Abstract
The emergence of antibiotic resistance to S. aureus and M. tuberculosis, particularly MRSA, VRSA, and drug-resistant tuberculosis, poses a serious threat to human health. Towards discovering new antibacterial agents, we designed and synthesized a series of new naphthalimide–thiourea derivatives and evaluated them against a panel of bacterial strains consisting of E. coli, S. aureus, K. pneumoniae, P. aeruginosa, A. baumannii and various mycobacterial pathogens. Compounds 4a, 4l, 4m, 4n, 4q, 9f, 9l, 13a, 13d, 13e, 17a, 17b, 17c, 17d, and 17e demonstrated potent antibacterial activity against S. aureus with MIC 0.03–8 μg mL−1. In addition, these compounds have also exhibited potent inhibition against MDR strains of S. aureus, including VRSA with MICs 0.06–4 μg mL−1. Compounds 4h, 4j, 4l, 4m, 4q, 4r, 9a, 9b, 9c, 9d, 9e, 9g, 9h, 9j, 13f and 17e also exhibited good antimycobacterial activity against M. tuberculosis with MIC 2–64 μg mL−1. The cytotoxicity assay using Vero cells revealed that all the compounds were non-toxic and exhibited a favorable selectivity index (SI >40). Time kill kinetics data indicated that compounds exhibited concentration-dependent killing. Furthermore, in silico studies were performed to decipher the possible mechanism of action. Comprehensively, these results highlight the potential of naphthalimide–thiourea derivatives as promising antibacterial agents.
Novel series of naphthalimide thiourea derivatives were synthesised and evaluated against bacterial pathogen panel and mycobacterial pathogen panel.
1. Introduction
Staphylococcus aureus is a nosocomial Gram-positive pathogen that causes skin infections, pneumonia, sepsis, septic arthritis, endocarditis, and toxic shock syndrome.1 Methicillin-resistant Staphylococcus aureus (MRSA) has acquired resistance to multiple antibiotics, such as β-lactam,2 macrolides,3 fluoroquinolones, vancomycin, etc.4 The emergence and spread of antimicrobial resistance (AMR) in Staphylococcus aureus, especially methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA), poses a serious threat to human health. As per the Centre for Disease Control and Prevention (CDC) report, due to MRSA infections, 323 700 people were admitted to hospitals, and 10 600 deaths were observed in 2017 in the United States alone.5 It is estimated that ∼10 million people will succumb to antimicrobial resistance (AMR) by 2050. According to the World Health Organization (WHO), methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Staphylococcus aureus (VRSA), and multidrug-resistant tuberculosis (MDR-TB) have been listed as high-priority pathogens.6 Hence, there is an urgent need to develop new antimicrobial drugs or therapies with new mechanisms of action to treat multidrug-resistant infections.
DNA gyrase is a well-known and validated target in antibacterial drug discovery. It introduces negative supercoils and relaxes positive supercoils which helps in DNA replication and transcription processes.7 Targeting DNA gyrase in bacterial pathogens offers a distinct advantage as it is not present in eukaryotes and is crucial for the survival of bacteria.8 Although, fluoroquinolone containing drugs such as Ciprofloxacin are widely used as potent antibacterial agents and are known to inhibit DNA gyrase, the emergence of resistance and side effects of fluoroquinolones prompted a shift towards the exploration of new scaffolds.9 Recently, the results of phase III clinical studies of new non quinolone DNA gyrase inhibitors such as zoliflodacin10 and gepotidacin11 revealed that they are against drug resistant infections caused by Neisseria gonorrhoea.
Thiourea is a bioisostere of urea and is reported to exhibit various biological activities such as anticancer,12,13 antimicrobial,14 antitubercular,15 antidiabetic,16etc. Some of the thiourea derivatives as listed in Fig. 1 were approved for therapeutic use.17,18 Various thiourea derivatives are being explored as potential antibacterial agents, such as 3-trifluoromethyl group on phenyl thiourea derivative A demonstrated potent anti-MRSA activity with minimum inhibitory concentration (MIC) ranging 0.25–2 μg mL−1,19 thiourea derivatives incorporated in hippuric acid moiety B inhibited S. aureus with MIC 3.12 μg mL−1,20 and pyrazolyl thiourea derivative C with NNSN motif (extended thiourea motifs enables binding of copper) showed MRSA inhibition with MIC 0.15 μM,21 Quinoline amino piperidine hybrid D exhibited potent inhibitory activity against M. tuberculosis with MIC 1.72 μg mL−1,22 some thiourea derivatives E showed potent inhibition against M. tuberculosis with MIC 0.78 μg mL−1 (ref. 23) and thiourea derivatives containing 1,3,4-thiadiazoles F,24 arylthiazole derivative appended with d-glucose G,25 and benzothiazole derivative appended with d-glucose H26 exhibited potent antibacterial activity against S. aureus with MIC 0.78 μg mL−1 (Fig. 2).
Fig. 1. Thiourea containing drugs approved for therapeutic use.
