Abstract
Tumor cells hijack the programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) pathway to suppress the immune response through overexpressing PD-L1 to interact with PD-1 of T cells. With in-depth ongoing research, tumor-intrinsic PD-L1 is found to play important roles in tumor progression without interaction with PD-1 expressed on T cells, which provides an additional important target and therapeutic approach for development of PD-L1 inhibitors. Existing monoclonal antibody (mAb) drugs against the PD-1/PD-L1 pathway generally behave by conformationally blocking the interactions of PD-1 with PD-L1 on the cell surface. Beyond general inhibition of the protein–protein interaction (PPI), inhibitors targeting PD-L1 currently focus on the functional inhibition of the interaction between PD-1/PD-L1 and degradation of tumor-intrinsic PD-L1. This perspective will clarify the evolution of PD-L1 inhibitors and provide insights into the current development of PD-L1 inhibitors, especially targeting internalization and degradation of PD-L1.
This review clarifies the evolution of PD-L1 inhibitors and provides insights into the current development of PD-L1 inhibitors, especially targeting internalization and degradation of PD-L1.
Introduction
Naive T cells are activated to become mature immunocompetent T cells through two independent signals, the interaction between an antigen–peptide–MHC complex (P-MHC) and antigen-presenting cell (APC) and the interaction of the T cell with co-stimulatory molecules on the surface of the APC.1–4 Immune checkpoints operate as negative regulators of T cell activation and are crucial for preserving immunological tolerance and homeostasis under physiologically normal circumstances.5–8 However, the aberrant expression and substantial participation of programmed cell death ligand-1 (PD-L1) in various malignant tumors hijacked the programmed cell death protein-1 (PD-1)/PD-L1 pathway to suppress the immune response.6–9 Current monoclonal antibody (mAb) drugs targeting PD-L1 all directly block the interaction of PD-L1 with PD-1.10 In addition to the interaction with PD-1, recent research studies have revealed that tumor-intrinsic PD-L1 could directly confer resistance to T cell-mediated tumor death without relying on the PD-1-dependent inhibition of T cells. Furthermore, tumor-intrinsic PD-L1 could control tumor survival pathways and enhance the resistance of cancer cells to PD-1/PD-L1 targeting therapy.11,12 Therefore, novel PD-L1 inhibitors are required against tumor-intrinsic PD-L1 beyond inhibiting the axis of PD-L1 and PD-1. Small-molecule inhibitors targeting PD-L1 are promising alternatives to monoclonal antibody drugs and have attracted great attention. Moreover, various reported small-molecule PD-L1 inhibitors exhibited unique mechanisms, which could functionally inhibit the PD-1/PD-L1 pathway by inducing dimerization and internalization of PD-L1 protein.13 Targeted protein degradation (TPD) has made important achievements recently. Inhibiting the immunosuppressive effect of PD-L1 through degradation of PD-L1 has become an attractive method for the development of PD-L1 inhibitors.14–16 In order to provide novel insights into the discovery of PD-L1 inhibitors towards tumor-intrinsic PD-L1, we prepared this perspective to discuss the evolution of PD-L1 inhibitors from the direct blocking of the protein–protein interaction (PPI) to the functional inhibition of the PD-1/PD-L1 axis and tumor-intrinsic PD-L1, especially PD-L1 inhibitors targeting PD-L1 internalization and PD-L1 degradation.
Mechanism of tumor-intrinsic PD-L1
PD-1 (also known as CD279), encoded by the PDCD1 gene, is a member of the immunoglobulin gene superfamily.17 PD-1 is expressed primarily on the surface of immune cells including T cells, B cells, and monocytes.2,18 PD-L1 (B7-H1, CD274), the ligand of PD-1, is rarely expressed in normal tissues and can be upregulated on the surface of various tumor cells.17–19,20 The binding of PD-1 and PD-L1 causes the phosphorylation of the immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) in the intracellular domain of PD-1, which recruits Src homology-2 domain-containing phosphatase 2 (SHP2) to suppress downstream signaling pathways, ultimately leading to the inhibition of T cells.21–23 This interaction results in tumor-specific T cell exhaustion and apoptosis, which enables tumor cells to evade immune surveillance.24,25 However, tumor cells can upregulate the expression of PD-L1 through two pathways, intrinsic immune resistance and acquired immune resistance (AIR). Immune cells and hematopoietic cells in the tumor microenvironment can activate oncogenic pathways such as the PI3K/AKT pathway26 and NPM/ALK pathway, which cause the increase of PD-L1 expression.27 The immune system expresses interferon-γ (IFN-γ) when killing tumor cells, and IFN-γ also causes upregulation of PD-L1 expression (acquired immune resistance).28
PD-L1 is present not only on the cell surface, but also in recycling endosomes (REs) and Golgi apparatus. These intracellular PD-L1s are complementary to the membrane PD-L1.29,30 In addition to inhibiting the immune system by interacting with PD-1, the tumor-intrinsic PD-L1 promotes the invasion, proliferation and chemoresistance of tumors (Fig. 1).31–33 Downregulating PD-L1 increases the apoptosis of MDA-MB-231 breast cancer cells and enhances the sensitivity of MDA-MB-231 cells to doxorubicin-induced apoptosis in vivo and in vitro.34 Additionally, PD-L1 in cytoplasm could bind to and stabilize mRNA, leading to increased expression of specific DNA damage repair proteins.35 Chemotherapy and radiation, such as cisplatin and ionizing radiation (IR), generally kill tumor cells by damaging the DNA. Tumor-intrinsic PD-L1 increases the resistance of tumor cells to some chemotherapies.36 In various triple-negative breast cancer (TNBC) cell lines, PD-L1 can activate mitogen-activated protein kinases (MAPKs) or extracellular regulated protein kinases (ERKs) to promote cell survival signaling and resistance to certain cytotoxic chemotherapy without relying on PD-1 or CD80.32 Nuclear PD-L1 binds to DNA and affects the expression of several immune response-related genes to modulate anti-tumor immune responses.37 In 2023, Fan's group found that the nuclear PD-L1 in uveal melanoma (UM) promotes tumor angiogenesis through the activation of transcriptional early growth response-1 (EGR1).38 Therefore, various oncogenic effects of tumor-intrinsic PD-L1 require novel PD-L1 inhibitors with effective and unique inhibition against PD-L1.
Fig. 1. The role of tumor-intrinsic PD-L1 in promoting the development of tumor cells.
Evolution of PD-L1 inhibitors
Currently, six mAbs targeting PD-L1 have been launched and achieved great clinical success.39–41 However, therapeutic antibodies have significant drawbacks, including the lack of oral bioavailability, inaccessibility to intracellular targets, complicated and expensive production, immune-related adverse effects (irAEs), and low tissue penetration, which limit the clinical usage of PD-1/PD-L1 antibody drugs.42–48 In contrast, small-molecule inhibitors have unparalleled benefits in addressing these challenging issues, such as excellent membrane and tumor permeability, oral administration and convenience for storage and transportation.49,50 Therefore, attention has been paid to the exploitation of small-molecule inhibitors against the PD-1/PD-L1 pathway and several PD-1/PD-L1 small-molecule inhibitors have been under clinical research (Table 1). However, the shallow, hydrophobic and extended binding pocket of PD-L1 with PD-1 protein hinders the development of small molecule inhibitors.51,52
Small-molecule PD-L1 inhibitors under clinical development.
| Generic name | Organization | Highest phase | Ref. |
|---|---|---|---|
| IMMH-010 | Tianjin ChaseSun Pharmaceuticals | I | 53 |
| ABSK-043 | Abbisko Therapeutics | I | 54 |
| AN-4005 | Adlai Nortye; Xiamen Biotime Biotechnology | I | 55 |
| ASC-61 | Ascletis | I | 56 |
| BPI-371153 | Betta Pharmaceuticals | I | 57 |
| INCB-099280 | Incyte | I | 58, 59 |
| INCB-099318 | |||
| MAX-10181 | Maxinovel Pharmaceuticals | I | 60 |
| CA-170 | Aurigene/Curis | II | 61 |
| INCB-086550 | Incyte | II | 62 |
With in-depth ongoing research about PD-L1 in tumors, inhibitors targeting PD-L1 currently focus on the functional inhibition of the interaction between PD-1/PD-L1 and degradation of tumor-intrinsic PD-L1. The perspective below will discuss the novel development of PD-L1 inhibitors in these past three years, especially inhibitors that induce PD-L1 internalization and multiple successful design strategies for PD-L1 degraders.
