Abstract
Excitotoxicity due to excessive activation of NMDARs is one of the main mechanisms of neuronal death during ischemic stroke. Previous studies have suggested that activation of either synaptic or extrasynaptic GluN2B-containing NMDARs results in neuronal damage, whereas activation of GluN2A-containing NMDARs promotes neuronal survival against ischemic insults. This study applied a systematic in silico, in vitro, and in vivo approach to the discovery of novel and potential GluN1/2A NMDAR positive allosteric modulators (PAMs). Ten compounds were obtained and identified as potential GluN1/2A PAMs by structure-based virtual screening and calcium imaging. The neuroprotective activity of the candidate compounds was demonstrated in vitro. Subsequently, compound 15 (aegeline) was tested further in the model of transient middle cerebral artery occlusion (tMCAO) in vivo, which significantly decreased cerebral infarction. The mechanism by which aegeline exerts its effect on allosteric modulation was revealed using molecular dynamics simulations. Finally, we found that the neuroprotective effect of aegeline was significantly correlated with the enhanced phosphorylation of cAMP response element-binding protein (CREB). Our study discovered the neuroprotective effect of aegeline as a novel PAM targeting GluN1/2A NMDAR, which provides a potential opportunity for the development of therapeutic agents for ischemic stroke.
The development of positive allosteric modulators targeting GluN1/2A is a new avenue for neuroprotection.
1. Introduction
Stroke is a leading cause of death and disability worldwide,1 of which ischemic stroke accounts for about 71%.2 For the past decades, excitotoxicity mediated by the N-methyl-d-aspartate (NMDA) type of glutamate receptors has been at the center stage of stroke research.3–5 According to the “NMDAR subtype” hypothesis, activating GluN2A-containing NMDARs promotes neuronal survival while activating GluN2B-containing NMDARs induces neuronal death.4–7 Targeting allosteric sites to enhance receptor function can avoid the neurotoxicity induced by direct and untimed overactivation of NMDARs.8,9 Therefore, the development of new positive allosteric modulators (PAMs) targeting GluN2A-containing NMDARs can be considered a promising neuroprotective strategy.10,11
NMDARs exist in a variety of subtypes with different subunit compositions. Assembled as tetramers, they contain two GluN1 subunits that are required along with either two GluN2 or GluN3 subunits, of which there are four (GluN2A–GluN2D) or two subtypes (GluN3A and GluN3B), respectively.12,13 Each NMDAR subunit has a modular architecture composed of three domains.14 Extracellularly, there are two clamshell-like bilobate domains including an amino-terminal domain (NTD) involved in allosteric modulation and a ligand-binding domain (LBD), where the agonists (glycine on GluN1 and glutamate on GluN2) bind. Besides, there is a transmembrane domain (TMD) which houses the ion channel pore (Fig. 1A). The NMDAR extracellular regions are tightly allosterically coupled as a result of close packing.15 Meanwhile, multiple protein/protein interfaces on tetramers provide a plethora of sites for allosteric modulation.16
Fig. 1. Conformational information on the amino terminal domain (NTD) of the GluN1–GluN2A NMDA receptor. (A) 3D surface map of the human GluN1–GluN2A receptor (model built from PDB: 7EOS). Viewed perpendicularly from the general two-fold y axis of symmetry, GluN1 and GluN2A are painted in wheat and light green, respectively. The corresponding segments of three domains on each individual GluN1 or GluN2A subunit are shown in the right panels. (B) Cartoon representation of the GluN1/N2A receptor in the activated and inhibited states. (C) Top view of the tube model for the NTD of the NMDAR. The valine at position 271 in each GluN2A subunit is labeled.
Recently, several structural biology studies provide mechanistic insights into NMDAR zinc and proton inhibition. It has been proposed that protonation causes the GluN2A–NTD to close, resulting in inhibited NMDA receptor activity.17 Similarly, the NTD clamshells of GluN2A subunits close upon zinc-binding. In the presence of zinc and protons, the closure of both NTD clamshells switches the channel from a high Po conformation (activation) to a low Po state (inhibition).18,19 In addition, holding the two NTD heterodimers in close proximity by engineering disulfide bonds significantly alters the allosteric transduction between the NTD layer and the downstream gating machinery in GluN2A receptors.20 These changes occur as the NTD clamshells close, causing the LBD clamshells to shake, bringing the lower LBD lobes together and relieving tension on the gate.18–20 (Fig. 1B). All of this has important implications for the development of small molecules that have allosteric modulation on the NMDAR.
Natural products are an important source for the discovery of drugs for major diseases. Growing interest in natural medicines has been sparked by the dearth of successful therapeutic approaches for stroke therapy.21 In this study, structure-based virtual screening (VS) strategies coupled with biological activity evaluation were carried out to discover positive allosteric modulators targeting the GluN1/N2A NMDAR NTD inter-dimer. After VS based on molecular docking methods with various degrees of precision and calcium influx assay, ten small molecules were identified as potential GluN1/N2A NMDAR PAMs. Among them, compound 15 (aegeline) showed significant neuroprotective activity in vitro and in vivo. And the way it exerts its allosteric effect on the target is revealed by flexible docking and molecular dynamics (MD). In addition, a preliminary exploration of the mechanism by which it exerts its neuroprotective effect was conducted. This research verified the protective effect of aegeline against ischemic stroke for the first time, providing a new reference for the treatment of stroke with natural products.
2. Results and discussion
2.1. Definition of the docking site on the GluN1/2A NTD inter-dimer
Lately, a gallery of cryo-EM structures of the human GluN1–GluN2A NMDA receptor, at an overall resolution of 4 Å in complex with distinct ligands or modulators, has been reported by Wang et al.22 Since the allosteric modulators prefer binding to holo (orthosteric site-bound) proteins,23 the holo state of the GluN1/GluN2A receptor (PDB code: 7EOS) was chosen for computational simulations. In a previous study, the cysteines introduced at the V217C in GluN2A NTD induced the formation of a disulfide link between the two GluN2 NTDs to prevent separation between the NTD dimer24 (Fig. 1C). The purpose of this study is to use small molecules to induce allosteric effects that will bring the NTD dimers closer to one another. Hence, we defined the two V217S inside the pocket so that the small molecules can act close to the interface of the two NTD dimers.
