Abstract
Numerous immunotherapy trials have been carried out in prostate cancer (PC) patients, with induction of antigen-specific T cells in some cases. Despite this capability, limited success is seen in terms of tumor regression or survival. In this review, we discuss the evidence for tumor escape strategies that may contribute to vaccine failure in the setting of PC. These include defects in antigen presentation, production of immunosuppressive substances, induction of T cell death, T cell receptor dysfunction, and the presence of tolerogenic dendritic cells and regulatory T cells inside prostate tumors. It is clear that novel strategies aimed at preventing tumor escape, such as small molecular weight inhibitors of immunosuppressive molecules, adoptive transfer of TCR transgenic T cells, removal of Tregs, combined with anti-androgen therapy and prostate-specific vaccines, need to be examined further in PC patients.
Keywords: Prostate cancer, Immunotherapy, T cell, Immune, Escape, Tumor
Introduction
Prostate cancer (PC) is currently the most commonly diagnosed cancer in men in Europe and the USA [1, 2]. Although the majority of cases are localized to the prostate, nearly one-third of newly diagnosed patients have advanced or metastatic PC. Radiation therapy and surgical resection can be curative in localized disease. However, no curative treatment currently exists when the disease spreads beyond the prostate. The identification of prostate tumor antigens that are recognized by T cells has created the opportunity to develop novel immunotherapeutic approaches, including tumor vaccines. Several antigens have already been identified, including prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), and prostate-specific membrane antigen (PSMA) [3]. This led to numerous immunotherapy trials, including peptide [4], DNA [5], virus-based [6], dendritic cell [7], and genetically-modified tumor cell vaccines [8], in which induction of tumor-specific T cells could be shown in some patients [9]. However, in most cases limited success has been seen in terms of tumor regression and survival.
Vaccine failure may be attributed to several potential tumor escape mechanisms such as defects in antigen presentation, production of immunosuppressive substances, T cell dysfunction, and the presence of regulatory T cells [10–12]. In the following sections the evidence for infiltration of prostate tumors by T cells and tumor escape mechanisms in PC are discussed.
Do T cells infiltrate prostate tumors?
Many studies have shown the presence of CD3+ T cells, including both CD4+ and CD8+ subtypes, inside prostate tumors. However, it is difficult to study the function of these cells for a number of reasons: prostate tumors are relatively small; surgery is only routinely carried out in early stage disease; the isolation of true tumor infiltrating lymphocytes (TILs) is problematic due to the infiltrative growth of PC within the prostate gland; low numbers of TILs are seen in PC [13]; the development of malignant ascites in PC is an extremely rare event with only a few published clinical case reports [14, 15]; and the generation of PC cell lines has proven difficult. Furthermore, the relatively few studies that have thus far looked at the relationship between lymphocytic infiltration and survival in PC have shown conflicting data. Vesalainen et al. [16] reported that tumors with a dense tumor lymphocyte infiltration were associated with higher survival rates than tumors with absent or decreased infiltrates. In contrast, Irani et al. [17] reported that an increased inflammatory cell infiltrate within the tumor was associated with an increased risk of tumor recurrence. More recently, McArdle et al. [18] reported that the presence of CD4+ T cell infiltrate was associated with poor cancer survival in patients with PC. One interpretation may be that patients who lack intratumoral T cells fail to mount an immune response to the tumor, while patients with intratumoral T cells are in the process of mounting an immune response, the success of which depends on the presence or absence of various tumor immune escape pathways.