Fig. 2. Thiourea motifs showing antimicrobial activity. MIC – minimum inhibitory concentration; MRSA – methicillin-resistant Staphylococcus aureus.
On the other hand, naphthalimide is a versatile scaffold well known for its DNA intercalating27 and other biological properties28 such as anticancer,29 anti-trypanosomal,30 antimalarial,31,32etc. Recently, naphthalimide derivative have gained attention due to their potent antimicrobial activity (Fig. 3). Zhang et al. identified naphthalimide corbelled aminothiazooxime derivative I which demonstrated antiMRSA activity with MIC 0.5 μg mL−1.33 Later, they reported another naphthalimide derivative naphthalimidopropanediol J which showed potent antibacterial activity against S. aureus with MIC 0.25 μg mL−1.34 Zhou et al. also synthesised naphthalimide azole derivative K with potent inhibitory activity against MRSA with a MIC 2 μg mL−1.35 Kang et al. demonstrated naphthalimide–metronidazole derivative L exhibiting potent inhibitory activity against MRSA with MIC 0.04 μmol mL−1.36 Chen et al. reported a novel naphthalimide–aminothiazole derivative M with potent antibacterial activity against MRSA with MIC 4 μg mL−1.37 Gong et al. identified novel series of Schiff base-linked imidazole naphthalimide N which exhibits anti-MRSA activity with MIC 0.003 μmol mL−1.38 Gupta et al. developed naphthalimidetriazines O as antibacterial agent against S. aureus with MIC 1.56 μg mL−1.39
Fig. 3. Naphthalimide derivatives showing antimicrobial activity. MIC – minimum inhibitory concentration; MRSA – methicillin-resistant Staphylococcus aureus.
Based on the structures of the above promising thiourea and naphthalimide motifs with potent antibacterial properties, we designed new naphthalimide–thiourea hybrids using molecular hybridisation approach which can potentially address multidrug resistance (Fig. 4). Herein, we report the design, synthesis, and biological evaluation of new naphthalimidethiourea hybrids as potent antimicrobial agents.
Fig. 4. Designed hybrids using molecular hybridisation concept. MIC – minimum inhibitory concentration; MRSA – methicillin-resistant Staphylococcus aureus.
2. Results and discussion
2.1. Chemistry
The synthetic route of target compounds 4a–4r is detailed in Scheme 1. The key intermediate 3 was synthesized by the condensation reaction of naphthalic anhydride 1 with aminoethyl piperazine 2. Then, the intermediate 3 was treated with substituted aryl/alkyl/heteroaryl isothiocyanates and isocyanates in presence of triethylamine in acetonitrile under reflux conditions for 1 h to give the final compounds 4a–4r.
Scheme 1. Reagents and conditions: (i) ethanol, reflux, 8 h, yield 80%; (ii) triethylamine, substituted isothiocyanates or isocyanates (RNCS or RNCO), acetonitrile, reflux, 1 h, yield 60–90%.
The synthetic route for synthesis of final compounds 9a–9l, 13a–g and 17a–e is shown in Scheme 2. Intermediates 5a–b were synthesised by the condensation reaction of naphthalic anhydride 1 with glycine/β-alanine in DMF heating at 110 °C for 8 h. Then, intermediates 5a–b were treated with Boc protected amines 6, 10, or 14 to give the corresponding intermediates 7a–b, 11a–b and 15, which upon further N-Boc deprotection using HCl in dioxane gave free amines 8a–b, 12a–b and 16. Compounds 9a–l, 13a–g and 17a–e were synthesised by reaction with different substituted aryl/alkyl/heteroaryl isothiocyanates in the presence of triethylamine in acetonitrile under reflux condition.
Scheme 2. Reagents and conditions: i) glycine/β-alanine, DMF, 110 °C, 8 h, yield 70–80%; ii) HATU, DIPEA, DMF, rt, 6 h yield 75–80%; iii) HCl in dioxane (4 M), DCM, 0 °C–rt 6 h, yield 60–80%; iv) triethylamine, substituted isothiocyanates, acetonitrile, reflux, 1 h, yield 60–85%.
2.2. Biological evaluation
2.2.1. Antibiotic susceptibility against bacterial and mycobacterial pathogen panel
The antibacterial potential of the synthesized compounds was evaluated against a panel of bacterial pathogens consisting of Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Klebsiella pneumonia (K. pneumoniae), Pseudomonas aeruginosa (P. aeruginosa) and Acinetobacter baumannii (A. baumannii) following Clinical and Laboratory Standards Institute (CLSI) guidelines. Levofloxacin was used as a reference drug. The MIC values obtained are provided in Table 1.
