Abstract
The PBj14 isolate of the simian immunodeficiency virus SIVsmmPBj14 is unique among primate lentiviruses in its ability to induce lymphocyte proliferation and acutely lethal disease. The studies reported here show that viral induction of T-cell proliferation requires accessory cells, such as primary monocytes or Raji B-lymphoma cells, as well as the presence of a putative immunoreceptor tyrosine-based activation motif within the viral Nef protein. Addition of CTLA4-immunoglobulin fusion protein or anti-B7 antibodies to virally infected T cells led to substantial, but not complete, inhibition of monocyte-costimulated T-cell proliferation—suggesting that both CD28/B7-dependent and non-CD28-dependent pathways may contribute to the costimulation of virally induced lymphoproliferation. Finally, cyclosporin A, a specific inhibitor of the calcium-calmodulin-regulated phosphatase activity of calcineurin, which influences activation of the transcription factor nuclear factor of activated T cells, was shown to block virally mediated T-cell proliferation. Taken together, these findings suggest that the effect of SIVsmmPBj14 on T-cell activation may be functionally analogous, at least in part, to the effect of engagement of the T-cell receptor.
Early events in human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) infection have been shown to be highly predictive of subsequent progression of virally induced disease (4, 6, 22, 25, 36). Thus, it is important to understand better the factors which influence the outcome of primary infection with HIV-1 and SIV. One model system for this critical phase of viral infection is the experimental infection of pig-tailed macaques with the PBj14 isolate of SIV (7, 12). SIVsmmPBj14 causes a very severe acute disease syndrome which is marked by extensive and rapid induction of T-cell proliferation, particularly in gut-associated lymphoid tissues (13, 15, 32). SIVsmmPBj14 also has the unique ability, among all known isolates of HIV-1 and SIV, to trigger the in vitro proliferation of unstimulated peripheral blood mononuclear cells (PBMCs) (11, 24). The molecular mechanisms which are responsible for SIVsmmPBj14-induced lymphoproliferation may offer insights into the massive T-cell activation that accompanies acute HIV-1 infection (4) and the chronic immune hyperactivation that frequently occurs thereafter in HIV-1 infected individuals (10, 26, 27).
It was previously determined that, like SIVsmmPBj14, a genetically modified variant of SIVmac239 containing a single mutation in Nef (R to Y at amino acid 17) can induce proliferation of resting PBMCs (8) and that proliferation required contact between lymphocytes and monocyte/macrophages (9). In light of these findings, we sought to confirm this result by using virus derived from a molecular clone of SIVsmmPBj14 (PBj6.6 [24]) and to define potential costimulatory pathways involved in SIV-induced T-cell proliferation. For all experiments, whole blood was collected from SIV-negative pig-tailed macaques housed at the Yerkes Regional Primate Research Center in Atlanta and immediately shipped to the University of Rochester. PBMCs were isolated from the blood within 24 h of phlebotomy by using lymphocyte separation medium (Organon Teknika), and cells were cultured in RPMI 1640 medium with 15% human AB serum and penicillin-streptomycin-glutamine (Gibco BRL). For assays requiring the use of separate populations of T cells and monocytes, PBMCs were cultured to allow monocytes to adhere. Nonadhering cells were collected and applied to human T-cell enrichment columns (HTCC 500; R&D Systems, Minneapolis, Minn.), which employ a negative selection method for isolation of T cells. PBMCs (2 × 105 cells per well) or T cells (0.5 × 105 to 1 × 105 cells per well) were plated in 96-well plates prior to inoculation with PBj6.6 virus (10 ng of SIV p27/106 cells) or stimulation with 10 ng of 1,3-phorbol myristate acetate (PMA) per ml. Cellular proliferation was quantified by measuring the incorporation of [3H]thymidine at 7 days postplating (24); all experiments were performed in triplicate.