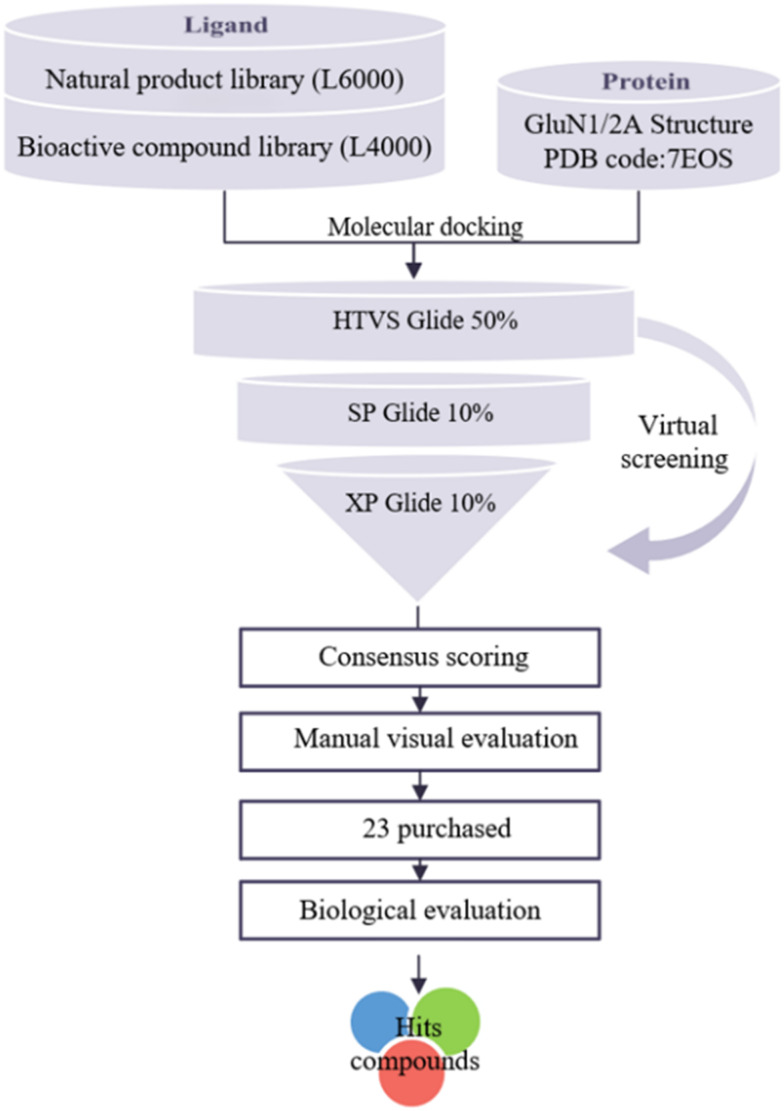
2.2. Structure-based virtual screening
Computer-aided drug design (CADD) is a widely used technique to reduce the cost and duration of the drug development process. Structure-based virtual screening (SBVS) attempts to predict the best interaction mode between two molecules to form a stable complex, and it uses scoring functions to estimate the force of non-covalent interactions between a ligand and molecular target.25 In this study, the SBVS strategy was used. The workflow is shown in Fig. 2. For drug repositioning and natural product action mining, conventionally recognized active pharmaceuticals and natural products were used for preliminary small-scale screening. Ligands from the prepared compound library were docked into the binding pocket defined at the GluN1/GluN2A NTD protein interface through all the three precision modes of Glide HTVS, SP, and XP. According to the results, a cut-off value of 7 kcal mol−1 was set and compounds with scores greater than 7 kcal mol−1 were defined as hits. After the consideration of the molecular diversity, stability, and ease of structural optimization, twenty-three compounds were manually selected for further molecular-level screening and in vitro activity evaluations. These compounds were from the TargetMol database and their docking scores and structures are provided in Table S1.†
Fig. 2. Workflow for the structure-based virtual screening (SBVS) in this work.
2.3. Candidate compounds selectively enhance agonist-induced calcium influx at GluN1/N2A receptors
Several distinctive characteristics distinguish NMDA receptors from other glutamate receptors, including voltage-dependent inhibition by extracellular Mg2+, high permeability to Ca2+, and the requirement for binding of coagonists, glutamate and glycine (or d-serine), for channel opening.14 In this light, measurement of calcium influx using fluorescent dyes has been widely used as a method to identify modulators of NMDAR activity in a microplate-based format.26 To verify whether the hit compounds as PAMs could enhance agonist-induced calcium influx to NMDA receptors, the twenty-three compounds were detected by calcium fluorescent assay in HEK-293 cells expressing GluN1/GluN2A or GluN1/GluN2B receptors, respectively. The results were expressed as the area under the curve of the fluorescence value (FMAX/F0). Ten compounds, at the concentration of 10 μM, significantly increased intracellular Ca2+ levels induced by Glu/Gly on the GluN1/N2A receptor (P < 0.05) (Fig. 3A). In contrast, there was a lack of detectable Ca2+ enhanced signals from GluN1/GluN2B receptors (Fig. 3B) in response to agonists. The results showed that these compounds had good selectivity on the GluN2A subunit.
Fig. 3. In vitro screening of candidate compounds for the modulation of NMDARs. Compounds 1 to 23, co-applied with Glu/Gly, selectively increased intracellular calcium in cell lines expressing GluN1/2A (A) and GluN1/2B (B). Data are presented as the mean ± SD from at least three independent experiments, and ***P < 0.005, **P < 0.01, *P < 0.05, test groups compared to the Glu/Gly group, one-way ANOVA, and Dunnett's multiple comparison test. Glu, glutamate; Gly, glycine.
The above ten compounds were identified as potential PAMs of the GluN1/N2A receptor. Among them, compounds 15 and 23 showed the greatest enhancement (P < 0.005). In addition, the compound 15 with the best performance was used for further studies. We identified the selectivity of compound 15 at the triheteromeric receptor (GluN1/N2A/N2B) (Fig. S1A†). Possibly because of the unique chemical spatial determination of our defined site located between the two GluN2A subunits on the GluN1/GluN2A receptor, no allosteric modulatory effect of the compounds was observed at the GluN1/GluN2A/GluN2B receptor. Compound 15 exhibits some degree of subunit selectivity and concentration dependence at the GluN1/2A receptor (Fig. S1B†). Interestingly, compound 8 exhibited a negative modulation of GluN1/GluN2B receptor activation. Fig. 4 displays the names corresponding to the number and structures of potential PAM compounds, more than half of which are natural products.
Fig. 4. Chemical structures and compound names of selected compounds.
2.4. In vitro neuroprotective activity evaluation
To further examine the phenotypic effects of the candidate compounds, these 10 compounds were evaluated for neuroprotective activity by glutamate-induced excitotoxicity models in PC-12 and HT-22 cells. The CCK-8 assay was used to detect the effect of compounds on the viability of model cells at three concentrations (0.1, 1 and 10 μM). Additionally, cytotoxicity tests were conducted at the concentration of 10 μM. As illustrated by the biological data in Table 1, compounds 2, 3, 5, and 20 displayed concentration-dependent cytoprotective activity in PC-12 cells. Compounds 2, 3, 4, 5, 19, 20, and 23 exerted neuroprotective activity at all the tested concentrations. What's more, compounds 15 and 23 were cytoprotective at three concentrations in HT-22 cells (Table 2). In general, the vast majority of compounds exhibited protective effects against glutamate-induced excitotoxicity at various concentrations.
