-10 can thus generate reactive CTL effector cells in vivo in normal subjects such that immune response generated is functionally of an anti-tumor Th1 type.
The findings of this study showed that in non-recurrent patients, IFN-γ had significant positive correlation with IL-2 and TNF-α, whereas a significant negative correlation was observed with IL-4 and IL-10 (Table 2). IL-2 producing Th1 cells are required for the generation and function of mature CTL [5]. The ability of Th1 cells to destroy the tumor cells in these patients seems to be due to the presence of IL-2, which allows the generation of specific reactive anti-tumor CTL and does not allow the tumor to locate and progress in the host These correlation data can therefore show that the non-recurrent patients may be able to mount direct anti-tumor responses, which can be linked primarily to synergistic effects of IFN-γ and TNF-α. Both these cytokines prevent bladder tumor proliferation as well as up-regulate the expression of major histocompatibility complex (MHC) class I and II antigens and adhesion molecules on bladder cancer cells following tumor resection [20, 38, 39]. This change in surface phenotype increases the likelihood that tumor cells will also be recognized by the CD4+T-cells or CD8+ cytotoxic T lymphocytes [21, 34]. TNF-α and IFN-γ have also been reported to induce Fas receptor expression on bladder cancer cells providing a mechanism for apoptotic destruction by Fas ligand positive activated T-cells that are not MHC-restricted but usually associated with a Th1 response [25, 19]. No such correlation was seen amongst any of the Th1/Th2 cytokine pairs in patients showing recurrence of the disease in this study (Table 2). Absence of any correlation among Th1/Th2 cytokines in recurrent patients, demonstrates the lack of capability of these patients to generate potent CTLs that can prevent the proliferation of bladder tumor cells thus resulting in recurrence of the disease. Significantly reduced percentage of CD4+T-helper cell subset in patients showing recurrence, as compared to the non-recurrent patients suggests greater immunodeficiency in recurrent patients who are therefore unable to mount potent anti-tumor immune responses, which may be responsible for recurrence of the tumor (Fig. 2). Although the exact mechanism of tumor clearance/recurrence in patients with superficial transitional cell carcinoma has not been clearly delineated, it appears to rely primarily on an up-regulated protective cell-mediated immunity and a down-regulated immunosuppressive humoral immunity.
Dysregulation of the immune response caused by the imbalance of Th1/Th2 cytokines observed in this study has also been reported in various other tumors also [35, 10, 26]. Lee et al., 1995 [22] reported that Th2 cells representing clones generated from tumor infiltrating lymphocytes of melanoma patients suppress CTL responses, while Agarwal et al., 2003 [1] reported a dysregulated expression of Th2 cytokine (IL-4 and IL-10) gene in oral cancer patients leading to over production of IL-4 and IL-10 mRNA with a concomitant downregulation of IFN-γ and IL-2. As IL-2 plays a crucial immunoregulatory role, its local presence in normals thus helps to initiate a complex multi-cell mediated anti-tumor response. In the present study patients with recurrence showed a reduction in the IFN-γ, IL-2 and TNF-α producing CD4+T-cells and a considerable increase in IL-4, IL-6 and IL-10 producing cells as compared to normal controls (Table 1). Therefore, possibly as a result of enhanced IL-4, and down-regulated IL-2 in the cells of peripheral blood of bladder cancer patients, there is a dysregulation in the functionality of Th1 and Th2 cells with an expansion in Th2 and a suppression in Th1. It appears that in patients showing recurrence, the immune system is functionally directed towards a Th2 type of immune response, which is unable to oppose and destroy bladder tumor cells.
Various studies in bladder cancer have similarly shown that patients whose lymphocytes show a depressed ability to make IL-2 mRNA have poor clinical responses, whereas those with high urinary IL-2 levels show more potent anti-tumor responses [16]. Also, high levels of urinary IFN-γ have been associated with a better prognosis [13], on the other hand, patients with elevated urinary IL-6, typical of Th2 immune response, are less likely to be cured of their bladder cancer [50]. The potent Th1 suppressing cytokine IL-10 detected in the urine of patients may play a role in shutting down the Th1 response [30]. However, these studies do not provide direct evidence of an altered cytokine profile in the PBMC or TILs of patients with superficial transitional cell carcinoma of the bladder. Moreover, the relationship between the various Th1/Th2 cytokines in anti-tumor immune responses in recurrent and non-recurrent patients has been incompletely defined. In the present study, we have also attempted to elucidate the difference in the immunoregulatory functions of PBMC of recurrent/non-recurrent bladder cancer patients.
