Abstract
Contingency (or ‘luck’) in early life plays an important role in shaping individuals’ development. When individuals live within larger societies, social experiences may cause the importance of early contingencies to be magnified or dampened. Here we test the hypothesis that competition magnifies the importance of early contingency in a sex-specific manner by comparing the developmental trajectories of genetically identical, free-living mice who either experienced high levels of territorial competition (males) or did not (females). We show that male territoriality results in a competitive feedback loop that magnifies the importance of early contingency and pushes individuals onto divergent, self-reinforcing life trajectories, while the same process appears absent in females. Our results indicate that the strength of sexual selection may be self-limiting, as within-sex competition increases the importance of early life contingency, thereby reducing the ability of selection to lead to evolution. They also demonstrate the potential for contingency to lead to dramatic differences in life outcomes, even in the absence of any underlying differences in ability (‘merit’).
Contingency (colloquially called ‘luck’ or ‘chance’) has long been recognized as an important determinant of outcomes in ecology and evolution (and to varying degrees in other fields, including philosophy, sociology, and economics (1–15)). The contingency hypothesis posits that an individual’s behavior, health, social position, or fitness are strongly dependent on unpredictable, uncontrollable events and experiences that occur across its life, and even in the lives of relatives and other social contacts (5, 16–20). Contingent outcomes in early life are often especially important, as they can set individuals onto divergent, self-reinforcing trajectories (1, 2, 5, 17, 18, 20). Recent evolutionary theory has argued that luck in an individual’s life largely swamps the importance of individual quality in determining lifetime reproductive success, and that luck in early life is especially important for such outcomes (1, 2). This heightened importance of contingency in early life is consistent with the theoretical and observed limitations of phenotypic plasticity: although some plasticity is maintained throughout life, plasticity is greatest during development, and developmental decisions can restrict individuals’ future phenotypic options (18, 21–23).
Many animals naturally live within larger social groups, such that contingency in outcomes is inextricably tied to individuals’ relationship to the behavior of others within societies (5, 24–28). Through repeated social interactions, individuals adopt a consistent set of social phenotypes (i.e., their ‘social niche’ (25, 27, 29)). We hypothesize that competitive social processes magnify the importance of contingency in early life. For example, animals that begin with zero or small differences in competitive ability may differ in their access to resources due to variation in contingent dominance or territorial interactions (19, 30–33). The resulting increased resource access for a subset of the population then improves those animals’ condition relative to those with reduced resource access, further entrenching the initial differences and magnifying the importance of early contingency (19, 24, 33–36). This process is analogous to the ‘Matthew effect’ in the social sciences, a phenomenon by which individuals or institutions that achieve early success tend to achieve ever greater success in the future (37–39).
Experimentally studying of the role of contingency in individual outcomes is achievable with the use of ‘replicate individuals’ that allow researchers to effectively ‘replay the tape of life’ for a single genotype under different circumstances (40, 41). Studies of genetically identical animals living in the lab have demonstrated the feasibility of this approach (e.g., inbred mice: (42–44), inbred fruit flies: (45, 46), naturally clonal Amazon mollies: (47, 48)). In these studies, small between-individual differences in early behavior increase in magnitude over time, despite animals’ sharing nearly identical genetics and macro-environments (42–48). Yet, assessing the ways in which competitive social processes magnify or dampen the impact of early contingency requires the study of replicate individuals living under realistic, complex, dynamic social conditions—requirements that cannot be readily met under standardized laboratory conditions (49–51).
Here we overcome this limitation by studying the development of spatial and social behavior in age-matched, genetically identical mice living outside in a large, shared macro-environment. From infancy through adulthood, we tracked the development of the magnitude of individual differences in ecologically relevant social and spatial behaviors among genetically identical mice living under semi-natural field conditions (Fig S1).
We make two contributions to understanding the development of individuality and the role that contingency and competitive processes play in that development. First, we present the most detailed data to date on the development of semi-natural spatial and social behaviors in the prototypical biomedical model mammal. We use this data to assess the developmental timing of the emergence of individuality in genetically identical animals across a wide range of spatial and social behaviors (Fig S2). Second, we show that small initial differences in males’ ability to acquire and defend territories causes males—but not females—to enter onto divergent, self-reinforcing behavioral trajectories, with downstream impacts on a wide range of male social and spatial behaviors (Figs. 1–2). These divergent trajectories cause individual males to assume their individually distinct adult behavioral phenotypes at an earlier age than do females. Using quantitative agent-based simulations we also show that these differences between males and females can be wholly explained by sex-specific social feedback loops, which magnify the importance of early contingent experiences and shape the timing of the development of individuality (Fig 1).
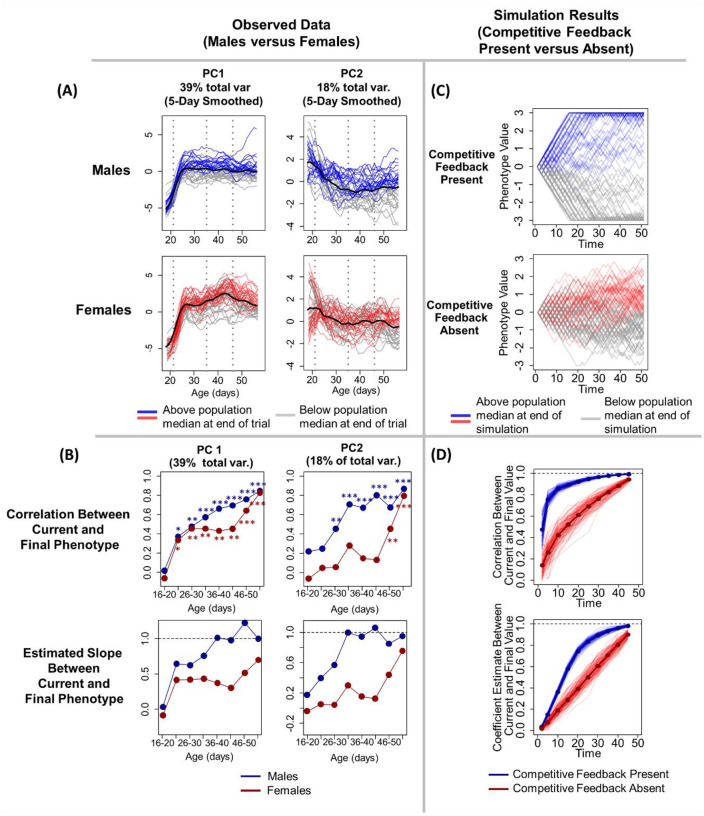
Figure 1. Males adopt their adult phenotypes earlier than females.
