Abstract
The human gut pathogen Clostridioides difficile displays extreme genetic variability and confronts a changeable nutrient landscape in the gut. We mapped gut microbiota inter-species interactions impacting the growth and toxin production of diverse C. difficile strains in different nutrient environments. Although negative interactions impacting C. difficile are prevalent in environments promoting resource competition, they are sparse in an environment containing C. difficile-preferred carbohydrates. C. difficile strains display differences in interactions with Clostridium scindens and the ability to compete for proline. C. difficile toxin production displays substantial community-context dependent variation and does not trend with growth-mediated inter-species interactions. C. difficile shows substantial differences in transcriptional profiles in the presence of the closely related species C. hiranonis or C. scindens. In co-culture with C. hiranonis, C. difficile exhibits massive alterations in metabolism and other cellular processes, consistent with their high metabolic overlap. Further, Clostridium hiranonis inhibits the growth and toxin production of diverse C. difficile strains across different nutrient environments and ameliorates the disease severity of a C. difficile challenge in a murine model. In sum, strain-level variability and nutrient environments are major variables shaping gut microbiota interactions with C. difficile.
INTRODUCTION
The human gut microbiome exists in a dynamic balance between homeostasis and disruption due to the contrasting evolutionary objectives of the host and the resident gut bacteria. Clostridioides difficile is an opportunistic human gut pathogen that can cause life-threatening damage to the colon. Antibiotics are the first-line treatment for C. difficile infection (CDI). However, they also damage the commensal gut microbiota that provides C. difficile colonization resistance and could cause the recurrence of CDI (rCDI) 1–3. Fecal microbiota transplantation (FMT) has proven to be effective for treating rCDI, but the effect of FMT on a patient can vary due to uncharacterized factors and donor microbiota variability 4. FMT can also result in the unintentional transfer of antibiotic-resistant bacteria, including other opportunistic pathogens 5,6. To overcome these limitations, defined communities of commensal bacteria can be designed to inhibit C. difficile. However, low richness communities do not display robustness of anti-C. difficile activity to changes in environmental contexts 7,8. This in turn could contribute to the variability in efficacy in clinical trials of certain living bacterial therapeutics for treating CDI 9. We lack an understanding of how environmental context, such as the genetics of C. difficile strains and nutrient environments, impacts the anti-C. difficile activity of human gut communities 10,11.
C. difficile has a diverse population structure comprising hundreds of strain types 12 that are distributed across at least 8 phylogenetic clades 13. This species is defined by a large pangenome 14, with an ultralow core genome (as low as 16% based on 73 genomes 15) and extreme levels of evolutionary plasticity that have been molded over long periods through frequent exchange with bacterial gene pools in multiple host environments via horizontal gene transfer 16–19. This substantial genetic variation among C. difficile strains has downstream impacts on the regulation of metabolic pathways and virulence 16,20–22. For instance, the emergence of the hypervirulent epidemic strain ribotype 027 has been proposed as the major driver of the increase in the prevalence of CDI 23,24. Notably, rCDI is not always due to infection with the same strain, where new strains were observed in 33–56% of recurrent episodes 25–29. This suggests that the degree of colonization resistance could vary across different C. difficile strains, potentially leading to differences in patient outcomes.
Interactions with gut microbiota are critical determinants of C. difficile colonization and toxin production, as evidenced by the colonization resistance variability of different microbiome compositions to C. difficile30. Previous studies have elucidated principles that influence C. difficile growth in human gut communities in vitro, such as a strong negative dependence on species richness 31, and identified specific mechanisms of C. difficile inhibition. For example, certain species compete with C. difficile for limiting resources, such as the consumption of specific mucus-derived sugars by Akkermansia muciniphila 32 or the utilization of Stickland metabolism amino acids by Clostridium species (e.g. Clostridium bifermentans 33,34 and Clostridium scindens 33). In addition, C. scindens can produce tryptophan-derived antibiotics that inhibit C. difficile growth 35. Clostridium hiranonis was shown to inhibit C. difficile in vitro through more than one mechanism in a single nutrient environment 31. However, the contribution of C. difficile strain-level variability to these interactions is currently unknown 31,36,37.
The bottom-up construction of synthetic microbiomes combined with computational modeling 38,39 and principled experimental design techniques 40 can be used to efficiently navigate large design landscapes of combinations of species. In addition, these bottom-up approaches can provide a deeper understanding of important molecular and ecological mechanisms. For example, a widely used dynamic ecological model referred to as generalized Lotka–Volterra (gLV) can be used to unravel growth-mediated microbial interactions shaping community assembly 41–43. By informing the model with properly collected experimental data, the gLV model can accurately forecast community dynamics as a function of the intrinsic growth of individual species and pairwise interactions with all constituent community members 38,44.
To understand how nutrient and strain-level variability shapes interaction networks with C. difficile, we used a bottom-up approach to build microbial communities combined with computational modeling. We elucidated strain-level differences in inter-species interactions at the transcriptional level using genome-wide transcriptional profiling. In addition, we discovered that the large variation in toxin production of C. difficile in communities was not correlated with growth-mediated inter-species interactions. Our workflow identifies Clostridium hiranonis as a “universal” C. difficile growth and toxin production inhibitor that is robust to variation in strain backgrounds and nutrient environments. This robust inhibition is consistent with its high metabolic niche overlap with C. difficile, which in turn could block the utilization of C. difficile-preferred substrates. Consistent with this notion, genome-wide transcriptional profiling reveals a unique massive alteration of C. difficile metabolism in the presence of C. hiranonis, which is not observed in co-culture with another closely related species, C. scindens. Furthermore, C. hiranonis ameliorated the C. difficile-induced disease severity of mice due to reduced abundance and toxin production. In sum, we demonstrate that strain-level variability and nutrient environments play an important role in shaping the interactions between C. difficile and human gut communities, and highlight C. hiranonis as a promising candidate to include in the design of robust anti-C. difficile defined consortia.
RESULTS
C. difficile strains display substantial phenotypic and genetic variability
To understand how the strain-level genetic variability influences C. difficile phenotypes, we characterized 18 C. difficile strains (9 from diseased patients that were diagnosed and treated for CDI and 9 from healthy individuals) and C. difficile DSM 27147 (R20291 reference strain of the epidemic ribotype 027). We individually profiled their growth in a chemically defined media supplemented with carbohydrate sources shown to promote colonization or virulence activities including succinate 45,46, trehalose 21,22, mannitol 46,47, sorbitol 46,47, and various mucus-derived sugars such as sialic acid and n-acetyl-D-glucosamine 36,48 (Fig. S1a–d; Table S1, 2). The growth of all C. difficile strains was supported in defined media without any carbohydrate source due to their ability to utilize amino acids through Stickland metabolism. In general, supplementation of glucose, mannitol, n-acetyl-D-glucosamine (GlcNAc), and sialic acid enhanced the growth of all C. difficile strains compared to media without carbohydrate sources.
In most single carbohydrate media, C. difficile displayed a unique growth profile that is distinct from the other commensal gut bacteria, where the culture grew rapidly at the beginning followed by a steep decline in OD600 during stationary phase at ~24 h of growth (i.e. non-monotonic growth response). While the variance in monoculture growth biological replicates is low in the first 24 h, this variability increases substantially at the time when OD600 declines in stationary phase (Fig. S1g–h). This implies that sporulation and cell lysis, in addition to halted cell division as observed by fluorescence microscopy contribute to the observed reduction and variability in OD600 (Fig. S2). To quantify the variability in growth profiles across C. difficile strains, we fit each growth curve to a logistic model to determine the growth rate (r) and carrying capacity (K) of each strain excluding data points with a >10% reduction in OD600 in the late stationary phase (Fig. 1a–b, S1e, see Methods). Overall, the logistic model displayed a high goodness of fit to the data (Pearson R=0.98, P<10E-05) (Fig. S1f).
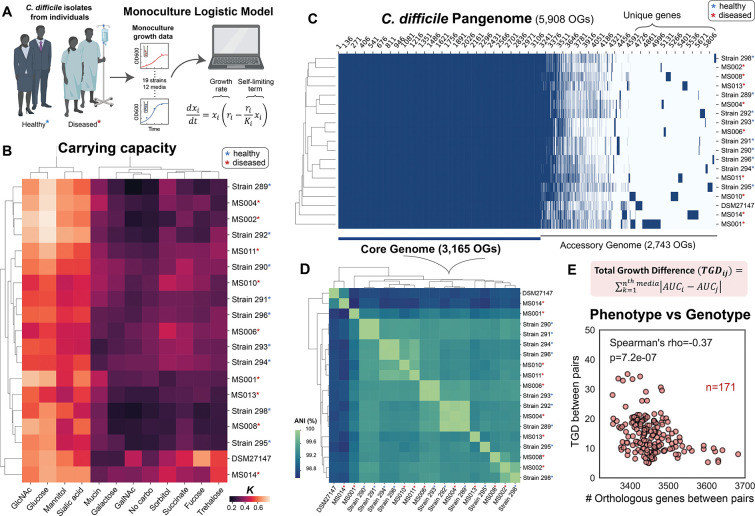
Figure 1. Phenotypic and genotypic characterization of diverse C. difficile isolates from diseased and healthy individuals.
a, Fitting of monoculture growth data from 19 C. difficile strains (including 18 C. difficile isolates from diseased and healthy individuals, Table S1) in 12 media containing different carbohydrate sources (Fig. S1a–b) to the logistic model. Mathematical description of the logistic growth model was shown, where is the absolute abundance of species , parameter is its maximum growth rate, and is the carrying capacity. When fitting the experimental data to the model, we cut time points where OD600 drops above 10% to exclude the highly variable phase. b, Biclustering heatmap of the carrying capacity () of the C. difficile isolates. Strains marked with red asterisks were isolated from diseased patients whereas the ones marked with blue asterisks were isolated from healthy individuals. c, Heatmap showing the presence and absence of all genes identified across the 19 C. difficile strains (pangenome). The columns indicate the genes, and the rows indicate the C. difficile strains. Blue means gene present and white means gene absent. Genes present in all of the 19 strains are the core genome, whereas genes present in a subset of the strains are the accessory genome. d, Biclustering heatmap of the Average Nucleotide Identity (ANI) of C. difficile isolate pairs based on their whole-genome sequence. The horizontal boxes indicate 100% ANI. e, Scatter plot of the Total Growth Difference (TGD) between isolate pairs and the number of orthologous genes between isolate pairs. Mathematical formula to calculate the TGD between isolate pairs is shown on the top, which is the sum of all AUC differences from 24 h of growth in the twelve media. Parts of the figure are generated using Biorender.
We performed whole-genome sequencing on each isolate to provide insights into the genetic variation driving the observed phenotypic variability. The C. difficile genome is comprised of a well-conserved core genome (3,165 orthologous genes) and substantial variation in its accessory genome (Fig. 1c). Metabolic genes varied substantially across the 19 C. difficile strains, where only ~63% of metabolic genes were shared (Fig. S3a). Clustering based on ANI, which represents the Average Nucleotide Identity of all orthologous genes shared between any two genomes, highlighted strains that are more genetically similar to each other (Fig. 1d), such as DSM 27147 and MS014. In addition to other genome similarities, these two strains possessed a mutation in the treR gene (L172I) that confers enhanced trehalose metabolism identified in hypervirulent C. difficile strains 22, consistent with their higher capability to utilize trehalose (Fig. 1b). Further, MS001 is clustered separately from the rest of the group based on ANI. MS001 has a much higher number of genes (4110) compared to the other strains (ranging from 3629 to 3892) (Table S3), and uniquely lacks the toxins TcdA and TcdB. Indeed, non-toxigenic C. difficile strains have distinct phenotypes compared to toxigenic strains, as a consequence of the variability in their genome 49. In general, there is no pattern between the C. difficile isolates from healthy and sick individuals in terms of their genotype.
To quantify if the genotypic variation displays an informative relationship with phenotypic variation in monoculture, we define the growth difference (GD) as the absolute value of the difference in the AUC of pairs of strains in a specific media. The total growth difference (TGD) is the sum of GD across the 12 media. The TGD and the number of orthologous genes (OGs) or ANI of pairs of C. difficile strains displayed a moderate negative correlation (Fig. 1e, S4a–b). In addition, growth in glucose, trehalose, galactose, and sorbitol was negatively correlated with ANI and the number of OGs (Fig. S4c–d). These results suggest that the genotypic variability quantified by these metrics displays an informative relationship with the utilization of certain carbohydrates.
Although the number of genes responsible for most core processes beyond metabolism is similar across isolates, there was large variability in the number of genes related to DNA recombination and integration, which are markers of mobile genetic elements (MGEs) (Fig. S3e). This suggests that MGEs play a major role in driving C. difficile genotypic differences, consistent with previous reports 50,51. To characterize the contribution of plasmids to the genome of C. difficile, we searched for high-coverage contigs within genome assemblies and discovered 11 of such instances in 7 of 19 genomes (Fig. S5a–c). These putative plasmids contained direct repeats on their termini indicative of being circular. In addition, the putative plasmids do not contain genes that could provide a selective advantage to these strains such as antibiotic resistance or virulence factors (Table S5). Interestingly, 4 of the 11 high-coverage contigs map to the same plasmid that is present in four different genetically distant C. difficile isolates from different patients. These isolates also have a highly variable number of conjugative systems and phages, covering 1.4–16.5% of their genomes (Fig. S3f, Table S6–7). In sum, the C. difficile isolates have highly diverse genomes with substantial variability in metabolic genes and mobile genetic elements.
Human gut communities containing different C. difficile isolates display differences in interaction networks
Since human gut microbiota interactions are critical determinants of C. difficile growth and colonization, we investigated how C. difficile genetic variation shapes gut microbiota interspecies interactions. To this end, we built human gut communities from the bottom up with one of 4 diverse C. difficile strains (DSM 27147, MS001, MS008, and MS014) and combinations of 7 gut species (C. scindens (CS), C. hiranonis (CH), Desulfovibrio piger (DP), Bacteroidetes thetaiotaomicron (BT), Phocaeicola vulgatus (PV), Bacteroidetes uniformis (BU), and Collinsella aerofaciens (CA)) (Fig. 2a). Many of these species are prevalent across individuals and span major phyla of the human gut microbiome. These species displayed variation in growth in media with different carbohydrates (Fig. S1a–b). The community features CS, previously shown to inhibit the growth of C. difficile in gnotobiotic mice 37, CH which can inhibit C. difficile growth through unknown mechanism 31, and Bacteroides species, which have the potential for C. difficile inhibition in different environments 36,45,52,53.
