Abstract
The phenomenon of active trans-membrane water cycling (AWC) has emerged in little over a decade. Here, we consider H2O transport across cell membranes from the origins of its study. Historically, trans-membrane water transport processes were classified into: A) compensating bidirectional fluxes (“exchange”), and B) unidirectional flux (“net flow”) categories. Recent literature molecular structure determinations and molecular dynamic (MD) simulations indicate probably all the many different hydrophilic substrate membrane co-transporters have membrane-spanning hydrophilic pathways and co-transport water along with their substrates, and that they individually catalyze category A and/or B water flux processes, although usually not simultaneously. The AWC name signifies that, integrated over the all the cell’s co-transporters, the rate of homeostatic, bidirectional trans-cytolemmal water exchange (category A) is synchronized with the metabolic rate of the crucial Na+,K+-ATPase (NKA) enzyme. A literature survey indicates the stoichiometric (category B) water/substrate ratios of individual co-transporters are often very large. The MD simulations also suggest how different co-transporter reactions can be kinetically coupled molecularly.
Is this (Na+,K+-ATPase rate-synchronized) cycling futile, or is it consequential? Conservatively representative literature metabolomic and proteinomic results enable comprehensive free energy analyses of the many transport reactions with known water stoichiometries. Free energy calculations, using literature intracellular pressure (Pi) values reveals there is an outward trans-membrane H2O barochemical gradient of magnitude comparable to that of the well-known inward Na+ electrochemical gradient. For most co-influxers, these gradients are finely balanced to maintain intracellular metabolite concentration values near their consuming enzyme Michaelis constants. The thermodynamic analyses include glucose, glutamate−, gamma-aminobutyric acid (GABA), and lactate− transporters. 2%−4% Pi alterations can lead to disastrous concentration levels. For the neurotransmitters glutamate− and GABA, very small astrocytic Pi changes can allow/disallow synaptic transmission. Unlike the Na+ and K+ electrochemical steady-states, the H2O barochemical steady-state is in (or near) chemical equilibrium. The analyses show why the presence of aquaporins (AQPs) does not dissipate the trans-membrane pressure gradient. A feedback loop inherent in the opposing Na+ electrochemical and H2O barochemical gradients regulates AQP-catalyzed water flux as an integral AWC aspect. These results also require a re-consideration of the underlying nature of Pi. Active trans-membrane water cycling is not futile, but is inherent to the cell’s “NKA system” - a new, fundamental aspect of biology.
INTRODUCTION:
One of the essential aspects of water in living tissue is its intra- extracellular compartmentalization. The kinetics of cellular, homeostatic trans-membrane water transport provides the basis of a new non-invasive, high-resolution MRI approach; metabolic activity diffusion imaging, MADI (1–3). It is providing novel views of cancer (4) and brain function (5) metabolism. Here, we inquire into kinetic, thermodynamic, and metabolic consequences of water co-transport phenomena in general.
Distinct Trans-membrane Water Transport Processes:
Category B unidirectional “flux” processes driven by trans-membrane osmotic gradients have been extensively investigated (6,7). These are characterized by cell volume (V) changes [cell swelling/shrinking; edema; tissue hypertrophy]. Their kinetics are quantified with the “osmotic” [flux, flow] water permeability coefficient, Pf.
However, the earliest studies of which we are aware also detected category A bidirectional trans-membrane water “exchange” (8,9). In such processes, there is no V change – the systems are homeostatic. The kinetics are quantified with the “diffusional” water permeability coefficient, Pd. Initial studies employed isotopic labeling (2HO1H), requiring very rapid, careful solution mixing – to perturb the system from isotopic equilibrium. As tracer methods, such approaches yield the Pd•ae product: ae = ρ〈A〉, where ρ is the cell [number] density [cells/volume(tissue)] and 〈A〉 is the tissue-averaged cell surface area (1). [In the older literature, ae is given as S, the total cell surface area per tissue [“ensemble”] volume.] Pd can be determined if ae can be estimated. Early Pd values were compiled (10). We (11) and others showed that, at steady-state, Pd confounds V and A with the homeostatic cellular water efflux rate constant, kio, Pd = (V/A)kio. It is kio that is a true measure of permeation probability (1). A nuclear magnetic resonance (NMR) approach was introduced in 1972 (12), which we later termed the “shutter-speed” (SS-NMR) method (13). Major SS-NMR advantages are it: 1.) yields kio un-confounded, and separated from (1 + ((ρ•V – 1)/fW)): fW is the tissue water volume fraction (1,14); 2.) does not require rapid solution mixing; and 3.) could, in principle, quantify the category B flux and category A exchange processes simultaneously. An extracellular paramagnetic agent labels extracellular 1H2O magnetization, not extracellular water molecules (15), and non-invasive radio frequency electromagnetic pulses perturb the system magnetization from equilibrium. A compilation of early SS-NMR results was reported in (16). (Acronyms and symbols are listed in the Appendix A1).
Molecular Aspects of Trans-membrane Water Transport:
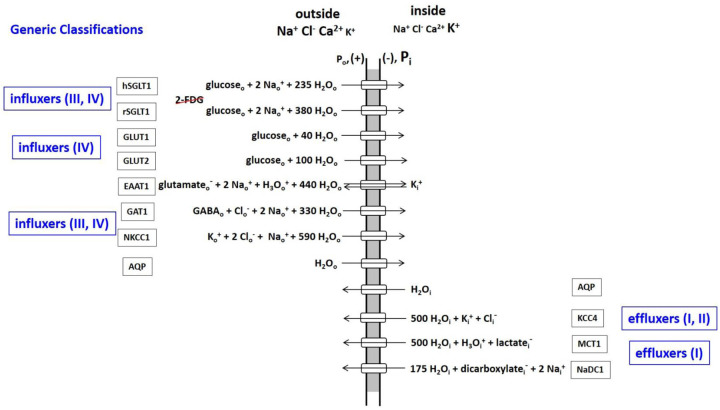
Over the years, many membrane-bound macromolecules have been found to transport water between intra- and extracellular spaces. In 1988, Agre and co-workers reported the selective water transport protein aquaporin (AQP) family (17). The impressive array of different AQP variants now known has been reviewed (6). During the 1990’s and 2000’s, many other proteins were found to co-transport water molecules, along with the metabolic substrates for which they are named (reviewed in (18,19)). Figure 1 shows AQP and the ten water co-transporters detailed by Zeuthen (19). Each of their reactions is reversible, but here they are classified by their tendencies for influx or efflux under normal cellular conditions. These proteins all catalyze crucial processes, and are profitably considered as enzymes.
Figure 1. An Inventory of water transporting membrane proteins.
The water stoichiometric values are taken from (19), and should be thought of as means of shot-to-shot variations (see text).
There are two glucose influx families: those co-transporting Na+ (SGLT), and those that do not (GLUT). (Interestingly, though both families transport glucose, it has been recently noted (20) the SGLT family does not transport 2-deoxy-2-18F-fluoro-D-glucose (2-FDG), the 18FDG-PET (positron emission tomography) glucose tracer. This can present interpretative problems.) The excitatory amino acid transporter (EAAT1) and the GABA transporter (GAT1) facilitate uptake of the principal excitatory and inhibitory neurotransmitters, glutamate− and GABA [the gamma-aminobutyric acid zwitterion], respectively. (In the Fig. 1 EAAT1 influx reaction, note the H3O+ co-influx, and the K+ counter-efflux components (21).) The Na+,K+,2Cl− co-transporter (NKCC1) facilitates K+ and Cl− influx (22).
The K+,Cl− co-transporter (KCC4) provides an important pathway for K+ and Cl− efflux (22). The monocarboxylic acid transporter (MCT1) extrudes the lactate− produced by cytoplasmic glycolytic-type metabolisms (HCO3− is the analogous product of mitochondrial oxidative phosphorylation (23).
One is struck by the large water stoichiometries in most co-transporter cases. These values should be considered averages. Surely, they fluctuate stochastically (shot-to-shot) with each enzyme cycle (see below). Also, they likely vary with the cellular environment and biological condition. It is important to realize the Fig. 1 stoichiometries were determined in model systems in vitro, and by inducing net category B unidirectional fluxes (19). Homeostatic exchange was not studied in this regard.
In 1989, Ye and Verkman presented an elaborate optical method to measure Pf and Pd for simultaneous water efflux from and exchange in liposomes and in (adenosine tri-phosphate) ATP-free erythrocyte ghosts (24). Two very important results from that study are the following. First, a given water transporter can be involved in both category B flux and category A exchange processes, but not necessarily in the same proportions. Using HgCl2 to inhibit AQP, the authors found while AQP contributes 90% of ghost water efflux, it accounts for only 45% of ghost exchange flux. Second, though Pf for ghost efflux is numerically four times larger than Pd for ghost exchange (in the same units), the time-course for water efflux is 50 times longer. The exchange process is much, much faster. A confusing aspect is that Pf and Pd are always reported with the same dimensions (length/time). However, the two permeability coefficients are defined by rate laws of very different natures ((7); (10), pp. 44 ff), and are not at all the same. The Pf quantity characterizes transporter efficiency only in the presence of an osmotic gradient, and with the system perturbed from the steady-state. It gives no information on Pd.
Active Trans-Membrane Water Cycling (AWC):
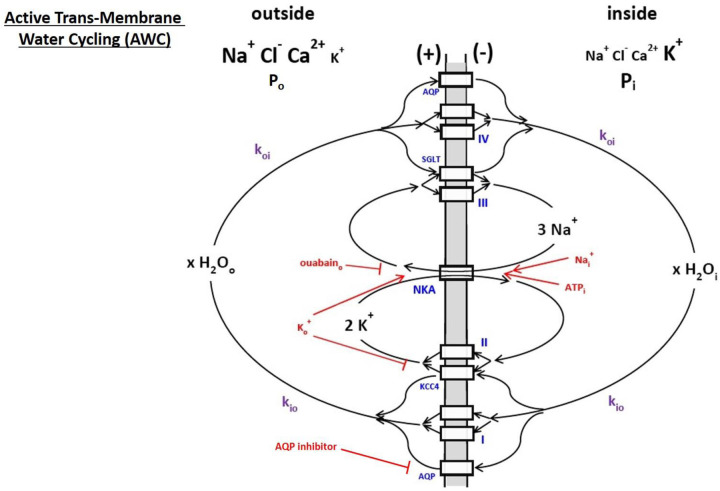
During most in vitro and in vivo studies, the tissue is in homeostasis: there is no substantial cell swelling or shrinking. Only category A trans-membrane water bidirectional exchange obtains. Evidence mounts for a homeostatic, metabolic AWC process in cells (reviewed (1,2,15,25)). This is a very fast water molecule exchange, the kinetics of which are driven by, and synchronized to, those of the rate-limiting plasma membrane Na+,K+-ATPase (NKA) “sodium pump.” The AWC phenomenon was first detected using water proton (1H2O) SS-NMR in 2011 (26). Figure 2 is a cartoon illustrating the AWC process. For each NKA cycle, one intracellular ATPi molecule is hydrolyzed, three intracellular Nai+ ions expelled, and two extracellular Ko+ ions imported. (First and second approximations of Fig 2 have appeared (2,15).) However, generic transporters II and III, respectively, allow K+ to re-exit and Na+ to re-enter exit the cell. Thus, NKA cycles extremely rapidly: depending on NKA expression, there can be 1010 (Na+ + K+) ions(cycled)/s/cell (15). These actions maintain the crucial trans-membrane ion concentration gradients, [Nao+] > [Nai+], [Ko+] < [Ki+]; and the membrane electrical potential (in more negative than out, Figs. 1,2). Explicit examples of II and III are KCC4 and SGLT, respectively. The NKA substrates intracellular ATP and Na+ and extracellular K+ (ATPi, Nai+, Ko+, respectively) are rendered in red in Fig. 2, as is the natural product ouabain, a specific extracellular NKA inhibitor (ouabaino).
Figure 2. A cartoon of active trans-membrane water cycling (AWC).
The rate constant for steady-state cellular water efflux is kio; that for steady-state cellular water influx is koi. The Na+,K+-ATPase enzyme is indicated as NKA, while AQP, KCC4, and SGLT are defined in Figure 1. The actions of NKA substrates intracellular Na+ and ATP (Nai+ and ATPi) and extracellular K+ (Ko+) are indicated in red, as are the NKA inhibitor extracellular ouabain (ouabaino) and an extracellular AQP inhibitor. Generic transporters I, II, III, and IV are exemplified in Figure 1: the actions of AQP, KCC4, and SGLT are shown here as specific examples. The quantity x is the AWC water stoichiometry integrated over the entire cell. Thus, for example, cMRH2O(influx) = cMRH2O(efflux) = xcMRNKA = x(cMRNa+(influx))/3 = x(cMRK+(efflux))/2, where cMR represents a cellular metabolic rate. First and second approximations of this cartoon have appeared in (15) and (2), respectively.
Generic water co-transporters I and IV represent secondary, active obligate water symporters for water to exit and enter cells, respectively. Most of the Fig. 1 enzymes fit into this classification: they share substrates (Nao+ and/or Ki+) with NKA, a primary, active transporter (hydrolyzes ATP directly). The first-order, unidirectional rate constants kio and koi are those for cellular, homeostatic water efflux and influx, respectively. In the differential first-order chemical trans-membrane water transport rate law, kio can be expressed as the sum of energetically active, kio(a) and passive, kio(p), contributions that are further elaborated in Equations (1), where: x is the overall, cellular AWC water
| (1) |
stoichiometric coefficient (H2O molecules(cycled)/NKA(cycle)/s/cell), [H2Oi] the intracellular water concentration, cMRNKA the cellular NKA metabolic rate (ATP(molecules hydrolyzed by NKA)/s/cell), and PW(p) the non-metabolic, passive diffusive water membrane permeability coefficient Pd(p) (1,2,15). The influx rate constant, koi, is ρ-dependent, and also has active and passive components that need not be in the same proportions as for kio. In healthy, living tissue, kio(p) and koi(p) seem negligible (2). There can be 1012 (H2O molecules actively cycled)/s/cell (15). The value of x may be of magnitude 106 (2).
The sodium pump is perhaps biology’s most vital enzyme: it is found in all mammalian cells, and it’s role in the evolution of life is thought crucial (27). Since NKA homeostatic activity has never before been accessible in vivo, medical MR imaging (MRI) applications of MADI are very promising (1,2,4,5). The water proton MR signal (1H2O) is by far the largest from tissue (28). The AWC-based MADI approach has been deemed “a new paradigm” (3).
Water-selective AQP transporters can also be involved in AWC as long there is a thermodynamic driving force for water flux (our calculations suggest their role in cellular homeostasis, see Discussion). AQP molecules themselves comprise simple, passive channels (29,30).
The existence of AWC has been demonstrated by many different, deliberate manipulations of NKA kinetics with, for example, ATPi, Ko+ and oubaino concentration alterations (Fig. 2, red), while monitoring kio. More than 20 independent studies, of a range of tissues, have been itemized (2,25). AWC can be mapped in vivo with non-invasive MADI, which estimates kio, V, and ρ separately (1,2). In awake, resting human brain gray matter [GM], MADI mapping of the cellular water flux [H2Oi]kioV = cMRAWC, an xcMRNKA estimate, correlates very well with the tissue metabolic rate of glucose consumption, tMRglu (glucose(consumed)/s/unit volume(tissue)) determined from quantitative 18FDG-PET (2). This is expected: overall ATP production (MRATP) and consumption remain balanced in the resting brain. The white matter [WM] cMRAWC/tMRglu ratio is larger than the GM cMRAWC/tMRglu slope (2), likely indicating a more oxidative metabolic mechanism.