(A) Traces of observed individual behavioral PC1 and PC2 values, smoothed over five days, across animals’ development. Lines are color-coded to indicate individuals’ behavior during the last three days of the experiment (age 56–58), with lines representing animals that displayed higher than median phenotype during this period in red or blue and animals that displayed lower than median phenotype in grey. (B, first row) The correlation between earlier and adult behavior is stronger in males for both PC1 (left column) and PC2 (right column). The y-axis represents the correlation coefficient between individuals’ behavior at the age-window on the x-axis and their behavior at the end of the experiment (age 56–68 days). Asterisks denote significance of the correlations depicted in each point (* p < 0.05, ** p < 0.01, *** p < 0.001). (B, second row) The slope of the relationship between earlier and adult behavior (y-axis). The slope of this relationship is consistently closer to 1 for males than for females. (C-D) Each of the observed results in (A) and (B) are closely mirrored by agent-based simulations in which simulated individuals’ phenotypes develop either in the presence (blue) or absence (red) of competitive feedback mechanisms. (C) Traces of individual phenotypes from a single run of the simulation, with shading of traces matching (A). (D) Results from 1,000 iterations of the simulation. Comparable to (B), relationships between current and future behavior are stronger when competitive feedback is present. Detailed description of the simulation can be found in the main text and methods.
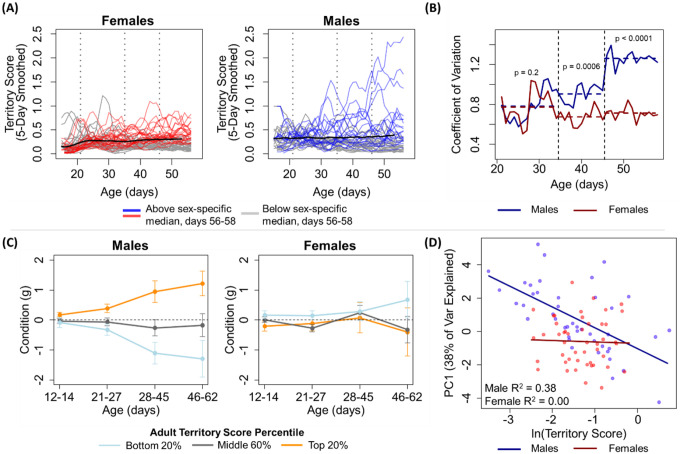
Figure 2. Territorial competition shapes males’ phenotypes through a sex-specific competitive feedback loop.
(A) Traces of individual territory score values, smoothed over five days, across animals’ development. Lines are color-coded to indicate individuals’ scores during the last three days of the experiment (age 56–58), with lines of animals that display higher than median scores during this period in red or blue and animals that display lower than median phenotype in grey. Black lines indicate sex-specific means. Vertical dashed lines represent indicate approximate ages of weaning (‘juvenility’, 21 days), sexual maturation (‘adolescence’, 35 days), and onset of conceptive mating (‘adulthood’, 46 days) (B) Male territory scores are more variable across individuals than female territory scores, a significant difference that emerges concurrent with sexual maturity and further increases following the onset of successful mating. Horizontal dashed line segments indicate the average coefficient of variation across the juvenile, adolescent, and adult stages. (C) Male adult territory scores are predicted by small differences in body mass in early life, a difference that is magnified over time. The relationship is absent in females. The y-axes represent deviations from age-predicted body mass. (D) Adult territory score strongly predicts an integrative measure (PC1) of the 16 other spatial and social phenotypes in males but not in females.
Males Develop Individual Adult Behavioral Phenotypes Earlier than Females.
We first assessed the developmental timing of individually distinct behavioral phenotypes and whether this timing differed in males and females. In free-living C57BL/6J lab mice, males compete for territorial control and resource access while females do not (52, 53). We hypothesized that this difference in competitive experiences should cause males to diverge onto self-reinforcing developmental trajectories as some males won competitive interactions and others lost. We expect this same dynamic to be absent in females.
Over seven weeks from May to June 2022, we allowed 16 litters (n = 104 pups, 90 survived to adulthood) of the C57BL/6J lab mouse strain to develop from infancy through adulthood outside in a large (~560 m2) enclosure that emulates the natural foraging and social environment of commensal house mice (Figure S1A–C). We placed litters of 2-week-old RFID microchipped pups and their mothers inside nest boxes within one of 16 “resource zones” monitored with RFID antennae. We inferred periods of social overlap using an established workflow to translate RFID positional data into estimates of the duration of social aggregations within each of the 16 monitored resource zones (Figure S1D, see (52) for details). Based on a total of 7.4 million RFID reads, we traced the development of 17 social and spatial phenotypes from infancy through adulthood (~6.5 weeks, 14–58 days of age, Table S1), after which we trapped out the mice and terminated the experiment.