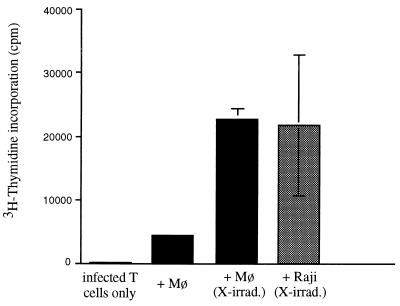
We first examined whether PBj6.6 virus-induced T-cell proliferation required the presence of accessory cells. The data presented in Fig. 1 show that either primary autologous simian monocyte-derived macrophages (Mø) or Raji cells, a B-cell lymphoma cell line, were capable of efficiently stimulating the proliferation of macaque T cells that had been infected with PBj6.6 virus, while infected T cells incubated in the absence of accessory cells failed to proliferate. Raji cells fixed in 0.4% paraformaldehyde were as efficient as irradiated cells in supporting proliferation of infected T cells (data not shown). Since neither irradiated nor fixed Raji cells would be expected to support SIV infection, these data provide evidence that productive viral infection of accessory cells is unnecessary for induction of lymphoproliferation and suggest that their function is in presentation of costimulatory molecules only. Interestingly, while fixed Raji cells were efficient in providing this costimulation, fixed autologous simian macrophages were not (data not shown)—the reason for this is uncertain but could relate to a differential effect of fixation on costimulatory molecules expressed by the two cell types. It is also intriguing to note that irradiation of primary simian macrophages led to an increase in costimulatory activity (Fig. 1). The basis for this effect is also unknown but could include changes in cytokine release and/or cell surface expression of costimulatory molecules. In any event, we are presently conducting studies to determine whether infection of simian macrophages with PBj6.6 virus, or coculturing them with infected T cells, leads to changes in their expression of costimulatory molecules or cytokines.
FIG. 1.
T cells were infected with PBj6.6 virus in the presence or absence of the indicated accessory cells. To prevent their proliferation, accessory cells were X- irradiated with 10,000 rads prior to plating. A total of 2.5 × 104 Raji cells or 5 × 104 autologous macrophages (cultured for 1 week prior to the experiment) were plated per well. The incorporation of [3H]thymidine by accessory cells alone was at background levels (not shown). Results shown are the mean values of three independent observations and are representative of three separate experiments yielding similar conclusions; error bars indicate the standard errors of mean values.
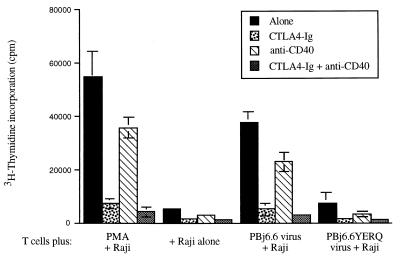
The requirement for accessory cells indicates that PBj6.6 virus-induced T-cell proliferation, like T-cell receptor (TCR)-mediated lymphoproliferation, requires activation of costimulatory pathways. Since primary monocyte-derived macrophages and Raji lymphoma cells express a number of costimulatory molecules, including B7-1 and B7-2 (14, 16, 19) and CD40 (2, 33), we wished to examine the relative contribution of these molecules to PBj6.6 virus-induced T-cell proliferation. Blocking experiments were therefore conducted by using (1) CTLA4- immunoglobulin (Ig) (a gift from P. Linsley), a soluble fusion protein which binds to both B7-1 and B7-2 with high affinity and blocks interaction of these molecules with CD28 (20), and (2) a soluble mouse monoclonal antibody directed against human CD40, which blocks its interaction with CD40L (clone G28-5, provided by R. Phipps and G. Sempowski). When Raji cells were used as accessory cells (Fig. 2), CTLA4-Ig largely blocked T-cell proliferation induced either by PMA or by PBj6.6 virus (87 or 86% inhibition of proliferation at 20 μg of CTLA4-Ig, per ml, respectively). The anti-CD40 monoclonal antibody had a lesser, but still significant, blocking effect (40% inhibition at 20 μg/ml antibody), consistent with the fact that Raji cells express high levels of CD40 and can stimulate T-cell proliferation via the CD40 or CD40L pathway (33). This finding may have significance with respect to costimulatory pathways that impact upon SIVsmmPBj14-induced lymphoproliferation in vivo, particularly in light of a recent report that HIV-1 can induce CD40 on endothelial cells (23). Experiments to evaluate whether SIVsmmPBj14 can also upregulate CD40 expression by endothelia (for example, in the gut) are ongoing. A combination of CTLA4-Ig and anti-CD40 virtually eliminated T-cell proliferation induced by either PBj6.6 virus or PMA, indicating that Raji cells support PBj6.6 virus-induced T-cell proliferation by providing costimulation predominantly through the B7-1/B7-2 costimulatory pathways, with a minor contributory effect from the CD40 pathway.
FIG. 2.
