Abstract
Bloom Syndrome helicase (Blm) is a RecQ family helicase involved in DNA repair, cell-cycle progression, and development. Pathogenic variants in human BLM cause the autosomal recessive disorder Bloom Syndrome, characterized by predisposition to numerous types of cancer. Prior studies of Drosophila Blm mutants lacking helicase activity or protein have shown sensitivity to DNA damaging agents, defects in repairing DNA double-strand breaks (DSBs), female sterility, and improper segregation of chromosomes in meiosis. Blm orthologs have a well conserved and highly structured RecQ helicase domain, but more than half of the protein, particularly in the N-terminus, is predicted to be unstructured. Because this region is poorly conserved across multicellular organisms, we compared closely related species to identify regions of conservation, potentially indicating important functions. We deleted two of these Drosophila-conserved regions in D. melanogaster using CRISPR/Cas9 gene editing and assessed the effects on different Blm functions. Each deletion had distinct effects on different Blm activities. Deletion of either conserved region 1 (CR1) or conserved region 2 (CR2) compromised DSB repair through synthesis-dependent strand annealing and resulted in increased mitotic crossovers. In contrast, CR2 is critical for embryonic development but CR1 is not as important. CR1 deletion allows for proficient meiotic chromosome segregation but does lead to defects in meiotic crossover designation and patterning. Finally, deletion of CR2 does not lead to significant meiotic defects, indicating that while each region has overlapping functions, there are discreet roles facilitated by each. These results provide novel insights into functions of the N-terminal disordered region of Blm.
Introduction
Bloom syndrome helicase (Blm in Drosophila; BLM in humans) is an ATP-dependent, RecQ family helicase (5–7). It is conserved across protists, plants, fungi, and animals, with roles in homology-directed DNA repair (HDR), cell-cycle progression, meiosis, and development (1, 3, 8–16). Pathogenic variants in BLM cause Bloom Syndrome, a rare autosomal recessive disorder characterized by a high predisposition to a broad range of cancers, sun sensitivity, short-stature, sterility, and immunodeficiency (5, 17, 18). BLM mutations have also been found in sporadic cancers (19–22). The high predisposition to cancer in individuals with Bloom Syndrome is associated with genome instability, including high rates of exchange between sister chromatids and homologous chromosomes (23, 24).
One important function of BLM/Blm in HDR is disassembly of DNA repair intermediates, which is done in concert with topoisomerase III alpha (TopIIIα) (25–28). BLM and TopIIIα, together with RMI1 (which Drosophila lacks (29)), unwind D-loops to promote dissociation of the invading strand in synthesis-dependent strand annealing (SDSA) (2, 7, 25, 27) and catalyzes dissolution of double Holliday junctions (dHJs) (13, 26, 30–32). These two functions prevent mitotic crossovers and therefore minimize loss of heterozygosity (LOH) and chromosome rearrangement. Blm orthologs also have functions in meiosis, but these include promoting crossovers (reviewed in 14). In Drosophila, loss of Blm results in decreased meiotic crossover rates, compromised crossover distribution and increased chromosome mis-segregation (non-disjunction) (1).
BLM/Blm also has functions in repair of stalled replication forks to promote an efficient S-phase (12, 25). BLM accumulates at stalled forks along with other DNA repair regulators, with in vitro studies suggesting BLM may act to regress stalled forks behind a DNA lesion to promote lesion removal by other repair pathways (33, 34). A second BLM cell cycle role is to resolve anaphase bridges to allow proper chromosome segregation during mitosis (35, 36). In human cells, this activity is mediated through interaction with topoisomerase IIα (TopIIα) (37). Micronuclei and aneuploidy are more prevalent in BLM-deficient cells, underscoring the importance of this BLM role to genome stability (38, 39). In Drosophila, embryos lacking Blm have increased anaphase bridges during rapid syncytial cell cycles, resulting in high rates of embryonic death (3, 16). These various functions suggest that BLM/Blm regulation is dependent on cell type and developmental timing.
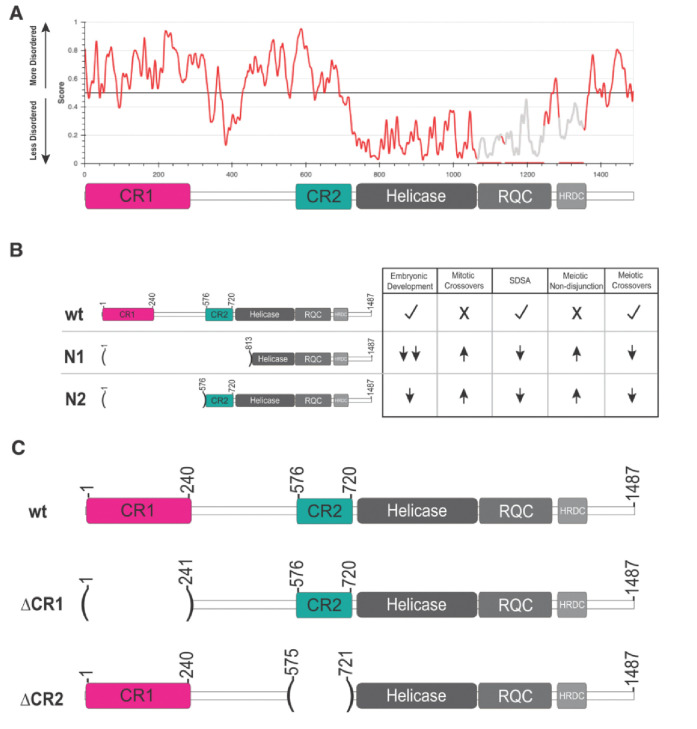
While BLM/Blm is best known by its RecQ helicase domain, there are large, intrinsically disordered regions (IDRs) both N- and C-terminal to this domain (Figure 1A). These regions, though poorly conserved in primary sequence, are likely candidates for both regulatory modifications and protein-protein interactions. TopIIIα is thought to bind in at least one of these regions, and other proteins’ interactions have been mapped to them as well (28, 37, 40). Despite this, the IDRs have been relatively poorly explored compared to the helicase domain, even though they make up more than half of the protein (Figure 1A). A study in Drosophila underscores the importance of these regions, with a Blm allele that deletes most of the unstructured N-terminus (BlmN2) compromising HDR and meiotic roles while only mildly affecting early embryonic functions (Figure 1B) (3).
Figure 1. Bloom syndrome helicase (Blm) predicted structural order and alleles used.
(A) IUPred3 (2) plot predicting the ordered and intrinsically disordered regions of Blm. A schematic of Blm domains is placed below for reference, illustrating that conserved regions 1 and 2 (CR1 and CR2) are predicted to be mostly disordered (>0.5). (B) Previously characterized alleles of Blm and their effects on Blm functions relative to wild-type (wt). A null allele, BlmN1 (N1) eliminates all well-characterized Blm functions, while the separation-of-function allele BlmN2 (N2) only moderately affects embryonic development. (C) Schematic of Blm deletions used compared to the wt Blm protein. ΔCR1 deletes amino acids 2–240, preserving amino acids 241–1487 in frame; ΔCR2 deletes amino acids 576–720, fusing amino acids 1–575 and 721–1487 together in frame.
