Abstract
Scots pine (Pinus sylvestris) is known to change its terpenoid metabolism in response to egg deposition by the sawfly Diprion pini (Hymenoptera, Diprionidae). Three days after egg deposition, parts of the pine twig adjacent to the egg-laden one are induced to emit volatiles, which attract egg parasitoids. In this study, we investigated whether egg deposition by this sawfly affects pine photosynthesis. Measurements of photosynthesis were taken from untreated control twigs and from pine twigs adjacent to egg-laden ones (i.e. systemically oviposition-induced twigs) for a period of 3 d starting after egg deposition. The net photosynthetic rate of oviposition-induced pine twigs was lower than that of untreated control twigs, whereas the respiration rate of pine twigs was not affected by egg deposition. CO2 response curves of oviposition-induced twigs tended to be lower than those of controls. The potential rate of electron transport (Jmax) and the maximum rate of Rubisco activity (Vcmax) were calculated from the data of the CO2 response curves. Jmax of oviposition-induced twigs was significantly lower than that of controls at day 1 after egg deposition, while the difference diminished from day 2 to day 3. A similar pattern was observed for Vcmax. Light response curves of oviposition-induced twigs were significantly lower than those of untreated ones during 3 d of measurements. Stomatal conductance was slightly lowered by egg deposition. When considering photosynthetic activity as a physiological currency to measure costs of induction of plant defense, the effects of insect egg deposition on gas exchange of pine are discussed with respect to known effects of insect feeding on the photosynthesis activity of plants.
Induced responses of plants to herbivore feeding damage have been studied extensively. These responses include changes in plant chemical composition, phenology, morphology, growth, and photosynthesis (for review, see Karban and Baldwin, 1997). Effects of herbivory on photosynthesis have been studied both on a local scale at the damaged leaf tissue and on a systemic scale by investigating photosynthetic activity of undamaged leaves adjacent to the damaged ones. Local effects measured at leaf tissue structurally damaged by mesophyll feeders, such as Diptera, Hemiptera, or Acari, or at leaf tissue right next to feeding holes produced by chewing insects often show that photosynthetic activity is reduced (Welter, 1989; Zangerl et al., 2002; Haile and Higley, 2003). Many studies on systemic effects of herbivory measured at undamaged leaves adjacent to the sites where chewing herbivores removed leaf material show an enhancement of the photosynthetic rate (Welter, 1989; Zangerl, 1999).
In comparison to the effects of insect feeding on plant metabolism, little is known about how a plant responds to insect egg deposition. Gall insects are known to disturb the inner architecture of a leaf by inserting their eggs (Hilker et al., 2002b). In a few cases, insect egg deposition was shown to induce a hypersensitive response of plant tissue, a well-known response of plants to phytopathogens (Shapiro and DeVay, 1987; Balbyshev and Lorenzen, 1997). Specific pea (Pisum sativum) lines were shown to form neoplasms in response to bruchid egg deposition (Doss et al., 1995, 2000). During the last years, several studies have shown that insect egg deposition induces a change of plant volatiles, thereby attracting egg parasitoids. This induction of volatiles by insect egg deposition is known to occur locally at the site of egg laying and systemically at plant tissue adjacent to the oviposition site (Meiners and Hilker, 2000; Hilker et al., 2002a; Colazza et al., 2004). In contrast to insect feeding, egg deposition of free-living herbivorous insects is investigated with respect to its effects on the plant's primary metabolism.
In this study, we investigated whether gas exchange of Scots pine (Pinus sylvestris) is affected by egg deposition of the sawfly Diprion pini, which often occurs in high population densities on pine (Pschorn-Walcher, 1982, 1988). Larvae of D. pini may heavily damage pine forests by their feeding activity, while adults do not feed upon the plant. The secondary metabolism of Scots pine is known to be changed by egg deposition of D. pini. Pine twigs carrying eggs are induced to emit volatiles that attract the eulophid egg parasitoid Chrysonotomia ruforum, which kills the eggs of the herbivore. Hilker et al. (2002a, 2002b) interpreted this oviposition-induced release of volatiles attracting egg parasitoids as a preventive induced defense strategy acting prior to feeding damage. The attractive volatiles are emitted both from parts of the pine twigs carrying eggs (local induction) and from parts free of eggs but adjacent to the egg-laden parts (systemic induction; Hilker et al., 2002a). The terpenoid volatile pattern of systemically oviposition-induced pine twigs changes quantitatively after an induction time of 3 d compared to controls (Mumm et al., 2003).
To examine the effect of egg deposition by D. pini on the primary metabolism of Scots pine, net photosynthesis activity of systemically oviposition-induced pine twigs and of untreated, egg-free ones were compared. To obtain information about the effect of egg deposition on the potential electron transport (Jmax), Rubisco activity (Vmax), and stomatal conductance, CO2 response curves were measured. In order to gain further information about the light response, pine twigs were also subjected to decreasing light intensities and light response curves were generated.
