Abstract
Tyrosinase or polyphenol oxidase (EC 1.14.18.1) is the key enzyme in melanin biosynthesis and in the enzymatic browning of fruits and vegetables. The role of tyrosinase in the secondary metabolism of plants still remains unclear, but its implication in betalain biosynthesis has been proposed. Betalains are an important class of water-soluble pigments, characteristic of plants belonging to the order Caryophyllales. In this article, the betaxanthins, tyrosine-betaxanthin (portulacaxanthin II) and dopaxanthin, are reported to be physiological substrates for tyrosinase. The direct activity of tyrosinase on selected betaxanthins is characterized in depth, and conversion of tyrosine-betaxanthin to dopaxanthin and its further oxidation to a series of compounds are described. Identity of the reaction products was studied by high-performance liquid chromatography and electrospray ionization-mass spectrometry. Masses determined for the reaction products were the same in all cases, 389 m/z ([M + H]+) and equal to that determined for betanidin. Data indicate that dopaxanthin-quinone is obtained and evolves to more stable species by intramolecular cyclization. Kinetic parameters for tyrosinase acting on dopaxanthin were evaluated, showing a high affinity for this substrate (Km = 84.3 μm). The biosynthetic scheme of betalains is reviewed and a branch is proposed based on the description of physiological substrates for tyrosinase. Lampranthus productus, Glottiphylum oligocarpum, and Glottiphylum pigmaeum are described as sources of stereopure (2S/S)-dopaxanthin.
Tyrosinase or polyphenol oxidase (monophenol, o-diphenol:oxygen oxidoreductase; EC 1.14.18.1) is a copper enzyme that catalyzes two different reactions using molecular oxygen: the hydroxylation of monophenols to o-diphenols (monophenolase activity) and the oxidation of the o-diphenols to o-quinones (diphenolase activity; Sánchez-Ferrer et al., 1995). This enzyme is widely distributed in plants, microorganisms, and animals where tyrosinase is responsible for melanization.
A wide variety of plant tyrosinase behaviors has been described and reviewed (Mayer and Harel, 1991; van Gelder et al., 1997). A molecular oxygen-scavenging function in the chloroplast (Vaughn et al., 1988) and a role in plant defense (Constabel et al., 1995) have been suggested. Its implication in the secondary metabolism of betalains has been proposed (Steiner et al., 1999), but the physiological function of tyrosinase in higher plants is yet to be fully determined.
Betalains are water-soluble nitrogen-containing pigments that are present in plants belonging to the order Caryophyllales (Strack et al., 2003) and in the fungal genera Amanita (Musso, 1979) and Hygrocybe (von Ardenne et al., 1974). All betalain pigments contain betalamic acid as the chromophore. Depending upon the nature of the betalamic acid addition residue, the betalains can be classified as either betacyanins or betaxanthins. Betacyanins contain a cyclo-3,4-dihydroxyphenylalanine (cyclo-DOPA; usually glycosylated) residue and exhibit a red/violet coloration, while the betaxanthins contain different amino acid or amine side chains and exhibit a yellow/orange coloration.
Betalains are located in different parts of plants, in roots (Hempel and Böhm, 1997), fruits (Wybraniec and Mizrahi, 2002), and flowers (Stintzing and Carle, 2004). The existence of betalains in flowers (Bougainvillea, Celosia, Gomphrena, Portulaca, Mirabilis, etc.) is especially interesting due to the importance of color in these structures, for which a variety of functions has been proposed (Stintzing and Carle, 2004).
Interest in betalains has grown since their antiradical activity was characterized (Escribano et al., 1998; Kanner et al., 2001; Pedreño and Escribano, 2001), and they are widely used as additives in the food industry because of their natural colorant properties and absence of toxicity, even at high concentrations (Schwartz et al., 1983).
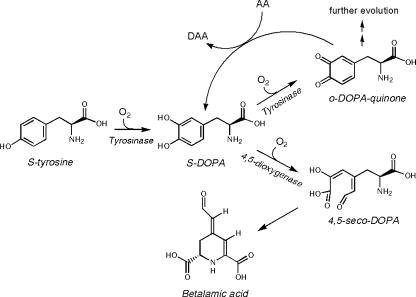
The involvement of the enzyme tyrosinase into the biosynthetic pathway of the betalains has been suggested (Piattelli, 1981; Steiner et al., 1999). The hydroxylation of Tyr to DOPA and the further oxidation of DOPA, both catalyzed by tyrosinase, are considered as the first steps in the biogenesis of betalamic acid (Fig. 1) and betacyanins (Fig. 2, steps 1 and 2). In the proposed pathway for betalamic acid biosynthesis, the amino acid (S)-Tyr is the precursor, and it is oxidized by tyrosinase to produce (S)-DOPA, which is then the substrate for a specific dioxygenase. This enzyme is able to cleave the aromatic ring to obtain the 4,5-seco-DOPA intermediate that spontaneously experiences an intramolecular condensation leading to attainment of betalamic acid (Chang et al., 1974). The enzyme 4,5-dioxygenase has been partially characterized in Amanita muscaria (Girod and Zrÿd, 1991) and recently in Portulaca grandiflora (Christinet et al., 2004). Figure 1 shows the biosynthetic steps transforming (S)-Tyr to betalamic acid.
Figure 1.
Biosynthetic scheme for betalamic acid formation. The structural unit of betalains is derived from the amino acid Tyr by the action of the enzymes tyrosinase and 4,5-dioxygenase (see text for details). The proposed detention of the oxidation process mediated by tyrosinase implies the existence of a reducing agent in the medium.
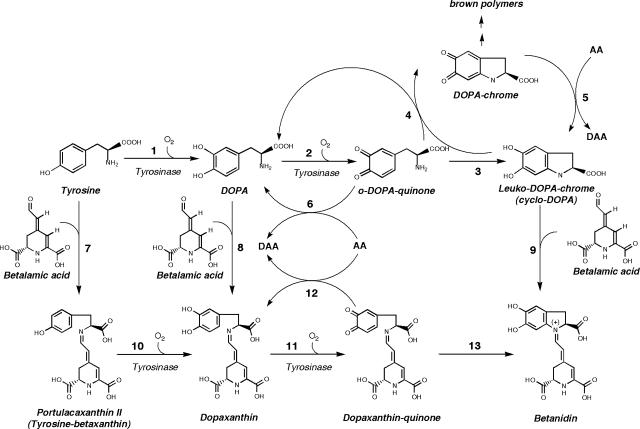
Figure 2.
Betalain biosynthesis. In this scheme, a general overview of the main steps proposed by several authors is provided. For an in-depth review, see Strack et al. (2003). Steps 10 to 13 are proposed here and imply a major role for tyrosinase and the fusion of the routes leading to betaxanthins and betacyanins.
According to the scheme shown in Figure 2, the biogenesis of betacyanins would start by the hydroxylation of Tyr to DOPA and its later oxidation to o-quinone (steps 1 and 2) by the action of the tyrosinase enzyme using molecular oxygen. The spontaneous cyclization (step 3) will lead to cyclo-DOPA (leuko-DOPA-chrome), and it has been proposed that this molecule would react with one molecule of betalamic acid to form betanidin (step 9; Piattelli, 1981). Later in this article, the feasibility of the proposed model will be discussed. The enzyme tyrosinase has been purified from betalain-containing extracts of A. muscaria (Mueller et al., 1996), P. grandiflora (Steiner et al., 1999), and Beta vulgaris (Gandía-Herrero et al., 2004). The detection of tyrosinase transcripts correlating with betacyanin accumulation in Phytolacca americana fruits has also been reported (Joy et al., 1995).
The involvement of tyrosinase in the biosynthetic pathway of betalains has been assumed but it has not been demonstrated conclusively. In this article, a role for tyrosinase in plants is suggested, and the function of tyrosinase in betalain biosynthesis will be approached following a different metabolic pathway from that proposed in the literature.