To further investigate the function of the intrinsically disordered N-terminal region, we characterized the impacts of deletions of two N-terminal regions conserved in closely related Drosophila species on embryonic development, HDR, and meiosis. We find that while deletion of the first 240 amino acids does not compromise Blm in meiotic chromosome segregation, it does affect embryonic development, HDR, and meiotic crossover distribution, albeit less severely than Blm null mutations. A deletion of the 146 amino acids just prior to the start of the structured RecQ helicase domain results in severe defects in cell division and development but has milder effects on HDR and apparently normal meiotic crossover distribution and segregation. These findings highlight the importance of investigating intrinsically disordered Blm regions to understanding function.
Results
Identification of N-terminal regions conserved among Drosophila species and deletion by CRISPR/Cas9 genome editing
Despite high conservation in the helicase domain of Blm, the roughly 720 amino acid N-terminal region is not well conserved among multicellular organisms. This region is predicted to be intrinsically disordered (Figure 1A). Prior studies the BlmN2 allele, which deletes the first 575 residues of the IDR but retains 146 residues upstream of the helicase domain pointed to a potential role of this helicase-adjacent N-terminal region in embryonic development (McVey, 2007). To further examine functions of the N-terminal region, we narrowed our focus to conservation among more closely related Drosophila species (Figure S1). Alignment of these species identified two regions of high similarity, which we term conserved region one (Figure 1C, CR1; amino acids 1–240) and conserved region two (Figure 1C; CR2; amino acids 533–720). CR1 may contain one of the two regions in human BLM found to interact with TopIIIα (28). We further narrowed CR2 to contain only the N-terminal amino acids predicted to present in the protein produced by the BlmN2 allele (amino acids 576–720), to compare their functions more directly. Using CRISPR/Cas9 genome editing, we separately deleted the sequences encoding amino acids 1–240 and 576–720 in the endogenous Blm gene (Figure S2). We refer to these alleles as BlmΔCR1 and BlmΔCR2.
Embryonic hatch rates are affected differently by each N-terminal Blm deletion
The absence of maternally supplied Blm results in frequent anaphase bridges and high rates of embryonic lethality (3, 16). To determine the effects of each deletion on Blm function in embryonic development, we conducted embryonic hatching assays. In agreement with prior results, embryos from females homozygous for the BlmN1 allele, which does not produce Blm transcript or protein (3), have severely reduced hatch rates. In contrast, there is much smaller, though significant, reduction in hatching of embryos from BlmN2 mothers (Figure 2). Functionality of the BlmN2 protein in embryogenesis likely requires the presence of the helicase, RecQ, and HRDC domains, but the predicted BlmN2 protein also has the last 146 residues of the N-terminal IDR that may contribute to function. We assessed the effects of deletion of this region (CR2) on embryogenesis (Figure 2). Strikingly, embryos from BlmΔCR2 females have a low hatch rate similar to that of embryos from BlmN1 mothers, consistent with this region being critical to Blm function in embryonic development.
Figure 2. Hatching of embryos from Blm mutant mothers.
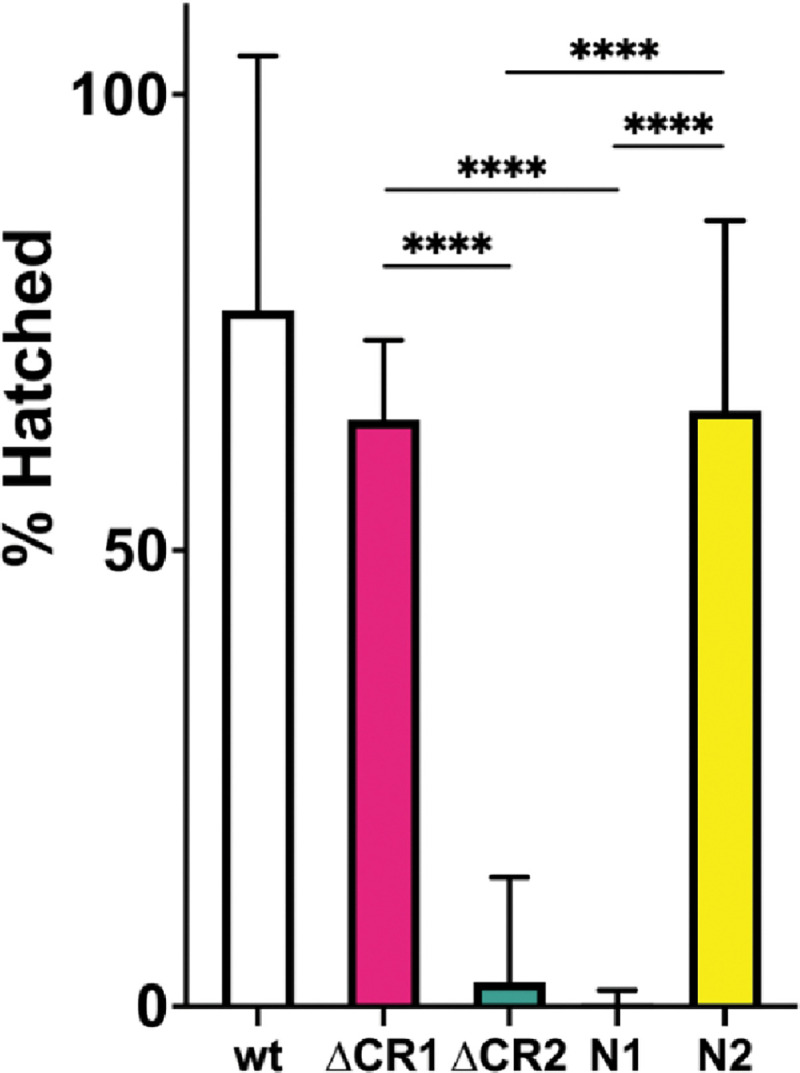
Virgin females homozygous for the Blm alleles indicated on the X-axis were crossed to Oregon-RM males and allowed to lay overnight on grape-juice agar. Embryos were transferred to fresh grape-juice agar plates and scored for hatching 48 hours later. Each experiment was repeated three times, with 100–250 embryos transferred each time. Embryos from BlmΔCR2 (ΔCR2) or BlmN1 (N1) females are rarely able to complete development. Embryos from BlmΔCR1 (ΔCR1) and BlmN2 (N2) have a modest but significant reduction in hatch rates. We conclude that the CR2 region is more critical for embryonic development but the CR1 region contributes only to a small degree. n = wt: 598; ΔCR1: 1080; ΔCR2: 743; N1: 706; N2: 700. **** p < 0.0001 by Fisher’s exact test.