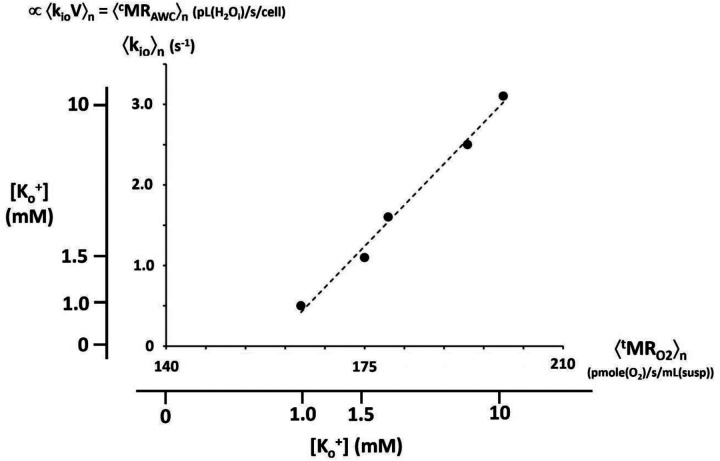
The most efficient MRATP comes from mitochondrial oxidative phosphorylation (Fig. 2 of ref (15)), and MRAWC should be even more sensitive to this. Indeed, in vitro model brain tissue studies indicate kioV correlates strongly with mitochondrial MRO2(consumption) (31). This is shown in Figure 3 in the context of MRNKA stimulation via [Ko+] titration (Fig. 2). The vertical axis measures the population-averaged 〈kio〉n for organotypic, cultured [spiking] rat somatosensory cortex superfused with a paramagnetic agent. This SS-NMR study also allowed the estimation that 〈V〉n was rather constant during the titration (Fig. 3D of ref. (31)): thus 〈kio〉n was proportional to 〈kioV〉n = 〈cMRAWC〉n. The horizontal axis measures 〈tMRO2〉n, directly determined in completely independent [Ko+] titrations of isolated rat brain synaptosome suspensions. It is the [Ko+] titrations that allow this correlation: these are shown as outer ordinate and abscissa scales. They are non-linearly (Michaelis-Menten) related to the inner 〈kio〉n and 〈tMRO2〉n scales, which are linear. The correlation is excellent over most of the range. (It is interesting O2 consumption continues in the synaptosome suspensions even when [Ko+] is zero. This is not the case for kio in the SS-NMR study of cultured cortex preparations.) The quantity kioV is probably best thought of as a high-resolution in vivo measure of mitochondrial function.
Figure 3. A correlation of kio with tMRO2.
Independent [Ko+] titrations of paramagnetic agent-superfused [SS-NMR] studies of organotypic, cultured [spiking] rat somatosensory cortex [ordinate] and direct studies of isolated rat brain synaptosome suspensions [abscissa] allow correlation of the population-averaged 〈kio〉n and 〈tMRO2〉n quantities, respectively. The outer [Ko+] scales are non-linearly related to the linear, inner 〈kio〉n and 〈tMRO2〉n scales due to their Michaelis-Menten relationships. The correlation of 〈kio〉n and 〈tMRO2〉n is very strong. The SS-NMR studies also indicate the mean cell volume 〈V〉n is rather constant. Thus, 〈kioV〉n = 〈cMRAWC〉n (pL(H2Oi)/s/cell) correlates with 〈tMRO2〉n (pmole(O2)/s/mL(suspension)). (This is a combination of Figures 3C and 3E of reference (31), where details are provided. The points are synaptosome measurements: the dashed line is the Michaelis-Menten fitting (Km = 4.5 mM) of the cortical culture measurements.)
In Figure 2, AWC participation of Fig. 1 enzymes is exemplified by SGLT (both a type III and a type IV transporter) and by KCC4 (a type II and type I enzyme). These transporters share substrates with NKA; Nao+ for SGLT and Ki+ for KCC4. The SGLT and KCC4 reactions are thereby coupled with the NKA reaction. As individual proteins, I and IV respectively are catalyzing unidirectional water effluxes and influxes. Working in concert with other transporters, and orchestrated by NKA activity however, they produce overall cellular homeostatic water bidirectional exchange. The cellular AWC fluxes are given in Equation (2). This is a “systems” aspect of the cell. Though none of the individual process metabolic
| (2) |
rates (cMR’s) are equal to cMRNKA, they rise or fall with the latter. For example, cMRK+(effl) = 2cMRNKA and cMRH2O(effl) = xcMRNKA = [H2Oi]kioV. Thus, cMRH2O(effl) = cMRH2O(infl) = xcMRNKA. NKA is the driver.
Molecular Dynamics (MD):
In silico simulations of Fig. 1 transporter mechanisms using MD (29,30,32–37) can be very informative. This is particularly so for studies of an SGLT enzyme: the bacterial transporter vSGLT forms a hydrophilic membrane-spanning channel (32–36). Generally, the enzyme exhibits spasmodic (tens of ns) bursts, some featuring net water unidirectional influx and some featuring bidirectional water exchange (33–35). That is, a given transporter can indeed facilitate the net flux (category B) and the exchange (category A) processes at different times (32).
For a period after sodium ion release in the vSGLT sugar-bound state, water molecules pass inward and outward, past the galactose molecule (a glucose stereoisomer) in the channel, in almost equal, small numbers (õne molecule/ns) - homeostatic exchange. The sugar is bound to its site midway through the channel, while a Na+ binding site is near the cytoplasmic mouth. In the ~100 ns after galactose is released, it proceeds through the channel into the cytoplasm, and pushes the channel-filling water molecules before it into the cell - net influx - in a quantity (33,35) generally consistent with the Fig. 1 SGLT water stoichiometries. Both such periods (exchange and net flux) are only transient (34). However, both seem triggered by Na+ release.
Since the common role of the Fig. 1 enzymes is to facilitate passage of hydrophilic substrates across the hydrophobic bilayer membrane, it is likely they all have somewhat similar hydrophilic pathways. The structure of the NKCC1 transporter channel has been determined (38). We surmise there are many more water co-transporting membrane enzymes, possibly including Na+,K+-ATPase (NKA) itself, as yet unstudied for this aspect. The NKA channel structure has been elucidated (39). Transient exchange periods simulated for AQP-1 (37) are very similar to those for vSGLT (34). It is likely each of the Fig. 1 transporters can accomplish what AQPs accomplish, but in addition the conduct of specific metabolites across the membrane. Water molecules will find their ways through any open hydrophilic pathway (“pore”).
The aforementioned vSGLT MD simulations also suggest possible molecular mechanisms for SGLT/NKA kinetic coupling. As stated, large tranches of net water molecule influx (category B) generally occur only upon galactose release from its site near the middle of the hydrophilic channel. But, the probability for this, and for exchange (category A), can depend on whether a Na+ ion is in a site: the Na+ can block the sugar (and apparently net water) ingress into the cell (35,36). More often than not, Na+ release occurs before sugar release (32). At least 80 ns after the Na+ release (35,36), galactose release can occur – unless the Na+ ion has re-bound or has been replaced by another Na+ ion. While galactose is still present, the duty cycle of the Na+ site remaining empty depends on the intracellular sodium concentration [Nai+]: this Na+ can come from only inside the cell. With a normal cMRNKA, [Nai+] is small (see below). However, [Nai+] increases as cMRNKA decreases (40). Thus, the probability of the Na+ site being re-occupied within 80 ns increases as cMRNKA decreases. As a consequence, the sugar-release frequency decreases as cMRNKA decreases. In this way, MRSGLT and MRNKA are positively correlated kinetically. (This pair-wise SGLT/NKA consideration assumes all other Figs. 1,2 enzyme activities remain unchanged.) Irrespective of the details, the sharing of any substrate with NKA should facilitate kinetic coupling. The correlation mechanisms of other Fig. 1 water co-transporter activities with MRNKA must bear some general similarity to this. Pore openings and closings are metabolically controlled.
A Futile Cycle?
When they were first realized, the Fig. 2 Na+ and K+ exchange processes may have been briefly viewed as “futile cycles.” This term is used to characterize a set of coupled biochemical reactions that consume ATP with no apparent benefit (41). Obviously, it is generally thought biological evolution discards futile cycles as “energy wasting.” However, it was quickly understood the trans-membrane ion concentration gradients enjoyed by Na+ and K+ serve to partially store chemical (Gibbs) free energy released by NKA-catalyzed ATP hydrolysis, ΔGATP = − 59 kJ/mole (42,43). As we will see below, ΔGNa(infl) is typically near – 14.5 kJ/mole, for Na+ influx, and ΔGK(effl) near 0 kJ/mole, for K+ efflux. Thus, though these cycles are homeostatic, they are not in equilibrium, but in steady-state. Energy is required to maintain them. Subsequently, the free energy thus stored in the [Na+] gradient is used to facilitate cellular uptake of many crucial molecules (Fig. 1), and these processes help complete the Na+ cycle. The free energy in the [K+] gradient is small only because of the cell’s membrane potential, which the homeostatic K+ efflux largely produces and maintains (see below).
The “pump and leak mechanism” term used for water (19,27) implies waste. However, a question naturally arises. Might the existence of active trans-membrane water cycling (Fig. 2) reveal an analogous metabolic energy storage function? Is AWC an actual example of a futile cycle, or does it have some evolutionary advantage?
THEORY:
Reaction Free Energy Changes (ΔG):
The issue of AWC futility is a chemical thermodynamic question. Thus, we must consider ΔG for each permeant as it crosses the membrane, for example, into the cell; ΔGper(infl), for permeant (per) influx (infl). For transport reactions (as in Fig. 1), the standard free energy change (ΔG0) is zero. There are potential chemogenic, electrogenic, and barogenic ΔG contributions. These are, respectively, the three terms on the right-hand-sides of Equations (3); where i and o are, respectively, the inside and outside compartmental indices, [per] is
| (3) |
the compartmental permeant concentration, Zper the (signed) permeant particle electrical charge, F is the Faraday constant (0.0965 kJ/(mV•mole) at physiological temperature, 310 K), Em,oi is the (signed) trans-membrane electrical potential (in minus out) in mV, Pi/Po is the intracellular/extracellular hydraulic pressure ratio (Figs. 1,2) ((44), p. 35), and RT = 2.58 kJ/mole at 310 K. For H2O as permeant, ZH2O = 0, and the second term makes no contribution.
Equations (3) can be written alternatively with permeant chemical potential changes; Δμper, where μper ≡ (φG/φnper)T,P,n(≠ per) ((44), p. 20). However, the ΔG notation is more inclusive, and more common in this field (42,43).
To obtain the free energy change for any Fig. 1 reaction, ΔGreac(infl), one must sum over all permeants, Equation (4); where sper is the permeant stoichiometric coefficient (Fig. 1). It is obvious from Eqs. (3,4) the chemogenic
| (4) |
terms require realistic compartmental permeant concentrations. Strictly, thermodynamic activity (a = the concentration•activity coefficient product) values should be used. Below, and in A2, we justify the first-order approximation that solute and solvent activity coefficients are each unity.
Concentration Scales:
In this paper, a concentration is indicated with square brackets, e.g., [permeant]. Sometimes, the molarity scale, with unit M = moles(permeant)/L(compartment) (activity coefficient yper ((45), pp. 26,27)) is used. However, first-order evaluation of chemogenic terms in Eqs. (3) and (4) consider only the entropy of mixing. This requires concentration scales with mole ratio- or mole fraction (X)-like quality. Molality, with unit m = moles(permeant)/kg(water) (activity coefficient γper ((45), pp. 26,27)), is one such scale. Biological aqueous solutions are sufficiently dilute in each solute that its m value is well-approximated by its M value; the infinite dilution assumption. The use of an entropy-centric concentration scale here is tantamount to assuming solution “ideality;” there are no specific solute/solute interactions. We will see below this is clearly not the case. The “volume molality” scale, Vm = moles(permeant)/L(solvent) ((44), p.37) will also be considered. (Using m or vm concentration values is tantamount to assuming the small molecule metabolites may sample the entire compartmental space or only aqueous spaces, respectively, (i.e., the water-excluding macromolecules are more or less mobile or immobile (14).) Since all the different neutral and ionic solutes (thus, osmolytes plural) need be counted, we will further use the OsMolarity (unit, OsM), or Osmolality (unit, Osm) concentration scales.
Since we also consider the solvent water as a permeant (Fig. 1), we must express its concentration. For understanding water thermodynamic activity – its “escaping tendency” - the dimensionless X (or a related) scale is best ((44), p. 56; (45), p.27; (46)). Thus, for water, XH2O = (moles(water))/(moles(osmolytes + water)), activity coefficient fH2O ((45), pp. 26,27)), is used.
One will further see milli- scales: mOsM, mOsm, and mOsX.
Chemogenic Contributions
Tissue Osmolyte Compartmental Concentrations.
For in vivo conditions, accurate determination of biological compartmental solute concentrations is difficult, particularly for intracellular metabolites. Recent metabolomic and proteomic advances (for example, using calibrated liquid chromatography/mass spectrometry techniques) have given realistic accountings. From such a study of cultured murine kidney epithelial cells, 101 different intracellular small molecule metabolites, and their concentrations, have been itemized (47). We compile an inventory of intra- and extra-cellular solute concentrations from a number of related studies (47–62) in Table 1. The inorganic ion (Na+, K+, H3O+, Ca2+, Mg2+, Cl−, HCO3−) concentrations are conventional. The units are mOsM (thus, mOsm for osmolytes). These should be considered representative of interstitial and intracellular compartment osmolytes. Of course, they are not the values for any actual cell. Concentrations surely differ from cell to cell, tissue to tissue, and in different biological conditions. Since we are calculating general thermodynamic trends, the Table 1 values suffice.
Table 1.
| interstitial | intracellular | |
|---|---|---|
| Na + | 135.0 | 15.0 |
| K + | 5.0 | 150.0 |
| H 3 o + | 0.00005 | 0.0001 |
| Ca 2+ | 1.5 | 0.0001 [free]; 0.01 [total] |
| Mg 2+ | 0,5 | 0.5 [free]; 10 [total] |
| other cations | 1.5 | 3.5 |
| Cl − | 105.0 | 15.0 |
| HC0 3 − | 30.0 | 10.0 |
| small molecule metabolite anions | 3.0 | 135.0a,b |
| protein anions | 7.5a | 9.5a,c,d |
| small molecule metabolites | 40.0a,b | |
| RNA | 0.5d | |
| lipids | 0.05d | |
| DIMA | 0.000005d | |
| total osmolytes | 289 | 379 |
| H 2 0 mole fraction (OsX w ) | 0.995 | 0.993 |
| H 2 0 millimolarity (mM) | 45,000e | 42,000f |
| f w | fw,o = 45/55 = 0.82 | fw,i = 42/55 = 0.76 |
| osmotic stoichiometry (H 2 O/osmolyte) | 45/0.289 = ~156 | 42/0.379 = ~111 |
We see the intracellular RNA, lipid assembly, and DNA mOsM concentrations are tiny. Though these occupy considerable intracellular volume (see below), because of their very large macromolecular masses they make negligible osmolal contributions. Since there are fewer (but non-zero: see below) extracellular macromolecules, we ignore their osmolal contributions here.