RESULTS
Extraction and Purification of Dopaxanthin from Yellow Flowers of Lampranthus productus
The first aim of this study was to obtain pure natural dopaxanthin from a natural source. For this purpose, the pigment contents in the petals of yellow flowers of different plants of the order Caryophyllales were analyzed by HPLC. It had been reported that dopaxanthin was the only pigment in Glottiphylum longum flowers (0.3 mg g−1; Impellizzeri et al., 1973). This single pigment composition is not usual in the distribution of betaxanthins, which are frequently reported to appear in mixtures (Piattelli et al., 1965; Kugler et al., 2004). The data published for Glottiphylum were reviewed by using the analytical tools available today, studying in this case the species Glottiphylum oligocarpum and Glottiphylum pigmaeum. The results obtained through HPLC analysis determined that dopaxanthin is the only pigment present in the flowers of these species, confirming the peculiarity of the genus Glottiphylum concerning the simplicity of pigment content in the flowers. The total amount of dopaxanthin was 1.35 mg g−1 fresh flower for G. oligocarpum and 1.38 mg g−1 for G. pigmaeum. The DOPA-derived betaxanthin has been reported as the main pigment responsible for the yellow coloration of flowers of P. grandiflora (Trezzini and Zrÿd, 1991a). Dopaxanthin is also the only pigment present in yellow flowers of L. productus, with a content of 4.17 mg g−1 fresh flower. Both genus Lampranthus and Glottiphylum belong to the Aizoaceae family. In all the cases analyzed, dopaxanthin existed as a single isomer (2S/S). The existence of isomers in betalains is frequently reported (Stintzing and Carle, 2004), attending to the two possibilities in the chiral carbon C-15 present in the betalamic acid moiety of the structure. The HPLC system used in this article is able to resolve both isomers for dopaxanthin, (2S/S) and (2S/R), and no trace of the second was detected in any of the natural extracts. All flower extracts were performed in both phosphate buffer and MeOH, with the same pigment compositions being obtained.
Therefore, the yellow flowers of L. productus were finally selected to obtain dopaxanthin on account of the high content and simplicity of pigment composition together with the higher floration rate compared to Glottiphylum.
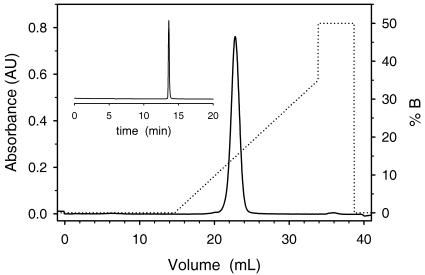
Dopaxanthin was extracted from the petals in buffer at pH 6.0 with 10 mm ascorbic acid (AA). Betalains are highly soluble in water and pH 6.0 favors their stability (von Elbe et al., 1974). AA was added in order to avoid any possible oxidation of the pigment by the extract. Once proteins were removed by ultrafiltration, dopaxanthin was submitted to purification in an automated system based on ionic exchange chromatography. The elution profile obtained for the extracted dopaxanthin can be seen in Figure 3. Pure (2S/S)-dopaxanthin, according to HPLC analysis (Fig. 3, inset), was obtained. No betalamic acid was detected. Ninety-nine percent of the starting dopaxanthin was recovered and submitted to C-18 chromatography in order to remove the NaCl used during the elution process. The yield for the last process was 92%, and the final sample was reduced to dust by freeze drying and used in further experiments.
Figure 3.
Chromatographic profile for the purification of dopaxanthin from extracts of L. productus yellow flowers. Conditions are described under “Materials and Methods.” One milliliter of an extract containing 25 μm dopaxanthin was injected. Elution was followed at 480 nm. Inset, HPLC elution profile of the extract showing the stereopure (2S/S)-dopaxanthin. Full scale is A480 = 0.9 absorbance units; 20 μL of 180 μm dopaxanthin was injected.
Monophenolase Activity: Conversion of Tyr-Betaxanthin to Dopaxanthin by Tyrosinase
Tyr is condensed spontaneously with betalamic acid to obtain Tyr-betaxanthin (portulacaxanthin II). The monophenolic nature of this pigment is apparently susceptible to being oxidized by the enzyme tyrosinase. The commercial mushroom tyrosinase was used as an enzyme source because it is readily available. Furthermore, the enzyme from mushroom is extensively used as a model for studies at molecular and kinetic levels (Seo et al., 2003).
The Tyr-betaxanthin purified by FPLC had a high NaCl content that needed to be removed in order to avoid the inhibition of tyrosinase by chloride (Martínez et al., 1986). The high polarity of the pigments makes separation by organic solvents difficult. Among the solvents assayed, diethyl ether, chloroform, dichloromethane, acetone, acetonitrile, 2-propanol, ethanol, methanol, and dimethyl sulfoxide, the last was the only one where the pigments could be totally solubilized and the salt precipitated. However, the solvent is hard to evaporate (boiling point, 189°C), and, finally, C-18 cartridges were used for removing the salt content by chromatography, with recovery of the target betaxanthins in yields of 92%.
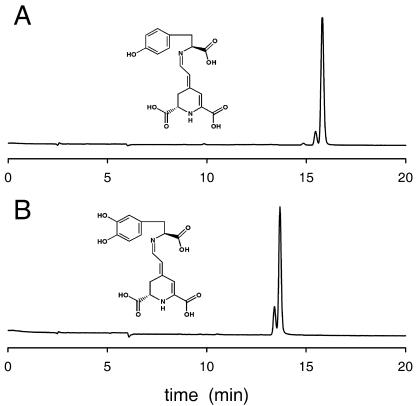
The synthesized and purified Tyr-betaxanthin was analyzed by HPLC, showing the existence of the isomers (2S/S) (major peak) and (2S/R) (minor peak), as can be seen in Figure 4A. The solution was stable under pH 6.0 at 25°C. However, when the enzyme tyrosinase was added (12.5 μg mL−1) and AA was initially present in the medium (20 mm), the pigment content evolved and the peaks corresponding to Tyr-betaxanthin in HPLC analysis disappeared, and two new peaks appeared. The peaks derived from the activity of tyrosinase on Tyr-betaxanthin were assigned to those corresponding to dopaxanthin by coelution and comparison of spectral properties with dopaxanthin directly synthesized from betalamic acid (Fig. 4B).
Figure 4.
HPLC elution profiles for (S)-Tyr (A) and (S)-DOPA (B) immonium conjugates of betalamic acid. (2S/S)-pigments (main peaks) are accompanied by the (2S/R)-diastereoisomeric forms (minor peaks); 20 μL of 9.0 μm solutions was injected. Full scale is the same in both cases (A480 = 0.035 absorbance units).
Interconversion between betaxanthins catalyzed by an enzyme is described. The attainment of the o-diphenol is in agreement with the general behavior of tyrosinase when acting on monophenols under reducing conditions (Valero et al., 2003).