T cells were plated with irradiated Raji cells in the presence or absence of 20 μg/ml of either CTLA4-Ig, anti-CD40, or both. Cells were mock-infected (+ Raji alone), stimulated with 10 ng of PMA per ml, infected with PBj6.6 virus, or infected with an ITAM-deleted mutant of PBj6.6 virus (PBj6.6YERQ; this virus is isogenic to PBj6.6, except for the substitution of RQ, the corresponding residues in SIVmac239, for YE at amino acids 17 and 18 of Nef [9, 30]). Results shown are the mean values of three independent observations; error bars indicate the standard errors of mean values. Data shown are representative of three separate experiments yielding similar conclusions (note that the experiment using the PBj6.6YERQ virus was performed only once, since this virus consistently fails to trigger lymphoproliferation in PBMC [30]).
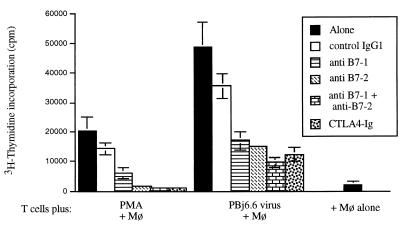
We next carried out similar blocking experiments using T cells costimulated by simian macrophages. In these studies, we focused on the B7/CD28-dependent costimulatory pathway, and we again used CTLA4-Ig to block proliferation of T cells infected in the presence of irradiated homologous monocyte/macrophages. Individual blocking monoclonal antibodies specific for the human B7-1 and B7-2 molecules (R&D Systems) were also used in these experiments to define better the costimulatory pathways at work in this cell system. When T cells were stimulated with PMA, and then cultured in the presence of primary simian macrophages, proliferation was completely blocked by CTLA4-Ig (>90% inhibition). Furthermore, a comparable level of inhibition was achieved by using a combination of monoclonal antibodies directed against B7-1 and B7-2 molecules (>90% inhibition), and substantial inhibition was also attained using either monoclonal antibody alone (70% for anti-B7-1 and 89% for anti-B7-2); in contrast, an isotype-matched control antibody (IgG1; R&D Systems) had little effect on lymphoproliferation (Fig. 3). These data confirm that CTLA4-Ig-mediated inhibition of PMA-driven T-cell proliferation was indeed due to an effect on CD28/B7-dependent signalling.
FIG. 3.
T cells were plated with 5 × 104 homologous macrophages (Mø) per well, in the presence of CTLA4-Ig (5 μg/ml) or of the indicated antibodies (5 μg/ml). Antibodies used in this experiment were as follows: anti-human B7-1 (clone 37711.11), anti-human B7-2 (clone 37301.11), and an isotype-matched normal mouse IgG1 (control IgG1); all antibodies were obtained from R&D Systems. Cells were mock-infected (+Mø alone), stimulated with 10 ng of PMA per ml, or infected with PBj6.6 virus. Results shown are the mean values of three independent observations and are representative of three separate experiments yielding similar conclusions; error bars indicate the standard errors of mean values.
Broadly similar results were obtained when T cells were infected with the PBj6.6 virus and then costimulated with primary macrophages (Fig. 3). In this case, proliferation was substantially, but not completely, blocked by CTLA4-Ig (75% inhibition) or by the combination of anti-B7 monoclonal antibodies (80% inhibition). The individual anti-B7 antibodies also partially inhibited lymphoproliferation (65% for anti-B7-1 and 69% for anti-B7-2), while the isotype-matched control antibody had only a minimal effect. In other experiments (not shown), similar results were obtained—although the degree of inhibition with the anti-B7-1 antibody was somewhat variable and typically less than that obtained with the anti-B7-2 antibody.
Overall, the results of this set of experiments suggest that costimulation of PBj6.6 virus-induced T-cell proliferation is achieved largely, but not exclusively, through a CD28-dependent, CTLA4-Ig-inhibitable pathway. This conclusion is further supported by the fact that macrophage-costimulated lymphoproliferation could not be fully inhibited, even upon addition of very high concentrations of CTLA4-Ig (up to 40 μg/ml; data not shown); anti-CD40 antibodies had no effect on lymphoproliferation in this cell system (data not shown). Further studies will be required to identify the cellular receptor-ligand interactions which may be involved in this second pathway, although possible candidates may include SLAM-, CDw101-, or OX-2-dependent mechanisms (1, 3, 28).