We first leveraged our detailed behavioral dataset to answer an outstanding question in social behavioral ecology—whether genetically identical animals display distinct, individually repeatable social behaviors under ecologically relevant contexts and, if so, when these differences emerge (40, 41, 47, 48, 54, 55). We measured repeatability as the proportion of a phenotype’s total variation in each sex that was explained by individual identity (56) over a sliding five-day age window, after controlling for maternal/litter identity. In total we assessed 17 phenotypes including basic measures of movement patterns (e.g., the number of nightly resource zones that an animal visited), measures of social phenotypes (e.g., the nightly number of opposite-sex animals encountered and territory scores), and derived social network measures (e.g., eigen vector centrality, see Table S1 for a description of phenotypes). We detect significant repeatability in all measured phenotypes, for both sexes, with repeatability emerging as early as age 21 days (Fig S2), roughly 1 month earlier than reported for spatial behavior of female populations of this strain in enriched lab vivaria (11, 30). Animals’ behavior was repeatable prior to sexual maturity (~age 35 days) for 15 of 17 phenotypes in males and 16 of 17 phenotypes in females (range = 21–55 days, median = 26 days, see Fig S2 G–H).
Having established that genetically identical mice still displayed strongly repeatable individual suites of behavior, we next assessed the developmental timing at which males and females assumed their individually distinct adult behavioral phenotypes. To generate an integrative measure of animals’ spatial and social behavior, we used principal component analysis to reduce the dimensionality of 16 of our 17 behavioral phenotypes into two principal components that accounted for a majority of the total variation in our dataset (57% total across PC1 and PC2; ‘time of first nightly transition’ phenotype could not be included because values were missing before animals began moving between zones). Many phenotypes were loaded onto PCs 1 and 2 without any one phenotype being particularly influential (see Table S2 for full loading information).
We identified animals’ final adult behavioral phenotypes by taking the average of each individuals’ PC1 and PC2 scores during the last three days of the experiment (age 56–58 days, Fig 1A). For each sex we then assessed the relationship between individuals’ phenotypes at earlier time points and these final adult behavioral phenotypes by building linear regressions between final adult phenotype and individuals’ average phenotypes over five-day, non-overlapping windows (e.g. age 21–25 days, 26–30 days, etc.). From each of these models we extracted the correlation between earlier and adult phenotype and the estimated slope of the relationship between earlier and later phenotypes (Fig 1B).
For both PC1 and PC2, males assumed their individually distinct adult behavioral phenotypes earlier than females. Male behavior became predictive (p < 0.05) of final adult behavior at or before females (PC1: 26 days vs. 26 days, PC2: 31 days vs 46 days, Fig 1B). The strength of the correlation between earlier and later behavior for the same individual was also substantially higher for males than for females across most or all of development (PC1: days 31–50; PC2: days 16–50, Fig 1B). Males’ behavior at earlier ages also more closely aligned with their adult behavior in absolute terms. That is, the slope of the linear relationship between earlier behavior and adult behavior was closer to 1 for males than for females, and this strong relationship developed at an earlier age for both PC1 and PC2 (Fig 1B). We performed this principal component analysis using all daily phenotype data for both males and females, but all of the above results hold if we instead generate separate sex-specific PC values (Figure S3)
To aid in interpretation of Figure 1B, we provide an example of one set of models (Figure S4), comparing the strength of the relationship between behavior immediately after sexual maturation (ages 36–40 days) and individuals future behavior in adulthood (days 55–58) for males and females. Here the strength of the relationship is much stronger for males for both PC1 (male R2 = 0.43; female R2 = 0.18) and PC2 (male R2 = 0.44; female R2 = 0.02). And the slopes of the relationship between earlier and later behavior is much closer to 1.00 for males than for females for both PC1 (male estimate = 1.01, 95% CI = 0.6–1.4; female estimate = 0.37, 95% CI = 0.1–0.6, non-overlapping with males) and for PC2 (male estimate = 0.95, 95% CI = 0.6–1.3; female estimate = 0.2, 95% CI = −0.2–0.5, non-overlapping with males).
We next assessed whether these sex differences in the developmental timing of individuality could be fully explained by sex-specific difference in the importance of competitive feedback in amplifying the importance of early contingency. To do so, we built a quantitative agent-based model to generate empirical predictions of how competitive processes shape the long-term phenotypic impacts of early contingency (Fig 1C–D). In this model we assumed that all individuals start with the same value of a phenotype. Individuals’ phenotypic value then changes at discrete timesteps across their lives, with the nature of that change depending on the presence or absence of competitive feedback. In simulations where competitive feedback was absent, the direction of change in phenotypic value was randomly chosen to be positive or negative. When competitive feedback was present, an individual’s phenotype increased in value if it won a competitive interaction with a randomly chosen individual and declined if they lost. The probability of winning the interaction was dependent on the relative phenotypic values of the two interactants (see methods for complete details).
The results of the simulation closely mirror our observations of differences in the development of individuality in males and females in our system. Specifically, when competitive feedback loops are present (i) the correlation between behavior at any given time and behavior at the end of the modeled period is stronger, and (ii) the slope of the relationship between earlier and later behavior is closer to 1.0. Thus, it appears that the sex-difference that we observe in the development of behavioral individuality could be entirely explained by differences in sex-specific competitive processes that amplify contingent early life differences in phenotype.