MIC (μg mL−1) values of synthesized derivatives against bacterial and mycobacterial pathogen panel.
| Code | MIC (μg mL−1) | Code | MIC (μg mL−1) | ||
|---|---|---|---|---|---|
| S. aureus ATCC 29213 | Mtb H37Rv ATCC 27294 | S. aureus ATCC 29213 | Mtb H37Rv ATCC 27294 | ||
| 4a | 2 | >64 | 9g | >64 | 32 |
| 4b | >64 | >64 | 9h | >64 | 64 |
| 4c | >64 | >64 | 9i | >64 | >64 |
| 4d | >64 | >64 | 9j | >64 | 8 |
| 4e | >64 | >64 | 9k | >64 | >64 |
| 4f | >64 | >64 | 9l | 1 | >64 |
| 4g | >64 | >64 | 13a | 4 | >64 |
| 4h | >64 | 64 | 13b | >64 | >64 |
| 4i | >64 | >64 | 13c | >64 | >64 |
| 4j | >64 | 64 | 13d | 0.25 | >64 |
| 4k | >64 | >64 | 13e | 0.5 | >64 |
| 4l | 0.125 | 2 | 13f | >64 | 64 |
| 4m | 0.125 | 4 | 13g | >64 | >64 |
| 4n | 0.25 | >64 | 17a | 0.5 | >64 |
| 4o | >64 | >64 | 17b | 0.03125 | >64 |
| 4p | >64 | >64 | 17c | 0.125 | >64 |
| 4q | 8 | 32 | 17d | 0.25 | >64 |
| 4r | >64 | 8 | 17e | 4 | 32 |
| 9a | >64 | 16 | Levofloxacin | 0.0625 | 0.6 |
| 9b | >64 | 8 | Isoniazid | — | 0.03 |
| 9c | >64 | 64 | Rifampicin | — | 0.03 |
| 9d | >64 | 64 | Streptomycin | — | 1 |
| 9e | >64 | 32 | Ethambutol | — | 2 |
| 9f | 0.25 | >64 | Amikacin | — | 0.5 |
In antimicrobial evaluation, compound 17b was found to possess the most potent antibacterial activity (MIC 0.03125 μg mL−1) with excellent activity against MRSA and VRSA (MIC 0.06 μg mL−1). Compounds 4l, 4m, and 17c also exhibited potent inhibition against S. aureus with MIC 0.125 μg mL−1. Compounds 4n, 9f, 13d and 17d also showed antibacterial inhibition with MIC 0.25 μg mL−1. Compounds 13e and 17a showed MIC 0.5 μg mL−1, whereas compounds 9l and 4a showed MIC 1 and 2 μg mL−1, respectively. Compounds 13a and 17e showed moderate inhibition with MIC 4 μg mL−1.
The synthesised derivatives were also tested against mycobacterial pathogen panel consisting of Mycobacterium tuberculosis, Mycobacterium abscessus, Mycobacterium fortuitum and Mycobacterium chelonae. Isoniazid, streptomycin, amikacin and ethambutol were used as the comparator drugs. The MIC values of all the tested compounds is summarized in Table 1. Most of the synthesized compounds have shown inhibition against M. tuberculosis (Mtb) H37Rv with MIC 2–64 μg mL−1. Compounds 4l and 4m showed potent activity against Mtb H37Rv with MIC 2 and 4 μg mL−1 respectively. Compounds 4r, 9b, and 9j exhibited good inhibitory activity against Mtb with MIC 8 μg mL−1, whereas compound 9a showed moderate inhibition against Mtb with MIC 16 μg mL−1. Compounds 4q, 9e, 9g and 17e demonstrated inhibitory activity against Mtb with MIC 32 μg mL−1. Compounds 4h, 4j, 9c, 9d, 9h, and 13f exhibited moderate inhibition against Mtb with MIC 64 μg mL−1. All the compounds were found to be inactive towards other bacterial and mycobacterial species (see Tables ST1 and ST2 in ESI†).
The antimicrobial screening results indicated that compound 4a exhibited potent antibacterial activity with MIC 2 μg mL−1 against S. aureus ATCC 29213. However, when substitutions were introduced on the phenyl ring in compounds 4b–4g led to loss of activity. The replacement of thiourea derivative with urea derivative (4q) resulted in a decrease in antibacterial activity to 8 μg mL−1. Additionally, substituting alkyl chains (4h, 4i, 4j) led to loss of antibacterial activity. In an effort to enhance potency, bioisosteric replacement of the phenyl group with cyclohexyl group or cyclopentyl group (4m, 4l) resulted in potent antibacterial activity with an MIC of 0.125 μg mL−1. However, increasing the ring size to cycloheptyl (4n) or adamantyl (4o) led to a decrease in antibacterial activity. Subsequently, decreasing the alicyclic ring size to cyclopropyl (4k) or replacement of alicyclic ring with heteroaromatic ring (4p), also led to loss of antibacterial activity.
Inserting a carbonyl group with a phenyl group (9a, 9g) or introducing substitution on phenyl group (9b–9e, 9h–9k) did not yield any potent antibacterial activity. However, replacing the phenyl group with cyclohexyl group (9f, 9l) reduced the antibacterial activity to 0.25 and 1 μg mL−1, respectively. Notably, the compounds with 3 carbon chain (9g–9k) showed a reduction in antibacterial activity. Compound with 3 carbon atoms with cyclohexyl group (9l), reduces the antibacterial activity to 1 μg mL−1.