Diphenolase Activity: Enzymatic Oxidation of Dopaxanthin by Tyrosinase and Analysis of the Oxidation Products
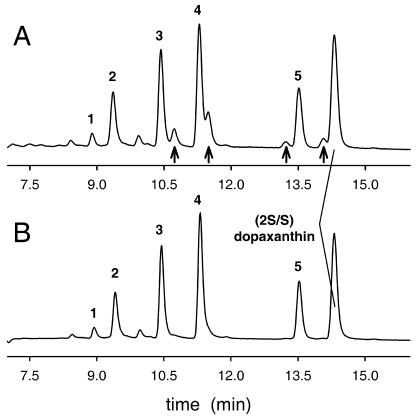
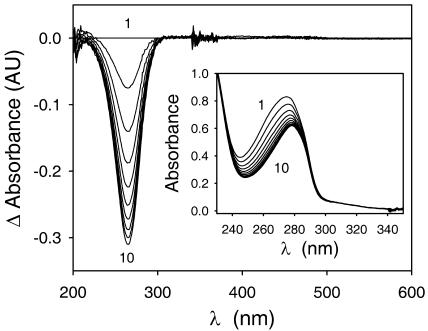
To evaluate the possibility that dopaxanthin was a substrate of the diphenolase activity of the enzyme, a solution of the pigment was prepared in phosphate buffer, pH 6.0, and tyrosinase was added. In the absence of the enzyme, the pigment content is stable, as HPLC analysis demonstrated. However, after its addition, a change in the chromatographic profile occurred. The peaks corresponding to dopaxanthin disappeared and a series of new peaks, previously undescribed, appeared. Figure 5 shows recordings for HPLC analysis corresponding to enzymatic action on natural dopaxanthin containing the isomer (2S/S) (Fig. 5B) and on the semisynthetic pigment that contains, furthermore, the (2S/R) isomer (Fig. 5A). Following the well-characterized mechanism of action of tyrosinase (Burton, 1994; Sánchez-Ferrer et al., 1995), the diphenol is converted into the o-quinone, and the species o-dopaxanthin-quinone is obtained. However, quinones are highly reactive, and they are known to evolve quickly to more stable molecules. In the case of DOPA, there is a nucleophilic attack of the amine on the ring involving an intramolecular cyclization and obtaining leuko-DOPA-chrome (Fig. 2, step 3). The corresponding “leuko” molecule derived from dopaxanthin-quinone is betanidin. However, this molecule could not be identified among the new peaks generated. Spectra and retention times were compared with a betanidin standard obtained by deglycosylation of purified betanin. HPLC retention times and maximum absorbance wavelengths are shown in Table I for the substrate, reaction products, and betanidin.
Figure 5.
Products obtained by the action of tyrosinase on dopaxanthin and discrimination between the products associated to the oxidation of the (2S/S)- and (2S/R)-forms. HPLC recordings show the analysis of the products derived from the action of 6 μg mL−1 of tyrosinase on 20 μm solutions of semisynthetic (A) and natural (B) dopaxanthin. Arrows indicate the products derived from (2S/R)-dopaxanthin, not present in B. Reaction was stopped in both cases after 50 min of reaction. Full scale is A480 = 0.020 absorbance units.
Table I.
Retention times and HPLC-PDA and mass spectrometry data of (2S/S)-dopaxanthin, oxidation products, and betanidin
Masses determined for betanidin and for the products obtained by the action of tyrosinase on dopaxanthin are equal and correspond to a quinone or leuko derivative of the latest. Rt, Retention time.
| Compound | Rt | λmax | [M + H]+ |
|---|---|---|---|
| min | nm | ||
| (2S/S)-Dopaxanthin | 14.46 | 472 | 391 |
| Oxidation product 1 | 8.89 | 480 | 389 |
| Oxidation product 2 | 9.36 | 472 | 389 |
| Oxidation product 3 | 10.43 | 464 | 389 |
| Oxidation product 4 | 11.30 | 464 | 389 |
| Oxidation product 5 | 13.51 | 488 | 389 |
| (2S/S)-Betanidin | 15.08 | 544 | 389 |
To ascertain whether the products were quinones or products derived from them, dopaxanthin was subjected to treatment with the enzyme peroxidase (EC 1.11.1.7; donor:H2O2 oxidoreductase). When this enzyme acts in the presence of H2O2 on an o-diphenolic structure, the result is the formation of the corresponding o-quinone but via a radical mechanism (Sawada et al., 1975; Rodríguez-López et al., 2000) that is completely different from that of tyrosinase. Thus, the oxidation of dopaxanthin by two different methods was achieved. The chromatographic pattern for the reaction medium after the action of peroxidase fit exactly the data registered for tyrosinase treatment (data not shown). Thus, dopaxanthin is converted to a quinone and then a multiplicity of substances is generated.
In an attempt to identify the compounds derived from dopaxanthin oxidation, AA (final concentration 20 mm) was added to the medium after the action of tyrosinase and once the enzyme had been removed by ultrafiltration. AA is a reducing agent able to convert o-quinones back to the diphenols, and any change in the HPLC profile should be considered as proof of the existence of quinones. However, no change was detectable, indicating that the original quinone derived from dopaxanthin was converted into other species not susceptible to AA reduction.
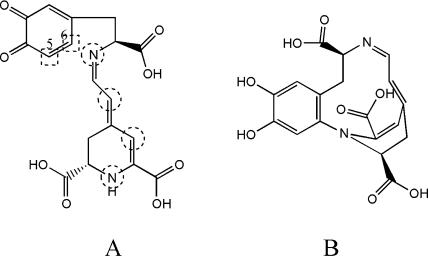
To further characterize the identity of the reaction products, the HPLC protocol described in “Materials and Methods” was applied using a mass spectrometry detector. The mass corresponding to dopaxanthin is 391 m/z ([M + H]+) and the masses determined for the reaction products were in all cases the same, 389 m/z ([M + H]+) and equal to that determined for the betanidin standard. The expected mass for dopaxanthin-quinone is 389 m/z ([M + H]+). All these data indicate that dopaxanthin-quinone evolves to more stable species by intramolecular cyclization rather than by intermolecular condensation since masses corresponding to dimers or other polymers were not detected. The only possibility of obtaining such a variety of nonquinoidal products derived from dopaxanthin-quinone with the masses determined is by following the same process as DOPA-quinone when it is converted into leuko-DOPA-chrome: internal nucleophilic cyclization. In dopaxanthin-quinone, the number of active sites susceptible to giving internal cyclization with positions 5 or 6 of the quinoidal ring (Dagnino-Subiabre et al., 2000) is increased to four by the resonance system present in the betalamic acid moiety of the molecule (Fig. 6). The product derived from the attack of the nitrogen closest to the favorable position 6 of the ring (betanidin) was not obtained in vitro in this work, although different conditions were assayed. A broad range of pH was tested (from pH 4.0 to pH 10.0) and, in an attempt to modify the environment of the enzyme, DMSO was added up to a final concentration of 20%. The temperature was also modified and the products were analyzed after incubation of dopaxanthin with tyrosinase at 25°C, 4°C, and −32°C (with the addition of 50% MeOH). No significant change was observed in the HPLC profile of the derived products with respect to controls. However, the attack of the closest nitrogen atom may be favored in vivo, and further experiments need to be performed to conduct the chemical cyclization stabilizing dopaxanthin-quinone through this nitrogen in order to validate definitively the proposed biosynthetic scheme and to obtain betanidin from dopaxanthin.
Figure 6.
Dopaxanthin-quinone evolution to more stable species implies intramolecular nucleophilic cyclization leading to structures similar to leuko-DOPA-chrome. A, Sites susceptible to giving internal cyclization are circled and the active positions of the ring are shown in squares. B, Suggested structure of the product derived from the attack of the distant nitrogen on position 6.
