Abstract
Background:
Steroid-resistant nephrotic syndrome is the second leading cause of chronic kidney disease among patients <25 years of age. Through whole exome sequencing, identification of >65 monogenic causes has rendered insights into disease mechanisms of nephrotic syndrome.
Methods:
To elucidate novel monogenic causes of NS, we combined homozygosity mapping with ES in a worldwide cohort of 1649 pediatric patients with NS.
Results:
We identified homozygous missense variants in MYO1C in two unrelated children with nephrotic syndrome (c.292C>T, p.R98W; c.2273 A>T, p.K758M). We evaluated publicly available kidney single-cell RNA sequencing datasets and found MYO1Cto be predominantly expressed in podocytes. We then performed structural modeling in molecular viewer PyMol using the super function aligning shared regions within both partial structures of MYO1C (4byf and 4r8g). In both structures, calmodulin, a common regulator of myosin activity, is shown to bind to the IQ motif. At both residue sites (K758; R98), there are ion-ion interactions stabilizing intradomain and ligand interactions: R98 binds to nearby D220 within the Myosin Motor Domain and K758 binds to E14 on a calmodulin molecule. Variants of these charged residues to non-charged amino acids could ablate these ionic interactions, weakening protein structure and function establishing the impact of these variants.
Conclusion:
We here identified recessive variants in MYO1C as a potential novel cause of nephrotic syndrome in children.
Introduction
Nephrotic syndrome (NS) is characterized by substantial proteinuria (> 40 mg/m2/hour), leading to hypoalbuminemia, edema, and hyperlipidemia. Approximately 80% of pediatric and young adult patients go into remission with standard steroid therapy, termed steroid-sensitive nephrotic syndrome (SSNS) [1]. Conversely, about 20% of individuals with steroid-resistant nephrotic syndrome (SRNS) are likely to progress to chronic kidney disease (CKD) and ultimately kidney failure [1]. The predominant renal histological feature of SRNS is focal segmental glomerulosclerosis (FSGS), which stands as the second most prevalent cause of kidney failure in the first two decades of life [2].
More than 65 genes have been identified as playing a role in monogenic SRNS, offering valuable insights into the complex mechanisms driving proteinuric kidney disease, SRNS, and podocyte biology [3]. Despite the relative rarity of these genetic variants, their discovery substantially deepens our understanding of podocytopathies. Furthermore, the identification of novel genes associated with SRNS expands the repertoire of genes incorporated into diagnostic gene panels, which are becoming increasingly accessible and utilized in clinical practice.
The glomerular filtration apparatus comprises a three-layered structure consisting of fenestrated endothelium, the glomerular basement membrane, and interconnected podocytes linked through the slit diaphragm. The actin network within podocytes is critical in maintaining foot process integrity, facilitating slit diaphragm turnover, and providing structural support amidst varying glomerular pressures. Genetic variants in genes encoding actin-interacting proteins are prevalent among the monogenic causes of SRNS, underscoring the central role of actin in podocyte physiology [4] [5].
Myosins serve as pivotal actin-based molecular motors, playing important roles in a wide array of cellular functions such as intracellular trafficking, cell adhesion, motility, and maintenance of membrane tension. Among them, MYO1C, a non-muscle myosin motor protein, exhibits high expression levels in glomerular podocytes [6]. It plays a crucial role in the function of the glomerular filter by interacting directly with nephrin and neph1, facilitating their transport to podocyte intercellular junctions [7]. Depletion of myo1c disrupts the localization of these slit diaphragm proteins at the podocyte cell membrane and leads to an edematous phenotype and abnormal podocyte morphology, as evidenced in zebrafish studies [7]. Moreover, podocyte-specific MYO1C knockout in mice highlights its critical role in TGF-β-signaling in podocyte disease pathogenesis [6].
Based on our prior investigations, we have established that whole exome sequencing (WES) can uncover a monogenic basis for steroid-resistant nephrotic syndrome (SRNS) in 11–45% of familial cases, often by detecting variants within one of the > 65 published genes [8–10].
Therefore, we hypothesized that previously unidentified single-gene causes of SRNS may be discovered in children and young adults affected by nephrotic syndrome. To investigate this hypothesis, we conducted ES on patients with SRNS/FSGS, leading to the identification of variants in MYO1C in two unrelated families.