Defects in antigen presentation
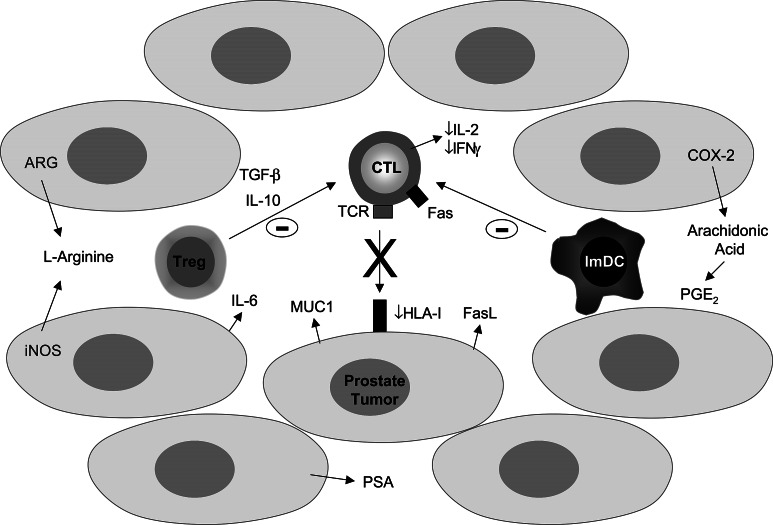
HLA class I antigens are critical for the recognition and lysis of tumor cells by cytotoxic T cells (CTLs), and defects in antigen presentation could allow the tumor to escape killing by CTLs (Fig. 1). Expression of HLA molecules and associated proteins required for efficient antigen processing, such as transporter associated with antigen processing (TAP), low molecular weight protein of the proteasome complex (LMP), and beta 2–microglobulin, were investigated in several human PC cell lines. HLA class I was under-expressed in two (LNCaP and PPC-1) PC cell lines [19, 20]. Furthermore, PPC-1 cells also under-expressed TAP-2 mRNA despite abundant HLA class I and beta 2-microglobulin message, and LNCaP cells had competent antigen transport but deficient HLA class I heavy-chain function despite abundant HLA class I RNA [19]. Increased shedding of beta 2-microglobulin was demonstrated in the PC-3 cell line, and increased levels of beta 2-microglobulin in the urine of PC patients was associated with a significantly shortened survival [21]. In contrast, no defects in the expression of class I antigen processing machinery (TAP-1, TAP-2, LMP-2, and LMP-7) were detected in a mouse PC cell line [22]. In primary human tissue, several studies report reduced or complete loss of HLA class I in prostate tumors and lymph node metastases, compared to the normal expression in benign tissue [20, 23–25]. However, other mechanisms for defects in antigen presentation, such as changes in the expression of TAP or LMP molecules, have not yet been reported in primary tumor tissue.
Fig. 1.
A schematic representation of the potential mechanisms of immune escape in prostate cancer. Abbreviations: ARG arginase, COX-2 cyclooxygenase-2, CTL cytotoxic T cell, FasL Fas ligand, iNOS inducible nitric oxide synthase, IFNγ interferon-gamma, imDC immature dendritic cell, IL interleukin, MUC1 epithelial mucin 1, PGE2 prostaglandin E2, PSA prostate specific antigen, TCR T cell receptor, TGF-β transforming growth factor-beta, Treg regulatory T cell
Production of immunosuppressive substances
Cytokines
It is postulated that an imbalance in Th1/Th2 cytokine production may be responsible for the development of cancer, with a shift toward a Th2 response and induction of immunosuppressive cytokines such as interleukin (IL)-4, IL-6, and IL-10 [26].
In the serum of PC patients elevated levels of several Th2 cytokines, such as IL-4, IL-6, and IL-10, were detected when compared to either patients with benign prostate hyperplasia or normal healthy controls [27–29]. Multiple studies have shown a role for IL-6 in PC progression and that increased serum levels of this cytokine are a significant indicator of poor prognosis in PC [30–32]. In prostate tumors it is postulated that IL-6 has a direct effect on tumor cell growth [33], and may also contribute to peripheral T cell dysfunction [11].
Basal expression of cytokines, measured in T cells from blood of PC patients, demonstrated a lower expression of both IL-10 and IFNγ [29]. In cultured peripheral blood T cells from PC patients stimulated with PMA and/or ionomycin an increase in IL-10 and a decrease in IL-2, compared to healthy controls was reported in one study [27], while higher ratios of IFNγ/IL-4, IFNγ/IL-10, and IL-2/IL-4, a favorable type-1 cytokine pattern, were observed in patients with lower serum PSA compared to patients with high serum PSA [34]. Furthermore, direct study of prostate TILs showed a significantly higher expression of IL-10, compared to matched blood T cells [35]. In addition, TILs isolated from a rare malignant ascites in a PC patient were tumor antigen reactive but predominantly secreted Th2 cytokines upon stimulation [15].
Transforming growth factor-beta (TGF-β) is a cytokine that suppresses the immune system, and also has a complex role in the regulation of PC cell growth [22]. TGF-β can modulate many immune processes including homing, cellular adhesion, chemotaxis and T cell activation, differentiation and apoptosis. Recent progress in this field also suggests a key role for TGF-β in regulatory T (Treg) cell-mediated suppression. Several studies report upregulation of TGF-β expression in PC patients, both in the serum and in the tumors, and an inverse correlation with survival [36–38]. Although prostate tumor cells may lose sensitivity to this cytokine, via downregulation of the receptors, TGF-β may still be able to function as a potent immunosuppressive agent against incoming immune cells.