PD-L1 inhibitors inducing the PD-L1 internalization
PD-L1 is typically upregulated on the surface of tumor cells. Inhibitors that induce PD-L1 internalization could block the PD-1 with PD-L1 on the tumor cell surface. Currently, the development of PD-L1 inhibitors, which regulate anti-tumor immunity through PD-L1 internalization, has attracted great attention (Fig. 2).
Fig. 2. The mechanism of PD-L1 internalization in tumor cells.
Membrane PD-L1 undergoes a continuous process of internalization and recycling, and the CKLF-like MARVEL transmembrane domain containing 6 (CMTM6) co-localizes with PD-L1 in the RE, thereby preventing PD-L1 targeting for lysosome-mediated degradation.30 Huntingtin-interacting protein 1-related (HIP1R) protein is identified as a negative regulator of PD-L1. HIP1R binds to the intracellular PD-L1 and mediates the transportation of PD-L1 to the lysosome. A rationally designed peptide (PD-LYSO) containing the PD-L1 binding sequence and lysosomal sorting of HIP1R effectively mediated degradation of PD-L1 in a lysosomal-dependent manner.63 Deacetylated PD-L1 by histone deacetylase 2 (HDAC2) at Lys263 interacts with HIP1R and undergoes the clathrin-dependent endocytosis.37 Besides, various small molecules and antibodies have been discovered as inhibitors inducing the internalization of PD-L1. The relevant research studies are discussed below.
Incyte disclosed a series of small-molecule PD-L1 inhibitors which could induce internalization of cell surface PD-L1.64 The most representative inhibitor 1 (Fig. 3) exhibited great activity against the PD-1/PD-L1 interaction in vitro, with an IC50 value of <1 nM. More importantly, 1 induced internalization of more than 90% cell-surface PD-L1 in CHO-PD-L1 cells.
Fig. 3. The structures of small-molecule PD-L1 inhibitors that induce PD-L1 internalization.
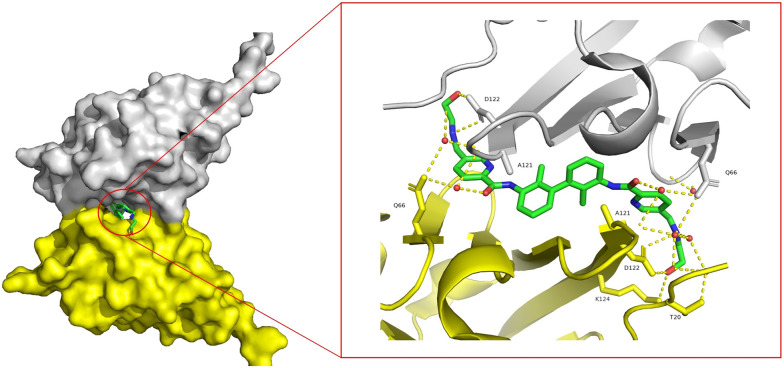
INCB086550 (2, Fig. 3), discovered by Incyte, is currently being tested in phase II clinical trials. INCB086550 could induce the dimerization and internalization of PD-L1 on the cell surface and then transfer to the nucleus to reduce the amount of PD-L1.65 Size-exclusion chromatography with multi-angle light scattering (SEC-MALS) and isothermal titration calorimetry (ITC) assay demonstrated the PD-L1 dimerization induced by INCB086550. INCB086550 reduced up to 70% cell-surface PD-L1. INCB086550 induced PD-L1 entry into Golgi vesicles, leading to subsequent translocation of PD-L1 to the nucleus. In the BALB/c-nu/nu mice bearing MDA-MB-231 xenograft model, PD-L1 levels in the tumor microenvironment decreased by 91% after administration of 200 mg kg−1 of INCB086550 twice daily. Doses of 2, 20 and 200 mg kg−1INCB086550 inhibited the growth of MC38-humanized PD-L1 tumors in vivo by 32%, 66% and 69%, respectively. The docking analysis of INCB086550/PD-L1 (Fig. 4) revealed that INCB086550 interacts with two PD-L1 units through various hydrogen bonds to stabilize the dimerization of PD-L1 proteins.
Fig. 4. (A) The docking analysis of INCB086550/PD-L1. Hydrogen bonds are indicated by the yellow dashed lines. (B) INCB086550 induces the formation of PD-L1 homodimers, as illustrated in the blue and pink space-filling models.
In 2021, Arbutus' group discovered ARB-272572 (3, Fig. 3) as a small-molecule PD-L1 inhibitor, which induced the dimerization and internalization of PD-L1.13 Native gel electrophoresis demonstrated that ARB-272572 could induce the dimerization of PD-L1. After treatment with ARB-272572 for 1 h, cell-surface PD-L1 was reduced completely as measured by flow cytometry. In humanized PD-1/PD-L1 mice inoculated with MC38-humanized PD-L1, the tumor volume was reduced by 60.4% compared with the control group after 7 days of treatment with ARB-272572 at 10 mg kg−1. Similar to INCB086550, the crystal structure of ARB-272572/PD-L1 reveals that the two pyridine rings participated in π–π stacking interactions with Tyr56 of chains A and B, respectively. The amide linker carboxylate interacts with Gln66 via a water molecule-mediated hydrogen bond. The polar tail forms hydrogen bonds with Asp122 of chains A and B (Fig. 5).
Fig. 5. Crystal structure of ARB-272572 with dimer PD-L1. The PDB code is 6VQN. Hydrogen bonds are indicated by the yellow dashed lines.
In 2022, Jiang's group discovered 4 (Fig. 3) as a PD-L1 inhibitor, with an IC50 value of 24.5 nM against the PD-1/PD-L1 interaction. 4 significantly activated the immune responses of peripheral blood cells (PBMCs). The SEC and X-ray structure of the 4/PD-L1 complex verified that 4 certainly induced the dimerization of PD-L1. Flow cytometry assay demonstrated that treatment with 4 reduced the amount of PD-L1 on the cell surface of MDA-MB 231 cells. Fluorescence microscopy assay indicated that 4 induced cell-surface PD-L1 into the cytoplasm in a time- and dose-dependent manner. Uniquely, 4 induced the degradation of PD-L1 through a lysosome-dependent pathway.66 In an in vivo experiment, 80 and 160 mg kg−1 per day of 4 reduced the tumor weights by 55% and 75%, respectively. Moreover, 4 could significantly induce PD-L1 degradation in a tumor environment in vivo. As shown in Fig. 6, 4 binds to the active sites of the two monomers. The unsubstituted phenyl ring and 1,3,4-oxadiazole form ATyr56 and π–π stacking with BTyr56, respectively.
Fig. 6. Crystal structure of 4 complexed with dimeric PD-L1. The PDB code is 6VQN. The hydrogen bond is shown as a yellow dashed line.
Yang's group discovered compound 5 as a highly potent and low-toxicity PD-1/PD-L1 inhibitor. Like the above-reported inhibitors, 5 could induce PD-L1 dimerization and was used to study the metabolic transit of PD-L1 protein and facilitated the internalization of PD-L1 protein into the endoplasmic reticulum (ER), which may result in subsequent ER-associated degradation.67
Apart from the compounds above, INCB090244 from Incyte Corporation,68BPI-371153 from Betta Pharmaceuticals,56 and ASC61 from Ascletis55 are also able to internalize surface PD-L1. Regrettably, the structure and detailed data are undisclosed yet.
In summary, the above small-molecule PD-L1 inhibitors can induce dimerization and internalization of membrane PD-L1. However, according to the patent of Incyte, it is not the dimerization that necessarily causes the internalization of PD-L1.64 The specific mechanism of action still needs to be further explored.
Glycosylated PD-L1 inhibits the T cell function in the tumor microenvironment. STM108 efficiently blocked the interaction of PD-1 with glycosylated PD-L1. Besides selectively targeting glycosylated PD-L1, STM108 could mediate PD-L1 internalization via caveolae-dependent endocytosis (CDE), which depends on the glycosylation of N192 and N200 in PD-L1.69 Western blot analysis demonstrated that STM108 was bound to PD-L1 and induced PD-L1 degradation in a lysosome-dependent manner, suggesting that STM108 is a good antibody-drug conjugate (ADC) candidate.
PD-L1 inhibitors inducing the PD-L1 degradation
As mentioned above, PD-L1 exists not only on the cell surface but also in the Golgi apparatus, RE, vesicles and cell nucleus.29,30 When cell-surface PD-L1 is reduced, internal PD-L1 will be constantly transported to the cell surface for renewal and supplementation via RE.70 Hence, targeted clearance of intracellular PD-L1 is an effective way to improve drug efficacy and block immune escape. Currently, many successes have been achieved in protein-targeted degradation. Antibody-based PROTACs (AbTACs) and lysosome-targeting chimaeras (LYTACs) degrade transmembrane and extracellular proteins, and proteolysis-targeting chimeras (PROTACs) allow the degradation of intracellular proteins.14,15,71–73 The following section describes the current research progress of PD-L1 degraders in terms of the dependent degradation pathways.