Materials and Methods
Results
To identify potential candidate genes for SRNS, we employed WES on an international cohort of 1649 individuals with NS. We discovered two distinct homozygous missense variants in the MYO1C gene, which encodes the motor protein Myosin 1C, in two unrelated families with proteinuric kidney disease.
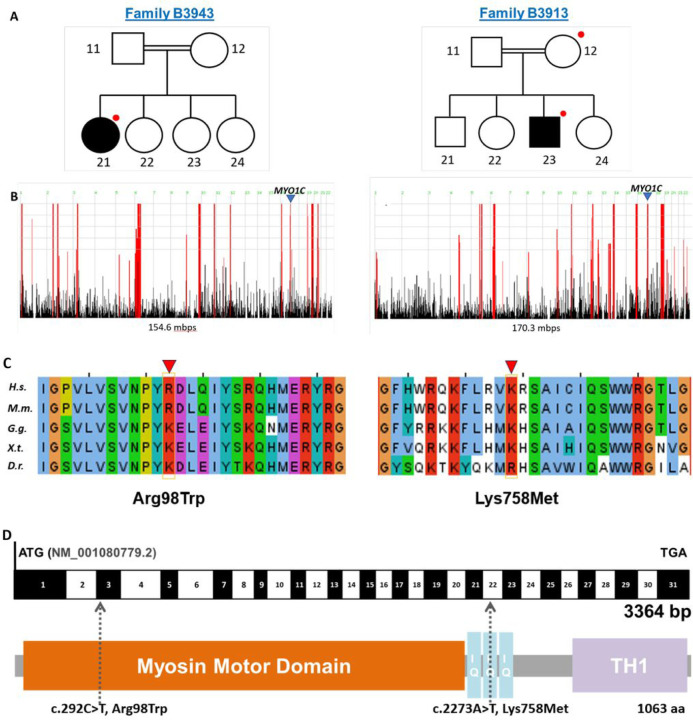
In family B3913, a homozygous missense variant c.292C > T (p.R98W) was identified, while in family B3934, a homozygous variant c.2273 A > T (p.K758M) was detected. We confirmed the presence of both variants by Sanger sequencing (Supp Fig.2). Importantly, neither variant was present homozygously in GnomAD, and only 5 and 14 heterozygous variants, respectively, were listed in the database of this control population (n = ~ 200,000 individuals) [17]. Evolutionary conservation analysis indicates that at the modified amino acid position, a positively charged amino acid—either arginine or lysine—is conserved across species down to Danio rerio. Two out of three bioinformatic prediction programs (SIFT, Mutation Taster and Polyphen-2) classified both variants as pathogenic (Table 1). The identified variants were located within well-defined functional domains, specifically the N-terminal myosin motor domain and the calmodulin-binding IQ domain, respectively (Fig. 1D). The affected individual in family B3913 is an Arabic male who developed steroid-dependent nephrotic syndrome at the age of 3. He has no extrarenal manifestation and has not had a kidney biopsy. In family B3943, an Arabic male developed steroid-resistant nephrotic syndrome at the age of 13, with no extrarenal manifestation, and a kidney biopsy revealed membranoproliferative glomerulonephritis. Consanguinity was reported in both families, and it was confirmed with homozygosity mapping showing runs of homozygosity spanning 154.6 Mb and 170.3 Mb respectively. Notably, the candidate variants in MYO1C coincided with a peak indicating a region of homozygosity by descent (Fig.1).
Figure 1.
WES identifies biallelic missense variants in the gene MYO1C encoding the motor protein Myosin 1C, in two unrelated families with nephrotic syndrome. (a) Pedigree of index families (B3913 and B3943). Squares represent males, circles females, double line denotes consanguinity, black shading indicates the affected individual, and red dots highlight individuals included in whole exome sequencing. (b) homozygosity mapping depicts high homozygosity in both families and confirms the reported consanguinity. A homozygous peak on chromosome 17 (arrowhead) that includes MYO1C is present in both families. (c) Evolutionary conservation across orthologues of MYO1C. Respective variants are indicated by the arrowhead. (d) Exon structure (black and white) and domain structure (colored) of MYO1C, depicting the location of the two MYO1C variants.