Amino acid metabolites
The amino acid l-arginine can be metabolized by the enzyme nitric oxide synthase (NOS), to generate the free radical nitric oxide (NO) and l-citrulline, or by the enzyme arginase (ARG), to generate urea and l-ornithine. In the prostate NO is reported to have a physiological role in smooth muscle tone and secretory functions [39, 40]. However, several studies in PC show that levels of the inducible form of NOS (iNOS) and ARG are increased inside the tumor [41–45], and that strong iNOS expression was a predictor for poor survival [46]. It is reported that increased l-arginine metabolism within tumors may contribute to tumor growth, angiogenesis, metastasis, and tumor-related immunosuppression [47, 48]. Recently, treatment of prostate tumors in culture with inhibitors of NOS (l-NMMA) or ARG (NOHA) resulted in upregulation of early activation markers on CD8+ TILs, prolonged their survival and restored their lytic capacity [44]. It is therefore possible that defective l-arginine metabolism is one mechanism by which T cells are immunosuppressed inside prostate tumors.
In addition, expression of the tryptophan degrading enzyme, indoleamine 2,3-dioxygenase (IDO) in dendritic cells, was shown to have profound effects on T cell proliferation, differentiation, effector functions, and viability [49]. Thus far, there are no studies of this enzyme or its expression in PC models or patients and research into this area is warranted.
Arachidonic acid metabolites
Cyclooxygenase-2 (COX-2) is an enzyme that converts arachidonic acid to various prostaglandins and other eicosanoids. It is induced under various conditions, such as inflammation and cancer. COX-2 expression has been studied extensively in PC [50] and found in smooth muscle cells of both the normal and cancerous prostate. Luminal epithelial cells surrounded by lymphocytes are induced to express the enzyme in prostatic inflammation, and COX-2 is expressed in the epithelial cells of high-grade prostatic intraepithelial neoplasia and cancer. Recently, COX-2 expression was found to correlate with a higher Gleason score, local chronic inflammation, and tumor neovascularization in human PC [51]. COX-2 is suggested to play a key role in tumorigenesis through stimulating epithelial cell proliferation, inhibiting apoptosis, stimulating angiogenesis, enhancing cell invasiveness, and importantly mediating immune suppression. Prostaglandin E2 (PGE2), a product of arachidonic acid metabolism by COX-2, may regulate immune function by acting as a negative feedback inhibitor for various processes, including T cell proliferation, lymphokine production and macrophage and natural-killer cell cytotoxicity [52]. Therefore, inhibition of COX activity in PC may be associated with an enhanced immune response and decreased tumorigenesis.
Other potentially immunosuppressive molecules
MUC1 glycoprotein is an epithelial associated mucin that is overexpressed, aberrantly glycosylated, shed from tumor cells during cancer transformation, and is implicated in the impairment of immune function in the vicinity of tumors. Depletion of soluble MUC1, by immunoprecipitation, from tumor supernatants neutralized the inhibitory effects on T cell proliferation [53]. Furthermore, human monocyte-derived DCs, when cultured in the presence of MUC1, displayed a modified phenotype with decreased expression of co-stimulatory molecules (CD86, CD40), antigen-presenting molecules (DR and CD1d), and differentiation markers (CD83) [54]. Several studies have shown MUC1 overexpression in PC and a correlation with advanced disease [55–57].
It is also suggested that PSA, found in high concentration in prostate tumor tissue, is immunosuppressive. One study demonstrated that PSA suppressed in vitro phytohemagglutinin- and alloantigen-stimulated lymphocyte proliferation in a dose-dependent manner [58]. Furthermore, the addition of active PSA to DC cultures significantly inhibited the generation and maturation of DC, as well as the ability of the DC to induce T cell proliferation [59].
Induction of T cell death
Fas ligand (FasL) is a type II transmembrane tumor necrosis factor family protein, known to trigger apoptosis in cells that bear the FasL receptor, Fas. The role of Fas in host-tumor interactions is complex, and Fas may have a beneficial role in the induction of tumor cell apoptosis, but may be a selective force influencing the escape of Fas-resistant aggressive tumor variants [60]. In PC patients soluble Fas levels are increased in serum [61], and LNCaP, DU145, and PC3 prostate carcinoma cells secrete soluble FasL into their local environment [62]. More recently, co-culture of T cells with exosomes derived from the LNCaP PC cell line dose-dependently inhibited T cell proliferation and induced apoptosis [63]. Addition of anti-FasL antibody blocked the T cell apoptosis induction by tumor exosomes. It is postulated that T cells themselves can commit suicide upon activation with antigen by secreting high amounts of FasL [64]. Indeed, TILs isolated from prostate tumors expressed higher amounts of FasL than their corresponding peripheral T cells [35]. However, the suggestion that immune cells can undergo apoptotic death at the tumor site through the Fas pathway has been received somewhat skeptically and awaits further studies to confirm this possibility [65].