RESULTS
Continuous Measurement of Gas Exchange
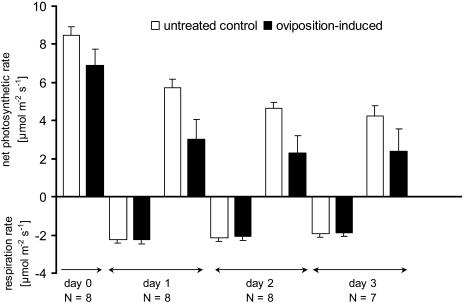
The net photosynthetic rate of Scots pine twigs was significantly affected by egg deposition of the sawfly D. pini (Fig. 1). Systemically oviposition-induced twigs showed a significantly lower rate than controls during all days of the measurements. Both in treated and control twigs, the net photosynthetic rate and respiration rate decreased during the 3-d measurement period. There was no interaction observed between treatment and time (days; Table I). Thus, the oviposition-induced twigs showed no stronger decrease of the net photosynthetic rate than the controls with increasing time. The respiration rate, which also decreased during the period of measurement, was not affected by the oviposition treatment.
Figure 1.
Net photosynthetic rate (mean ± se) of systemically oviposition-induced twigs of Scots pine and untreated controls. Continuous measurements during 3 d. Light saturation of approximately 1,100 μmol m−2 s−1 during the light period (18 h), no light during the dark period (6 h), and a CO2 concentration of 350 μmol mol−1 were given.
Table I.
Repeated-measures ANOVA of continuously measured gas exchange
Rate of photosynthesis and respiration of systemically oviposition-induced twigs of Scots pine and untreated controls were compared (Fig. 1). df, Degrees of freedom; MS, mean squares; F, F value, F test.
| Measurement | Source | df | MS | F | P |
|---|---|---|---|---|---|
| Photosynthetic rate | Treatment | 1 | 45.277 | 7.133 | 0.037 |
| Day | 3 | 57.676 | 13.721 | 0.001 | |
| Treatment × day | 3 | 1.0208 | 1.625 | 0.219 | |
| Respiration rate | Treatment | 1 | 0.002 | 0.004 | 0.953 |
| Day | 2 | 0.307 | 8.789 | 0.004 | |
| Treatment × day | 2 | 0.010 | 0.726 | 0.504 |
CO2 Response Curves
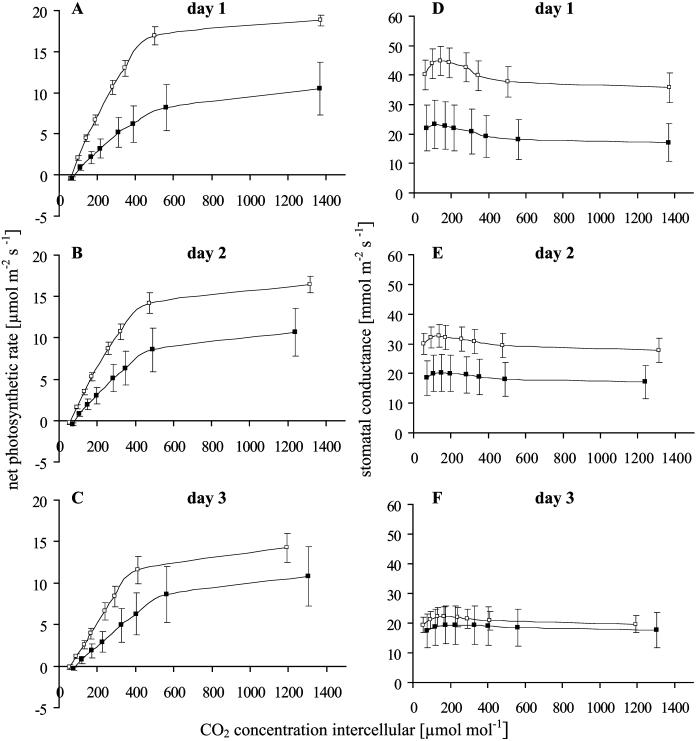
Both oviposition-induced and control pine twigs significantly responded to increasing CO2 concentrations by enhancing their net photosynthetic rates (Fig. 2). However, the CO2 response of oviposition-induced twigs was slightly lower than that of controls (P = 0.055; Table II). The CO2 response of the pine twigs did not change from day 1 to day 3. A combined effect of treatment and time was detected, i.e. the slight difference of oviposition-induced and control twigs decreased from day 1 to day 3.
Figure 2.
CO2 response (net photosynthetic rate) of systemically oviposition-induced (black squares) twigs of Scots pine and untreated controls (white squares) at day 1 to day 3 (A–C) and stomatal conductance calculated from these CO2 response curves at these three measurement days (D–F). Mean values ± se are given. PPFD, 1,100 μmol m−2 s−1 with increasing CO2 concentrations raised in eight steps (each lasting 10 min) from 50 to 2,000 μmol mol−1. Days 1 and 2 with n = 8; day 3 with n = 7.
Table II.
Repeated-measures ANOVA of CO2 response and light response
Net photosynthetic rate (CO2 and light response curves) during a 3-d measurement period of systemically oviposition-induced twigs of Scots pine and untreated controls were compared (Figs. 2 and 3). df, Degrees of freedom; MS, mean squares; F, F value, F test.
| Measurement | Source | df | MS | F | P |
|---|---|---|---|---|---|
| CO2 response curve | Treatment | 1 | 619.129 | 5.617 | 0.055 |
| Day | 2 | 103.613 | 2.581 | 0.117 | |
| CO2 | 7 | 1,105.203 | 47.706 | 0.001 | |
| Treatment × day | 2 | 45.027 | 4.128 | 0.043 | |
| Treatment × CO2 | 7 | 36.322 | 4.943 | 0.001 | |
| Light response curve | Treatment | 1 | 107.226 | 6.582 | 0.043 |
| Day | 2 | 0.791 | 0.602 | 0.563 | |
| Light | 7 | 225.907 | 44.136 | 0.001 | |
| Treatment × day | 2 | 3.576 | 3.343 | 0.070 | |
| Treatment × light | 7 | 11.138 | 6.207 | 0.001 |
Amax, A350, Jmax, and Vcmax
The parameters Amax, A350, and Jmax calculated from the CO2 response curves differed significantly at day 1 between oviposition-induced twigs and controls, while Vcmax only tended to be lower in oviposition-induced twigs than in untreated controls. At day 2, differences between oviposition-induced and control twigs decreased with respect to these four parameters and vanished at day 3. The Vcmax/Jmax ratio remained statistically unchanged on all days (Table III).