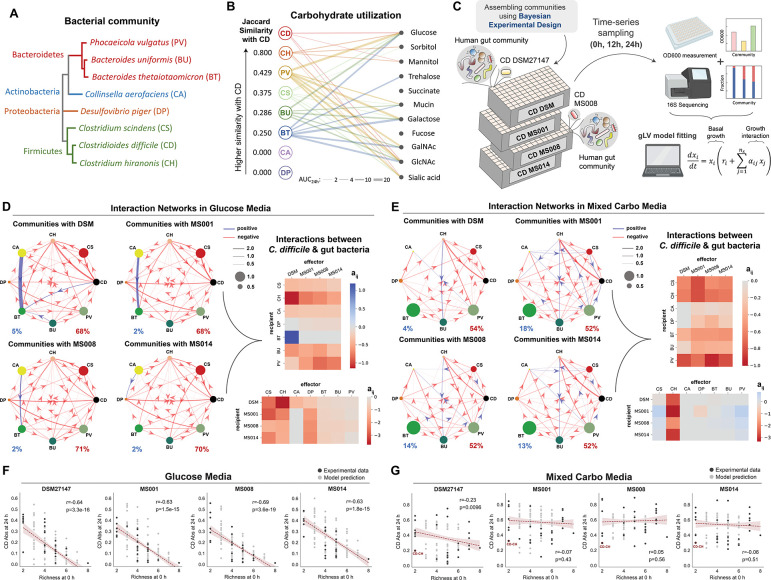
Figure 2. Interspecies interactions between C. difficile strains and the human gut bacteria in different nutrient environments.
a, Phylogenetic tree of the 7-member resident synthetic gut community and C. difficile. The phylogenetic tree was generated from the 16S rRNA sequence of each species using the Clustal Omega multiple sequence alignment tool. b, Bipartite network of carbohydrate utilization by C. difficile and gut bacteria based on their monoculture growth profiles in Fig. S1a–b. The edge thickness indicates the AUC24h of the gut species grown in specific carbohydrates subtracted by the AUC24h of the gut species grown in media without any carbohydrates. Only edges with a magnitude larger than 2 are shown. For C. difficile, the growth profile of the DSM27147 strain is used as a representative. The Jaccard Similarity values of each gut species with C. difficile were computed based on the number of carbohydrates being utilized, where higher Jaccard Similarity values mean larger niche overlap with C. difficile. Different colors represent different species. c, Schematic of the experimental workflow to assess interactions between different C. difficile strains and human gut bacteria in the glucose media. Experimental communities were assembled using the Bayesian experimental design by utilizing monoculture growth data as prior information (See Methods). A total of 147 subcommunities (2 to 8 species) containing combinations of gut species and one of the C. difficile strains were cultured at an equal absolute abundance ratio in the glucose media. Cultures were grown in microtiter plates in anaerobic conditions and incubated at 37°C. After 12 h and 24 h of growth, aliquots of the culture were taken for multiplexed 16S rRNA sequencing to determine community composition and cell density measurement at 600 nm (OD600) to calculate the absolute abundance of each species. Absolute abundance data are used to infer the parameters of a generalized Lotka–Volterra (gLV) model and elucidate the interaction networks of the communities. d-e, Inferred interspecies interaction networks between the 7 gut species and each of the representative C. difficile strains when grown in the glucose media (d) or the mixed carbohydrates media (e). Node size represents species carrying capacity in monoculture (mean of all biological replicates) and edge width represents the magnitude of the interspecies interaction coefficient (aij). Edges represent parameters whose absolute values were significantly constrained to be non-zero based on the Wald test (Fig. S8 for glucose media and Fig. S10 for mixed carbohydrates media). Percentage of positive (blue) and negative (red) interactions for each community are shown. The right panel shows the heatmap of interspecies interaction coefficients of the gLV model between the different C. difficile strains and the 7 gut species in the glucose media (d) or the mixed carbohydrates media (e). f-g, Scatter plots of C. difficile absolute abundance at 24 h as a function of initial species richness in all possible subcommunities of 2–8 species simulated by the gLV (gray data points) and in experimentally measured subcommunities (mean value of biological replicates, black data points). Panel f are model predictions and experimental data of communities grown in the glucose media, whereas Panel g are those grown in the mixed carbohydrates media. Red dashed line indicates the linear regression between the species richness at 0 h and C. difficile absolute abundance at 24 h, with the 95% confidence bounds shown as red shading. Pearson’s correlation coefficient (r) and p-values are shown, which were computed using the pearsonr from the scipy package in Python. Parts of the figure are generated using Biorender.
To infer the inter-species interaction networks, we down selected a set of representative C. difficile strains based on their genotypic and phenotypic variations. Strains that have similar genotypes and metabolic genes may display similar interaction networks, whereas interactions may be divergent for strains with large differences in genotype. MS014 shows a similar genotype to DSM 27147 and thus might evolve from the same ancestor, but MS014 was more recently isolated. By contrast, the non-toxigenic strain MS001 has the most different genotype than the other strains, suggesting potentially larger differences in inter-species interactions. Finally, MS008 is genotypically and phenotypically distinct from the other 3 strains (Fig. 1b–d). In addition, MS008 clustered differently from MS014, DSM 27147 and MS001 based on metabolic genes, suggesting divergent metabolic capabilities (Fig. S3a).
Given the key role of resource competition in the ecology of C. difficile 32–34,54, the extent of metabolic niche overlap with C. difficile may be a major variable influencing interactions with human gut bacteria. To quantify the extent of metabolic niche overlap between each gut species and C. difficile, we calculated the Jaccard Similarity of carbohydrate utilization based on the change in growth in the presence and absence of the given carbohydrate (Fig. 2b). Notably, CH displayed the largest metabolic niche overlap of carbohydrate utilization with C. difficile (Jaccard Index=0.8). In addition to the similarities in carbohydrate utilization, CH has been shown to use amino acids via Stickland metabolism, similar to C. difficile 33.
To study community inter-species interactions in a gut environment with high resource competition, we used a defined media containing glucose as the sole carbohydrate source. Glucose can support the growth of most species in monoculture including C. difficile, and thus promotes inter-species competition (Fig. S6a–c). To quantify the differences in the inter-species interaction networks, we cultured different combinations of species with one of the four C. difficile strains (DSM 27147, MS001, MS008, and MS014) (Fig. 2c). Since there are too many community combinations to be comprehensively explored (635 combinations), we used a Bayesian experimental design approach to select combinations of bacteria that would maximize information content as quantified by the expected Kullback-Leibler divergence between the posterior and prior parameter distributions (see Methods and Supplementary text) 40. Briefly, a preliminary gLV model was fit to the monoculture growth in glucose media. We used a Bayesian inference approach to approximate the posterior parameter distribution as a multivariate Gaussian. The parameter distribution inferred for the preliminary model was used as a prior to guide the design of 147 combinations of 2 to 8-member sub-communities containing one of the four C. difficile strains (DSM 27147, MS001, MS008, and MS014). Species absolute abundance was determined by multiplying the relative abundance fraction via multiplexed 16S rRNA sequencing by the total biomass obtained by OD600 as previously described 31,38. The parameters of the gLV model were inferred based on time-series data of species abundances (0, 12, and 24 h) (Fig. S7a, DATASET001 in Table S8). Based on the parameter posterior distributions, we analyzed parameters with absolute values that were significantly constrained to be non-zero based on the Wald test 55 (Fig. S8, Supplementary text). The Wald test compares the parameter mean to its standard deviation to evaluate whether the peak of the posterior parameter distribution is significantly higher or lower than zero compared to the width of the distribution. The percentage of constrained parameters is 76.6%, 73.4%, 75%, and 75% for communities containing DSM, MS001, MS008, or MS014 respectively. To evaluate model prediction performance on held-out data, we performed 10-fold cross-validation where only community samples were subjected to testing (see Methods). Using a 10-fold cross-validation, the model prediction exhibited good agreement with the measured species abundance in all communities with different C. difficile strains (Pearson’s R=0.93–0.95, P<10E-05), demonstrating that our model can capture and predict the trends in species abundance (Fig. S7b).
Consistent with a high competition resource environment, the interaction networks for distinct C. difficile strains displayed a high fraction of negative interactions (68–71%) and inhibition of C. difficile by all species (Fig. 2d). CS and CH display a high magnitude of negative inhibition towards C. difficile, consistent with their ability to compete for amino acids via Stickland fermentation. Notably, the C. difficile DSM 27147 hypervirulent strain exhibits the largest differences in interaction profile from other C. difficile strains (e.g. BT, DP, and CH).
In addition to the observed changes in pairwise interactions with C. difficile, other inter-species interactions displayed strain-specific differences. A higher order interaction (HOI) is defined as a substantial change in a pairwise interaction due to the presence of a third community member 56,57. Changes in pairwise interactions due to the presence of different C. difficile strains may suggest HOI. For instance, the interaction coefficients between CA and BT are substantially impacted by the specific C. difficile strain that is present in the community (Fig. S7c). To further explore whether C. difficile strain variations could impact CA-BT interactions, we cultured the CA-BT pairwise community in the sterilized spent media of C. difficile (Fig. S7d). The abundances of CA and BT in the community were statistically different when cultured in the sterile conditioned media of the different C. difficile strains. This implies that different strains of C. difficile differentially altered the chemical environment, which in turn impacted the interactions between CA and BT. In sum, inferred inter-species interaction networks containing distinct C. difficile strains displayed infrequent direct and indirect differences.
Human gut bacteria infrequently inhibit C. difficile in the presence of preferred carbohydrates
Antibiotic treatments lead to massive gut bacterial mortality, alternations in the resource landscape, and changes in community composition. This new environment can be exploited by C. difficile 45,48,58–61. To explore community interactions in media that mirrors post-antibiotic environments, we designed a media containing multiple carbohydrates that could be utilized by C. difficile (mixed carbohydrates media) (Fig. S9a). In this media, C. difficile strains displayed substantial growth and a diminished decline in OD600 in late stationary phase than glucose media (Fig. S9b). In pairwise communities, the relative abundance of C. difficile was high in all communities (>50% in all cases) except when grown with BT. The absolute abundance of C. difficile remained high after three 24 h growth cycles, except for the community containing PV (Fig. S9c–d). In the 7-member community, C. difficile displayed a relative abundance of ~20–50% following 24 h of growth (Fig. S9e). This contrasts with the low abundance of C. difficile in the glucose media (~1 to 5%) (Fig. S7a).
To determine the inter-species interaction network in the presence of multiple preferred carbohydrates, we built a gLV model using a design-test-learn (DTL) cycle (Fig. S9f). A DTL cycle was used to account for potentially more complex interactions in the presence of a complex resource environment, which may require additional data to constrain the model parameters. Each cycle consisted of (i) Bayesian experimental design informed by prior experimental observations to select combinations of species that minimize parameter uncertainty (design), (ii) experimental characterization of sub-communities (test), and (iii) updates to the gLV model parameters based on new experimental data (learn) (Methods and Supplementary text) 44. In the initial experiment, we constructed 82 communities consisting of all possible pairwise, leave-one-out, and full communities containing the gut bacteria and individual C. difficile strains (Table S8, DATASET002). Using 10-fold cross-validation, the model displayed a low to moderate prediction performance of individual species (Fig. S9g). To select informative experimental conditions for the second DTL cycle, Bayesian experimental design based on the inferred parameter uncertainties guided the design of 94 new combinations of medium richness communities (3–6 members) (Table S8, DATASET003). Using these data, the prediction performance of most individual species was improved (Pearson’s R=0.90 to 0.91, P<10E-05) (Fig. S9g). The parameter uncertainty distributions are shown in Fig. S10. In comparison to the media with glucose, the constrained non-zero parameters are lower in the mixed carbohydrates media (60.9%, 71.8%, 68.8%, and 67.2% for communities containing DSM, MS001, MS008, and MS014 respectively). To determine whether species predictive performance could be improved with additional data, we performed a sensitivity analysis of the model’s prediction performance by varying how the training and validation data was partitioned (k in k-fold) (Fig. S11). The model prediction performance increased with k and saturated for most species. This implies that additional data for moderately predicted species (e.g. CH and DP) will not substantially improve the model prediction performance. Poor or moderate prediction performance could be due to insufficient variation of the particular species abundance across communities or limited flexibility of the gLV model to capture complex interaction modalities 39.
The inferred interaction networks in the mixed carbohydrates media display a higher frequency of positive interactions (4–18%) compared to media containing only glucose (2–5%) (Fig. 2e), and C. difficile displayed higher absolute abundance across communities (Fig. S12). While DSM 27147 exhibited the most different interaction profile in glucose media, this strain displayed similar interaction patterns to MS008 and MS014 in the mixed carbohydrates media. By contrast, MS001 displayed the largest differences in inter-species interactions in the mixed carbohydrates media than the other C. difficile strains. Thus, the differential interaction profiles between the C. difficile strains and human gut microbiota are nutrient dependent. Of 7 diverse human gut species, only CH displayed negative interactions with each C. difficile strain. Several communities used to train the model (3–6 members) containing CH displayed a higher magnitude of C. difficile inhibition than the C. difficile–CH pairwise community (Fig. 2g, S13a). In particular, CS, DP, CA, and PV are enriched in these communities. This suggests that the inhibitory activity of CH can be further enhanced by the presence of specific gut bacteria.
To further investigate inter-species interactions in the mixed carbohydrate media, we cultured different C. difficile strains in the sterilized spent media of the gut bacteria and fresh media as a control (Fig. S14a–b). Overall, the qualitative effects of the pH-adjusted conditioned media were largely consistent with the signs of the inferred gLV pairwise interaction coefficients (71% agreement compared to 32% in the non-pH-adjusted conditioned media) (Fig. S14c). Without pH adjustment, C. difficile growth was substantially reduced in Bacteroides spp. conditioned media due to the acidification of the environment (pH of 5.0–5.2), and this inhibition was eliminated in the pH-adjusted Bacteroides spp. conditions. Since pH changes over time in co-culture, the large variation in the initial pH of the spent media may not be physiologically relevant to microbial community interactions. Notably, C. difficile growth was reduced in CS-conditioned media but not in co-culture with CS. This inconsistency suggests that the feedback of metabolite exchange and/or metabolic niche partitioning plays a role in the C. difficile-CS pair in the mixed carbohydrates media. Although CS can utilize many of the same carbohydrates as C. difficile, CS has a wider range of carbohydrate utilization capabilities than C. difficile in the tested media (Fig. 2b). This implies that C. difficile and CS may prefer utilizing similar resources in monoculture and display distinct metabolic niches in co-culture.
Model accurately predicts C. difficile inhibition potential in human gut communities
Using the model trained on all data, we forecasted the abundance of C. difficile at 24 h in all possible communities (Fig. 2f–g). A previous study showed a strong negative dependence between C. difficile growth and species richness in a rich media 31, consistent with a negative relationship between these variables in glucose media. However, this trend was not present in the presence of mixed carbohydrates. This suggests that high-richness communities may not universally inhibit C. difficile in environments with C. difficile preferred substrates, and the identity of the species in the community may be more impactful than the number of species.
To determine if our model could design communities to inhibit C. difficile, we used our gLV model trained on community data in the mixed carbohydrates media (Table S8, DATASET003) to predict C. difficile abundance in all possible 2 to 8-member communities (Fig. S15a). Based on the model prediction, we selected a 3-member weak inhibitory community (WIC, consisting of BU, CA, and DP) and a strong inhibitory community (SIC, consisting of CH, CS, and DP). The WIC was selected due to its low inhibition potential of C. difficile, whereas the SIC was selected for its high inhibition potential against diverse C. difficile strains. Although CH was the only species that could strongly inhibit C. difficile in the mixed carbohydrates media, CH, CS, and DP were the three most inhibitory species in the glucose media (Fig. 2d–e). The interaction networks revealed sparse and almost negligible incoming negative interactions towards C. difficile in the WIC. By contrast, the SIC displayed stronger negative interactions towards C. difficile, especially from CH (Fig. S15b). To validate the model predictions, we cultured WIC and SIC in the absence and presence of different C. difficile strains (Fig. S15c). We observed that the abundance of all C. difficile strains in SIC was significantly lower than those in the WIC (~2.1 to 4.2-fold lower), corroborating the differential inhibitory potential of the SIC and WIC communities and highlighting that the inhibition of the SIC is robust to strain-level variability. This indicates that the model could predict the C. difficile inhibition potential of different communities.