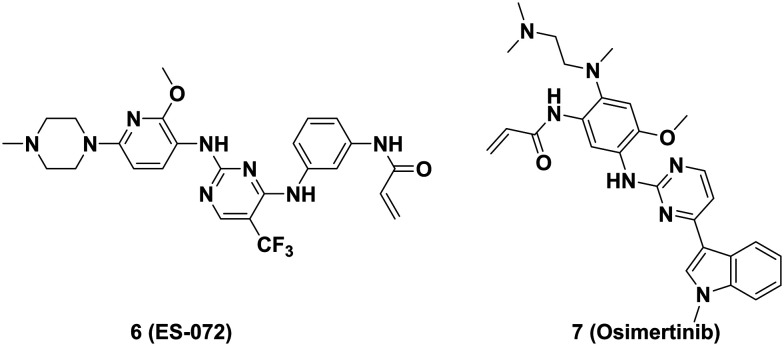
Activation of the epidermal growth factor receptor (EGFR) upregulates the expression of PD-L1 through the Janus kinase/signal transducer and activator of the transcription 3 (JAK/STAT3) signaling pathway and inhibits PD-L1 degradation by phosphorylation of glycogen synthase kinase 3β (GSK3β).74 Xia's group reported that ES-072 (6, Fig. 9), a third-generation EGFR inhibitor, induced effective degradation of PD-L1 at 10 μM. ES-072 induced phosphorylation at S279 and S283 in intracellular segments of PD-L1 by activating the EGFR-GSK3α pathway and enhanced the interaction of PD-L1 with the E3 ubiquitin ligase Ariadne-1 homolog (ARIH1), then ubiquitinating PD-L1 and targeting it for proteasomal degradation (Fig. 8).75 In 2021, Sun's group elucidated that osimertinib (7, Fig. 9) elevated the expression of E3 ligase membrane associated RING-CH8 (MARCH8), suppressed PD-L1 mRNA transcription and induced PD-L1 degradation in EGFR-mutant non-small-cell lung carcinoma (NSCLC) cells at <100 nM. By constructing harboring PD-L1 and MARCH8WT co-transfected HEK cells, it was demonstrated that MARCH8 could interact with N-terminal and ubiquitinated PD-L1 proteins, leading to proteasomal degradation of PD-L1 (Fig. 8).76
Fig. 9. EGFR inhibitors induce PD-L1 degradation.
Fig. 8. PD-L1 degradation through the proteasomal pathway. Inhibitors targeting PD-L1 degradation through the proteasome pathway.
Li's group from the University of Texas MD Anderson Cancer Center reported that the tumor suppressing factor WSX1, which is downregulated in hepatocellular carcinoma (HCC) cells and associated with poor prognosis, could regulate the PI3Kδ/AKT/GSK3β pathway and further promoted the E3 ligase-mediated K48 ubiquitination and subsequent proteasomal degradation of PD-L1 (Fig. 8).77
Asparagine-linked glycosylation is a co- and post-translational modification that occurs in the ER, which is vital for protein modification and degradation.78,79 PD-L1 protein is extensively glycosylated, with four N-glycosylation sites in its extracellular structure (N35, N192, N200, and N219). Following the process of glycosylation, PD-L1 is transformed into a fully functional protein and transported to the cell surface.69,80 Yu's group identified that BMS1166 (8, Fig. 10), a small-molecule PD-L1 inhibitor developed by Bristol Myers Squibb, partially but specifically inhibited the N-glycosylation of PD-L1 and prevented the transfer of under-glycosylated PD-L1 protein to the Golgi apparatus, leading to accumulation of PD-L1 in ER. The accumulation of PD-L1 induced by BMS1166 was eventually degraded through the proteasome pathway.81
Fig. 10. Small-molecule compounds that induced PD-L1 abnormal glycosylation and the structure of berberine.
In 2018, Hung's group reported that metformin (9, Fig. 10) activated AMP-activated protein kinase (AMPK). AMPK could phosphorylate Ser195 of PD-L1 protein, leading to abnormal PD-L1 glycosylation and blocking its transfer from ER to Golgi, thus resulting in accumulated PD-L1 in the ER and eventual degradation by ER-associated degradation (ERAD) (Fig. 8).82 Liu's group demonstrated that 4 mM metformin could promote the binding of AMPK protein to PD-L1 protein, and reduce the abundance of PD-L1 in endometrial cancer cells.83 Regrettably, metformin achieves this effect only at super-pharmacological concentrations. Lv's group discovered that d-mannose (10, Fig. 10) activated AMPK phosphorylated Ser195 of PD-L1, and degraded PD-L1 via proteasomes (Fig. 7). This effect of d-mannose improved the therapeutic efficacy of triple-negative breast cancer immunotherapy and radiotherapy.84
Fig. 7. Common pathways of PD-L1 internalization and degradation induced by the above small molecule inhibitors.
Thyroid adenoma associated gene (THADA) has been proven to be involved in the coat protein complex II (COPII) transport mechanism, mediating transport of PD-L1 from the ER to the Golgi. THADA promotes PD-L1 bonding to COPII and enables the PD-L1-specific vesicle transporting from the ER to the Golgi complex. Silence of THADA led to the absence and accumulation of PD-L1 in the ER and further degradation of PD-L1 by ERAD.85
Deng's group found that berberine (11, Fig. 10), an isoquinoline alkaloid extracted from Coptis chinensis, promoted the ubiquitin–proteasome degradation of PD-L1 by selectively binding to the Glu76 of constitutive photomorphogenic-9 signalosome5 (CSN5) (Fig. 8).86,87 Hu's group revealed that docosahexaenoic acid (DHA) reduced the expression of PD-L1, promoted PD-L1 degradation through the ubiquitin–proteasome pathway, and reverted PD-L1-mediated T-cell inactivation in a co-culture model. It was shown by western blot experiments that DHA inhibited palmitoylation of PD-L1 through repression of the fatty acid synthase (FASN)/DHHC5 axis. Inhibiting CNS5 expression enhanced ubiquitination of PD-L1 and degraded PD-L1 (Fig. 8).88
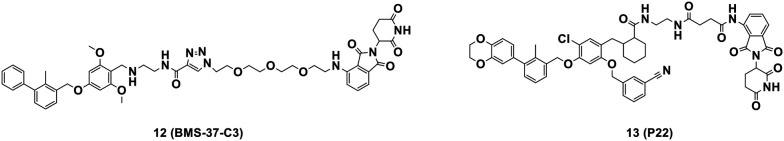
Compared with small-molecule inhibitors, PROTACs are more selective to target proteins, especially non-druggable targets, and provide more differentiated pharmacological results.89,90 To date, PROTACs are one of the most popular technologies of pharmaceutical research. Chen's group from Shenyang Pharmaceutical University reported a series of PROTAC-based PD-L1 degraders, combining different PD-L1 small molecule inhibitors with VHL, cereblon (CRBN), MDM2 or cIAP E3 ligase systems. BMS-37-C3 (12, Fig. 11) was identified as a PD-L1 degrader with the most potential with CRBN as the E3 ligase. It induced PD-L1 degradation via the ubiquitin–proteasome pathway in a dose- and time-dependent manner and enhanced T cell killing activity against melanoma. In addition, BMS-37-C3 inhibited the growth of A375 and B16-F10 with IC50 values of 8.07 ± 1.82 μM and 14.02 ± 0.29 μM, respectively.91 Chen's group from Southern Medical University designed and synthesized resorcinol diphenyl ether-based PROTAC-like molecules, which were bifunctional molecules using BMS series degraders as PD-L1 ligands and pomalidomide as CRBN E3 ligase. P22 (13, Fig. 11) is one of the best compounds with IC50 of 39.2 nM. However, different from the conventional PROTAC molecule degradation by the ubiquitin–proteasome system (UPS), P22 degraded PD-L1 via the lysosome pathway.92 In 2022, Cai's group designed a series of high-potency peptide-induced PROTACs. The peptide-PROTACs consisted of a cell-penetrating peptide (CPP) sequence, targeting protein recognition (TPR) peptide sequence, linker, and E3 recruitment peptide (ERP) sequence. The peptide (CGIQDTNSKKQSDTHLEETGSGSALAPYIPRRRRRRRR) could target and degrade palmitoyltransferase ZDHHC3 (DHHC3) through the proteasomal pathway. This peptide further inhibited the palmitoylation of PD-L1 and attenuated the PD-L1 expression in tumors.93
Fig. 11. PROTAC-based PD-L1 degraders.