Table III.
Different parameters calculated from CO2 and light response curves
Calculation of parameters after the C3 photosynthesis model (Farquhar et al., 1980; Medlyn et al., 2002). See Figure 2, A to C, and Figure 3. Mean values ± se of systemically oviposition-induced twigs of Scots pine and untreated controls are given. P levels evaluated by the Wilcoxon matched pairs test are presented. Amax, Light-saturated (PPFD 1,100 μmol m−2 s−1) rate of net photosynthesis, measured at CO2-saturated (2,000 μmol mol−1) concentration; A350, light-saturated rate of net photosynthesis, measured at CO2 concentration of 350 μmol mol−1; Jmax, potential electron transport rate at Amax conditions; Vmax, maximum rate of Rubisco activity at Amax conditions.
| Measurement
|
Parameters
|
Day 1, n = 8
|
P
|
Day 2, n = 8
|
P
|
Day 3, n = 7
|
P
|
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Noninduced | Oviposition-Induced | Noninduced | Oviposition-Induced | Noninduced | Oviposition-Induced | |||||
| μmol m−2 s−1 | ||||||||||
| CO2 response curve | Amax | 18.9 ± 0.62 | 10.5 ± 3.22 | 0.049 | 16.5 ± 1.01 | 10.7 ± 2.88 | 0.093 | 14.3 ± 1.74 | 10.9 ± 3.57 | 0.129 |
| A350 | 6.7 ± 0.56 | 3.3 ± 1.17 | 0.017 | 5.4 ± 0.50 | 3.1 ± 0.99 | 0.069 | 4.0 ± 0.66 | 3.0 ± 1.24 | 0.237 | |
| Jmax | 91.4 ± 3.94 | 51.7 ± 15.30 | 0.036 | 77.2 ± 5.84 | 53.6 ± 13.86 | 0.093 | 66.9 ± 7.77 | 52.3 ± 17.04 | 0.129 | |
| Vcmax | 60.3 ± 6.48 | 28.9 ± 9.04 | 0.069 | 51.5 ± 5.47 | 29.2 ± 8.45 | 0.093 | 45.7 ± 8.09 | 37.2 ± 12.80 | 0.237 | |
| Vcmax/Jmax | 0.68 ± 0.11 | 0.50 ± 0.04 | 0.116 | 0.70 ± 0.12 | 0.67 ± 0.17 | 0.575 | 0.70 ± 0.13 | 0.81 ± 0.21 | 0.499 | |
| Light response curve | A350 | 6.1 ± 0.72 | 2.75 ± 1.01 | 0.017 | 5.5 ± 0.52 | 2.9 ± 1.09 | 0.050 | 4.7 ± 0.73 | 3.0 ± 1.29 | 0.128 |
Light Response Curves
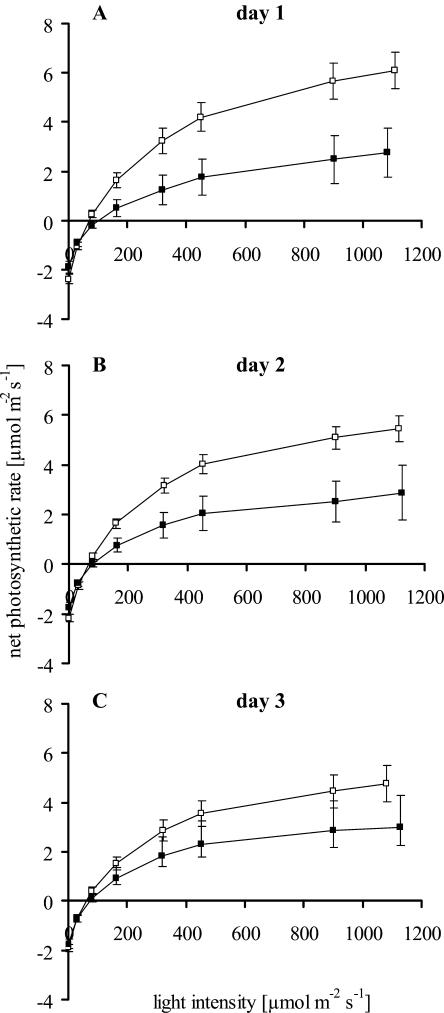
Both treated and control twigs significantly raised their net photosynthetic rates with increasing light intensity (Fig. 3). However, the light response of oviposition-induced twigs was significantly lower than that of untreated twigs. Light response did not significantly change from day 1 to day 3, indicating that the responsiveness of the twigs to light was stable during the measurement. A slight combined effect of treatment and time was detected, i.e. the difference of oviposition-induced twigs and controls (P = 0.07; Table II) tended to decrease from day 1 to day 3. The light response of oviposition-induced and untreated twigs was significantly different on day 1 when comparing data measured at light saturation and an ambient CO2 concentration of 350 μmol mol−1 (A350; Table III).