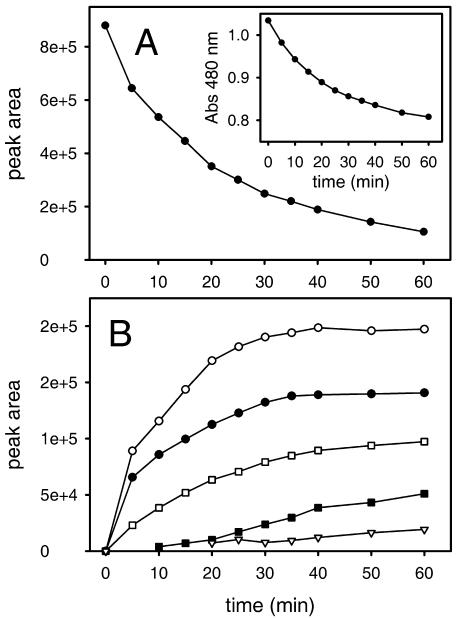
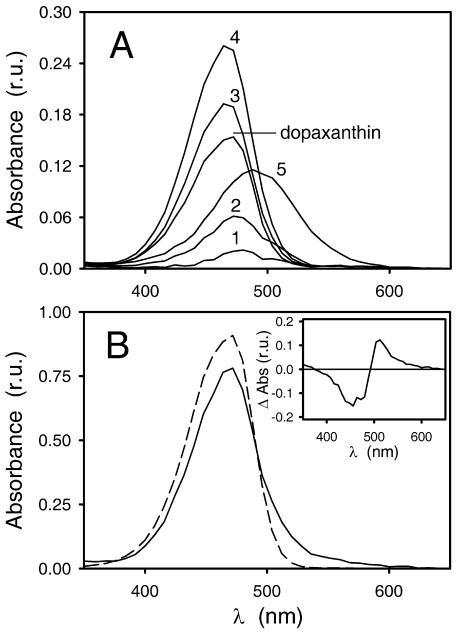
The depletion of substrate (Fig. 7A) and the appearance of the reaction products (Fig. 7B) with time were followed by HPLC. Dopaxanthin oxidation by tyrosinase was also followed spectrophotometrically at 480 nm (Fig. 7A, inset). It can be seen that a hyperbolic-type curve was obtained for the depletion of dopaxanthin in the presence of tyrosinase. Photodiode array (PDA) spectra of the compounds present in the reaction medium during HPLC analysis after 50 min of reaction are shown in Figure 8A.
Figure 7.
Progress curves for (2S/S)-dopaxanthin oxidation by tyrosinase: 6 μg mL−1 of enzyme was added to a 20 μm solution. A, Depletion of the substrate followed by HPLC and spectrophotometry (inset). B, Appearance of reaction products. Products are identified by means of retention times as follows: ▿, 8.89 min; ▪, 9.36 min; •, 10.43 min; ○, 11.30 min; and □, 13.51 min.
Figure 8.
A, PDA spectra of the compounds present in the reaction medium during HPLC analysis after 50 min of reaction. Identification numbers correspond to that of Table I. B, The combination of the individual spectra of products leads to a global spectrum (solid line) that is compared with that obtained for the substrate (2S/S)-dopaxanthin before the reaction started (dotted line). Inset, Subtraction of the dopaxanthin spectrum to the combination of products.
Spectroscopic Analysis of the Oxidation of Dopaxanthin by Tyrosinase
Dopaxanthin oxidation by tyrosinase was also monitored by observing changes in the UV-visible spectrum with time at pH 6.0 (Fig. 9A). Maximal spectral changes were observed at 476 nm (decrease in absorbance), and an isosbestic point was observed in the visible range of the electromagnetic spectrum (λ = 500 nm). Another lower maximum at 514 nm could be detected better by subtracting the initial recording from the rest of the recordings (differential spectra; Fig. 9A, inset). Because these changes were not observed in the absence of the enzyme, they were considered to be results of tyrosinase activity. The results were as expected after comparison of the spectrum of the substrate with that resulting from the addition of the products (Fig. 8B).
Figure 9.
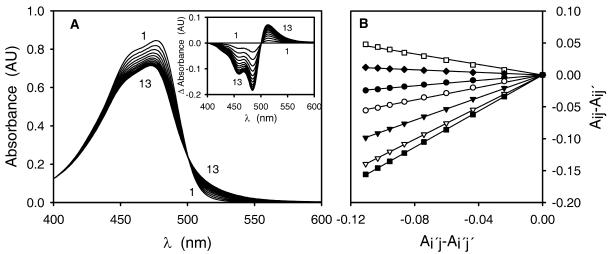
A, Consecutive scan spectra of (S)-dopaxanthin oxidation by tyrosinase. The assay medium contained 17.5 μm dopaxanthin. The reaction was started by addition of the enzyme (12.5 μg mL−1). Scan speed was 1,000 nm min−1 at 3-min intervals. Inset, Differential spectra derived from the previous scans, subtracting the first recording from the rest. B, Coleman graphic analysis of the consecutive spectra shown in A. The test of two species with restrictions was applied. In this analysis, Aij is the absorbance at a wavelength i obtained during tracing j, where i′ = 480 nm, i = • (426 nm), i = ○ (444 nm), i = ▾(470 nm), i = ▿ (478 nm), i = ▪ (487 nm), i = □ (523 nm), i = ♦ (555 nm), and j′ = first tracing.
The existence of an isosbestic point is often considered proof of the presence of only two absorbing species in the analyzed solution. Moreover, the Coleman graphic analysis procedure (Coleman et al., 1970) was conducted in order to determine the number of absorbing species in solution. The convergence of the straight lines at the origin of the coordinate axis (Fig. 9B) predicted the existence of only two absorbing species. However, for the case of dopaxanthin, the presence of at least five different reaction products has been demonstrated by HPLC. The occurrence of an isosbestic point when more than two absorbing species are present and wrong estimation applying the Coleman analysis are possible under the general condition that the changes in the concentrations of the various components are linearly related (Pouët et al., 2004). In the case of dopaxanthin, the concomitant appearance of the products makes the existence of an isosbestic point possible and all the compounds derived from the pigment can be considered as a single absorbing substance. Thus, the final absorbance can be written at a given wavelength λ as the combination of absorbances of the different products (Aa–e):
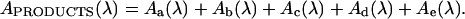 |
(1) |
At a spectral level, the result of the reaction can be considered as a single product with an absorbance spectrum formed as the adjusted addition of the different species. Each individual product contributes to the overall absorbance at a given wavelength according to a fixed factor. At the isosbestic point (λip):
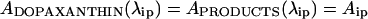 |
(2) |
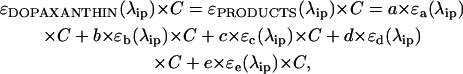 |
(3) |
where a to e are factors relating the concentration between the five products identified and C is the initial concentration of substrate. During the reaction, on transforming a proportion α of dopaxanthin, the absorbance value at λip remains fixed:
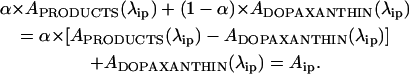 |
(4) |
Hence, an isosbestic point can be maintained with six different absorbing species in the medium.
Kinetic Characterization of Dopaxanthin Oxidation by Tyrosinase
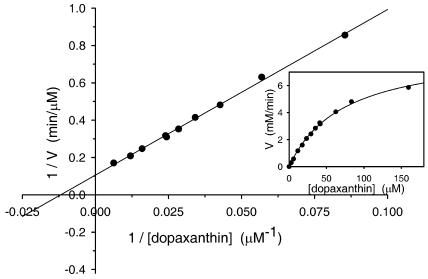
Dopaxanthin oxidation rate by tyrosinase was dependent on substrate concentration (Fig. 10). When the concentration of dopaxanthin was varied in the reaction medium and the resulting steady-state rates were fit by nonlinear regression to the Michaelis-Menten equation, the kinetic parameters were evaluated as Vm = 9.5 μm min−1, Km = 84.3 μm. The value for Km is very low and it is one-half the value reported for the oxidation of the substrate DOPA (Ros et al., 1994), from which dopaxanthin is derived. Another related substrate, 3,4-dihydroxyphenylacetic acid, evaluated against mushroom tyrosinase yielded a Km of 4 mm (Kahn et al., 2000). The high affinity of mushroom tyrosinase for dopaxanthin may be indicative of a physiological role and is related to the data reported for tyrosinases from other sources acting on flavonoids. A Km = 200 μm was obtained for the oxidation of catechin catalyzed by peach tyrosinase (Jiménez-Atiénzar et al., 2004) and Km = 646 μm for the oxidation of quercitin by the enzyme from broad bean (Jiménez and García-Carmona, 1999).
Figure 10.
Lineweaver-Burk plot for the kinetic analysis of the action of tyrosinase on (2S/S)-dopaxanthin. Inset, Dependence of dopaxanthin oxidation on its concentration. The reaction medium contained different substrate concentrations in 50 mm sodium phosphate buffer, pH 6.0, at 25°C with 12.5 μg mL−1 of tyrosinase.