Carbon dots (CDs) are novel carbon-based nanomaterials that possess low toxicity, good biocompatibility, modifiability, and photostability, and are widely used in biomedicine.94 In 2023, Wang's group reported a carbon-dot-based PROTAC (CDTAC) technology (Fig. 12). Using the special properties of carbon dots, BMS1166 and E3 ubiquitin ligase thalidomide were conjugated to CDs to recruit CRBN and PD-L1, then promoted the degradation of PD-L1 via the UPS. CDTACs concentration-dependently degraded PD-L1 in CT26 cells and B16-F10 tumor cells with DC50 values of 0.35 ± 0.03 μM and 0.39 ± 0.06 μM and Dmax of 97.5 ± 0.5% and 75.7 ± 3.8%, respectively.95
Fig. 12. The CDTACs targeting PD-L1.
Inhibitors targeting PD-L1 degradation through the lysosome pathway
PROTACs require small-molecule ligands with cytoplasmic binding domains, which restrict the degradation activity targeting the membrane proteins.96 Currently, the advent of degradation technologies, such as LYTACs and AbTACs, has covered the shortage of PORTACs. In 2021, Wells' group from the University of California converted the small molecules of PROTACs into an antibody form to construct AbTACs. AbTACs are genetically encoded IgG bispecific antibodies that specifically recruit membrane-bound E3 ligases (e.g., RNF43) on one aim and recognize the protein to be targeted on the other aim, enabling effective degradation of proteins of interest (POIs) through internalization to lysosome. This degradation technology has been successfully applied on PD-L1 degradation and the construction of RNF43/atezolizumab (Atz) bispecific antibody AC-1. AC-1 effectively degraded PD-L1 with a DC50 value of 3.4 nM and Dmax of 63% in MDA-MB-231 cells.15 Furthermore, Z18/Atz AbTAC, with ZNRF3 as the E3 ligase ligand, degraded PD-L1 in the T24 cell line overexpressing WT ZNRF3 significantly.71
As adhesion receptors on the cell surface, integrin αvβ3 is highly expressed in tumor cells and integrin-related tumor targeted therapy has gained great attention. In 2022, Fang's group reported an integrin-facilitated lysosomal degradation (IFLD) strategy for the degradation of PD-L1. The αvβ3 integrin-recognition motif Arg–Gly–Asp (RGD) sequence was chosen as an integrin-recognition ligand, and PD-L1 inhibitor BMS-8 was conjugated to the integrin-recognizing ligand to afford BMS-L1-RGD (15, Fig. 14). BMS-L1-RGD showed great activity of inducing PD-L1 degradation at 25 nM and significantly suppressed tumor growth in xenograft C57BL/6J mice.72
Fig. 14. Integrin-facilitated lysosomal degrader BMS-L1-RGD.
In 2020, Bertozzi's group reported LYTACs. LYTACs are composed of specific antibodies or small molecules targeting the substrate protein, an oligosaccharide targeting cell-surface lysosome-targeting receptors (LTRs), and a chemically synthesized linker. LYTACs were bound to POIs and a cation-independent mannose-6-phosphate receptor (CI-M6PR) to form the LTR–LYTAC-target protein complexes, which were endocytosed and transported to the lysosome (Fig. 13). After 48 h treatment, Atz-LYTAC with PD-L1 antibody Atz successfully induced 70% PD-L1 degradation in HDLM-2 cells.14 However, LYTACs face some challenges, such as bad pharmacokinetic properties, long duration of action in vivo, and safety risk of LYTACs caused by the immunogenic synthesized LTR ligand structure.
Fig. 13. PD-L1 lysosomal degradation mechanism.
Song's group discovered a novel aloperine derivative SA-49 (16, Fig. 15). SA-49 promoted the nuclear translocation of the microphthalmia transcription factor (MIFT) by activating protein kinase Cα (PAKα) and inhibiting GSK3β activity. SA-49 further induced lysosome biogenesis and finally translocated PD-L1 to lysosomes for degradation (Fig. 13). Moreover, SA-49 increased the cytotoxicity of co-cultured T cells and natural killer (NK) cells.97 Third-generation dihydropyridine-calcium channel blockers lercanidipine (17, Fig. 15) was identified as a PD-L1 degrader. However, the calcium influx antagonistic activity of lercanidipine suppressed its further development.98,99 In 2022, Dong's group designed and synthesized F4 (18, Fig. 15) through structure–activity relationship (SAR) exploration based on lercanidipine. F4 could diminish calcium influx block activity, and enhance the T cell-mediated killing of tumor cells. The flow cytometry experiment proved that the degradation rate of PD-L1 by F4 was 66.99 ± 10.79% at 10–20 μM concentration.100
Fig. 15. Small-molecule compounds that induce degradation of PD-L1 through the lysosome pathway.
In 2019, Xu's group reported that palmitoyltransferase DHHC3 could palmitoylate the Cys272 site of PD-L1 and stabilize PD-L1 by deubiquitination. Then, a competitive palmitoylation peptide inhibitor CPP-S1 (YGRKKRRQRRR MMDVKKCGIQDTNS) was synthesized. It is evaluated that CPP-S1 could induce the PD-L1 ubiquitination and promote PD-L1 degradation in a lysosomal-dependent manner (Fig. 13).101
Apart from inducing abnormal glycosylation of PD-L1, AMPK could also hamper the interaction of PD-L1 with CMTM4 and subsequently promote PD-L1 degradation in a lysosome-dependent manner (Fig. 13).102
MAPK inhibitor (MAPKi) treatment could induce the accumulation of PD-L1/L2 on the surface of tumor cells, causing immune evasion and accelerating acquired drug resistance. In 2022, Lo and co-workers discovered that E3 ligase ITCH could regulate the expression of PD-L1 in MAPKi therapy in the treatment of melanoma cells. ITCH induced the ubiquitination of PD-L1 at K46 and K162. Moreover, ITCH promoted the internalization of cell-surface PD-L1 and lysosomal-dependent degradation of PD-L1 (Fig. 13). Immunoprecipitation experiments and western blot indicated that AK087, a small-molecule ITCH activator (19, Fig. 15), treatment in melanoma cells adapted to BRAF inhibition (M238 R1) enhanced the ubiquitination of ITCH. Furthermore, AK087 effectively inhibited the acquired MAPKi resistance in YUMM1.7ER melanoma mice.103
Xu group's found that calcium channel blocker amlodipine (20, Fig. 16) potently degraded PD-L1. Intracellular calcium promoted the Beclin-1 cleavage and blocked the autophagic degradation of PD-L1 in the recycling endosome. The blockade of the calcium flux by amlodipine prevented the cleavage of Beclin-1 by calpain and thus promoted the degradation of PD-L1 in an autophagic-dependent manner. Moreover, amlodipine increased the infiltration of CD8+ T cells in tumor tissues (Fig. 13).104 Chen's group from Central South University discovered that RTK inhibitor sunitinib (21, Fig. 16) could activate the interaction of p62 with PD-L1, inducing PD-L1 degradation via p62-mediated selective autophagy.105 Sigma1 could interact with glycosylated PD-L1 and maintained PD-L1 stability in tumor cells. Sigma1 inhibitor IPAG (22, Fig. 16) significantly induced the selective autophagy-dependent degradation of PD-L1 in PC3 and MDA-MB-231 cells and thereby enhanced the T-cell activity (Fig. 13).106
Fig. 16. Inhibitors that induce autophagy degradation of PD-L1.
Conclusion and future perspectives
Immune checkpoint blockade therapy represented by PD-1/PD-L1 antibody drugs has made great breakthroughs over the last decade. Recent research studies have revealed that PD-L1 in tumor cells is involved in multiple oncogenic pathways without relying on the interaction with PD-1 on T cells, which provides additional challenges for the development of PD-L1 inhibitors. Discovery of novel PD-L1 inhibitors from the direct blocking of the binding of PD-L1 with PD-1 to functional inhibition of the PD-1/PD-L1 axis received more attention.
Small-molecule PD-L1 inhibitors, alternatives to PD-1/PD-L1 mAbs, were discovered to remedy the inherent defects of mAbs. Interestingly, in addition to the blocking of the PD-1/PD-L1 interaction, small-molecule PD-L1 inhibitors were reported to functionally inhibit the PD-1/PD-L1 axis through inducing the dimerization and internalization of PD-L1. Due to the unique mechanism, INCB086550, which has entered phase II clinical trials, exhibited remarkable in vivo antitumor efficacy compared with clinical PD-L1 antibody drugs.