When the piperazine ring was replaced with 4-amino-1-Boc-piperidine ring, compound 13d with cyclohexyl group and 13e with cycloheptyl ring, exhibited a decrease in antibacterial activity with an MIC of 0.25 μg mL−1 and 0.5 μg mL−1 respectively. Upon replacement of 4-amino-1-Boc-piperidine with 4-(N-Boc-amino)piperidine having cyclopentyl group (17b), the most potent antibacterial activity was observed with MIC 0.03125 μg mL−1. However, compound 17c with cyclohexyl and compound 17d with cycloheptyl ring gave equipotent antibacterial activity with MIC of 0.125 μg mL−1 and 0.25 μg mL−1 as compound 4m and 4n. Compound 17e with adamantly ring gave moderate antibacterial activity with MIC 4 μg mL−1. The structure–activity relationship is summarized in Fig. 5.
Fig. 5. Structure–activity relationship (SAR) of designed compounds.
2.2.2. Cytotoxicity against Vero cells
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was employed to assess the cytotoxicity of active compounds 4a, 4l, 4m, 4n, 9f, 9l, 13a, 13d, 13e, 17a, 17b, 17c and 17d against Vero cells, a cell line derived from African green monkey kidneys (ATCC CCL-81). The CC50 value (μg mL−1), representing the concentration at which a 50% decrease in cell viability occurs, was determined. Levofloxacin and doxorubicin were used as positive controls, and each experiment was performed in triplicate. The cytotoxicity results indicate that compounds 4a, 4l, 4m, 4n, 9f, 9l, 13a, 13d, 13e, 17a, 17b, 17c and 17d exhibited no toxicity towards Vero cells, with CC50 values greater than 10 and demonstrate a favourable selectivity index (SI) exceeding 20 as shown in Table 2. Compound 17b exhibited the highest selectivity index >3200 and CC50 >100.
CC50 (μg mL−1) against Vero cells and selectivity index.
| S. no | Code | MIC against S. aureus ATCC 29213 | Mtb H37Rv ATCC 27294 | CC50 (μg mL−1) | Selectivity index |
|---|---|---|---|---|---|
| 1 | 4a | 2 | >64 | >100 | >50 |
| 2 | 4l | 0.125 | 2 | >20 | >160 |
| 3 | 4m | 0.125 | 4 | >20 | >160 |
| 4 | 4n | 0.25 | >64 | >20 | >80 |
| 5 | 9f | 0.25 | >64 | >100 | >400 |
| 6 | 9l | 1 | >64 | >50 | >50 |
| 7 | 13a | 4 | >64 | >200 | >50 |
| 8 | 13d | 0.25 | >64 | >10 | >40 |
| 9 | 13e | 0.5 | >64 | >100 | >200 |
| 10 | 17a | 0.5 | >64 | >100 | >200 |
| 11 | 17b | 0.03125 | >64 | >100 | >3200 |
| 12 | 17c | 0.125 | >64 | >100 | >800 |
| 13 | 17d | 0.25 | >64 | >100 | >400 |
2.2.3. Determination of activity against MDR S. aureus
To assess their efficacy against multiple strains of multidrug resistant (MDR) S. aureus, compounds 4a, 4l, 4m, 4n, 9f, 9l, 13a, 13d, 13e, 17a, 17b, 17c and 17d were tested against various clinical isolates of MRSA and VRSA with methicillin, vancomycin, levofloxacin and meropenem as reference controls. The results were summarized in Table 3. From the results, it is observed that the compound 17b exhibited equipotent antibacterial activity against various clinical isolates of MRSA and VRSA with MIC 0.06 μg mL−1. Compounds 4l, 4m, 4n, 9f, 13d, 13e, 17a, 17c, and 17d also exhibited potent antibacterial activity with MIC ranging from 0.125–1 μg mL−1 against clinical isolates of MRSA and VRSA.
MIC (μg mL−1) of compounds 4a, 4l, 4m, 4n, 9f, 9l, 13a, 13d, 13e, 17a, 17b, 17c and 17d against clinical MRSA & VRSA isolates.