Interestingly, we see the total osmolyte concentrations are ~0.3 OsM for the interstitial space and ~0.4 OsM for the intracellular space. It is widely assumed intracellular osmolarity is near 0.3 (52), and thus similar to that in the extracellular space. The implications of this trans-membrane osmolarity gradient are explored in the section on intracellular pressure (Pi) below.
Tissue Water Compartmental Concentrations.
Experimentally determining biological compartmental water contents is even harder than for solutes (2). For many years, it has been stated, usually anecdotally, that cell water content is 70% (v/v) [or (w/w), assuming unit density, d] (53). Vinnakota and Bassingthwaighte presented a comprehensive review and tabulation of rigorous, quantitative determinations of myocardial tissue compartmental densities, volumes, volume fractions, and mass fractions (51). These derive from direct ex vivo mass analyses and in vivo tracer studies, and yield the cell water content value 75 % (w/w). A more recent optical determination yields 85 % (v/v) for cultured mammalian cell controls (54).
If we use the 75 % value (51) and take d = 1.1 g/mL, we obtain [H2Oi] = 42 M; i.e., 42 moles(H2O)/L(cell). This is the most parsimonious interpretation of the data, and includes water in the cytoplasm, mitochondria, and sarcoplasmic reticula. The magnitude is considerably reduced from the value for pure water, 55 M. The intracellular water volume fraction, fW,i, estimated from this is 42/55 = 0.76 (Table 1). This is in good agreement with quantitative MRI in vivo measures (55). (Note: fW is different from fH2O.) Rand states, without attribution, [H2Oi] = 54 M (56).
From tracer (15OH2) studies (reviewed in (51)) and NMR (1H2O) studies (reviewed in (2)), essentially all intracellular water is effectively “well-mixed,” at homeostasis. There has been much consideration of “bound” water in tissue (reviewed in (25,57–59)). There is no doubt water near macromolecule and membrane surfaces is thermodynamically different from bulk water – it has at least lower entropy (see below) – but kinetically (tumbling- and diffusion-wise) it remains in very rapid communication with all the water in the cell. The fraction of water molecules in “buried” intra-macromolecular sites inside cells is miniscule. Though the latter have significant NMR consequences (reviewed in (25)), even these water molecules have labile access to the cytoplasm (60).
Because of its small volume fraction (typically, 0.2) the extracellular compartmental water content in parenchymal tissue has been even more difficult to determine. Vinnakota and Bassingthwaighte report myocardial interstitial water content 92 % (w/w) and d = 1.0 g/mL, but this has a significantly greater uncertainty (51) and seems excessively large. The water content of cartilage can be more directly determined, and this might be a good extracellular model: it is an essentially acellular matrix of collagen fibrils (61). Lu and Mow imply a cartilage water content value of 78 % to 85 % (61). Maroudas and co-workers carefully measured the value 81 % for unloaded human femoral head cartilage (62). Again assuming unit density, this yields [H2Oo] = 45 M, also significantly reduced from 55 M, though not as much as [H2Oi]. The corresponding fW,o is 45/55 = 0.82 (Table 1).
In Table 1, we give intra- and extracellular “osmotic stoichiometry” values; the overall ratio of water molecules to osmolyte particles in the compartment. We see the intra- and extracellular values are near 100 and 150 water molecules per osmolyte, respectively. The fact that in Fig. 1 transporter osmotic stoichiometries for the reactants are either above or below these values is consistent with the water stoichiometry being principally determined by the hydrophilic channel volumes (35). The molecular action of any particular transporter has nothing to do with the overall “osmotic stoichiometry.” It is only the net water transported by all influxers and effluxers in the cell that must be zero for homeostasis. However, even if values of 100 or 150 did obtain, this would be irrelevant for the calculations below.
The large macromolecular volumes are the reason the 42 M intracellular H2O concentration (Table 1) used below is so much less than that of pure water (55 M). These large molecules exclude considerable water (Fig. 8 of (15)). Obviously, there are also water-displacing extracellular macromolecules: 45 M (Table 1) is also smaller than 55 M.
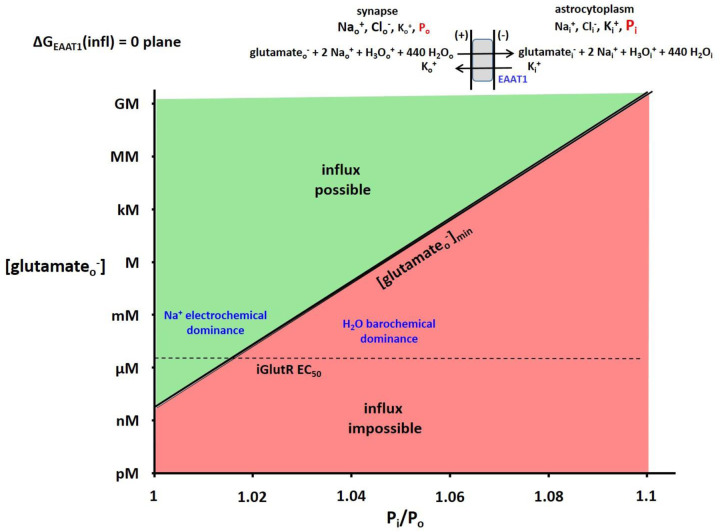
Figure 8. The 2D plot of the ΔGEAAT1(influx) = 0 plane from the 3D plot analogous to Figure 4.
The (in this case) astrocytic glutamate− concentration, [glutamatei−], the membrane potential, Em,oi, and Po were fixed at 1.4 mM (the Km value for glutamine synthetase), −91 mV, and 1 atm, respectively (T = 310 K). The surface is colored green when influx is thermodynamically possible and red when it is impossible. Thus, the intersection of the ΔG surface with the ΔG = 0 plane traces the trajectory of the (Pi/Po)-dependence of the minimum synaptic glutamate− concentration, [glutamateo−]min, value required for astrocyte uptake. The EC50 value of the iGlutR receptor for synaptic glutamateo− (2.3 μM) is indicated with a horizontal dashed line. It is clear glutamateo− is well-cleared from the synapse when Pi is small, and not when Pi is only a few percentage points greater. In the latter case, the receptors would be saturated, and synaptic transmission would be interrupted.
However, the molar concentration scale is useful mainly for molecular counting (1 mole = 6 × 1023 molecules). As justified above, we use the dimensionless mole fraction concentration scale for water (XH2O). (This differs from the tissue compartmental water mole fractions (“populations”) pi and po (important in SS-NMR).) For most practical aqueous solutions, including those in biological compartments, however, XH2O is always very nearly unity. “In dilute solutions of electrolytes, the activities, and even the activity coefficients (fH2O), of the solvent are little different from unity” ((63), p.12). Very important consequences of this are considered below.
One kg of water is (1000/18.0154) 55.508 moles H2O. Dividing this by (55.508 + the sum of osmolyte concentrations) in each of the cytosolic and interstitial compartments yields corresponding water mole fractions OsXH2O,i = 0.993 and OsXH2O,o = 0.995 (Table 1). OsXH2O,i is only 0.2% smaller than OsXH2O,o, and each value is nearly that for pure water. Given this situation, following Pitzer ((63), p.12), it is not unreasonable to assume equal activity coefficients (fH2O,i = fH2O,o = 1) and thus use these mole fraction values in the Eqs. (3) and (4) terms for water as permeant. Inserting {OsXH2O,i/OsXH2O,o} = 0.998 yields ΔGH2O(infl) = − 0.00517 kJ/mole for the chemogenic contribution.
Therefore, unlike the other Table 1 species, water is almost (but not exactly) in trans-membrane chemical equilibrium. (In this paper, “chemical” equilibrium is defined as: [peri] = [pero].) There is (only) ~5 J/mole favoring cellular water influx. Strictly, this chemogenic ΔGH2O(infl) value might be considered not significantly different from zero (chemical equilibrium). However, analyses of surrogate solutions in A2 give water activity values: aH2O,i = 0.994 and aH2O,o = 0.995. These correspond to chemogenic ΔGH2O(infl) = − 0.003 kJ/mole. Henceforth, we will use – 3 J/mole. As we will see, this has no bearing on the important metabolic consequences detailed below. For co-transported water, the barogenic contribution will be much greater than the chemogenic term.
It is probably unreasonable that chemogenic ΔGH2O(infl) be positive. First-order osmotic considerations more strongly favor water influx. However, the first-order is inadequate, see below.
Electrogenic Contributions
We make the common approximation that K+ efflux permeabillity dominates over Na+ and Cl− permeabilities (in the Pf sense) in cellular homeostasis. Thus, we use a version of the Nernst Equation (5) ((44), p. 113) to calculate Em,oi,
| (5) |
where: RT = 2.58 kJ/mole and the Faraday constant F = 0.0965 kJ/mV•mole (both at 310 K) and insert the Table 1 [K+] values. Z must be the value for the potential-producing ion, ZK+ = 1. It is important to note the actual number of excess Ko+ ions (and an equal number of uncompensated intracellular anions) is insufficient to significantly alter the Table 1 [K+] values. An excess of only 0.02 mM intracellular anionic charges (0.014% = ((100×0.02)/145); Table 1) produces Em,oi ≈ − 100 mV for a small (1 fL) spherical cell ((53), p. 198.)
Barogenic Contributions
Intracellular pressure (Pi) can be counteracted with an externally applied (macroscopically uniform) mechanical pressure, in principle ((44), p.32), and elegantly transduced into an isotropic, hydraulic (mechanical) pressure with cellular insertion of a pressure-sensitive “osmotic” microelectrode (64,65). The trans-membrane pressure difference (Pi – Po) force is oriented roughly vectorially radial to the cell surface. Experimentally, most mammalian cells have (Pi - Po) values under 0.1 atm (27). However, an osmotic microelectrode study of perfused ex vivo murine lens (featuring relatively simple lens cells) found (Pi - Po) values up to 0.5 atm (66). Most Po values are near 1 atm: even “high” tumor interstitial Po is only 1.02 atm. (67). For water, the standard state (fH2O truly 1) pressure, P0, is 1.0 atm.
RESULTS:
Free Energy Calculations
Permeant Influx Free Energy Changes, Eq. (3).
In Table 2, we list many potential non-metabolite permeant (per) species, and calculate chemogenic, electrogenic, and barogenic ΔG(infl) contributions (kJ/mole) for their influx. For these calculations, we use the Table 1 water OsXW and solute mOsm concentrations for the chemogenic terms. We calculate the barogenic contributions for Pi/Po ratios of 1.00 (blue) and 1.05 (red).
Table 2.
Free Energy Change for Influx, Eq. (3).
| potential permeant | ΔGper(infl) (kJ/mole) | |||
|---|---|---|---|---|
| chemogenic | electrogenic | barogenic | sum | |
| Pi/Po = 1.00 || 1.05 | Pi/Po = 1.00 || 1.05 | |||
| H 2 O o | −0.003 | 0 | 0 || 0.13 | −0.003 || 0.13 |
| Na o + | −5.67 | −8.78 | 0 || 0.13 | −14.5 || −14.3 |
| K o + | 8.78 | −8.78 | 0 || 0.13 | 0 || 0.13 |
| H 3 O o + | 1.79 | −8.78 | 0 || 0.13 | −6.99 || −6.86 |
| Ca o 2+ | −24.8 | −17.6 | 0 || 0.13 | −42.4 || −42.3 |
| Cl o − | −5.02 | 8.76 | 0 || 0.13 | 3.74 || 3.87 |
| HCO 3,o − | −2.83 | 8.76 | 0 || 0.13 | 5.93 || 6.06 |
Concentrations in Table 1. Em,oi = −91 mV
It is clear that, compared with those of the other permeants, the chemogenic and electrogenic contributions for individual H2O molecule transport are negligible. We see the slight chemogenic tendency for water molecule influx when Pi = Po is reversed when Pi ≥ 1.05 Po. In fact, for ΔGH2O(infl) = − 0.003 kJ/mole, any Pi > 1.001 Po would result in water efflux if there were no other considerations.
Let us consider two other potential permeants, Nao+ and Ko+ as examples. The electrogenic terms for their influxes are identical, − 8.78 kJ/mole. Electrostatically, influx of each is favored. However, while the chemogenic Nao+ contribution (− 5.67 kJ/mole) also favors influx, the chemogenic Ko+ term (8.78 kJ/mole) opposes influx. Thus, the electrochemical potential gradient for Nao+ influx is very favorable, − 14.5 kJ/mole (− 5.67 – 8.78), but zero for Ko+ influx (or Ki+ efflux) when Pi = Po. The barogenic contributions for Nao+ and Ko+ are identical, but this has very small effect for only Nao+. However, compared with Nao+, and indeed with all the other Table 2 permeants except Ko+, the barogenic contribution for H2O is proportionally much greater than the chemo- and electrogenic terms. (For example, a Pi of 1.05 atm causes a more than 40-fold increase in ΔGH2O, but only a 1% change in ΔGNa.) This has very important consequences.
Transport Reaction Free Energy Changes, Eq. (4).
We use Eq. (4) to calculate the total ΔGtransporter(infl) for each of the Fig. 1 transporter reactions - utilizing the stoichiometries seen there. The “metabolite” substrates (glucose, glutamate−, GABA, and lactate−) are omitted because we study below how their transport is affected by the thermodynamic contributions of the other “ancillary” (support) substrates.
Influx Reactions.
Reactions that normally run in the influx direction (IV, III, or both, in Figs. 1,2) are presented in Table 3a (Em,oi is fixed at − 91 mV). Each is favorable (negative ΔG) when Pi = Po (blue). However, they each become less favorable as Pi/Po increases, and all become unfavorable (positive ΔG) when Pi has reached 1.05 Po (red). This is due to Pi opposition to water co-influx (the factors that are − 0.003 when Pi = Po become positive when Pi exceeds 1.001 Po). Because of the large water stoichiometries, the barogenic contribution can have a significant influence on secondary active water co-transporter thermodynamics. This is due to essentially only the water co-transport. For the other permeants, the blue/red values generally have very similar magnitudes.
Table 3a.
