Abstract
Exposure of Lipofectin-DNA complexes to the partially purified G glycoprotein of the vesicular stomatitis virus envelope (VSV-G) results in loss of serum-mediated inhibition and in enhanced efficiency of gene transfer. Sucrose density gradient sedimentation analysis indicated that the VSV-G associates physically with the DNA-lipid complex to produce a VSV-G liposome. The ability to incorporate surrogate viral or cellular envelope components such as VSV-G into liposomes may allow more-efficient and possibly targeted gene delivery by lipofection, both in vitro and in vivo.
Cationic lipid-mediated gene transfer (lipofection) is one of the standard transfection methods for introducing foreign genes into mammalian cells (7). The simplicity of this technique, its lack of toxicity, and its largely nonimmunogenic properties, as well as the ability of liposomes to deliver large pieces of DNA, make lipofection an attractive alternative under some conditions to viral gene delivery not only in vitro but also in vivo (8, 10). For instance, Zhu et al. have shown that liposome-mediated gene delivery to a large number of organs is feasible after intravenous injection, although under these conditions DNA delivery and transgene expression were most efficient for the macrophage populations in lung or spleen tissue (29). Nabel and colleagues have also demonstrated that direct intratumoral injection of DNA-lipid complex containing the HLA-B7 gene can provoke an immune response that can in turn lead to some degree of tumor stabilization or even regression around the injected region, as well as at distant metastases, with no evidence of detectable toxicity associated with the treatment (19).
Although liposome-mediated gene transfer has advanced to the point of justifying clinical trials, gene transfer by liposomes is still much less efficient than viral vector-mediated transfer. Zabner et al. have suggested that the inefficiency of lipid-mediated DNA transfer may be due to events subsequent to endocytosis of the DNA-lipid complexes into cells, especially to inefficient release into the cytoplasm of intact DNA from endocytosed vesicles and inefficient nuclear localization (26).
It is well known that the envelope spike G glycoprotein of vesicular stomatitis virus (VSV-G) can be incorporated into the envelopes of other viruses to produce pseudotyped particles with new host ranges and cell tropisms (27). Our laboratory has reported general and efficient methods for pseudotyping murine leukemia virus (MLV)-derived retrovirus vectors with VSV-G (4–6, 25). More recently, the tools and techniques of VSV-G pseudotyping have been used for the production of lentivirus-based vectors (21). Because of the pantropic characteristics of the VSV-G envelope, VSV-G-pseudotyped vectors allow efficient gene transfer into many cell types refractory to other methods of gene transfer. It has been reported that purified VSV-G can spontaneously reconstitute into the lipid bilayer; however, the conditions required for the conservation of its fusogenic activity in such lipids have not been fully defined, and the role of VSV-G in producing efficient gene transfer liposomes has not been reported (14, 17, 22).
We have recently demonstrated that VSV-G particles are efficiently released into culture medium from cells expressing VSV-G and that VSV-G prepared from such conditioned medium can be introduced into the membranes of spikeless, immature, noninfectious MLV-based retrovirus-like particles in a cell-free system to generate infectious virus particles in vitro (2, 23). In the present study, we report that VSV-G prepared from conditioned medium of VSV-G-expressing cells can also be introduced into lipofection complexes to produce fusogenic VSV-G liposomes that demonstrate a markedly enhanced lipofection efficiency and that abrogate the serum inhibition of gene transfer by conventional lipofection complexes.
VSV-G purification.