C. difficile strains have a differential ability to compete with C. scindens over proline
Although CS can inhibit the growth of C. difficile via competition for limiting pools of amino acids via Stickland metabolism 33, inhibition of most C. difficile strains by CS was not observed in the mixed carbohydrates media (Fig. 2e). This suggests that these C. difficile strains occupied alternative metabolic niches in co-culture with CS. The inferred interaction from CS to MS001 was larger in magnitude than to MS008 or MS014. By contrast, CS moderately inhibited the growth of the DSM strain. Model predictions of co-cultures of CS and individual C. difficile strains displayed consistent trends with independent in vitro experiments that did not inform the gLV model (Fig. 3a).
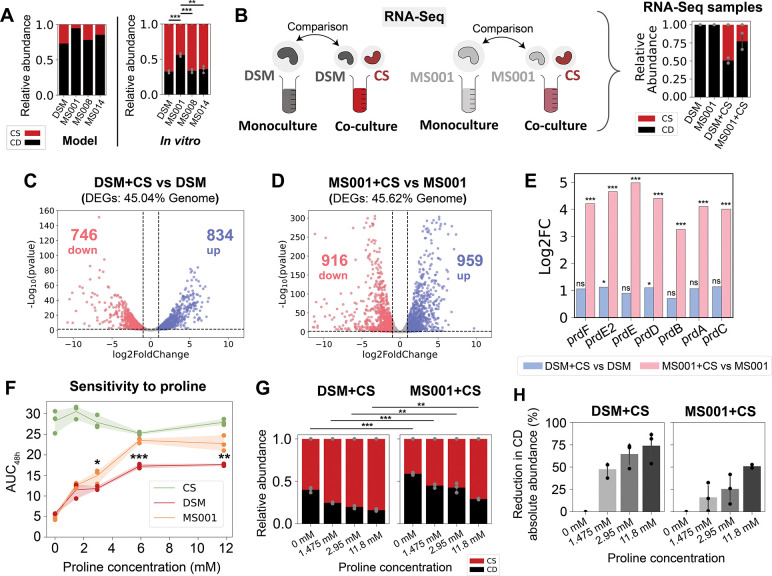
Figure 3. Genome-wide transcriptional profiling of C. difficile DSM27147 and C. difficile MS001 in the presence of C. scindens.
a, Model prediction and independent experimental validation (not included in model fitting) of the relative abundance of pairwise communities containing CS and one of the four C. difficile strains. Each bar represents the average absolute abundance of each species, and the error bars on the in vitro data represent s.d. (n=3). Asterisks above the bars indicate the p-value from unpaired t-test of species relative abundance between co-cultures: ** indicates p<0.01, *** indicates p<0.001. b, Schematic of the genome-wide transcriptional profiling experiment of two C. difficile strains in the presence of C. scindens. Monocultures of C. difficile DSM and MS001 strain, and cocultures of DSM+CS and MS001+CS were grown in the mixed carbohydrates media for ~7 h until they reached exponential phase. Aliquots were taken for DNA extraction for next-generation sequencing to determine the cocultures’ composition, and aliquots were taken for RNA extraction for RNA-Seq. Transcriptomes of C. difficile in cocultures (DSM+CS and MS001+CS) were compared to the C. difficile monocultures’ transcriptome. The panel on the right shows the stacked bar plot of the composition of the samples subjected to RNA-Seq as determined by 16S sequencing. c-d, Volcano plots of log-transformed transcriptional fold changes for C. difficile DSM27147 (c) and MS001 strain (d) in the presence of C. scindens. Vertical dashed lines indicate 2-fold change, and the horizontal dashed line indicates the statistical significance threshold (p = 0.05). Blue indicates up-regulated genes and red indicates down-regulated genes. e, Bar plot of the log-transformed fold changes of the proline reductase (prd) genes of the DSM strain in the presence of CS compared to monoculture (blue) and the MS001 strain in the presence of CS compared to monoculture (pink). Asterisks above the bars indicate the adjusted p-value from DESeq2 differential gene expression analysis: * indicates p<0.05, *** indicates p<0.001, ns indicates not significant (p>0.05). f, Sensitivity of C. difficile DSM27147, MS001, and C. scindens monoculture growth towards proline concentration in the mixed carbohydrates media. AUC48h was calculated from the growth curves in Fig. S17a. Data were shown as mean and 95% c.i. (shading), n = 3 biological replicates. Asterisks indicate the p-value from unpaired t-test of the AUC48h between DSM and MS001 strain at specific proline concentration: * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001. g, Stacked bar plot of the relative abundance of C. difficile DSM27147 or MS001 grown with CS in media supplemented with different proline concentrations. Each bar represents the average relative abundance of each species, and the error bars represent s.d. (n=3). Asterisks above the bars indicate the p-value from unpaired t-test of the relative abundance between MS001-CS and DSM-CS coculture at a specific proline concentration: ** indicates p<0.01, *** indicates p<0.001. h, Percentage reduction of C. difficile abundance in media supplemented with different concentrations of proline compared to media without proline. Percentage reduction was calculated based on data from Fig. S17d. Error bars represent s.d. (n=3).
To provide insights into the transcriptional activities that mediate the observed differences in inter-species interactions, we performed genome-wide transcriptional profiling of C. difficile strains DSM27147 and MS001 in the presence and absence of CS (Fig. 3b, S16a). For both DSM and MS001 strains, ~45% of transcripts were differentially expressed in the presence of CS than in monoculture, indicating that the presence of CS caused a global shift in the transcriptome of C. difficile (Fig. 3c–d, Table S9–10).
To identify significant changes in transcriptional activities, we performed gene set enrichment analysis (GSEA) using Kyoto Encyclopedia of Genes and Genomes (KEGG) modules. Many biological pathways such as the amino-acid transport system, pimeloyl-ACP biosynthesis, and iron complex transport system displayed similar patterns in DSM and MS001 (Fig. S16b–e). In addition, both C. difficile strains up-regulated genes for mannitol utilization, consistent with the inability of CS to utilize mannitol (Fig. 2b). This implies that C. difficile and CS display niche partitioning in co-culture, thus reducing competition for limiting substrates. In addition, both strains down-regulated the grd operon which is involved in glycine utilization via Stickland metabolism. Notably, only the MS001 strain up-regulated the proline reductase (prd) genes for Stickland metabolism via the proline pathway (~10 to 32-fold) (Fig. 3e). This implies that these C. difficile strains display differential utilization of proline in the presence of CS.
The growth of C. difficile increased with supplemented proline (Fig. 3f, S17a). The MS001 strain displayed a significantly larger increase in growth than the DSM strain in the presence of intermediate proline concentrations. Although there are some variations in the sequence of the prd operon genes among C. difficile isolates, their protein-coding sequences are largely similar (Fig. S17b–c). By contrast, variation in supplemented proline did not alter the growth of CS. This demonstrates that proline metabolism via the Stickland pathway is crucial for C. difficile growth, but not a major resource utilized by CS in monoculture. However, we observed an opposite trend in co-cultures where increasing proline concentrations reduced C. difficile growth in the community (Fig. 3g, S17d). These results suggest that CS competed more efficiently with C. difficile over proline in co-culture, which was distinct from its metabolic niche in monoculture. The absolute abundance of CS increased with supplemented proline only in co-culture with the MS001 strain, but not the DSM strain (Fig. S17d). Consistent with the monoculture data, the MS001 strain displayed higher growth than DSM in co-culture with CS (Fig. S17d), and its abundance was reduced to a lower degree as a function of proline compared to the DSM strain (Fig. 3h). These data suggest that MS001 can compete better with CS over limited proline to perform Stickland metabolism than DSM, consistent with the higher fold change in the expression of the prd operon (Fig. 3e). These trends are consistent with the stronger inhibition of CS by MS001 compared to DSM in the inferred gLV interaction network (Fig. 2e).
C. difficile toxin production in communities is not explained by growth-mediated inter-species interactions
A myriad of environmental factors including specific nutrients 62–66, pH 67, and environmental stressors including alteration of the redox potential, antibiotic exposure, and temperature increase 68 shape the production of toxins in C. difficile. By modifying the environment, certain bacterial species may impact the toxin production of C. difficile 69,70. However, we lack an understanding of how toxin production is shaped by diverse human gut species. To investigate this question, we characterized C. difficile toxin expression in the presence of 25 individual diverse human gut species. Many of these species are prevalent and abundant in the human gut microbiome and are linked to human health and disease 44 (Fig. 4a, S18a–b). Individual species were co-cultured with distinct C. difficile strains that we previously used to study community-level interactions (DSM27147, MS008, and MS014), as well as two other C. difficile strains isolated from healthy individuals (Strain 292 and Strain 296) which are clustered differently from the previous strains in terms of genotype and phenotype (Fig. 1b–d). We measured OD600 and performed 16S sequencing to determine species absolute abundances, and endpoint toxin quantification using ELISA (Fig. 4b). A gLV model was fit to the time-resolved absolute abundance data (0, 12, 24 h) to infer inter-species interactions (Fig. S18c–d, DATASET004 in Table S8). The inferred interaction parameters using this dataset displayed an informative relationship with the parameters inferred in Fig. 2e (DATASET003) (Fig. S18e).
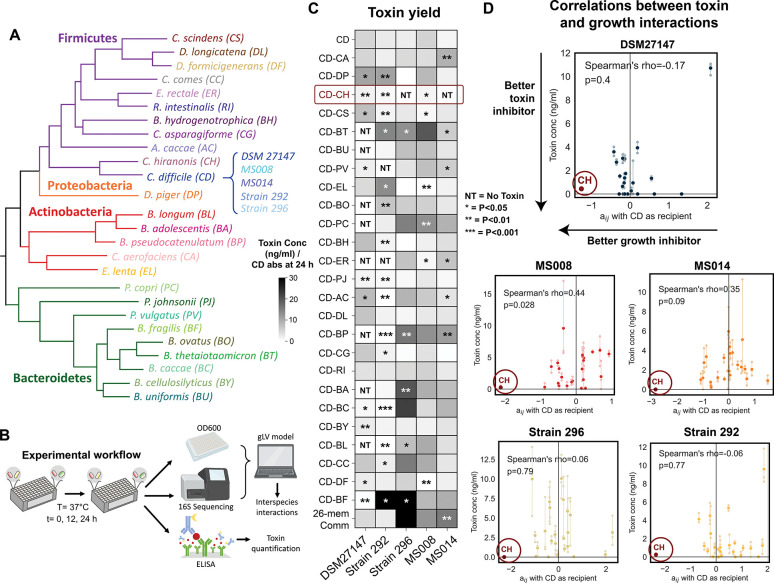
Figure 4. C. hiranonis inhibits the growth and toxin production of diverse C. difficile strains.
a, Phylogenetic tree of 25-member resident synthetic gut community and C. difficile. b, Schematic of the experimental workflow. C. difficile was grown with gut communities and samples were taken at 12 and 24 h. Samples were subjected to OD600 measurement and 16S sequencing to determine species absolute abundance. Time series abundance measurements were fitted to the gLV model to obtain the interaction parameters of the community members. Samples at 24 h were subjected to toxin quantification via ELISA. c, Heatmap of toxin yield (toxin production per C. difficile abundance at 24 h) of different C. difficile strains when grown in pairwise and 26-member communities with human gut bacteria in the mixed carbohydrates media. Toxin concentrations (TcdA and TcdB) were measured in monocultures or communities after 24 h of growth using ELISA (n=3) (Fig. S19a). Asterisks on the heatmap indicate the p-value from unpaired t-test of the toxin yield in cocultures compared to C. difficile monocultures: * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, NT indicates No Toxin (toxin concentration per CD absolute abundance = 0 ng/ml). d, Scatter plots of the interspecies interaction coefficients (aij where C. difficile is the recipient) versus toxin production in cocultures. Solid data points indicate the mean of the biological replicates which are represented by transparent data points connected to the mean with transparent lines. The lower the toxin concentration indicates a better toxin inhibitor and the more negative the aij indicates a better C. difficile growth inhibitor. Spearman’s rho and p-value are shown, which were computed using the spearmanr from the scipy package in Python. Parts of the figure are generated using Biorender.
Toxin yield (toxin concentration normalized by the C. difficile absolute abundance at 24 hr) provides insight into context-dependent changes in toxin production, whereas the toxin concentration may be more physiologically relevant. In 16.2% of conditions, toxin yields were enhanced in communities than in monoculture (36.2% for toxin concentration) (Fig. 4c, S19a). Meanwhile, in 26.2% of conditions, toxin yields were reduced in communities compared to monoculture (25.4% for toxin concentration). Genotype and toxin production did not display an informative relationship since the similar hypervirulent strains DSM27147 and MS014 displayed very different toxin production profiles in communities. Overall, C. difficile strains exhibited substantial variability in toxin production with Strain 296, MS008, and MS014 displaying greater similarity to each other than the other strains (Spearman’s rho=0.53–0.75, P=5.4E-03 to 1.1E-05) (Fig. 4c, S19b). These strains displayed higher toxin production in many pairwise communities (e.g. BT, BU, PV, PC, BP, BA, BC, BL, CC, and BF) and the 26-member community. The similarities in toxin production profiles were not explained by toxin protein-coding sequences (Fig. S19c). While Strain 296 and MS014 clustered together based on their metabolic genes, MS008 has distinct metabolic genes (Fig. S3a). These imply that toxin production in communities is likely impacted by regulatory networks and other cellular processes 71–73 that are shaped by gut microbiota inter-species interactions.
Some stresses including nutrient limitations have been reported to induce C. difficile toxin production 72,74. Strong negative inter-species interactions may activate stress response networks leading to an increase in toxin production. However, our results revealed that toxin production and the inferred pairwise gLV interaction coefficients impacting C. difficile growth in communities did not display an informative relationship (Fig. 4d). For instance, although the abundance of C. difficile Strain 296 was lower than DSM, MS008, and MS014 in the 26-member community (Fig. S18d), this strain displayed the highest toxin expression level (Fig. S19a, d). In sum, C. difficile strain-level variability and human gut microbiota inter-species interactions beyond growth were major variables shaping toxin production.
C. difficile metabolism, growth, and toxin production are substantially impacted by C. hiranonis
Based on the inferred inter-species interaction network, CH inhibited distinct C. difficile strains regardless of whether the nutrient environment favored competition or C. difficile growth (Fig. 2d–e). Of 25 diverse gut bacteria, CH is the only species that robustly inhibited both C. difficile growth and toxin production of diverse C. difficile strains (Fig. 4c–d), highlighting its potential as a “universal” C. difficile inhibitor. This robustness of inhibitory interaction across the two nutrient environments and strain background may be attributed to the substantial metabolic niche overlap for carbohydrate utilization (Fig. 2b) and capability for amino acid Stickland metabolism. In addition, introducing C. hiranonis into communities with specific human gut species enhanced C. difficile growth and toxin inhibition than in co-culture with only C. hiranonis (Fig. S13a–b).
To provide insights into the mechanisms by which CH inhibits C. difficile, we performed genome-wide transcriptional profiling of C. difficile DSM27147 in the presence and absence of CH (Fig. 5a, S16a, f). In the presence of CH, 36% of C. difficile genes were differentially expressed compared to monoculture (Fig. 5b, Table S11). The transcriptional profile of C. difficile in the presence of CH was largely different compared to the co-culture with CS (17% of genes have an opposite sign of fold change) (Fig. 5c).