Neuroprotective activity of the screened compounds on PC-12 cells.
| Compounds | Cell viability (%) ± SD | Toxicity (10 μM) | ||
|---|---|---|---|---|
| 0.1 μM | 1 μM | 10 μM | ||
| Control | 100.0 | |||
| Model | 44.89 ± 2.08 | 43.19 ± 2.98 | 43.75 ± 2.04 | |
| 2 | 62.56 ± 1.50** | 69.46 ± 3.50** | 77.77 ± 6.33** | 95.53 ± 4.15 |
| 3 | 63.89 ± 1.07** | 68.16 ± 1.05** | 87.52 ± 6.31** | 94.79 ± 6.61 |
| 4 | 64.66 ± 7.93** | 66.68 ± 2.34** | 60.94 ± 7.82** | 93.18 ± 6.58 |
| 5 | 66.10 ± 3.93** | 76.13 ± 1.76** | 101.50 ± 8.02** | 103.9 ± 3.52 |
| 6 | 66.20 ± 2.42** | 57.29 ± 9.91** | 35.25 ± 7.15 | 98.35 ± 5.95 |
| 15 | 54.34 ± 5.72 | 62.44 ± 4.68** | 46.03 ± 10.22 | 97.60 ± 4.55 |
| 18 | 74.06 ± 1.58** | 75.17 ± 0.57** | 53.59 ± 1.38 | 69.64 ± 12.22 |
| 19 | 64.15 ± 8.14** | 66.47 ± 1.74** | 62.75 ± 3.66** | 96.62 ± 5.27 |
| 20 | 67.39 ± 3.80** | 69.05 ± 3.30** | 70.10 ± 3.25** | 74.71 ± 7.99 |
| 23 | 66.05 ± 7.24** | 52.41 ± 3.93** | 66.55 ± 0.50** | 73.31 ± 3.82 |
Neuroprotective activity of the screened compounds on HT-22 cells.
| Compounds | Cell viability (%) ± SD | Toxicity (10 μM) | ||
|---|---|---|---|---|
| 0.1 μM | 1 μM | 10 μM | ||
| Control | 100.0 | |||
| Model | 30.31 ± 2.02 | 35.56 ± 2.12 | 34.80 ± 4.02 | |
| 2 | 24.82 ± 2.69 | 71.03 ± 1.94** | 52.41 ± 7.34** | 89.66 ± 3.33 |
| 3 | 37.73 ± 4.60* | 73.81 ± 2.45** | 37.43 ± 1.85 | 93.03 ± 1.77 |
| 4 | 39.89 ± 1.26** | 76.53 ± 6.53** | 15.99 ± 1.99 | 86.82 ± 4.66 |
| 5 | 32.62 ± 3.76 | 95.37 ± 1.46** | 43.33 ± 3.79* | 55.80 ± 4.62 |
| 6 | 36.45 ± 3.47 | 87.83 ± 6.32** | 24.50 ± 1.69 | 81.71 ± 2.96 |
| 15 | 41.11 ± 2.11** | 71.13 ± 3.03** | 42.92 ± 4.01** | 90.29 ± 2.70 |
| 18 | 46.26 ± 7.11** | 80.44 ± 4.86** | 25.64 ± 2.01 | 22.38 ± 1.67 |
| 19 | 42.66 ± 7.32** | 77.57 ± 7.65** | 39.58 ± 4.43 | 86.01 ± 5.84 |
| 20 | 34.92 ± 7.57 | 69.00 ± 4.73** | 41.38 ± 3.12* | 76.87 ± 2.34 |
| 23 | 41.44 ± 5.05* | 102.9 ± 5.01** | 42.92 ± 4.01* | 92.06 ± 3.50 |
Additionally, compound 18 did not show protective effects at high concentrations because of the higher toxicity in both cell lines (cell viability < 70%). In HT-22 cells, some of the compounds did not show a rescue effect under good cytocompatibility (cell viability > 80%). Possibly because of the higher receptor expression abundance, the drug also unselectively upregulates GluN2B subunit function, producing a detrimental effect. Meanwhile, highly differentiated PC-12 cells have neuron-like characteristics and are now widely used to study the neurotoxicity of drugs and neuronal damage and protection.27–29 Higher homology of NMDAR sequences between the Rattus norvegicus and Homosapiens was also the reason for choosing this cell line. Meanwhile, to further analyse the quantitative relationship of the compounds, we measured the EC50 of compound 15, which was the focus of the subsequent experiments, on a model of glutamate-induced excitotoxicity in PC-12 cells (Fig. S2†).
2.5. ADME property prediction
ADME analysis was performed to check the drug-likeness and biological properties of the newly discovered GluN1/GluN2A positive allosteric modulator. The properties predicted using Qikprop are listed in Table 3. Colorectal carcinoma cells are a model for the gut–blood barrier. The predicted Caco-2 cell permeability values of these three compounds were 3975.158, 1359.356 and 2035.374 nm s−1, showing that compounds 6, 15 and 20 have great cell permeability (>1000). Moreover, the majority of CNS drugs are small molecules that cross the BBB via the transcellular passive diffusion route. MDCK cells are considered to be a good mimic for the BBB (blood–brain barrier). The predicted cell permeability values of these three compounds based on the MDCK cell were 2198.777, 689.394 and 1066.493, indicating that compounds 6, 15 and 20 may have good brain exposure (>500). Meanwhile their affinity for brain tissue (QPlogBB) and the ability to be active in the central nervous system were predicted. The results showed that compounds 6, 15 and 20 have good brain/blood partition coefficients, and the corresponding predicted brain/blood partition coefficients are −0.123, −0.842 and −0.635.
The properties predicted using Qikprop of the ten studied compounds.
| Compounds | Log Pa | Log Swb | QPPCacoc | QPPMDCKd | QPlogBBe | CNSf |
|---|---|---|---|---|---|---|
| 2 | 3.035 | −4.257 | 100.126 | 1233.656 | −0.070 | 1 |
| 3 | 3.992 | −4.215 | 85.841 | 54.712 | 0.033 | 1 |
| 4 | 2.699 | −2.724 | 69.091 | 1030.22 | −0.734 | −1 |
| 5 | 3.553 | −4.305 | 740.790 | 593.642 | −1.127 | −2 |
| 6 | 3.257 | −3.497 | 3975.158 | 2198.777 | −0.123 | 0 |
| 15 | 3.346 | −4.247 | 1359.356 | 689.394 | −0.842 | −1 |
| 18 | 1.637 | −4.356 | 18.230 | 6.523 | −3.261 | −2 |
| 19 | 2.255 | −3.724 | 123.815 | 51.730 | −2.129 | −2 |
| 20 | 4.668 | −5.865 | 2035.374 | 1066.493 | −0.635 | 0 |
| 23 | 3.704 | −5.077 | 252.020 | 111.525 | −2.049 | −2 |
Predicted octanol/water partition coefficient.