We also assayed the effects of the CR1 deletion on hatching. While the fraction of embryos from BlmΔCR1 females that hatched was significantly lower that of embryos from wild-type females, it was significantly higher than that of embryos from either BlmΔCR2 or BlmN1 females (Figure 2), indicating that this region is less important to Blm roles in embryonic development. This was consistent with the high hatch rate of embryos from BlmN2 mothers, which also significantly lower than that of wild-type but higher than that of BlmΔCR2 and BlmN1, in line with previous findings (CITE McVey 2007).
Mitotic crossovers are moderately elevated in BlmΔCR1 and BlmΔCR2 mutants
Flies with the BlmN1 or BlmN2 deletion have elevated spontaneous mitotic crossovers, probably due at least in part to compromised SDSA and/or dHJ dissolution functions (Figure 1B) (3, 11). We assayed BlmΔCR1 and BlmΔCR2 mutants and found they also have elevated mitotic crossovers (Figure 3), but at rates (0.28% and 0.61%, respectively) that are significantly lower than those of BlmN1 and BlmN2 alleles (2.3% and 2.4%, respectively). This suggests that loss of CR1 or CR2 allows some non-crossover repair or some other function that prevents lesions that can be repaired as crossovers.
Figure 3. Meiotic non-disjunction (NDJ).
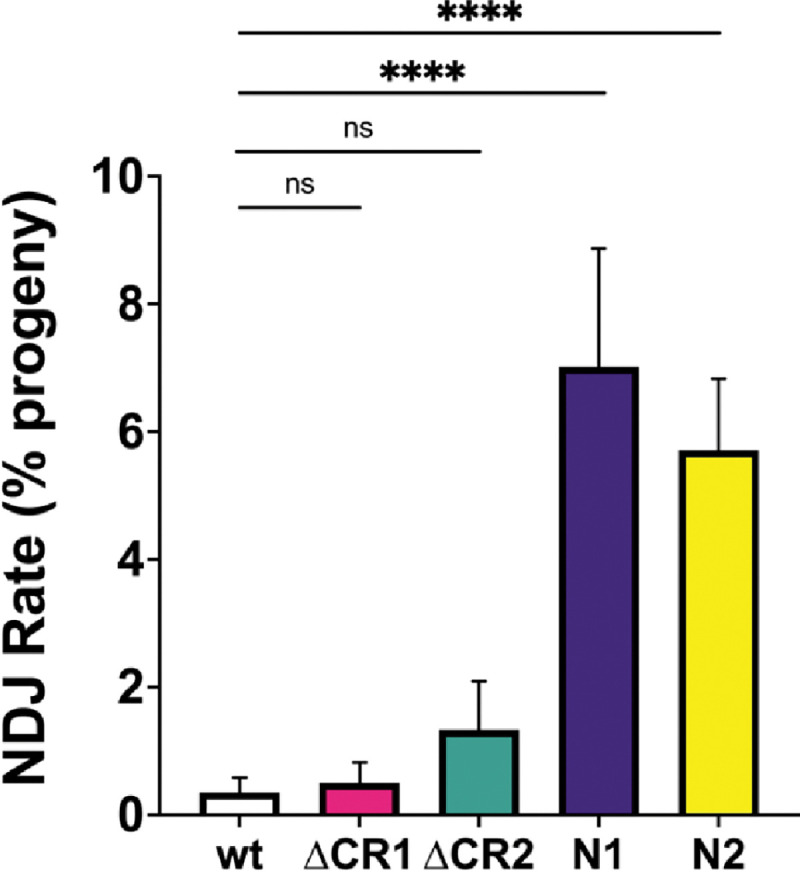
Virgin females with the Blm alleles indicated on the X-axis over the BlmN1 null allele (BlmN1 was over BlmD2) were crossed to y sc cv v g f / Dp(1;Y)BS males in at least 15 vials, each serving as a biological replicate. Progeny were scored for non-disjunction (NDJ), indicated by bar eyes in daughters (XXY) and non-bar-eyes in sons (X0) genotypes. The number of NDJ progeny was doubled to correct for genotypes that do not progress to adulthood (XXX and Y0), then NDJ rate was determined as a ratio of the number of corrected NDJ individuals to total progeny for each genotype. Neither BlmΔCR1 (ΔCR1) nor BlmΔCR2 (ΔCR2) had a significant increase in NDJ compared to wild type (wt). In agreement with prior studied, both BlmN1 and BlmN2 females have significantly elevated NDJ. Number of progeny = wt: 6900; ΔCR1: 3593; ΔCR2: 777; N1: 2959; N2: 1906. **** p < 0.0001; ns: p > 0.05 by the methods described in Zeng et al. (4).
CR1 and CR2 are required for repair of DSBs by SDSA
Blm has a key role in SDSA, where it is thought to promote dissociation of D-loops during or after synthesis (7, 41). To determine whether the lower number of mitotic crossovers in the BlmΔCR1 and BlmΔCR2 mutants relative to null mutants is due to better capabilities of these alleles to complete SDSA, we conducted the P{wa} SDSA assay (7, 41). In this assay, effectiveness of SDSA in the male germline is determined by scoring progeny for a red eye color that indicates synthesis of >4000 bp from each end of gap generated by transposase-mediated excision, followed by dissociation of nascent strands and annealing of an internal repeat (the long terminal repeat of a copia retrotransposon). This outcome is greatly reduced in BlmN1 and BlmN2 mutants, demonstrating inability to complete SDSA (3, 7). We found a similar reduction in BlmΔCR1 and BlmΔCR2, (Figure 4), revealing a requirement for both CR1 and CR2 in SDSA repair.
Figure 4. Mitotic crossovers in Blm mutants.