T cell receptor dysfunction
T cells recognize antigen via the heterodimeric T cell receptor (TCR) molecule, which is noncovalently associated with the CD3 molecular complex. Reduced or aberrant expression of TCR-associated signal-transduction molecules is reported in many types of cancer and may contribute to tumor escape [10]. In PC, diminished expression of TCR-beta, CD3-epsilon, and CD3-zeta was observed on intratumoral T cells isolated from a TRAMP-C1P3 mouse model of PC [66]. Furthermore, expression of the zeta-chain was decreased in peripheral T cells of some PC patients, but normalized after treatment with a PSA vaccine [67, 68]. In addition to a functional TCR, a complex network of co-stimulatory and co-inhibitory signals regulate the T cell immune response [69]. Several studies in murine PC models have shown improved anti-tumor immune responses with the addition of co-stimulatory signals, such as B7.1 ligand, or with blockade of co-inhibitory signals, such as CTLA-4 [70–72]. Collectively, these data suggest that manipulation of T cell co-stimulatory and inhibitory signals may improve PC immunotherapy.
Tolerogenic dendritic cells
Dendritic cells (DCs) are phenotypically distinct and extremely potent antigen-presenting cells that are critical for initiation of specific immune responses [73]. DCs take up and process antigen as peptides within the major histocompatibility complex and present these to T cells. They also deliver additional co-stimulatory signals facilitating activation and expansion of antigen-specific T cells. Different types of DC exist which have different functional capacities with respect to the T cell responses that are induced, with tolerogenic immature and immunogenic mature differentiation stages [74]. In cancer, disabled DC differentiation, maturation, migration, and function have been proposed as one mechanism for tumor escape [75]. Indeed, in a murine model of PC, DCs isolated from TRAMP-C1P3 tumors were found to be phenotypically immature (CD11c+, B7.2+/−, I-A−, CD40−) [66]. Furthermore, in human prostate tumors DC were found to represent only a small subset of leukocytes present in both benign and malignant prostatic tissue. Statistically there were significantly less DC in PC compared with normal prostatic tissue, and these cells were minimally activated [76]. The presence of PC cells significantly inhibited the generation of DC, and these cells were weak stimulators of T cell proliferation, suggesting that DC generated in the PC microenvironment are functionally inhibited [77, 78]. Fortunately, many studies have demonstrated that good quality mature DCs can still be generated from the blood of PC patients, thus allowing their use as immunotherapeutic vaccines [79–81].
Regulatory T cells
Recently, it was suggested that the presence of regulatory CD4+CD25+ T cells may explain the poor clinical efficacy of immunotherapeutic protocols in human tumors [82]. It is now well established that immunoregulatory CD4+CD25+ T cells control key aspects of immunologic tolerance to self-antigens [83, 84]. These cells constitutively express CD25 (IL-2 receptor α-chain) on their surface and constitute 5–10% of CD4+ T cells in humans and rodents. Although the precise mechanisms of suppression by Tregs remain to be determined, these cells inhibit immune-cell functions either directly through cell–cell contact or indirectly through the secretion of anti-inflammatory mediators, such as IL-10 and TGF-β.
Tien et al [85] demonstrated an increased frequency of tumor infiltrating CD4+CD25+ Tregs in the tumor mass of a 12T-7s murine spontaneous model of PC [85]. Furthermore, blockade of the CD4+CD25+ T cells using an anti-CD25 mAb reduced PC cell growth both in a prostate tumor transplant model (TRAMP-C2) and in the spontaneous prostate tumor model (12T-7s). Recently, we have found that CD4+CD25high T cells are increased in the peripheral blood and tumor tissue of patients with early stage PC (unpublished data). Furthermore, in this study supernatants from primary prostate tumors, malignant ascites and PC cell lines, attracted CD4+CD25+ T cells in an in vitro migration assay and contained the chemokine CCL22, suggesting one possible mechanism for the increased numbers of Tregs inside prostate tumors.
Concluding remarks
In general, cancer immunotherapy has not yet fulfilled its expectations and the knowledge of the immunobiology of PC is seriously lacking behind some other cancers, such as ovarian cancer and melanoma. Induction of tumor-specific T cells was demonstrated in some PC immunotherapy trials but it has been difficult to correlate this data with tumor regression. It is likely that a number of tumor escape pathways play a role in limiting the effectiveness of PC vaccines.