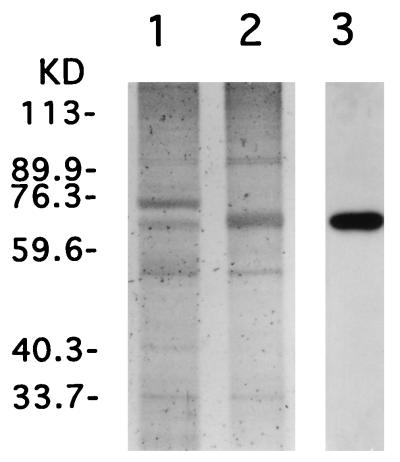
VSV-G protein was prepared from conditioned medium of 293 cells transfected with plasmid pCMV-G expressing VSV-G by the calcium phosphate coprecipitation method as previously described (25). The conditioned medium was centrifuged at 24,000 rpm with a Beckman SW28 rotor for 90 min, and the pelleted VSV-G was resuspended with phosphate-buffered saline (PBS) (pH 7.5). To partially purify the VSV-G, the suspension of the pellet was layered onto 5 to 30% continuous sucrose gradients in PBS for velocity sedimentation. The gradients were centrifuged at 30,000 rpm in an SW41Ti rotor for 25 min, and fractions containing VSV-G were collected, diluted with PBS, and centrifuged at 30,000 rpm for 90 min. The resulting VSV-G pellet was resuspended with PBS, and the amount of protein was quantified with the bicinchoninic acid protein detection kit (Pierce, Rockford, Ill.). The proteins were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on a 7.5% gel and were visualized with the silver staining kit (Bio-Rad, Richmond, Calif.). Identification of the VSV-G band was confirmed by Western blot analysis with anti-VSV-G monoclonal antibody P5D4 (Sigma, St. Louis, Mo.) (1). The VSV-G in pellets of transfected cells was detected as a band of approximately 68 kDa in size, with significant amounts 75 kDa, probably representing bovine serum albumin (Fig. 1, lane 1). After velocity sucrose gradient sedimentation and repelleting, the purity of the VSV-G was substantially improved, as judged by silver staining (Fig. 1, lane 2). Approximately 20 to 30 μg of VSV-G protein from 200 ml of conditioned medium from 293 cells transfected with pCMV-G was routinely obtained.
FIG. 1.
SDS-polyacrylamide gel electrophoresis of partially purified VSV-G. The conditioned medium of 293 cells transfected with pCMV-G was centrifuged, and the resulting pellet was examined by electrophoresis (lane 1). The pellet suspension was subjected to velocity sucrose gradient, and VSV-G-containing fractions were pooled, diluted with PBS, and repelleted. The repurified pellet suspension was analyzed as described above (lanes 2 and 3). About 100 ng of protein in each VSV-G preparation was loaded and visualized by silver staining (lanes 1 and 2) and by Western blot analysis with anti-VSV-G monoclonal antibody, P5D4 (lane 3).
VSV-G-induced enhancement of Lipofectin-mediated DNA transfer.
The effect of VSV-G on lipofection was examined by using Lipofectin (GIBCO BRL/Life Technologies, Grand Island, N.Y.). The DNA-lipid complexes were prepared according to the manufacturer’s instructions and were mixed with VSV-G just before transfection. All transfections were performed on cell cultures at approximately 80% confluency. BHK and 293 cells grown in six-well plates were washed twice with Dulbecco modified Eagle medium (DMEM), were maintained in fresh DMEM containing 10% fetal bovine serum (FBS), and were incubated with the DNA-lipid complex. The DNA-lipid complex was prepared with reduced volume as follows. A 5-μg amount of Lipofectin was diluted with 100 μl of DMEM for each well. After incubation for 30 min at room temperature, the diluted Lipofectin was mixed with 100 μl of DMEM containing 1 μg of plasmid pCMV-luc expressing the firefly luciferase gene, and the mixture was incubated for 15 min at room temperature and subjected to transfection. Culture medium was changed with fresh DMEM with 10% FBS after 12 h of incubation. Luciferase activity in the transfected cells was