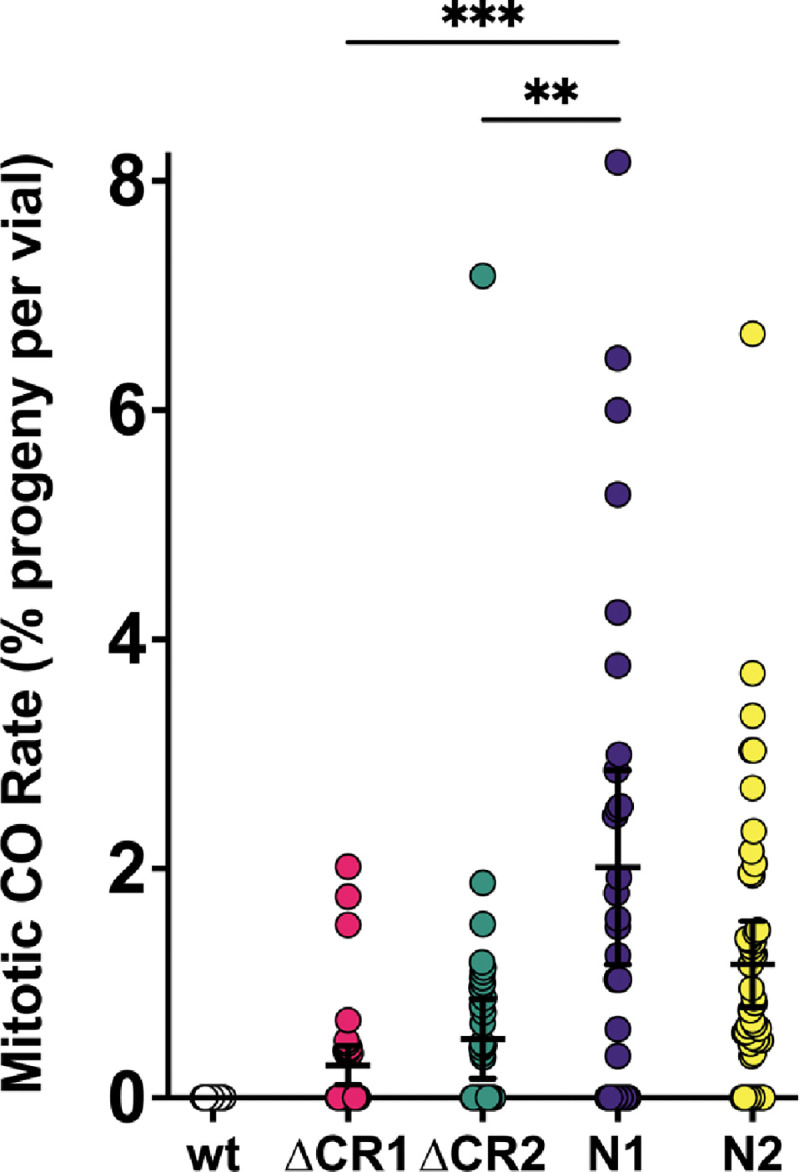
Single males with the Blm alleles indicated on the X-axis over a the BlmN1 null allele (or BlmD2 for BlmN1) were crossed to homozygous net dppho dpy b pr cn recessive phenotypic marker virgin females, with each vials serving as a biological replicate. Progeny were then scored for mitotic crossovers occurring in the parental male’s germline, indicated by mixed presence and/or absence of recessive phenotypes. To obtain the mitotic crossover rate per vial, the number of mitotic crossover progeny was divided by the total number of progeny in that vial. Rates for each vial were then pooled to obtain a mean mitotic crossover rate for each genotype. Crossovers are extremely rare in wild-type males (3), so these are excluded from statistical analyses. While both BlmΔCR1 (ΔCR1) and BlmΔCR2 (ΔCR2) have mitotic crossovers, the rates in both mutants are significantly less than that of the BlmN1 null mutants (N1). ***p<0.001 and **p < 0.01 by ANOVA with Tukey’s Post Hoc. Compared to the separation-of-function BlmN2 (N2; n = 7390) mutant, ΔCR1 mutants had significantly fewer mitotic crossovers (p < 0.05 by ANOVA with Tukey’s Post Hoc), but ΔCR2 was not significantly different. n = wt: 37 vials, 7091 progeny; ΔCR1: 54 vials, 9284 progeny; ΔCR2: 44 vials, 7174 progeny; N1: 88 vials, 9368 progeny; N2: 54 vials, 7390 progeny.
BlmΔCR1 and BlmΔCR2 mutants have distinct meiotic phenotypes compared to BlmN1 null mutants
Loss of Blm causes meiotic non-disjunction (NDJ; Figure 1B) (1, 3). To assess this function in our Blm deletion alleles, we performed an X chromosome NDJ assay. The rates of NDJ in BlmΔCR1 (0.5%) and BlmΔCR2 females (1.33%) were not significantly different from that of wild-type females (Figure 5), indicating that the regions deleted in CR1 and CR2 are dispensable for Blm functions that prevent NDJ. BlmΔCR1 and BlmΔCR2 each also had significantly lower NDJ rates than BlmN1 and BlmN2 females (7.02% and 5.71%, respectively).
Figure 5. Repair of DNA gaps by SDSA.
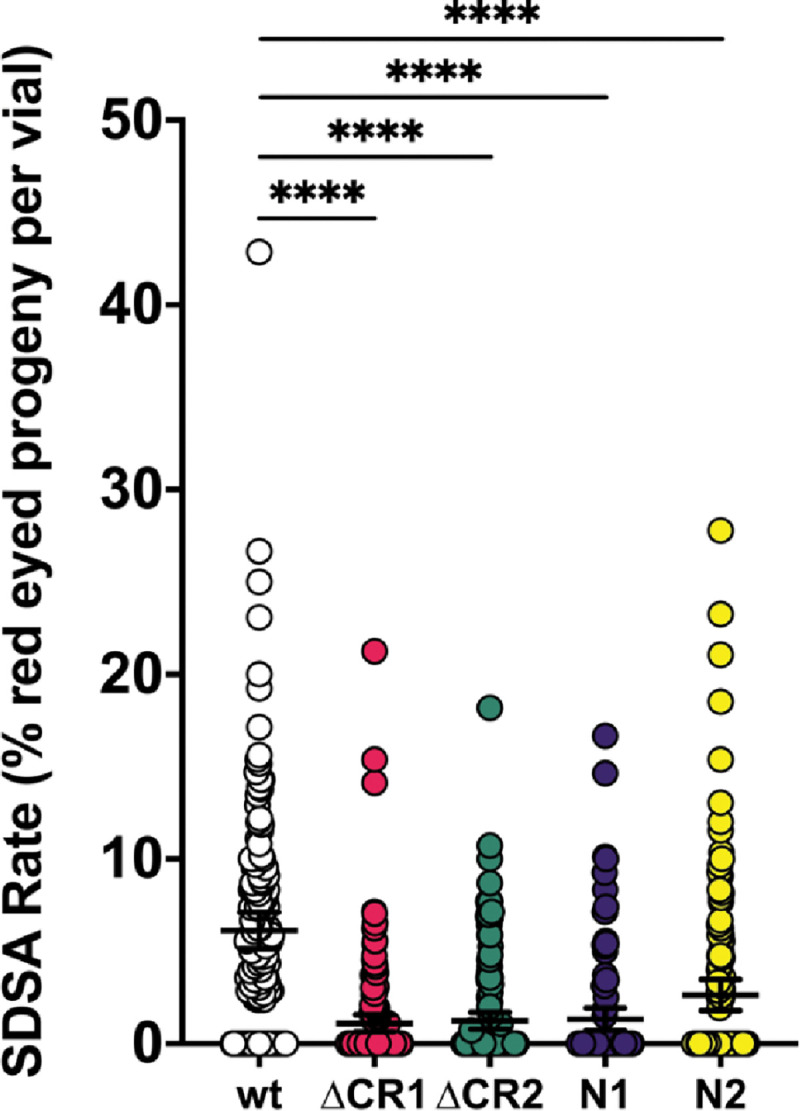
Single males with the Blm alleles indicated on the X-axis (in trans to a BlmD2 null allele) and the Δ2–3 transposase were crossed to homozygous P{wa} virgin females, with each vial serving as a biological replicate. Progeny without the Δ2–3 transposase were scored for the type of repair that occurred in the parental male’s germline, with red eyes indicating completed SDSA, yellow or white eyes indicating end-joining, and apricot eyes indicating either no excision or repair that restored the complete P{wa}. SDSA frequency is the percentage of proteny with red eyes. All mutants had significantly lower numbers of red-eyed progeny than wild-type. ****p<0.0001 by ANOVA with Tukey’s Post Hoc and Kruskal-Wallis with Dunn’s Multiple comparisons. n =wt: 151 vials, 4675 progeny; ΔCR1: 45 vials, 6393 progeny; ΔCR2: 148 vials, 4328 progeny; N1: 106 vials, 4197 progeny; N2: 133 vials, 3860 progeny.