Predicted aqueous solubility, Sw in mol L−1.
Predicted Caco-2 cell permeability in nm s−1 (<25, poor; >500, great).
Predicted apparent MDCK cell permeability in nm s−1. (<25, poor; >500, great).
Predicted brain/blood partition coefficient, default is −3.0 to +1.2.
Central nervous system (CNS) activity, default is −2 to +2.
Many potential therapeutic agents fail to the extent of the clinical trials due to their unfavorable absorption, distribution, metabolism, and elimination (ADME) parameters. The physicochemical properties required for brain penetration have been studied in-depth by a number of researchers in an attempt to define the characteristics of successful CNS drugs and drug candidates using plenty of approaches.30 We filtered the small molecules by Lipinski's rule of five before the virtual screening, after which compounds with allosteric agonistic effects on the receptor were selected for ADME prediction. Their predicted ADME properties are suitable for CNS drugs.
2.6. In vivo neuroprotective activity evaluation
Subsequent literature research on the above ten compounds, based on their relevant studies in the central nervous system, revealed that compound 15 has not been reported in the field of neuroprotection. Then compound 15 (aegeline) was selected for in vivo neuroprotective activity evaluation by corroborating the two cellular activity data with in vitro calcium fluorescence screening results. As shown in Fig. 5, the infarct volume was calculated using 2,3,5-triphenyltetrazolium chloride (TTC) staining on 1 mm brain sections 24 h after tMCAO. A white area on the brain sections of a model mouse represented an infarction brought on by MCAO. A considerable reduction (about 30.68% and 42.70%, P < 0.005 vs. vehicle) in the infarct volume was observed with a treatment of 5 mg kg−1 (90.53 ± 4.92 mm3) and 10 mg kg−1aegeline (74.83 ± 10.29 mm3) compared with the vehicle group (130.60 ± 9.93 mm3). Meanwhile, edaravone (10 mg kg−1) as the positive drug significantly reduced ischemic cerebral infarction (68.04 ± 4.53 mm3, P < 0.005 vs. vehicle). In vivo neuroprotective effects of edaravone and aegeline were comparable at the same dose (10 mg kg−1). Matching the histological staining results, we observed a recovery in neurological deficit scores both in the 10 mg kg−1aegeline-treated group and 10 mg kg−1edaravone-treated groups (P < 0.005 vs. vehicle). The 5 mg kg−1aegeline-treated group also reduced neurological scores to some extent (P < 0.01 vs. vehicle). Therefore, aegeline had a beneficial effect on cerebral infarction and neurologic deficit improvement (Fig. 5D).
Fig. 5. Aegeline attenuated nerve injury of tMCAO mice. (A) Experimental protocol and timeline for the evaluation of in vivo neuroprotective activity. (B) Representative TTC-stained brain slices of mice injected intraperitoneally in the control, model/vehicle, edaravone (10 mg kg−1), and aegeline (1, 5, and 10 mg kg−1) treatment groups; magenta: healthy tissue, white: damaged tissue. (C) Quantification and statistical analysis of the infarct volume after tMCAO in different groups. (D) Statistical analysis of the neurological scores after tMCAO in different groups. Data are presented as the mean ± SD, and each measurement was repeated 6 times, and ***P < 0.005, **P < 0.01, vs. vehicle-treated MCAO group, one-way ANOVA, and Dunnett's multiple comparison test.
2.7. Molecular docking and complex MD simulation analysis
Compared with rigid receptor docking, the induced fit docking (IFD) method may provide a more plausible spatial conformation of the receptor in the presence of ligands and evaluate a more reliable complex in more detail.31 The highest-scoring docking pose of aegeline was used to analyze the binding pattern of the ligand in the binding pocket of the GluN1/N2A receptor (PDB ID: 7EOS). As depicted in Fig. 6, the hydroxyanisole and styrene groups are linked by amide bonds to occupy a protein pocket with hydrophobic sides and hydrophilic middle. Plenty of hydrophobic interactions with the residues, including Ile 204, Leu219, Ile 222, Ile 227, and Ile 239, hold aegeline in the pockets. What's more, aegeline acts as a hydrogen bond donor and a hydrogen bond acceptor to form a hydrogen bond with Ser224.
Fig. 6. Docking pose of aegeline in the NMDAR binding pocket (PDB code: 7EOS). (A) Vertical clipping of the ligand-binding pocket coloring with molecular lipophilicity potential. The surface coloring ranges from dark goldenrod for the most hydrophobic potentials, through white, to dark cyan for the most hydrophilic. (B) Top view of local information on receptor binding in the pocket. Residues are shown in gray, ligands in red, and hydrogen bond interactions in yellow.
Starting from the docking outcomes, aegeline was put through a 100 ns all-atom explicit water MD simulation. The protein–ligand complexes that corresponded to the optimal docking score were assembled into the MD system. According to the root-mean-square deviation (RMSD) of the Cα atoms (Fig. S3A†), the systems reach equilibria from ∼60 ns of the MD simulations. By keeping track of the RMSD value of the ligand's heavy atoms, we can approximately confirm that the ligand can stably bind to the protein. It also can be seen that the protein chain root-mean-square fluctuation (RMSF) in the residues of the binding sites is significantly rigid compared to most other regions (Fig. S3B†). At the same time, to determine the stabilities of the chosen ligand in the binding pocket during the MD simulation, 5 properties were examined. As displayed in Fig. S4,† it was monitored that the RMSD of aegeline exhibited fluctuation but later ended up stable throughout the simulation with the overall RMSD less than 1.5 Å. The rGyr variation of aegeline in the binding pocket was found to remain stable at the range of 3.75 Å to 4.75 Å. And MolSA, SASA, PSA also remained steady during the entire MD simulation period.
The interactions throughout the trajectories showed conserved interactions of aegeline with residues of the GluN2A subunit (Fig. S5A†). Trp222 and Ile255 are significantly important for hydrophobic interactions, and Ser171, Val226 and Ser224 mainly contribute for water bridges, while Ser224 also contributes to H-bond interactions (Fig. S5B†).