The oxidation rate was calculated taking into account that the coefficients for the products derived from dopaxanthin-quinone are constant, as demonstrated by the existence of the isosbestic point described and the Coleman analysis. Under this condition, and considering that substrate and products absorb in the same range of wavelengths, an apparent molar extinction coefficient (ɛapp) can be defined. This ɛapp relates the increments of absorbance at a given wavelength to the concentration of transformed dopaxanthin that causes it. The wavelength chosen for the determination of ɛapp was λ = 480 nm because the differences between the starting spectrum of dopaxanthin and the final global spectrum obtained for the products were maximum, as can be seen in the differential records (Fig. 9A, inset). The resulting value was ɛapp = 9,580 m−1 cm−1.
DISCUSSION
Formation of cyclo-DOPA: The Previous Model
The hydroxylation of Tyr to DOPA and further oxidation to o-quinone by tyrosinase is considered to be the first step in the biosynthesis of betacyanins (Strack et al., 2003). Spontaneous cyclization leads to the formation of cyclo-DOPA, which is able to react with one molecule of betalamic acid to form betanidin (Fig. 2, steps 1–3 and 9), as suggested by Piattelli (1981).
However, the pathway has not been demonstrated experimentally with clarity because betanidin has only been obtained in vitro through the oxidation of Tyr or DOPA to DOPA-chrome, and its later reduction by high concentrations of AA (Fig. 2, step 5) and further addition to betalamic acid (Schliemann et al., 1998). This mechanism is not likely to occur at a biological level because, if the AA is present during the Tyr or DOPA oxidation, this would reduce dopaquinone to the diphenol (step 6), as has been widely demonstrated (Golan-Goldhirsh and Whitaker, 1984), and cyclo-DOPA or DOPA-chrome could never be obtained. Moreover, the presence of betalamic acid during the first steps should imply its condensation with Tyr or DOPA, leading spontaneously to the corresponding betaxanthins (steps 7 and 8). Therefore, this pathway does not seem likely to lead to the biosynthesis, in vivo, of betacyanins.
Betaxanthins had not been considered as taking part in the biosynthesis of betacyanins. Two different pathways are assumed and only share the formation of the structural unit betalamic acid. Moreover, the direct enzymatic action of tyrosinase on betaxanthins had not been reported, and the formation of betacyanins via condensation with cyclo-DOPA (Fig. 2, steps 1–3 and 9) has been assumed.
Betaxanthins as Substrates for Tyrosinase: A Biosynthetic Scheme
An alternative pathway is proposed based on the addition of betalamic acid to Tyr and/or DOPA to form the corresponding betaxanthins (Fig. 2, steps 7 and 8). According to Figure 2, tyrosinase catalyzes the ortho-hydroxylation of the monophenol Tyr-betaxanthin to the diphenol dopaxanthin (monophenolase activity). Dopaxanthin is oxidized in a subsequent reaction also catalyzed by tyrosinase (diphenolase activity) to give rise to the corresponding o-quinone (dopaxanthin-quinone), with both steps occurring in the presence of molecular oxygen (Fig. 2, reactions 10 and 11). In the absence of any reducing agent in the reaction medium, the o-quinone evolves nonenzymatically to yield a more stable product, such as betanidin (Fig. 2, step 13), in line with the behavior experienced by DOPA (Cánovas et al., 1982). Oxidation to dopaxanthin-quinone by tyrosinase and the evolution of this compound to cycled forms related to betanidin have been demonstrated. The masses determined for the products, together with their insensibility to AA treatment and the well-established mechanism of the action of tyrosinase (Burton, 1994; Sánchez-Ferrer et al., 1995), make the proposal of internal cyclization the only satisfactory explanation for the multiplicity of products derived from the action of tyrosinase on dopaxanthin.
Conditions for the formation of betanidin from dopaxanthin-quinone are still to be fully determined, but this pathway is more plausible at a biological level than the previous one since AA is not necessary and therefore would not interfere in the process of internal cyclization. The formation of betanidin from dopaxanthin-quinone would make tyrosinase the decisive enzyme responsible for the change of color from yellow to violet.
Under the perspective of the proposed scheme, Tyr-betaxanthin should be the first betalain to exist in the pathway and the only one to be formed via direct condensation with betalamic acid. In the presence of a reducing agent such as AA in the reaction medium, the system cannot reach the steady state, and the o-quinone product of the catalytic activity (dopaxanthin-quinone) is continuously reduced to the diphenol concomitant with the oxidation of AA to dehydroascorbic acid (DAA; Fig. 2, step 12). The pathway stops at this point and dopaxanthin is the final product.
In recent years, the biosynthetic scheme of another family of plant pigments has been elucidated: aurones (Nakayama, 2002). The final steps for the formation of the structural unit of this small class of flavonoids from chalcones are catalyzed by the enzyme aureusidin synthase, which has been described as an oxidase with high homology with tyrosinase (Nakayama et al., 2000; Sato et al., 2001). Hydroxylation and oxidative cyclization of the chalcone substrates are in accordance with the mechanism proposed in this article for betanidin biosynthesis and may be considered as a general model for the evolution of pigments after tyrosinase activity.
An Endogenous Reducing Agent Is Necessary in Yellow Flowers
According to the scheme proposed in this article, if dopaxanthin is present providing yellow coloration to flowers, a reducing agent is necessary (Fig. 2, step 12). Among the reducing agents present in plants, AA (vitamin C) is considered to be the most abundant (Smirnoff, 2000). The effect of AA on the tyrosinase-mediated catalysis is the reduction of the o-quinone products of the enzymatic reaction back to their corresponding o-diphenols; several authors (Varoquaux and Sarris, 1979; Ros et al., 1993) have proposed that AA and DAA neither activate nor inhibit the enzyme. AA has been widely used as the model for reducing conditions in diverse studies, including those performed on the biosynthesis of betacyanins (Schliemann et al., 1999), reducing DOPA-chrome to leuko-DOPA-chrome (Fig. 2, step 5). Despite the relevance of the presence of a reducing agent in the biosynthetic scheme of betalains, the occurrence of endogenous AA has not been demonstrated in flowers of plants belonging to the Caryophyllales.
AA shows a characteristic absorbance spectrum with a maximum placed at 265 nm, and its evolution can be followed spectrophotometrically. In order to evaluate the presence of endogenous AA in dopaxanthin-containing flowers of L. productus, extracts without the addition of AA were performed. Endogenous AA was removed from the extract enzymatically through treatment with the enzyme ascorbate oxidase. Ascorbate oxidase (EC 1.10.3.3; l-ascorbate: O2 oxidoreductase) is an enzyme that catalyzes the oxidation of l-AA to l-DAA in the presence of molecular oxygen. Figure 11 shows the evolution of the extract after the addition of the enzyme to the medium. The evolution of the specific depletion of AA is better shown when differential spectra are represented. The level of AA determined was 0.8 mg g−1 petal fresh weight, which is more than 4 times higher than the levels reported for beet roots (0.15 mg g−1; Jiratanan and Liu, 2004) or cactus pears (0.18–0.23 mg g−1; Stintzing et al., 2001). AA could therefore be the reducing agent needed for dopaxanthin biosynthesis.
Figure 11.
Depletion of endogenous AA from L. productus flower extract after the addition of ascorbate oxidase (0.07 units mL−1). Differential spectra show negative recordings of the characteristic absorbance spectrum of AA derived from consecutive spectra (inset). Scan speed was 1,000 nm min−1 at 1-min intervals.
Moreover, the presence of AA would also be necessary for the biosynthesis of betalamic acid from Tyr since DOPA needs to be accumulated in the medium to be the substrate of 4,5-dioxygenase (Fig. 1). Without AA and in the presence of tyrosinase (the pathway previously proposed), Tyr evolves to dopaquinone and DOPA-chrome, and the level of DOPA under these conditions is negligible (Cabanes et al., 1987), making the biosynthesis of betalamic acid improbable.