It is clear that vaccination alone will not be sufficient to create an effective immunotherapy for PC. An improved understanding of the mechanisms mediating tumor escape from immune recognition will lead to more effective vaccination protocols. Prospects for future investigation in PC could include combination of prostate-specific vaccines with low molecular weight inhibitors of immunosuppressive molecules or their receptors, improved immunological adjuvants, adoptive transfer of TCR transgenic T cells, or the removal of suppressive Tregs.
Acknowledgements
Experimental work in our laboratory is supported partly by grants from the Cancer Society in Stockholm, the Swedish Cancer Society, Karolinska Institute Funds, the EU 6-FP “ALLOSTEM” (LSHB-CT-2004-503319), the EU 6-FP “ENACT”, and U.S. Department of Defense Prostate Cancer Research Program (PC030958).
Footnotes
This article is a symposium paper from the conference Progress in Vaccination against Cancer 2005 (PIVAC 5), held in Athens, Greece, on 20–21 September 2005.
References
- 1.Black RJ, Bray F, Ferlay J, Parkin DM. Cancer incidence and mortality in the European Union: cancer registry data and estimates of national incidence for 1990. Eur J Cancer. 1997;33:1075–1107. doi: 10.1016/S0959-8049(96)00492-3. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer Statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Harada M, Noguchi M, Itoh K. Target molecules in specific immunotherapy against prostate cancer. Int J Clin Oncol. 2003;8:193–199. doi: 10.1007/s10147-003-0332-x. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, Noda S. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 5.Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, Bergman B, Egevad L, Hellstrom M, Kiessling R, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91:688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 7.Pandha HS, John RJ, Hutchinson J, James N, Whelan M, Corbishley C, Dalgleish AG. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int. 2004;94:412–418. doi: 10.1111/j.1464-410X.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 8.Simons JW, Mikhak B, Chang JF, DeMarzo AM, Carducci MA, Lim M, Weber CE, Baccala AA, Goemann MA, Clift SM, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 9.Vieweg J, Dannull J. Technology insight: vaccine therapy for prostate cancer. Nature Clinical Practice Urology. 2005;2:44–51. doi: 10.1038/ncpuro0079. [DOI] [PubMed] [Google Scholar]
- 10.Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjoberg J, Pisa P, Petersson M. Tumor-induced immune dysfunction. Cancer Immunol Immunother. 1999;48:353–362. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawelec G. Tumour escape: antitumour effectors too much of a good thing? Cancer Immunol Immunother. 2004;53:262–274. doi: 10.1007/s00262-003-0469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53:844–854. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey P (2003) Prostate pathology: American society clinical Pathology
- 14.Megalli MR, Gursel EO, Veenema RJ. Ascites as an unusual presentation of carcinoma of the prostate. J Urol. 1973;110:232–234. doi: 10.1016/s0022-5347(17)60173-3. [DOI] [PubMed] [Google Scholar]
- 15.Ozenci V, Miller AM, Palmborg A, Egevad L, Jaremko GA, Kalkner KM, Pisa P. Presence and specificity of tumor associated lymphocytes from ascites fluid in prostate cancer. Prostate. 2005;65:20–26. doi: 10.1002/pros.20229. [DOI] [PubMed] [Google Scholar]
- 16.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A:1797–1803. doi: 10.1016/0959-8049(94)E0159-2. [DOI] [PubMed] [Google Scholar]
- 17.Irani J, Goujon JM, Ragni E, Peyrat L, Hubert J, Saint F, Mottet N. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Pathologist Multi Center Study Group. Urology. 1999;54:467–472. doi: 10.1016/S0090-4295(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 18.McArdle PA, Canna K, McMillan DC, McNicol AM, Campbell R, Underwood MA. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer. 2004;91:541–543. doi: 10.1038/sj.bjc.6601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM, Simons JW. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bander NH, Yao D, Liu H, Chen YT, Steiner M, Zuccaro W, Moy P. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate. 1997;33:233–239. doi: 10.1002/(SICI)1097-0045(19971201)33:4<233::AID-PROS2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Abdul M, Hoosein N. Changes in beta-2 microglobulin expression in prostate cancer. Urol Oncol. 2000;5:168–172. doi: 10.1016/S1078-1439(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 22.Lee HM, Timme TL, Thompson TC. Resistance to lysis by cytotoxic T cells: a dominant effect in metastatic mouse prostate cancer cells. Cancer Res. 2000;60:1927–1933. [PubMed] [Google Scholar]