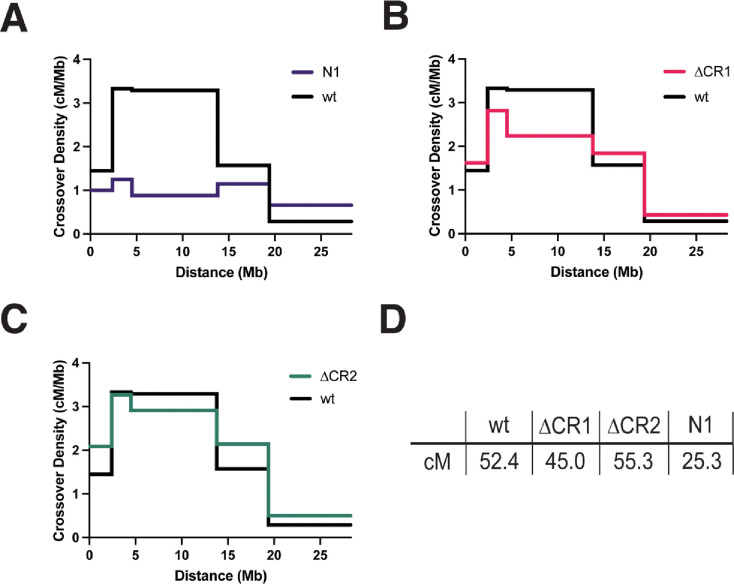
We also wanted to examine crossing over in BlmΔCR1 and BlmΔCR2 mutants. Based on results from the NDJ assay, we hypothesized that both BlmΔCR1 and BlmΔCR2 mutants would have normal meiotic crossovers. Surprisingly, crossovers were significantly reduced in BlmΔCR1 mutants (total genetic length of the region assayed was 44.8 cM in BlmΔCR1 vs. 52.4 cM in wild-type, p < 0.0001), particularly in the middle of the chromosome arm assayed (Figure 6). Also surprising was that BlmΔCR2 mutants had significantly more crossovers (55.3 cM vs. 52.4 cM in wild-type females), with a similar distribution (Figure 6). These results suggest that CR1 and CR2 have different functions in meiosis, contributing to meiotic crossover distribution in opposing ways.
Figure 6. Meiotic Crossovers in Blm Mutants.
Virgin females with the Blm alleles indicated on the X axis (in trans so the BlmN1 null allele or, for BlmN1, BlmD2) and heterozygous for the net dppho dpy b pr cn chromosome were test crossed and progeny were scored for recessive phenotypes. Graphs show crossover density (cM/Mb) for each genetic interval. (A) BlmN1 (N1) had a significant reduction in crossovers and an altered distribution, in agreement with a prior study (1). (B) Crossovers were significantly reduced in BlmΔCR1 (p < 0.01 by Fisher’s exact test). (C) BlmΔCR2 mutants had a modest but statistically significant increase in crossovers (p < 0.01 by Fisher’s exact test. (D) Both BlmΔCR1 and BlmΔCR2 mutants had significantly higher crossing over than BlmN1 (p < 0.0001 fof each, Fisher’s exact test for each. n =4031 for wt; 4049 for BlmΔCR1; 5088 for BlmΔCR2.
Discussion
CR2 is required for embryonic development
We have shown here that two previously uncharacterized regions of Drosophila Blm have distinct functional roles. Embryos from BlmΔCR2 homozygous mutant females show compromised hatching, to a similar degree as null mutants. This is likely due to the accumulation of anaphase bridges resulting from defects in rapid replication and/or an inability to resolve sister chromatid entanglements during anaphase. Russell and colleagues (37) mapped a TopIIα interaction with human BLM to the region that may correspond to CR2 of Drosophila Blm, but this interaction has not been mapped in Drosophila Blm.It is possible that this region is regulated to either promote or prevent such interaction. Phosphorylation by ataxia-telengiectasia and rad3+ related and mutated (ATR/ATM) kinases might be one way to promote interaction with TopIIα as part of the DNA damage response, both in stalled fork repair and resolution of anaphase bridges. Human ATR phosphorylates BLM at two residues to promote the recovery of replication forks after stalling by hydroxyurea, and mutation of these residues to alanine results in cell cycle arrest (42). Tangeman and colleagues (43) found that additional predicted ATR/ATM phosphorylation sites are important for BLM nucleolar localization and TopI interaction. The CR2 region has several S/T-Q sites that are possible targets of ATR/ATM phosphorylation, but a Drosophila phosphoproteomic analysis did not identify any phosphopeptides from this region in embryos (44).
Harris-Behnfeldt and colleagues (45) showed a potential requirement for phosphorylation of the human BLM region analogous to Drosophila Blm CR2, identifying several residues that when mutated to alanine increase ultra-fine anaphase bridges and DNA double-strand breaks while decreasing colocalization of BLM and TopIIα. While some of these residues were predicted to be phosphorylated by ATR/ATM, others were not, suggesting that regulation may be distinct in different species. Regardless of the kinase, regulation and specifically phosphorylation of this region is important to BLM/Blm interaction with TopIIα and function in replication fork repair and resolution of anaphase bridges, even if the residues and kinases involved differ.
CR1 and CR2 are required for SDSA and prevention of mitotic crossovers
Both BlmΔCR1 and BlmΔCR2 mutants showed defects in DSB repair. SDSA rates were compromised to the same extent as in BlmN1 null mutants, but the frequency of spontaneous mitotic crossovers was not as high as in null mutants. One possibility is that CR1 and CR2 are required for SDSA but not for dHJ dissolution. It is not possible to test this possibility in vivo due to the lack of a dHJ dissolution assay. CR1 may be analogous to the major TopIIIα-interacting region of human BLM (28). In vitro, dHJ dissolution requires TopIIIα, which might suggest that BlmΔCR1 mutants would be defective for dissolution; however, human TopIIIα also interacts with the C-terminus of BLM (28). Interactions between Drosophila Blm and TopIIIα have not been mapped. Furthermore, although BLM can disassemble short D-loops in vitro, it is likely that disassembly of D-loops in vivo, where the ends are not free to rotate, requires topoisomerase activity, so loss of this interaction may impair both SDSA and dHJ dissolution.