Molecular dynamics (MD) simulation is a much-validated process used to observe the real-time dynamics and analyze the changes imparted by the ligand binding to protein.32 To explore the conformational dynamics upon the aegeline binding, RMSDs of the tetrameric NTDs of the GluN1–GluN2A receptor were used for further analysis. Considering the computational efficiency, the TMDs were removed and the LBDs were retained to constrain the NTD conformation due to the conformational conduction and interactions with the NTDs. The results showed that all the NTDs of GluN1 and GluN2A underwent large conformational changes (Fig. 7B). The chain C (GluN2A–NTD) bound to aegeline experienced even larger mobility than the other chains of NTDs (Fig. 7A). Notably, the conformations within the two heterodimers were comparatively constrained, in contrast to the whole tetrameric NTDs, which initially showed a noticeable mobility before tending to obstruct large-scale mobilities (Fig. 7B).
Fig. 7. Analysis of molecular dynamics simulation results. RMSD trajectories for each chain (A) and for the NTD tetramer or two NTD dimers (B) were calculated on Ca atoms based on the initial model coordinate (the docking complex) within the whole simulation time of 100 ns. (C) Center-of-mass distance changes between the two chains and R2–R2 lobes averaged of the GluN2A subunits. (D) Top-down view shows the conformational change of the NTD tetramer by aligning the initial receptor structure (bound agonist but not aegeline). (E) After combining with aegeline and running a 100 ns MD simulation, the distance between the two V217s'Cα atoms was shortened.
After MD simulation, we measured the center-of-mass (COM) distance changes between the two GluN2A chains and between the GluN2A–R2 lobes. The distance between NTDs of GluN2A subunits remained constant, while the distance between their respective R2 lobes shortened by 2 Å (Fig. 7C). Then, the receptor binding aegeline was superimposed with the apo state as shown in Fig. 7D. We discovered that the combination of aegeline flipped R2 lobes inward and upward, leading to an approach on the GluN2A space. Meanwhile, the distance between the Val217s on α5 in the two GluN2A chains was reduced by 1.52 Å, which is also consistent with the reduction of the distance between the entire R2 lobes (Fig. 7E). In summary, perturbation of the receptor by the aegeline drives the opening of the GluN2A clamshells, resulting in a more convergent plane between GluN2A NTDs. The spatial proximity of GluN2A NTDs to each other facilitates the stabilization of the receptor activation state. Therefore, we speculate that aegeline upregulates the receptor function by the mechanism of allosteric regulation as mentioned above.
2.8. Detection of the activation state of the pro-survival-related protein CREB
CREB (cAMP-response element binding protein) is the key downstream pro-survival molecule of GluN2A. The activation of CREB-mediated transcription is a prerequisite for NMDAR-mediated neuronal survival. GluN2A activation protects neurons against lethal ischemia via upregulating CREB signaling and subsequent expression of CREB target genes,33–35 including anti-apoptotic BTG2, apoptotic p53 suppressor BCL6, and survival-promoting neurotrophin BDNF (brain-derived neurotrophic factor).36,37
To determine CREB activity, we examined phosphorylated CREB at Ser-133 (pCREB–S133), an activated form of CREB.38NVP-AAM077, a highly selective NMDA receptor antagonist of GluN2A,39 blocked the phosphorylation of intracellular CREB under glutamate-induced excitotoxicity (P < 0.05 vs. Glu alone). Administration of aegeline significantly enhanced CREB phosphorylation (P < 0.05 vs. Glu alone), and NVP-AAM077 reversed this increased effect (P < 0.05 vs. Glu + NVP), suggesting that the neuroprotective effect of aegeline was achieved by enhancing downstream CREB phosphorylation mediated by activation of GluN2A subunits (Fig. 8).
Fig. 8. Aegeline increases the phosphorylation of CREB in PC-12 cells under glutamate exposure. Glutamate (Glu; 20 mM) was used to induce excitotoxicity in the presence and absence of the GluN2A preferential antagonist NVP-AAM077 (NVP; 0.4 μM) or aegeline (1 μM) and treated PC-12 cells for 24 h. (A) Protein levels of phosphorylated CREB (p-CREB), CREB, and tubulin were determined. The intensity of each band was quantified using ImageJ. (B) Statistical analysis of densitometric data from indicated experimental groups. Data obtained from 3 individual experiments are displayed as mean ± SD normalized to total CREB protein or normalized to tubulin. *P < 0.05, one-way ANOVA, and Dunnett's multiple comparison test.
3. Conclusions
Through molecular simulations, this study confirms the potential of developing PAMs based on the GluN1/2A NTD inter-dimer. The data presented in this study provide the first evidence of the neuroprotective effect of aegeline as a novel allosteric modulator targeting GluN1/N2A NMDARs. Its neuroprotective activity was demonstrated in the excitotoxic cell model as well as the ischemic stroke mouse model, which is associated with the upregulation of GluN2A subunit function and enhances downstream pro-survival signaling. In addition, aegeline was predicted to have good ADME properties for central nervous system drugs. In this way, aegeline could be an interesting molecule for the development of new therapeutic strategies for stroke.
4. Methods and materials
4.1. Structure-based virtual screening and ADME prediction
The initial structural model of the GluN1/GluN2A NMDA receptor was acquired from the RCSB Protein Data Bank (PDB code: 7EOS). In this step, only the N-terminal structural domain (NTD) was kept for simulation in order to increase screening efficiency. Protein Preparation Wizard Workflow from Schrödinger 2020 was used to prepare the protein models. Binding pockets with dimensions of 10 Å × 10 Å × 10 Å were then defined for each complex by using Glide's receptor grid generation component, with the pockets encapsulating two residues (Val217s) selected from the above-prepared protein structures. Grid generation was carried out using Glide's default settings.
The ligand databases for VS include the TargetMol Database L6000 and L4000. The database L6000 is a natural product library for HTS containing 3760 natural product monomers, and the database L4000 is a bioactive compound library containing a collection of 10 799 compounds with biological activity that can elicit biological responses in cells, tissues and even individuals. The 3D structure of each compound is generated using the LigPrep module in the Schrödinger 2020 software package (Schrödinger 2020-2, New York, USA). The protonation states and tautomers were handled using Epik at pH = 7.0 ± 2.0. For each compound, a maximum of 32 stereoisomers were allowed, and the other parameters of Ligprep were left at their default settings. To filter out ligands with suitable pharmacological properties, protocols such as QikProp and Lipinski's rule-of-five filters were incorporated. The blood–brain barrier (BBB) penetration (QPlogBB), Caco-2 cell permeability (QPPCaco), water partition (log P), water solubility (log Sw), blood–brain barrier (BBB) activity (QPPCaco), and central nervous system (CNS) activity of the chemical are all calculated using the Schrödinger module QikProp.40
The Virtual Screening Workflow (Maestro, Schrödinger 2020) was adopted to identify the potential small molecule allosteric modulator binding the putative active site of GluN1/GluN2A NTD dimer. The prepared compound databases were used in VS. The Glide41 module in Schrödinger 2020 was utilized for docking-based VS. For the VS, three different types of docking with varying degrees of precision were gradually used. Firstly, after the HTVS, 50% of the best scoring structure is reserved for SP docking. From this, the top 10% of structures in the SP docking were kept and incorporated into the XP docking.42 Lastly, the best 10% of the XP docking findings were reserved for further inspection.