Territoriality acts as a sex-specific competitive feedback loop
We next assessed whether males and females displayed differences in the strength of resource competition in a fashion that would support this putative sex-biased competitive feedback loop indicated by the model analysis in Figure 1. To do so, we estimated individuals’ nightly resource access by calculating a nightly territory score for each animal (see methods). Consistent with males and females experiencing different levels of competition for resource access, territory score varied more among males than it did among females (Fig 2A). This difference emerged concurrently with the onset of sexual maturity, the period when we expect intrasexual competition to increase in intensity. Although variation in territory score is comparable for males and females during the juvenile period (ratio of variance = 1.5, p = 0.2, two-sided F test), following the onset of sexual maturation (~age 35 days), males displayed significantly higher variation than did females (ratio = 2.9, p = 0.0006), a difference that further increased following the onset of successful mating (~age 46 days, ratio = 3.6, p < 0.0001, Fig2A).
Two additional pieces of evidence are consistent with males, but not females, experiencing strong competitive feedback that set them on self-reinforcing divergent life trajectories. First, small individual differences in early body mass (measured, days 21–27) predicted adult (days 46–58) territory scores for males (p < 0.05, see Fig 2C, Fig S5) but not for females. Prior to release into the enclosure, very minor differences in infant body condition did not predict future territory access in either sex (days 12–14 compared to adult, p > 0.05, Fig S5), consistent with individuals starting out on an approximately ‘even playing field’. The magnitude of the differences in body mass between males with differential resource access then increased over development (age × adult territory score interaction: p = 0.004, Fig 3C, S5), consistent with males experiencing a competitive feedback loop that increased the condition of winners relative to losers. This developmental pattern was absent in females (p = 0.19, Fig 3C). In adulthood, this mass measure in females is partially confounded by pregnancy status, but at no point (even before any pregnancies began) was there a relationship between body condition in females and adult territory status (unlike in males, Fig 2C).
Second, male territory scores strongly predicted the rest of males’ behavioral phenotypes, while the same was not true of females, indicating that our measure of competition in males has major impacts on males’ daily behaviors and ability to reproduce. Specifically, we performed a principal component analysis using the other 16 phenotypes that we measured in adulthood (i.e., excluding territory score) to obtain a single integrative measure of animals’ other behavior (PC1 explained 41% of the total variation in this dataset). Males’ adult territory scores strongly predicted this adult PC1 value (Fig 2D, p < 0.0001, R2 = 0.38), while females’ territory scores did not (Fig 2D, p = 0.86, R2 = 0.00). The same conclusion holds if we assess individual adult phenotypes, rather than this principal component measure (see Table S3 for all 16 comparisons). Individuals’ access to members of the opposite sex provides a particularly striking, fitness-relevant example from this more granular analysis: for males, the average number of females met on a given night is very strongly, positively associated with territory score (R2 = 0.67, p < 0.0001), while territory score does not predict the number of males met by females (R2 = 0, p = 0.7).
Thus, male territorial competition appears to act as a sex-specific competitive feedback loop. Small initial differences in male body mass became magnified over time, depending on territorial control. Male territorial control then had downstream implications for a wide range of ecologically and fitness relevant spatial and social behaviors.
Discussion:
Our results provide empirical support for the hypothesis that contingency (or ‘luck’) in early life can have a major and sex-specific impact on the development of animals’ individual differences in social and fitness-relevant phenotypes. Sex-specific competitive feedback loops magnify the importance of contingency experienced early in life, such that young free-living male lab mice enter onto divergent, self-reinforcing developmental trajectories. Our interpretation of our empirical results is supported by their match to an agent-based simulation of expected differences in the developmental timing of individuality in the presence and absence of competitive feedback. We expect the sex-specificity of such competitive feedback loops to vary across different species, depending on a given species’ specific social behavioral ecology. For example, in hyenas and other female-dominant species, we would expect the reverse pattern to be present, such that females’ outcomes to be more dependent on early luck (e.g. the relative social status of the matriline into which they were born, (57, 58)) than males’ outcomes.
Our results suggest an inevitable limitation of sexual selection to shape behaviors. Intrasexual selection relies on within-sex competition resulting in differential reproductive success, and for variation in this success to be heritable (59–61). But here we have shown that intrasexual competition also magnifies the importance of contingency in later life outcomes in the sex expressing that competition. As the importance of luck in determining individual phenotypic outcomes increases, selection’s ability to cause evolution declines (1, 2). Thus, intrasexual selection may be self-limiting, as an increase in the importance of competition in a single sex leads in turn to an increase in the importance of contingency in determining individual outcomes. And to the extent that intersexual choice is at least partially dependent on intrasexual competition, we expect the increased importance of luck to act as a limit on the effectiveness of intersexual selection as well. This increased importance of luck in systems with intrasexual competition may help to explain why sexual selection fails to fully deplete genetic variation, despite strong selection imposed by mate choice and intrasexual competition (i.e., the lek paradox (62–64)).
Our results provide a strong biological analog to the Matthew Effect, an often-observed phenomenon in social science whereby small individual advantages earlier in life are correlated with ever larger advantages over time (37–39). Such processes are understood to be the result of social feedback mechanisms, by which an individual’s initial success improves their opportunities for future success as well as the perception by other members of society of the individual’s potential for success (39, 65). The extent to which Matthew Effects are specific to human societies has remained an open question (39). Our results suggest that Matthew Effects (i) may have a biological origin, (ii) are especially likely to occur in highly competitive environments or among groups that face high levels of competition, and (iii) may emerge even in the absence of any variation in underlying individual quality or ability.
The sources of inequality in human society are of central interest to both moral philosophy and public policy (66–68). As with reproductive success in non-human animals, human outcomes are likely to be partially explained by differences in genotypes (69). However, we show here that even among isogenic animals, individuals still attain dramatically different phenotypic and fitness outcomes. Our results add to sociological and biological literature that underscore the potential importance of unpredictable, uncontrollable experiences in generating differences in outcomes even when differences in underlying quality (or ‘talent’) are small or non-existent (12, 15, 16).