| Bacterial strains | MIC (μg mL−1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4a | 4l | 4m | 4n | 9f | 9l | 13a | 13d | 13e | 17a | 17b | 17c | 17d | LVX | MEM | VAN | ||
| MSSA | ATCC 29213 | 4 | 0.125 | 0.25 | 0.5 | 0.125 | 2 | 4 | 0.25 | 1 | 1 | 0.06 | 0.125 | 0.25 | 0.25 | 0.125 | 1 |
| MRSA | NRS 100 | 4 | 0.25 | 0.25 | 0.5 | 0.5 | 4 | 4 | 0.25 | 0.5 | 1 | 0.06 | 0.125 | 0.25 | 0.125 | 32 | 2 |
| NRS 119 | 4 | 0.125 | 0.25 | 1 | 0.25 | 2 | 2 | 0.125 | 0.5 | 0.25 | 0.06 | 0.125 | 0.25 | 16 | 64 | 2 | |
| NRS 129 | 4 | 0.25 | 0.25 | 0.5 | 0.25 | 2 | 4 | 0.5 | 1 | 1 | 0.06 | 0.125 | 0.25 | 0.25 | 2 | 1 | |
| NRS 186 | 4 | 0.25 | 0.25 | 1 | 0.125 | 1 | 2 | 0.125 | 0.5 | 0.25 | 0.06 | 0.125 | 0.25 | 4 | 4 | 1 | |
| NRS 191 | 2 | 0.125 | 0.25 | 1 | 0.25 | 2 | 2 | 0.125 | 0.5 | 0.25 | 0.06 | 0.125 | 0.25 | 16 | 64 | 1 | |
| NRS 192 | 2 | 0.125 | 0.25 | 0.5 | 0.125 | 1 | 1 | 0.25 | 1 | 0.25 | 0.06 | 0.125 | 0.25 | 8 | 4 | 1 | |
| NRS 193 | 2 | 0.125 | 0.25 | 1 | 0.25 | 4 | 2 | 0.125 | 0.5 | 0.25 | 0.06 | 0.125 | 0.25 | 32 | 64 | 2 | |
| NRS 194 | 4 | 0.125 | 0.25 | 0.5 | 0.125 | 2 | 4 | 0.25 | 1 | 1 | 0.06 | 0.125 | 0.25 | 0.125 | 1 | 1 | |
| NRS 198 | 2 | 0.125 | 0.25 | 1 | 0.25 | 1 | 2 | 0.25 | 1 | 0.25 | 0.06 | 0.125 | 0.25 | 32 | 64 | 2 | |
| VRSA | VRS 1 | 4 | 0.125 | 0.25 | 1 | 0.25 | 2 | 2 | 0.5 | 1 | 0.5 | 0.06 | 0.125 | 0.25 | 32 | 64 | >64 |
| VRS 4 | 2 | 0.125 | 0.25 | 0.5 | 0.25 | 1 | 2 | 0.25 | 1 | 0.25 | 0.06 | 0.125 | 0.25 | >64 | 64 | >64 | |
| VRS 12 | 4 | 0.25 | 0.5 | 1 | 0.25 | 2 | 4 | 0.25 | 1 | 0.5 | 0.06 | 0.125 | 0.25 | 32 | 8 | >64 | |
2.2.4. Time kill kinetics
The bacteriostatic/ bactericidal activity of hybrids 4l, 4m, 4n and 17b against S. aureus ATCC 29213 was determined by the time-kill assay. Initially, S. aureus ATCC 29213 cells were diluted up to ∼105 cfu mL−1 and incubated at 37 °C with lead compound 4l, 4m, 4n, 17b and vancomycin in Mueller–Hinton broth (MHB) at 1× and 10× MIC. After the time intervals of 0 h, 1 h, 6 h and 24 h, 0.1 mL of samples were collected, diluted in phosphate-buffered saline (PBS) and plated on TSA followed by incubation at 37 °C for 18–24 h. Plotting of kill curves based on the count of cfu mL−1 of remnant bacterial cells on plate at the specified time intervals in the presence as well as absence of compound. As can be seen in Fig. 6, the compounds 4l, 4m, 4n and 17b reduce ∼5 log10 cfu mL−1 as compared to untreated in 24 at 10× MIC whereas vancomycin at 10× MIC completely eliminates all culture. Thus, compounds 4l, 4m, 4n and 17b showed concentration-dependent bactericidal reduction which is comparable to vancomycin.
Fig. 6. Time kill kinetics of compounds 4l, 4m, 4n and 17b along with comparator antibiotic.
2.3. Molecular modelling studies
2.3.1. Molecular docking
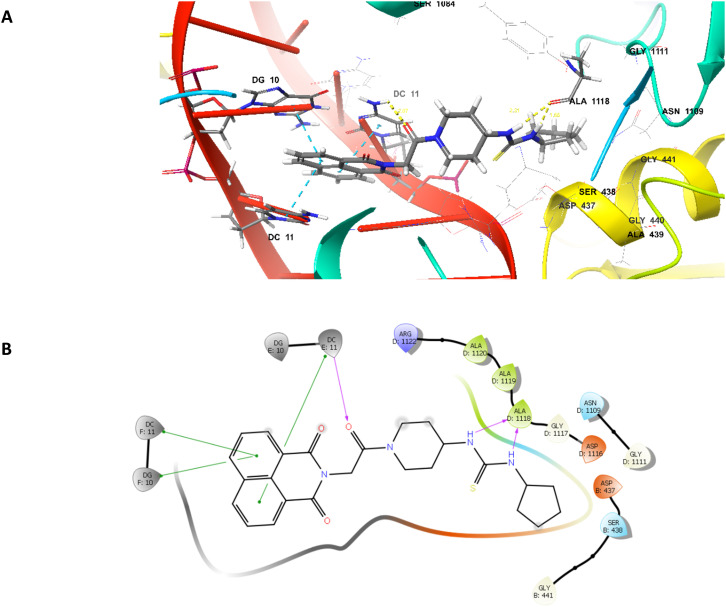
As naphthalimide37 and thiourea derivatives22,25,40 are explored as DNA gyrase inhibitors and in order to understand the binding pattern and interaction of compound 17b, molecular docking was performed at the active site of the enzyme S. aureus DNA gyrase complexed with GSK299423 (PDB ID 2XCS) using Schrodinger 2022-1 Maestro version 13.1.141. Validation of docking protocol was done by redocking the cocrystal and rmsd was found to be 0.752. Compound 17b showed hydrogen bonding with ALA1118 via nitrogen atom of thiourea group with a distance of 2.31 and 1.66 Å. It also showed hydrogen bonding between oxygen atom of carbonyl group and the DC11 with a distance of 2.07 Å. The naphthalimide ring of the compound undergoes DNA intercalation with DG10 and DC11 through π–π stacking interactions as shown in Fig. 7.