- 23.Natali PG, Nicotra MR, Bigotti A, Venturo I, Marcenaro L, Giacomini P, Russo C. Selective changes in expression of HLA class I polymorphic determinants in human solid tumors. Proc Natl Acad Sci USA. 1989;86:6719–6723. doi: 10.1073/pnas.86.17.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blades RA, Keating PJ, McWilliam LJ, George NJ, Stern PL. Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology. 1995;46:681–686. doi: 10.1016/S0090-4295(99)80301-X. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Melamed J, Wei P, Cox K, Frankel W, Bahnson RR, Robinson N, Pyka R, Liu Y, Zheng P. Concordant down-regulation of proto-oncogene PML and major histocompatibility antigen HLA class I expression in high-grade prostate cancer. Cancer Immun. 2003;3:2. [PubMed] [Google Scholar]
- 26.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 27.Filella X, Alcover J, Zarco MA, Beardo P, Molina R, Ballesta AM. Analysis of type T1 and T2 cytokines in patients with prostate cancer. Prostate. 2000;44:271–274. doi: 10.1002/1097-0045(20000901)44:4<271::AID-PROS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Wise GJ, Marella VK, Talluri G, Shirazian D. Cytokine variations in patients with hormone treated prostate cancer. J Urol. 2000;164:722–725. doi: 10.1016/S0022-5347(05)67289-8. [DOI] [PubMed] [Google Scholar]
- 29.Elsasser-Beile U, Gierschner D, Jantscheff P, Schultze-Seemann W, Katzenwadel A, Wetterauer U. Different basal expression of type T1 and T2 cytokines in peripheral lymphocytes of patients with adenocarcinomas and benign hyperplasia of the prostate. Anticancer Res. 2003;23:4027–4031. [PubMed] [Google Scholar]
- 30.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. doi: 10.1016/S0022-5347(01)62092-5. [DOI] [PubMed] [Google Scholar]
- 31.Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate. 1999;41:127–133. doi: 10.1002/(SICI)1097-0045(19991001)41:2<127::AID-PROS7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, Murai M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–2706. [PubMed] [Google Scholar]
- 33.Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95:497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- 34.Perambakam SM, Srivastava R, Peace DJ. Distinct cytokine patterns exist in peripheral blood mononuclear cell cultures of patients with prostate cancer. Clin Immunol. 2005;117:94–99. doi: 10.1016/j.clim.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Elsasser-Beile U, Przytulski B, Gierschner D, Grussenmeyer T, Katzenwadel A, Leiber C, Deckart A, Wetterauer U. Comparison of the activation status of tumor infiltrating and peripheral lymphocytes of patients with adenocarcinomas and benign hyperplasia of the prostate. Prostate. 2000;45:1–7. doi: 10.1002/1097-0045(20000915)45:1<1::AID-PROS1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 36.Cardillo MR, Petrangeli E, Perracchio L, Salvatori L, Ravenna L, Di Silverio F. Transforming growth factor-beta expression in prostate neoplasia. Anal Quant Cytol Histol. 2000;22:1–10. [PubMed] [Google Scholar]
- 37.Stravodimos K, Constantinides C, Manousakas T, Pavlaki C, Pantazopoulos D, Giannopoulos A, Dimopoulos C. Immunohistochemical expression of transforming growth factor beta 1 and nm-23 H1 antioncogene in prostate cancer: divergent correlation with clinicopathological parameters. Anticancer Res. 2000;20:3823–3828. [PubMed] [Google Scholar]
- 38.Shariat SF, Kattan MW, Traxel E, Andrews B, Zhu K, Wheeler TM, Slawin KM. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin Cancer Res. 2004;10:1992–1999. doi: 10.1158/1078-0432.CCR-0768-03. [DOI] [PubMed] [Google Scholar]
- 39.Burnett AL, Maguire MP, Chamness SL, Ricker DD, Takeda M, Lepor H, Chang TS. Characterization and localization of nitric oxide synthase in the human prostate. Urology. 1995;45:435–439. doi: 10.1016/S0090-4295(99)80012-0. [DOI] [PubMed] [Google Scholar]