Transport Reaction Free Energy Changes, ΔG(infl) (kJ/mol); Eq. (4) (Pi/P0 = 1.00 || 1.05) influxer reactions [III, IV, or both, in Fig. 1) w/o metabolic substrates
| enzyme | substrates and free energies | net | |||
|---|---|---|---|---|---|
| hSGLT1 | giucoseo + | 2 Nao+ + | 235 H2Oo → | ||
| 2(−14.5 || −14.3) (−29 || −28.6) + | 235(−0.003 || 0.13) (−0.71 || 31) = | (−29.71 || 2.4) | |||
| rSGLT1 | giucoseo + | 2 Nao+ + | 380 H2Oo → | ||
| 2(−14.5 || −14.3) (−29 || −28.6) + | 380(−0.003 || 0.13) (−1.1 || 49) = | (−30 || 20.4) | |||
| GLUT1 | glucoseo + | 40 H2Oo | |||
| 40(−0.003 || 1 0.13) (−0.12 || 5 2) = | (−0.12 || 5.2) | ||||
| GLUT2 | glucoseo + | 100 H2Oo → | |||
| 100(−0.003 | I 0.13) (−0.30 || 13) = | (−0.30 || 13) | ||||
| EAAT1 | glutamateo + | 2 Nao+ + | 440 H2Oo + | −k+ + H3Oo+→ | |
| 2(−14.5 || −14.3) (−29 || −26 8) + | 440(−0.003 || 0.13) (−1.3 || 57) = | (0 || −0.13)+ (−6.99 || −6.86) = | (−37 || 23) | ||
| GAT1 | GABAo + | 2 Nao+ + | 330 H2Oo + | Clo → | |
| 2(−14.5 || −14.3) (−29 || −26.8) + | 330(−0.003 || 0.1B) (−0.99 || 43) = | (3.74 || 3.87) = | (−26 || 20) | ||
| NKCC1 | 2CIo + | Nao+ + | 590 H2Oo + | Ko+ → | |
| 2(3.74 || 3.87) (7.48 || 7.74) | (−14.5 || −14.3) | 590(−0.003 || 0.13) (−1.77 || 77) | (0 || 0.13) | (−8.8 || I 71) | |
Glucose Uptake at Fixed [glucosei].
For the rSGLT1 influx reaction as an example: ΔG = − 30 kJ/mole when Pi = Po, but 20.4 kJ/mole when Pi = 1.05 Po. (Pi = 1.03 Po is sufficient to make influx unfavorable.) To illustrate the consequences of this on glucose uptake, we fix the intracellular glucose concentration, [glci], at 2 μM. (The intracellular enzyme that phosphorylates glucose, hexokinase, has a glucoseo Km value reported as 1.7 μM (20). A principle of central carbon (glucose-related) metabolism is that any intracellular metabolite steady-state concentration should be near the Km value of the enzyme that consumes it (47).) Thus, we write Eq. (4) explicitly for the 2Na+/glucose co-transporter rSGLT1 influx free energy change, ΔGrSGLT1(infl), in Equation (6). The first term represents the glucose barochemical contribution
| (6) |
with [glci] fixed at 2 μM. The second and third terms are due to, respectively, the Na+ electro- and barochemical and the H2O barochemical potential gradients. The former favors influx, while the latter opposes it.
If we fix Pi = 1.05 Po, and neglect the water co-transport, ΔG becomes positive (i.e., influx ceases) only when the extracellular glucose concentration [glco] decreases below 0.33 nM. On the other hand, if we keep Pi = 1.05 Po but retain the water co-transport, the reaction becomes unfavorable when [glco] decreases below 2.4 mM – three orders of magnitude greater than [glci]. The large electrochemical ΔGNa+ favoring influx (− 28.6 kJ/mole) is counteracted by the even larger barochemical ΔGH2O(infl) favoring efflux (49 kJ/mole). A number of significant consequences of this will be considered in the Discussion section.
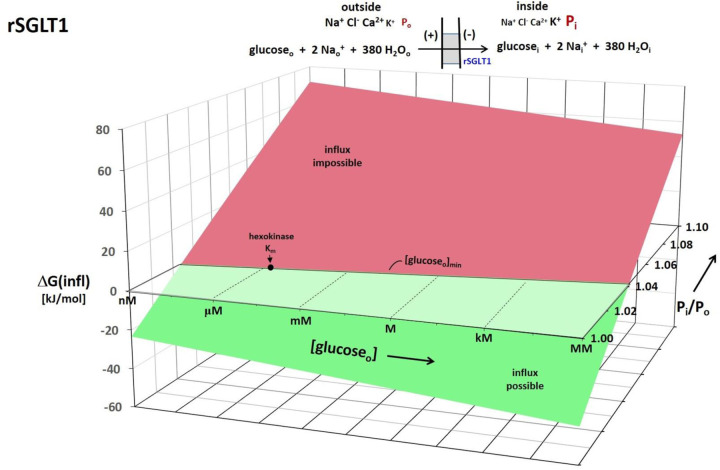
To partially generalize these considerations, Figure 4 shows a 3D plot of Eq. (6) for the rSGLT1 reaction. For this, the [Nao+] and [Nai+] values were fixed at those in Table 1, [glci] at 2 μM, and Em,oi at − 91 mV. The vertical axis plots the rSGLT1 influx reaction ΔGrSGLT1(infl), while the (logarithmic) oblique axes increment [glco] and Pi/Po. The free energy surface is colored green when influx is possible (ΔGrSGLT1(infl) < 0), and red when impossible (ΔGrSGLT1(infl) > 0). The intersection of the ΔGrSGLT1(infl) surface with the horizontal ΔGrSGLT1(infl) = 0 plane defines the trajectory of the (Pi/Po)-dependence of the minimum extracellular glucose concentration required for influx. While the exponential nature of the (Pi/Po)-dependence is hardly noticeable, that of [glco] is very evident.
Figure 4. A 3D plot for the rSGLT1 reaction.
The vertical axis measures the Gibbs free energy change (ΔG) for the influx direction shown, and calculated with Eq. (6). The logarithmic oblique axes plot the extracellular glucose concentration, [glucoseo], and the intracellular/extracellular hydraulic pressure ratio (Pi/Po) over the experimentally measured range. For this calculation, the Table 1 concentrations and the Tables 2 and 3a free energy terms were used (chemogenic ΔGH2O(infl) = − 3 J/mole). The intracellular glucose concentration, [glucosei], the membrane potential, Em,oi, and Po were held fixed at 2 μM, − 91 mV, and 1 atm, respectively (T = 310 K). The surface is colored green when influx is thermodynamically possible and red when it is impossible. Thus, the intersection of the ΔG surface with the ΔG = 0 plane traces the trajectory of the (Pi/Po)-dependence of the minimum, [glucosei]min, value. The value of the intracellular hexokinase Km (1.7 μM) for glucosei is indicated.
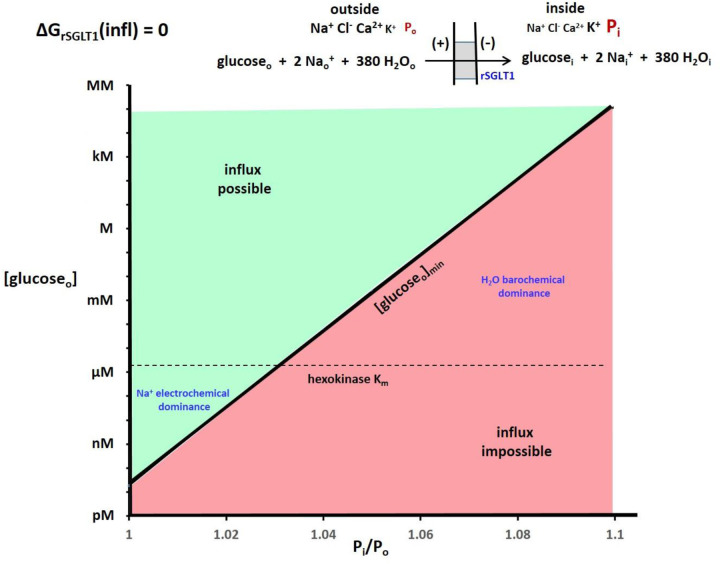
To make these effects clearer, Figure 5 shows the 2D plot of the Fig. 4 ΔGrSGLT1(infl) = 0 plane. With H2O co-influx, increasing Pi/Po strongly increases [glco]min. For Pi = 1.04 Po, [glco]min is already ~10 μM (greater than [glci] here) for rSGLT1. The location of the hexokinase glucosei Km is shown as a horizontal dashed line. Of course, the position of the surface depends also on the Em,oi, [glci], and [Nai+] values, among other quantities. For example, making ΔGNa(infl) less negative (say, by increasing [Nai+]) would increase [glco]min, all other factors being equal – a given Pi/Po, for example.
Figure 5. The 2D plot of the Figure 4 ΔGrSGLT1(influx) = 0 plane.
The regions where the Na+ electrochemical gradient dominate and the H2O barochemical gradient dominate are indicated. The dependence is so strong that small percentage changes of Pi cause very large changes of the minimum [glucoseo] required for glucose uptake. The hexokinase Km for glucosei (1.7 μM) is indicated with a horizontal line.
Plausible water chemogenic ΔGH2O(infl) valies (− 5 to 5 J/mole) are small compared with a typical barogenic magnitude (2.58ln(Pi/Po) = 2.58ln(1.03) = 76 J/mole), which favors efflux. Thus, it is relatively inconsequential whether water is in chemical equilibrium (OsXH2O,i = OsXH2O,o) or not. The larger steady-state water barochemical potential gradient is mostly barogenic.
Since it is likely almost all cells have Pi 1.01 atm or greater, it seems that each of the Table 3a reactions will have similar behaviors. The water barochemical contributions provide crucial counterbalances for other influx transporters as well.
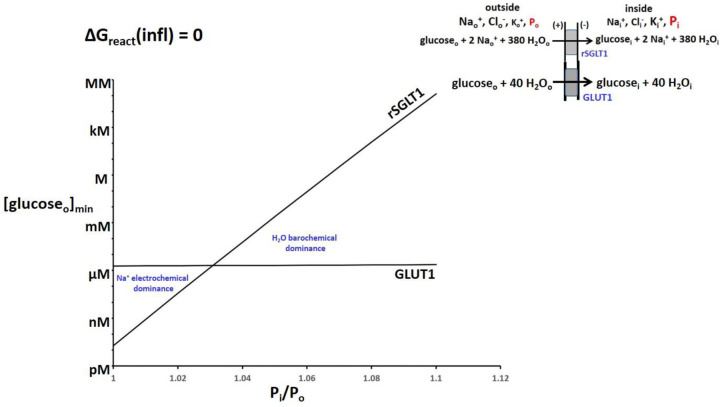
Though the GLUT family of glucose influxers lacks the large SGLT Na+ electrochemical potential gradient favoring influx, it also has a smaller H2O barochemical force opposing influx when Pi > Po: the GLUT H2O stoichiometries are smaller (Fig. 1). Figure 6 contrasts the 2D plot of GLUT1 with that of rSGLT1 (from Fig. 5) and makes obvious for the SGLT family the Na+ electrochemical dominance at small Pi values and the H2O barochemical dominance at large Pi.
Figure 6. The (Pi/Po)-dependences of [glucoseo]min for rSGLT1 (from Fig. 5) and for GLUT1.
Equation (4) was used, with the intracellular glucose concentration, [glucosei], the membrane potential, Em,oi, and Po held fixed at 2 μM, −91 mV, and 1 atm, respectively (T = 310 K). The regions of Na+ electrochemical and H2O barochemical dominance are very evident. Influx through the rSGLT1 transporter is much more sensitive to Pi/Po than that (barely noticeable) through the GLUT1 transporter. This is due to the much greater water stoichiometry of the former (Fig. 1).
Glucose Uptake at Fixed [glucoseo].
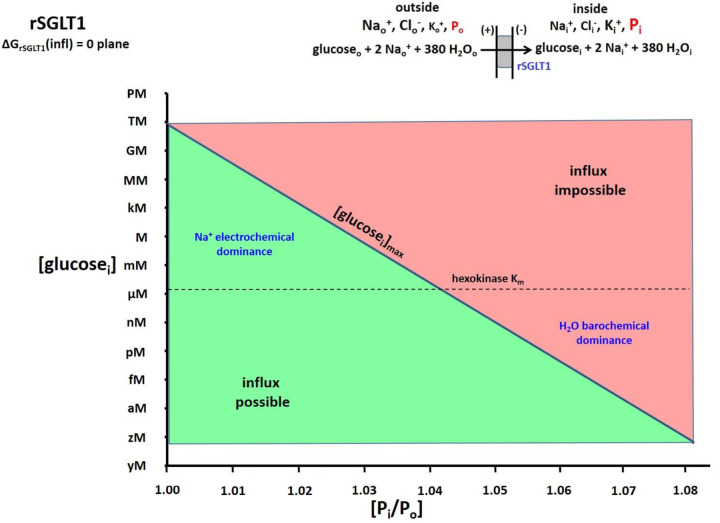
For an alternative perspective, we take the extracellular glucose concentration, [glco], to be maintained at 5 mM (a large, but reasonable (68,69), value). With rSGLT1, if we fix Pi = 1.05 Po and neglect water co-transport, ΔG remains negative until the intracellular glucose concentration, [glci], reaches the absurdly large value of 300 M. On the other hand, if we keep Pi = 1.05 Po but retain the water co-transport, the reaction becomes unfavorable when [glci] reaches only 4.1 μM. More generally for rSGLT1 with H2O transport, Figure 7 shows the (Pi/Po)-dependence of [glci]max, with [glco] fixed at 5 mM. The location of the hexokinase glucosei Km is shown as a horizontal dashed line. For such a plot, in the lower portion of the graph, the influx reaction is now possible (green), while it is impossible (red) in the upper portion. The maximum value of [glci] is greatly suppressed by increasing Pi/Po.
Figure 7. The 2D plot of the ΔGrSGLT1(influx) = 0 plane from a 3D plot similar to Figure 4.
Equation (6) was used and, in this case, the extracellular glucose concentration, [glucoseo], the membrane potential, Em,oi, and Po were held fixed at 5 mM, −91 mV, and 1 atm, respectively (T = 310 K). The surface is colored green when influx is thermodynamically possible and red when it is impossible. Thus, the intersection of the ΔG surface with the ΔG = 0 plane traces the trajectory of the (Pi/Po)-dependence of the (in this case) maximum intracellular glucose− concentration, [glucosei]max, value allowing uptake. The Km value of the cytoplasmic hexokinase for glucosei (1.7 μM) is indicated with a horizontal dashed line. It is clear when Pi is small, tremendous values of [glucosei] are allowed, which would easily saturate hexokinase. However, this is not the case when Pi is only a few percentage points greater.
For GLUT2 at Pi = 1.04 Po and [glco] = 5 mM, [glci] could accumulate to 4.8 mM if there was no water co-influx, but only 110 μM with H2O co-influx.
Neurotransmitter Uptake and Clearance.
There are considerable similarities of the rSGLT and excitatory amino acid transporter (EAAT1) influx reactions – with the exceptions of the H3O+ co-influx and Ki+ co-efflux of the latter. Glutamate− influx is greatly aided by the Na+ electrochemical gradient, but opposed by the H2O barochemical gradient. The analogous EAAT1 3D plot (not shown) is very similar to that for rSGLT (Fig. 4). However, the roles of these two reactions are quite different. While the purpose of rSGLT is mainly to deliver glucose into cells, that of EAAT1 is principally to clear glutamate− from synapses (via astrocytic uptake) after action potential transmission.