CONCLUSION
Evidence of the involvement of tyrosinase in the metabolism of betalains is provided. Tyrosine-betaxanthin and dopaxanthin are described as substrates of tyrosinase. Based on the results of the enzymatic activity and considering the mechanism of action of tyrosinase on simple phenolic substrates, a mechanism for betanidin (the structural unit of violet betacyanins) synthesis is provided.
It has long been assumed that tyrosinase is not directly involved in the biosynthesis of low-Mr phenolics in plants (Vaughn et al., 1988). Our findings on the betalain biosynthetic scheme, together with the research of other authors (Strack et al., 2003) and the recent studies on aurones (Nakayama, 2002), provide evidence for a biological role of tyrosinase in the biosynthesis of plant pigments and flower coloration.
MATERIALS AND METHODS
Chemicals
Mushroom tyrosinase (2,590 units mg−1 solid, lot 092K70491), other enzymes, chemicals, and reagents were purchased from Sigma (St. Louis). Solvents came from Merck Chemicals (Dorset, UK). HPLC-grade acetonitrile was purchased from Labscan (Dublin). Distilled water was purified using a Milli-Q system (Millipore, Bedford, MA).
Plant Material and Sample Preparation
Glottiphylum oligocarpum and Glottiphylum pigmaeum plants were obtained from private collections from the association of Friends of the Cacti and Other Succulents (ACYS; Botanical Garden of the University of Valencia, Valencia, Spain). Lampranthus productus plants were grown by the authors in Murcia, Spain. Yellow flower samples were carefully collected, and the petals were removed and washed. Pigments were extracted in 10 mm phosphate buffer, pH 6.0, containing 10 mm AA in a polytron homogenizer (5 s, 2 pulses at medium speed; Kinematica AG, Littau, Switzerland). The homogenate was filtered through nylon cloth and centrifuged at 120,000g for 40 min. The supernatant was then filtered through Centriplus YM-10 membranes (Millipore) to remove proteins and the filtrate was used for pigment analysis or further purification. The whole process was carried out at 4°C. Flower extracts were also carried out in pure MeOH and filtrated through 0.45-μm PVDF filters (Millipore) before analysis.
Betaxanthin (Dopaxanthin and Tyr-Betaxanthin) Semisynthesis
Betaxanthin standards were necessary for the identification of the natural pigments of the different flower extracts and for the study of the behavior of tyrosinase in an alternative mechanism proposed for betalain biosynthesis. Synthetic betaxanthins were obtained as immonium condensation products of betalamic acid with (S)-forms of the amino acids DOPA and Tyr, as described by Wyler et al. (1965), with some modifications. Briefly, aliquots of a 0.15 mm betanin:isobetanin mixture (95:5) purified from red beet extracts were used as a source of betalamic acid. Basic hydrolysis (pH 11.4) of betanin released the acid (Huang and von Elbe, 1985), which was condensed with the amino acid after reaching pH 5.0. Seven hundred micromoles of the selected amino acid per micromole of betalamic acid were added to the solution prior to basic hydrolysis. The corresponding betaxanthin was obtained, accompanied by a color change from pale yellow (betalamic acid, λm = 424 nm) to deep yellow (betaxanthins, λm = 480 nm). Immediately after synthesis was achieved, an automated system was used for purification to remove the excess substrates and secondary products affecting stability. This procedure is described in the FPLC purification section.
Betanin was obtained from commercial red beet. Extraction was performed in 10 mm phosphate buffer, pH 6.0, in a model 230 Omnimixer (Sorvall, Norwalk, CT) at maximum speed for 10 s. The homogenate was filtered through cheesecloth and centrifuged at 120,000g. The supernatant was then filtered using a YM-10 membrane (Millipore) to remove proteins. All steps were carried out at 4°C. The pigment was then purified according to the method described by Escribano et al. (1998). A Sephadex G-25 (Sigma) gel was conditioned and used in a 30-mL column. Elution was performed with a volume of 40 mL of water, and 1-mL fractions were collected. The elution process was followed at 536 and 480 nm. Fractions containing purified betanin were pooled.
FPLC Purification
Anionic exchange chromatography of synthetic betaxanthins and natural (2S/S)-dopaxanthin was performed in an Äkta purifier apparatus (Amersham Biosciences, Uppsala). The equipment was operated completely via a PC using Unikorn software version 3.00. Elutions were followed at 280, 480, and 536 nm. The purification process was aimed at obtaining the betaxanthin free from the corresponding amino acid and without cyclo-DOPA-glucoside.
Solvents used were BisTris, 20 mm, pH 6.0 (solvent A), and BisTris 20 mm, pH 6.0, with NaCl 2 m (solvent B). A 25- × 7-mm, 1-mL Q-Sepharose fast flow column (cross-linked agarose with quaternary ammonium as exchanger group, 90 μm of particle size; Amersham Biosciences) was used. After sample injection, the elution process was as follows: 0% B from beginning to 15 mL; after washing, a linear gradient was developed from 0% B to 35% B in 20 mL, with 1-mL fractions being collected. Cleaning (7 mL, 50% B) and reequilibration (7 mL, 100% A) steps were performed between each elution. Injection volume was 1 mL and the flow rate was 0.5 mL min−1. Amino acids were unable to interact with the column under the working conditions and were totally washed out as an unbound fraction (followed by A280). As previously stated, in the starting material for synthetic betaxanthins there is 5% of betanin present in the isomeric form isobetanin. As isobetanin is processed in the same way as betanin, the synthesized and purified betaxanthins have 5% in the “iso” form (2S/R).
C-18 Chromatography
One-milliliter C-18 cartridges (Waters, Milford, MA) were conditioned with 5 mL of methanol followed by 10 mL of purified water. Betaxanthins dissolved in water were injected and bound to the column. Salts and buffers eluted first and were washed off by rinsing the column with water. Dopaxanthin and Tyr-betaxanthin were eluted isocratically with water. Samples were freeze-dried and compounds obtained as powders. All samples were stored at −80°C until use.
HPLC Analysis
PDA Detection
A Shimadzu LC-10A apparatus equipped with a SPD-M10A PDA detector was used for analytical HPLC purifications. Reverse-phase chromatography was performed with a 250- × 4.6-mm Kromasil 100 C-18 column packed with 5-μm particles (Teknokroma, Barcelona). Gradients were formed between two helium-degassed solvents. Solvent A was composed of water with 0.05% trifluoroacetic acid, and solvent B was composed of acetonitrile with 0.05% trifluoroacetic acid. A linear gradient was performed in 20 min from 0% B to 28% B. The flow rate was 1 mL min−1, operated at 25°C. Injection volume was 20 μL. Pigments from natural samples and standards had the same retention times and superimposable spectra.
HPLC-Mass Spectrometry
For HPLC-electrospray ionization-mass spectrometry analyses, an Agilent VL 1100 apparatus with a liquid chromatography-mass selective detector trap (Agilent Technologies, Palo Alto, CA) was used. Elution conditions were as described above using a Zorbax SB-C18 (30- × 2.1-mm, 3.5 μm) column (Agilent Technologies) with a flow rate of 0.3 mL min−1. Vaporizer temperature was 350°C and voltage was maintained at 3.5 kV. Sheath gas was nitrogen operated at a pressure of 35 psi. Samples were ionized in positive mode. Ion-monitoring mode was full scan in the range m/z 60 to 600. For detection, the electron multiplier voltage was 1,350 V.
Before analysis, all samples derived from enzymatic activities were ultrafiltrated through YM-10 membranes (Millipore) in order to remove the catalyst and stop the reaction.
Absorbance Spectroscopy
For absorbance spectroscopy, a Kontron Uvikon 940 spectrophotometer was used.
Quantification of Betalains
Pigment concentration was evaluated through absorbance, taking a molar extinction coefficient of ɛ = 48,000 m−1 cm−1 at 480 nm for betaxanthins (Trezzini and Zrÿd, 1991b; Schliemann et al., 1999) and ɛ = 65,000 m−1 cm−1 and ɛ = 54,000 m−1 cm−1 at 536 nm for betanin and betanidin, respectively (Schwartz and von Elbe, 1980). Measurements were made in water at 25°C.