- 40.Bloch W, Klotz T, Loch C, Schmidt G, Engelmann U, Addicks K. Distribution of nitric oxide synthase implies a regulation of circulation, smooth muscle tone, and secretory function in the human prostate by nitric oxide. Prostate. 1997;33:1–8. doi: 10.1002/(SICI)1097-0045(19970915)33:1<1::AID-PROS1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 41.Uotila P, Valve E, Martikainen P, Nevalainen M, Nurmi Harkonen M P. Increased expression of cyclooxygenase-2 and nitric oxide synthase-2 in human prostate cancer. Urol Res. 2001;29:23–28. doi: 10.1007/s002400000148. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Torbenson M, Wang Q, Ro JY, Becich M. Expression of inducible nitric oxide synthase in paired neoplastic and non-neoplastic primary prostate cell cultures and prostatectomy specimen. Urol Oncol. 2003;21:117–122. doi: 10.1016/s1078-1439(02)00208-9. [DOI] [PubMed] [Google Scholar]
- 43.Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–1903. doi: 10.1002/(SICI)1097-0142(19980515)82:10<1897::AID-CNCR12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 44.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201(8):1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris BE, Pretlow TP, Bradley EL, Jr, Whitehurst GB, Pretlow TG., II Arginase activity in prostatic tissue of patients with benign prostatic hyperplasia and prostatic carcinoma. Cancer Res. 1983;43:3008–3012. [PubMed] [Google Scholar]
- 46.Aaltoma SH, Lipponen PK, Kosma VM. Inducible nitric oxide synthase (iNOS) expression and its prognostic value in prostate cancer. Anticancer Res. 2001;21:3101–3106. [PubMed] [Google Scholar]
- 47.Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- 48.Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 49.Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. 2005;338:20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- 50.Kirschenbaum A, Liu X, Yao S, Levine AC. The role of cyclooxygenase-2 in prostate cancer. Urology. 2001;58:127–131. doi: 10.1016/S0090-4295(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005;11:3250–3256. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- 52.Badawi AF. The role of prostaglandin synthesis in prostate cancer. BJU Int. 2000;85:451–462. doi: 10.1046/j.1464-410x.2000.00507.x. [DOI] [PubMed] [Google Scholar]
- 53.Chan AK, Lockhart DC, von Bernstorff W, Spanjaard RA, Joo HG, Eberlein TJ, Goedegebuure PS. Soluble MUC1 secreted by human epithelial cancer cells mediates immune suppression by blocking T-cell activation. Int J Cancer. 1999;82:721–726. doi: 10.1002/(SICI)1097-0215(19990827)82:5<721::AID-IJC16>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 54.Rughetti A., Pellicciotta I., Biffoni M., Backstrom M., Link T., Bennet E.P., Clausen H., Noll T, Hansson GC, Burchell JM, et al. Recombinant tumor-associated MUC1 glycoprotein impairs the differentiation and function of dendritic cells. J Immunol. 2005;174:7764–7772. doi: 10.4049/jimmunol.174.12.7764. [DOI] [PubMed] [Google Scholar]
- 55.Kirschenbaum A, Itzkowitz SH, Wang JP, Yao S, Eliashvili M, Levine AC. MUC1 expression in prostate carcinoma: correlation with grade and stage. Mol Urol. 1999;3:163–168. [PubMed] [Google Scholar]
- 56.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arai T, Fujita K, Fujime M, Irimura T. Expression of sialylated MUC1 in prostate cancer: relationship to clinical stage and prognosis. Int J Urol. 2005;12:654–661. doi: 10.1111/j.1442-2042.2005.01112.x. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy-Smith AG, McKenzie JL, Owen MC, Davidson PJ, Vuckovic S, Hart DN. Prostate specific antigen inhibits immune responses in vitro: a potential role in prostate cancer. J Urol. 2002;168:741–747. doi: 10.1016/S0022-5347(05)64738-6. [DOI] [PubMed] [Google Scholar]
- 59.Aalamian M, Tourkova IL, Chatta GS, Lilja H, Huland E, Huland H, Shurin GV, Shurin MR. Inhibition of dendropoiesis by tumor derived and purified prostate specific antigen. J Urol. 2003;170:2026–2030. doi: 10.1097/01.ju.0000091264.46134.b7. [DOI] [PubMed] [Google Scholar]
- 60.Abrams SI. Positive and negative consequences of Fas/Fas ligand interactions in the antitumor response. Front Biosci. 2005;10:809–821. doi: 10.2741/1575. [DOI] [PubMed] [Google Scholar]
- 61.Furuya Y, Fuse H, Masai M. Serum soluble Fas level for detection and staging of prostate cancer. Anticancer Res. 2001;21:3595–3598. [PubMed] [Google Scholar]
- 62.Liu QY, Rubin MA, Omene C, Lederman S, Stein CA. Fas ligand is constitutively secreted by prostate cancer cells in vitro. Clin Cancer Res. 1998;4:1803–1811. [PubMed] [Google Scholar]