The results obtained in our study indicate that the Th2 type of immune response can be induced by a number of factors. One of the important factors could be the effect of cytokine microenvironment in the tumor-bearing host. Ectopic production of Th2 cytokines like IL-4, IL-6 and IL-10 by tumor cells can influence hierarchical immunosuppression most profoundly seen in cancer patients. This can affect the type of immune response at either the local or systemic level, or both together, by suppressive effects on IL-2 mechanisms. The absence of Th1 cell co-stimulator signals also induces an unresponsive anergy state in these cells and a transition from Th1 to a Th2-like signaling phenotype. The existence of the mechanism by which IL-4 down-regulates IL-2 production is confirmed by the results of other studies [45, 41] which have shown that, in several infectious disease models, IL-4 inhibits the generation of protective cell-mediated immunity and that the environmental IL-4 is able to down-regulate IL-2 production by the inhibition of IL-2 gene transcription. In fact, an IL-4 dependent mechanism by which CD8 cytolytic response can be turned into non-cytolytic ones [8] was found, which probably allows a pathogen to escape elimination. IL-4 down-regulates IFN-γ production [37] and has suppressive effects on macrophages which further diminishes the biological consequences of Th1 activation.
It has also been speculated that tumor cells may also escape immune attack by modulating the immune response such that activation of T-helper cells and CTL is directed in favor of the Th2 cells [11], possibly with the consequence of activation induced apoptosis of Th1 sub-populations [48]. Expression of a cell death signal cascade like CD95L or TNF-α, has been found to be expressed in different tumor cells [3]. IFN-γ, which is produced by Th1, activates monocytes to induce TNF-α [12]. TNF-α is a cytokine that exhibits a pleiotropic activity, including an immune response against cancer. Tripp et al., 1993 [47] has revealed that TNF-α is a co-stimulator with IL-12 of IFN-γ production by NK cells. These observations suggest that it is associated with a type 1 immune response. The results of the present study also showed that the level of TNF-α was much lower in patients than in healthy subjects (Table 1), as shown by Zielinski [49] in breast cancer.
To allow the generation of a specific and productive immune response which destroys the tumor, it is very vital to elucidate how these mechanisms should be manipulated in vivo, to shift the cytokine balance to provide a therapeutic benefit to bladder cancer patients. Therefore, immunotherapy in bladder cancer patients showing recurrence should be directed toward reversal of anergy and modulation of a Th2-like immunosuppressive response that probably exists in these patients towards a Th1-like protective cell-mediated immune response.
Measurement of various cytokines released from CD4+ T-cells as reported in this study, provides a more sensitive and valuable tool for evaluating the status of cell mediated immune response in bladder cancer patients. In conclusion, the CD4+T-helper cell subset was significantly reduced in recurrent patients as compared to non-recurrent patients. Our findings also revealed that IL-2 values were also reduced in recurrent cases, while the Th2 cytokines like IL-4 and IL-10, which are immunosuppressive, were enhanced in recurrent cases. Although the expression of these cytokines were not statistically significant, perhaps with the increase in number of cases studied the same may reveal statistical significance. Assessing the Th1/Th2 balance in peripheral blood lymphocytes may prove to be useful to monitor cancer therapy, including immunotherapy. Confirmation of these findings in larger sample groups may be useful to detect recurrences in patients of superficial transitional cell carcinoma of the bladder. Development of new strategies attempting to manipulate the equilibrium between Th1 and Th2 cells would be beneficial in the management of superficial transitional cell carcinoma in future. Further experiments addressing the expression of cytokines after TURBT and intravesical immunotherapy may also provide an insight in understanding the role these cytokines play in tumor recurrence and progression.
The progression of tumors despite the presence of substantial lymphocytic infiltrates suggests that the local immune response to control tumor growth is impaired. A gradient of functional impairment exists from tumor infiltrating lymphocytes to peripheral blood mononuclear cells suggesting that immunosuppressive effects of the tumor extend beyond its microenvironment as shown in the study where the TCC patients primarily showed a Th2 immunosuppressive response, whereas the normal control showed a Th1 protective cell-mediated immune response. Although these results do not directly address the roles of IL-4 and IL-10 as mediators of Th2 inhibition of Th1 responses in vivo, they are consistent with the view that IL-4 and IL-10 are important components of such inhibition. This may suggest that the tumor exerts a systemic suppressive effect on immune cells.