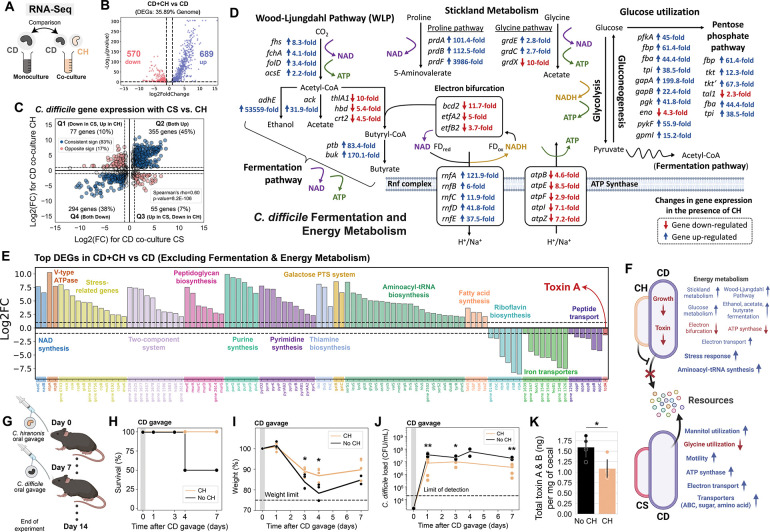
Figure 5. C. hiranonis altered C. difficile metabolism and other important cellular processes.
a, Schematic of the genome-wide transcriptional profiling experiment of C. difficile DSM 27147 strain in the presence of C. hiranonis. Monocultures of C. difficile DSM and cocultures of DSM+CH were grown in the mixed carbohydrates media for ~7 h until they reached the exponential phase. Aliquots were taken for DNA extraction for next-generation sequencing to determine the cocultures’ composition, and aliquots were taken for RNA extraction for RNA-Seq. Transcriptome of C. difficile in coculture is compared to the C. difficile monocultures’ transcriptome. b, Volcano plot of log-transformed transcriptional fold changes for C. difficile DSM strain in the presence of C. hiranonis. Vertical dashed lines indicate a 2-fold change, and the horizontal dashed line indicates the statistical significance threshold (p = 0.05). Blue indicates up-regulated genes and red indicates down-regulated genes. c, Scatter plot of fold changes of C. difficile DSM 27147 differentially expressed genes in the presence of CS and CH. Only genes with p-value less than 0.05 are shown. Blue indicates a consistent sign of fold changes whereas red indicates an opposite sign of fold changes. Grey indicates genes that are not differentially expressed in the presence of CS and CH (less than 2-fold change marked by the dashed lines). Spearman’s rho and p-value are shown, which were computed using the spearmanr from the scipy package in Python. d, Differentially expressed genes that are involved in C. difficile’s fermentation and energy metabolism. The fold changes were shown next to the gene annotations. Blue indicates up-regulated genes and red indicates down-regulated genes. e, Bar plot of the log-transformed fold changes of selected highly differentially expressed genes of C. difficile DSM in the presence of C. hiranonis. Horizontal dashed lines indicate a 2-fold change. f, Schematic highlighting substantial transcriptional changes in C. difficile in the presence of CH compared to CS. g, Schematic of the mice experiment. Mice were orally gavaged with CH for one week prior to C. difficile DSM27147 challenge (n=5). As a control, one group of mice without CH was challenged C. difficile (n=4). h, Percent survival of the mice after C. difficile gavage. i, Percent of initial weight after C. difficile gavage. Data points indicate individual mice, and the line indicates the average of all mice in the group. The horizontal dashed line indicates the weight limit of 75%. Mice with weights that dropped below the limit were sacrificed. Asterisks indicate the p-value from unpaired t-test between the weight of mice gavaged with CH and mice without CH: * indicates p<0.05. j, C. difficile abundance in the fecal and cecal content over time as determined by CFU counting on C. difficile selective plates. The horizontal dashed line indicates the limit of detection. Asterisks indicate the p-value from unpaired t-test between the CFU of C. difficile of mice gavaged with CH and mice without CH: ** indicates p<0.01, * indicates p<0.05. k, Total amount of C. difficile toxin per mg of cecal content. Data were shown as mean ± s.d. (n=5). Asterisks indicate the p-value from unpaired t-test between the toxin amount from the cecal samples of mice gavaged with CH and mice without CH: * indicates p<0.05. Parts of the figure are generated using Biorender.
Notably, co-culturing with CH yielded a massive alteration in the expression of fermentation and energy metabolism genes in C. difficile (Fig. 5d). Many genes involved in glycolysis, pentose phosphate pathway, Stickland metabolism, Wood-Ljungdahl Pathway (WLP), and fermentation pathway were highly up-regulated in the presence of CH. Since ATP synthases were down-regulated, it is possible that the cells were forced to generate ATP through the aforementioned pathways to perform essential cellular functions. C. difficile couples certain fermentation pathways, such as the butyrate fermentation, to the generation of a sodium/proton gradient using electron bifurcation in combination with the membrane-spanning Rnf complex 75. Electron bifurcation couples the NADH-dependent reduction of a substrate to the reduction of ferredoxin. The free energy resulting from the redox potential difference between ferredoxin and NAD+ is used to transport ions across the membrane through the Rnf complex, generating NADH in the process. Since electron bifurcating enzymes were down-regulated, Rnf complex genes were up-regulated, and glycolysis genes were highly up-regulated, C. difficile likely needed to generate NAD+ in the presence of CH. This could be achieved by the reductive Stickland metabolism or the WLP coupled to fermentation pathways. C. difficile heavily relies on Stickland reactions for reductive pathways 76. When there are abundant preferred electron acceptor substrates such as proline and glycine, the WLP is not used by C. difficile. However, C. difficile uses WLP as its terminal electron sink to support growth on glucose when C. difficile lacks Stickland amino acid acceptors 76. Therefore, the concomitant up-regulation of the proline and glycine reductases and genes involved in the WLP suggests that C. difficile competed with CH over proline and glycine and thus resorted to the WLP as an alternative electron-accepting pathway.
In addition to altering C. difficile’s metabolism, CH impacted the expression of genes involved in various important cellular pathways such as stress responses (Fig. 5e, S16g). For instance, genes related to two-component systems that enable bacteria to adapt to diverse environmental changes, and many stress response genes including recA and relA were highly up-regulated. Consistent with the inhibition of C. difficile’s toxin production in the presence of CH as measured by ELISA, the toxin A (tcdA) gene was down-regulated in the presence of CH. Since C. difficile toxin expression is tightly linked with metabolic activity 72, toxin inhibition by CH could be associated with the massive changes in C. difficile’s metabolism. In sum, CH blocked access of C. difficile to alternative resource niches and led to a global alteration in the metabolic activities of C. difficile, providing insights into mechanisms that could mediate inhibitory inter-species interactions that are robust to strain and nutrient variability (Fig. 5f). In contrast, another closely related species, CS, loses its inhibitory activity in the presence of multiple carbohydrates since C. difficile can utilize mannitol, which is not utilized by CS.
C. hiranonis ameliorates the effects of C. difficile in germ-free mice
To examine whether CH could inhibit C. difficile in vivo, we used gnotobiotic mice and orally gavaged them with CH for one week to allow time for colonization and immune development 77 (Fig. 5g). After one week, the mice were orally gavaged with the hypervirulent C. difficile DSM27147. As a control, we also gavaged germ-free mice with C. difficile (no CH group). Four days after C. difficile inoculation, 50% of the mice from the control group (no CH) died (Fig. 5h). Although mice orally gavaged with CH also exhibited a decreasing trend in weight during the first few days of C. difficile gavage, the relative reduction in weight was lower than the control group (5.3% and 8.5% higher after 3 and 4 days of C. difficile challenge respectively) (Fig. 5i). While CH has a low relative abundance when co-cultured with C. difficile DSM27147 in vitro (~11% in the glucose media and ~18% in the mixed carbohydrates media after 24 h of growth), CH was highly abundant in mice (~72% after 7 days of C. difficile challenge) (Fig. S20). The mice harboring CH also displayed lower C. difficile abundance and toxin concentration than in the absence of CH (Fig. 5j–k). Thus, CH ameliorates the disease severity of a C. difficile challenge in a murine model.
DISCUSSION
Defined communities that have been optimized to inhibit C. difficile hold tremendous promise to overcome the limitations of FMT for treating CDI. For instance, oral consortia from VE303 (Vedanta Biosciences) has passed the phase 2 clinical trial for rCDI 11 and is currently undergoing phase 3. Robustness of anti-C. difficile activity to environmental variability is not typically considered in the design process. This potential lack of robustness may contribute to the failure of the community to successfully treat a fraction of patients (~14% after a few months) 78,79. The C. difficile inhibitory activity of defined communities may be more variable than fecal communities used during FMT due to their reduced functional redundancy, richness, and diversity 7,8. Therefore, there is a need to understand how anti-C. difficile activity of human gut communities varies in response to diverse C. difficile strain backgrounds and environmental contexts (e.g. variations in diet).
Systems biology approaches combining experiments and computational modeling have been used to understand C. difficile metabolism and virulence 80, study interactions with human gut communities 31, and design a bacterial consortium that protects against CDI 81. For instance, genome-scale metabolic models were used to guide the design of communities with enriched amino acid metabolism pathways associated with successful FMTs for rCDI treatment 81. However, the robustness of the designed communities to environmental context was not evaluated, and thus it is unknown whether they are effective across different strain or nutrient contexts. We used a data-driven approach to dissect interspecies interactions and toxin production of genotypically diverse C. difficile strains in human gut communities under different nutrient environments. We combined high-throughput in vitro experiments with computational modeling to deduce interaction networks impacting each C. difficile strain in different media conditions. We showed that C. difficile strain variation could directly or indirectly shape interspecies interactions of human gut microbiota. In addition, strain-level variability has a major impact on toxin production in communities, adding another layer of complexity to the design of robust anti-C. difficile consortia. The nutrient environment also plays a key role in shaping the interactions between C. difficile and the gut communities. Although it has been reported that C. difficile inhibition is prevalent in media that promote resource competition 31, we showed that it is sparse when there are multiple preferred carbohydrates for C. difficile. Our study showcases our quantitative systems-biology approach to map context-dependent interactions and provides insights into the mechanisms that could enhance the robustness of inhibition across strains and environments. Based on our results, interactions that lead to global shifts in metabolism and other cellular processes may exhibit greater robustness to environmental variability. More broadly, this framework considering robustness as a feature could be applied to the design of anti-pathogen bacterial therapeutics.
Of the 7 gut bacteria used to study community interactions, CS and CH are the only two species that can utilize amino acids to perform Stickland metabolism, similar to C. difficile. In the media supplemented with only glucose as a sole carbohydrate, CS and CH have a stronger magnitude of C. difficile inhibition compared to the other species (Fig. 2d). These inhibitory interactions may stem from competition over Stickland amino acids in addition to glucose, whereas the other gut bacteria only compete for glucose. Previous work has shown that introducing Stickland amino acid competitors can protect mice from CDI 33. In sum, competition over Stickland amino acids is an attractive strategy to enhance inhibition against C. difficile. However, in the media containing multiple carbohydrates, CH is the only species that can inhibit C. difficile whereas CS lost this inhibition capability (Fig. 2e). In a different rich media, CH inhibition of C. difficile was proposed to arise partially from resource competition and not via external pH change or extracellular protein release 31. Our results go beyond this study by demonstrating that CH suppresses the growth and toxin production of diverse C. difficile strains in two distinct nutrient environments, yields a massive change in the metabolic activity of C. difficile, and improves disease severity in germ-free mice (Fig. 5). Although, to our knowledge there is no evidence regarding the role of CH on CDI outcomes in humans, the presence of CH is negatively associated with C. difficile colonization in dogs and cats 82–84.
A key question is how CH maintains its inhibitory effect on C. difficile when provided with multiple C. difficile-preferred carbohydrates, whereas the inhibitory capability is abolished for CS. Since CH and CS are closely related, we would expect a similar transcriptional response in C. difficile in the presence of these two species. Genome-wide transcriptional profiling revealed that C. difficile exhibited a substantial difference in gene expression in the presence of CH and CS (Fig. 5c). These data provided insights into the unique transcriptional signature of CH’s inhibition mechanism, which was not observed in the presence of CS. Although our results support the hypothesis that C. difficile competes for Stickland amino acids with CS, C. difficile could switch to mannitol as an alternative nutrient source, which cannot be utilized by CS (Fig. 5f). By contrast, CH and C. difficile share highly similar metabolic niches, which may substantially limit the available resources for C. difficile. Therefore, C. difficile increased expression of enzymes in core energy-generating metabolic pathways in the presence of CH, including glycolysis, pentose phosphate pathway, Stickland metabolism, Wood-Ljungdahl Pathway (WLP), and fermentation (acetate, ethanol, and butyrate production) (Fig. 5d), which were not observed when CS was present. Because C. difficile normally favors Stickland fermentation over WLP as their main electron-accepting pathway, the activation of WLP suggests that CH successfully competes for reductive Stickland amino acids and forces C. difficile to use WLP as their alternative electron sink 76. These massive alterations in C. difficile core metabolism also impact virulence such as toxin production. Further, C. difficile upregulated stressed-related pathways (Fig. 5e), which were not observed in the presence of CS (Fig. S16d–e). Beyond resource competition, CH may produce an antimicrobial targeting C. difficile as previously hypothesized 31 that contributes to this unique transcriptional response. Future work could mine the biosynthetic gene clusters in CH for potential antimicrobial compounds and perform targeted and untargeted metabolomics to provide deeper insights into the mechanisms of inter-species interaction.
Certain bacteria in the gut have been reported to increase C. difficile toxin production and enhance their fitness and virulence in vivo, such as the opportunistic pathogen Enterococcus faecalis 70. Some metabolites produced by gut microbes such as butyrate could also increase C. difficile toxin, albeit moderately 85. However, we found that the enhancement of C. difficile toxin is sparse among human gut commensals (toxin production per unit biomass is enhanced in only ~16% of all communities compared to monocultures). In addition, strain-level variability played a larger role in toxin production in communities than inferred gLV growth-mediated inter-species interactions. Since toxin production is tightly linked with metabolism 73,86, genotypic variations among C. difficile strains would impact their toxin production profiles. The lack of an informative relationship between growth-mediated inter-species interactions and toxin production suggests that inhibiting C. difficile growth may not always protect against CDI unless C. difficile is excluded from the community. Thus, the identification of C. difficile inhibitors should consider both inhibition of growth and toxin production. Further, we discovered that C. difficile strains with similar hypervirulent genotypes (DSM 27147 and MS014) have different toxin production profiles in communities. By contrast, an isolate from a healthy individual (Strain 296) has a similar toxin production profile with genetically distinct isolates from patients with CDI (MS008 and MS014) (Fig. 4c, S19a–b). This indicates that rather than the genotype of C. difficile alone, community context is a major variable shaping C. difficile toxin production.