Classically, T-cell proliferation requires two distinct signals— antigen-major histocompatibility complex binding to the TCR, followed by a second, CD28-mediated, signal (5, 34, 35). Viewed in this context, our data suggest that SIVsmmPBj14 perturbs the T cell in a manner that is at least partially analogous to the normal activation event(s) which occurs in response to TCR signaling. This is not unlikely, since SIVsmmPBj14 Nef is known to contain an immunoreceptor tyrosine-based activation motif (ITAM) ([YxxL] xxxxxxx [YxxL]) close to its amino terminus (9). This motif has been implicated in the ability of the virus to trigger lymphoproliferation (8, 9, 29), and elimination of this motif is sufficient to abolish the ability of the virus to trigger T-cell proliferation (Fig. 2) (30).
The ITAM from SIVsmmPBj14 Nef is not only essential for virally induced lymphoproliferation, but it has also been shown to increase the activity of the transcription factor nuclear factor of activated T cells (NFAT), which is a major downstream target of T-cell activation pathways (21). In order to examine whether activation of NFAT is necessary for induction of T-cell proliferation by SIVsmmPBj14, we conducted experiments using cyclosporin A (CsA). CsA is a potent immunosuppressive drug that is a specific inhibitor of the calcium-calmodulin-dependent phosphatase activity of calcineurin, which is required for the dephosphorylation and subsequent activation of NFAT (17, 31).
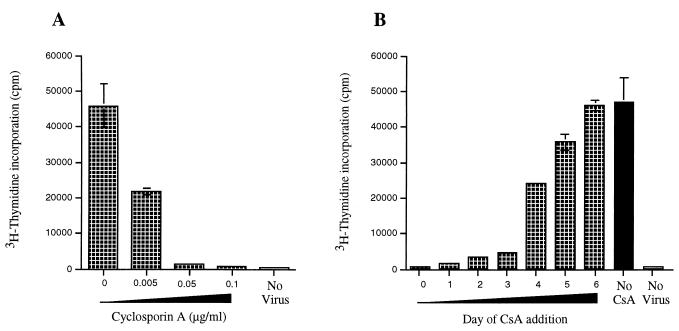
Addition of CsA to PBj6.6 virus-infected PBMCs resulted in a potent inhibition of virally driven lymphoproliferation, with a 50% inhibitory concentration of <0.005 μg/ml (Fig. 4A). This finding shows that activation of NFAT is indeed required for the mitogenic effect of SIVsmmPBj14. Additional experiments revealed that protein tyrosine kinase inhibitors (genistein, herbimycin A) also inhibited the induction of lymphocyte proliferation by SIVsmmPBj14 (data not shown). These observations are consistent with the fact that the ITAM from SIVsmmPBj14 Nef can recruit ZAP-70, a T-cell-specific tyrosine kinase which is involved in cellular activation and that Lck tyrosine kinase is required for this effect (21).
FIG. 4.
(A) PBMCs were infected with PBj6.6 virus, or mock-infected, and incubated in the presence of the indicated concentrations of cyclosporin A. (B) PBMCs were infected with PBj6.6 virus, or mock-infected, and CsA (0.1 μg/ml) was then added to the cultures at the indicated times after virus infection (days); proliferation was measured 7 days after initial exposure of the cells to PBj6.6 virus. Values shown represent the means from a single experiment that was performed in triplicate; the standard errors of mean values are marked by bars. The results shown are representative of two experiments that yielded similar results; in addition, the experiment whose results are shown in Panel A was repeated on three occasions, with similar results, with a different molecular clone of SIVsmmPBj14—PBj 1.9 (7, 12).
Finally, we examined the time course of the effect of CsA on virally induced lymphoproliferation. In these experiments, CsA was added to resting simian PBMCs either at the same time as the virus (day 0), or at 24-h intervals thereafter; 7 days after addition of virus, cellular proliferation was assessed. As shown in Fig. 4B, the inhibitory effect of CsA on virally induced lymphoproliferation was dependent upon addition of the drug within the first 3 days after exposure to the virus (days 0 to 3). At later times, CsA had little or no effect on cellular proliferation (days 4 to 6). One interpretation of these data is that NFAT activation is required only during the initial phases of SIVsmmPBj14-induced lymphoproliferation, whereas at later stages, proliferation may proceed independently of NFAT.