Materials and Methods:
Field Enclosure and RFID Data Collection:
A detailed description of the enclosures at Cornell University’s Liddell Field Station can be found elsewhere (52), so here we only describe those elements critical to the success of this experiment. The enclosure is 15m × 38m, approximately 9,000 times the area of a typical mouse cage. Within the enclosure we set up 16 plastic tubs (31 gallon storage totes, Rubbermaid, USA), placed into four neighborhoods of (Figure S1E). Each tub (hereafter “resource zones”) contained ad libitum food access along with a nestbox that provided insulation and shelter from adverse weather conditions. We equipped each zone with a single joint entrance/exit made out of a 6-inch-long PVC pipe (2” in diameter). These resources and the single entrance made the resource zones highly valuable, defendable areas that are meant to mimic the foraging landscape of commensal mice. To track the comings and goings of mouse visitors to each zone, we placed a 10-inch RFID antennae (Biomark, USA), beneath the entrance tube of each zone. The antennas were connected to a central monitoring system (Small Scale System, Biomark, USA) and transmitted RFID reads at a rate of 2–3 Hz.
Study Subjects:
We bred 16 litters of C57BL/6J mice by pairing 9-week old virgin males and females that we ordered from Jackson Laboratory (Bar Harbor, ME). We timed pregnancies such that all litters were born within 48 hours of each other, allowing infants to be approximately age-matched. Males were removed from breeding cages two weeks after pairing to prevent mothers from becoming pregnant again following parturition.
When pups were 8–10 days of age, we anesthetized litters and their mothers using isoflurane and injected either 1 (pups) or 2 (mothers) PIT tags (Biomark Mini HPT10) subcutaneously. When animals were 12–14 days old (12: n = 49 pups, 13: n = 51 pups, 14: n = 4 pups) we placed litters along with their mothers and their nesting material in cardboard transfer containers. We then transferred litters and mothers to our field enclosures and placed them inside of nest boxes within resource zones. We balanced litter sizes across the four neighborhoods, placing 26 pups and 4 mothers in each neighborhood. 3 neighborhoods had litter sizes of 5, 6, 7 and 8 pups. The final neighborhood had litter sizes of 4, 6, 7, and 9 pups.
We then allowed animals to develop and live largely undisturbed (but see “Mass Measures” below) for 46 days, at which point we terminated the experiment. We selected this length of time to prevent animals from giving birth in our enclosure. In total, 85 of the 104 pups that we placed outside survived until the last three days prior to the end of the experiment (survival rate = 82%). These animals’ data were included in all analyses. 5 other animals survived until at least 46 days of age (the onset of conceptive mating). These animals (n = 90 total) were included in all analyses except those appearing in Figure 4. An additional 5 animals (n = 95 total) survived until 30 days of age, and we included these animals’ data in the repeatability analyses presented in Figure 2.
Mass Measures:
When pups were all 21–23 days of age, we caught by hand all living animals in the enclosure to measure weaning mass. At this time, we also removed half of the mothers living within the enclosure. This manipulation was performed to assess whether our animals relied on post-weaning maternal care. Though we do not discuss these results in detail here, there was no impact of this manipulation on dispersal, survival, juvenile behavior, or adult behavior. Following mass measures, all other animals were returned to the resource zone in which they were found.
For the remainder of the experiment, we opportunistically caught animals by hand and took mass measures. For five days a week we caught all animals present within and immediately below resource zones from a single neighborhood. We rotated neighborhoods each day to try to prevent discouraging animals from using the resource zones as a result of frequent disturbance.
On two occasions we re-captured an animal who had lost its PIT tag. To prevent these animals from making unmeasured contributions to the social environment, we humanely euthanized these animals. On one occasion we re-captured an animal that appeared to be in poor physical condition. We humanely euthanized this animal to prevent future suffering. All other animals were returned to the resource zone in which we found them.
At the conclusion of the experiment, we placed 48 Sherman live traps in resource zones for three nights until all animals were successfully caught (all animals aged 61–64 days at time of trapping). In the mornings following trapping we humanely euthanized animals, took their body masses for a final time and then dissected and preserved a range of tissues for future analysis. We placed fewer traps than there were animals due to logistical constraints on the number of daily dissections that we could perform.
Contingency Model (Fig 1C–D):
To assess how the presence or absence of competitive feedback magnifies or dampens the importance of early-life contingency on later life outcomes we built a probabilistic, agent-based model.
We simulated populations of 100 replicate individuals that each began with an identical value (0) of a given phenotype. We assumed that the phenotype had a range of possible values, which we took to be [−3,3]. Individuals’ phenotypes then changed contingently over a series of time steps. At each time step, phenotypic values for each individual increased or decreased by a value drawn from a normal distribution centered around 0.2 with a standard deviation of 0.02. Individuals continued to change their phenotypic value for 50 time steps, at which point the simulation ended.
At each timestep, half of the individuals in our populations increased their phenotypic value and the other half decreased their phenotypic value. Deciding the direction of these changes proceed by the following steps. First, at each timestep the 100 individuals in a population were randomly placed into pairs. Deciding the direction of phenotypic change for each member of the pair depended on the presence or absence of competitive feedback in the population.
In the absence of a role for competition in the development process, the individual whose value increased was selected at random. This approach is meant to simulate the impact of short-term contingency, with individuals increasing or decreasing their phenotype as a result of recent differences in the animal’s internal state or by recent non-social environmental experiences (24).
In the presence of a competitive feedback loop, the identity of the animal whose phenotype increased was determined by a contest. The probability of an animal winning the contest was dependent on the difference in phenotypic values of the two animals in the pair.