Now, besides Em,oi = − 91 mV as before, we fix intracellular [glutamatei−] at 1.4 mM, the glutamatei− Km for glutamine synthetase (70), which converts astrocytic glutamatei− to glutaminei. Figure 8 depicts the 2D ΔGEAAT1(infl) = 0 plane of the unshown 3D plot. The vertical axis measures log extracellular [glutamateo−], while the horizontal axis plots Pi/Po. The intersection of the ΔGEAAT1(infl) surface with the ΔGEAAT1(infl) = 0 plane traces the trajectory of the logarithm of the minimum glutamateo− concentration ([glutamateo−]min) necessary to initiate influx, as a function of Pi/Po. Though strictly bi-exponential, the plot is almost a single exponential function (the exponential nature of the Pi/Po axis is very weak). For a member of the ionotropic glutamate receptor (iGlutR) family, the position of the L-glutamateo− EC50 value, 2.3 μM (71), is shown as a horizontal dashed line.
There is an influxer for which representative concentrations for all substrates are found in Table 2. Ko+ influx via NKCC1 is not affected by the concentration of a metabolite substrate.
Inclusion of the effects of Em,oi and ΔGNa variation would increase the dimensionalities of Figure 4-type plots. Making ΔGNa(infl) less negative, for example, would (non-linearly) shift the lines to the left in Figs. 5–8. Making Em,oi less negative (by increasing [Ko+] or by [Ki+] reduction) should do the same. In this paper, however, we focus on the novel (and large) effects of Pi variation, consequent to water co-transport.
Efflux Reactions.
The ΔGtransporter(effl) values for the normal efflux reactions (II, I, or both, in Figs. 1,2) are found in Table 3b (again, Em,oi is fixed at − 91 mV). (Note: the calculations are for ΔG(effl), not ΔG(infl).) In contrast to the Table 3a influx reactions, except for that of KCC4 the other efflux reactions are unfavorable at Pi/Po = 1.00 (blue). But, they are made much more favorable as Pi/Po increases (red), because of water co-efflux.
Table 3b,
Transport Reaction Free Energy Changes, ΔG(effl) (kJ/mol), Efflux Version of Eq. (4) (Pi/Po = 1.00 || 1.05) effluxer reactions (I. II. or both, in Fig. 1) w/o metabolic substrates
| KCC4 | Ki+ + | Cli− + | 500. H2Oi → | net | |
|---|---|---|---|---|---|
| (0 || −0.13) + | (−3.74 || −3.87) + | 500(0.003 || −0.13) (1.5 || −65) = | (−2.2 || −69) | ||
| MCT1 | lactatei− + | H3Oi+ + | 500 H2Oi → | ||
| (6.99 || 6.86) + | 500(0.003 || −0.13) (1.5 || −65) = | (8.5 || −58) | |||
| NaDCl | dicarboxylatei2− + | 175 H2Oi + | 2 Nai+ → | ||
| 175(0.003 || −0.13) (0.53 || −23) + | 2(14.5 || 14.3) (29 || 29) = | (30 || 6.0) |
There is an efflux reaction for which representative concentrations for all substrates are found in Table 2; KCC4, the [K+,Cl−,500H2O] effluxer. For this, we see the efflux favorability is greatly enhanced by water co-efflux, even though it is slightly favorable even w/o H2O co-transport. Thus, it will always operate in efflux mode. Ki+ efflux via KCC4 is not limited by the concentration of a metabolite substrate.
We focus on the lactic acid exporter, MCT1. The reaction is unfavorable when Pi/Po = 1.00, ΔGMCT1(effl) = 8.5 kJ/mole (blue), but very favorable, − 58 kJ/mole (red), when Pi/Po = 1.05. Again, this is due to H2O co-efflux. At Pi/Po = 1.05 atm, the [laco−]/[laci−] ratio could reach only two without water, but can attain 3 × 1010 with the 500 H2O molecules co-effluxed.
DISCUSSION:
Biochemical Roles for Water Co-Transport.
The analyses above suggest co-transported water can exert significant thermodynamic effects on its process via the cytoplasmic pressure (Pi). We elaborate some examples.
Figure 1 Glucose Influxers.
Small [glucoseo].
Commonly, the SGLT transporter family is thought of in terms of its potential for catalyzing glucose influx against its concentration gradient, chemogenic ΔGglc(infl) (72,73). However, Figs. 5 and 6 show this is true only when the Pi value is relatively small. For rSGLT1, “uphill” glucose influx is impossible for any Pi above ~1.03 Po. A 1 % Pi increase to 1.04 atm requires a [glco] value of at least ~10 μM for influx. This is five times larger than the 2 μM [glci] fixed for Figs. 5–6. Perhaps cells using exclusively SGLT transporters always have small Pi values. Maybe Pi fluctuation serves to control glucose influx. The GLUT family of transporters, with its smaller H2O stoichiometries (Fig. 1), does not suffer this severe (Pi/Po)-dependence (Fig. 6). For GLUT1, the H2O/glucose transport ratio is much smaller than for rSGLT1, and its [glco]min value is almost Pi-insensitive (Fig. 6). Perhaps cells with larger Pi values, and in situations with smaller [glco] values, employ GLUT enzymes. On the other hand, if Pi values are small, cells with SGLT transporters will take up glucose at smaller [glci] values than cells with GLUT transporters. When rSGLT1 Pi is small, small [glco] can insure sufficient [glci]. Also, when Pi is small, besides carrying glucose uphill, SGLT transporters are “pumping” water up a barochemical hill. They are maintaining the H2O barochemical steady-state.
Large [glucoseo].
If there is a situation where extracellular glucose is maintained at a relatively large value (e.g., 5 mM), the role of Pi can be viewed differently. Setting Pi = Po, or ignoring co-transported water, would allow absurdly large [glci]max values for cells with SGLT enzymes (Fig. 7). This would surely amount to a sugar overload. Even if that was not cytotoxic, it could cause cells in a tissue to partake differentially of any available glucose charge – some cells initially reached by a bolus taking up more (or even all) sugar than others. This would mean a heterogeneous cellular [glci] spatial distribution. However with H2O co-transport, representative experimental Pi values (say, 4% larger than Po) suppress the [glci]max value to near the hexokinase Km, 1.7 μM for glucose (Fig. 7). Cells with even larger Pi values will take up less glucose than those with smaller Pi. For rSGLT1 when Pi/Po = 1.05 and [glco] = 5 mM, [glci]max is near 1 nM, insufficient for metabolism. With no H2O co-transport, [glci]max would increase more than ten orders of magnitude; many orders greater than the hexokinase Km for glucose.
For a given extracellular glucose concentration, cells with exclusively GLUT transporters will take up more glucose than cells with exclusively SGLT enzymes at larger Pi values. Perhaps GLUT transporters are found in cells with larger Pi values also in environments where [glco] is large.
As shown in Fig. 1, both SGLT and GLUT transporters deliver glucose. It is interesting a switch from GLUT1- to SGLT-mediated cellular glucose uptake during lung cancer progression has been reported (20). More generally, GLUT1 expression (along with other genetic changes) is found to promote the cancer cell Warburg State (74). However, among these families, only the GLUTs transport the 18FDG-PET tracer, (2-fluorodeoxyglucose) 2-FDG (20). This produces an interpretation problem for the metabolic rate of glucose uptake and consumption determined by quantitative 18FDG-PET.
Because of H2O co-transport, cells seem never allowed much steady-state free glucose. The role of water co-influx, common to all the glucose influxers [hSGLT1, rSGLT1, GLUT1, GLUT2], appears dominant in controlling [glci]. It seems water co-transport via an SGLT generally guarantees a [glci] near the hexokinase Km for glucose. Glucose is effectively metabolized immediately upon entering the cell. This protects cells from too much glucose, and tissues from excessive cellular glucose uptake inequality. A large ([glco] - [glci]) difference makes [glci] hard to measure, even with modern methods (47). Also, glci likely does not contribute to the glucoCEST NMR signal (75,76). On the other hand, GLUT1 generally keeps [glci] near [glco] (Fig. 6). (GLUT2 will keep it somewhat smaller.)
Figure 1 Neurotransmitter Influxers: astrocyte uptake / synaptic clearance.
Similar considerations must also obtain for the Table 3a influx reactions of the principal excitatory and inhibitory neurotransmitters glutamateo− and GABAo, respectively. Once inside the astrocyte, these must be processed immediately by their metabolizing enzymes. EAAT1 (Fig. 8) and GAT1 will not allow them to build-up. (Note: for EAAT1, Zglt = − 1, and there are H3Oo+ co-influx and Ki+ co-efflux terms (21).)
However, an even more crucial aspect of the EAAT and GAT roles is the effective clearance of neurotransmitter species from synaptic junctions in time to enable the next action potential. Figure 8 shows this for EAAT1 and glutamateo−. It is clear the astrocyte Pi must be relatively small (< 1.02 atm) to ensure [L-glutamateo−] does not much exceed 2.3 μM, its EC50 value for the iGlutR receptor enzyme. At the same time, the astrocyte Pi must be large enough (1.035 atm) to ensure astrocytosolic [L-glutamatei−] does not much exceed 1.4 mM, its Kd value for glutamine synthetase (analogous to Fig. 7). The large H2O stoichiometries for EAAT1 (and GAT1) transport (similar to that of rSGLT1, Fig. 1) guarantee high sensitivity to astrocyte Pi.
The astrocytic uptake of synaptic glutamate− is particularly interesting. If astrocyte Pi/Po is even ~1.03, [glutamateo−] would have to reach ~100 μM (almost two orders of magnitude over 2.3 μM) to initiate uptake (Fig. 8). This could be sufficient to saturate iGlutR, and quench further synaptic transmission. Perhaps this is a physicochemical mechanism whereby astrocytes serve as “gatekeepers” for synaptic activity. Increases of Pi to only 3 or 4% above Po could allow receptor saturation, and the interruption of neuronal firing. Very small astrocyte Pi fluctuations could enable or disable synaptic function. For excitatory glutamatergic synapses this means turning off or on neural excitation. A glance at the Fig. 1 GAT1 influx stoichiometry indicates very similar considerations would obtain for GABAergic synapses. In that case, neuronal inhibition could be halted by increased intra-astrocyte pressures – resulting in increased excitation.
Astrocyte swelling, a sign of possible Pi change, has been implicated in the glymphatic processes occurring during sleep (commentary (77)). Also, it has been suggested some therapeutic interventions can disturb the glutamate−/glutamine cycle leaving interstitial glutamine, which can act as a nutrient for brain cancer cells (78). In non-neural cells, [glutamatei−] values can become very large, 64 mM (47). Perhaps this is because there is no glutamine synthetase.
The Figure 1 NKCC1 Influxer.
The function of NKCC1 (reviewed in (22)) is to effect cellular Ko+ and H2Oo uptake against their respective chemical and barochemical gradients. Because of the huge H2O stoichiometry (Fig. 1), this process is predicted to be strongly Pi-dependent.
Figure 1 Effluxers.
For the Table 3b effluxers, each of the reactions are made significantly more favorable by increased intracellular pressure (only KCC4 is favorable when Pi = Po, and NaDC1 requires Pi/Po > 1.05). Again, this is due to the pressure effect on the free energy caused by the large numbers of co-transported H2O molecules.
We use the monocarboxylate transporter [MCT1] as an example. It’s main role is ridding the cell of lactic acid build-up from glycolytic-type metabolism. This is common in cancer cells (the Warburg Effect). Lactic acid can be sufficiently cytotoxic that MCT1 inhibition has been considered as a cancer therapy (79). Equation (7) expresses
| (7) |
the free energy change for the lactic acid efflux reaction: the first, second, and third terms represent the lactate−, H3O+, and H2O contributions, respectively. Taking the Table 1 H3O+ and OsXH2O concentrations, Em,oi = −0.91 mV, {Po/Pi} = {1/1.05} = 0.95, and letting ΔGMCT1(effl) = 0, we solve for the maximum {[laco−]/[laci−]} value that can be achieved. We find {[laco−]/[laci−]} = 2.6 × 1010 when 500 H2O molecules are co-transported, compared with only 2 if there was no H2O co-transport. This ten order-of-magnitude increase insures essentially all lactic acid is expelled from the cell – because of the barochemical water contribution. Once again, water co-transport is protecting the cells – even if they are malignant. A consequence is the extracellular acidification common in tumors (79). This phenomenon is also found for other cell types (e.g., muscle tissue cramping).
We now scrutinize the nature of intracellular hydraulic pressure (Pi).
The Nature of Intracellular Pressure.
It seems clear the crucial thermodynamic role played by co-transported water is effectuated by the pressure difference across the cell membrane. The intracellular pressure (Pi) is commonly considered “osmotic” in nature. That is, it is thought to result from the phenomenon of water molecules crossing the membrane. The simple first-order picture (H2O permeable; osmolytes (Na+, K+, Cl−) impermeable in the Pf(p) sense) invokes selective water permeability, with influx and efflux becoming the same when ΔP (≡ Pi - Po) equals RT times the trans-membrane osmotic gradient (7): at T = 310 K, RT is 25.4 L•atm/mole. Strictly, osmotic effects are entropic in nature (the entropy of mixing (27)).
The first-order expression for the trans-cytolemmal osmotic gradient (Δπoi) in terms of osmolyte concentrations is given in Equation (8), where [osmolytesi] and [osmolyteso] are the intra- and extracellular concentrations,
| (8) |
respectively. This equation assumes ideal solutions ((44), p 37; (80), p. 302 ff): i.e., entropy only. In Eq. (8), the osmolyte concentrations are given on the osmolarity scale. We use the representative values listed in Table 1. There, total [osmolytesi] = 0.379 OsM and total [osmolyteso] = 0.289 OsM. (At such magnitudes, these are essentially identical to their molal concentrations. We neglect the tiny mOsM intracellular RNA, lipid, and DNA concentrations.) Inserting these into Eq. (8) yields Δπoi = 2.29 atm. With the Table 1 compartmental water contents, we can also estimate volume molal (Vm = OsM/fW) osmolyte concentrations. We obtain: Vmi = 0.38/0.76 = 0.50 mole(osmolytes)/L(cell water), and Vmo = 0.29/0.82 = 0.35 mole(osmolytes)/L(extracellular water). Inserting these into Eq. (8) gives: Δπoi = 25.4 (Vmi - Vmo) = 25.4(0.50 – 0.35) = 3.8 atm, an even greater value.
If there were only first-order mixing entropy contributions, Pi would equal 2.3 or 3.8 atm at osmotic “equilibrium” (really steady-state; assuming Po = 1 atm). A molecular mechanism for selective water influx and efflux could be free (i.e., unregulated) bidirectional aquaporin-mediated transport, as has been suggested (81). However, the largest experimental Pi value (1.5 atm (66)) is 1.5 times smaller than even 2.3 atm. The most pertinent Pi values (1.02 to 1.05; Figs. 4–8) are more than 2.2 times smaller. A (Pi - Po) of 1.03 would correspond to Δ[osmolytes] = 0.04 OsM (1.03/25.4). This is 2.5 times smaller than the 0.1 OsM value we estimate in Table 1. Of course, the Table 1 Δ[osmolytes] value is not found for any real cell. However, it is a reasonably representative magnitude: it is very unlikely to be less than 1.5 times any real value. If anything, 0.1 OsM may even be small (52). It is not clear any real cytoplasmic lipid bilayer membrane could even survive ΔP of 2.3 atm (the hydraulic pressure ca. 38 ft below sea level) if the internal pressure (Pi) is the greater, let alone 3.8 atm. Even if it could, a ΔP value of even 2.29 atm would yield a barogenic Eq. (8) term: ΔG(infl) = 2.58ln(2.29) = 2.14 kJ/mole. This is so large, it would dominate the values in Tables 2 - 4, and obliterate the realistic estimations of the last section.