Mbps, mega base pairs; aa, amino acids; H.s., Homo sapiens; M.m., Mus musculus; G.g., Gallus gallus; X.t., Xenopus tropicalis; D.r., Danio rerio
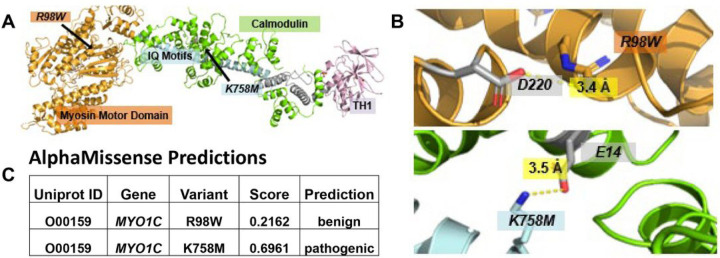
We examined kidney single-cell RNA sequencing datasets available publicly and identified strong podocyte expression of MYO1C in comparison to positive (nephrin and podocin) and negative controls (ACTA2) ([26]) (Supp Fig.1). Next, using the molecular viewer PyMol software, we conducted structural modeling to align shared regions within both partial structures of MYO1c. (4byf and 4r8g), aligned with the common regulator of myosin activity, calmodulin (Fig.2). Calmodulin was observed to bind to the IQ motif in both structures. At residue sites (K758, R98), ion-ion interactions were discerned, stabilizing intradomain and ligand interactions: specifically, R98 forms a bond with nearby D220 within the Myosin Motor Domain, while K758 interacts with E14 on a calmodulin molecule. We analyzed the effects of the variants on protein tertiary structure utilizing the AlphaMissense software [27]. The R98W exchange in the myosin domain (Fig.2A) was predicted to be benign, whereas K758M, which interacts with glutamate E14 on calmodulin was predicted to ablate this ionic bond and was deemed likely pathogenic (Fig.2C).
Figure 2.
Crystallized structures of MYO1C (PDB: 4byf, 4r8g). Structural modeling was performed in molecular viewer PyMol using the super function aligning shared regions within both partial structures. In both 4byf and 4r8g structures, calmodulin, a common regulator of myosin activity, is shown to bind to the IQ motif. (A) Complete MYO1C protein structure modeled next to calmodulin. (B) Close-up view of molecular residue interactions at both mutation sites. At both residue sites (K758, R98), there are ion-ion interactions stabilizing intradomain and ligand interactions: R98 binds to nearby D220 within the Myosin Motor Domain and K758 binds to E14 on a calmodulin molecule. Mutations of these charged residues to non-charged amino acids could ablate these ionic interactions, weakening protein structure and function. (C) AlphaMissense analyzes the effects of missense variants on protein tertiary structure. It predicts K758M to be a pathogenic mutation with a score of 0.69
Discussion
In this study, we identified two homozygous missense variants in the gene MYO1C in two unrelated families with proteinuric kidney disease. Reported consanguinity and measured homozygosity in both families suggest a recessive cause of disease. Indeed, homozygous MYO1C variants were detected in peaks on homozygosity mapping. Both variants are missense alterations resulting in a positively charged amino acid, arginine or lysine, substituted by a neutral amino acid. Evolutionary conservation, pathogenicity prediction by in-silico programs and 3D structure prediction programs, and position within protein domain structures, support the pathogenic role of these MYO1C variants.
The majority of causative SRNS genes exhibit high level expression in glomerular podocytes, strongly suggesting podocytes to be the main site of injury in NS and underscoring its crucial role in upholding the filtration barrier. MYO1C is predominantly expressed in podocytes as evidenced by single-cell RNA sequencing datasets [26] (Supp Fig.1). This supports a potential pathogenic role of MYO1C in SRNS. Moreover, 3D structural modeling revealed ion-ion interactions at both residue sites (K758, R98), which likely play an important role in stabilizing intradomain and ligand interactions. Modifying these charged residues to non-charged amino acids could potentially disrupt these ionic interactions, leading to a weakening of protein structure and its function. Published functional data further supports the pivotal role of MYO1C in podocyte physiology. MYO1C was shown to be a direct interactor of the podocyte slit diaphragm structural proteins, nephrin and neph1, and a mediator of their transport to the podocyte intercellular junction [7]. Arif et al. demonstrated abnormal developmental phenotype of myo1c knockout zebrafish, characterized by pericardial edema and dilated renal tubules [6]. Subsequent analysis of the glomerular ultrastructure in myo1c depleted zebrafish revealed absence of the slit diaphragm and abnormal podocyte morphology. Furthermore, their research highlighted the central role of Myo1c-mediated regulation of TGF-b in the pathogenesis of podocyte injury, as evidenced by findings from a Myo1c knockout mouse model [7].