measured 2 days after lipofection and was presented as relative light units (RLU) per microgram of cellular protein, as previously described (15). To examine the stable transformant, 293 cells were transfected with plasmid pcDNA3 (Invitrogen, Carlsbad, Calif.), which contains the neomycin resistance gene driven by the simian virus 40 promoter, serially diluted cells were spread on 10-cm plates 24 h after transfection, and G418 selection was started 48 h after transfection. G418-resistant colonies were counted after 2 weeks of selection (15). As shown in Table 1, addition of VSV-G into the DNA-lipid complex increased the lipofection efficiency by approximately 10-fold in the presence of 10% FBS in the culture medium, as estimated by luciferase activities in both BHK and 293 cells or by the numbers of stable, G418-resistant colonies of 293 cells. In all cases, this enhanced transfection was inhibited by anti-VSV-G neutralizing antibody I1 (4). We also examined the enhancing effect with a fusion-defective VSV-G mutant, VSV-G-P127L. The mutant VSV-G-P127L contains a mutation encoding a proline-to-leucine substitution at amino acid 127 and was made by use of the MORPH in vitro mutagenesis kit (5prime→3prime, Inc., Boulder, Colo.). The mutagenesis primer 5′-ATATCCACAACTCTGCAGAGGGAACCCGGGATTCAGCCA-3′ also includes a number of silent mutations (underlined bases). An identical mutant was previously reported by Zhang and Ghosh (28). Although VSV-G-P127L is expressed well on the cell surface and is released efficiently into culture medium (2), its cell fusion function is less than 5% of that of wild-type VSV-G (9, 16, 28). The VSV-G-P127L that was prepared and used by the method used for wild-type VSV-G in the lipofection experiment did not show any enhancing effect on lipofection (Table 1). Therefore, we conclude that the enhancement of lipofection by VSV-G requires the fusogenic function of VSV-G. Neither luciferase activity nor G418 resistance was seen after exposure of the cells to DNA or VSV-G without Lipofectin (data not shown).
TABLE 1.
Effects of VSV-G on lipofectiona
| Complex | Luciferase activity (RLU/μg)
|
No. of stable transformants of 293 cells (no. of colonies/2 × 103 cells) | |
|---|---|---|---|
| BHK cells | 293 cells | ||
| DNA-lipid | 40,900 ± 800 | 16,700 ± 700 | 8 |
| DNA-lipid + VSV-G | 442,000 ± 11,100 | 125,000 ± 5,300 | 62 |
| DNA-lipid + anti-VSV-G (I1) | 32,000 ± 700 | 13,300 ± 800 | 10 |
| DNA-lipid + VSV-G + anti-VSV-G (I1) | 26,700 ± 800 | 12,400 ± 500 | 11 |
| DNA-lipid + VSV-G–P127L | 28,300 ± 900 | 13,600 ± 700 | 12 |
VSV-G pellets from 200 μl of conditioned medium from 293 cells transfected with pCMV-G or pCMV-G-P127L were resuspended in 10 μl of PBS, and the suspension was added to the DNA-lipid complex consisting of Lipofectin (5 μg) and pCMV-luc (1 μg) just before transfection. In some cases, a neutralizing antibody against VSV-G, I1, was added into the culture medium before transfection. Transfection was performed with six-well plates in DMEM with 10% FCS. Luciferase activity in the transfected cells was measured 48 h after transfection, and results are RLU per microgram of cellular protein. The experiment was performed in triplicate, and the data are means ± standard deviations. For examination of stable gene transfer, cells were transfected with pcDNA3 and G418-resistant colonies derived from 2 × 103 cells reseeded 24 h after transfection were counted.
Optimization of VSV-G-dependent increase in Lipofection efficiency.