How then might each of the Blm deletions studied lead to compromised SDSA? For CR1, it may be that Blm-TopIIIα interaction with both N- and C-terminal regions of Blm together are necessary for effective SDSA, with loss of either leading to disrupted repair. This could be further explored with a C-terminal deletion in examination of SDSA and mitotic crossovers. We attempted to pursue such a mutant, deleting both the final 100 and 150 amino acids in the unstructured C-terminal region of Blm, but both deletions were homozygous lethal, which is unexpected given that Blm null mutants are viable. Future studies could also target ATR/ATM predicted phosphorylation residues within CR1 to attempt to characterize the role of regulation of this region in effective SDSA.
As for the role of CR2 in SDSA, it may be that an interaction with TopII is required for this process. While TopIIIα is likely the primary topoisomerase involved in dissolution of D-loops in SDSA, TopII may be necessary to decatenate more complex DNA structures resulting from errors or disrupted repair. Future directions will also work to characterize the effects of regulation of CR2 on SDSA, with positive regulation potentially promoting additional interaction and/or stabilization.
CR1 and CR2 contribute to distinct meiotic processes
The two deletions caused different meiotic phenotypes. BlmΔCR1 mutants had a significant reduction in meiotic COs, whereas BlmΔCR2 mutants had an increase. Neither mutant had increased NDJ. These are both different from Blm null mutants, which have decreased meiotic COs, altered CO distribution, and elevated NDJ.
CR1 appears to play a role in meiotic CO distribution, but in a way that is not required for proper segregation of meiotic chromosomes. How loss of this region impacts crossovers but not segregation is unknown. While many of the components involved in meiotic and mitotic DNA repair are conserved, their regulation does often differ in each process. CR1 would be hypothesized to be involved in the resolution of meiotic DSBs as COs, but not in their repair as NCOs. This would be explained by a higher incidence of meiotic NCOs in BlmΔCR1 mutants. This might be detectable in whole-genome sequencing of progeny to quantify NCOs. Ability of BlmΔCR1 to resolve any bridged chromosomes during meiotic anaphases could explain the normal NDJ numbers. We should note too that while BlmΔCR1 CO numbers were significantly lower than wild-type, they were much higher than Blm null mutants, so the effects on COs may be mild enough to lead to normal meiotic chromosome segregation.
BlmΔCR2 meiotic activities are also unusual, with a significant increase of meiotic COs yet normal meiotic disjunction. We speculate that this may be due to an inability of BlmΔCR2 to resolve DSBs as NCOs, sending more of them into a CO pathway. This would be consistent with CR2, but not CR1, being required for meiotic SDSA and/or dHJ dissolution. Consistent with this hypothesis, overall numbers and patterning of crossovers would not be disrupted, possibly due to BlmΔCR2 having an intact CR1.
Conclusion
We have assessed genetic functions of N-terminal, unstructured regions of Drosophila Blm helicase. We show that deletion of the first 240 amino acids (CR1) does not impair embryonic development or meiotic chromosome segregation but disrupts mitotic DNA repair and meiotic crossover distribution. Deletion of the 146 amino acids upstream of the helicase domain (CR2) leads to severely disrupted embryonic development and aberrant mitotic DNA repair but allows normal meiotic crossover distribution and chromosome segregation. Through this characterization, we have begun to assign distinct Blm functions to different regions of the N-terminus, leading to a better understanding of how this complex protein works to promote development, meiosis, and genome stability.
Methods
CRISPR/Cas9 Deletion of CR1 and CR2
The endogenous CR1 and CR2 region of the Blm gene (chromosome 3L, cytological region 86E17) were deleted in-frame using CRISPR/Cas9 genome engineering similar to that described in Lamb et al., 2017 (Figure S2). A plasmid containing DNA homologous to 5’ and 3’ Blm flanking sequence of either CR1 or CR2 (pSL1180 ΔCR1 5’+3’ Homology Arms and pSL1180 ΔCR2 5’+3’ Homology Arms, respectively) and another plasmid containing 5’ and 3’ Blm gRNAs for CR1 or CR2 were (pCFD4 Blm 1+240 gRNA and pCFD4 Blm 576+720 gRNA, respectively) were simultaneously injected into Drosophila embryos expressing Cas9 in their germline stem cells under control of the nanos promoter (Genetivision, Houston, TX). Upon eclosion of these embryos, single male progeny were crossed to TM3,Sb/TM6B, Hu Tb females to balance their potentially edited chromosomes. Once balanced, subsequent single male progeny were again mated to TM3,Sb/TM6B, Hu Tb females. After being allowed to mate for 3–4 days at 25 °C, these single males were collected, frozen, and had their genomic DNA isolated to screen for successful deletions by PCR. For vials in which parental males contained the deletion (indicated by a smaller DNA band after PCR compared to wild-type flies), progeny were then mated to siblings to establish a stock. Each deletion stock was then further screened via genomic extraction, PCR, and sequencing of homozygous flies within the resulting stock to confirm the deletion resulted in the correct sequence and that there were no frameshifts. All homozygotes sequenced from each resulting stock contained the correct deletion, flanking sequence, and were not frameshifted, indicating that CR1 and CR2 were successfully deleted.
Embryonic Hatching Assay
20–30 virgin females homozygous for each Blm allele were crossed to 15 Oregon-RM (wild-type) males and allowed to acclimate to grape-juice agar plates with yeast paste for 24–36 hrs at 25 °C. Plates were then changed and embyros were collected overnight (16 hrs) at 25 °C. 150–300 embryos were then transferred to a gridded grape juice agar plate (10/grid) and scored for hatching after 48 hours at 25 °C. Hatch assays were completed in three replicates for each allelic condition, with a minimum of 550 total embryos assayed per condition.