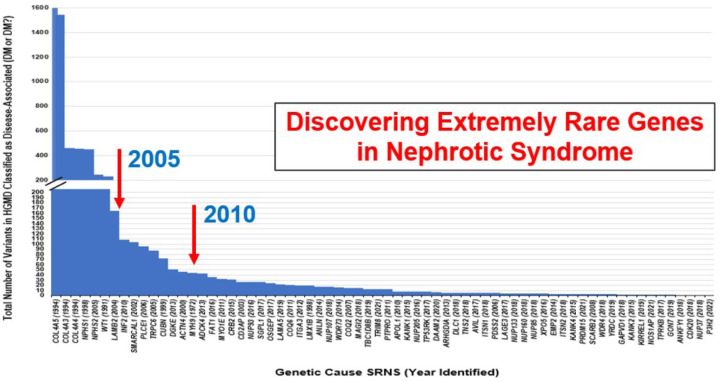
Although the number of known genes associated with SRNS has increased from 27 to 69 over the last decade, the impact on the solve rate has been limited [Supp Table 1]. This can be explained by the rarity of the newly discovered genes as shown in Fig.3. Analyzing this chart reveals a discernible trend: there appears to be a correlation between the year a gene was discovered as being causative of SRNS, and its prevalence. Notably, the genes implicated latest as monogenic causes of SRNS are exceptionally rare, being present in only a few families.
Figure 3.
The number of disease-causing alleles currently listed in the HGMD database for the 67 monogenic causes of steroid-resistant nephrotic syndrome. The more frequently mutated genes were discovered first, such as nephrin, podocin, WT1 and LAMB2. Genes like INF2 and PLCE1 were discovered before 2010, and thereafter newly discovered genes were extremely rare
Based on our prior experience, an initial discovery of a rare variant becomes the basis for identifying additional variants in the candidate gene. With sufficient genetic evidence, usually when four different families with distinct alleles in the same gene are discovered, functional studies are, then, warranted to support the biological impact of disease-causing variants. Nevertheless, when lacking this threshold, we still find it crucial to report these discoveries, once genetic evidence, computational methods, and existing literature strongly support a potential role of the gene-product in the pathogenesis of SRNS. For instance, KANK1 and CRB2, initially identified by our lab in one and four families respectively [28, 29], have now been reported in seven and thirty-one families in Human Gene Mutation Database, indicating the evolving landscape of genetic discoveries in nephrotic syndrome.
In conclusion, we here present variants in MYO1C in two families, which may point to MYO1C as a potential new candidate gene for SRNS. Discovery of further families with NS who carry variants in the MYO1C gene, together with functional evidence to support their pathogenicity, are necessary to assess the role of MYO1C as a candidate gene for SRNS.
Acknowledgments
The authors thank individuals, families, and their physicians who contributed to this study. We thank Wanxia Wu, Anja E. H. Nordstrom, Maria Ericsson, and Louise M. Trakimas for their technical assistance.
Funding
F.H. is the William E. Harmon Professor of Pediatrics at Harvard Medical School. This research was supported by grants from the National Institutes of Health to F.H. (RC-2-DK1222397), the Office of Faculty Development to R.S, the German Research Foundation to C.M.K. (Project No.: 499462148).
Funding Statement
F.H. is the William E. Harmon Professor of Pediatrics at Harvard Medical School. This research was supported by grants from the National Institutes of Health to F.H. (RC-2-DK1222397), the Office of Faculty Development to R.S, the German Research Foundation to C.M.K. (Project No.: 499462148).
Footnotes
Ethics Declaration
This study was approved by the institutional review board (IRB) of the University of Michigan (#2003 − 0116) and of Boston Children’s Hospital (#IRB-P00006200) and of institutions where families were recruited. Informed consent of each individual or its legal guardian was obtained.
Disclosure
All authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
Table 1 is available in the Supplementary Files section.
Supplementary Files
Contributor Information
Izzeldin Elmubarak, Boston Children’s Hospital.
Shirlee Shril, Boston Childrens Hospital: Boston Children’s Hospital.
Bshara Mansour, Boston Childrens Hospital: Boston Children’s Hospital.