Fig. 7. Binding interactions of compound 17b at the active site of S. aureus DNA gyrase (PDBid-2XCS). (A) 3D representation of compound 17b (grey), (B) 2D representation of compound 17b. Magenta dotted lines represents hydrogen bonding interactions, and blue dotted lines represents π–π stacking interactions.
2.3.2. Molecular dynamics simulations
To validate the molecular docking results, molecular dynamic studies were performed to comprehend the stability of protein ligand complex of compound 17b in the binding site over a timeframe of 100 ns, using Desmond. Initially, system was built using buffer box size calculation method where compound 17b with protein complexes was kept in an orthorhombic box consisting of TIP3P water molecules. Then the system was neutralized by adding 0.15 M NaCl. For this, OPLS4 force field method was used. After the minimization process, MD simulation was initiated using NPT ensemble maintaining 300 K temperature and pressure 1 bar. Then, the simulation results were analysed using simulation interaction diagram. The obtained results were assessed using 2 important key parameters root mean square deviation (RMSD) and protein–ligand contacts. The RMSD value of the protein was utilized to study the protein's stability when the ligand is present in the active site. The observed RMSD value of the protein falls in the range of 1–2.2 Å (Fig. 8A), suggesting that the compound 17b in the active site of the protein did not affect the stability of the protein backbone. Additionally, the RMSD of ligand was calculated to assess the binding stability of ligand within the protein's binding pocket. This value was found to be in the range of 1.5–3 Å indicating the relative stability of ligand. Furthermore, the root mean square fluctuations (RMSF) of protein was used for characterizing the fluctuations along the protein chain, as shown in Fig. 8B. It also showed interactions of residues with ligand. The RMSF changes observed were also below 2 Å, indicating the stability of the protein in the presence of ligand. During simulation, it was observed that compound 17b showed hydrogen bonding with ALA1118 through water while with SER438, it showed both hydrogen bonding with and without water mediation as shown in protein ligand contact diagram (Fig. 8C).
Fig. 8. A) RMSD plot of the protein–ligand complex of compound 17b where, the blue line indicates protein RMSD and pink line indicates ligand RMSD; B) protein RMSF during 100 ns simulation. C) Protein–ligand contact diagram-blue color shows hydrogen bonding through water bridges; green color shows hydrogen bonding.
2.3.3. ADME studies
In silico ADME profiling was done to investigate the druggability profile of the most potent compound using Qikprop. It consists of molecular weight, number of hydrogen bond donors, number of hydrogen bond acceptors, dipole moment, and other parameters. According to Lipinski's rule of five, the compound should have hydrogen bond donor <5, hydrogen bond acceptor <10, molecular weight <500, and log P value <5 for drug-like properties. In silico ADME results demonstrated that the compound 17b did not violate the Lipinski's rule of five and has druggable properties. It also predicted 100% oral absorption. ADME results are given as ST3 in supplementary information.
3. Conclusion
In summary, a series of novel naphthalimide–thiourea derivatives were designed, synthesised and evaluated against ESKAP panel and mycobacterial pathogen panel. Antibacterial evaluation of the synthesized compounds led to the identification of compound 17b as the most potent antibacterial agent against S. aureus (MIC 0.03125 μg mL−1). Compounds 4l and 4m also exhibited potent inhibitory activity against S. aureus (MIC 0.125 μg mL−1) as well as M. tuberculosis (MIC 2 and 4 μg mL−1). Compound 17b was non-toxic to Vero cells with an excellent SI of >3200. In addition, compound 17b also showed equipotent activity against MDR strains of S. aureus, including VRSA with MIC 0.06 μg mL−1. Moreover, compound 17b showed bacteriostatic profile in a concentration-dependent manner throughout the tested time of 24 h. Through in silico studies, it is indicated that the compound 17b exhibits antibacterial activity by inhibiting DNA gyrase enzyme. Molecular docking results were further validated by molecular dynamic studies which stated that the compound 17b is stable in the active site and has shown active interactions throughout the simulation period of 100 ns. ADME studies confirmed that the compound did not violate Lipinski rule of five and found to be druggable. These results suggest that these compounds exhibit potential for further development as potent antibacterial agents.