Tyrosinase Assays
Unless otherwise stated, the reaction medium (1.0 mL) contained 50 mm sodium phosphate buffer, pH 6.0, and 12.5 μg mL−1 of the enzyme. Other conditions and reagents are detailed in the text and in the figure legends. All experiments were performed in triplicate. Cuvettes of 0.5 and 1 cm of light path were used.
The apparent molar extinction coefficient corresponding to dopaxanthin oxidation at 480 nm was determined by an end-point method, carrying out a set of experiments at initial 10, 17.5, and 25 μm concentrations and at sufficiently high enzyme concentration and incubation time, and allowing the reaction to proceed until all the substrate had been converted to product. The data thus obtained were fit by least-squares linear regression.
Absorption spectra were also recorded with the above instrument. Kinetic data analysis was carried out by using linear and nonlinear regression fitting (Marquardt, 1963) using SigmaPlot Scientific Graphing for Windows version 8.0 (2001; SPSS, Chicago).
Peroxidase Assay
The medium for peroxidase-mediated oxidation of dopaxanthin (1.0 mL) contained 50 mm sodium acetate buffer, pH 5.0, 0.45 mm H2O2, and 17.5 μm (2S/S)-dopaxanthin. The reaction started with the addition of horseradish peroxidase, type VI (1 unit mL−1), and was followed spectrophotometrically at 480 nm, at 25°C. Controls without H2O2 or horseradish peroxidase were made to ascertain pigment stability. Products were analyzed by HPLC following the standard protocol.
Betanin Deglycosylation
Betanidin was obtained enzymatically from purified betanin through β-glucosidase (EC 3.2.1.21; β-d-glucoside glucohydrolase) treatment. A 4 μm betanin solution was incubated for 30 min with 14 units mL−1 of β-glucosidase (Sigma) in 50 mm acetate buffer, pH 5.0, at 25°C. The enzyme was removed by ultrafiltration through YM-10 membranes. Transformation was complete according to HPLC analysis.
Ascorbate Oxidase Treatment
L. productus extract was carried out in 10 mm phosphate buffer, pH 6.0, under the same conditions described above but without the addition of AA. Endogenous AA was depleted from the extract enzymatically through treatment with the enzyme ascorbate oxidase. Fresh extract aliquots were diluted to give A265 below 1 unit and incubated with 0.07 units mL−1 of the enzyme (provided by Sigma) in 10 mm phosphate buffer, pH 6.0, at 25°C. The process was followed by UV-visible spectrophotometry until it was completed. The concentration of oxidized AA was calculated from the decrease in A265 using the molar extinction coefficient of 16,500 m−1 cm−1 for AA (Davies et al., 1991). The experiment was repeated three times with different extracts.
Acknowledgments
The authors are grateful to J. Lozano, president of the ACYS (Botanical Garden of the University of Valencia, Valencia, Spain) for kindly providing Glottiphylum plants and to Dr. M.D. Alcázar for growing them.
This work was supported by two grants from the Ministerio de Ciencia y Tecnología, Madrid, and FEDER (project no. AGL2003–05647) and the Fundación Séneca, Consejería de Agricultura, Agua y Medio Ambiente, Murcia, Spain, and FEDER (project no. AGR/11/FS/02). F.G.-H. holds a fellowship from the Fundación Séneca, Murcia, Spain.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.057992.
References
- Burton SG (1994) Biocatalysis with polyphenol oxidase—a review. Catal Today 22: 459–487 [Google Scholar]
- Cabanes J, García-Cánovas F, Lozano JA, García-Carmona F (1987) A kinetic study of the melanization pathway between L-tyrosine and dopachrome. Biochim Biophys Acta 923: 187–195 [DOI] [PubMed] [Google Scholar]
- Cánovas FG, García-Carmona F, Sánchez JV, Pastor JLI, Teruel JAL (1982) The role of pH in the melanin biosynthesis pathway. J Biol Chem 257: 8738–8744 [PubMed] [Google Scholar]
- Chang C, Kimler L, Mabry TJ (1974) Biogenesis of betalamic acid. Phytochemistry 13: 2771–2775 [Google Scholar]
- Christinet L, Burdet FX, Zaiko M, Hinz U, Zrÿd JP (2004) Characterization and functional identification of a novel plant 4,5-extradiol dioxygenase involved in betalain pigment biosynthesis in Portulaca grandiflora. Plant Physiol 134: 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JS, Varga LP, Mastin SH (1970) Graphical method for determining the number of species in solution from spectrophotometric data. Inorg Chem 9: 1015–1020 [Google Scholar]
- Constabel CP, Bergey DR, Ryan CA (1995) Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc Natl Acad Sci USA 92: 407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnino-Subiabre A, Cassels BK, Baez S, Johansson A-S, Mannervik B, Segura-Aguilar J (2000) Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochem Biophys Res Commun 274: 32–36 [DOI] [PubMed] [Google Scholar]
- Davies MB, Austin J, Partridge DA (1991) Vitamin C. Its Chemistry and Biochemistry. Thomas Grahan House, Cambridge, pp 36, 117
- Escribano J, Pedreño MA, García-Carmona F, Muñoz R (1998) Characterization of the antiradical activity of betalains from Beta vulgaris L. roots. Phytochem Anal 9: 124–127 [Google Scholar]
- Gandía-Herrero F, García-Carmona F, Escribano J (2004) Purification and characterization of a latent polyphenol oxidase from beet root (Beta vulgaris L.). J Agric Food Chem 52: 609–615 [DOI] [PubMed] [Google Scholar]
- Girod PA, Zrÿd JP (1991) Biogenesis of betalains: purification and partial characterization of DOPA 4,5-dioxygenase from Amanita muscaria. Phytochemistry 30: 169–174 [Google Scholar]
- Golan-Goldhirsh A, Whitaker JR (1984) Effect of ascorbic acid, sodium bisulfite, and thiol compounds on mushroom polyphenol oxidase. J Agric Food Chem 32: 1003–1009 [Google Scholar]
- Hempel J, Böhm H (1997) Betaxanthin pattern of hairy roots from Beta vulgaris var lutea and its alteration by feeding of amino acids. Phytochemistry 44: 847–852 [Google Scholar]
- Huang AS, von Elbe JH (1985) Kinetics of the degradation and regeneration of betanine. J Food Sci 50: 1115–1120 [Google Scholar]
- Impellizzeri G, Piattelli M, Sciuto S (1973) A new betaxanthin from Glottiphylum longum. Phytochemistry 12: 2293–2294 [Google Scholar]
- Jiménez M, García-Carmona F (1999) Oxidation of the flavonol quercetin by polyphenol oxidase. J Agric Food Chem 47: 56–60 [DOI] [PubMed] [Google Scholar]
- Jiménez-Atiénzar M, Cabanes J, Gandía-Herrero F, García-Carmona F (2004) Kinetic analysis of catechin oxidation by polyphenol oxidase at neutral pH. Biochem Biophys Res Commun 319: 902–910 [DOI] [PubMed] [Google Scholar]
- Jiratanan T, Liu RH (2004) Antioxidant activity of processed table beets (Beta vulgaris var, conditiva) and green beans (Phaseolus vulgaris L.). J Agric Food Chem 52: 2659–2670 [DOI] [PubMed] [Google Scholar]
- Joy RW IV, Sugiyama M, Fukuda H, Komamine A (1995) Cloning and characterization of polyphenol oxidase cDNAs of Phytolacca americana. Plant Physiol 107: 1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn V, Ben-Shalom N, Zakin V (2000) p-Hydroxyphenylacetic acid and 3,4-dihydroxyphenylacetic acid as substrates for mushroom tyrosinase. J Food Biochem 24: 1–19 [Google Scholar]