Such first-order considerations carry the implicit assumption of solution ideality; no specific osmolyte/osmolyte molecular interactions. Osmotic pressure is generally considered one of the “colligative” properties: the identity of the osmolyte is irrelevant. This is why the mole fraction concentration scale, mOsX (or the related mOsm scale), for solution components is the most appropriate for osmotic considerations ((44), p. 56; (45), p.27; (46)). But, since the Table 1 osmolyte mOSM values are sufficiently small (in the absolute sense) that mOsm values can be used, this suggests their activity coefficients (γosmolyte, or yosmolyte) are near unity. However, such reasoning does “fail to emphasize the departure from ideality indicated by the activity of the solute” ((45), p.29).
Furthermore, we see from Table 1 intra- and extracellular water mole fractions OsXH2O,i = 0.993 and OsXH2O,o = 0.995, respectively. OsXH2O,i is only 0.2% smaller than OsXH2O,o, and each is nearly the value for pure water. Given this situation, it is not unreasonable to also assume the water activity coefficients are equal, fH2O,i = fH2O,o (63). In A.2, we present calculations of H2O activities for surrogate interstitial and cytosolic solutions that support this contention. Table 2 shows inserting the calculated values aH2O,i = 0.994 and aH2O,o = 0.995 into Eq. (3) or (4), leads to a water chemogenic contribution to ΔGH2O(infl) of only ~3 J/mole favoring cellular water influx. Also, we showed above that chemogenic ΔGH2O(infl) =− 0.003 kJ/mole is counteracted by a Pi/Po ratio of only 1.001. This is only one tenth of a conservatively small typical intracellular pressure, Pi/Po = 1.01 (27,66). This adds to the suggestion the entropic contribution to Pi is actually small, usually less than 10%.
All these considerations indicate the mixing entropic contribution (− TΔSH2O(infl)) to water ΔGH2O(infl) is small. By definition, a thermodynamically non-ideal solution means there is an enthalpic (ΔHH2O(infl)) contribution. A representative Pi/Po value of 1.05 corresponds to barochemical ΔGH2O(infl) = − 0.13 kJ/mole. A Pi/Po ratio of 1.001 implies ~90% ΔHH2O(infl); i.e., − 0.12 kJ/mole. The conclusion must be that enthalpic (ΔHH2O(infl)) contributions dominate Pi. The aqueous solutions inside (and, for that matter, outside) cells deviate greatly from ideality. There are highly specific molecular interactions between the various solutes, and with water. It has long been known the “hydration” of biological solutes is extremely important for many different cellular processes (57–59). If the solute-interacting water entropy is smaller than in pure water, this could even give rise to a (not mixing) entropic driving force for efflux. Whatever the actual case, the intracellular osmolytes do not seem to lower the water “escaping tendency” nearly as much as the first-order osmotic pressure equation (8) would predict.
There is a long history of considering higher order contributions to Eq. (8) ((82), p. 210 ff; (83)). However, it is probably not realistic to expect an environment as complex as the intracellular milieu to be well-modeled as a homogeneous solution.
Recent reports suggest particular intracellular species contribute to regulating intracellular hydraulic pressure. These include: the mechanosensitive transcriptional regulator YAP (Yes-associated protein) (84), tropomyosins 1.6 and 2.1 (Tpm 1.6 and Tpm 2.1) (85), and the capsaicin-activatable transient receptor potential vanilloid 1 (TRPV1) cation channel, that interestingly, stimulates the Fig. 1 NKCC1 water co-influxer (86) as well as NKA. An especially intriguing proposal is that intracellular macromolecular polyanion electrostatic interactions dominate Pi, while allowing modulation by extracellular osmolality (52).
It may be often assumed tissue homeostasis means Δπ is zero, and is regulated to that point: i.e., [osmolytesi] = [osmolyteso] in Eq. (8): i.e., “isosmolality.” However, the word “isotonal” (6) suggests only equal pressures, and is preferred. A 290 mOsm NaCl solution is often considered “isotonic.” Bathing solutions hyper- or hypo-tonic relative to this cause in vitro cells (19) and ex vivo tissue cells (87) to shrink or swell due to net water efflux or influx, respectively. Bolus blood infusions of “hypertonic” solutions are used clinically to transiently open the blood-brain-barrier by shrinking capillary endothelial cells – and thus deliver otherwise non-extravasating therapeutic drugs to the cerebral parenchyma (88). In cell cultures and to some degree in perfused tissues, the investigator can specify and control [osmolyteso]. When such a system reaches homeostasis, a common presumption may be that [osmolytesi] = [osmolyteso]. From this, it is usually assumed [osmolytesi] ≈ 0.3 OsM, the Table 1 [osmolyteso] value (52). However, our estimated [osmolytesi] ≈ 0.4 OsM (Table 1) indicates this may be rarely (never) true.
Likely further evidence of cytoplasmic non-ideality is that different tissues seem to require different bathing solution osmolalities to achieve isotonicity. While ~320 mOsm suffices for the retina and olfactory bulb, almost 600 mOsm is required for cerebral cortex (87). Inhibition of NKA causes cortical cells to swell considerably; i.e., a net category B water influx (89).
Whatever the actual Pi physicochemical nature, the calculations in the last section remain valid because they employ experimental Pi values.
Aquaporin Role in Cellular Homeostasis.
The specific water-transporting membrane aquaporin (AQP) molecules are found in almost all tissues (6,90,91). Like all the Fig. 1 transporters, they are capable of catalyzing category B unidirectional (flux, Pf-type) or category A bidirectional (exchange, Pd-type) water transport. The famously large “single channel” AQP4 water volume flux, 0.25 fL/s/AQP4 (8.3 H2O molecules/ns/AQP4), was determined at 10°C and with a very large 4.4 atm osmotic gradient (92). Like all Pf measurements, this is derived from the asymptotic volume change when transport is initiated away from osmotic “equilibrium.” However, AQP efficiency is minimal when there is no pressure gradient (ΔP = 0) (37), i.e., the Pd-type condition. This is consistent with molecular considerations (the water single-file nature of the AQP channel structure (30) is not optimal for exchange). Thus, AQP’s by themselves have been thought of as intrinsically passive transporters. They can facilitate very large net water influxes or effluxes, but only when the latter are driven by independent forces. Thus, though their involvement in non-homeostatic cell swelling or shrinking has been widely investigated (6,90,91), their roles in homeostasis has been less clear. We are not aware a “single channel” category A exchange magnitude has ever been experimentally determined.
The results presented here suggest there is thermodynamic (metabolic) energy stored in tissue water compartmentalization itself, helping maintain the system in homeostasis. Thus, we have inserted AQPs explicitly into the active trans-membrane water cycling (AWC) scheme, rate-limited by cMRNKA (Fig. 2). Since AWC is, by definition, homeostatic, MRH2O(infl) = MRH2O(effl). However, it is unlikely AQP’s contribute equally to water influx and efflux. As stated above, if ΔP was actually osmotic in nature, unregulated dominant AQP activity would lead to ΔP values many times those measured. Thus, it is likely MRAQP(infl) ≠ MRAQP(effl).
In cell suspensions (and in vivo) astroglial aquaporin AQP4 expression has been shown to contribute to category A water exchange (81). Furthermore, studies with human C6 glioma (cancer) cell suspensions and TGN020 (a specific AQP4 inhibitor) are informative. The kio rate constant ratios (TGN020kio)/(C6kio) is 0.69, while (ouabainkio)/(C6kio) is 0.65 (81). Inhibiting water exchange with extracellular TGN020 reduces kio to the same extent as inhibiting it with extracellular ouabain, the specific NKA inhibitor. This indicates AQP4 contributes to AWC. Cancer cells in suspension may not be in the Warburg state (2,4).
The metabolite influxers (Fig. 1) can provide significant MRH2O(infl) values. The cellular glucose consumption rate, MRglc(consump), has been determined to be 38 μM(glc)/s/cell for murine kidney epithelial cells (47) and for brain tissue cells, assuming reasonable cell density and volume values (68). The glucose influx rate, MRglc(infl) must be at least MRglc(consump). But GLUT1 and rSGLT1 have different stoichiometries (Fig. 1). Thus, MRH2O(infl) would range from 1.8×109 to 17×109 H2O/s/cell for 2pL cells with, respectively, exclusively GLUT1 or exclusively rSGLT1 transporters. Using the ratio of the glutamate−-to-glutamine conversion flux, MRglu-gln(cycle) to MRglc(consump) (93), we estimate MRH2O(infl) provided by the EAAT1 transporter to be 4 × 107 H2O/s/cell for a 2 pL astrocyte. This would be in addition to MRH2O(infl) provided by a glucose influxer.
Some so-called “metabolic water” is generated within the cell after glucose is imported. However, there are at most six H2O molecules produced per glucose molecule metabolized, depending on the glycolysis and oxidative phosphorylation proportions (94). This is small when compared with even the smallest Fig. 1 glucose influxer H2O stoichiometries.
Our most recent estimate of brain x, the MRH2O(AWC)/MRNKA ratio, is > 106 (2). We opined “such large stoichiometries suggest aquaporin participation in active trans-membrane water cycling.” Given the large cellular water influx rates, and the Pi > Po pressure gradient, it seems likely MRAQP(effl) > MRAQP(infl). That is, in homeostasis AQP is likely mainly catalyzing water efflux. It is working in parallel with the KCC effluxer. This is why we indicate the inhibitor blocking MRAQP(effl) in Fig. 2.
Water should be considered a substrate for active trans-membrane water cycling. It has thermodynamic consequences. If it turns out NKA itself has its own water stoichiometry, AQPs could be then considered as also secondary active enzymes. If not, they are only tertiary active transporters – sharing water as a substrate with only the secondary active water co-transporters.
An interesting analogy with potassium ion-specific channels (PCs) (reviewed in (29); (95)) might obtain. It is thought the formally “passive” Ki+ efflux through PCs dominates the production of Em,oi. The idea is Ki+ efflux driven by the chemogenic term proceeds until the latter is balanced by the electrogenic term (Table 2), which actually requires very few effluxed K+ ions. After that, an “electrochemical” steady-state condition is reached, and homeostatic Ki+ efflux is synchronized with cMRNKA. If it is water transport that dominates the Pi value, perhaps there is an analogous H2Oi efflux driven by the barochemical gradient. This would tend to decrease Pi. However, diminished Pi would allow the Na+ electrochemical gradient a greater role. This would tend to increase the water co-influxes of the many transporters that also bring in Na+ (e.g., NKCC- and SGLT-catalyzed influxes; Figs. 1–8), which would tend to increase Pi. This “feedback loop” would then establish an NKA-maintained steady-state, and AQP-catalyzed water efflux is also synchronized with cMRNKA. Thus, we have AQP playing a vital role in active trans-membrane water cycling (Fig. 2).
A trans-membrane pressure difference could also affect transporter kinetics. This is discussed in A.3.
CONCLUSION:
The Na+,K+-ATPase (NKA) enzyme function has long been known to maintain Na+ and K+ in trans-membrane electrochemical steady-states, which are far from chemical equilibria ([Nai+] = [Nao+], [Ki+] = [Ko+]). Here, we find metabolic energy released by NKA-catalyzed ATP hydrolysis is also required to maintain (and is thus stored in) in a trans-cytolemmal water barochemical steady-state. This is an inherent part of NKA function. An important difference from the Na+ and K+ cases is the trans-membrane water distribution is in (or near) chemical equilibrium ([H2Oi] ≈ [H2Oo]). Crucially, the barochemical H2O steady-state is significant, and enzymatic water co-transport has very important thermodynamic metabolic consequences. Active trans-membrane water cycling does not represent a futile cycle.
SYNOPSIS:
Via intracellular pressure, membrane co-transported water influences thermodynamic control of cell metabolite maintenance.
ACKNOWLEDGEMENTS:
The authors especially thank Professor Daniel Zuckerman for critical readings of manuscript drafts and very insightful suggestions, and Dr. August George for accomplishing initial calculations. They also thank Professors Joseph Ackerman, Valerie Anderson, James Balschi, James Bassingthwaighte, Cynthia Burrows, Deborah Burstein, Kieran Clarke, Ira Cohen, Michael Grabe, Fahmeed Hyder, Joanne Ingwall, Christopher Kroenke, Kenneth Krohn, Philip Kuchel, Xin Li, Daniel Liefwalker (for ref. (74)), Jeffrey Maki, Silvia Mangia, Richard Mathias, Anusha Mishra, William Rooney, Douglas Rothman, Suzanne Scarlatta, William Skach, and Mark Woods, and Drs. Joseph Armstrong, Mauro DiNuzzo, Bassam Haddad, Audrey O’Connor, and Gregory Wilson, and Messers. Eric Baetscher, Eric Baker, Brendan Moloney, and Joshua Schlegel for stimulating discussions.
Support:
• OHSU Brenden-Colson Center for Pancreatic Care; Sheppard (PI), Springer (PI)
• NIH UL1TR02369; Ellison, Morris (PIs); awarded by the Oregon Clinical and Translation Institute, Biomedical Innovation Program; Pike (PI)
Appendix A.1. Acronyms and Symbols.