A grand challenge for microbiome engineering is the rational design of microbial communities as living therapeutics for treating multiple human diseases involving alterations in the human gut microbiome. For CDI, a potential driver of the efficacy of FMT is the high richness and diversity of species in the fecal samples, which could repopulate the gut flora and restore colonization resistance. This is further supported by the fact that most of the products with successful outcomes in clinical trials so far are communities derived from stool samples, thus having high species richness 87. However, due to heavy reliance on donors, these stool-derived communities suffer from batch-to-batch variations and are designed without any knowledge of molecular mechanisms of C. difficile inhibition. This could be overcome by using defined communities that are standardized and optimized to inhibit C. difficile. However, the number of strains in a bacterial therapeutic currently scales with manufacturing cost. Our study shows that in the media with multiple carbohydrates preferred by C. difficile mimicking a perturbed gut condition, species richness is no longer a strong determinant of C. difficile inhibition, but rather the identity of the species in the community (Fig. 2g). Therefore, it is conceivable that small bacterial communities with high anti-C. difficile activity that is robust to environmental variability could be identified. We identified CH as a “universal” C. difficile growth and toxin inhibitor of genotypically diverse C. difficile strains and nutrient environments. Therefore, CH may represent a unique class of species that could be used to build a robust anti-C. difficile bacterial therapeutics to environmental variability. Future work will elucidate how to expand the number of species communities containing CH to further enhance the anti-C. difficile activity and robustness to environmental variability in the mammalian gut.
METHODS
Strain, media, and growth conditions
The strains used in this work were obtained from the sources listed in Table S1. There are a total of 18 C. difficile isolates. Nine of them were obtained from diseased patients who were diagnosed and treated for C. difficile infection (CDI) in the UW-Madison Hospital 88. These isolates were subjected to C. difficile nucleic acid amplification test (NAAT) (GeneXpert) via admission stool sample, and bacterial identification was confirmed via sequencing of the 16S rRNA gene. The other nine isolates were obtained from healthy individuals from the Winning the War on Antibiotic Resistance (WARRIOR) project 89. Briefly, the WARRIOR project collects biological specimens, including nasal, oral, and skin swabs and saliva and stool samples, along with extensive data on diet and MDRO risk factors, as an ancillary study of the Survey of the Health of Wisconsin (SHOW) 90. WARRIOR participants include 600 randomly selected Wisconsin residents aged 18 and over, and C. difficile isolates were identified by anaerobic inoculation of stool samples in prereduced C. difficile Brucella Broth and then plated on Brucella agar plates. Colonies with correct morphology were identified using Gram staining and catalase testing. The presence of toxin genes is assessed using an in-house PCR assay and bacterial identification is confirmed via sequencing of the 16S rRNA gene.
Single-use glycerol stocks were prepared as described previously 44. The media used in this work are anaerobic basal broth (ABB, Oxoid) for growing starter cultures, and in-house defined media (DM) for all of the experiments. DM29 is the defined media without any carbohydrate source (recipe listed in Table S2), which was formulated to support the growth of phylogenetically diverse human gut bacteria 44 and has been used to study inter-species interactions of human gut communities 91,92. For supplementation of single carbohydrate sources to DM29, the carbohydrates were added to a final concentration of 5 g/L. For mixed carbohydrates media that mimics a perturbed gut condition, we modified DM29 by adding carbohydrate sources that are preferred by C. difficile and could increase in abundance upon antibiotic treatment 45,48,58–61, which are glucose, sorbitol, mannitol, trehalose, succinate, galactose, GalNAc, GlcNAc, and sialic acid at a concentration of 2 g/L each.
For all experiments, cells were cultured in an anaerobic chamber (Coy Lab products) with an atmosphere of 2.0 ± 0.5% H2, 15 ± 1% CO2, and balance N2 at 37 °C. Starter cultures were inoculated by adding 200 μL of a single-use 25% glycerol stock to 5 mL of anaerobic basal broth media (ABB) and grown at 37 °C without shaking.
Growth characterization in media with different carbohydrate sources
Starter cultures of C. difficile isolates and gut commensal bacteria were prepared. The cell pellets from starter cultures were collected by centrifugation at 3,000 × g for 10 min, and then washed with DM29 media. The washed cell pellets were resuspended into DM29 media to a final OD600 of approximately 0.1. These cultures were inoculated into a 96-well plate (Greiner Bio-One) containing DM29 supplemented with specific carbohydrate sources at a concentration of 5 g/L to an initial OD600 of 0.01 (3 biological replicates for each strain). These plates were covered with a gas-permeable seal (Breathe-Easy® sealing membrane) and incubated at 37 °C anaerobically. Cell growth determined by OD600 was monitored using Tecan F200 plate reader every 3 h using robotic manipulator arm (RoMa) integrated with our Tecan Freedom Evo 100 instrument.
Logistic growth model
The logistic growth model was used to describe C. difficile population growth dynamics in monoculture experiments. The logistic growth model for species takes the following form:
where is the absolute abundance of species , parameter is its maximum growth rate, and is its carrying capacity. Due to the unique growth profile of C. difficile isolates, we cut time points where the OD600 drops below > 10% to exclude the highly variable phase. Thus, the steady-state solution of the model is the carrying capacity (i.e. the value of when equals 0). We also excluded data points less than 120% of the initial OD600 (OD600 at t=0) to exclude the lag phase which is not captured in the logistic model. A custom MATLAB script is used to estimate the parameters in the logistic growth model. For each species , the model is fitted to experimental data with L2 regularization. Specifically, given a series of experimental OD600 measurements, , and a series of OD600 simulated using parameter at the same time intervals, , the optimization scheme minimizes the cost function:
where is the L2 regularization parameter, which was set to be 0.02 for all species, and indicates vector 2-norm. Solutions to the logistic growth model were obtained using the ode15s solver and the optimization problem was solved using FMINCON in MATLAB (R2022a).
Fluorescence microscopy of C. difficile
Starter cultures of several C. difficile strains were prepared. The cell pellets from starter cultures were collected by centrifugation at 3,000 × g for 10 min, and then washed with DM29 media. The washed cell pellets were resuspended into DM29 media to a final OD600 of approximately 0.1. These cultures were inoculated into new culture tubes containing either DM29 media or DM29 supplemented with 5 g/L glucose to an initial OD600 of 0.01 by adding 500 μl of washed starter cultures to 4.5 mL media. After 16 h and 40 h of growth, 100 μl aliquots were taken, stained with SYBR Green dye, and viewed with a microscope (Nikon Eclipse Ti-E inverted microscope) at 20× dry objective with appropriate filter sets. Images were captured with Photometrics CoolSNAP Dyno CCD camera and associated software (NIS-Elements Ver. 4.51.00).
Whole genome sequencing of C. difficile isolates
C. difficile DSM 27147 and the 18 isolates used in this study were subjected to whole-genome sequencing. Strains were grown from a single colony to OD600 of 0.3, and then centrifuged to obtain the cell pellets. Genomic DNA was extracted using Qiagen DNeasy Blood and Tissue Kit according to the manufacturer’s protocol. The harvested DNA was detected by the agarose gel electrophoresis and quantified by a Qubit fluorometer. The genomic DNA was sent to SeqCenter (Pittsburgh, PA, USA) for paired-ends Illumina sequencing. Sample libraries were prepared using the Illumina DNA Prep kit and IDT 10 bp UDI indices, and sequenced on an Illumina NextSeq 2000, producing 2 × 151 bp reads. Demultiplexing, quality control, and adapter trimming were performed with bcl-convert (v3.9.3) Illumina software. The clean bases of each sample are ~1 billion bp. The WGS raw data was submitted and is accessible in BioProject PRJNA902807.
Whole-genome sequencing data analysis
SPAdes Genome Assembler 93 is used to assemble contigs from the whole-genome sequencing data with the following parameters: spades.py --pe1–1 (forward read fastq file) --pe1–2 (reverse read fastq file) --isolate -o (output name). The --isolate option was used due to the high-coverage sequencing data. We then used FastANI 94 to compute the whole-genome Average Nucleotide Identity (ANI) values between pairs of isolates, which is defined as the mean nucleotide identity of orthologous gene pairs shared between two microbial genomes. The following parameters are used: fastANI --ql (list of contigs.fasta files of all isolates from SPAdes) --rl (list of contigs.fasta files of all isolates from SPAdes) –matrix -o (output name). The newly sequenced genomes are high-quality drafts with a low number of contigs (median 61 [range 40–458]) and high N50 (median 285,062 [range 146,596–782,135]) (Table S3).
Annotation of the contigs was performed using DFAST 95. For further comparative genomic analyses, the gene content across 19 C. difficile strains was analyzed by clustering all predicted coding sequences into orthologous groups 96 (Fig. S3b). Clustering of gene orthologs was carried out using ProteinOrtho6 96 across variable coverage and identity settings using BlastP for alignment. Distributions of OGs show a high degree of strain variability with many genes in a limited subset of strains (Fig. S3c).
To get Gene Ontology (GO) information, we used BlastP of the NCBI Blast Suite 97 against the proteins from all C. difficile strains that exist in UniProt database (downloaded from UniProtKB on 10th November 2022) at 1E-3 E-value cutoff. Following BlastP, GO information such as biological process, molecular function, and cellular compartment of each protein was extracted from UniProt. To align the sequence of specific genes such as Toxin A (tcdA), Toxin B (tcdB), RNA polymerase (rpoB, rpoB’), we used Clustal Omega multiple sequence alignment tool 98.
We evaluated the genetic diversity of our C. difficile strains in the context of the other 118 publicly available C. difficile genomes (Table S4). Phylogenomic analysis was performed using GToTree 99 on the C. difficile isolates dataset along with 118 public strains. SPAdes FASTA files were used as inputs to GToTree analysis and the resulting tree was visualized using the Interactive Tree of Life web-based tool 100. Our isolates span 64% of the C. difficile phylogeny of this dataset (9 of 14 major tree branches) (Fig. S3d).
To get the relative copy number of the genes in each isolate, the Illumina paired-end reads were aligned to the gene list from DFAST using Bowtie2 101. The detection of conjugative systems was performed using CONJScan 102 module of MacSyFinder. The detection of phages was performed using VirSorter 103.
Construction of strain-specific genome-scale metabolic models to assess variations in metabolism
Raw sequencing data was first preprocessed using fastp 0.22.0 104, trimming the first 5bp at the 5’ end and trimming the 3’ end with a sliding window approach, maintaining a minimum quality score of 20. Reads shorter than 60bps were omitted. 85%–95% of reads passed all filters across samples, yielding 2.9M to 7.1M reads per sample. Preprocessed reads were assembled using MEGAHIT 1.2.9 105 using default k-mer sizes and a minimum contig length of 1000bps. Completeness and contamination were assessed using CheckM2 1.0.1 106 yielding completeness of >99.9% for all assemblies while maintaining contamination below 1.5%. Bacterial species identity was verified using the GTDB toolkit 2.1.0 107 using the database version 207. All assemblies were classified as Clostridioides difficile by average nucleotide identity and placement in the GTDB reference tree.
De novo gene predictions of the assemblies were performed by Prodigal 2.6.3 108. Metabolic draft models were built using CarveMe 1.5.2 109 from the isolate gene predictions using DIAMOND 2.1.6 110 with additional options of “--more-sensitive –top 10 ”. Media composition was translated by manual mapping to the BiGG database 111. Salts were decomposed into their aqueous phase ions to mimic the effect of hydrolysis in the translated medium. Draft models were then gapfilled to be able to grow on the mapped media. During gapfilling, no more than 10 new reactions and 6 new metabolites were added to each model. Model quality was assessed using MEMOTE 0.13.0 112. Metabolic reaction content was assessed using the “metabolic_dist” function from MICOM 0.32.5 113 where metabolic distances were calculated by the Jaccard distance of metabolic reaction absence/presence (1 - shared reactions / total reactions) for each pair of reconstructed models.
Bacterial genome DNA extraction for 16S amplicon sequencing
All the genomic DNA (gDNA) extraction and next-generation sequencing sample preparation were performed as described previously 31,44. Bacterial gDNA extractions were carried out using a modified version of the Qiagen DNeasy Blood and Tissue Kit protocol in 96-well plates. Briefly, cell pellets were resuspended in 180-μl enzymatic lysis buffer containing 20 mg/ml lysozyme (Sigma-Aldrich), 20 mM Tris–HCl pH 8 (Invitrogen), 2 mM EDTA (Sigma-Aldrich), and 1.2% Triton X-100 (Sigma-Aldrich), and then incubated at 37°C at 600 RPM for 30 min. Samples were treated with 25 μL 20 mg/ml Proteinase K (VWR) and 200 μL buffer AL (Qiagen), mixed by pipette, and then incubated at 56°C at 600 RPM for 30 min. Samples were treated with 200 μL 200 proof ethanol (Koptec), mixed by pipette, and transferred to 96-well nucleic acid binding plates (Pall). After washing with 500 μL buffer AW1 and AW2 (Qiagen), a vacuum was applied for 10 min to dry excess ethanol. Genomic DNA was eluted with 110 μL buffer AE (Qiagen) preheated to 56°C and then stored at −20°C.
Genomic DNA concentrations were measured using the Quant-iT™ dsDNA Assay Kit (Invitrogen) with a 6-point DNA standard curve (0, 0.5, 1, 2, 4, 6 ng/μL biotium). 1 μL of samples and 5 μL of standards were diluted into 95 μL of 1× SYBR green (Invitrogen) in TE buffer and mixed by pipette. Fluorescence was measured with an excitation/emission of 485/535 nm (Tecan Spark). Genomic DNA was then normalized to 2 ng/μL by diluting in molecular grade water (VWR International) using a Tecan Evo Liquid Handling Robot.
Dual-indexed primers for multiplexed amplicon sequencing of the V3-V4 region of the 16S rRNA gene were designed as described previously 38,44. PCR was performed using the normalized gDNA as template and Phusion High-Fidelity DNA Polymerase (Thermo Fisher) for 25 cycles with 0.05 μM of each primer. Samples were pooled by plate, purified using the DNA Clean & Concentrator™-5 kit (Zymo) and eluted in water, quantified by NanoDrop, and combined in equal proportions into a library. The library was quantified using Qubit 1× HS Assay (Invitrogen), diluted to 4.2 nM, and loaded at 10 pM onto Illumina MiSeq platform for 300-bp paired-end sequencing using MiSeq Reagent Kit v2 (500-cycle), or loaded at 21 pM using MiSeq Reagent Kit v3 (600-cycle) depending on the desired sequencing reads.
16S amplicon sequencing data analysis to determine community composition
Sequencing data were analyzed as described previously 31,38. Briefly, reads were demultiplexed with Basespace FastQ Generation, and the FastQ files were analyzed using custom Python scripts. Paired reads were merged using PEAR (Paired-End reAd mergeR) v0.9.0 114. A reference database containing 16S V3-V4 region of each species in the study was created by assembling consensus sequence based on sequencing results of each monospecies. Reads were mapped to the reference database using the mothur v1.40.5 command classify.seqs using the Wang method with bootstrap cutoff value of 60% 115,116. Relative abundance was calculated by dividing the read counts mapped to each organism by the total reads in the sample. Absolute abundance was calculated by multiplying the relative abundance of an organism by the OD600 of the sample. Samples were excluded from further analysis if > 1% of the reads were assigned to a species not expected to be in the community (indicating contamination).
Parameter estimation of generalized Lotka-Volterra models
The generalized Lotka-Volterra (gLV) model is a set of coupled ordinary differential equations that describe the growth of interacting species over time,
where is the abundance of species and is the total number of species. Model parameters that need to be estimated from data include the species growth rate, denoted as , and coefficients that determine how species affects the growth of species , denoted as . The data used for parameter estimation is the growth of species over time under different inoculation conditions. For monoculture growth data, we use OD600 measurements only, whereas for community data, this was obtained by multiplying the relative abundance obtained from 16S sequencing by the total OD600.