Specifically, the probability of the first animal in a pair winning a contest was:
With p(win1) being truncated at 0 and 1. Thus, any contest between animals whose difference in phenotypic values was greater than or equal to 1 had a deterministic outcome. Contests that had smaller differences in values between contestants were probabilistic.
This approach is meant to model a competitive phenotype that determines access to resources. The probability of an individual’s phenotype increasing or decreasing depends on the outcome of a contest, which in turn depends on the value of its phenotype compared to another individual in the population. The winner of this contest acquires additional access to resources, which in turn increases its competitive ability and its phenotypic value (thus generating a competitive feedback loop (19, 34)).
To generate the data in Figure 1D, we built linear models in which each individual’s final (time = 50) phenotype value was the response variable and the predictor variable was each individual’s phenotype value at an earlier point in time (times = {2, 5, 10, 15, 20, 25, 30, 35, 40, 45}). We then extracted the correlation coefficients and slope estimates from the relationship between phenotype values at these different points in time and individuals’ final phenotypes.
Statistical Analyses
Analysis of Acquisition of Adult Behavior in Males and Females (Fig 1, S2–S3).
We first performed a principal components analysis to reduce the number of behavioral phenotypes in our population to two variables that explained a majority of the total variation in our dataset (PC1: 39% of variation explained, PC2 18% of variation explained). In this principal component analysis, we included 16 of our 17 measured daily phenotypes for all individuals from ages 14–58 days.
We then assessed the relationship between individuals’ behavior during a given period and their eventual final behavior in the experiment (measured during the last three days for which the youngest animals were present, age 56–58 days).
As in the agent-based simulation (above), we built a series of linear models for each sex where the response variable was each individual’s average PC1 or PC2 value during age 56–58 days and the predictor variable was each individual’s average value during a series of non-overlapping 5 day periods (16–20, 21–25, 26–30, 31–35, 36–40, 41–45, 46–50 and 51–55 days). We extracted the correlation coefficients and slopes of the relationships between earlier and later behavior.
Repeatability Analyses (Fig 3, S2):
We used the function rptGaussian (packaged ‘rptR’ (70)) to calculate repeatability estimates for behavioral measures. We calculated sex-specific repeatability across sliding 5-day age windows. We included pup age as a fixed effect and maternal/litter identity as a random effect in models with which repeatability was estimated. Thus, our repeatability estimates are the proportion of the variance in a five-day dataset of a given behavioral measure that is explained by pup identity, after controlling for pup age and maternal/litter identity.
To measure the age at which repeatability in a phenotype emerged (Figure 2C), we identified the earliest age at which animals displayed significant (p < 0.05) repeatability in a given phenotype and then continued to be repeatable thereafter for the rest of the experiment.
Territoriality Analysis (Figure 2, S5):
We calculated a nightly territory score for each animal. To do so, we calculated the proportion of sex-specific nightly RFID reads at a given resource zone that originated from each animal. We then summed these values across each of the sixteen resource zones for each animal to calculate a measure of total nightly resource access. For example, if there were 5000 male-sourced RFID reads at Resource Zone 1 on night 30 of the experiment and 4500 of them came from Male 1, Male 1 was assigned a value of 0.9 for Resource Zone 1 for that night. If Male 1 also visited exactly one other zone, where he accounted for 40% of the male-sourced RFID reads at that zone, his total value of this measure for the night (hereafter ‘territory score’) would be 1.30.
We measured coefficients of variation in territory scores for each day from age 15 to 58. To assess differences in variance in males’ and females’ territory scores we calculated each individuals’ average territory score across three different periods: (i) age 21–34 days, (ii) age 35–45 days, and (iii) age 46–58 days. We then performed a difference in variance test for square-root transformed average territory score values for the two sexes during each of these three periods (‘var.test’ function).
For each sex we then built a mixed effects linear model (function ‘glmmTMB’ (71)) with body condition as the response variable, predicted by the interaction between the age at which mass was measured and the log of animals’ average territory score during age 46–58 days (Fig 3C). We also included a random effect of animal ID, with age nested within ID as a random slope.
Finally, we performed a principal components analysis using all behavioral data from the 46–58 day-old period, excluding territory score. We then built linear models for each sex in which the response variable was an individual’s first principal component value (PC 1 explained 38% of the total variance in the dataset) and the predictor variable was the natural log of the individual’s average territory score in adulthood.
Supplementary Material
Acknowledgments:
We gratefully acknowledge our sources of funding that made this work possible. MNZ has been supported by an NSF postdoctoral fellowship in biology (award # 2109636) and a Klarman postdoctoral research fellowship from Cornell University. CCV is supported by a Mong Neurotechnology Fellowship from Cornell University. This work was also supported by Pilot and Feasibility awards to MNZ and MJS from the Animal Models for the Social Dimensions of Health and Aging Network (project #5R24AG065172-03). The costs of care for the mouse colony were supported in part by R35 GM138284 to Andrew Moeller.