4.2. Cell culture and plasmid transfection
Human embryonic kidney 293 (HEK293) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS) at 37 °C with 5% CO2. When HEK293 cells achieved 90% confluence, plasmids of either GluN1 & GluN2A or GluN1 & GluN2B were co-transfected into the cells using Sinofection (Sino Biological). The transfection ratio with these combinations of NMDAR subunits was all 1 : 1 (GluN1/GluN2A or GluN1/GluN2B).
4.3. Measurement of intracellular calcium in fluorescence imaging plate reader FlexStation3 assay
Ca2+ indicator Cal-520 AM (AAT Bioquest, USA) was used to measure the change of intracellular calcium in a collection of cells with the Multi-Mode Microplate Reader in FlexStation 3 assay (Molecular Devices). HEK293 cells were transiently transfected with cDNAs of the NMDAR before being seeded at a density of 40 000 cells per well in poly-d-lysine-treated black-backed translucent 96-well plates (Corning Costar) for an overnight culture at 37 °C with 5% CO2. On the next day, cells were incubated with the calcium fluorescent dye at 37 °C for 1.5 h, and the Glu (2 mM)/Gly (100 μM) induced responses were measured in a modified HEPES-buffered Tyrode's solution (HBTS) containing (mM): 135 NaCl, 5 KCl, 2.5 CaCl2, 10 HEPES, 10 glucose (pH = 7.2). The values of the relative fluorescence unit (RFU) were measured using FlexStation3 at the wavelength of 485 nm (excitation) and 515 nm (emission) with an interval of 1.6 s.
4.4. In vitro neuroprotection assay and cytotoxicity assay
PC-12 cells (high differentiation) (NCACC, Shanghai, China) and HT-22 cells (iCell Bioscience Inc, Shanghai, China) were cultured under the same conditions as HEK293 cells and were planted into 96-well plates at a density of 5000 cells per well for 24 h. The viability of cells was measured using CCK8 (Targetmol, USA) assays. The cells were incubated with different concentrations (0.1, 1 and 10 μM) of compounds for 4 h. The glutamate was then added to give a final concentration (20 mM in PC-12 cells and 10 mM in HT-22 cells), and was co-incubated with cells for 24 h. After treatment, the medium was added with 10 μL CCK8 agent solution, and then cells were incubated for an additional 2 h at 37 °C in the dark. Absorbance at 450 nm was measured with a microplate reader (BioTek) and the data were normalized to untreated cells. The same assay protocol was used for the cytotoxicity assay. Cell viability was assayed after co-incubation with cells for 24 h by adding only 10 μM of the compound to be tested.
4.5. Animal and treatments
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Qingdao University and approved by the Animal Ethics Committee of “QDU-AEC-2023441”. With a 12/12 h light/dark cycle and unrestricted access to food and water, C57BL/6 mice were maintained under standard conditions. Male C57BL/6 mice that were six to eight weeks old and weighed 18 to 22 g were utilized in the experiment and were randomly assigned to each group. Aegeline and edaravone were dissolved in 5% DMSO, 20% PEG300, and 5% Tween-80 with saline for i.p. treatments. The University Policies on the usage and Care of Animals were followed for all animal usage operations. Everything was done at room temperature.
4.6. tMCAO model
Briefly, the left common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) of the mice were exposed through a midline neck incision after isoflurane anesthesia. The middle cerebral artery (MCA) origin through the proximal external carotid artery was blocked by inserting a monofilament nylon suture into the left internal carotid artery. After 1.5 h of occlusion, the suture was removed to allow for blood flow restoration (reperfusion). Mice were moved into an intensive-care chamber following the procedure, where a constant temperature of 37 °C was maintained until the animals fully recovered. The body temperature of all mice was maintained at 37.0 ± 0.5 °C throughout the perioperative period.
Experimental groups and drug administration: mice were randomly divided into four groups as follows: Sham group (saline), mode/vehicle group (5% DMSO, 20% PEG300, and 5% Tween-80 in saline), compound 15 group (1, 5, and 10 mg kg−1), and edaravone group (1, 5, and 10 mg kg−1). At 2 h after reperfusion began, the medications were infused intravenously (i.p.).
Evaluation of neurological scores: the examiner, who was unaware of the treatment circumstances, reviewed the neurological scores after 24 hours after reperfusion to determine the state of the mouse's neurological impairment. The following scale was used to assign the scores: 0: no symptoms of neurological deficits; 1: not extending the right forepaw fully; 2: moving in a right-circle; 3: falling to the right; and 4: lack of spontaneous movement and low awareness levels.43
Infarct volume analysis: the mice were sacrificed after a neurological assessment by being injected with 10% chloral hydrate. Brain tissues were dissected and stored at 80 °C for 10 min. In the event that a subarachnoid hemorrhage was noticed, the mice were removed from the research. The brain tissue was then taken out and placed in a matrix for the brain slicer, where it was cut into seven 1.0 mm-thick coronal pieces. The brain slices were incubated for 20 min at 37 °C in 2% TTC solution and were immersed in 4% paraformaldehyde overnight. Following fixation, digital images were captured and subjected to image analysis (Image-Pro Plus).
4.7. Molecular docking and molecular dynamics simulation
Aegeline was positioned into the GluN1/N2A NMDAR active site using the Induced Fit Docking module of Schrödinger's Glide.44 No hydrogen bond constraints were applied. Side chains within 5.0 Å of docked ligands were refined with Prime. XP Precision was used to score poses in the final redocking step. The optimally-docked complex was submitted into the Desmond (version 3.8) module in the Schrödinger package for MD simulations. Systems were built for the complexes using an orthorhombic box shape whose edges were 10 Å away from the protein, and the box was solvated using the TIP3P water model. The system was neutralized with sodium ions. Molecular dynamics was used to set the simulation parameters. The energy of the system was first minimized using the steepest descent algorithm for 50 000 steps; then the position of the heavy atoms was restricted to run NVT equilibrium and NPT equilibrium for 50 000 steps; the system temperature was kept at 300 K and the system pressure was kept at 1 atm. After completing the two equilibrium phases, an unrestricted kinetic simulation was performed for 100 ns. We set the energy and coordinates of the trajectory to be saved once every 10 ps. RMSD calculations were then performed for each chain, the entire NTD of the protein, and the AB and CD dimers, respectively, followed by the calculation of the R2–R2 center-of-mass distance of GluN2A. The images were made with ChimeraX.45
4.8. Western blot analysis
Proteins from cell samples were extracted on ice in RIPA lysis buffer containing protease inhibitors and phosphatase inhibitors. Next, the protein concentrations were determined using a BCA protein assay kit. Protein samples were separated by electrophoresis in SDS-polyacrylamide gel and transferred to nitrocellulose filters. Either 5% non-fat dry milk or bovine serum albumin (BSA) was diluted in TBST (20 mM Tris-Cl, 140 mM NaCl, pH 7.5, 0.05% Tween-20) and used for blocking. Then, primary antibodies were added, and the membrane was incubated overnight at 4 °C. The primary antibodies were as follows: rabbit anti-pCREB (Ser133) (1 : 1000, Cell Signaling Technology, 9198) or rabbit anti-CREB (1 : 1000, Cell Signaling Technology, 9197). Internal control was performed using rabbit anti-tubulin beta antibody (1 : 5000, Affinity Biosciences, AF7011). After washing three times in TBST, membranes were incubated with secondary antibody (goat anti-rabbit IgG (H + L) HRP) for 1 h at room temperature. Lastly, the membranes were detected using the ChampChemi 610 chemiluminescence imager (SinSage Technology). The density of bands was analyzed using ImageJ software.