Aaron Bao, Boston Childrens Hospital: Boston Children’s Hospital.
Caroline Kolvenbach, Boston Childrens Hospital: Boston Children’s Hospital.
Sherif El Desoky, King Abdulaziz University Faculty of Medicine.
mohamed shalaby, King Abdulaziz University Faculty of Medicine.
Jameela Kari, King Abdulaziz University Faculty of Medicine.
Friedhelm Hildebrandt, Boston Childrens Hospital: Boston Children’s Hospital.
Ronen Schneider, Boston Childrens Hospital: Boston Children’s Hospital.
References
- 1.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int 20:765–771. [DOI] [PubMed] [Google Scholar]
- 2.Leonard MB, Donaldson LA, Ho M, Geary DF (2003) A prospective cohort study of incident maintenance dialysis in children: an NAPRTC study. Kidney international 63:744–755. [DOI] [PubMed] [Google Scholar]
- 3.Kopp JB, Anders H-J, Katalin S, Podestà MA, Giuseppe R, Friedhelm H, Paola R (2020) Podocytopathies (Primer). Nature Reviews: Disease Primers 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schell C, Sabass B, Helmstaedter M, Geist F, Abed A, Yasuda-Yamahara M, Sigle A, Maier JI, Grahammer F, Siegerist F, Artelt N, Endlich N, Kerjaschki D, Arnold HH, Dengjel J, Rogg M, Huber TB (2018) ARP3 Controls the Podocyte Architecture at the Kidney Filtration Barrier. Dev Cell 47:741–757 e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perico L, Conti S, Benigni A, Remuzzi G (2016) Podocyte-actin dynamics in health and disease. Nat Rev Nephrol 12:692–710. [DOI] [PubMed] [Google Scholar]
- 6.Arif E, Kumari B, Wagner MC, Zhou W, Holzman LB, Nihalani D (2013) Myo1c is an unconventional myosin required for zebrafish glomerular development. Kidney Int 84:1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arif E, Solanki AK, Srivastava P, Rahman B, Tash BR, Holzman LB, Janech MG, Martin R, Knolker HJ, Fitzgibbon WR, Deng P, Budisavljevic MN, Syn WK, Wang C, Lipschutz JH, Kwon SH, Nihalani D (2019) The motor protein Myo1c regulates transforming growth factor-beta-signaling and fibrosis in podocytes. Kidney Int 96:139–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warejko JK, Tan W, Daga A, Schapiro D, Lawson JA, Shril S, Lovric S, Ashraf S, Rao J, Hermle T, Jobst-Schwan T, Widmeier E, Majmundar AJ, Schneider R, Gee HY, Schmidt JM, Vivante A, van der Ven AT, Ityel H, Chen J, Sadowski CE, Kohl S, Pabst WL, Nakayama M, Somers MJG, Rodig NM, Daouk G, Baum M, Stein DR, Ferguson MA, Traum AZ, Soliman NA, Kari JA, El Desoky S, Fathy H, Zenker M, Bakkaloglu SA, Muller D, Noyan A, Ozaltin F, Cadnapaphornchai MA, Hashmi S, Hopcian J, Kopp JB, Benador N, Bockenhauer D, Bogdanovic R, Stajic N, Chernin G, Ettenger R, Fehrenbach H, Kemper M, Munarriz RL, Podracka L, Buscher R, Serdaroglu E, Tasic V, Mane S, Lifton RP, Braun DA, Hildebrandt F (2018) Whole Exome Sequencing of Patients with Steroid-Resistant Nephrotic Syndrome. Clin J Am Soc Nephrol 13:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan W, Lovric S, Ashraf S, Rao J, Schapiro D, Airik M, Shril S, Gee HY, Baum M, Daouk G, Ferguson MA, Rodig N, Somers MJG, Stein DR, Vivante A, Warejko JK, Widmeier E, Hildebrandt F (2018) Analysis of 24 genes reveals a monogenic cause in 11.1% of cases with steroid-resistant nephrotic syndrome at a single center. Pediatr Nephrol 33:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, Vega-Warner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Group SS, Hildebrandt F (2015) A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 26:1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovric S, Ashraf S, Tan W, Hildebrandt F (2016) Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol Dial Transplant 31:1802–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacArthur D, Manolio T, Dimmock D, Rehm H, Shendure J, Abecasis G, Adams D, Altman R, Antonarakis S, Ashley E (2014) Guidelines for investigating causality of sequence variants in human disease. Nature 508:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedhammer KM, Braunisch MC, Gunthner R, Wagner M, Hemmer C, Strom TM, Schmaderer C, Renders L, Tasic V, Gucev Z, Nushi-Stavileci V, Putnik J, Stajic N, Weidenbusch M, Uetz B, Montoya C, Strotmann P, Ponsel S, Lange-Sperandio B, Hoefele J (2020) Exome Sequencing and Identification of Phenocopies in Patients With Clinically Presumed Hereditary Nephropathies. Am J Kidney Dis 76:460–470. [DOI] [PubMed] [Google Scholar]