| 〈A〉 tissue or voxel mean cell surface area |
| AQP aquaporin |
| AQP4 aquaporin 4 |
| AQP4KO aquaporin 4 knock-out |
| ATP adenosine triphosphate |
| ATPi intracellular ATP |
| AWC active trans-membrane water cycling |
| ae ρ〈A〉 |
| aH2O water thermodynamic activity |
| CEST chemical exchange saturation transfer |
| Δ G Gibbs free energy change |
| ΔG0 Standard AG |
| ΔGyyy ΔG for substrate, enzyme, reaction; YYY |
| ΔGYYY(effl) ΔG for YYY efflux |
| ΔGiYY(infl) ΔG for YYY influx |
| ΔHYYY(infl) enthalpy change for YYY influx |
| ΔTToi trans-cytolemmal osmotic gradient (in - out) |
| ΔSYYY(infl) entropy change for YYY influx |
| ΔV†† activation volume |
| d density (mass/volume) |
| EAAT1 excitatory amino acid transporter 1 |
| Em,oi trans-membrane electrical potential (in - out) |
| effl an efflux process |
| FDG 2-deoxy-2-18Fluoro-D-glucose |
| fH2O water activity coefficient on the mole fraction scale (X) |
| fW tissue water volume fraction |
| fW,i intracellular fW |
| fW,o extracellular fW |
| ϕ osmotic coefficient |
| G Gibbs free energy |
| GABA γ-amino butyric acid |
| GAT 1 GABA transporter 1 |
| GLUT1 glucose transporter 1 |
| glc glucose |
| gln glutamine |
| glu glutamate− |
| γH2O water activity coefficient on the molality scale (m) |
| 1H2O water proton MR signal |
| H2Oi intracellular water molecule |
| H2Oo extracellular water molecule |
| H3Oi+ intracellular hydronium ion |
| H3Oo+ extracellular hydronium ion |
| iGlutR an ionotropic glutamate’receptor |
| infl an influx process |
| Ki+ intracellular K+ |
| Ko+ extracellular K+ |
| KCC4 potassium, chloride transporter 4 |
| k kinetic rate constant |
| kio cellular water efflux k (1/τi) |
| kio(a) active kio contribution |
| kio(p) passive kio contribution |
| koi(p) passive cellular water influx k contribution |
| M molarity concentration scale |
| MADI metabolic activity diffusion imaging |
| MCT1 mono-carboxylate transporter |
| MD molecular dynamics |
| MRI magnetic resonance imaging |
| MRyyy YYY metabolic rate |
| MRglu(consump) MR of glucose consumption |
| MRglu(infl) MR of glucose influx |
| MRGlu-gln(cycle) MR of glutamate’-glutamine cycling |
| MRO2 MR of O2 consumption |
| cMRYYY cellular metabolic rate for YYY process = tMRYYY/ρ |
| tMRYYY tissue metabolic rate for YYY process = /ρcMRYYY |
| MWH2O molecular mass (“weight”) of water |
| m molality concentration scale |
| mOsM milli-osmolarity concentration scale |
| mOsm milli-osmolality concentration scale |
| mOsX milli-osmole fraction concentration scale |
| μYYY chemical potential of YYY; (∂G/∂nYYY)T,p,n(≠ nYYY) |
| Nai+ intracellular Na+ |
| Nao+ extracellular Na+ |
| NKA Na+,K+-ATPase (sodium pump) |
| NKCC1 sodium, potassium, chloride transporter 1 |
| NMR nuclear magnetic resonance |
| n number of moles |
| OsM osmolarity concentration scale |
| OsMYYY YYY OsM |
| Osm osmolality concentration scale |
| OsmYYY YYY Osm |
| OsX osmole fraction concentration scale |
| OsXYYYi intracellular YYY OsX |
| OsXYYY,o extracellular YYY OsX |
| P hydraulic (mechanical) pressure |
| P0 standard state P |
| Pd diffusional permeability coefficient (exchange) |
| Pd(p) passive Pd |
| PdYYY YYY Pd |
| Pf flux (flow) permeability coefficient |
| PfYYY YYY Pf |
| Pi intracellular P |
| Po extracellular P |
| PC potassium channel |
| PET positron emission tomography |
| p tissue water mole fraction (“population”) |
| pi intracellular p |
| po extracellular p |
| per permeant particle |
| R ideal gas constant |
| ρ cell (number) density |
| SYYY stoichiometric coefficient of YYY |
| SGLT sodium/glucose co-transporter |
| SS trans-cytolemmal NMR shutter-speed |
| TGN020 specific AQP4 inhibitor |
| τi mean H2Oi molecule lifetime (1/kio) |
| 〈V〉 tissue or voxel mean cell volume |
| Vm volume molality concentration scale |
| Vmi intracellular Vm |
| Vmo extracellular Vm |
| v tissue volume fraction |
| ve extracellular v (ECV) (= 1 - vi) |
| vi intracellular v [pV] |
| X mole fraction concentration scale |
| XYYY YYY X |
| XYYY,i intracellular YYY X |
| XYYY,o extracellular YYY X |
| x water cycling stoichiometry [H2O/ATP] |
| x′ x for individual water co-transporter |
| [YYY] YYY concentration |
| [YYYc] compartmental YYY concentration |
| [YYYi] intracellular YYY concentration |
| [YYYo] extracellular YYY concentration |
| [YYYt] tissue YYY concentration = vi[YYYi] = ve[YYYo] |
| yL yocto liter (= 10−24 L = 1 (nm)3)) |
| yH2O water activity coefficient on the molarity scale (M) |
| ZYYY signed electrical charge of YYY |
A.2. Compartmental Water Thermodynamic Activities.
Solvent mole fraction values as large as OsXH2O,o = 0.995 and OsXH2O,i = 0.993 (Table 1) “fail to emphasize the departure from ideality indicated by the activity coefficient of the solute” ((45), p.29). However, here the solvent activity coefficient difference is also very small. Since the water activity aH2O (dimensionless on this scale) is always so close to unity, it requires sophisticated apparatus for high-precision direct water vapor pressure determinations to measure (45). Using these, it has been found aH2O is inversely, and exponentially, related to the solute osmolality, Osmsolute, via the empirical osmotic coefficient, φ, Equation (A.2.1) (19,45,46; (63), p.12). MWH2O is the water molecular
| (A.2.1) |
weight (mass), 18.0154 g/mole(H2O). The dimensionless product (MWH2O•Osmsolute/1000) is the solute/solvent mole ratio (1000 is (g/kg)), and essentially the solute mole fraction, OsXsolute. The latter is also dimensionless, and since the exponent must be dimensionless, φ is also dimensionless. Equation (A.2.1) provides a way to evaluate aH2O. (When Osmsolute is zero (pure water), aH2O is exactly one.)
How might we more fruitfully evaluate Eq. (4) when per = H2O? Solutions as complex as those in Table 1 have never been subjected to precise measurements such as those of the last paragraph. However, pure NaCl and KCl solutions have been so studied, at 298 K ((45), p.476). We could take as a surrogate interstitium 0.290 Osm NaCl, and a surrogate cytosol 0.375 Osm KCl. (A glance at the metabolomics study mentioned above (47) indicates a K+glutamate− solution would be a better intracellular surrogate. However, that has never been studied.) Their respective osmotic coefficients are φo = 0.9249 and φi = 0.9141 ((45), p.476) and, via Eq. (A.2.1), give aH2O,o = 0.995 and aH2O,i = 0.994. Using these activities in Eq. (4) gives ΔG(infl) = − 0.003 kJ/mole for H2O influx. This free energy change is again so small that correcting for temperature and actual solute content would make no difference. The conclusion is inescapable: there is a very small trans-membrane chemical potential difference (~3 J/mole) for water to enter the cell.
A.3. Intracellular Pressure and Kinetics (Reaction Activation Volumes).
Mechanical pressure can affect transporter free energies (as above), and macromolecular structures (96), but can also exert an effect on chemical reaction kinetics. This is characterized by the volume of activation, ΔV††, the difference between the partial molar volume of the reaction transition state and the partial molar volume of the reactants (97–99). The pressure dependence of a rate constant, k, is expressed in Equations (A.3.1) and (A.3.2),
| (A.3.1) |
| (A.3.2) |
where RT is 25.4 L•atm/mole at 310 K. If a process has ΔV†† = 6 L/mole, a 10 atm pressure increase will cause its rate constant to decrease by 90%. Six L/mole is 10−23 L/molecule, or 10 yL/molecule (1 yocto liter = 1 yL = 10−24 L = 1 (nm)3). The plasma membrane NKA is a macromolecular complex of mass 145 kDa (145,000 g/mole). Thus (assuming d = 1 g/mL), it has a partial molar volume of approximately 242 yL/molecule. A 6 yL ΔV†† would represent only a 2.5 % volume change. For Pi = 1.5 atm (the maximum Pi reported), Po = 1 atm, and ΔV†† = 6 L/mole, Eq. (A.3.2) yields k1.5 atm = 0.89 k1 atm, an 11% decrease.
It is easy to imagine ΔV†† values of this magnitude, or greater. In a molecular dynamics study of a small 6.5 kDa protein, Persson and Halle report a relatively constrained, 2 kDa domain undergoes numerous spontaneous 3% conformational volume fluctuations in a 1000 μs period (60). Most membrane transporters are rather large macromolecular complexes. It is quite likely they undergo significant absolute volume changes in reaching their transition states. Thus, their enzyme activities could be quite sensitive to intracellular pressure development. Some would slow down, and some would speed up: ΔV†† quantities can be negative (97–99). Furthermore, the activities of most membrane transporters are coupled to each other via common substrates or products (Fig. 1). Since the cell has evolved to have trans-membrane fluxes in homeostatic balance, it is likely a Pi change would disturb the latter. The consequent flux changes could serve an auto-regulatory function.
Footnotes
CONFLICTS OF INTEREST:
CSS and TMB are co-inventors on U.S. patent 11,728,038, “Activity MRI” (issued 15 August, 2023), which describes the MADI approach.
REFERENCES:
- 1.Springer C. S., Baker E. M., Li X., Moloney B., Wilson G. J., Pike M. M., Barbara T. M., Rooney W. D., Maki J. H., Metabolic activity diffusion imaging [MADI]: I. Metabolic, cytometric modeling and simulations. NMR Biomed. 36, e4781 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Springer C. S., Baker E. M., Li X., Moloney B., Wilson G. J., Anderson V. C., Sammi M. K., Pike M. M., Garzotto M. G., Kopp R. P., Coakley F. V., Rooney W. D., Maki J. H., Metabolic activity diffusion imaging [MADI]: II. Non-invasive, high-resolution human brain imaging of sodium pump flux and cell metrics. NMR Biomed. 36, e4782 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Neil J. J., Ackerman J. J. H., Metabolic activity diffusion imaging (MADI): A new paradigm. NMR in Biomed. 36, e4841 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Schlegel J., Baker E., Holland S., Stoller J., Packwood W., Li X., Barajas R., Springer C., Pike M., Metabolic activity diffusion imaging [MADI] of rat brain glioma. Proc. Int. Soc. Magn. Reson. Med. 31, 3924 (2023). [Google Scholar]
- 5.Pike M. M., Li X., Baetscher E., Barbara T. M., Sammi M. K., Stevens A. A., Springer C. S., Does MADI detect temporal brain metabolic activity changes?” Proc. Int. Soc. Magn. Reson. Med. 31, 5177 (2023). [Google Scholar]
- 6.Day R. E., Kitchen P., S Owen D., Bland C., Marshall L., Conner A. C., Bill R. M., Conner M. T., Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta 1840, 1492–1506 (2014). [ 10.1016/j.bbagen,2013,09,033] [DOI] [PubMed] [Google Scholar]
- 7.Verkman A. S., Water permeability measurements in living cells and complex tissues. J. Membrane Biol. 173, 73–87 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Zeuthen E., A sensitive ‘Cartesian Diver’ balance. Nature 159, 440–441 (1947). [DOI] [PubMed] [Google Scholar]
- 9.Kedem O., Katchalsky A., Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim. Biophys. Acta 27, 229–246 (1958). [DOI] [PubMed] [Google Scholar]
- 10.House C. R., Water transport in cells and tissues. (Edward Arnold, London, 1974), p. 156. [Google Scholar]
- 11.Chen S-T., Springer C. S., Ionophore-catalyzed cation transport between phospholipid inverted micelles manifest in DNMR. Biophys. Chem. 14, 375–388 (1981). [DOI] [PubMed] [Google Scholar]
- 12.Conlon T., Outhred R., Water diffusion permeability of erythrocytes using an NMR technique. Biochim. Biophys. Acta 288, 354–361 (1972). [DOI] [PubMed] [Google Scholar]
- 13.Landis C. S., Li X., Telang F. W., Molina P. E., Pályka I., Vétek G., Springer C. S., Equilibrium transcytolemmal water-exchange kinetics in skeletal muscle in vivo. Magn. Reson. Med. 42, 467–478 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Li X., Rooney W. D., Springer C. S., A unified magnetic resonance imaging pharmacokinetic theory: Intravascular and extracellular contrast reagents. Magn. Reson. Med. 54, 1351–1359 (2005). [DOI 10.1002/mrm.20684] [DOI] [PubMed] [Google Scholar]
- 15.Springer C. S., Using 1H2O to measure and map sodium pump activity in vivo. J. Magn. Reson. 291, 110–126 (2018). [ 10.1016/j/jmr.2018.02.018] [DOI] [PubMed] [Google Scholar]
- 16.Hills B. P., Belton P. S., NMR studies of membrane transport. Ann. Reports NMR Spectroscopy 21, 99–159 (1989). [Google Scholar]
- 17.Denker B. M., Smith B. L., Kuhajda F. P., Agre P., Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 263, 15634–15642 (1988). [PubMed] [Google Scholar]
- 18.MacAulay N., Molecular mechanisms of brain water transport. Nat. Rev. Neurosci. 22, 326–344 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Zeuthen T., Water-transporting proteins. J. Membrane Biol. 234, 57–73 (2010). [DOI 10.1007/s00232-009-9216-y] [DOI] [PubMed] [Google Scholar]
- 20.Barrio J. R., Huang S. C., Satyamurthy N., Scafoglio C. S., Yu A. S., Alavi A., Krohn K. A., Does 2-FDG-PET accurately reflect quantitative in vivo glucose utilization. J. Nucl. Med. 61, 931–937 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacAulay N., Gether U., Klaerke D. A., Zeuthen T., Water transport by the human Na+-coupled glutamate cotransporter expressed in Xenopus oocytes. J. Physiol. 530, 367–378 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaila K., Price T. J., Payne J. A., Puskarjov M., Voipio J., Cation-chloride cotransporters in neuronal development, plasticity and disease. Nature Rev. Neurosci. 15, 637–654 (2014). [doi: 10.1038/nrn3819] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Autry A. W., Gordon J. W., Chen H-Y., LaFontaine M., Bok R., Van Criekinge M., Slater J. B., Carvajal L., Villanueva-Meyer J. E., Chang S. M., Clarke J. L., Lupo J. M., Xu D., Larson P. E. Z., Vigneron D. B., Li Y., Characterization of serial hyperpolarized 13C metabolic imaging in patients with glioma. NeuroImage: Clin. 27, 102323 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye R., Verkman A. S., Simultaneous optical measurement of osmotic and diffusional water permeability in cells and liposomes. Biochem. 28, 824–829 (1989). [DOI] [PubMed] [Google Scholar]
- 25.Li X., Mangia S., Lee J-H., Bai R., Springer C. S., NMR shutter-speed elucidates apparent population inversion of 1H2O signals due to active transmembrane water cycling. Magn. Reson. Med. 82, 411–424 (2019). [DOI: 10.1002/mrm.27725] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Poirier-Quinot M., Springer C. S., Balschi J. A., Active trans-plasma membrane water cycling in yeast is revealed by NMR. Biophys. J. 101, 2833–2842 (2011). [DOI: 10.1016/j.bpj.2011.10.035] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Konstantopoulos K., Zhao R., Mori Y., Sun S. X., The importance of water and hydraulic pressure in cell dynamics. J. Cell. Sci. 133, jcs240341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Springer C. S., Measurement of metal cation compartmentalization in tissue by high resolution metal cation NMR. Ann. Rev. of Biophys. and Biophys. Chem., 16, 375–399 (1987). [DOI] [PubMed] [Google Scholar]