References
- 1.Agarwal A, Rani M, Saha GK, Valarmathi TM, Bahadur S, Mohanti BK, Das SN. Disregulated expression of the Th2 cytokine gene in patients with intraoral squamous cell carcinoma. Immunol Invest. 2003;32:17–30. doi: 10.1081/IMM-120019205. [DOI] [PubMed] [Google Scholar]
- 2.Assenmacher M, Schmitz J, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-gamma and in interleukin-4-expressing cells. Eur J Immunol. 1994;24:1097–101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- 3.Banat GA, Christ O, Cochlovius B, Pralle HB, Zoller M. Tumour-induced suppression of immune response and its correction. Cancer Immunol Immunother. 2001;49:573–86. doi: 10.1007/s002620000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest. 1968;97:31–50. [PubMed] [Google Scholar]
- 5.Chen LK, Tourvieille B, Burns GF, Bach FH, Mathieu-Mahul D, Sasportes M, Bensussan A. Interferon: a cytotoxic T lymphocyte differentiation signal. Eur J Immunol. 1986;16:767. doi: 10.1002/eji.1830160709. [DOI] [PubMed] [Google Scholar]
- 6.Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987;138:3688–94. [PubMed] [Google Scholar]
- 7.Elson LH, Nutman TB, Metcalfe DD, Prussin C. Flow cytometric analysis for cytokine production identifies T helper 1, T helper 2, and T helper 0 cells within the human CD4+CD27- lymphocyte subpopulation. J Immunol. 1995;154:4294–301. [PubMed] [Google Scholar]
- 8.Erard F, Wild MT, Garcia-Sanz JA, Le Gros G. Switch of CD8 T cells to noncytolytic CD8-CD4-cells that make TH2 cytokines and help B cells. Science. 1993;260:1802–5. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 9.Gajewski TF, Fitch FW. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–52. [PubMed] [Google Scholar]
- 10.Goto S, Sato M, Kaneko R, Itoh M, Sato S, Takeuchi S. Analysis of Th1 and Th2 cytokine production by peripheral blood mononuclear cells as a parameter of immunological dysfunction in advanced cancer patients. Cancer Immunol Immunother. 1999;48:435–42. doi: 10.1007/s002620050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halak BK, Maguire HC, Jr, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911–7. [PubMed] [Google Scholar]
- 12.Hernandez-Pando R, Rook GA. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994;82:591–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson AM, Ivshina AV, Senko O, Kuznetsova A, Sundan A, O’Donnell MA, Clinton S, Alexandroff AB, Selby PJ, James K, Kuznetsov VA. Prognosis of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer by immunological urinary measurements:statistically weighted syndrome analysis. J Urol. 1998;159:1054–63. doi: 10.1016/S0022-5347(01)63835-7. [DOI] [PubMed] [Google Scholar]
- 14.Jung T, Lack G, Schauer U, Uberuck W, Renz H, Gelfand EW, Rieger CH. Decreased frequency of interferon-gamma- and interleukin-2-producing cells in patients with atopic diseases measured at the single cell level. J Allergy Clin Immunol. 1995;96:515–27. doi: 10.1016/S0091-6749(95)70296-2. [DOI] [PubMed] [Google Scholar]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Kaempfer R, Gerez L, Farbstein H, Madar L, Hirschman O, Nussinovich R, Shapiro A. Prediction of response to treatment in superficial bladder carcinoma through pattern of interleukin-2 gene expression. J Clin Oncol. 1996;14:1778–86. doi: 10.1200/JCO.1996.14.6.1778. [DOI] [PubMed] [Google Scholar]
- 17.Kavanaugh DY, Carbone DP. Immunologic dysfunction in cancer. Hematol Oncol Clin North Am. 1996;10:927–51. doi: 10.1016/S0889-8588(05)70376-2. [DOI] [PubMed] [Google Scholar]
- 18.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–80. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein LT, Miller MI, Ikeguchi E, Buttyan R, Connor JP, Katz A, Raffo AJ, Olsson C. Anti-fas antibody mediated apoptosis in bladder tumor cells: a potential intravesical therapeutic agent. Proc Annu Meet Amer Assoc cancer Res. 1996;37:A103. [Google Scholar]
- 20.Kurisu H, Matsuyama H, Ohmoto Y, Shimabukuro T, Naito K. Cytokine-mediated antitumor effect of bacillus Calmette-Guerin on tumor cells in vitro. Cancer Immunol Immunother. 1994;39:249–53. doi: 10.1007/BF01525988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lattime EC, Gomella LG, McCue PA. Murine bladder carcinoma cells present antigen to BCG-specific CD4+ T-cells. Cancer Res. 1992;52:4286–90. [PubMed] [Google Scholar]