Figure 3.
Light response (net photosynthesis rate) of systemically oviposition-induced (black squares) twigs of Scots pine and untreated controls (white squares) at day 1 to day 3 (A–C). Mean values ± se are given. Ambient CO2 concentration, 350 μmol mol−1 with decreasing PPFD in eight steps (each lasting 10 min) from 1,100 to 0 μmol m−2 s−1. Days 1 and 2 with n = 8; day 3 with n = 7.
Stomatal Conductance
Stomatal conductance was slightly, but not significantly, lower in oviposition-induced twigs than in controls (Table IV; Fig. 2). From day 1 to day 3, stomatal conductance decreased significantly in both treated and control twigs. A combined effect of treatment and time was detected, i.e. the slight difference of oviposition-induced twigs and untreated controls decreased from day 1 to day 3.
Table IV.
Repeated-measures ANOVA of stomatal conductance
Stomatal conductance during a 3-d measurement period of systemically oviposition-induced twigs of Scots pine and untreated controls were compared (Fig. 2, D–F). df, Degrees of freedom; MS, mean squares; F, F value, F test.
| Source | df | MS | F | P |
|---|---|---|---|---|
| Treatment | 1 | 9,107.085 | 1,681.567 | 0.059 |
| Day | 2 | 5,011.298 | 822.155 | 0.015 |
| CO2 | 7 | 118.707 | 31.989 | 0.003 |
| Treatment × day | 2 | 1,994.224 | 211.947 | 0.003 |
| Treatment × CO2 | 7 | 5.138 | 2.717 | 0.095 |
DISCUSSION
This study investigates the effect of egg deposition by a free-living herbivorous insect on plant photosynthesis activity. Our data clearly show that insect egg deposition on Scots pine induces a decrease of photosynthetic activity in parts of the pine twig immediately adjacent to the site of egg deposition. These data raise numerous physiological and ecological questions. How can this reduction of photosynthetic activity be explained from a physiological perspective? Which factors cause this reduction? From an ecological point of view, is there a causal link between the reduction of photosynthetic activity and the induction of terpenoid volatiles by egg deposition? Can insects detect the differences of gas exchange of oviposition-induced and noninduced pine twigs and exploit the detected differences?
Physiological processes caused by water deficiency might have affected the photosynthetic activity of oviposition-induced pine twigs. The female pine sawfly slits the pine needle tangentially prior to egg laying, thereby causing desiccation of egg-laden pine foliage (Codella and Raffa, 2002). This water loss might explain the slightly lower stomatal conductance of oviposition-induced pine twigs compared to egg-free ones and thus contribute to the down-regulation of photosynthesis activity in egg-laden pine twigs. The difference between stomatal conductance of egg-laden pine twigs and egg-free ones diminished from day 1 to day 3. This may be due to the fact that we studied cut pine twigs. It is well known that cutting twigs leads to accelerated aging, water deficiency, and subsequent decrease of photosynthesis (e.g. Moldau et al., 1993; Richardson and Berlyn, 2002). Aging and water deficiency of both test and control twigs are indicated by the decrease in the net photosynthetic rate and stomatal conductance during the 3-d measurement period. Thus, effects on stomatal conductance and photosynthetic activity induced by egg deposition might interfere with effects caused by cutting the twigs.
Even though egg deposition by sawflies may cause desiccation of pine needles and thus affect stomatal conductance and photosynthetic activity, water deficiency does not seem to be the only factor causing a decrease in photosynthetic activity of egg-laden pine twigs. Rubisco amount, activity, or kinetic properties (as described by Vcmax; Sage, 1994) and the regeneration capacity of this enzyme (as described by Jmax; Long, 1991) affect photosynthetic activity. Insect egg deposition on Scots pine led to a significant decrease in Jmax at day 1 of the measurements. Also, Vcmax tended to be lower in oviposition-induced twigs compared to controls 1 d after egg deposition (Table III). However, the effects of egg deposition on Rubisco diminished on days 2 and 3 of the measurements. While feeding damage by herbivores is known to affect expression of genes related to photosynthesis (Arimura et al., 2000), it is unknown so far whether insect egg deposition and the specific plant wounding associated with oviposition are able to influence expression of genes involved in the regulation of photosynthesis.
Methyl jasmonate can down-regulate genes involved in photosynthesis such as Rubisco, whereas genes encoding enzymes of secondary metabolism are up-regulated (Reinbothe et al., 1994; Hermsmeier et al., 2001; Cheong and Yang, 2003). Secondary metabolism of terpenoids in pine and numerous other plants is well known to be up-regulated by methyl jasmonate (Mumm et al., 2003, and refs. therein). Application of methyl jasmonate to Scots pine has the same effect on egg parasitoids as oviposition. Also, treatment of pine twigs with methyl jasmonate induces the emission of specific terpenoid volatiles that render pine odor attractive to the egg parasitoid C. ruforum (Hilker et al., 2002a), even though the volatile pattern induced by methyl jasmonate is not fully identical with the volatile pattern induced by insect egg deposition (Mumm et al., 2003). We still do not know whether the reduction of photosynthesis activity in systemically oviposition-induced pine twigs is also mediated by methyl jasmonate.