A prior over the parameter distribution is set so that growth rates have a mean of 0.3, self-interaction terms have a mean of −1, and inter-species interaction terms have a mean of −0.1. Given a dataset of measured species abundances over time after inoculating different combinations of species, the model parameters are determined by minimizing a cost function given by a weighted squared difference between model-predicted species abundances and measured abundances and a penalty for deviations from the prior mean. Using the fitted parameter estimates, the covariance of the posterior parameter distribution is approximated as the inverse of the Hessian (matrix of second derivatives) of the cost function with respect to the model parameters. The Expectation-Maximization (EM) algorithm is used to optimize the precision of the prior parameter distribution and the precision of the noise distribution, which collectively determine the degree to which estimated parameters are penalized for deviations from the prior mean 117. In other words, the precision of the prior and noise are hyperparameters that determine the degree of regularization. To evaluate model prediction performance on held-out data, we performed 10-fold cross validation where the degree of regularization was optimized using the EM algorithm and only community samples were subjected to testing (i.e. monoculture data was reserved only for model training). See Supplementary Text for a more detailed description of parameter estimation and the EM algorithm.
Bayesian experimental design to guide community experiments
We define an experimental design as a set of unique inoculation conditions, where in each condition each species may be present or absent, and the total inoculation density sums to OD600 of 0.01. We used a Bayesian experimental design approach to select experimental conditions that were expected to collectively minimize parameter uncertainty as quantified by the expected Kullback-Leibler (KL) divergence between the posterior parameter distribution and the prior parameter distribution (See Equation 20 in Supplementary Text).
Growth of synthetic gut communities with C. difficile isolates
Starter cultures of all C. difficile isolates and commensal gut bacteria were prepared. The cell pellets from starter cultures were collected by centrifugation at 3,000 × g for 10 min, and then washed with DM29 media. The washed cell pellets were resuspended into DM29 media to a final OD600 of approximately 0.1.
For the growth experiment of each of the 19 C. difficile strains with 8-member gut bacteria at a single timepoint (Fig. S6b–c), the monocultures of individual C. difficile strains and each gut species were mixed in equal proportions based on OD600 and inoculated into 2 mL 96-deep-well plates (Nest Scientific) containing DM29 supplemented with specific carbohydrate sources (glucose, mannitol, galactose, or mucin) to an initial OD600 of 0.01. The initial OD600 of each species is therefore 0.0011 (0.01 divided by 9, the number of species in the community). As a control, we also inoculated a mixture of gut species without C. difficile to the same initial OD600 of 0.01. There are a total of 4 plates for media with different carbohydrate sources, each containing 20 communities (18 for different C. difficile isolates, 1 for C. difficile DSM 27147 strain, and 1 for the gut community without C. difficile), with 3 biological replicates for each community. These plates were covered with a gas-permeable seal (Breathe-Easy® sealing membrane) and incubated at 37 °C anaerobically for 24 hours to capture C. difficile growth prior to the highly variable late stationary phase response. At the end of the experiment, OD600 was measured with a Tecan F200, and cell pellets were collected for DNA extraction, PCR amplification, and NGS sequencing.
For the growth experiment of each of the 19 C. difficile strains with 7-member gut bacteria at a single time point in the mixed carbohydrates media (Fig. S9e), the monocultures of individual C. difficile strains and each gut species were mixed in equal proportions based on OD600 and inoculated into 2 mL 96-deep-well plate (Nest Scientific) containing the mixed carbohydrates media to an initial OD600 of 0.01. The initial OD600 of each species is therefore 0.00125 (0.01 divided by 8, the number of species in the community). As a control, we also inoculated a mixture of gut species without C. difficile to the same initial OD600 of 0.01. The deep-well plate was covered with a gas-permeable seal (Breathe-Easy® sealing membrane) and incubated at 37 °C anaerobically for 24 hours. At the end of the experiment, OD600 was measured with a Tecan F200, and cell pellets were collected for DNA extraction, PCR amplification, and NGS sequencing.
For time-course growth experiment of 4 different C. difficile strains with 7 gut bacteria in the glucose media (Fig. 2d) or mixed carbohydrates media (Fig. 2e), C. difficile and gut bacteria were mixed and grown in 2–8 member communities. The community combinations were generated from the Bayesian experimental design (Table S8). The monocultures of C. difficile strains and each gut species were mixed in equal proportions based on OD600 and inoculated into 2 mL 96-deep-well plates (Nest Scientific) containing the glucose media (Fig. 2d), or the mixed carbohydrates media (Fig. 2e) to an initial OD600 of 0.01. For instance, the initial OD600 of each species in a 2-member community is therefore 0.005 (0.01 divided by 2, the number of species in the community). These plates were covered with a gas-permeable seal (Breathe-Easy® sealing membrane) and incubated at 37 °C anaerobically. After 12 and 24 hours of growth, OD600 was measured with a Tecan F200, and cell pellets were collected for DNA extraction, PCR amplification, and NGS sequencing. For longer-term growth experiments in Fig. S9c–d, the communities were grown for 72 hours and passaged using a 1:20 dilution at 24 and 48 h to observe community assembly over three batch culture growth cycles and capture the longer-term behavior of the consortia.
For time-course growth experiment of 5 different C. difficile strains with 25 gut bacteria in the mixed carbohydrates media (Fig. 4), individual C. difficile strain and gut bacteria were mixed and grown in pairwise and full 26-member communities. The monocultures of C. difficile strains and each gut species were mixed in equal proportions based on OD600 and inoculated into 2 mL 96-deep-well plates (Nest Scientific) containing the mixed carbohydrates media to an initial OD600 of 0.01. For pairwise communities, the initial OD600 of each species is 0.005 (0.01 divided by 2), and for 26-member communities, the initial OD600 of each species is 0.000385 (0.01 divided by 26). These plates were covered with a gas-permeable seal (Breathe-Easy® sealing membrane) and incubated at 37 °C anaerobically. After 12 and 24 hours of growth, OD600 was measured with a Tecan F200, and cell pellets were collected for DNA extraction, PCR amplification, and NGS sequencing. Supernatants of communities at 24 hours of growth were collected for toxin quantification using ELISA.
C. difficile toxin measurements using ELISA
Toxin (both TcdA and TcdB) concentrations in C. difficile monocultures or co-cultures, and toxin titer in mice cecal samples were determined by comparison to a standard curve using ELISA (tgcBiomics, Germany). The blank media used to grow the cultures were also included in the assay to measure any background noise. Samples subjected to toxin measurements in this study were processed in parallel at the same time using the same batch of ELISA kits to minimize batch-to-batch variations and ensure comparable results.
Growth of C. aerofaciens and B. thetaiotaomicron in the sterilized spent media of different C. difficile strains
Starter cultures of C. difficile DSM27147, MS001, MS008, and MS014 were prepared. The cell pellets from starter cultures were collected by centrifugation at 3,000 × g for 10 min, and then washed with DM29 media. The washed cell pellets were resuspended into DM29 media to a final OD600 of approximately 0.1. Each of the C. difficile strains was inoculated into new culture tubes containing DM29 media supplemented with 5g/L glucose to an initial OD600 of 0.01. Culture tubes were incubated at 37°C with no shaking. After an incubation time of 24 h, cultures were spun down aerobically at 3,000 × g for 20 min and sterile filtered using Steriflip 0.2-μM filters (Millipore-Sigma) before returning to the anaerobic chamber.
Then, starter cultures of C. aerofaciens and B. thetaiotaomicron were prepared. The cell pellets from starter cultures were collected by centrifugation at 3,000 × g for 10 min, and then washed with DM29 media. The washed cell pellets were resuspended into DM29 media to a final OD600 of approximately 0.1. CA-BT coculture was inoculated in the sterilized spent media of each C. difficile strain mixed with fresh media (DM29 supplemented with 5g/L glucose) at an equal ratio to replenish the nutrients. CA and BT were inoculated at an equal initial abundance to a final OD600 of 0.01 in 2 mL 96-deep-well plates (Nest Scientific) that were covered with gas-permeable seals (BreatheEasy), and incubated at 37°C with shaking. After 24 h, OD600 of the cultures were measured and the cell pellets were collected for DNA extraction, PCR amplification, and NGS sequencing.
Growth of C. difficile strains in the sterilized spent media of gut bacteria
Starter cultures of commensal gut bacteria were prepared. The cell pellets from starter cultures were collected by centrifugation at 3,000 × g for 10 min, and then washed with DM29 media. The washed cell pellets were resuspended into DM29 media to a final OD600 of approximately 0.1. Each of the gut bacteria was inoculated into new culture tubes containing the mixed carbohydrates media to an initial OD600 of 0.01. Culture tubes were incubated at 37°C with no shaking. After an incubation time of 24 h, cultures were spun down at 3,000 × g for 20 min and sterile-filtered using Steriflip 0.2-μM filters (Millipore-Sigma). Media control (mixed carbohydrates media) was spun down and filtered in parallel with samples. The pH of the sterilized spent media was adjusted to the same value as the media control.
Then, starter cultures of C. difficile strains were prepared. The cell pellets from starter cultures were collected by centrifugation at 3,000 × g for 10 min, and then washed with DM29 media. The washed cell pellets were resuspended into DM29 media to a final OD600 of approximately 0.1. The C. difficile strains were inoculated in the sterilized spent media of each gut bacteria (and the mixed carbohydrates media as a control) to a final OD600 of 0.01 in 96-well microplates that were covered with gas-permeable seals (BreatheEasy). The plates were incubated at 37°C with shaking, and OD600 was measured every 3 h (Tecan Infinite Pro F200).
Transcriptome profiling
C. difficile DSM27147 monoculture, C. difficile MS001 monoculture, CD DSM-CS coculture, CD MS001-CS coculture, and CD DSM-CH coculture conditions were inoculated from starter cultures into individual culture tubes containing the mixed carbohydrates media. For monoculture conditions, C. difficile was inoculated to an OD600 of 0.01. For cocultures, C. difficile and CS or CH were inoculated to an equal ratio (OD600 of 0.005 each). The cultures were incubated anaerobically at 37°C with no shaking for ~7 h until the culture reached the exponential phase (OD600 ~0.2). 1000 μL of the culture was taken for OD600 measurement and total DNA extraction for next-generation sequencing, and 2000 μL of the culture was taken for total RNA extraction for transcriptomics. 4000 μL of RNAprotect (Qiagen) was added to 2000 μL of culture and incubated for 5 min at room temperature. Cultures were then centrifuged at room temperature for 10 min at 3000 g and the supernatant was carefully removed. Cell pellets were immediately subjected to RNA extraction using acidic phenol bead-beating method. Pellets were resuspended in 500 μL 2× Buffer B (200 mM sodium chloride, 20 mM ethylenediaminetetraacetic acid) and transferred to 2 mL microcentrifuge tubes containing 500 μL Phenol:Chloroform:IAA (125:24:1, pH 4.5) and 210 μL 20% sodium dodecyl sulfate and were bead-beated with acid washed beads (Sigma G1277) for 3 min. All solutions used for RNA extraction were RNAse-free. Samples were centrifuged at 4°C for 5 min at 7,200 g, and 600 μL of the upper aqueous phase was added to 60 μL 3 M sodium acetate and 660 μL cold isopropanol and chilled on ice for 5 min before freezing for 5 min at −80°C. Samples were centrifuged at 4°C for 15 min at 18,200 g, the supernatant was decanted, and the pellet was washed with cold 100% ethanol. The pellets were dried in a biosafety cabinet for 15 min and then resuspended in 100 μL RNAse-free water. Samples were purified using RNeasy Mini Kit (Qiagen) and genomic DNA was removed using RNAse-Free DNase Set (Qiagen). Two replicates of each condition were sent to Novogene Corporation Inc (Sacramaneto, CA, United States of America) for rRNA depletion, cDNA library preparation, and sequencing on Illumina NovaSeq. Data was de-multiplexed using Illumina’s bcl2fastq 2.17 software, where one mismatch was allowed for index sequence identification.
The compressed FASTQ files were quality-checked using the FastQC tool v0.12.1 118. The BBDuk, BBSplit, and BBMap tools from BBTools suite (v38.42) 119 were used to trim adapters, deplete rRNA, and map the remaining mRNA reads to the reference genomes. For monoculture or cocultures containing C. difficile DSM27147, the reference genome was obtained from GenBank (FN545816.1). For monoculture or cocultures containing C. difficile MS001 isolate, the reference genome was obtained from the whole-genome sequencing data that was assembled and annotated using SPAdes Genome Assembler 93. The feature-Counts package v1.6.4 120 from the SubRead suite was used to map reads to features on the genome and quantify raw counts for each transcript. Reads per kilobase million (RPKM) values were computed using a custom Python script to see the agreement of gene expression between biological replicates. The gene expression (represented by RPKM values) shows a good correlation between biological replicates (Pearson’s R=0.95–0.98, P<10E-05) (Fig. S16a). The DESeq2 Bioconductor library v4.0.3 121 was used in R v4.0.4 to quantify differential gene expression using a negative binomial generalized linear models with apeglm shrinkage estimator 122. When calculating RPKM of C. difficile genes in the CD-CS and CD-CH coculture, the “reads mapped” in the denominator was the number of reads mapped to the C. difficile genome. Similarly, when quantifying differential gene expression for C. difficile genes in the CD-CS and CD-CH coculture, only reads mapped to the C. difficile genome were provided to DeSeq2. We define differentially expressed genes (DEGs) as those with >2-fold change and a p-value less than 0.05. The RNA-seq data was submitted and is accessible in BioProject PRJNA983758.
Gene Set Enrichment Analysis (GSEA)
GSEA was performed using the GSEA method of the ClusterProfiler R package (v4.2.2) 123. KEGG modules for C. difficile were used as gene sets and were supplied as a user-defined annotation with the TERM2GENE field. The analysis was run with the log2FCs calculated by DeSeq2. The p-value cutoff used was 0.05 and the minimum gene set size used was 3.
Gnotobiotic mouse experiments
All germ-free mouse experiments were performed following protocols approved by the University of Wisconsin-Madison Animal Care and Use Committee. We used 10-week-old C57BL/6 gnotobiotic male mice (wild-type) and a regular diet (Chow diet, Purina, LabDiet 5021). All strains were grown at 37 °C anaerobically in Anaerobe Basal Broth (ABB, Oxoid) to stationary phase. C. hiranonis and C. difficile DSM27147 strain for oral gavage was diluted to ~10,000 CFU/mL, and these cultures were transferred to Hungate tubes (Chemglass) on ice prior to oral gavage. On day 0, 0.2 mL of C. hiranonis culture was introduced into the mice by oral gavage inside a Biological Safety Cabinet (BSC) and the mice were housed in biocontainment cages (Allentown Inc.) for the duration of the experiment. After one week, 0.2 mL of C. difficile (~2,000 CFU) was introduced into the mice by oral gavage. Mice were maintained for a total of two weeks after the first colonization with the core community (day 0). Groups of mice (4–5 mice) with the same core community and C. difficile were co-housed in a single cage. Mice were weighed and fecal samples were collected at specific time points after oral gavage for NGS sequencing. Cecal contents from mice that were dead or sacrificed in the middle of the experiment were collected for NGS sequencing.