Meiotic Non-disjunction Assay
Female meiotic non-disjunction (NDJ) of the X chromosome was measured by first crossing w; BlmN1/TM3, Sb virgin females to Oregon-RM (wild-type) males or males with the Blm allele of interest (BlmN2, BlmΔCR1, or BlmΔCR2) balanced over either TM3, Sb or TM6B, Hu Tb to generate heteroallelic Blm females (e.g., BlmN1 / BlmN2). For experiments with BlmN1 only, BlmN1 ry e P{UASp::Blm} / TM6B, Hu Tb virgin females were crossed meiP22103 st BlmD2 ry rec1 Ubx P{matα::GAL4} / TM6B, Hu Tb males to generate heteroallelic Blm null females that could rescue Blm expression after meiotic chromosome segregation to prevent maternal-effect lethality. BlmD2 is another null allele of Blm that contains a premature stop codon in the helicase domain (Kusano, 2001). Heteroallelic Blm females were then crossed to y sc cv v g f / Dp(1;Y)BS males. The duplication on the Y chromosome carries a dominant mutation causing bar-shaped eyes. Normal progeny resulting from this cross are females whose eyes are Bar+ and males whose eyes are Bar−. Non-disjoined ova that are diplo-X result in XXY females (and XXX progeny who do not survive) whose eyes are Bar−. Non-disjoined ova that are nullo-X result in X0 males (and Y0 progeny who do not survive) whose eyes are Bar+. X NDJ is calculated as the percentage of progeny that arose from NDJ (Bar− females and Bar+ males), correcting for the loss of half of the diplo- and nullo-X ova by multiplying this percentage by two. Crosses were set up as ten females and four males/vial for Oregon-RM (wild-type), BlmΔCR1, and BlmN2 genotypes and thirty females and eight males for BlmΔCR2 and BlmN1 genotypes. Data were pooled from between 15–60 vials and at least 1000 total progeny to determine the mean NDJ rate for each genotype.
Mitotic Crossover Assay
Pre-meiotic mitotic crossovers were measured in the male germline using the net dppho dpy b pr cn recessive phenotypic marker chromosome. Virgin females with net dppho dpy b pr cn/ SM6a and wild-type or various Blm alleles (BlmN2, BlmΔCR1, or BlmΔCR2) balanced over TM6B, Hu Tb were crossed to w; BlmN1/ TM3, Sb to generate single males heteroallelic (e.g. BlmN2/BlmN1) for Blm and heterozygous for recessive phenotypic markers for mitotic crossover analysis. For BlmN1 only, virgin females were instead crossed to w; BlmD2/TM3, Sb. Single males for each genotype were then crossed to homozygous net dppho dpy b pr cn females and scored for mitotic crossovers indicated by the mixed presence and/or absence of recessive phenotypic markers in progeny. Progeny for each single male was scored as a ratio of crossover progeny to total progeny to generate a mitotic crossover rate for each vial. Data for each genotype were pooled from at least 38 vials and 7000 progeny to determine the mean mitotic crossover rate.
P{wa} Assay
The P{wa} was performed as described previously (Adams et al., 2003), with minor modifications. First, y2 wΔ P{wa} virgin females with wild-type or various Blm alleles (BlmN1, BlmN2, BlmΔCR1, or BlmΔCR2) balanced over TM6B, Hu Tb were crossed to st P{Δ2–3} BlmD2 Sb/TM6B, Hu Tb males to generate single males that were heteroallelic for Blm (e.g. BlmN1 / BlmD2) with the P{wa} insertion and the Δ2–3 transposase. Single males for each genotype were then crossed to y2 wΔ P{wa} and progeny were scored for efficiency of repair by resulting eye color: red indicating efficient SDSA, white/yellow indicating end-joining, and apricot indicating no cutting or perfect repair. Progeny from each single male was scored as a ratio of red-eyed progeny to total progeny as a measure of SDSA repair rate. Data for each genotype were pooled from at least 160 vials and 3800 progeny to determine the mean SDSA repair rate.
Meiotic Crossover Assay
Meiotic crossovers were measured in the female germline using the net dppho dpy b pr cn recessive phenotypic marker chromosome. Virgin females with net dppho dpy b pr cn/ SM6a and wild-type or various Blm alleles (BlmΔCR1 or BlmΔCR2) combined with P{matα::GAL4} for maternal-effect lethality rescue were crossed to BlmN1 ro e P{UASp::Blm}/ TM6B, Hu Tb to generate females heteroallelic for Blm and heterozygous for recessive phenotypic markers for meiotic crossover analysis. For BlmN1 only, net dppho dpy b pr cn/CyO; BlmN1 r e P{UASp::Blm}/ TM6B, Hu Tb virgin females were instead crossed to mei-P22103 st BlmD2 ry rec1 Ubx P{matα::GAL4} / TM6B, Hu Tb. Virgin females for each genotype were then crossed to homozygous net dppho dpy b pr cn males and scored for meiotic crossovers indicated by the mixed presence and/or absence of recessive phenotypic markers in progeny. Progeny was scored as a ratio of crossover progeny to total progeny to generate a meiotic crossover rate. Data for each genotype were pooled from at least 38 vials and 7000 progeny to determine the mean mitotic crossover rate.
Acknowledgements
We thank members of the Sekelsky lab for helpful comments on the manuscript. This work was supported by a grant from the National Institute of General Medical Sciences to JS under award 1R35GM118127. EBD was supported in part by a grants from the National Cancer Institute (T32 CA217824). The funders did not play any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Hatkevich T, Kohl KP, McMahan S, Hartmann MA, Williams AM, Sekelsky J. Bloom syndrome helicase promotes meiotic crossover patterning and homolog disjunction. Curr Biol. 2017;27(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheok CF, Wu L, Garcia PL, Janscak P, Hickson ID. The Bloom’s syndrome helicase promotes the annealing of complementary single-stranded DNA. Nucleic Acids Res. 2005;33(12):3932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McVey M, Andersen SL, Broze Y, Sekelsky J. Multiple functions of Drosophila BLM helicase in maintenance of genome stability. Genetics. 2007;176(4):1979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng Y, Li H, Schweppe NM, Hawley RS, Gilliland WD. Statistical analysis of nondisjunction assays in Drosophila. Genetics. 2010;186(2):505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, et al. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83(4):655–66. [DOI] [PubMed] [Google Scholar]
- 6.Larsen NB, Hickson ID. RecQ helicases: Conserved guardians of genomic integrity. Adv Exp Med Biol. 2013;767:161–84. [DOI] [PubMed] [Google Scholar]
- 7.Adams MD, McVey M, Sekelsky J. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science. 2003;299(5604):265–7. [DOI] [PubMed] [Google Scholar]
- 8.Dutertre S, Ababou M, Onclercq R, Delic J, Chatton B, Jaulin C, et al. Cell cycle regulation of the endogenous wild type Bloom's syndrome DNA helicase. Oncogene. 2000;19(23):2731–8. [DOI] [PubMed] [Google Scholar]
- 9.Imamura O, Fujita K, Shimamoto A, Tanabe H, Takeda S, Furuichi Y, et al. Bloom helicase is involved in DNA surveillance in early S phase in vertebrate cells. Oncogene. 2001;20(10):1143–51. [DOI] [PubMed] [Google Scholar]