- 63.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP. Tumor exosomes expressing Fas ligand mediate CD8(+) T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999;162:3273–3279. [PMC free article] [PubMed] [Google Scholar]
- 65.Restifo NP. Not so Fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med. 2000;6:493–495. doi: 10.1038/74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciavarra RP, Holterman DA, Brown RR, Mangiotti P, Yousefieh N, Wright GL, Jr, Schellhammer PF, Glass WF, Somers KD. Prostate tumor microenvironment alters immune cells and prevents long-term survival in an orthotopic mouse model following flt3-ligand/CD40-ligand immunotherapy. J Immunother. 2004;27:13–26. doi: 10.1097/00002371-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Healy CG, Simons JW, Carducci MA, DeWeese TL, Bartkowski M, Tong KP, Bolton WE. Impaired expression and function of signal-transducing zeta chains in peripheral T cells and natural killer cells in patients with prostate cancer. Cytometry. 1998;32:109–119. doi: 10.1002/(SICI)1097-0320(19980601)32:2<109::AID-CYTO6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 68.Meidenbauer N, Gooding W, Spitler L, Harris D, Whiteside TL. Recovery of zeta-chain expression and changes in spontaneous IL-10 production after PSA-based vaccines in patients with prostate cancer. Br J Cancer. 2002;86:168–178. doi: 10.1038/sj.bjc.6600039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peggs KS, Allison JP. Co-stimulatory pathways in lymphocyte regulation: the immunoglobulin superfamily. Br J Haematol. 2005;130:809–824. doi: 10.1111/j.1365-2141.2005.05627.x. [DOI] [PubMed] [Google Scholar]
- 70.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 72.Hull GW, McCurdy MA, Nasu Y, Bangma CH, Yang G, Shimura S, Lee HM, Wang J, Albani J, Ebara S, et al. Prostate cancer gene therapy: comparison of adenovirus-mediated expression of interleukin 12 with interleukin 12 plus B7–1 for in situ gene therapy and gene-modified, cell-based vaccines. Clin Cancer Res. 2000;6:4101–4109. [PubMed] [Google Scholar]
- 73.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 74.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/S1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 75.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 76.Troy A, Davidson P, Atkinson C, Hart D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol. 1998;160:214–219. doi: 10.1016/S0022-5347(01)63093-3. [DOI] [PubMed] [Google Scholar]
- 77.Aalamian M, Pirtskhalaishvili G, Nunez A, Esche C, Shurin GV, Huland E, Huland H, Shurin MR. Human prostate cancer regulates generation and maturation of monocyte-derived dendritic cells. Prostate. 2001;46:68–75. doi: 10.1002/1097-0045(200101)46:1<68::AID-PROS1010>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 78.Shurin GV, Aalamian M, Pirtskhalaishvili G, Bykovskaia S, Huland E, Huland H, Shurin MR. Human prostate cancer blocks the generation of dendritic cells from CD34+ hematopoietic progenitors. Eur Urol 39 Suppl. 2001;4:37–40. doi: 10.1159/000052584. [DOI] [PubMed] [Google Scholar]
- 79.Tjoa BA, Simmons SJ, Bowes VA, Ragde H, Rogers M, Elgamal A, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, et al. Evaluation of phase I/II clinical trials in prostate cancer with dendritic cells and PSMA peptides. Prostate. 1998;36:39–44. doi: 10.1002/(SICI)1097-0045(19980615)36:1<39::AID-PROS6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 80.Barrou B, Benoit G, Ouldkaci M, Cussenot O, Salcedo M, Agrawal S, Massicard S, Bercovici N, Ericson ML, Thiounn N. Vaccination of prostatectomized prostate cancer patients in biochemical relapse, with autologous dendritic cells pulsed with recombinant human PSA. Cancer Immunol Immunother. 2004;53:453–460. doi: 10.1007/s00262-003-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 82.Antony PA, Restifo NP. Do CD4+ CD25+ immunoregulatory T cells hinder tumor immunotherapy? J Immunother. 2002;25:202–206. doi: 10.1097/00002371-200205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 84.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 85.Tien AH, Xu L, Helgason CD. Altered immunity accompanies disease progression in a mouse model of prostate dysplasia. Cancer Res. 2005;65:2947–2955. doi: 10.1158/0008-5472.CAN-04-3271. [DOI] [PubMed] [Google Scholar]