Footnotes
Data and Code Availability:
While this manuscript is under review, all data and code supporting this manuscript and its analyses can be found in this Box folder: https://cornell.box.com/s/9jz4hp4kl1wnxhb5u3q275m650kfub7x
References:
- 1.Snyder R. E., Ellner S. P., Hooker G., Time and chance: using age partitioning to understand how luck drives variation in reproductive success. American Naturalist 197 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Snyder R. E., Ellner S. P., Pluck or luck: Does trait variation or chance drive variation in lifetime reproductive success? American Naturalist 191 (2018). [DOI] [PubMed] [Google Scholar]
- 3.da Col G., Introduction: Natural philosophies of fortune-luck, vitality, and uncontrolled relatedness. Social Analysis 56 (2012). [Google Scholar]
- 4.Losos J. B., Improbable Destinies: Fate, Chance, and the Future of Evolution (Riverhead Books, New York, 2017). [Google Scholar]
- 5.Bengtson N. M., Elder V. L. Jr, H. G., & Putney, “The life course perspective on ageing: Linked lives, timing, and history. Adult lives: A life course perspective.” in Adult Lives: A Life Course Perspective. (2012). [Google Scholar]
- 6.Gould S. J., Wonderful Life: The Burgess Shale and the Nature of History (WW Norton & Co, New York, 1989). [Google Scholar]
- 7.Kimura M., The Neutral Theory of Molecular Evolution (Cambridge University Press, Cambridge, 1983). [Google Scholar]
- 8.Snyder R. E., Ellner S. P., We happy few: Using structured population models to identify the decisive events in the lives of exceptional individuals. American Naturalist 188 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Johnson C., Darwin’s Dice: The Idea of Chance in the Thought of Charles Darwin (Oxford University Press, Oxford, 2014). [Google Scholar]
- 10.Rescher N., Luck: The Brilliant Randomness of Everyday Life (University of Pittsburgh Press, Pittsburgh, PA, 2001). [Google Scholar]
- 11.Frank R. H., Success and Luck: Good Fortune and the Myth of Meritocracy (Princeton University Press, Princeton, NJ, 2016). [Google Scholar]
- 12.Sauder M., A Sociology of Luck. Sociol Theory 38 (2020). [Google Scholar]
- 13.Chetty R., Hendren N., Katz L. F., The effects of exposure to better neighborhoods on children: New evidence from the moving to opportunity experiment. American Economic Review 106 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Chetty R., Hendren N., Kline P., Saez E., Where is the land of opportunity? The geography of intergenerational mobility in the United States. Quarterly Journal of Economics 129 (2014). [Google Scholar]
- 15.Gladwell M., The Tipping Point: How Little Things Can Make a Big Difference (2002)vol. 15. [Google Scholar]
- 16.Sapolsky R., Behave: The Biology of Humans at Our Best and Worst (Penguin Press, New York, 2017). [Google Scholar]
- 17.Lindström J., Early development and fitness in birds and mammals. Trends Ecol Evol 14 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Monaghan P., Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 1635–1645 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith J. E., Natterson-Horowitz B., Mueller M. M., Alfaro M. E., Mechanisms of equality and inequality in mammalian societies. Philosophical Transactions of the Royal Society B: Biological Sciences 378 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sapolsky R., Scars and PARS in a close relative. Proceedings of the National Academy of Sciences 121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West-Eberhard M. J., Developmental Plasticity and Evolution (Oxford University Press, Oxford, 2003). [Google Scholar]
- 22.Uller T., Developmental plasticity and the evolution of parental effects. Trends Ecol Evol 23, 432–438 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Kuzawa C. W., Which environments matter in studies of early life developmental plasticity? Evol Med Public Health 2017, 188–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sih A., Mathot K. J., Moirón M., Montiglio P. O., Wolf M., Dingemanse N. J., Animal personality and state-behaviour feedbacks: A review and guide for empiricists. Trends Ecol Evol 30 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Laskowski K. L., Chang C. C., Sheehy K., Aguin tild aga J., Consistent Individual Behavioral Variation: What Do We Know and Where Are We Going? Annu Rev Ecol Evol Syst 53 (2022). [Google Scholar]
- 26.Wolf J. B., Brodie E. D., Moore A. J., Interacting phenotypes and the evolutionary process. II. Selection resulting from social interactions. American Naturalist 153 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Bergmüller R., Taborsky M., Animal personality due to social niche specialisation. Trends Ecol Evol 25 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Gartland L. A., Firth J. A., Laskowski K. L., Jeanson R., Ioannou C. C., Sociability as a personality trait in animals: methods, causes and consequences. Biological Reviews 97 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Saltz J. B., Geiger A. P., Anderson R., Johnson B., Marren R., What, if anything, is a social niche? Evol Ecol 30 (2016). [Google Scholar]
- 30.Ostfeld R. S., The ecology of territoriality in small mammals. Trends Ecol Evol 5 (1990). [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann J. H., On the definitions and functions of dominance and territoriality. Biological Reviews 58 (1983). [Google Scholar]
- 32.Ord T. J., Costs of territoriality: a review of hypotheses, meta-analysis, and field study. Oecologia 197 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Smith J. E., Natterson-Horowitz B., Alfaro M. E., The nature of privilege: Intergenerational wealth in animal societies. Behavioral Ecology 33 (2022). [Google Scholar]
- 34.Strauss E. D., Shizuka D., The ecology of wealth inequality in animal societies. Proceedings of the Royal Society B: Biological Sciences 289 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dugatkin L. A., Earley R. L., Individual recognition, dominance hierarchies and winner and loser effects. Proceedings of the Royal Society B: Biological Sciences 271 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutte C., Taborsky M., Brinkhof M. W. G., What sets the odds of winning and losing? Trends Ecol Evol 21 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Merton R. K., The matthew effect in science. Science (1979) 159 (1968). [PubMed] [Google Scholar]
- 38.Bol T., De Vaan M., Van De Rijt A., The Matthew effect in science funding. Proc Natl Acad Sci U S A 115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigney D., The Matthew Effect: How Advantage Begets Further Advantage (Columbia University Press, New York, 2010). [Google Scholar]