- 22.Lee KY, Goedegebuure PS, Linehan DC, Eberlein TJ. Immunoregulatory effects of CD4+ T helper subsets in human melanoma. Surgery. 1995;117:365–72. doi: 10.1016/S0039-6060(05)80054-6. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, C.E Detecting cytokine production at the single-cell level. Cytokine. 1991;3:184–8. doi: 10.1016/1043-4666(91)90014-5. [DOI] [PubMed] [Google Scholar]
- 24.Lord EM, Frelinger JG. Tumor immunotherapy: cytokines and antigen presentation. Cancer Immunol Immunother. 1998;46:75–81. doi: 10.1007/s002620050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Y, Szilvasi A, Chen X, DeWolf WC, O’Donnell MA. A novel method for monitoring Mycobacterium bovis BCG trafficking with recombinant BCG expressing green fluorescent protein. Clin Diagn Lab Immunol. 1996;3:761–8. doi: 10.1128/cdli.3.6.761-768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155:4926–32. [PubMed] [Google Scholar]
- 27.Manson LA. Does antibody-dependent epitope masking permit progressive tumor growth in the face of cell-mediated cytotoxicity. Immunol Today. 1991;12:352–5. doi: 10.1016/0167-5699(91)90065-2. [DOI] [PubMed] [Google Scholar]
- 28.Meyaard L, Hovenkamp E, Keet IP, Hooibrink B, de Jong IH, Otto SA, Miedema F. Single cell analysis of IL-4 and IFN-gamma production by T cells from HIV-infected individuals: decreased IFN-gamma in the presence of preserved IL-4 production. J Immunol. 1996;157:2712–18. [PubMed] [Google Scholar]
- 29.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-gamma in response to bacillus Calmette-Guerin. J Immunol. 1999;163:4246–52. [PubMed] [Google Scholar]
- 31.Old LJ. Tumour necrosis factor. Polypeptide mediator network. Nature. 1987;326:330–1. doi: 10.1038/326330a0. [DOI] [PubMed] [Google Scholar]
- 32.Olsson CA, Rao CN, Menzoian JO, Byrd WE. Immunologic unreactivity in bladder cancerpatients. J Urol. 1972;107:607–9. doi: 10.1016/s0022-5347(17)61090-5. [DOI] [PubMed] [Google Scholar]
- 33.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O’Garra A Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;2876:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 35.Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42:1–8. doi: 10.1007/s002620050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picker LJ, Singh MK, Zdraveski Z, Treer JR, Waldrop SL, Bergstresser PR, Maino VC. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 37.Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:3043–9. doi: 10.1002/eji.1830231147. [DOI] [PubMed] [Google Scholar]
- 38.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol. 1992;147:1636–42. doi: 10.1016/s0022-5347(17)37668-1. [DOI] [PubMed] [Google Scholar]
- 39.Pryor K, Stricker P, Russell P, Golovsky D, Penny R. Antiproliferative effects of bacillus Calmette-Guerin and interferon alpha 2b on human bladder cancer cells in vitro. Cancer Immunol Immunother. 1995;41:309–16. doi: 10.1007/BF01517219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyrah LN, Raper FP, Thomas, GM Report of a follow-up of papillary tumours of the bladder. Br J Urol. 1964;36:14–25. doi: 10.1111/j.1464-410X.1964.tb09475.x. [DOI] [PubMed] [Google Scholar]
- 41.Scott, P IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–55. [PubMed] [Google Scholar]
- 42.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 43.Silverberg E, Lubere JA. Cancer statstics 1988. CA Cancer J clin. 1988;38:5–22. doi: 10.3322/canjclin.38.1.5. [DOI] [PubMed] [Google Scholar]
- 44.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 45.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–806. [PubMed] [Google Scholar]
- 46.Torti FM, Lum BL. Superficial carcinoma of bladder: natural history and the role of interferons. Semin oncol. 1986;13:57–60. [PubMed] [Google Scholar]
- 47.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993;90:3725–9. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999;162:3273–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Zielinski CC, Mueller C, Tyl E, Tichatschek E, Kubista E, Spona J. Impaired production of tumor necrosis factor in breast cancer. Cancer. 1990;66:1944–8. doi: 10.1002/1097-0142(19901101)66:9<1944::AID-CNCR2820660916>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 50.Zlotta AR, Drowart A, Huygen K, De Bruyn J, Shekarsarai H, Decock M, Pirson M, Jurion F, Palfliet K, Denis O, Mascart F, Simon J, Schulman CC, Van Vooren JP. Humoral response against heat shock proteins and other mycobacterial antigens after intravesical treatment with bacille Calmette-Guerin (BCG) in patients with superficial bladder cancer. Clin Exp Immunol. 1997;109:157–65. doi: 10.1046/j.1365-2249.1997.4141313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]