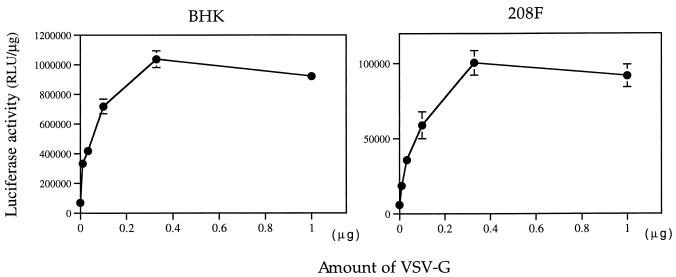
To optimize the composition of the Lipofectin–DNA–VSV-G complexes during lipofection, we examined the effect of increasing amounts of VSV-G added to constant amounts (2.5 μg) of the lipid-DNA complex immediately prior to addition to the cells. As shown in Fig. 2, when 2.5 μg of lipid-DNA was mixed just before transfection with amounts of partially purified VSV-G ranging from 10 ng to 1 μg, the efficiency of lipofection on both BHK and 208F cells increased in a dose-dependent manner and reached a maximum efficiency at approximately 300 to 400 ng of VSV-G. Amounts of VSV-G that were greater than 400 ng slightly decreased the transfection efficiency, although the decrease was not correlated with the known toxic effect of VSV-G, i.e., the formation of syncytium.
FIG. 2.
The effect of increasing amounts of the partially purified VSV-G on lipofection efficiency in BHK and 208F cells (6). Increasing amounts of partially purified VSV-G in the form of pelleted conditioned medium from transfected 293 cells were added to the DNA-lipid complex (2.5 μg of Lipofectin and 0.5 μg of pCMV-luc per well) immediately prior to addition to cells. Transfection was performed with 12-well plates in DMEM with 10% FBS. The experiment was performed in triplicate, and the data are means ± standard deviations.
Effect of serum in culture medium on transfection efficiency.
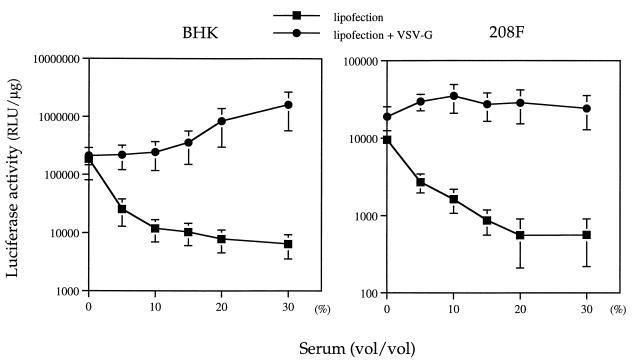
In many cell lines, the maximum efficiency of lipofection is known to require serum-depleted or serum-free medium, even though such conditions are themselves toxic to many cell types. For such cell lines, it would be advantageous to have lipofection conditions that allow efficient gene transfer in the presence of serum. Therefore, we examined the effect of serum on lipofection in the presence of VSV-G. Cells grown in 12-well plates were washed twice with DMEM and were maintained in fresh DMEM containing various concentrations of FBS, and the plates were incubated with the DNA-lipid complex or the DNA–lipid–VSV-G complex. As shown in Fig. 3, lipofection efficiency was, as expected, dramatically inhibited by serum in the absence of VSV-G. However, in the presence of VSV-G, serum-mediated inhibition of lipofection was completely abrogated. In the case of BHK cells, high concentrations of serum may even have a further enhancing effect on lipofection efficiency.
FIG. 3.
Effect of the concentration of serum in culture medium on VSV-G-induced lipofection efficiency. Aliquots of partially purified VSV-G (200 ng) were added to the DNA-lipid complex immediately before addition to cells in 12-well plates in DMEM supplemented with various concentrations of FBS. The experiment was performed in triplicate, and the data are means ± standard deviations.
Physical association between VSV-G and DNA-lipid complexes.