- 40.Stamps J., Groothuis T. G. G., The development of animal personality: Relevance, concepts and perspectives. Biological Reviews 85 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Stamps J. A., Groothuis T. G. G., Developmental perspectives on personality: Implications for ecological and evolutionary studies of individual differences. Philosophical Transactions of the Royal Society B: Biological Sciences 365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freund J., Brandmaier A., Lewejohann L., Kirste I., Kritzler M., Krüger A., Sachser N., Lindenberger U., Kempermann G., Emergence of individuality in genetically identical mice. Science (1979) 340, 756–759 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Zocher S., Schilling S., Grzyb A. N., Adusumilli V. S., Lopes J. B., Günther S., Overall R. W., Winter Y., Kempermann G., Early-life environmental enrichment generates persistent individualized behavior in mice. Sci Adv 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kempermann G., Lopes J. B., Zocher S., Schilling S., Ehret F., Garthe A., Karasinsky A., Brandmaier A. M., Lindenberger U., Winter Y., Overall R. W., The individuality paradigm: Automated longitudinal activity tracking of large cohorts of genetically identical mice in an enriched environment. Neurobiol Dis 175, 105916 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Kain J. S., Stokes C., De Bivort B. L., Phototactic personality in fruit flies and its suppression by serotonin and white. Proc Natl Acad Sci U S A 109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Bivort B., Buchanan S., Skutt-Kakaria K., Gajda E., Ayroles J., O’Leary C., Reimers P., Akhund-Zade J., Senft R., Maloney R., Ho S., Werkhoven Z., Smith M. A. Y., Precise Quantification of Behavioral Individuality From 80 Million Decisions Across 183,000 Flies. Front Behav Neurosci 16 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bierbach D., Laskowski K. L., Wolf M., Behavioural individuality in clonal fish arises despite near-identical rearing conditions. Nat Commun 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laskowski K. L., Bierbach D., Jolles J. W., Doran C., Wolf M., The emergence and development of behavioral individuality in clonal fish. Nat Commun 13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lahvis G., Point of view: Unbridle biomedical research from the laboratory cage. Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lahvis G., Animal welfare: Make animal models more meaningful. Nature 543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zipple M., Vogt C., Sheehan M., Re-wilding Model Organisms: Opportunities to test causal mechanisms in social determinants of health and aging. Neurbiology and BioBehavioral Reviews (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogt C. C., Zipple M. N., Sprockett D. D., Miller C. H., Hardy S. X., Arthur M. K., Greenstein A. M., Colvin M. S., Michel L. M., Moeller A. H., Sheehan M. J., Spatial and social structure of rewilded laboratory mice. bioRxiv, 2022.04.19.488643 (2023). [Google Scholar]
- 53.Zipple M., Vogt C., Sheehan M., Genetically Identical Mice Express Alternative Reproductive Tactics Depending on Social Conditions in the Field. bioRxiv (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fisher D. N., Brachmann M., Burant J. B., Complex dynamics and the development of behavioural individuality. Anim Behav 138 (2018). [Google Scholar]
- 55.Trillmich F., Müller T., Müller C., Understanding the evolution of personality requires the study of mechanisms behind the development and life history of personality traits. Biol Lett 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bell A. M., Hankison S. J., Laskowski K. L., The repeatability of behaviour: a meta-analysis. Anim Behav 77 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strauss E. D., Holekamp K. E., Social alliances improve rank and fitness in convention-based societies. Proc Natl Acad Sci U S A 116, 8919–8924 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strauss E. D., Shizuka D., Holekamp K. E., Juvenile rank acquisition is associated with fitness independent of adult rank. Proceedings of the Royal Society B: Biological Sciences, doi: 10.1098/rspb.2019.2969 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersson M., Sexual Selection. (Princeton University Press, Princeton, 1994). [Google Scholar]
- 60.West-Eberhard M., Sexual selection, social competition, and evolution. Proc Am Philos Soc 123 (1979). [Google Scholar]
- 61.Emlen S., Orring L., Ecology, sexual selection, and the evolution of mating systems. Science (1979) 197, 215–223 (1977). [DOI] [PubMed] [Google Scholar]
- 62.Kotiaho J. S., LeBas N. R., Puurtinen M., Tomkins J. L., On the resolution of the lek paradox. Trends Ecol Evol 23 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Kotiaho J. S., Simmons L. W., Tomkins J. L., Towards a resolution of the lek paradox. Nature 410 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Pomiankowski A., Moller A. P., A resolution of the lek paradox. Proceedings of the Royal Society B: Biological Sciences 260 (1995). [Google Scholar]
- 65.Hedström P., Swedberg R., Eds., Social Mechanisms: An Analytical Approach to Social Theory (Cambridge University Press, Cambridge, 1998). [Google Scholar]
- 66.Rawls J., Justice as Fairness: A Restatement (Belknap Press, Boston, MA, 2001). [Google Scholar]
- 67.Smeeding T. M., Public policy, economic inequality, and poverty: The United States in comparative perspective. Soc Sci Q 86 (2005). [Google Scholar]
- 68.Nico M., Pollock G., The Routledge Handbook of Contemporary Inequalities and the Life Course (2021). [DOI] [PMC free article] [PubMed]
- 69.Harden K. P., The Genetic Lottery: Why DNA Matters for Social Equality (Princeton University Press, Princeton, NJ, 2021). [Google Scholar]
- 70.Stoffel M. A., Nakagawa S., Schielzeth H., rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8, 1639–1644 (2017). [Google Scholar]
- 71.Magnusson A., Skaug H., Nielsen A., Berg C., Kristensen K., Maechler M., van Bentham K., Bolker B., Brooks M., glmmTMB: generalized linear mixed models using a template model builder. R package version 0.1 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
While this manuscript is under review, all data and code supporting this manuscript and its analyses can be found in this Box folder: https://cornell.box.com/s/9jz4hp4kl1wnxhb5u3q275m650kfub7x