- 29.Öster C., Kendriks K., Kopec W., Chevelkov V., Shi C., Michl D., Lange S., Sun H., de Groot B. L., Lange A., The conduction pathway of potassium channels is water free under physiological conditions. Sci. Adv. 5, eeaw6756 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Groot B. L., Grubmüller H., Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science 294, 2353–2357 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Bai R., Springer C. S., Plenz D., Basser P. J., Brain active trans-membrane water cycling measured by MR is associated with neuronal activity. Magn. Reson. Med. 81, 1280–1295 (2019). [DOI: 10.1002/mrm.27473] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adelman J. L., Ghezzi C., Bisignano P., Loo D. D. F., Choe S., Abramson J., Rosenberg J. M., Wright E. M., Grabe M., Stochastic steps in secondary active sugar transport. Proc. Nat. Acad. Sci. 113, E3960–E3966 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adelman J. L., Sheng Y., Choe S., Abramson J., Wright E. M., Rosenberg J. M., Grabe M., Structural determinants of water permeation through the sodium-galactose transporter vSGLT. Biophys. J. 106, 1280–1289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Shaikh S. A., Enkavi G., Wen P-C., Huang Z., Tajkhorshid E., Transient formation of water-conducting states in membrane transporters. Proc. Nat. Acad. Sci. 110, 7696–7701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choe S., Rosenberg J. M., Abramson J., Wright E. M., Grabe M., Water permeation through the sodium-dependent galactose cotransporter vSGLT.” Biophys. J. 99, L56–L58 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe A., Choe S., Chaptal V., Rosenberg J. M., Wright E. M., Grabe M., Abramson J., The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature 468, 988–991 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu F., Tajkhorshid E., Schulten K., Theory and simulation of water permeation in aquaporin-1. Biophys. J. 86, 50–57 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S., Zhou J., Zhang Y., Liu T., Friedel P., Zhou W., Somasekharan S., Roy K., Zhang L., Liu Y., Meng X., Deng H., Zeng W., Li G., Forbush B., Yang M., The structural basis of function and regulation of neuronal cotransporters NKCC1 and KCC2. Comm. Biol. 4, 226 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogawa H., Shinoda T., Cornelius F., Toyoshima C., Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and sodium.” Proc. Nat. Acad. Sci. 106, 13742–13747 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pike M. M., Frazer J. C., Dedrick D. F., Ingwall J. S., Allen P. D., Springer C. S., Smith T. W., 23Na and 39K nuclear magnetic resonance studies of perfused rat hearts. Biophys. J. 48, 159–173 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehninger A. L., “Principles of Biochemistry.” (Worth Pub., New York, 1982), p. 571. [Google Scholar]
- 42.Bers D. M., Barry W. H., Despa S., Intracellular Na+ regulation in cardiac myocytes. Cardiovas. Res. 57, 897–912 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Kadir A. A., Stubbs B. J., Chong C-R, Lee H., Cole M., Carr C., Hauton D., McCullagh J, Evans R. D., Clarke K., On the interdependence of ketone body oxidation, glycogen content, glycolysis, and energy metabolism in the heart. J. Physiol. 601.7, 1207–1224 (2023). [doi: 10.1113/JP284270] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall A. G., “Biophysical Chemistry: Principles, Techniques, and Applications” (Wiley, New York, 1978). [Google Scholar]
- 45.Robinson R. A., Stokes R. H., in “Electrolyte Solutions” (Dover, Mineola, ed. 2nd rev., 1970). [Google Scholar]
- 46.Blandamer M. J., Engberts J. B. F. N., Gleeson P. T., Reis J. C. R., Activity of water in aqueous systems: A frequently neglected property.” Chem. Soc. Rev. 34, 440–458 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Park J. O., Rubin S. A., Xu Y-F., Amador-Noguez D., Fan J., Shlomi T., Rabinowitz J. D., Metabolite concentrations, fluxes, and free energies imply efficient enzyme usage. Nat. Chem. Biol. 12, 482–489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersen O. S., “Cellular electrolyte metabolism,” in Enclycopedia of Metalloproteins, Kretsinger R. H., Uversky V. N., Permiakov A., Eds. (Springer, 2013), pp. 580–587. [Google Scholar]
- 49.Milo R., What is the total number of protein molecules per cell volume? A call to rethink some published values. Bioessays. 35, 1050–1055 (2013). [DOI 10.1002/bies.201300066] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neidhardt F. C., Umbarger H. E., in Escherichia coli and Salmonella: Cellular and Molecular Biology, Neidhardt F. C., Ed. (Am. Soc. Microbiol. Press, New York, ed. 2, 1996), vol. 1, chapt. 3. [Google Scholar]
- 51.Vinnakota K. C., Bassingthwaighte J. B., Myocardial density and composition: A basis for calculating intracellular metabolite concentrations. Am. J. Physiol. Heart Circ. Physiol. 286, H1742–H1749 (2004). [ 10.1152/ajpheart.00478-2003] [DOI] [PubMed] [Google Scholar]
- 52.Wennerström H., Oliveberg M., On the osmotic pressure of cells. QRB Discovery. 3, e12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milo R., Phillips R., Orme N., “Cell Biology By the Numbers.” (Garland Science, New York, 2016), pp. 70, 92, 93, 96, 106, 130, 198. [Google Scholar]
- 54.Model M. A., Schonbrun E., Optical determination of intracellular water in apoptotic cells. J. Physiol. 591.23, 5843–5849 (2013). [DOI: 10.1113/jphysiol.2013.263228] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rooney W. D., Johnson G., Li X., Cohen E. R., Kim S-G., Ugurbil K., Springer C. S., Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn. Res. Med. 57, 308–318 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Rand R. P., Probing the role of water in protein conformation and function. Phil. Trans. R. Soc. Lond. B 359, 1277–1285 (2004). [DOI 10.1098/rstb.2004.1504] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ball P., Water is an active matrix of life for cell and molecular biology. Proc. Nat. Acad. Sci. 114: 13327–13335 (2017). [doi/ 10.1073/pnas.1703781114] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaplin M., Do we underestimate the importance of water in biology. Nat. Rev. Mol. Cell Biol. 7, 862–866 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Garlid K. D., The state of water in biological systems. Int. Rev. Cytology 192, 281–302 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Persson F., Halle B., Transient access to the protein interior: Simulation versus NMR. J. Am. Chem. Soc. 135, 8735–8748 (2013). [ 10.1021/ja403405d] [DOI] [PubMed] [Google Scholar]
- 61.Lu X. L., Mow V. C., Biomechanics of articular cartilage and determination of material properties. Med. Sci. Sports Exerc. 40, 193–199 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Nimer E., Schneiderman R., Maroudas A., Diffusion and partition of solutes in cartilage under static load. Biophys. Chem. 106, 125–146 (2003). [doi: 10.1016/S0301-4622(03)00157-1] [DOI] [PubMed] [Google Scholar]
- 63.Pitzer K. S., in “Activity Coefficients in Electrolyte Solutions” (CRC Press, Boca Raton, ed. 2, 1991). [Google Scholar]
- 64.Gao J., Sun X., Moore L. C., White T. W., Brink P. R., Mathias R. T., Lens intracellular pressure is generated by the circulation of sodium and modulated by gap junction coupling. J. Gen. Physiol. 137, 507 520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrie R. J., Koo H., Direct measurement of intracellular pressure. Curr. Protoc. Cell Biol. 63, 12.9.1–12.9.9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao J., Sun X., White T. W., Delamere N. A, Mathias R. T., Feedback regulation of intracellular hydrostatic pressure in surface cells of the lens. Biophys. J. 109, 1830–1839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LoCastro E., Paudyal R., Mazaheri Y., Hatzoglou V., Oh J. H., Lu Y., Konar A. S., vom Eigen K., Ho A., Ewing J. R., Lee N., Deasy J. O., Shukla-Dave A., Computational modeling of interstitial fluid pressure and velocity in head and neck cancer based on dynamic contrast-enhanced magnetic resonance imaging: Feasibility analysis. Tomog. 6, 129–138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DiNuzzo M., Dienel G. A., Behar K. L., Petroff O. A., Benveniste H., Hyder F., Giove F., Michaeli S., Mangia S., Herculano-Houzel S., Rothman D. L., Neurovascular coupling is optimized to compensate for the increase in proton production from nonoxidative glycolysis and glycogenolysis during brain activation and maintain homeostasis of pH, pCO2, and pO2. J. Neurochem. 00, 1–31 (2023). [DOI: 10.1111/jnc.15839] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chowdhury H. H., Differences in cytosolic glucose dynamics in astrocytes and adipocytes measured by FRET-based nanosensors. Biophys. Chem. 261, 106377 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Schomburg I., Chang A., Placzek S., Sohnigen C., Rother M., Lang M., Munaretto C., Ulas S., Stelzer M., Grote A., Scheer M., Schomberg D., BRENDA in 2013: Integrated reactions, kinetic data, enzyme function data, improved disease classification: New options and content in BRENDA. Nucleic Acids Res. 41, D764–D772 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patneau D. K., Mayer M. L., Structure-activity relationships for amino acid transmitter candidates acting at N-Methyl-D-aspartate and quisqualate Receptors. J. Neurosci. 10, 2385–2399 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bisignano P., Ghezzi C., Jo H., Polizzi N. F., Athoff T., Kalyanaraman C., Friemann R., Jacobson M. P., Wright E. M., Grabe M., Inhibitor binding mode and allosteric regulation of Na+-glucose symporters. Nature Comm. 9, 5245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jurcovicova J., Glucose transport in brain – Effect of inflammation. Endo. Reg. 48, 35–48 (2014). [doi: 10.4149/endo_2014_01_35] [DOI] [PubMed] [Google Scholar]
- 74.Lee J-H., Liu R., Li J., Wang Y., Tan L., Li X-J., Qian X., Zhang C., Xia Y., Xu D., Guo W., Ding Z., Du L., Zheng Y., Chen Q., Lorenzi P. L., Mills G. B., Jiang T., Lu Z., EGFR-phosphorylated platelet isoform of phosphofructokinase 1 promotes PI3K activation. Mol. Cell 70, 197–210 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu X., Sehgal A. A., Yadav N. N., Laterra J., Blair L., Blakeley J., Seidemo A., Coughlin J. M., Pomper M. G., Knutsson L., van Zijl P. C. M., D-glucose weighted chemical exchange saturation transfer (glucoCEST)-based dynamic glucose enhanced (DGE) MRI at 3T: Early experience in healthy volunteers and brain tumor patients. Magn. Reson. Med. 84, 247–262 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nasrallah F. A., Pagès G., Kuchel P. W., Golay X., Chaung K-H., Imaging brain deoxyglucose uptake and metabolism by GlucoCEST MRI. J. Cerebr. Blood Flow & Metabol. 33, 1270–1278 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith A. J., Jin B-J., Verkman A. S., Muddying the water in brain edema? Trends Neurosci. 38, 331–332 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seyfried T. N., Shelton L., Arismendi-Morillo G., Kalamian M., Elsakka A., Maroon J., Mukherjee P., Provocative question: Should ketogenic metabolic therapy become the standard of care for glioblastoma? Neurochem. Res. 44, 2392–2404 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Nath K., Nelson D. S., Heitjan D. F., Zhou R.. Leeper D. B., Glickson J. D., Effects of hyperglycemia on lonidamine-induced acidification and de-energization of human melanoma xenografts and sensitization to melphalan. (2015). NMR Biomed. 28, 395–403. [DOI: 10.1002/nbm.3260] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Callen H. B., “Thermodynamics and an introduction to thermostatistics.” (Wiley, New York, ed. 2, 1985). [Google Scholar]
- 81.Jia Y., Xu S., Han G., Wang B., Wang Z., Lan C., Zhao P., Gao M., Zhang Y., Jiang W., Qiu B., Liu R., Hsu Y-C., Sun Y., Liu C., Liu Y., Bai R., Transmembrane water-efflux rate measured by magnetic resonance imaging as a biomarker of the expression of aquaporin-4 in gliomas. Nat. Biomed. Eng. 7, 236–252 (2023).[ 10.1038/s41551-022-00960-9] [DOI] [PubMed] [Google Scholar]
- 82.Tanford C., “Physical Chemistry of Macromolecules.” (Wiley, New York, 1961). [Google Scholar]
- 83.Naito H. R., Okamoto R., Sumi T., Koga K., Osmotic second virial coefficients for hydrophobic interactions as a function of solute size. J. Chem. Phys. 156, 221104 (2022). [DOI] [PubMed] [Google Scholar]
- 84.Perez-Gonzalez N. A., Rochman N. D., Yao K., Tao J., Le M-T. T., Flanary S., Sablich L., Toler B., Crentsil E., Takaesu F., Lambrus B., Huang J., Fu V., Chengappa P., Jones T. M., Holland A. J., An S., Wirtz D., Petrie R. J., Guan K-L., Sun S. X., YAP and TAZ Regulate Cell Volume. J. Cell Biol. 218, 3472–3488 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sao K., Jones T. M., Doyle A. D., Maity D., Schevzov G., Chen Y., Gunning P. W., Petrie R. J., Myosin II governs intracellular pressure and traction by distinct tropomyosin-dependent mechanisms. Mol. Biol. Cell 30, 1170–1181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shahidullah M., Mandal A., Mathias R. T., Gao J., Križaj D., Redmon S., Delamere N. A., TRPV1 activation stimulates NKCC1 and increases hydrostatic pressure in the mouse lens. Am. J. Physiol. Cell Physiol. 318, C969–C980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pallotto M., Watkins P. V., Fubara B., Singer J. H., Briggman K. L., Extracellular space preservation aids the connectomic analysis of neural circuits. eLife 4, e08206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neuwelt E. A., Mechanisms of disease: The blood-brain barrier. Neurosurgery 54, 131–142 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Bai R., Springer C. S., Plenz D., Basser P. J., Fast, Na+/K+ pump driven, steady-state transcytolemmal water exchange in neuronal tissue: A study of rat brain cortical cultures. Magn. Reson. Med. 79, 3207–3217 (2018). [DOI: 10.1002/mrm.26980] [DOI] [PubMed] [Google Scholar]
- 90.Meli R., Pirozzi C., Pelagalli A., New perspectives on the potential role of aquaporins (AQPs) in the physiology of inflamation. Front. Physiol. 9, 101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King L. S., Kozono D., Agre P.. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 5, 687–698 (2004). [DOI] [PubMed] [Google Scholar]
- 92.Yang B., Verkman A. S., Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 272, 16140–16146 (1997). [DOI] [PubMed] [Google Scholar]
- 93.Hertz L., The glutamate-glutamine (GABA) cycle: Importance of late postnatal development and potential reciprocal interactions between biosynthesis and degration. Front. Endochrinol. 4, 59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koch G. W., Schwartz E., Isotopic labeling of metabolic water with 18O2. Rapid Comm. Mass Spectrom. 37, e9447 (2023). [DOI] [PubMed] [Google Scholar]
- 95.Kuang Q., Purhonen P., Hebert H., Structure of potassium channels. Cell. Mol. Life Sci. 72, 3677–3693 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rand R. P., Parsegian V. A., Rau D. C., Intracellular osmotic action. Cell. Mol. Life Sci. 57, 1018–1032 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wiebe H., Spooner J., Boon N., Deglint E., Edwards E., Dance P., and Weinberg N., Calculation of molecular volumes and volumes of activation using molecular dynamics simulations. J. Phys. Chem. C 116, 2240–2245 (2012). [ 10.1021/jp209088u] [DOI] [PubMed] [Google Scholar]
- 98.Hubbard C. D., van Eldik R., Mechanistic information on some inorganic and bioinorganic reactions from volume profile analysis. Inorg. Chim. Acta 363, 2357–2374 (2010). [doi: 10.1016/j.ica.2009.09.042] [DOI] [Google Scholar]
- 99.Drljaca A., Hubbard C. D., van Eldik R., Asano T., Basilevsky M. V., le Noble W. J., Activation and reaction volumes in solution. 3. Chem. Revs. 98, 2167–2289 (1998). [DOI] [PubMed] [Google Scholar]