Author contributions
Preeti Rana – conceptualization, designing, synthesis, data curation, writing-original draft. Ramulu Parupalli – writing review and editing. Abdul Akhir – biological studies. Deepanshi Saxena – biological studies Rahul Maitra – biological studies. Mohmmad Imran – biological studies, Pradip Malik – biological studies. Shaik Mahammad Ghouse – writing – review and editing. Swanand Vinayak Joshi – writing – review and editing. Danaboina Srikanth – writing – review and editing. Y. V. Madhavi – writing – review and editing, Arunava Dasgupta – formal analysis. Sidharth Chopra – supervision, writing review, and editing. Srinivas Nanduri – Conceptualization, supervision, writing – review and editing.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
PR, RP, SMG, SVJ and DS thank DoP, Ministry of Chemicals & Fertilizers, Govt. of India, for the award of NIPER fellowships. AA, DS thank UGC, RM thank DST-INSPIRE, MI and PM thank CSIR for their fellowship.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4md00062e
References
- Paterson G. Harrison E. Holmes M. Trends Microbiol. 2014;22:42–47. doi: 10.1016/j.tim.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F. N. Engl. J. Med. 2005;352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- Moran G. J. Krishnadasan A. Gorwitz R. J. Fosheim G. E. McDougal L. K. Carey R. B. Talan D. A. N. Engl. J. Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- O'Neil J., Antimicrobial resistance: tackling a crisis for the health and wealth of nations, Review on Antimicrobial Resistance, 2014, pp. 1–16 [Google Scholar]
- CDC, Antibiotic Resistance Threats in the United States, 2019, U.S. Department of Health and Human Services, CDC, Atlanta, GA, 2019 [Google Scholar]
- WHO: Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics, https://scholar.google.com/scholar_lookup?title=GlobalPriorityListofAntibiotic-ResistantBacteriatoGuideResearch%2CDiscovery%2CandDevelopmentofNewAntibiotics&publication_year=2017&author=WHO, (accessed 1 June 2023)
- Pommier Y. ACS Chem. Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin F. Karkare S. Maxwell A. Appl. Microbiol. Biotechnol. 2011;92:479–497. doi: 10.1007/s00253-011-3557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave L. S. Sutton S. B. Webber M. A. Piddock L. J. V. Trends Microbiol. 2014;22:438–445. doi: 10.1016/j.tim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Morgan H. Lipka-Lloyd M. Warren A. J. Hughes N. Holmes J. Burton N. P. Mahenthiralingam E. Bax B. D. Int. J. Mol. Sci. 2023;24:1634. doi: 10.3390/ijms24021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. Hossain M. Powell M. Raychaudhuri A. Scangarella-Oman N. Tiffany C. Xu S. Dumont E. Janmohamed S. Infect. Dis. Ther. 2022;11:2297–2310. doi: 10.1007/s40121-022-00706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula S. N. Malleshappa Noolvi N. Vipan Parihar K. Manohara Reddy S. A. Ramani V. Gadad A. K. Singh G. Gopalan Kutty N. Mallikarjuna Rao C. Eur. J. Med. Chem. 2009;44:2923–2929. doi: 10.1016/j.ejmech.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Saeed S. Rashid N. Jones P. G. Ali M. Hussain R. Eur. J. Med. Chem. 2010;45:1323–1331. doi: 10.1016/j.ejmech.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Lafzi F. Kilic D. Yildiz M. Saracoglu N. J. Mol. Struct. 2021;1241:130566. doi: 10.1016/j.molstruc.2021.130566. [DOI] [Google Scholar]
- Tapera M. Kekeçmuhammed H. Sahin K. Siva V. Lherbet C. Homberset H. Chebaiki M. Mourey L. Zorlu Y. Tapera M. Kekeçmuhammed H. Sahin K. Krishna V. S. Lherbet C. J. Mol. Struct. 2022;1270:133899. doi: 10.1016/j.molstruc.2022.133899. [DOI] [Google Scholar]
- Faidallah H. M. Al-Mohammadi M. M. Alamry K. A. Khan K. A. J. Enzyme Inhib. Med. Chem. 2016;31:157–163. doi: 10.1080/14756366.2016.1180594. [DOI] [PubMed] [Google Scholar]
- Ronchetti R. Moroni G. Carotti A. RSC Med. Chem. 2021:1046–1064. doi: 10.1039/D1MD00058F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel A. Altaf A. A. Qureshi A. M. Badshah A. Journal of Drug Design and Medicinal Chemistry. 2016;2:10–20. doi: 10.11648/j.jddmc.20160201.12. [DOI] [Google Scholar]