Using intrinsic intracellular protein degradation pathways to degrade the target proteins has great advantages in inhibiting specific pathways and overcoming drug resistance. Recently, a great deal of related research studies reported inhibitor-induced PD-L1 degradation (Fig. 8 and 13). Due to the location on the cell surface of tumor cells, small-molecule PD-L1 degraders with general PROTAC technology exhibited limited effect on PD-L1 degradation. Therefore, other TPD technologies based on intrinsic intracellular protein degradation pathways have been applied for the discovery of PD-L1 degraders. Among these PD-L1 degraders, LYTACs and AbTACs with PD-L1 antibody drugs as probes of PD-L1 protein demonstrated excellent activity for inducing PD-L1 degradation.
Since the first PD-L1 mAb drug atezolizumab was launched in 2016, anti-PD-L1 mAbs have demonstrated the clinical value of PD-L1 as a cancer immunotherapy target. Along with the understanding of PD-L1's oncogenic effects on tumor cells, discovery of novel PD-L1 inhibitors beyond direct inhibition against the PD-1/PD-L1 pathway will be promising and challenging. We hope that this perspective could be helpful for medicinal chemists to expand the future development of PD-L1 inhibitors.
Abbreviations used
- AbTACs
Antibody-based protacs
- ADC
Antibody-drug conjugate
- AIR
Acquired immune resistance
- AMPK
AMP-activated protein kinase
- APC
Antigen-presenting cell
- ARIH1
Ariadne-1 homolog
- Atz
Atezolizumab
- CDE
Caveolae-dependent endocytosis
- CDs
Carbon dots
- CDTACs
Carbon-dot (CD)-based PROTACs
- CI-M6PR
Cation-independent mannose-6-phosphate receptor
- CMTM6
CKLF-like MARVEL transmembrane domain containing 6
- COPII
Coat protein complex II
- CPP
Cell-penetrating peptide
- CRBN
Cereblon
- CSN5
Constitutive photomorphogenic-9 signalosome5
- DHA
Docosahexaenoic acid
- EGFR
Epidermal growth factor receptor
- EGR1
Early growth response-1
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- ERK
Extracellular regulated protein kinase
- ERP
E3 recruitment peptide
- FASN
Fatty acid synthase
- GSK3β
Glycogen synthase kinase 3β
- HCC
Hepatocellular carcinoma
- HDAC2
Histone deacetylase 2
- HIP1R
Huntingtin-interacting protein 1-related
- IFLD
Integrin-facilitated lysosomal degradation
- IFN-γ
Interferon-γ
- IR
Ionizing radiation
- irAEs
Immune-related adverse effects
- ITC
Isothermal titration calorimetry
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- ITSM
Immunoreceptor tyrosine-based switch motif
- JAK/STAT3
Janus kinase/signal transducer and activator of transcription 3
- LTRs
Lysosome-targeting receptors
- LYTACs
Lysosome-targeting chimaeras
- mAbs
Monoclonal antibodies
- MAPKs
Mitogen-activated protein kinases
- MAPKi
MAPK inhibitor
- MARCH8
Membrane associated RING-CH8
- MIFT
Microphthalmia transcription factor
- NK cells
Nature killer cells
- NSCLC
Non-small-cell lung carcinoma
- PAKα
Protein kinase Cα
- PBMCs
Peripheral blood cells
- PD-1
Programmed death-1
- PD-L1
Programmed death-1 ligand
- p-MHC
Peptide–MHC complex
- POIs
Proteins of interest
- PPIs
Protein–protein interactions
- PROTACs
Proteolysis-targeting chimeras
- RE
Recycling endosome
- SAR
Structure–activity relationship
- SEC-MALS
Size-exclusion chromatography with multiangle light scattering
- SHP2
Src homology-2 domain-containing phosphatase 2
- THADA
Thyroid adenoma associated gene
- TNBC
Triple-negative breast cancer
- TPD
Targeted protein degradation
- TPR
Targeting protein recognition
- UM
Uveal melanoma
- UPS
Ubiquitin-proteasome system
- ZDHHC3
Zinc finger dhhc-type palmitoyltransferase 3
Author contributions
Conceptualization: Sheng Jiang and Tianyu Wang; writing the manuscript: Jiazheng Guo and Fengyi Yu; review & editing: Xiangyu Zhang, Kuojun Zhang and Tianyu Wang.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (22107117, 22001267 and 22207125), the China Postdoctoral Science Foundation (2021M693516 and 2022M713480), the Postdoctoral Research Program of Jiangsu Province (2021K218B) and the Jiangsu Funding Program for Excellent Postdoctoral Talent.
Notes and references
- Marelli-Berg F. M. Okkenhaug K. Mirenda V. A two-signal model for T cell trafficking. Trends Immunol. 2007;28(6):267–273. doi: 10.1016/j.it.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Keir M. E. Butte M. J. Freeman G. J. Sharpe A. H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008;26(1):677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedoeem A. Azoulay-Alfaguter I. Strazza M. Silverman G. J. Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014;153(1):145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Guerder S. Flavell R. A. T-Cell Activation: Two for T. Curr. Biol. 1995;5(8):866–868. doi: 10.1016/S0960-9822(95)00175-8. [DOI] [PubMed] [Google Scholar]
- He X. Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. Gao R. Li Y. Zeng C. Regulation of PD-1 in T cells for cancer immunotherapy. Eur. J. Pharmacol. 2020;881:173240. doi: 10.1016/j.ejphar.2020.173240. [DOI] [PubMed] [Google Scholar]
- Rezaei M. Tan J. Zeng C. Li Y. Ganjalikhani-Hakemi M. TIM-3 in Leukemia; Immune Response and Beyond. Front. Oncol. 2021;11:753677. doi: 10.3389/fonc.2021.753677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Acevedo J. A. Kimbrough E. O. Lou Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021;14(1):45. doi: 10.1186/s13045-021-01056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H. T. Ahmed R. Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr. Top. Microbiol. Immunol. 2011;350:17–37. doi: 10.1016/j.ejphar.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon H. Holvey-Bates E. G. McGrail D. J. Stark G. R. PD-L1 sustains chronic, cancer cell–intrinsic responses to type I interferon, enhancing resistance to DNA damage. Proc. Natl. Acad. Sci. U. S. A. 2021;118(47):e2112258118. doi: 10.1073/pnas.2112258118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escors D. Gato-Cañas M. Zuazo M. Arasanz H. García-Granda M. J. Vera R. Kochan G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduction Targeted Ther. 2018;3(1):26. doi: 10.1038/s41392-018-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. J. Thi E. P. Carpio V. H. Bi Y. Cole A. G. Dorsey B. D. Fan K. Harasym T. Iott C. L. Kadhim S. et al. Checkpoint inhibition through small molecule-induced internalization of programmed death-ligand 1. Nat. Commun. 2021;12(1):1222. doi: 10.1038/s41467-021-21410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik S. M. Pedram K. Wisnovsky S. Ahn G. Riley N. M. Bertozzi C. R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature. 2020;584(7820):291–297. doi: 10.1038/s41586-020-2545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton A. D. Nguyen D. P. Gramespacher J. A. Seiple I. B. Wells J. A. Development of Antibody-Based PROTACs for the Degradation of the Cell-Surface Immune Checkpoint Protein PD-L1. J. Am. Chem. Soc. 2021;143(2):593–598. doi: 10.1021/jacs.0c10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B. Xiao Y. Xue M. Cao H. Chen J. Recent Advances in the Development of PD-L1 Modulators: Degraders, Downregulators, and Covalent Inhibitors. J. Med. Chem. 2020;63(24):15389–15398. doi: 10.1021/acs.jmedchem.0c01362. [DOI] [PubMed] [Google Scholar]