Taken together, our results show that the CD28/B7 costimulatory pathway plays an important role in the induction of lymphocyte proliferation by SIVsmmPBj14—much as it does in the T-cell proliferative response that occurs during infection with human T-lymphotropic virus type 1 or 2 (18). However, since monocyte-costimulated proliferation of virally infected cells was only partially blocked by CTLA4-Ig or by anti-B7 antibodies, non-CD28-dependent alternate pathways may be also involved; studies to identify these pathways are ongoing. Finally, our findings suggest that SIVsmmPBj14-mediated induction of T-cell activation is dependent on a previously identified ITAM motif within the viral Nef protein, which may be capable of delivering an activation signal(s) that is at least partially analogous to the signal provided by engagement of the TCR.
Acknowledgments
We thank Peter Linsley for providing CTLA4-Ig and Gregory Sempowski and Richard Phipps for providing the G28-5 monoclonal antibody. We also thank Pia Challita-Eid, Renu Lal, Gregory Sempowski, and Richard Phipps for helpful discussions and advice on experiments; Ellen Lockwood and Selena Taylor for obtaining and shipping blood samples from macaques; and Sanjay Maggirwar for critical review of the manuscript.
This work was supported by NIH grants to S.D. (RO1 AI39397, KO4 AI01240) and to F.J.N. (RO1 CA67364) and by grant RR-00165.
REFERENCES
- 1.Aversa G, Chang C C, Carballido J M, Cocks B G, de Vries J E. Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J Immunol. 1997;158:4036–4044. [PubMed] [Google Scholar]
- 2.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi J P, van Kooten C, Liu Y J, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 3.Borriello F, Lederer J, Scott S, Sharpe A H. MRC OX-2 defines a novel T cell costimulatory pathway. J Immunol. 1997;158:4548–4554. [PubMed] [Google Scholar]
- 4.Cossarizza A, Ortolani C, Mussini C, Borghi V, Guaraldi G, Mongiardo N, Bellesia E, Franceschini M G, De Rienzo B, Franceschi C. Massive activation of immune cells with an intact T cell repertoire in acute human immunodeficiency virus syndrome. J Infect Dis. 1995;172:105–112. doi: 10.1093/infdis/172.1.105. [DOI] [PubMed] [Google Scholar]
- 5.Croft M, Dubey C. Accessory molecule and costimulation requirements for CD4 T cell response. Crit Rev Immunol. 1997;17:89–118. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- 6.De Rossi A, Masiero S, Giaquinto C, Ruga E, Comar M, Giacca M, Chieco-Bianchi L. Dynamics of viral replication in infants with vertically acquired human immunodeficiency virus type 1 infection. J Clin Invest. 1996;97:323–330. doi: 10.1172/JCI118419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewhurst S, Embretson J E, Anderson D C, Mullins J I, Fultz P N. Sequence analysis and acute pathogenicity of molecularly cloned SIVSMM-PBj14. Nature. 1990;345:636–640. doi: 10.1038/345636a0. [DOI] [PubMed] [Google Scholar]
- 8.Du Z, Ilyinskii P O, Sasseville V G, Newstein M, Lackner A A, Desrosiers R C. Requirements for lymphocyte activation by unusual strains of simian immunodeficiency virus. J Virol. 1996;70:4157–4161. doi: 10.1128/jvi.70.6.4157-4161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskii P O, Daniel M D, Jung J U, Desrosiers R C. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 10.Fauci A S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 11.Fultz P N. Replication of an acutely lethal simian immunodeficiency virus activates and induces proliferation of lymphocytes. J Virol. 1991;65:4902–4909. doi: 10.1128/jvi.65.9.4902-4909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fultz P N, McClure H M, Anderson D C, Switzer W M. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM) AIDS Res Hum Retrovir. 1989;5:397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- 13.Fultz P N, Zack P M. Unique lentivirus-host interactions: SIVsmmPBj14 infection of macaques. Virus Res. 1994;32:205–225. doi: 10.1016/0168-1702(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 14.Guinan E C, Gribben J G, Boussiotis V A, Freeman G J, Nadler L M. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261–3282. [PubMed] [Google Scholar]