- 14.Vivante A, Hildebrandt F (2016) Exploring the genetic basis of early-onset chronic kidney disease. Nature Reviews Nephrology 12:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Yang W, Ying D, Cherny SS, Hildebrandt F, Sham PC, Lau YL (2011) Homozygosity mapping on a single patient: identification of homozygous regions of recent common ancestry by using population data. Hum Mutat 32:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nature methods 7:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudmundsson S, Singer-Berk M, Watts NA, Phu W, Goodrich JK, Solomonson M, Genome Aggregation Database C, Rehm HL, MacArthur DG, O’Donnell-Luria A (2022) Variant interpretation using population databases: Lessons from gnomAD. Hum Mutat 43:1012–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature protocols 4:1073–1081. [DOI] [PubMed] [Google Scholar]
- 19.Machuca E, Hummel A, Nevo F, Dantal J, Martinez F, Al-Sabban E, Baudouin V, Abel L, Grunfeld JP, Antignac C (2009) Clinical and epidemiological assessment of steroid-resistant nephrotic syndrome associated with the NPHS2 R229Q variant. Kidney Int 75:727–735. [DOI] [PubMed] [Google Scholar]
- 20.Ng PC, Henikoff S (2001) Predicting deleterious amino acid substitutions. Genome Res 11:863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Committee ALQA (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz JM, Cooper DN, Schuelke M, Seelow D (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nature methods 11:361–362. [DOI] [PubMed] [Google Scholar]
- 23.Seelow D, Schuelke M, Hildebrandt F, Nurnberg P (2009) HomozygosityMapper--an interactive approach to homozygosity mapping. Nucleic Acids Res 37:W593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, Banks E, Garimella KV, Altshuler D, Gabriel S, DePristo MA (2013) From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 43:11 10 11–11 10 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Ding X, Sun X, Tsang SY, Xue H (2015) SAMSVM: A tool for misalignment filtration of SAM-format sequences with support vector machine. J Bioinform Comput Biol 13:1550025. [DOI] [PubMed] [Google Scholar]
- 26.Witt K, Milner A, Spittal MJ, Hetrick S, Robinson J, Pirkis J, Carter G (2019) Population attributable risk of factors associated with the repetition of self-harm behaviour in young people presenting to clinical services: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry 28:5–18. [DOI] [PubMed] [Google Scholar]
- 27.Cheng J, Novati G, Pan J, Bycroft C, Zemgulyte A, Applebaum T, Pritzel A, Wong LH, Zielinski M, Sargeant T, Schneider RG, Senior AW, Jumper J, Hassabis D, Kohli P, Avsec Z (2023) Accurate proteome-wide missense variant effect prediction with AlphaMissense. Science 381:eadg7492. [DOI] [PubMed] [Google Scholar]
- 28.Gee HY, Zhang F, Ashraf S, Kohl S, Sadowski CE, Vega-Warner V, Zhou W, Lovric S, Fang H, Nettleton M, Zhu JY, Hoefele J, Weber LT, Podracka L, Boor A, Fehrenbach H, Innis JW, Washburn J, Levy S, Lifton RP, Otto EA, Han Z, Hildebrandt F (2015) KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest 125:2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebarasi L, Ashraf S, Bierzynska A, Gee HY, McCarthy HJ, Lovric S, Sadowski CE, Pabst W, Vega-Warner V, Fang H, Koziell A, Simpson MA, Dursun I, Serdaroglu E, Levy S, Saleem MA, Hildebrandt F, Majumdar A (2015) Defects of CRB2 cause steroid-resistant nephrotic syndrome. Am J Hum Genet 96:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]