- Francisco L. M. Sage P. T. Sharpe A. H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236(1):219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agata Y. Kawasaki A. Nishimura H. Ishida Y. Tsubat T. Yagita H. Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8(5):765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- Dong H. Strome S. E. Salomao D. R. Tamura H. Hirano F. Flies D. B. Roche P. C. Lu J. Zhu G. Tamada K. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Zou W. Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- Wu Q. Jiang L. Li S. C. He Q. J. Yang B. Cao J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2021;42(1):1–9. doi: 10.1038/s41401-020-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. A. Wu D. C. Cheung J. Navarro A. Xiong H. Cubas R. Totpal K. Chiu H. Wu Y. Comps-Agrar L. et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer. 2020;1(7):681–691. doi: 10.1038/s43018-020-0075-x. [DOI] [PubMed] [Google Scholar]
- Latchman Y. Wood C. R. Chernova T. Chaudhary D. Borde M. Chernova I. Iwai Y. Long A. J. Brown J. A. Nunes R. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Chen M. Nie H. Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum. Vaccines Immunother. 2019;15(5):1111–1122. doi: 10.1080/21645515.2019.1571892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. Yang L. Xiao M. Zhang Z. Shen J. Anuchapreeda S. Tima S. Chiampanichayakul S. Xiao Z. PD-L1 regulates cell proliferation and apoptosis in acute myeloid leukemia by activating PI3K-AKT signaling pathway. Sci. Rep. 2022;12(1):11444. doi: 10.1038/s41598-022-15020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa A. T. Waldron J. S. Panner A. Crane C. A. Parney I. F. Barry J. J. Cachola K. E. Murray J. C. Tihan T. Jensen M. C. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- Marzec M. Zhang Q. Goradia A. Raghunath P. N. Liu X. Paessler M. Wang H. Y. Wysocka M. Cheng M. Ruggeri B. A. et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc. Natl. Acad. Sci. U. S. A. 2008;105(52):20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-K. Seo S.-H. Kim B.-S. Kim C.-D. Lee J.-H. Kang J.-S. Maeng P. J. Lim J.-S. IFN-gamma regulates the expression of B7-H1 in dermal fibroblast cells. J. Dermatol. Sci. 2005;40(2):95–103. doi: 10.1016/j.jdermsci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Zhu L. Kuang X. Zhang G. Liang L. Liu D. Hu B. Xie Z. Li H. Liu H. Ye M. et al. Albendazole induces immunotherapy response by facilitating ubiquitin-mediated PD-L1 degradation. J. Immunother. Cancer. 2022;10(5):e003819. doi: 10.1136/jitc-2021-003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr M. L. Sparbier C. E. Chan Y. C. Williamson J. C. Woods K. Beavis P. A. Lam E. Y. N. Henderson M. A. Bell C. C. Stolzenburg S. et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549(7670):101–105. doi: 10.1038/nature23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. Reyes R. M. Osta E. Kari S. Gupta H. B. Padron A. S. Kornepati A. V. R. Kancharla A. Sun X. Deng Y. et al. Bladder cancer cell-intrinsic PD-L1 signals promote mTOR and autophagy activation that can be inhibited to improve cytotoxic chemotherapy. Cancer Med. 2021;10(6):2137–2152. doi: 10.1002/cam4.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornepati A. V. R. Vadlamudi R. K. Curiel T. J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer. 2022;22(3):174–189. doi: 10.1038/s41568-021-00431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta H. B. Clark C. A. Yuan B. Sareddy G. Pandeswara S. Padron A. S. Hurez V. Conejo-Garcia J. Vadlamudi R. Li R. et al. Tumor cell-intrinsic PD-L1 promotes tumor-initiating cell generation and functions in melanoma and ovarian cancer. Signal Transduction Targeted Ther. 2016;1:16030. doi: 10.1038/sigtrans.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebeh H. Lehe C. Barhoush E. Al-Romaih K. Tulbah A. Al-Alwan M. Hendrayani S. F. Manogaran P. Alaiya A. Al-Tweigeri T. et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010;12(4):R48. doi: 10.1186/bcr2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X. Qin B. Zhang Y. Zhang C. Kahila M. Nowsheen S. Yin P. Yuan J. Pei H. Li H. et al. PD-L1 (B7-H1) Competes with the RNA Exosome to Regulate the DNA Damage Response and Can Be Targeted to Sensitize to Radiation or Chemotherapy. Mol. Cell. 2019;74(6):1215–1226.e1214. doi: 10.1016/j.molcel.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudan S. Hickson I. D. DNA repair inhibition: a selective tumour targeting strategy. Trends Mol. Med. 2005;11(11):503–511. doi: 10.1016/j.molmed.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Gao Y. Nihira N. T. Bu X. Chu C. Zhang J. Kolodziejczyk A. Fan Y. Chan N. T. Ma L. Liu J. et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol. 2020;22(9):1064–1075. doi: 10.1038/s41556-020-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. Zhuang A. Gu X. Hua Y. Yang L. Ge S. Ruan J. Chai P. Jia R. Fan X. Nuclear PD-L1 promotes EGR1-mediated angiogenesis and accelerates tumorigenesis. Cell Discovery. 2023;9(1):33. doi: 10.1038/s41421-023-00521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham A. Atezolizumab: First Global Approval. Drugs. 2016;76(12):1227–1232. doi: 10.1007/s40265-016-0618-8. [DOI] [PubMed] [Google Scholar]
- Joseph J. Zobniw C. Davis J. Anderson J. Trinh V. Avelumab: A Review of Its Application in Metastatic Merkel Cell Carcinoma. Ann. Pharmacother. 2018;52(9):928–935. doi: 10.1177/1060028018768809. [DOI] [PubMed] [Google Scholar]
- Spigel D. R. Faivre-Finn C. Gray J. E. Vicente D. Planchard D. Paz-Ares L. Vansteenkiste J. F. Garassino M. C. Hui R. N. Quantin X. et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022;40(12):1301. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou M. Zarganes-Tzitzikas T. Magiera-Mularz K. Holak T. A. Dömling A. Immune Checkpoint PD-1/PD-L1: Is There Life Beyond Antibodies? Angew. Chem., Int. Ed. 2018;57(18):4840–4848. doi: 10.1002/anie.201710407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann H. Cancer Immunotherapy: Selected Targets and Small-Molecule Modulators. ChemMedChem. 2016;11(5):450–466. doi: 10.1002/cmdc.201500566. [DOI] [PubMed] [Google Scholar]
- Zhan M. M. Hu X. Q. Liu X. X. Ruan B. F. Xu J. Liao C. From monoclonal antibodies to small molecules: the development of inhibitors targeting the PD-1/PD-L1 pathway. Drug Discovery Today. 2016;21(6):1027–1036. doi: 10.1016/j.drudis.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Lin X. Lu X. Luo G. Xiang H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2020;186:111876. doi: 10.1016/j.ejmech.2019.111876. [DOI] [PubMed] [Google Scholar]
- Vladimer G. I. Snijder B. Krall N. Bigenzahn J. W. Huber K. V. M. Lardeau C. H. Sanjiv K. Ringler A. Berglund U. W. Sabler M. et al. Global survey of the immunomodulatory potential of common drugs. Nat. Chem. Biol. 2017;13(6):681. doi: 10.1038/nchembio.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A. Wolchok J. D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchett A. L. Darby J. M. Tovar C. Lyons A. B. Woods G. M. The Immunomodulatory Small Molecule Imiquimod Induces Apoptosis in Devil Facial Tumour Cell Lines. PLoS One. 2016;11(12):e0168068. doi: 10.1371/journal.pone.0168068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Lee L. F. Fisher T. S. Jessen B. Elliott M. Evering W. Logronio K. Tu G. H. Tsaparikos K. Li X. et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol. Res. 2015;3(2):149–160. doi: 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- Di L. Kerns H. E. Carter T. G. Drug-Like Property Concepts in Pharmaceutical Design. Curr. Pharm. Des. 2009;15(19):2184–2194. doi: 10.2174/138161209788682479. [DOI] [PubMed] [Google Scholar]