- 10.Brady MM, McMahan S, Sekelsky J. Loss of Drosophila Mei-41/ATR alters meiotic crossover patterning. Genetics. 2018;208(2):579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaFave MC, Andersen SL, Stoffregen EP, Korda Holsclaw J, Kohl KP, Overton LJ, et al. Sources and structures of mitotic crossovers that arise when BLM helicase is absent in Drosophila. Genetics. 2014;196(1):107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L. Role of the BLM helicase in replication fork management. DNA Repair (Amst). 2007;6(7):936–44. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Hickson ID. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426(6968):870–4. [DOI] [PubMed] [Google Scholar]
- 14.Hatkevich T, Sekelsky J. Bloom syndrome helicase in meiosis: Pro-crossover functions of an anti-crossover protein. Bioessays. 2017;39(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox RL, Hofley CM, Tatapudy P, Patel RK, Dayani Y, Betcher M, et al. Functional conservation of RecQ helicase BLM between humans and Drosophila melanogaster. Scientific reports. 2019;9(1):17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruchert JM, Brady MM, McMahan S, Lacey KJ, Latta LC, Sekelsky J, et al. Blm helicase facilitates rapid replication of repetitive DNA sequences in early Drosophila development. Genetics. 2022;220(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ababou M. Bloom syndrome and the underlying causes of genetic instability. Molecular genetics and metabolism. 2021;133(1):35–48. [DOI] [PubMed] [Google Scholar]
- 18.Payne M, Hickson ID. Genomic instability and cancer: lessons from analysis of Bloom's syndrome. Biochem Soc Trans. 2009;37(Pt 3):553–9. [DOI] [PubMed] [Google Scholar]
- 19.Luo G, Santoro IM, McDaniel LD, Nishijima I, Mills M, Youssoufian H, et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat Genet. 2000;26(4):424–9. [DOI] [PubMed] [Google Scholar]
- 20.Goss KH, Risinger MA, Kordich JJ, Sanz MM, Straughen JE, Slovek LE, et al. Enhanced tumor formation in mice heterozygous for Blm mutation. Science. 2002;297(5589):2051–3. [DOI] [PubMed] [Google Scholar]
- 21.Gruber SB, Ellis NA, Scott KK, Almog R, Kolachana P, Bonner JD, et al. BLM heterozygosity and the risk of colorectal cancer. Science. 2002;297(5589):2013. [DOI] [PubMed] [Google Scholar]
- 22.Lindor NM, Larson MC, DeRycke MS, McDonnell SK, Baheti S, Fogarty ZC, et al. Germline miRNA DNA variants and the risk of colorectal cancer by subtype. Genes Chromosomes Cancer. 2017;56(3):177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaganti RS, Schonberg S, German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci USA. 1974;71(11):4508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.German J, Schonberg S, Louie E, Chaganti RS. Bloom's syndrome. IV. Sister-chromatid exchanges in lymphocytes. Am J Hum Genet. 1977;29(3):248–55. [PMC free article] [PubMed] [Google Scholar]
- 25.Bachrati CZ, Borts RH, Hickson ID. Mobile D-loops are a preferred substrate for the Bloom's syndrome helicase. Nucleic Acids Res. 2006;34(8):2269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karow JK, Constantinou A, Li JL, West SC, Hickson ID. The Bloom's syndrome gene product promotes branch migration of holliday junctions. Proc Natl Acad Sci USA. 2000;97(12):6504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Brabant AJ, Ye T, Sanz M, German IJ, Ellis NA, Holloman WK. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39(47):14617–25. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Davies S, North P, Goulaouic H, Riou J, Turley H, et al. The Bloom's syndrome gene product interacts with topoisomerase III. J Biol Chem. 2000;275(13):9636 – 44. [DOI] [PubMed] [Google Scholar]
- 29.Sekelsky J. DNA repair in Drosophila: Mutagens, models, and missing genes. Genetics. 2017;205(2):471–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plank JL, Wu J, Hsieh TS. Topoisomerase IIIa and Bloom's helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proc Natl Acad Sci USA. 2006;103(30):11118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raynard S, Bussen W, Sung P. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIalpha, and BLAP75. J Biol Chem. 2006;281(20):13861–4. [DOI] [PubMed] [Google Scholar]
- 32.Wu L, Chan KL, Ralf C, Bernstein DA, Garcia PL, Bohr VA, et al. The HRDC domain of BLM is required for the dissolution of double Holliday junctions. EMBO J. 2005;24(14):2679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ralf C, Hickson ID, Wu L. The Bloom's syndrome helicase can promote the regression of a model replication fork. J Biol Chem. 2006. [DOI] [PubMed] [Google Scholar]
- 34.Davies SL, North PS, Hickson ID. Role for BLM in replication-fork restart and suppression of origin firing after replicative stress. Nat Struct Mol Biol. 2007;14(7):677–9. [DOI] [PubMed] [Google Scholar]
- 35.Chan KL, North PS, Hickson ID. BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J. 2007;26(14):3397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seki M, Nakagawa T, Seki T, Kato G, Tada S, Takahashi Y, et al. Bloom helicase and DNA topoisomerase IIIalpha are involved in the dissolution of sister chromatids. Mol Cell Biol. 2006;26(16):6299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell B, Bhattacharyya S, Keirsey J, Sandy A, Grierson P, Perchiniak E, et al. Chromosome breakage is regulated by the interaction of the BLM helicase and topoisomerase IIalpha. Cancer Res. 2011;71(2):561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann MB, Hodges CA, Barnes E, Vogel H, Hassold TJ, Luo G. Defective sister-chromatid cohesion, aneuploidy and cancer predisposition in a mouse model of type II Rothmund-Thomson syndrome. Hum Mol Genet. 2005;14(6):813–25. [DOI] [PubMed] [Google Scholar]
- 39.Naim V, Rosselli F. The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat Cell Biol. 2009;11(6):761–8. [DOI] [PubMed] [Google Scholar]
- 40.Grierson PM, Acharya S, Groden J. Collaborating functions of BLM and DNA topoisomerase I in regulating human rDNA transcription. Mutat Res. 2013;743–744:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McVey M, Larocque JR, Adams MD, Sekelsky JJ. Formation of deletions during double-strand break repair in Drosophila DmBlm mutants occurs after strand invasion. Proc Natl Acad Sci USA. 2004;101(44):15694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies SL, North PS, Dart A, Lakin ND, Hickson ID. Phosphorylation of the Bloom's syndrome helicase and its role in recovery from S-phase arrest. Mol Cell Biol. 2004;24(3):1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tangeman L, McIlhatton MA, Grierson P, Groden J, Acharya S. Regulation of BLM nucleolar localization. Genes. 2016;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhai B, Villen J, Beausoleil SA, Mintseris J, Gygi SP. Phosphoproteome analysis of Drosophila melanogaster embryos. Journal of proteome research. 2008;7(4):1675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Behnfeldt JH, Acharya S, Tangeman L, Gocha AS, Keirsey J, Groden J. A tri-serine cluster within the topoisomerase IIalpha-interaction domain of the BLM helicase is required for regulating chromosome breakage in human cells. Hum Mol Genet. 2018;27(7):1241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]