Genomic DNA extraction from fecal and cecal samples
The DNA extraction for fecal and cecal samples was performed as described previously with some modifications 124. Fecal samples (~50 mg) were transferred into solvent-resistant screw-cap tubes (Sarstedt Inc) with 500 μL 0.1 mm zirconia/silica beads (BioSpec Products) and one 3.2 mm stainless steel bead (BioSpec Products). The samples were resuspended in 500 μL of Buffer A (200 mM NaCl (DOT Scientific), 20 mM EDTA (Sigma) and 200 mM Tris·HCl pH 8.0 (Research Products International)), 210 μL 20% SDS (Alfa Aesar) and 500 μL phenol/chloroform/isoamyl alcohol (Invitrogen). Cells were lysed by mechanical disruption with a bead-beater (BioSpec Products) for 3 min twice, while being placed on ice for 1 min in between to prevent overheating. Next, cells were centrifuged for 7 min at 8,000 × g at 4°C, and the supernatant was transferred to an Eppendorf tube. We added 60 μL 3M sodium acetate (Sigma) and 600 μL isopropanol (LabChem) to the supernatant and incubated on ice for 1 h. Next, samples were centrifuged for 20 min at 18,000 × g at 4°C, and the supernatant was decanted. The harvested DNA pellets were washed once with 500 μL of 100% ethanol (Koptec), and the remaining trace ethanol was removed by air drying the samples. Finally, the DNA pellets were resuspended into 300 μL of AE buffer (Qiagen). The crude DNA extracts were purified by a Zymo DNA Clean & Concentrator™-5 kit (Zymo Research) prior to PCR amplification and NGS sequencing.
C. difficile colony-forming unit counting from fecal and cecal samples
C. difficile selective plates were prepared by autoclaving C. difficile agar (Oxoid CM0601) and adding defibrinated horse blood (Lampire 7233401, 70 mL/1L media), norfloxacin (Santa Cruz 215586, 120 μg/mL), moxalactam (Santa Cruz 250419, 320 μg/mL), and erythromycin (Santa Cruz 204742, 100 μg/mL) after the media is cooled to ~55°C. Right after mice fecal or cecal collection, around 1μL of fresh fecal samples were taken using an inoculating loop and mixed with PBS. The samples were then serially diluted (1:10 dilution) using PBS. Four dilutions of each sample were spotted on C. difficile selective agar plates, with 2 technical replicates per sample. Plates were incubated at 37°C for 48 h at which point colonies were counted in the dilution spot containing between 5 and 100 colonies. The CFU/mL for each sample was calculated as the average of the 2 technical replicates times the dilution factor. The lower limit of detection for the assay was 20,000 CFU/mL.
Supplementary Material
Acknowledgments
We would like to thank Jun Feng, Yiyi Liu, Freeman Lan, Tyler D. Ross, Erin O. Loss, Yu-Yu Cheng, and Alex Carr for their helpful advice on this project. This research was supported by the National Institutes of Allergy and Infectious Diseases under grant number R21AI156438 and R21AI159980 for O.S.V, R35GM124774 for O.S.V. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Competing interests
J.E.S. and O.S.V. have filed a U.S. nonprovisional patent application 63/621,370. The other authors declare that they have no competing interests.
Code availability
Codes for processing sequencing data, fitting the gLV models, and performing Bayesian experimental design will be available through Github prior to publication. Until then, we have provided the code as a supplementary file.
Data availability
Whole-genome sequence data of the C. difficile strains will be deposited in the NCBI database. Mapped growth media and strain-specific genome scale metabolic models in SBML format can be found at https://github.com/gibbons-lab/2023_cdiff_venturelli. Nextflow pipelines for assembly and metabolic model building can be found at https://github.com/gibbons-lab/pipelines. RNA-seq data used in this study will be deposited in the NCBI database. Raw DNA sequencing data and processed sequencing data to determine community composition will be made available via Zenodo prior to publication.
References
- (1).Cornely O. A.; Miller M. A.; Louie T. J.; Crook D. W.; Gorbach S. L. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clinical infectious diseases 2012, 55, S154–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Tsigrelis C. Recurrent Clostridioides difficile infection: Recognition, management, prevention. Cleveland Clinic Journal of Medicine 2020, 87, 347–359. [DOI] [PubMed] [Google Scholar]
- (3).Song J. H.; Kim Y. S. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut and liver 2019, 13, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kazemian N.; Ramezankhani M.; Sehgal A.; Khalid F. M.; Kalkhoran A. H. Z.; Narayan A.; Wong G. K.-S.; Kao D.; Pakpour S. The trans-kingdom battle between donor and recipient gut microbiome influences fecal microbiota transplantation outcome. Scientific reports 2020, 10, 18349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).DeFilipp Z.; Bloom P. P.; Torres Soto M.; Mansour M. K.; Sater M. R.; Huntley M. H.; Turbett S.; Chung R. T.; Chen Y.-B.; Hohmann E. L. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. New England Journal of Medicine 2019, 381, 2043–2050. [DOI] [PubMed] [Google Scholar]
- (6).Wang S.; Xu M.; Wang W.; Cao X.; Piao M.; Khan S.; Yan F.; Cao H.; Wang B. Systematic review: adverse events of fecal microbiota transplantation. PloS one 2016, 11, e0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Van Elsas J. D.; Chiurazzi M.; Mallon C. A.; Elhottovā D.; Krištůfek V.; Salles J. F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proceedings of the National Academy of Sciences 2012, 109, 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Shade A.; Peter H.; Allison S. D.; Baho D. L.; Berga M.; Bürgmann H.; Huber D. H.; Langenheder S.; Lennon J. T.; Martiny J. B. Fundamentals of microbial community resistance and resilience. Frontiers in microbiology 2012, 3, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ratner M. Seres’s pioneering microbiome drug fails mid-stage trial. Nature biotechnology 2016, 34, 1004–1006. [DOI] [PubMed] [Google Scholar]
- (10).Feuerstadt P.; Louie T. J.; Lashner B.; Wang E. E.; Diao L.; Bryant J. A.; Sims M.; Kraft C. S.; Cohen S. H.; Berenson C. S. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. New England Journal of Medicine 2022, 386, 220–229. [DOI] [PubMed] [Google Scholar]
- (11).Dsouza M.; Menon R.; Crossette E.; Bhattarai S. K.; Schneider J.; Kim Y.-G.; Reddy S.; Caballero S.; Felix C.; Cornacchione L. Colonization of the live biotherapeutic product VE303 and modulation of the microbiota and metabolites in healthy volunteers. Cell Host & Microbe 2022, 30, 583–598. e588. [DOI] [PubMed] [Google Scholar]
- (12).Stubbs S. L.; Brazier J. S.; O’Neill G. L.; Duerden B. I. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. Journal of clinical microbiology 1999, 37, 461–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Knight D. R.; Imwattana K.; Kullin B.; Guerrero-Araya E.; Paredes-Sabja D.; Didelot X.; Dingle K. E.; Eyre D. W.; Rodríguez C.; Riley T. V. Major genetic discontinuity and novel toxigenic species in Clostridioides difficile taxonomy. Elife 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Scaria J.; Ponnala L.; Janvilisri T.; Yan W.; Mueller L. A.; Chang Y.-F. Analysis of ultra low genome conservation in Clostridium difficile. PloS one 2010, 5, e15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Janvilisri T.; Scaria J.; Thompson A. D.; Nicholson A.; Limbago B. M.; Arroyo L. G.; Songer J. G.; Gröhn Y. T.; Chang Y.-F. Microarray identification of Clostridium difficile core components and divergent regions associated with host origin. Journal of Bacteriology 2009, 191, 3881–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Knight D. R.; Elliott B.; Chang B. J.; Perkins T. T.; Riley T. V. Diversity and evolution in the genome of Clostridium difficile. Clinical microbiology reviews 2015, 28, 721–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Juhas M. Horizontal gene transfer in human pathogens. Critical reviews in microbiology 2015, 41, 101–108. [DOI] [PubMed] [Google Scholar]
- (18).He M.; Sebaihia M.; Lawley T. D.; Stabler R. A.; Dawson L. F.; Martin M. J.; Holt K. E.; Seth-Smith H. M.; Quail M. A.; Rance R. Evolutionary dynamics of Clostridium difficile over short and long time scales. Proceedings of the National Academy of Sciences 2010, 107, 7527–7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Brouwer M. S.; Roberts A. P.; Hussain H.; Williams R. J.; Allan E.; Mullany P. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nature communications 2013, 4, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kulecka M.; Waker E.; Ambrozkiewicz F.; Paziewska A.; Skubisz K.; Cybula P.; Targoński Ł.; Mikula M.; Walewski J.; Ostrowski J. Higher genome variability within metabolism genes associates with recurrent Clostridium difficile infection. BMC microbiology 2021, 21, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Collins J.; Danhof H.; Britton R. A. The role of trehalose in the global spread of epidemic Clostridium difficile. Gut Microbes 2019, 10, 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Collins J.; Robinson C.; Danhof H.; Knetsch C.; Van Leeuwen H.; Lawley T.; Auchtung J.; Britton e. R. Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature 2018, 553, 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Guh A. Y.; Mu Y.; Winston L. G.; Johnston H.; Olson D.; Farley M. M.; Wilson L. E.; Holzbauer S. M.; Phipps E. C.; Dumyati G. K. Trends in US burden of Clostridioides difficile infection and outcomes. New England Journal of Medicine 2020, 382, 1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lessa F. C.; Mu Y.; Bamberg W. M.; Beldavs Z. G.; Dumyati G. K.; Dunn J. R.; Farley M. M.; Holzbauer S. M.; Meek J. I.; Phipps E. C. Burden of Clostridium difficile infection in the United States. New England Journal of Medicine 2015, 372, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Barbut F.; Petit J. Epidemiology, risk factors and prevention of Clostridium difficile nosocomial infections. Pathologie-biologie 2000, 48, 745–755. [PubMed] [Google Scholar]
- (26).Johnson S.; Adelmann A.; Clabots C. R.; Peterson L. R.; Gerding D. N. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. The Journal of infectious diseases 1989, 159, 340–343. [DOI] [PubMed] [Google Scholar]
- (27).O’neill G.; Beaman M.; Riley T. Relapse versus reinfection with Clostridium difficile. Epidemiology & Infection 1991, 107, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Tang-Feldman Y.; Mayo S.; Silva J. Jr; Cohen S. H. Molecular analysis of Clostridium difficile strains isolated from 18 cases of recurrent Clostridium difficile-associated diarrhea. Journal of clinical microbiology 2003, 41, 3413–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wilcox M.; Fawley W.; Settle C.; Davidson A. Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection? Journal of Hospital Infection 1998, 38, 93–100. [DOI] [PubMed] [Google Scholar]
- (30).Buffie C. G.; Pamer E. G. Microbiota-mediated colonization resistance against intestinal pathogens. Nature Reviews Immunology 2013, 13, 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hromada S.; Qian Y.; Jacobson T. B.; Clark R. L.; Watson L.; Safdar N.; Amador-Noguez D.; Venturelli O. S. Negative interactions determine Clostridioides difficile growth in synthetic human gut communities. Molecular systems biology 2021, 17, e10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Pereira F. C.; Wasmund K.; Cobankovic I.; Jehmlich N.; Herbold C. W.; Lee K. S.; Sziranyi B.; Vesely C.; Decker T.; Stocker R. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nature Communications 2020, 11, 5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Aguirre A. M.; Yalcinkaya N.; Wu Q.; Swennes A.; Tessier M. E.; Roberts P.; Miyajima F.; Savidge T.; Sorg J. A. Bile acid-independent protection against Clostridioides difficile infection. PLoS Pathogens 2021, 17, e1010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Girinathan B. P.; DiBenedetto N.; Worley J. N.; Peltier J.; Arrieta-Ortiz M. L.; Immanuel S. R. C.; Lavin R.; Delaney M. L.; Cummins C. K.; Hoffman M. In vivo commensal control of Clostridioides difficile virulence. Cell Host & Microbe 2021, 29, 1693–1708. e1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kang J. D.; Myers C. J.; Harris S. C.; Kakiyama G.; Lee I.-K.; Yun B.-S.; Matsuzaki K.; Furukawa M.; Min H.-K.; Bajaj J. S. Bile acid 7α-dehydroxylating gut bacteria secrete antibiotics that inhibit Clostridium difficile: role of secondary bile acids. Cell chemical biology 2019, 26, 27–34. e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pereira F. C.; Wasmund K.; Cobankovic I.; Jehmlich N.; Herbold C. W.; Lee K. S.; Sziranyi B.; Vesely C.; Decker T.; Stocker R. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nature communications 2020, 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Buffie C. G.; Bucci V.; Stein R. R.; McKenney P. T.; Ling L.; Gobourne A.; No D.; Liu H.; Kinnebrew M.; Viale A. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Venturelli O. S.; Carr A. V.; Fisher G.; Hsu R. H.; Lau R.; Bowen B. P.; Hromada S.; Northen T.; Arkin A. P. Deciphering microbial interactions in synthetic human gut microbiome communities. Molecular systems biology 2018, 14, e8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Baranwal M.; Clark R. L.; Thompson J.; Sun Z.; Hero A. O.; Venturelli O. S. Recurrent neural networks enable design of multifunctional synthetic human gut microbiome dynamics. Elife 2022, 11, e73870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Thompson J. C.; Zavala V. M.; Venturelli O. S. Integrating a tailored recurrent neural network with Bayesian experimental design to optimize microbial community functions. PLOS Computational Biology 2023, 19, e1011436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Marino S.; Baxter N. T.; Huffnagle G. B.; Petrosino J. F.; Schloss P. D. Mathematical modeling of primary succession of murine intestinal microbiota. Proceedings of the National Academy of Sciences 2014, 111, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Gonze D.; Coyte K. Z.; Lahti L.; Faust K. Microbial communities as dynamical systems. Current opinion in microbiology 2018, 44, 41–49. [DOI] [PubMed] [Google Scholar]
- (43).Qian Y.; Lan F.; Venturelli O. S. Towards a deeper understanding of microbial communities: integrating experimental data with dynamic models. Current opinion in microbiology 2021, 62, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Clark R. L.; Connors B. M.; Stevenson D. M.; Hromada S. E.; Hamilton J. J.; Amador-Noguez D.; Venturelli O. S. Design of synthetic human gut microbiome assembly and butyrate production. Nature communications 2021, 12, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Ferreyra J. A.; Wu K. J.; Hryckowian A. J.; Bouley D. M.; Weimer B. C.; Sonnenburg J. L. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell host & microbe 2014, 16, 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ghimire S.; Roy C.; Wongkuna S.; Antony L.; Maji A.; Keena M. C.; Foley A.; Scaria J. Identification of Clostridioides difficile-inhibiting gut commensals using culturomics, phenotyping, and combinatorial community assembly. Msystems 2020, 5, e00620–00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Theriot C. M.; Koenigsknecht M. J.; Carlson P. E.; Hatton G. E.; Nelson A. M.; Li B.; Huffnagle G. B.; Z Li J.; Young V. B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nature communications 2014, 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Ng K. M.; Ferreyra J. A.; Higginbottom S. K.; Lynch J. B.; Kashyap P. C.; Gopinath S.; Naidu N.; Choudhury B.; Weimer B. C.; Monack D. M. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 2013, 502, 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Camorlinga M.; Sanchez-Rojas M.; Torres J.; Romo-Castillo M. Phenotypic characterization of non-toxigenic Clostridioides difficile strains isolated from patients in Mexico. Frontiers in Microbiology 2019, 10, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Sebaihia M.; Wren B. W.; Mullany P.; Fairweather N. F.; Minton N.; Stabler R.; Thomson N. R.; Roberts A. P.; Cerdeno-Tárraga A. M.; Wang H. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nature genetics 2006, 38, 779–786. [DOI] [PubMed] [Google Scholar]