- Ste K. Napi A. Bielenica A. Stefa J. Augustynowicz-kope E. Sanna G. Madeddu S. Boi S. Giliberti G. Struga M. Eur. J. Med. Chem. 2015;101:111–125. doi: 10.1016/j.ejmech.2015.06.027. [DOI] [PubMed] [Google Scholar]
- Abbas S. Y. El-sharief M. A. M. S. Basyouni W. M. Fakhr I. M. I. El-gammal E. W. Eur. J. Med. Chem. 2013;64:111–120. doi: 10.1016/j.ejmech.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Delpe-acharige A. Zhang M. Eschliman K. Dalecki A. Covarrubias-zambrano O. Minjarez-almeida A. Shrestha T. Lewis T. Al-ibrahim F. Leonard S. Roberts R. Tebeje A. Malalasekera A. P. Wang H. Kalubowilage M. Wolschendorf F. Kutsch O. Bossmann S. H. ACS Omega. 2021;6:6088–6099. doi: 10.1021/acsomega.0c04513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medapi B. Renuka J. Saxena S. Sridevi J. P. Medishetti R. Kulkarni P. Yogeeswari P. Sriram D. Bioorg. Med. Chem. 2015;23:2062–2078. doi: 10.1016/j.bmc.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Doğan Ş. D. Gündüz M. G. Doğan H. Krishna V. S. Lherbet C. Sriram D. Eur. J. Med. Chem. 2020;199:112402. doi: 10.1016/j.ejmech.2020.112402. [DOI] [PubMed] [Google Scholar]
- Thanh N. D. Toan V. N. Giang N. T. K. Van H. T. K. Hai D. S. Tri N. M. Toan D. N. RSC Med. Chem. 2023;14:2751–2767. doi: 10.1039/D3MD00508A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh N. D. Lan P. H. Hai D. S. Anh H. H. Giang N. T. K. Van H. T. K. Toan V. N. Tri N. M. Toan D. N. RSC Med. Chem. 2023;14:1114–1130. doi: 10.1039/D3MD00010A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh N. D. Do S. H. Giang N. T. K. Toan V. N. Van H. T. K. Tri N. M. Toan D. N. New J. Chem. 2023;47:22360–22376. doi: 10.1039/D3NJ04443B. [DOI] [Google Scholar]
- Chen Z. Liang X. Zhang H. Xie H. Liu J. Xu Y. Zhu W. Wang Y. Wang X. Tan S. Kuang D. Qian X. J. Med. Chem. 2010;53:2589–2600. doi: 10.1021/jm100025u. [DOI] [PubMed] [Google Scholar]
- Kamal A. Bolla N. R. Srikanth P. S. Srivastava A. K. Expert Opin. Ther. Pat. 2013;23:299–317. doi: 10.1517/13543776.2013.746313. [DOI] [PubMed] [Google Scholar]
- Tomczyk M. D. Walczak K. Z. Eur. J. Med. Chem. 2018;159:393–422. doi: 10.1016/j.ejmech.2018.09.055. [DOI] [PubMed] [Google Scholar]
- Muth M. Hoerr V. Glaser M. Ponte-Sucre A. Moll H. Stich A. Holzgrabe U. Bioorg. Med. Chem. Lett. 2007;17:1590–1593. doi: 10.1016/j.bmcl.2006.12.088. [DOI] [PubMed] [Google Scholar]
- Shalini Legac J. Adeniyi A. A. Kisten P. Rosenthal P. J. Singh P. Kumar V. ACS Med. Chem. Lett. 2020;11:154–161. doi: 10.1021/acsmedchemlett.9b00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana S. Keshri S. K. Shukla J. Vikramdeo K. S. Mondal N. Mukhopadhyay P. Dhar S. K. ACS Omega. 2016;1:318–333. doi: 10.1021/acsomega.6b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P. Gopala L. Zhang S. Cai G. Eur. J. Med. Chem. 2022;229:114050. doi: 10.1016/j.ejmech.2021.114050. [DOI] [PubMed] [Google Scholar]
- Zhang P. Hind M. Li Y. Gao W. Lin J. Zhou C. Eur. J. Med. Chem. 2022;241:114657. doi: 10.1016/j.ejmech.2022.114657. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y. Zhou C. H. Bioorg. Med. Chem. Lett. 2011;21:4349–4352. doi: 10.1016/j.bmcl.2011.05.042. [DOI] [PubMed] [Google Scholar]
- Kang J. Tangadanchu V. K. R. Gopala L. Gao W. W. Cheng Y. Liu H. B. Geng R. X. Li S. Zhou C. H. Chin. Chem. Lett. 2017;28:1369–1374. doi: 10.1016/j.cclet.2017.04.002. [DOI] [Google Scholar]
- Chen Y. Y. Gopala L. Bheemanaboina R. R. Y. Liu H. B. Cheng Y. Geng R. X. Zhou C. H. ACS Med. Chem. Lett. 2017;8:1331–1335. doi: 10.1021/acsmedchemlett.7b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H. H. Baathulaa K. Lv J. S. Cai G. X. Zhou C. H. MedChemComm. 2016;7:924–931. doi: 10.1039/C5MD00574D. [DOI] [Google Scholar]
- Gupta S. Paul K. Eur. J. Med. Chem. 2023;258:115551. doi: 10.1016/j.ejmech.2023.115551. [DOI] [PubMed] [Google Scholar]
- Hashem H. E. Amr A. E.-G. E. Nossier E. S. Elsayed E. A. Azmy E. M. Molecules. 2020;25:2766. doi: 10.3390/molecules25122766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.