4.9. Statistical analyses
Data from at least three separate experiments are expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used in a statistical analysis, followed by Dunnett's test or Tukey's test for multiple comparisons, with P < 0.05 considered statistically significant. GraphPad Prism 9 software was used for all statistical analyses.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Material
Acknowledgments
Thanks to Professor Shujia Zhu of CAS Center for Excellence in Brain Science and Intelligence Technology for the gift of human NMDAR plasmids. This work was supported by the Qingdao Science and Technology Plan [grant number: 22-3-3-hygg-25-hy].
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3md00455d
References
- Feigin V. L. Forouzanfar M. H. Krishnamurthi R. Mensah G. A. Connor M. Bennett D. A. Moran A. E. Sacco R. L. Anderson L. Truelsen T. O'Donnell M. Venketasubramanian N. Barker-Collo S. Lawes C. M. Wang W. Shinohara Y. Witt E. Ezzati M. Naghavi M. Murray C. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–254. doi: 10.1016/S0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. C. V. De Silva D. A. Macleod M. R. Coutts S. B. Schwamm L. H. Davis S. M. Donnan G. A. Ischaemic stroke. Nat. Rev. Dis. Primers. 2019;5(1):70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- Szydlowska K. Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Lai T. W. Zhang S. Wang Y. T. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog. Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Wu Q. J. Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol. Brain. 2018;11(1):15. doi: 10.1186/s13041-018-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y. Chen W. Axerio-Cilies P. Wang Y. T. NMDARs in Cell Survival and Death: Implications in Stroke Pathogenesis and Treatment. Trends Mol. Med. 2020;26(6):533–551. doi: 10.1016/j.molmed.2020.03.001. [DOI] [PubMed] [Google Scholar]
- Lai T. W. Shyu W. C. Wang Y. T. Stroke intervention pathways: NMDA receptors and beyond. Trends Mol. Med. 2011;17(5):266–275. doi: 10.1016/j.molmed.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgraf M. Sellers B. D. Jiang Y. Wu G. Ly C. Q. Villemure E. Pastor R. M. Yuen P. W. Lu A. Luo X. Liu M. Zhang S. Sun L. Fu Y. Lupardus P. J. Wallweber H. J. Liederer B. M. Deshmukh G. Plise E. Tay S. Reynen P. Herrington J. Gustafson A. Liu Y. Dirksen A. Dietz M. G. Liu Y. Wang T. M. Hanson J. E. Hackos D. Scearce-Levie K. Schwarz J. B. Discovery of GluN2A-Selective NMDA Receptor Positive Allosteric Modulators (PAMs): Tuning Deactivation Kinetics via Structure-Based Design. J. Med. Chem. 2016;59(6):2760–2779. doi: 10.1021/acs.jmedchem.5b02010. [DOI] [PubMed] [Google Scholar]
- Chen X. Zhu H. Liu X. Li Q. Dong M. Design and synthesis of novel GluN2A NMDAR positive allosteric modulators via scaffold hopping strategy as anti-stroke therapeutic agents. Bioorg. Med. Chem. 2023;83:117236. doi: 10.1016/j.bmc.2023.117236. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Le G. N. T. Ge Y. Tang X. Chen X. Ejim L. Bordeleau E. Wright G. D. Burns D. C. Tran S. Axerio-Cilies P. Wang Y. T. Dong M. Woolley G. A. Isomerization of bioactive acylhydrazones triggered by light or thiols. Nat. Chem. 2023;15(9):1285–1295. doi: 10.1038/s41557-023-01239-5. [DOI] [PubMed] [Google Scholar]
- Hansen K. B. Wollmuth L. P. Bowie D. Furukawa H. Menniti F. S. Sobolevsky A. I. Swanson G. T. Swanger S. A. Greger I. H. Nakagawa T. McBain C. J. Jayaraman V. Low C. M. Dell'Acqua M. L. Diamond J. S. Camp C. R. Perszyk R. E. Yuan H. Traynelis S. F. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021;73(4):298–487. doi: 10.1124/pharmrev.120.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P. Bellone C. Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F. Wollmuth L. P. McBain C. J. Menniti F. S. Vance K. M. Ogden K. K. Hansen K. B. Yuan H. Myers S. J. Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel D. Paoletti P. Architecture and function of NMDA receptors: an evolutionary perspective. J. Physiol. 2021;599(10):2615–2638. doi: 10.1113/JP279028. [DOI] [PubMed] [Google Scholar]
- Geoffroy C. Paoletti P. Mony L. Positive allosteric modulation of NMDA receptors: mechanisms, physiological impact and therapeutic potential. J. Physiol. 2022;600(2):233–259. doi: 10.1113/JP280875. [DOI] [PubMed] [Google Scholar]
- Zhang J. B. Chang S. Xu P. Miao M. Wu H. Zhang Y. Zhang T. Wang H. Zhang J. Xie C. Song N. Luo C. Zhang X. Zhu S. Structural Basis of the Proton Sensitivity of Human GluN1-GluN2A NMDA Receptors. Cell Rep. 2018;25(13):3582–3590.e4. doi: 10.1016/j.celrep.2018.11.071. [DOI] [PubMed] [Google Scholar]
- Jalali-Yazdi F. Chowdhury S. Yoshioka C. Gouaux E. Mechanisms for Zinc and Proton Inhibition of the GluN1/GluN2A NMDA Receptor. Cell. 2018;175(6):1520–1532.e15. doi: 10.1016/j.cell.2018.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen M. Siegler Retchless B. Mony L. Johnson J. W. Paoletti P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459(7247):703–707. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M. Stroebel D. Piot L. David M. Ye S. Paoletti P. GluN2A and GluN2B NMDA receptors use distinct allosteric routes. Nat. Commun. 2021;12(1):4709. doi: 10.1038/s41467-021-25058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T. Liu M. Chen M. Luo Y. Wang C. Xu T. Jiang Y. Guo Y. Zhang J. H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Ther. 2020;216:107695. doi: 10.1016/j.pharmthera.2020.107695. [DOI] [PubMed] [Google Scholar]