- (51).Mullany P.; Allan E.; Roberts A. P. Mobile genetic elements in Clostridium difficile and their role in genome function. Research in microbiology 2015, 166, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Ghimire S.; Roy C.; Wongkuna S.; Antony L.; Maji A.; Keena M. C.; Foley A.; Scaria J. Identification of Clostridioides difficile-inhibiting gut commensals using culturomics, phenotyping, and combinatorial community assembly. Msystems 2020, 5, 10.1128/msystems. 00620–00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Hassall J.; Cheng J. K.; Unnikrishnan M. Dissecting individual interactions between pathogenic and commensal bacteria within a multispecies gut microbial community. Msphere 2021, 6, 10.1128/msphere.00013-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Britton R. A.; Young V. B. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 2014, 146, 1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Wasserman L.: All of statistics: a concise course in statistical inference; Springer Science & Business Media, 2013. [Google Scholar]
- (56).Wootton J. T. Indirect effects and habitat use in an intertidal community: interaction chains and interaction modifications. The American Naturalist 1993, 141, 71–89. [Google Scholar]
- (57).Billick I.; Case T. J. Higher order interactions in ecological communities: what are they and how can they be detected? Ecology 1994, 75, 1529–1543. [Google Scholar]
- (58).Fletcher J. R.; Erwin S.; Lanzas C.; Theriot C. M. Shifts in the gut metabolome and Clostridium difficile transcriptome throughout colonization and infection in a mouse model. Msphere 2018, 3, e00089–00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Battaglioli E. J.; Hale V. L.; Chen J.; Jeraldo P.; Ruiz-Mojica C.; Schmidt B. A.; Rekdal V. M.; Till L. M.; Huq L.; Smits S. A. Clostridioides difficile uses amino acids associated with gut microbial dysbiosis in a subset of patients with diarrhea. Science translational medicine 2018, 10, eaam7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Jenior M. L.; Leslie J. L.; Young V. B.; Schloss P. D. Clostridium difficile colonizes alternative nutrient niches during infection across distinct murine gut microbiomes. MSystems 2017, 2, e00063–00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Theriot C. M.; Koenigsknecht M. J.; Carlson P. E. Jr; Hatton G. E.; Nelson A. M.; Li B.; Huffnagle G. B.; Z. Li J.; Young V. B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nature communications 2014, 5, 3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Karasawa T.; Maegawa T.; Nojiri T.; Yamakawa K.; Nakamura S. Effect of arginine on toxin production by Clostridium difficile in defined medium. Microbiology and immunology 1997, 41, 581–585. [DOI] [PubMed] [Google Scholar]
- (63).Ikeda D.; Karasawa T.; Yamakawa K.; Tanaka R.; Namiki M.; Nakamura S. Effect of isoleucine on toxin production by Clostridium difficile in a defined medium. Zentralblatt für Bakteriologie 1998, 287, 375–386. [DOI] [PubMed] [Google Scholar]
- (64).Karlsson S.; Burman L. G.; Åkerlund T. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology 1999, 145, 1683–1693. [DOI] [PubMed] [Google Scholar]
- (65).Karlsson S.; Lindberg A.; Norin E.; Burman L. G.; Åkerlund T. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infection and immunity 2000, 68, 5881–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Dupuy B.; Sonenshein A. L. Regulated transcription of Clostridium difficile toxin genes. Molecular microbiology 1998, 27, 107–120. [DOI] [PubMed] [Google Scholar]
- (67).Wetzel D.; McBride S. M. The impact of pH on Clostridioides difficile sporulation and physiology. Applied and environmental microbiology 2020, 86, e02706–02719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Onderdonk A.; Lowe B.; Bartlett J. Effect of environmental stress on Clostridium difficile toxin levels during continuous cultivation. Applied and environmental microbiology 1979, 38, 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Wilson K. H. The microecology of Clostridium difficile. Clinical infectious diseases 1993, 16, S214–S218. [DOI] [PubMed] [Google Scholar]
- (70).Smith A. B.; Jenior M. L.; Keenan O.; Hart J. L.; Specker J.; Abbas A.; Rangel P. C.; Di C.; Green J.; Bustin K. A. Enterococci enhance Clostridioides difficile pathogenesis. Nature 2022, 611, 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Majumdar A.; Govind R. Regulation of Clostridioides difficile toxin production. Current opinion in microbiology 2022, 65, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Martin-Verstraete I.; Peltier J.; Dupuy B. The regulatory networks that control Clostridium difficile toxin synthesis. Toxins 2016, 8, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Powers D. A.; Jenior M. L.; Kolling G. L.; Papin J. A. Network analysis of toxin production in Clostridioides difficile identifies key metabolic dependencies. PLOS Computational Biology 2023, 19, e1011076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Voth D. E.; Ballard J. D. Clostridium difficile toxins: mechanism of action and role in disease. Clinical microbiology reviews 2005, 18, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Neumann-Schaal M.; Jahn D.; Schmidt-Hohagen K. Metabolism the difficile way: the key to the success of the pathogen Clostridioides difficile. Frontiers in microbiology 2019, 10, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Gencic S.; Grahame D. A. Diverse energy-conserving pathways in Clostridium difficile: growth in the absence of amino acid Stickland acceptors and the role of the Wood-Ljungdahl pathway. Journal of bacteriology 2020, 202, 10.1128/jb.00233-00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Round J. L.; Mazmanian S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nature reviews immunology 2009, 9, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Sims M. D.; Khanna S.; Feuerstadt P.; Louie T. J.; Kelly C. R.; Huang E. S.; Hohmann E. L.; Wang E. E.; Oneto C.; Cohen S. H. Safety and tolerability of SER-109 as an investigational microbiome therapeutic in adults with recurrent Clostridioides difficile infection: a Phase 3, open-label, single-arm trial. JAMA Network Open 2023, 6, e2255758–e2255758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Louie T.; Golan Y.; Khanna S.; Bobilev D.; Erpelding N.; Fratazzi C.; Carini M.; Menon R.; Ruisi M.; Norman J. M. VE303, a defined bacterial consortium, for prevention of recurrent Clostridioides difficile infection: a randomized clinical trial. JAMA 2023, 329, 1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Jenior M. L.; Papin J. A. Computational approaches to understanding Clostridioides difficile metabolism and virulence. Current opinion in microbiology 2022, 65, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Jenior M. L.; Leslie J. L.; Kolling G. L.; Archbald-Pannone L.; Powers D. A.; Petri W. A.; Papin J. A. Systems-ecology designed bacterial consortium protects from severe Clostridioides difficile infection. bioRxiv 2023, 2023.2008. 2008.552483. [Google Scholar]
- (82).Werner M.; Ishii P. E.; Pilla R.; Lidbury J. A.; Steiner J. M.; Busch-Hahn K.; Unterer S.; Suchodolski J. S. Prevalence of Clostridioides difficile in Canine Feces and Its Association with Intestinal Dysbiosis. Animals 2023, 13, 2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Dickson R. P. The microbiome and critical illness. The Lancet Respiratory Medicine 2016, 4, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Takáčová M.; Bomba A.; Tóthová C.; Micháľová A.; Turňa H. Any future for faecal microbiota transplantation as a novel strategy for gut microbiota modulation in human and veterinary medicine? Life 2022, 12, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Baldassare M. A.; Bhattacharjee D.; Coles J. D.; Nelson S.; McCollum C. A.; Seekatz A. M. Butyrate enhances Clostridioides difficile sporulation in vitro. bioRxiv 2023, 2023.2004. 2027.538596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Jenior M. L.; Leslie J. L.; Powers D. A.; Garrett E. M.; Walker K. A.; Dickenson M. E.; Petri W. A. Jr; Tamayo R.; Papin J. A. Novel drivers of virulence in Clostridioides difficile identified via context-specific metabolic network analysis. Msystems 2021, 6, e00919–00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Dieterle M. G.; Rao K.; Young V. B. Novel therapies and preventative strategies for primary and recurrent Clostridium difficile infections. Annals of the New York Academy of Sciences 2019, 1435, 110–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Watson L.; Zimbric M. L.; Shaughnessy C.; Kale S.; Debad J.; Navaratnam M.; Safdar N. In Tilte2019; Oxford University Press. [Google Scholar]
- (89).Eggers S.; Malecki K. M.; Peppard P.; Mares J.; Shirley D.; Shukla S. K.; Poulsen K.; Gangnon R.; Duster M.; Kates A. Wisconsin microbiome study, a cross-sectional investigation of dietary fibre, microbiome composition and antibiotic-resistant organisms: rationale and methods. BMJ open 2018, 8, e019450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Nieto F. J.; Peppard P. E.; Engelman C. D.; McElroy J. A.; Galvao L. W.; Friedman E. M.; Bersch A. J.; Malecki K. C. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC public health 2010, 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Feng J.; Qian Y.; Zhou Z.; Ertmer S.; Vivas E. I.; Lan F.; Hamilton J. J.; Rey F. E.; Anantharaman K.; Venturelli O. S. Polysaccharide utilization loci in Bacteroides determine population fitness and community-level interactions. Cell host & microbe 2022, 30, 200–215. e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Ostrem Loss E.; Thompson J.; Cheung P. L. K.; Qian Y.; Venturelli O. S. Carbohydrate complexity limits microbial growth and reduces the sensitivity of human gut communities to perturbations. Nature ecology & evolution 2023, 7, 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Prjibelski A.; Antipov D.; Meleshko D.; Lapidus A.; Korobeynikov A. Using SPAdes de novo assembler. Current protocols in bioinformatics 2020, 70, e102. [DOI] [PubMed] [Google Scholar]
- (94).Jain C.; Rodriguez-R L. M.; Phillippy A. M.; Konstantinidis K. T.; Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nature communications 2018, 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Tanizawa Y.; Fujisawa T.; Nakamura Y. DFAST: a flexible prokaryotic genome annotation pipeline for faster genome publication. Bioinformatics 2018, 34, 1037–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Lechner M.; Findeiß S.; Steiner L.; Marz M.; Stadler P. F.; Prohaska S. J. Proteinortho: detection of (co-) orthologs in large-scale analysis. BMC bioinformatics 2011, 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Camacho C.; Coulouris G.; Avagyan V.; Ma N.; Papadopoulos J.; Bealer K.; Madden T. L. BLAST+: architecture and applications. BMC bioinformatics 2009, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Sievers F.; Wilm A.; Dineen D.; Gibson T. J.; Karplus K.; Li W.; Lopez R.; McWilliam H.; Remmert M.; Söding J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular systems biology 2011, 7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Lee M. D. GToTree: a user-friendly workflow for phylogenomics. Bioinformatics 2019, 35, 4162–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Letunic I.; Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic acids research 2019, 47, W256–W259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Langmead B.; Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nature methods 2012, 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Cury J.; Abby S. S.; Doppelt-Azeroual O.; Néron B.; Rocha E. P.: Identifying conjugative plasmids and integrative conjugative elements with CONJscan. In Horizontal Gene Transfer; Springer, 2020; pp 265–283. [DOI] [PubMed] [Google Scholar]
- (103).Roux S.; Enault F.; Hurwitz B. L.; Sullivan M. B. VirSorter: mining viral signal from microbial genomic data. PeerJ 2015, 3, e985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Chen S.; Zhou Y.; Chen Y.; Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Li D.; Liu C.-M.; Luo R.; Sadakane K.; Lam T.-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [DOI] [PubMed] [Google Scholar]
- (106).Chklovski A.; Parks D. H.; Woodcroft B. J.; Tyson G. W. CheckM2: a rapid, scalable and accurate tool for assessing microbial genome quality using machine learning. Nature Methods 2023, 20, 1203–1212. [DOI] [PubMed] [Google Scholar]
- (107).Chaumeil P.-A.; Mussig A. J.; Hugenholtz P.; Parks D. H. GTDB-Tk v2: memory friendly classification with the genome taxonomy database. Bioinformatics 2022, 38, 5315–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Hyatt D.; Chen G.-L.; LoCascio P. F.; Land M. L.; Larimer F. W.; Hauser L. J. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC bioinformatics 2010, 11, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Machado D.; Andrejev S.; Tramontano M.; Patil K. R. Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic acids research 2018, 46, 7542–7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Buchfink B.; Reuter K.; Drost H.-G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nature methods 2021, 18, 366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Norsigian C. J.; Pusarla N.; McConn J. L.; Yurkovich J. T.; Dräger A.; Palsson B. O.; King Z. BiGG Models 2020: multi-strain genome-scale models and expansion across the phylogenetic tree. Nucleic acids research 2020, 48, D402–D406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Lieven C.; Beber M. E.; Olivier B. G.; Bergmann F. T.; Ataman M.; Babaei P.; Bartell J. A.; Blank L. M.; Chauhan S.; Correia K. MEMOTE for standardized genome-scale metabolic model testing. Nature biotechnology 2020, 38, 272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Diener C.; Gibbons S. M.; Resendis-Antonio O. MICOM: metagenome-scale modeling to infer metabolic interactions in the gut microbiota. MSystems 2020, 5, 10.1128/msystems.00606-00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Zhang J.; Kobert K.; Flouri T.; Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Wang Q.; Garrity G. M.; Tiedje J. M.; Cole J. R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and environmental microbiology 2007, 73, 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Schloss P. D.; Westcott S. L.; Ryabin T.; Hall J. R.; Hartmann M.; Hollister E. B.; Lesniewski R. A.; Oakley B. B.; Parks D. H.; Robinson C. J. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and environmental microbiology 2009, 75, 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Bishop C.: Pattern Recognition and Machine Learning Chris Bishop. Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- (118).Andrews S.: FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics, Babraham Institute, Cambridge, United Kingdom, 2010. [Google Scholar]
- (119).Bushnell B. BBMap: a fast, accurate, splice-aware aligner 2014. [Google Scholar]
- (120).Liao Y.; Smyth G. K.; Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [DOI] [PubMed] [Google Scholar]
- (121).Love M. I.; Huber W.; Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology 2014, 15, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Zhu A.; Ibrahim J. G.; Love M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Wu T.; Hu E.; Xu S.; Chen M.; Guo P.; Dai Z.; Feng T.; Zhou L.; Tang W.; Zhan L. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. The Innovation 2021, 2, 100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Goodman A. L.; Kallstrom G.; Faith J. J.; Reyes A.; Moore A.; Dantas G.; Gordon J. I. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proceedings of the National Academy of Sciences 2011, 108, 6252–6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Whole-genome sequence data of the C. difficile strains will be deposited in the NCBI database. Mapped growth media and strain-specific genome scale metabolic models in SBML format can be found at https://github.com/gibbons-lab/2023_cdiff_venturelli. Nextflow pipelines for assembly and metabolic model building can be found at https://github.com/gibbons-lab/pipelines. RNA-seq data used in this study will be deposited in the NCBI database. Raw DNA sequencing data and processed sequencing data to determine community composition will be made available via Zenodo prior to publication.