From an ecological perspective, photosynthesis activity is one currency among several others to measure costs of plant defense (Cipollini et al., 2003, and refs. therein). Several studies address costs and benefits of plant responses induced by herbivore feeding damage (Dicke and Sabelis, 1992; Karban et al., 1997; Agrawal et al., 1999; Heil and Baldwin, 2002; Dicke and Hilker, 2003; Zangerl, 2003); this study investigates what oviposition-induced plant responses cost. Gershenzon (1994) considered the metabolic costs of terpenoid accumulation in higher plants. Scots pine is known to emit increased amounts of (E)-β-farnesene after egg deposition of D. pini (Mumm et al., 2003). This change of the terpenoid volatile pattern attracts egg parasitoids, killing the eggs (Hilker et al., 2002a; Mumm and Hilker, 2005). Is this defense costly?
A greater allocation of resources to defense may lead to a reduced allocation of resources to tolerance, and vice versa, because defense is expected to involve metabolic costs at the expense of growth (Herms and Mattson, 1992). Induced plant resistance to herbivores has been described by two basic forms, defense and tolerance. Induced defense is a trait that reduces damage involving biochemical, physiological, and morphological responses to herbivores. Induced tolerance is a trait that reduces the negative fitness impact of damage involving increases of growth and photosynthesis to compensate for the herbivore damage (Karban and Baldwin, 1997; Nabeshima et al., 2001; Agrawal et al., 2004). In these terms, induction of defense in Scots pine by egg deposition of D. pini might be considered to be paid by reduced tolerance toward eggs of this species. This interpretation of a trade-off between defense and photosynthesis activity requires that diversion of resources to defense is adaptive. However, we cannot fully exclude that reduction in photosynthesis is just a nonadaptive consequence of egg deposition and the associated tissue wounding, leading to water stress and closure of stomata.
Also, herbivore feeding damage is well known to lead to desiccation at the site of damage (Ferree and Hall, 1980; Brito et al., 1986; Morrill et al., 1995; Haile and Higley, 2003). However, while our results show that insect egg deposition on pine leads systemically to a reduction of photosynthetic activity, herbivore feeding often induces systemically or at the canopy level an increase of photosynthesis (Welter, 1989; Zangerl, 1999; Nykänen and Koricheva, 2004). A systemic enhancement of photosynthesis activity close to sites damaged by chewing herbivores may be due to extrinsic and intrinsic factors. An important extrinsic factor is the greater exposure of the remaining leaves to light because of the removal of other leaf tissue. Intrinsically, the change of source/sink relationships by leaf damage may result in increased photosynthesis rates (Zangerl, 1999). Herbivores that remove leaf tissue alter the amount of source tissue without affecting the amount of sink tissue, e.g. roots and stems. Photosynthesis of the remaining tissue of undamaged leaves adjacent to damaged leaves may increase to compensate for the demands of the sink tissue (Welter, 1989).
A possible adaptation to reduction of photosynthesis in oviposition-induced pine twigs does not only need consideration from the plant's perspective, but also from that of the insect. Could it be possible that the egg parasitoid or the herbivorous sawfly uses the photosynthesis changes for orientation or host location? Some herbivorous insects have been shown to be very sensitive toward CO2 gradients. For example, the moth Cactoblastis cactorum is able to detect CO2 concentration 5 mm above the host plant surface. Female moths probe the plant surface with their CO2 sensor, thus probably examining the suitability of the host plant. Most eggs are laid on the most vigorous plants (Stange et al., 1995). Furthermore, Thom et al. (2004) showed that CO2 plays an important role in the foraging behavior of the nectar-feeding moth Manduca sexta, which prefers surrogate flowers that emit CO2 concentrations characteristic of a flowering plant. However, studies on the oviposition behavior of the butterfly Pieris rapae could not detect any preferences for plants with higher gas exchange activities (Langan et al., 2001, 2004). For parasitoids of eggs of herbivorous insects, no sensitivity for CO2 has been reported. The elucidation of the use of reduced CO2 assimilation in systemically oviposition-induced pine twigs will need further study, as well as the sensitivity of egg parasitoids for CO2.
MATERIALS AND METHODS
Plants and Insects
Branches of Scots pine (Pinus sylvestris) were detached from 15- to 30-year-old trees in a forest near Berlin, placed in water, and brought into the laboratory where the stems were cleaned and sterilized according to the method of Moore and Clark (1968) prior to measurement. The sawfly Diprion pini was reared continuously in the laboratory on cut pine twigs as described by Bombosch and Ramakers (1976) and Eichhorn (1976) at 25° ± 1°C, 18-h-light/6-h-dark cycles.
Plant Treatment
Two small pine twigs (about 20 cm in length) were cut from a branch. The cut end was placed in water. One twig was used for induction by oviposition (treatment); the other was kept untreated as a control. Since a test and a control twig were always cut from the same branch, they were considered a paired sample.
For treatment, females of D. pini laid eggs on the lower half of a twig for a period of 1 d at the abiotic rearing conditions given above. When at least four egg masses had been laid onto the lower half of the twig, the upper, egg-free half of the twig was used to start measurements of photosynthesis (i.e. day 0; see description of measurements below). Thus, the upper, systemically oviposition-induced part of the twig was used for measurements (for further treatment details, see Hilker et al., 2002a; Mumm et al., 2003). Control twigs were kept at the same conditions as treated twigs, but without any contact with sawflies. For measurements, the upper halves of control twigs were also used.