- Kanner J, Harel S, Granit R (2001) Betalains, a new class of dietary cationized antioxidants. J Agric Food Chem 49: 5178–5185 [DOI] [PubMed] [Google Scholar]
- Kugler F, Stintzing FC, Carle R (2004) Identification of betalains from petioles of differently colored Swiss chard (Beta vulgaris L. ssp. cicla [L.] alef. cv. bright lights) by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem 52: 2975–2981 [DOI] [PubMed] [Google Scholar]
- Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11: 431–441 [Google Scholar]
- Martínez JH, Solano F, Peñafiel R, Galindo JD, Iborra JL, Lozano JA (1986) Comparative study of tyrosinases from different sources: relationship between halide inhibition and the enzyme active site. Comp Biochem Physiol B 83: 633–636 [DOI] [PubMed] [Google Scholar]
- Mayer AM, Harel E (1991) Phenoloxidases and their significance in fruit and vegetables. In PF Fox, ed, Food Enzymology, Ed 1, Vol 1. Elsevier, London, pp 373–398
- Mueller LA, Hinz U, Zrÿd JP (1996) Characterization of a tyrosinase from Amanita muscaria involved in betalain biosynthesis. Phytochemistry 42: 1511–1515 [Google Scholar]
- Musso H (1979) Pigments of fly agaric, Amanita muscaria. Tetrahedron 35: 2843–2853 [Google Scholar]
- Nakayama T (2002) Enzymology of aurone biosynthesis. J Biosci Bioeng 94: 487–491 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Yonekura-Sakakibara K, Sato T, Kikuchi S, Fukui Y, Fukuchi-Mizutani M, Ueda T, Nakao M, Tanaka Y, Kusumi T, et al (2000) Aureusidin synthase: a polyphenol oxidase homolog responsible for flower coloration. Science 290: 1163–1166 [DOI] [PubMed] [Google Scholar]
- Pedreño MA, Escribano J (2001) Correlation between antiradical activity and stability of betanine from Beta vulgaris L roots under different pH, temperature and light conditions. J Sci Food Agric 81: 627–631 [Google Scholar]
- Piattelli M (1981) The betalains: structure, biosynthesis, and chemical taxonomy. In EE Conn, ed, The Biochemistry of Plants, Vol 7. Academic Press, New York, pp 557–575
- Piattelli M, Minale L, Nicolaus RA (1965) Betaxanthins from Mirabilis jalapa L. Phytochemistry 4: 817–823 [Google Scholar]
- Pouët M-F, Baures E, Vaillant S, Thomas O (2004) Hidden isosbestic point(s) in ultraviolet spectra. Appl Spectrosc 58: 486–490 [DOI] [PubMed] [Google Scholar]
- Rodríguez-López JN, Gilabert MA, Tudela J, Thorneley RNF, García-Cánovas F (2000) Reactivity of horseradish peroxidase compound II toward substrates: kinetic evidence for a two-step mechanism. Biochemistry 39: 13201–13209 [DOI] [PubMed] [Google Scholar]
- Ros JR, Rodríguez-López JN, García-Cánovas F (1993) Effect of L-ascorbic acid on the monophenolase activity of tyrosinase. Biochem J 295: 309–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros JR, Rodríguez-López JN, García-Cánovas F (1994) Tyrosinase: kinetic analysis of the transient phase and the steady state. Biochim Biophys Acta 1204: 33–42 [DOI] [PubMed] [Google Scholar]
- Sánchez-Ferrer A, Rodríguez-López JN, García-Cánovas F, García-Carmona F (1995) Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta 1247: 1–11 [DOI] [PubMed] [Google Scholar]
- Sato T, Nakayama T, Kikuchi S, Fukui Y, Yonekura-Sakakibara K, Ueda T, Nishino T, Tanaka Y, Kusumi T (2001) Enzymatic formation of aurones in the extracts of yellow snapdragon flowers. Plant Sci 160: 229–236 [DOI] [PubMed] [Google Scholar]
- Sawada Y, Iyanagi T, Yamazaki I (1975) Relation between redox potentials and rate constants in reactions coupled with the system oxygen-superoxide. Biochemistry 14: 3761–3764 [DOI] [PubMed] [Google Scholar]
- Schliemann W, Kobayashi N, Strack D (1999) The decisive step in betaxanthin biosynthesis is a spontaneous reaction. Plant Physiol 119: 1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliemann W, Steiner U, Strack D (1998) Betanidin formation from dihydroxyphenylalanine in a model assay system. Phytochemistry 49: 1593–1598 [DOI] [PubMed] [Google Scholar]
- Schwartz SJ, von Elbe JH (1980) Quantitative determination of individual betacyanin pigments by high-performance liquid chromatography. J Agric Food Chem 28: 540–543 [Google Scholar]
- Schwartz SJ, von Elbe JH, Pariza MW, Goldsworthy T, Pilot HC (1983) Inability of red beet betalain pigments to initiate or promote hepatocarcinogenesis. Food Chem Toxicol 21: 531–535 [DOI] [PubMed] [Google Scholar]
- Seo S-Y, Sharma VK, Sharma N (2003) Mushroom tyrosinase: recent prospects. J Agric Food Chem 51: 2837–2853 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-faceted molecule. Curr Opin Plant Biol 3: 229–235 [PubMed] [Google Scholar]
- Steiner U, Schliemann W, Böhm H, Strack D (1999) Tyrosinase involved in betalain biosynthesis of higher plants. Planta 208: 114–124 [Google Scholar]
- Stintzing FC, Carle R (2004) Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci Technol 15: 19–38 [Google Scholar]
- Stintzing FC, Schieber A, Carle R (2001) Phytochemical and nutritional significance of cactus pear. Eur Food Res Technol 212: 396–407 [Google Scholar]
- Strack D, Vogt T, Schliemann W (2003) Recent advances in betalain research. Phytochemistry 62: 247–269 [DOI] [PubMed] [Google Scholar]
- Trezzini GF, Zrÿd JP (1991. a) Two betalains from Portulaca grandiflora. Phytochemistry 30: 1897–1899 [Google Scholar]
- Trezzini GF, Zrÿd JP (1991. b) Characterization of some natural and semi-synthetic betaxanthins. Phytochemistry 30: 1901–1903 [Google Scholar]
- Valero E, Lozano MI, Varón R, García-Carmona F (2003) Enzymatic synthesis of 3′-hydroxyacetaminophen catalyzed by tyrosinase. Biotechnol Prog 19: 1632–1638 [DOI] [PubMed] [Google Scholar]
- van Gelder CWG, Flurkey WH, Wichers HJ (1997) Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 45: 1309–1323 [DOI] [PubMed] [Google Scholar]
- Varoquaux P, Sarris J (1979) Influence de l'acide ascorbique sur la cinetique de lo-diphenoloxydase du champignon de Paris (Agaricus bisporus). Lebensm-Wiss Technol 12: 318–320 [Google Scholar]
- Vaughn KC, Lax AR, Duke SO (1988) Polyphenol oxidase: the chloroplast oxidase with no established function. Physiol Plant 72: 659–665 [Google Scholar]
- von Ardenne R, Döpp H, Musso H, Steiglich W (1974) Über das vorkommen von muscaflavin bei hygrocyben (agaricales) und seine dihydroazepin-struktur. Z Naturforsch 29c: 637–639 [Google Scholar]
- von Elbe JH, Maing I-Y, Amundson CH (1974) Color stability of betanin. J Food Sci 39: 334–337 [Google Scholar]
- Wybraniec S, Mizrahi Y (2002) Fruit flesh betacyanin pigments in Hylocereus cacti. J Agric Food Chem 50: 6086–6089 [DOI] [PubMed] [Google Scholar]
- Wyler H, Wilcox ME, Dreiding AS (1965) Umwandlung eines betacyans in ein betaxanthin. Synthese von indicaxanthin aus betanin. Helv Chim Acta 48: 361–366 [Google Scholar]