- Zak K. M. Grudnik P. Magiera K. Domling A. Dubin G. Holak T. A. Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2. Structure. 2017;25(8):1163–1174. doi: 10.1016/j.str.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Zak K. M. Kitel R. Przetocka S. Golik P. Guzik K. Musielak B. Domling A. Dubin G. Holak T. A. Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1. Structure. 2015;23(12):2341–2348. doi: 10.1016/j.str.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Chen X., Ma C., Yang Y., Lai F., Wang Y., Ji M. and Guo K., Maleate of nicotinyl alcohol ether derivative, crystal form thereof, and application thereof, WO2021047528, 2021
- Cooper A. Andelkovic V. Wilkinson K. Ganju V. Lundy J. Hong M. Airey S. Meng L. L. Shen B. Li H. et al. 700P First-in-human dose-escalating study of ABSK043, a novel and oral small-molecule inhibitor of PD-L1, in patients with advanced solid tumors. Ann. Oncol. 2023;34:S487. doi: 10.1016/j.annonc.2023.09.1886. [DOI] [Google Scholar]
- Yu Z., Li P., Xu B., Zhou Y., Pang W., Wen Q., Shi Y., Sun Z. and Lv M., PD-L1 antagonist compound, WO2021129584, 2019
- Wu J. J. He H. Abstract 5529: In vivo efficacy evaluation of ASC61, an oral PD-L1 inhibitor, in two tumor mouse models. Cancer Res. 2022;82(12_Supplement):5529–5529. doi: 10.1158/1538-7445.AM2022-5529. [DOI] [Google Scholar]
- Wang Y. Jing X. Chen H. Zhang H. Ma T. Zhang Y. Zhang C. Zhang G. Liu X. Yan D. et al. Abstract 5444: BPI-371153, an orally bioavailable small molecule PD-L1 inhibitor. Cancer Res. 2022;82(12_Supplement):5444. doi: 10.1158/1538-7445.AM2022-5444. [DOI] [Google Scholar]
- Prenen H. Lesimple T. Robert M. Delafontaine B. Machiels J.-P. Meniawy T. Cutsem E. V. Kotecki N. Piha-Paul S. Schweizer M. et al. 734 A phase 1 study exploring the safety and tolerability of the small-molecule PD-L1 inhibitor, INCB099280, in patients with select advanced solid tumors. J. Immunother. Cancer. 2022;10(Suppl 2):A766. [Google Scholar]
- Pinato D. Plummer R. Gutierrez M. Yachnin J. Schiza A. Hojgaard M. Smeland K. Edenfield W. Prenen H. Ny L. et al. 622 A phase 1 study exploring the safety and tolerability of the small-molecule PD-L1 inhibitor, INCB099318, in patients with select advanced solid tumors. J. Immunother. Cancer. 2022;10(Suppl 2):A654. [Google Scholar]
- Wang Y. Zhang N. Wang F. Zhao Q. Li Z. Abstract LB-018: Orally active small molecule PD-L1 inhibitor demonstrating similar efficacy as Durvalumab in human knock-in MC38 model. Cancer Res. 2019;79(13_Supplement):LB-018. doi: 10.1158/1538-7445.AM2019-LB-018. [DOI] [Google Scholar]
- Radhakrishnan V. Banavali S. Gupta S. Kumar A. Deshmukh C. D. Nag S. Beniwal S. K. Gopichand M. Naik R. Lakshmaiah K. C. et al. 1209P - Excellent CBR and prolonged PFS in non-squamous NSCLC with oral CA-170, an inhibitor of VISTA and PD-L1. Ann. Oncol. 2019;30:v494. doi: 10.1093/annonc/mdz253.035. [DOI] [Google Scholar]
- Koblish H. K. Wu L. Wang L. S. Liu P. C. C. Wynn R. Rios-Doria J. Spitz S. Liu H. Volgina A. Zolotarjova N. et al. Characterization of INCB086550: A Potent and Novel Small-Molecule PD-L1 Inhibitor. Cancer Discovery. 2022;12(6):1482–1499. doi: 10.1158/2159-8290.CD-21-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Yao H. Li C. Shi H. Lan J. Li Z. Zhang Y. Liang L. Fang J.-Y. Xu J. HIP1R targets PD-L1 to lysosomal degradation to alter T cell–mediated cytotoxicity. Nat. Chem. Biol. 2019;15(1):42–50. doi: 10.1038/s41589-018-0161-x. [DOI] [PubMed] [Google Scholar]
- Liu P. C. V. A., Wynn R., Zolotarjova N., Wu L., Xiao K., Mei S., Lu L., Zhu W., Ye Y., Wang H., Qian D.-Q. and Yao W., Tetrahydro imidazo [4,5-c] pyridine derivatives as PD-L1 internalization inducers, WO2018119224A1, 2018
- Koblish H. K. Wu L. Wang L.-C. S. Liu P. C. C. Wynn R. Rios-Doria J. Spitz S. Liu H. Volgina A. Zolotarjova N. et al. Characterization of INCB086550: A Potent and Novel Small-Molecule PD-L1 Inhibitor. Cancer Discovery. 2022;12(6):1482–1499. doi: 10.1158/2159-8290.CD-21-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. Cai S. Cheng Y. Zhang W. Wang M. Sun H. Guo B. Li Z. Xiao Y. Jiang S. Discovery of Small-Molecule Inhibitors of the PD-1/PD-L1 Axis That Promote PD-L1 Internalization and Degradation. J. Med. Chem. 2022;65(5):3879–3893. doi: 10.1021/acs.jmedchem.1c01682. [DOI] [PubMed] [Google Scholar]
- Sun C. Yin M. Cheng Y. Kuang Z. Liu X. Wang G. Wang X. Yuan K. Min W. Dong J. et al. Novel Small-Molecule PD-L1 Inhibitor Induces PD-L1 Internalization and Optimizes the Immune Microenvironment. J. Med. Chem. 2023;66(3):2064–2083. doi: 10.1021/acs.jmedchem.2c01801. [DOI] [PubMed] [Google Scholar]
- Rios-Doria J. Volgina A. Gokhale P. Liu H. Stevens C. Zolotarjova N. DiMatteo D. Kapilashrami K. Behshad E. Thekkat P. et al. INCB090244, a potent small molecule that inhibits the PD-L1/PD-1 axis and functions similarly to PD-L1 antibodies. J. Immunother. Cancer. 2021;9(Suppl 2):A247. doi: 10.1136/jitc-2021-SITC2021.232. [DOI] [Google Scholar]
- Li C. W. Lim S. O. Chung E. M. Kim Y. S. Park A. H. Yao J. Cha J. H. Xia W. Chan L. C. Kim T. et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell. 2018;33(2):187–201 e110. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S. Veyhl J. Jessa F. Polyakova O. Alenzi A. MacMillan C. Ralhan R. Walfish P. G. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget. 2016;7(22):32318–32328. doi: 10.18632/oncotarget.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramespacher J. A. Cotton A. D. Burroughs P. W. W. Seiple I. B. Wells J. A. Roadmap for Optimizing and Broadening Antibody-Based PROTACs for Degradation of Cell Surface Proteins. ACS Chem. Biol. 2022;17(5):1259–1268. doi: 10.1021/acschembio.2c00185. [DOI] [PubMed] [Google Scholar]
- Zheng J. He W. Li J. Feng X. Li Y. Cheng B. Zhou Y. Li M. Liu K. Shao X. Zhang J. Li H. Chen L. Fang L. Bifunctional Compounds as Molecular Degraders for Integrin-Facilitated Targeted Protein Degradation. J. Am. Chem. Soc. 2022;144(48):21831–21836. doi: 10.1021/jacs.2c08367. [DOI] [PubMed] [Google Scholar]
- Sakamoto K. M. Kim K. B. Kumagai A. Mercurio F. Crews C. M. Deshaies R. J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. U. S. A. 2001;98(74):8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N. Zeng Y. Du W. Zhu J. Shen D. Liu Z. Huang J.-A. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int. J. Oncol. 2016;49(4):1360–1368. doi: 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- Wu Y. Zhang C. Liu X. He Z. Shan B. Zeng Q. Zhao Q. Zhu H. Liao H. Cen X. Xu X. Zhang M. Hou T. Wang Z. Yan H. Yang S. Sun Y. Chen Y. Wu R. Xie T. Chen W. Najafov A. Ying S. Xia H. ARIH1 signaling promotes anti-tumor immunity by targeting PD-L1 for proteasomal degradation. Nat. Commun. 2021;12(1):2346. doi: 10.1038/s41467-021-22467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian G. Guo J. Vallega K. A. Hu C. Chen Z. Deng Y. Wang Q. Fan S. Ramalingam S. S. Owonikoko T. K. et al. Membrane-Associated RING-CH 8 Functions as a Novel PD-L1 E3 Ligase to Mediate PD-L1 Degradation Induced by EGFR Inhibitors. Mol. Cancer Res. 2021;19(10):1622–1634. doi: 10.1158/1541-7786.MCR-21-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. Xia X. Hu J. Fowlkes N. W. Li S. WSX1 act as a tumor suppressor in hepatocellular carcinoma by downregulating neoplastic PD-L1 expression. Nat. Commun. 2021;12(1):3500. doi: 10.1038/s41467-021-23864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak K. M. Grudnik P. Guzik K. Musielak B. Zieba B. J. Dömling A. Dubin G. Holak T. A. Structural basis for small molecule targeting of the programmed death ligand 1 (PD-L1) Oncotarget. 