Gas Exchange Analyzer
Gas exchange was measured using compact mini cuvette systems (CMS 400; Walz, Effeltrich, Germany) equipped with input humidity control (KF-18/2 and RSV-42; Walz) measuring gas cooler and lighting units (FL 440; Walz) in constant environmental conditions (25°C, vapor pressure deficit 1.4 kPa, photosynthetic photon flux density [PPFD] approximately 1,100 μmol m−2 s−1, wind speed 1.9 m s−1). Two mini cuvette systems were available. With one of the systems, gas exchange of the treated twig was measured, and with the other, measurements of the control were conducted simultaneously. The systems were connected to differential nondispersive infrared gas analyzers (IRGA) for water vapor and CO2 (BINOS 100; Fisher-Rosemount, Hasselroth, Germany), respectively.
Peltier-controlled climate units (GK 022; Walz) with flanged Plexiglas cuvettes (MK-022/A; Walz) were provided with air taken from outside the laboratory. Relative humidity (55%) inside the Plexiglas cuvette (500 cm3) was controlled by passing saturated air with water vapor through an input humidity control (dew-point temperature 15.4°C). The CO2 concentration was controlled by passing air over soda lime columns, retaining the naturally occurring CO2, and adding the concentration needed from a CO2 gas container. CO2 partial pressure was varied to eight CO2 concentrations (50, 150, 250, 350, 550, 700, 1,000, and 2,000 μmol mol−1 CO2) by using a CO2/N2 gas-mixing system (GMA-2; Walz). Nonlinearity of the differential IRGA systems to background CO2 concentration was accurately described with nonlinear equations. Calibration was accomplished with precision mixing pumps (Type 1 SA 27/2a; Wösthoff, Bochum, Germany).
The flow rate through the cuvettes was regulated by thermal mass flow meters (1,000 cm3 min−1). The setup was illuminated by a halogen lamp (FL 440; Walz) providing about 1,100 μmol m−2 s−1 during the light phase (18 h/day). Environmental conditions inside the cuvette and leaf temperature were monitored continuously with a microprocessor-controlled data acquisition system.
A mini cuvette was placed over the upper, egg-free part of the treated twig. The lower, egg-laden part of the twig was left outside the cuvette. The opening where the upper half of the twig entered the mini cuvette was closed by a sealant (Terostat; Teroson GmbH, Heidelberg). Accordingly, the upper half of the untreated control twig was similarly placed into the cuvette of the second measurement system with the lower half left outside. The cut ends of the twigs were supplied with tap water during measurement.
Continuous Measurements of Gas Exchange
Measurements of a systemically oviposition-induced twig (n = 8) and the respective control (n = 8) taken from the same branch were conducted simultaneously. The day when oviposition-induced and untreated control pine twigs were placed in the mini cuvette systems is referred to as day 0. From this time on, the gas exchange was continuously measured for a period of further 3 d. Changes in the difference between the controlled input of CO2 and water partial pressures into the cuvette and outputs from the cuvettes were monitored continuously with the IRGA. At day 3, one of the control twigs no longer showed photosynthesis. Since test and control twigs were considered paired samples, this pair was removed from further statistical analyses (thus, n = 7 at day 3 of the measurements).
CO2 and Light Response Curves
On days 1 to 3, each morning 3 to 4 h after the onset of the light cycle, a light response curve was determined. For this purpose, the PPFD was lowered in eight steps (each lasting 10 min), from 1,100 to 0 μmol m−2 s−1 with a constant CO2 concentration of 350 μmol mol−1. The measurement was conducted at the end of each 10-min period. After measuring these light responses, plants were provided with light of approximately 1,100 μmol m−2 s−1 and a CO2 concentration of 350 μmol mol−1 for 1 h. After this acclimatization period, measurements for the CO2 response curve were conducted. For this purpose, the CO2 concentration was changed in eight steps (each lasting 10 min) from 50 to 2,000 μmol mol−1 with light saturation of approximately 1,100 μmol m−2 s−1. Again, the measurement was conducted at the end of each 10-min period. After measurements for light and CO2 response curves, the continuous measurements of water vapor and CO2 were restarted.
Data Calculation and Statistics
All data were calculated on the basis of projected leaf area measured with a leaf area meter (model Li-3100; LI-COR, Lincoln, NE). The net photosynthetic rates were calculated after von Caemmerer and Farquhar (1981) and Field et al. (1989). The potential electron transport rate (Jmax) and maximum rate of Rubisco activity (Vmax) were calculated from data from the CO2 response curves without stomatal influences (Farquhar et al., 1980; Forstreuter, 2002; Medlyn et al., 2002). The stomatal conductance to CO2 was calculated from gas exchange data from the CO2 response curves according to Farquhar and von Caemmerer (1982).
Data obtained from simultaneously conducted measurements of a treated twig and its respective control from the same branch were considered paired samples. Data on photosynthesis and respiration rates of continuous measurements, data on light and CO2 response curves, and stomatal conductance were statistically analyzed by repeated-measures ANOVA for single effects of treatment and time, and for combined effects. The calculated parameters Jmax, Vmax, Amax, and A350 were compared for each day separately using the Wilcoxon matched-pairs test. All analyses were performed using Statistica 4.5 scientific software (StatSoft, Hamburg, Germany). Results are given as the mean ± se, and P < 0.05 was used to indicate statistical significance. The cuvette systems used for treated and control twigs were changed after measuring a pair of samples to control for possible effects of the measurement systems.