- 15.Gummuluru S, Novembre F J, Lewis M, Gelbard H A, Dewhurst S. Apoptosis correlates with immune activation in intestinal lymphoid tissue from macaques acutely infected by a highly enteropathic simian immunodeficiency virus, SIVsmmPBj14. Virology. 1996;225:21–32. doi: 10.1006/viro.1996.0571. [DOI] [PubMed] [Google Scholar]
- 16.Hathcock K S, Laszlo G, Pucillo C, Linsley P, Hodes R J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree G R. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 18.Lal R B, Rudolph D L, Dezzutti C S, Linsley P S, Prince H E. Costimulatory effects of T cell proliferation during infection with human T lymphotropic virus types I and II are mediated through CD80 and CD86 ligands. J Immunol. 1996;157:1288–1296. [PubMed] [Google Scholar]
- 19.Linsley P S, Ledbetter J A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 20.Linsley P S, Wallace P M, Johnson J, Gibson M G, Greene J L, Ledbetter J A, Singh C, Tepper M A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 21.Luo W, Peterlin B M. Activation of the T-cell receptor signaling pathway by Nef from an aggressive strain of simian immunodeficiency virus. J Virol. 1997;71:9531–9537. doi: 10.1128/jvi.71.12.9531-9537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellors J W, Kingsley L A, Rinaldo C R, Jr, Todd J A, Hoo B S, Kokka R P, Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Moses A V, Williams S E, Strussenberg J G, Heneveld M L, Ruhl R A, Bakke A C, Bagby G C, Nelson J A. HIV-1 induction of CD40 on endothelial cells promotes the outgrowth of AIDS-associated B-cell lymphomas. Nat Med. 1997;3:1242–1247. doi: 10.1038/nm1197-1242. [DOI] [PubMed] [Google Scholar]
- 24.Novembre F J, Johnson P R, Lewis M G, Anderson D C, Klumpp S, McClure H M, Hirsch V M. Multiple viral determinants contribute to pathogenicity of the acutely lethal simian immunodeficiency virus SIVsmmPBj variant. J Virol. 1993;67:2466–2474. doi: 10.1128/jvi.67.5.2466-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak M A, Lloyd A L, Vasquez G M, Wiltrout T A, Wahl L M, Bischofberger N, Williams J, Kinter A, Fauci A S, Hirsch V M, Lifson J D. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J Virol. 1997;71:7518–7525. doi: 10.1128/jvi.71.10.7518-7525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E. Immune hyperactivation of HIV- 1-infected T cells mediated by Tat and the CD28 pathway. Science. 1997;275:1481–1485. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 27.Pantaleo G, Fauci A S. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 28.Russell G J, Parker C M, Sood A, Mizoguchi E, Ebert E C, Bhan A K, Brenner M B. p126 (CDw101), a costimulatory molecule preferentially expressed on mucosal T lymphocytes. J Immunol. 1996;157:3366–3374. [PubMed] [Google Scholar]
- 29.Sasseville V G, Du Z, Chalifoux L V, Pauley D R, Young H L, Sehgal P K, Desrosiers R C, Lackner A A. Induction of lymphocyte proliferation and severe gastrointestinal disease in macaques by a nef gene variant SIVmac239. Am J Pathol. 1996;149:163–176. [PMC free article] [PubMed] [Google Scholar]
- 30.Saucier, M., S. Hodge, S. Dewhurst, T. Gibson, J. P. Gibson, H. M. McClure, and F. J. Novembre. The tyrosine-17 residue of Nef in SIVsmmPBj14 is required for acute pathogenesis and contributes to replication in macrophages. Virology, in press. [DOI] [PubMed]
- 31.Schreiber S L, Crabtree G R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 32.Schwiebert R, Fultz P N. Immune activation and viral burden in acute disease induced by simian immunodeficiency virus SIVsmmPBj14: correlation between in vitro and in vivo events. J Virol. 1994;68:5538–5547. doi: 10.1128/jvi.68.9.5538-5547.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sempowski G D, Chess P R, Phipps R P. CD40 is a functional activation antigen and B7-independent T cell costimulatory molecule on normal human lung fibroblasts. J Immunol. 1997;158:4670–4707. [PubMed] [Google Scholar]
- 34.Shaw A S, Dustin M L. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 35.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 36.Wong M T, Dolan M J, Kozlow E, Doe R, Melcher G P, Burke D S, Boswell R N, Vahey M. Patterns of virus burden and T cell phenotype are established early and are correlated with the rate of disease progression in human immunodeficiency virus type 1-infected persons. J Infect Dis. 1996;173:877–887. doi: 10.1093/infdis/173.4.877. [DOI] [PubMed] [Google Scholar]