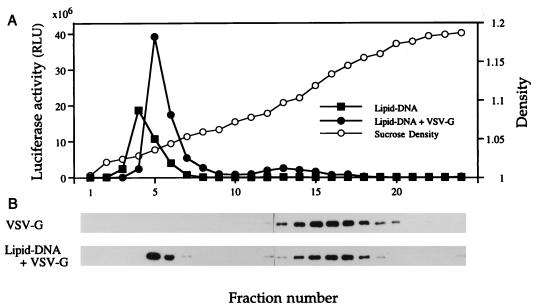
To examine the mechanism of the VSV-G-mediated enhancement of lipofection, we analyzed the association of VSV-G with the lipid-DNA complex by equilibrium buoyant density sucrose gradient sedimentation. Partially purified VSV-G (2.5 μg), DNA-lipid complex (50 μg of lipid and 10 μg of pCMV-luc), or VSV-G–DNA–lipid conjugate (2.5 μg of VSV-G, 50 μg of lipofectin, and 10 μg of pCMV-luc) was layered onto 5 to 40% continuous sucrose gradients prepared in PBS (pH 7.5). The gradients were centrifuged at 35,000 rpm in an SW41Ti rotor for 16 h at 4°C. Fractions were collected from the top of the gradient. Transfection efficiency was measured by determining luciferase activity under the serum-free conditions described above. VSV-G was detected by established Western blotting methods (1) by using anti-VSV-G monoclonal antibody P5D4. As shown in Fig. 4, the buoyant density of uncomplexed VSV-G is approximately 1.10 to 1.15 g/ml. The formation of the lipid–DNA–VSV-G complex results in a shift of the buoyant density of some of the VSV-G to approximately 1.05 g/ml, a position that corresponds to the lipofection maximum of the sample. Interestingly, the position of maximal infectivity of the VSV-G liposome was several fractions heavier than that of the Lipofectin-DNA complex itself, which is a result consistent with the presence of added VSV-G protein in the VSV-G liposome.
FIG. 4.
Buoyant-density analysis by sucrose density gradient centrifugation of uncomplexed VSV-G, Lipofectin-DNA complex, and Lipofectin–DNA–VSV-G complexes. (A) Luciferase activities (represented by the total activity of each fraction) in BHK cells transfected with gradient fractions containing the Lipofectin-DNA complex or the Lipofectin–DNA–VSV-G complex; (B) Western blot analysis of fractions from the gradient containing pelleted VSV-conditioned medium from transfected, VSV-G-producing 293 cells and from a gradient containing the Lipofectin–DNA–VSV-G liposome. VSV-G protein was detected with the anti-VSV-G monoclonal antibody P5D4. Sucrose densities are indicated as the densities in parallel gradients.
These studies demonstrate that the addition to a Lipofectin-DNA complex of VSV-G in the form of pelleted particles from the conditioned medium of transfected VSV-G-producing cells markedly enhances the lipofection efficiency of the complex and abrogates the serum-mediated inhibition of lipofection in both transient and stable gene transfer. Because this enhancement was completely abolished by the neutralizing antibody against VSV-G and was not seen with the fusion-defective VSV-G mutant G-P127L, we conclude that the enhancement requires the fusogenic function of the VSV-G glycoprotein. The enhanced lipofection seems to be due not merely to the presence of a fusogenic material, since sedimentation analysis in a sucrose gradient demonstrates that a significant amount of the VSV-G becomes physically associated with the DNA-lipid complex, indicating the formation of a more-complex lipid–DNA–VSV-G structure, i.e., the VSV-G liposome.
While the inhibitory effect of FBS on lipid-mediated gene transfer in some cases certainly depends on the nature of the target cells, serum inhibition represents one of the major obstacles to efficient use of lipofection for the transfer of potentially therapeutic genes, especially in in vivo gene transfer models. Because most workers have found the presence of serum to be highly detrimental to lipofection, a great deal of effort has been put into overcoming the problems of serum inhibition of lipofection by modifying the lipid components or the methods and conditions used to make the DNA-lipid complex (11–13, 18, 24). Several previous studies have reported improved stability and gene transferring capability with regard to liposomes in the presence of serum. Brunette et al. reported that lipofection does not require the removal of serum, but their system was limited to some specific cell types, such as the CV1 simian kidney cell line and MEL murine erythroleukemia cells (3).