Acknowledgments
We thank Ute Braun (Freie Universität Berlin, Germany) for her technical assistance when rearing Diprion pini and Dr. Martti Varama (Finnish Forest Research Institute, Vantaa, Finland) for his assistance and helpful comments. We are grateful to Prof. Dieter Overdieck (Technische Universität, Berlin) for permitting us to conduct the measurements in his laboratory. Many thanks to Dr. Bernhard Götz and Prof. Harald Schill (Forest Botanical Garden, Eberswalde, Germany) for their technical support by providing one of the gas exchange systems.
This work was supported by the German National Science Foundation (GRK 837/1–03).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.059915.
References
- Agrawal AA, Conner JK, Stinchcombe JR (2004) Evolution of plant resistance and tolerance to frost damage. Ecol Lett 7: 1199–1208 [Google Scholar]
- Agrawal AA, Strauss SY, Stout M (1999) Costs of induced responses and tolerance to herbivory in male and female fitness components of wild radish. Evolution 53: 1093–1104 [DOI] [PubMed] [Google Scholar]
- Arimura GI, Tashiro K, Kuhara S, Nishioka T, Ozawa R, Takabayashi J (2000) Gene responses in bean leaves induced by herbivory and by herbivore-induced volatiles. Biochem Biophys Res Commun 22: 305–310 [DOI] [PubMed] [Google Scholar]
- Balbyshev NF, Lorenzen JH (1997) Hypersensitivity and egg drop, a novel mechanism of host-plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 90: 652–657 [Google Scholar]
- Bombosch S, Ramakers PMJ (1976) Zur Dauerzucht von Gilpinia hercyniae Htg. Z Pflanzenkr Pflanzenschutz 83: 40–44 [Google Scholar]
- Brito RM, Stern VM, Sances FV (1986) Physiological response of cotton plants to feeding of three Tetranychus spider mite species (Acari: Tetranychidae). J Econ Entomol 79: 1217–1220 [Google Scholar]
- Cheong J-J, Yang DC (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19: 409–413 [DOI] [PubMed] [Google Scholar]
- Cipollini D, Purrington CB, Bergelson J (2003) Costs of induced responses. Basic Appl Ecol 4: 79–85 [Google Scholar]
- Codella SG, Raffa KF (2002) Desiccation of Pinus foliage induced by conifer sawfly oviposition: effect on egg viability. Ecol Entomol 27: 618–621 [Google Scholar]
- Colazza S, Fucarino A, Peri E, Salerno G, Conti E, Bin F (2004) Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J Exp Biol 207: 47–53 [DOI] [PubMed] [Google Scholar]
- Dicke M, Hilker M (2003) Induced plant defences: from molecular biology to evolutionary ecology. Basic Appl Ecol 4: 3–14 [Google Scholar]
- Dicke M, Sabelis MW (1992) Costs and benefits of chemical information conveyance: proximate and ultimate factors. In BD Roitberg, MB Isman, eds, Insect Chemical Ecology: An Evolutionary Approach. Chapman and Hall, New York, pp 31–60
- Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy SR, Clement SL, Williamson RT, Carney JR, Devilbiss ED (2000) Bruchins—insect derived plant regulators that stimulate neoplasm formation. Proc Natl Acad Sci USA 97: 6218–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss RP, Proebsting WM, Potter SW, Clement SL (1995) Response of Np mutant of pea (Pisum sativum) to pea weevil (Bruchus pisorum) oviposition and extracts. J Chem Ecol 21: 97–106 [DOI] [PubMed] [Google Scholar]
- Eichhorn O (1976) Dauerzucht von Diprion pini L. (Hym.: Diprionidae) im Laboratorium unter Berücksichtigung der Fotoperiode. Anz Schaedlkd Pflanzenschutz Umweltschutz 49: 38–41 [Google Scholar]
- Farquhar GD, von Caemmerer S (1982) Modeling of photosynthetic response to environmental conditions. In O Lange, P Nobel, CB Osmond, H Ziegler, eds, Encyclopaedia of Plant Physiology, Vol 12B, Physiological Plant Écology II: Water Relations and Carbon Assimilation. Springer-Verlag, Berlin, pp 549–587
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation on leaves of C3 species. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Ferree DC, Hall FR (1980) Effects of soil water stress and two-spotted spider mites on net photosynthesis and transpiration of apple leaves. Photosynth Res 1: 189–197 [DOI] [PubMed] [Google Scholar]
- Field CB, Ball JT, Berry JT, Berry JA (1989) Photosynthesis: principles and field techniques. In RW Pearcy, J Ehleringer, HA Mooney, PW Rundel, eds, Plant Physiological Ecology. Field Methods and Instrumentation. Chapman and Hall, London, pp 209–253
- Forstreuter M (2002) Auswirkungen globaler Klimaänderungen auf das Wachstum und den Gaswechsel (CO2/H2O) von Rotbuchenbeständen (Fagus sylvatica L.). In Technische Universität Berlin, Fakultät Architektur, Umwelt, Gesellschaft, ed, Landschaftsentwicklung und Umweltforschung, Vol 119. Technische Universität Berlin, pp 1–307
- Gershenzon J (1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20: 1281–1328 [DOI] [PubMed] [Google Scholar]
- Haile FJ, Higley LG (2003) Changes in soybean gas-exchange after moisture stress and spider mite injury. Environ Entomol 32: 433–440 [Google Scholar]
- Heil M, Baldwin IT (2002) Fitness costs of induced resistance—the emerging experimental support for a slippery concept. Trends Plant Sci 7: 645–654 [DOI] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67: 283–335 [Google Scholar]