2016;7:30323–30335. doi: 10.18632/oncotarget.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sambrooks C. Shrimal S. Khodier C. Flaherty D. P. Rinis N. Charest J. C. Gao N. Zhao P. Wells L. Lewis T. A. et al. Oligosaccharyltransferase inhibition induces senescence in RTK-driven tumor cells. Nat. Chem. Biol. 2016;12(12):1023–1030. doi: 10.1038/nchembio.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. W. Lim S. O. Xia W. Y. Lee H. H. Chan L. C. Kuo C. W. Khoo K. H. Chang S. S. Cha J. H. Kim T. W. et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. F. Li Z. Ma D. Yu Q. Small-molecule PD-L1 inhibitor BMS1166 abrogates the function of PD-L1 by blocking its ER export. OncoImmunology. 2020;9(1):1831153. doi: 10.1080/2162402X.2020.1831153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J. H. Yang W. H. Xia W. Wei Y. Chan L. C. Lim S. O. Li C. W. Kim T. Chang S. S. Lee H. H. Hsu J. L. Wang H. L. Kuo C. W. Chang W. C. Hadad S. Purdie C. A. McCoy A. M. Cai S. Tu Y. Litton J. K. Mittendorf E. A. Moulder S. L. Symmans W. F. Thompson A. M. Worms H. P. Chen C. H. Khoo K. H. Hung M. C. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell. 2018;71(4):606–620 e607. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J. Li L. Li N. Li F. Qin X. Li T. Liu M. Metformin suppresses cancer cell growth in endometrial carcinoma by inhibiting PD-L1. Eur. J. Pharmacol. 2019;859:172541. doi: 10.1016/j.ejphar.2019.172541. [DOI] [PubMed] [Google Scholar]
- Zhang R. Yang Y. Dong W. Lin M. He J. Zhang X. Tian T. Yang Y. Chen K. Lei Q. Y. Zhang S. Xu Y. Lv L. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc. Natl. Acad. Sci. U. S. A. 2022;119(8):e2114851119. doi: 10.1073/pnas.2114851119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Chi H. Deng S. Wang H. Yao H. Wang Y. Chen D. Guo X. Fang J. Y. He F. et al. THADA drives Golgi residency and upregulation of PD-L1 in cancer cells and provides promising target for immunotherapy. J. Immunother. Cancer. 2021;9(8):e002443. doi: 10.1136/jitc-2021-002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. M. Vetizou M. Daillere R. Roberti M. P. Yamazaki T. Routy B. Lepage P. Boneca I. G. Chamaillard M. Kroemer G. et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44(6):1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Liu Y. Liu X. Zhang N. Yin M. Dong J. Zeng Q. Mao G. Song D. Liu L. Deng H. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm. Sin. B. 2020;10(12):2299–2312. doi: 10.1016/j.apsb.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Chen H. Yin S. Fan L. Jin C. Zhao C. Hu H. Docosahexaenoic acid reverses PD-L1-mediated immune suppression by accelerating its ubiquitin-proteasome degradation. J. Nutr. Biochem. 2022;112:109186. doi: 10.1016/j.jnutbio.2022.109186. [DOI] [PubMed] [Google Scholar]
- Neklesa T. K. Winkler J. D. Crews C. M. Targeted protein degradation by PROTACs. Pharmacol. Ther. 2017;174:138–144. doi: 10.1016/j.pharmthera.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Luh L. M. Scheib U. Juenemann K. Wortmann L. Brands M. Cromm P. M. Prey for the Proteasome: Targeted Protein Degradation-A Medicinal Chemist's Perspective. Angew. Chem., Int. Ed. 2020;59(36):15448–15466. doi: 10.1002/anie.202004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Zheng M. Ma Z. Zhou Y. Huo J. Zhang W. Liu Y. Guo Y. Zhou X. Li H. Chen L. Design, synthesis, and evaluation of PD-L1 degraders to enhance T cell killing activity against melanoma. Chin. Chem. Lett. 2022;34(5):107762. doi: 10.1016/j.cclet.2022.107762. [DOI] [Google Scholar]
- Cheng B. Ren Y. Cao H. Chen J. Discovery of novel resorcinol diphenyl ether-based PROTAC-like molecules as dual inhibitors and degraders of PD-L1. Eur. J. Med. Chem. 2020;199:112377. doi: 10.1016/j.ejmech.2020.112377. [DOI] [PubMed] [Google Scholar]
- Dai M. Y. Shi Y. Y. Wang A. J. Liu X. L. Liu M. Cai H. B. High-potency PD-1/PD-L1 degradation induced by Peptide-PROTAC in human cancer cells. Cell Death Dis. 2022;13(11):924. doi: 10.1038/s41419-022-05375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Li R. Yang B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020;6(12):2179–2195. doi: 10.1021/acscentsci.0c01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W. Tan M. Wang Z. Zhang J. Huang W. Song H. Wang X. Ran H. Gao Y. Nie G. et al. Targeted Degradation of PD-L1 and Activation of the STING Pathway by Carbon-Dot-Based PROTACs for Cancer Immunotherapy. Angew. Chem. 2023;62(11):e202218128. doi: 10.1002/anie.202218128. [DOI] [PubMed] [Google Scholar]
- Bond M. J. Crews C. M. Proteolysis targeting chimeras (PROTACs) come of age: entering the third decade of targeted protein degradation. RSC Chem. Biol. 2021;2(3):725–742. doi: 10.1039/D1CB00011J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N. Dou Y. Liu L. Zhang X. Liu X. Zeng Q. Liu Y. Yin M. Liu X. Deng H. Song D. SA-49, a novel aloperine derivative, induces MITF-dependent lysosomal degradation of PD-L1. EBioMedicine. 2019;40:151–162. doi: 10.1016/j.ebiom.2019.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X. Li R. Guo H. Zhang W. Xu X. Chen X. Ding L. Dihydropyridine Calcium Channel Blockers Suppress the Transcription of PD-L1 by Inhibiting the Activation of STAT1. Front. Pharmacol. 2021;11:539261. doi: 10.3389/fphar.2020.539261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchinda P. Kulthanan K. Khankham S. Jongjarearnprasert K. Dhana N. Cutaneous adverse reactions to calcium channel blockers. Asian Pac. J. Allergy Immunol. 2014;32(3):246–250. doi: 10.12932/AP0380.32.3.2014. [DOI] [PubMed] [Google Scholar]
- Pan C. Luo M. Lu Y. Pan X. Chen X. Ding L. Che J. He Q. Dong X. Design, synthesis and biological evaluation of new dihydropyridine derivatives as PD-L1 degraders for enhancing antitumor immunity. Bioorg. Chem. 2022;125:105820. doi: 10.1016/j.bioorg.2022.105820. [DOI] [PubMed] [Google Scholar]
- Yao H. Lan J. Li C. Shi H. Brosseau J. P. Wang H. Lu H. Fang C. Zhang Y. Liang L. Zhou X. Wang C. Xue Y. Cui Y. Xu J. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat. Biomed. Eng. 2019;3(4):306–317. doi: 10.1038/s41551-019-0375-6. [DOI] [PubMed] [Google Scholar]
- Dai X. Bu X. Gao Y. Guo J. Hu J. Jiang C. Zhang Z. Xu K. Duan J. He S. et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol. Cell. 2021;81(11):2317–2331 e2316. doi: 10.1016/j.molcel.2021.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Wang Y. Liu S. Deng W. Lomeli S. H. Moriceau G. Wohlschlegel J. Piva M. Lo R. S. Enhancing PD-L1 Degradation by ITCH during MAPK Inhibitor Therapy Suppresses Acquired Resistance. Cancer Discovery. 2022;12(8):1942–1959. doi: 10.1158/2159-8290.CD-21-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Yao H. Wang H. Fang J. Y. Xu J. Repurposing screen identifies Amlodipine as an inducer of PD-L1 degradation and antitumor immunity. Oncogene. 2021;40(6):1128–1146. doi: 10.1038/s41388-020-01592-6. [DOI] [PubMed] [Google Scholar]
- Li H. Kuang X. Liang L. Ye Y. Zhang Y. Li J. Ma F. Tao J. Lei G. Zhao S. Su J. Yang N. Peng C. Xu X. Hung M. C. Han L. Liu H. Liu J. Chen X. The Beneficial Role of Sunitinib in Tumor Immune Surveillance by Regulating Tumor PD-L1. Adv. Sci. 2021;8(2):2001596. doi: 10.1002/advs.202001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher C. M. Thomas J. D. Haas D. A. Longen C. G. Oyer H. M. Tong J. Y. Kim F. J. Small-Molecule Sigma1 Modulator Induces Autophagic Degradation of PD-L1. Mol. Cancer Res. 2018;16(2):243–255. doi: 10.1158/1541-7786.MCR-17-0166. [DOI] [PubMed] [Google Scholar]