Other investigators have used viral envelope glycoproteins as liposome components. Nakanishi et al. have reported that the HVJ liposome, in which Sendai virus is fused with liposomes, demonstrates a marked increase in DNA transfer efficiency that is not significantly inhibited by serum (18, 20). However, preparation of the HVJ liposomes is time-consuming and requires the inactivation by UV radiation of the wild-type Sendai virus used for production of the complex. In the present study, we demonstrate that the incorporation of the VSV-G glycoprotein is accomplished by simple mixing of the Lipofectin-DNA complex with VSV-G derived from conditioned medium of transfected cells in the absence of all other viral components and that the procedure, therefore, does not require potentially complicating virus inactivation steps. It is known that detergent-purified VSV-G protein can be incorporated in vitro into the lipid bilayers of synthetic liposomes to produce agents capable of fusing target cells (14, 17, 22). However, the use of detergent and long-term dialysis to remove the residual detergent can disrupt the fusogenic activity of VSV-G, and the gene transferring capability of such VSV-G-containing liposomes has not previously been demonstrated (17). We have also attempted to construct VSV-G liposomes with detergent-purified VSV-G, but without reproducible success. In contrast, the method for the preparation of VSV-G liposomes presented in the present study is simple and reproducible and conserves the fusogenic function of the VSV-G glycoprotein needed for the enhanced lipofection effect. The VSV-G liposome represents an efficient and simple modification of the current lipid-mediated DNA transfer tools and also facilitates study of the incorporation of modified envelope glycoproteins into liposomes for tissue-specific targeting. Further optimization of the methods for fusogenic liposome production and its use for gene transfer, both in vitro and especially in vivo, will eventually establish the extent of its utility as an approach to the transfer of therapeutic genes for the purpose of gene therapy.
Acknowledgments
These studies were supported by grants Dk49023 and HL53680 from the National Institutes of Health and by a grant from the Del Webb Foundation. A.A. is a visiting scholar supported by a grant from the Sankyo Foundation of Life Science.
We thank Henrik Steinberg for excellent and skillful technical assistance.
REFERENCES
- 1.Abe A, Emi N, Taji H, Kasai M, Kohno A, Saito H. Induction of humoral and cellular anti-idiotypic immunity by intradermal injection of naked DNA encoding a human variable region gene sequence of an immunoglobulin heavy chain in a B cell malignancy. Gene Ther. 1996;3:988–993. [PubMed] [Google Scholar]
- 2.Abe, A., S.-T. Chen, A. Miyanohara, and T. Friedmann. Submitted for publication.
- 3.Brunette E, Stribling R, Debs R. Lipofection does not require the removal of serum. Nucleic Acids Res. 1992;20:1151. doi: 10.1093/nar/20.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S-T, Iida A, Guo L, Friedmann T, Yee J K. Generation of packaging cell lines for pseudotyped retroviral vectors of the G protein of vesicular stomatitis virus by using a modified tetracycline inducible system. Proc Natl Acad Sci USA. 1996;93:10057–10062. doi: 10.1073/pnas.93.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emi N, Friedmann T, Yee J K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65:1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felgner P L, Gadek T R, Holm M, Roman R, Chan H W, Wenz M, Northrop J P, Ringold G M, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felgner P L, Tsai Y J, Sukhu L, Wheeler C J, Manthorpe M, Marshall J, Cheng S H. Improved cationic lipid formulations for in vivo gene therapy. Ann N Y Acad Sci. 1995;772:126–139. doi: 10.1111/j.1749-6632.1995.tb44738.x. [DOI] [PubMed] [Google Scholar]