- Wang H. Lv S. Stroebel D. Zhang J. Pan Y. Huang X. Zhang X. Paoletti P. Zhu S. Gating mechanism and a modulatory niche of human GluN1-GluN2A NMDA receptors. Neuron. 2021;109(15):2443–2456.e5. doi: 10.1016/j.neuron.2021.05.031. [DOI] [PubMed] [Google Scholar]
- An X. Lu S. Song K. Shen Q. Huang M. Yao X. Liu H. Zhang J. Are the Apo Proteins Suitable for the Rational Discovery of Allosteric Drugs? J. Chem. Inf. Model. 2019;59(1):597–604. doi: 10.1021/acs.jcim.8b00735. [DOI] [PubMed] [Google Scholar]
- Lu S. He X. Ni D. Zhang J. Allosteric Modulator Discovery: From Serendipity to Structure-Based Design. J. Med. Chem. 2019;62(14):6405–6421. doi: 10.1021/acs.jmedchem.8b01749. [DOI] [PubMed] [Google Scholar]
- Maia E. H. B. Assis L. C. de Oliveira T. A. da Silva A. M. Taranto A. G. Structure-Based Virtual Screening: From Classical to Artificial Intelligence. Front. Chem. 2020;8:343. doi: 10.3389/fchem.2020.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. Camargo L. M. Yeboah F. Digan M. E. Niu H. Pan Y. Reiling S. Soler-Llavina G. Weihofen W. A. Wang H. R. Shanker Y. G. Stams T. Bill A. A NMDA-receptor calcium influx assay sensitive to stimulation by glutamate and glycine/D-serine. Sci. Rep. 2017;7(1):11608. doi: 10.1038/s41598-017-11947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. Wei E. Q. Zhang W. P. Ge Q. F. Liu J. R. Wang M. L. Huang X. J. Hu X. Chen Z. Minocycline protects PC12 cells against NMDA-induced injury via inhibiting 5-lipoxygenase activation. Brain Res. 2006;1085(1):57–67. doi: 10.1016/j.brainres.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Dai S. H. Qin N. Chen T. Luo P. Zhang L. Rao W. Yang Y. F. Jiang X. F. Fei Z. Activation of mGluR5 attenuates NMDA-induced neurotoxicity through disruption of the NMDAR-PSD-95 complex and preservation of mitochondrial function in differentiated PC12 cells. Int. J. Mol. Sci. 2014;15(6):10892–10907. doi: 10.3390/ijms150610892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. Y. Piletz J. E. Halaris A. Regunathan S. Effect of agmatine against cell death induced by NMDA and glutamate in neurons and PC12 cells. Cell. Mol. Neurobiol. 2003;23(4–5):865–872. doi: 10.1023/A:1025069407173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic Z. CNS drug design: balancing physicochemical properties for optimal brain exposure. J. Med. Chem. 2015;58(6):2584–2608. doi: 10.1021/jm501535r. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Tiwary P. Borrelli K. Feng S. Miller E. B. Abel R. Friesner R. A. Berne B. J. Prediction of Protein-Ligand Binding Poses via a Combination of Induced Fit Docking and Metadynamics Simulations. J. Chem. Theory Comput. 2016;12(6):2990–2998. doi: 10.1021/acs.jctc.6b00201. [DOI] [PubMed] [Google Scholar]
- Hollingsworth S. A. Dror R. O. Molecular Dynamics Simulation for All. Neuron. 2018;99(6):1129–1143. doi: 10.1016/j.neuron.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. Lu T. J. Chen X. J. Zhou Y. Chen Q. Feng X. Y. Xu L. Duan W. H. Xiong Z. Q. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke. 2008;39(11):3042–3048. doi: 10.1161/STROKEAHA.108.521898. [DOI] [PubMed] [Google Scholar]
- Terasaki Y. Sasaki T. Yagita Y. Okazaki S. Sugiyama Y. Oyama N. Omura-Matsuoka E. Sakoda S. Kitagawa K. Activation of NR2A receptors induces ischemic tolerance through CREB signaling. J. Cereb. Blood Flow Metab. 2010;30(8):1441–1449. doi: 10.1038/jcbfm.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Hu B. Wang F. Du L. Huang B. Li L. Qi J. Wang X. Glycine bidirectionally regulates ischemic tolerance via different mechanisms including NR2A-dependent CREB phosphorylation. J. Neurochem. 2015;133(3):397–408. doi: 10.1111/jnc.12994. [DOI] [PubMed] [Google Scholar]
- Hardingham G. E. Fukunaga Y. Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hardingham G. E. Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem. Soc. Trans. 2009;37(Pt 6):1147–1160. doi: 10.1042/BST0371147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A. Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Liu Y. Wong T. P. Aarts M. Rooyakkers A. Liu L. Lai T. W. Wu D. C. Lu J. Tymianski M. Craig A. M. Wang Y. T. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007;27(11):2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. Gong X. Liu H. Yao X. Virtual Screening and Biological Activity Evaluation of New Potent Inhibitors Targeting LRRK2 Kinase Domain. ACS Chem. Neurosci. 2021;12(17):3214–3224. doi: 10.1021/acschemneuro.1c00399. [DOI] [PubMed] [Google Scholar]
- Friesner R. A. Banks J. L. Murphy R. B. Halgren T. A. Klicic J. J. Mainz D. T. Repasky M. P. Knoll E. H. Shelley M. Perry J. K. Shaw D. E. Francis P. Shenkin P. S. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Friesner R. A. Murphy R. B. Repasky M. P. Frye L. L. Greenwood J. R. Halgren T. A. Sanschagrin P. C. Mainz D. T. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Zhang H. Yu P. Lin H. Jin Z. Zhao S. Zhang Y. Xu Q. Jin H. Liu Z. Yang W. Zhang L. The Discovery of Novel ACA Derivatives as Specific TRPM2 Inhibitors that Reduce Ischemic Injury Both In Vitro and In Vivo. J. Med. Chem. 2021;64(7):3976–3996. doi: 10.1021/acs.jmedchem.0c02129. [DOI] [PubMed] [Google Scholar]
- Sherman W. Day T. Jacobson M. P. Friesner R. A. Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006;49(2):534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- Goddard T. D. Huang C. C. Meng E. C. Pettersen E. F. Couch G. S. Morris J. H. Ferrin T. E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018;27(1):14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.