- Hermsmeier D, Schittko U, Baldwin IT (2001) Molecular interactions between specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large scale changes in the accumulation of growth and defense-related plants mRNAs. Plant Physiol 125: 683–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker M, Kobs C, Varama M, Schrank K (2002. a) Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J Exp Biol 205: 455–461 [DOI] [PubMed] [Google Scholar]
- Hilker H, Rohfritsch O, Meiners T (2002. b) The plant's response towards insect egg deposition. In M Hilker, T Meiners, eds, Chemoecology of Insect Eggs and Egg Deposition. Blackwell Scientific Publications, Berlin, pp 205–233
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. The University of Chicago Press, Chicago
- Karban R, Agrawal AA, Mangel M (1997) The benefits of induced defenses against herbivores. Ecology 78: 1351–1355 [Google Scholar]
- Langan AM, Wheater CP, Dunleavy PJ (2001) Does the small white butterfly (Pieris rapae L.) aggregate eggs on plants with greater gas exchange activity? J Insect Behav 14: 459–468 [Google Scholar]
- Langan AM, Wheater CP, Dunleavy PJ (2004) Biogenic gradients of CO2 and H2O and oviposition by the small white butterfly (Pieris rapae L.) in cages. Appl Entomol Zool 39: 55–59 [Google Scholar]
- Long SP (1991) Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant Cell Environ 14: 729–739 [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF, le Roux X, Montpied P, Strassemeyer J, Walcroft A, et al (2002) Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ 25: 1167–1179 [Google Scholar]
- Meiners T, Hilker M (2000) Induction of plant synomones by oviposition of a phytophagous insect. J Chem Ecol 26: 221–232 [Google Scholar]
- Moldau H, Wong SC, Osmond CB (1993) Transient depression of photosynthesis in bean leaves during rapid water loss. Aust J Plant Physiol 20: 45–54 [Google Scholar]
- Moore GF, Clark EW (1968) Suppressing microorganisms and maintaining turgidity in coniferous foliage used to rear insects in the laboratory. J Econ Entomol 61: 1030–1031 [Google Scholar]
- Morrill WL, Shepard BM, Rida GS, Parducho M (1995) Damage by the Malayan black bug (Heteroptera: Pentatomidae) in rice. J Econ Entomol 88: 1466–1468 [Google Scholar]
- Mumm R, Hilker M (2005) The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem Senses 30: 1–7 [DOI] [PubMed] [Google Scholar]
- Mumm R, Schrank K, Wegener R, Schulz S, Hilker M (2003) Chemical analysis of volatiles emitted by Pinus sylvestris after induction by insect oviposition. J Chem Ecol 29: 1235–1252 [DOI] [PubMed] [Google Scholar]
- Nabeshima E, Murakami M, Hiura T (2001) Effects of herbivory and light conditions on induced defense in Quercus crispula. J Plant Res 114: 403–409 [Google Scholar]
- Nykänen H, Koricheva J (2004) Damage-induced changes in woody plants and their effects on insect herbivore performance: a meta-analysis. Oikos 104: 247–268 [Google Scholar]
- Pschorn-Walcher H (1982) Symphyta. In W Schwenke, ed, Forstschädlinge Europas, Vol 4, Ed 2. Parey, Hamburg, Germany, pp 66–103
- Pschorn-Walcher H (1988) Die Parasitenkomplexe europäischer Diprionidae in ökologisch-evolutionsbiologischer Sicht. Z Zool Syst Evolutionsforsch 26: 89–103 [Google Scholar]
- Reinbothe S, Mollenhauer B, Reinbothe C (1994) JIPs and RIPs: the regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell 6: 1197–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AD, Berlyn GP (2002) Changes in foliar spectral reflectance and chlorophyll fluorescence of temperate species following branch cutting. Tree Physiol 22: 499–506 [DOI] [PubMed] [Google Scholar]
- Sage RF (1994) Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynth Res 39: 590–596 [DOI] [PubMed] [Google Scholar]
- Shapiro AM, DeVay JE (1987) Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kill eggs of Pieris butterflies (Lepidoptera: Pieridae). Oecologia 71: 631–632 [DOI] [PubMed] [Google Scholar]
- Stange G, Monro J, Stowe S, Osmond CB (1995) The CO2 sense of the moth Cactoblastis cactorum and its probable role in the biological control of the CAM plant Opuntia stricta. Oecologia 102: 341–352 [DOI] [PubMed] [Google Scholar]
- Thom C, Guerenstein PG, Mechaber WL, Hildebrand JG (2004) Floral CO2 reveals flower profitability to moth. J Chem Ecol 30: 1285–1288 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- Welter SC (1989) Arthropod impact on plant gas exchange. In EA Bernays, ed, Insect-Plant Interaction. CRC Press, Boca Raton, FL, pp 135–151
- Zangerl AR (1999) Locally-induced responses in plants: the ecology and evolution of restrained defense. In AA Agrawal, S Tuzun, E Bent, eds, Induced Plant Defenses against Pathogens and Herbivores. APS Press, St. Paul, pp 231–249
- Zangerl AR (2003) Evolution of induced plant responses to herbivores. Basic Appl Ecol 4: 91–103 [Google Scholar]
- Zangerl AR, Hamilton JG, Miller TJ, Crofts AR, Oxborough K, Berenbaum MR, de Lucia EH (2002) Impact of folivory on photosynthesis is greater than the sum of its holes. Proc Natl Acad Sci USA 99: 1088–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]