- 9.Fredericksen B L, Whitt M A. Vesicular stomatitis virus glycoprotein mutations that affect membrane fusion activity and abolish virus infectivity. J Virol. 1995;69:1435–1443. doi: 10.1128/jvi.69.3.1435-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X, Huang L. Cationic liposome-mediated gene transfer. Gene Ther. 1995;2:710–722. [PubMed] [Google Scholar]
- 11.Gao X, Huang L. Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry. 1996;35:1027–1036. doi: 10.1021/bi952436a. [DOI] [PubMed] [Google Scholar]
- 12.Hofland H E, Shephard L, Sullivan S M. Formation of stable cationic lipid/DNA complexes for gene transfer. Proc Natl Acad Sci USA. 1996;93:7305–7309. doi: 10.1073/pnas.93.14.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong K, Zheng W, Baker A, Papahadjopoulos D. Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. FEBS Lett. 1997;400:233–237. doi: 10.1016/s0014-5793(96)01397-x. [DOI] [PubMed] [Google Scholar]
- 14.Hug P, Sleight R G. Fusogenic virosomes prepared by partitioning of vesicular stomatitis virus G protein into preformed vesicules. J Biol Chem. 1994;269:4050–4056. [PubMed] [Google Scholar]
- 15.Li X, Yee J K, Wolff J A, Friedmann T. Factors affecting long-term stability of Molony murine leukemia virus-based vectors. Virology. 1989;171:331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Drone C, Sat E, Ghosh H P. Mutational analysis of the vesicular stomatitis virus glycoprotein G for membrane fusion domains. J Virol. 1993;67:4070–4077. doi: 10.1128/jvi.67.7.4070-4077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metsikko K, van Meer G, Simmons K. Reconstitution of the fusiogenic activity of vesicular stomatitis virus. EMBO J. 1986;5:3429–3435. doi: 10.1002/j.1460-2075.1986.tb04665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuguchi H, Nakagawa T, Nakanishi M, Imazu S, Nakagawa S, Mayumi T. Efficient gene transfer into mammalian cells using fusogenic liposome. Biochem Biophys Res Commun. 1996;218:402–407. doi: 10.1006/bbrc.1996.0070. [DOI] [PubMed] [Google Scholar]
- 19.Nabel G J, Nabel E G, Yang Z Y, Fox B A, Plautz G E, Gao X, Huang L, Shu S, Gordon D, Chang A E. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci USA. 1993;90:11307–11311. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakanishi M, Uchida T, Sugawa H, Ishiura M, Okada Y. Efficient introduction of contents of liposomes into cells using HVJ (Sendai virus) Exp Cell Res. 1985;159:399–409. doi: 10.1016/s0014-4827(85)80013-6. [DOI] [PubMed] [Google Scholar]
- 21.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 22.Petri W A J, Wagner R R. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biochem. 1979;254:4313–4316. [PubMed] [Google Scholar]
- 23.Sharma S, Murai F, Miyanohara A, Friedmann T. Noninfectious virus-like particles produced by Moloney murine leukemia virus-based retrovirus packaging cells deficient in viral envelope become infectious in the presence of lipofection reagents. Proc Natl Acad Sci USA. 1997;94:10803–10808. doi: 10.1073/pnas.94.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephan D J, Yang Z Y, San H, Simari R D, Wheeler C J, Felgner P L, Gordon D, Nabel G J, Nabel E G. A new cationic liposome DNA complex enhances the efficiency of arterial gene transfer in vivo. Hum Gene Ther. 1996;7:1803–1812. doi: 10.1089/hum.1996.7.15-1803. [DOI] [PubMed] [Google Scholar]
- 25.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zabner J, Fasbender A J, Moninger T, Poellinger K A, Welsh M J. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 27.Zavada J. The pseudotype paradox. J Gen Virol. 1982;63:15–24. doi: 10.1099/0022-1317-63-1-15. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Ghosh H P. Characterization of the putative fusogenic domain in vesicular stomatitis virus glycoprotein G. J Virol. 1994;68:2186–2193. doi: 10.1128/jvi.68.4.2186-2193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu N, Liggitt D, Liu Y, Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993;261:209–211. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]