Abstract
Background:
The evaluation of volume status is essential to clinical decision-making, yet multiple studies have shown that physical exam does not reliably estimate a patient’s intravascular volume. Venous excess ultrasound score (VExUS) is an emerging volume assessment tool that utilizes inferior vena cava (IVC) diameter and pulse-wave Doppler waveforms of the portal, hepatic and renal veins to evaluate venous congestion. A point-of-care ultrasound exam initially developed by Beaubein-Souligny et al., VExUS represents a reproducible, non-invasive and accurate means of assessing intravascular congestion. VExUS has recently been validated against RHC—the gold-standard of hemodynamic evaluation for volume assessment. While VExUS scores were shown to correlate with elevated cardiac filling pressures (i.e., right atrial pressure (RAP) and pulmonary capillary wedge pressure (PCWP)) at a static point in time, the ability of VExUS to capture dynamic changes in volume status has yet to be elucidated. We hypothesized that paired VExUS examinations performed before and after hemodialysis (HD) would reflect changes in venous congestion in a diverse patient population.
Methods:
Inpatients with end-stage renal disease undergoing intermittent HD were evaluated with transabdominal VExUS and lung ultrasonography before and following HD. Paired t-tests were conducted to assess differences between pre-HD and post-HD VExUS scores, B-line scores and dyspnea scores.
Results:
Fifty-six patients were screened for inclusion in this study. Ten were excluded due to insufficient image quality or incomplete exams, and forty-six patients (ninety-two paired ultrasound exams) were included in the final analysis. Paired t-test analysis of pre-HD and post-HD VExUS scores revealed a mean VExUS grade change of 0.82 (p<0.001) on a VExUS scale ranging from 0 to 4. The mean difference in B-line score following HD was 0.8 (p=0.001). There was no statistically significant difference in subjective dyspnea score (p=0.41).
Conclusions:
Large-volume fluid removal with HD was represented by changes in VExUS score, highlighting the utility of the VExUS exam to capture dynamic shifts in intravascular volume status. Future studies should evaluate change in VExUS grade with intravenous fluid or diuretic administration, with the ultimate goal of evaluating the capacity of a standardized bedside ultrasound protocol to guide inpatient volume optimization.
Keywords: End-stage renal disease (ESRD), hemodialysis (HD), point-of-care ultrasonography (POCUS), venous congestion, venous excess ultrasonography (VExUS), volume status
BACKGROUND
The evaluation of volume status is essential to clinical decision-making in the intensive care unit, yet multiple studies have shown that physical exam does not reliably estimate a patient’s volume. Bedside assessments of intravascular volume remain subjective and often vary significantly between providers.1–5 Invasive methods (i.e., the direct measurement of central venous pressure via right heart catheterization (RHC)) remain the gold-standard for the evaluation of intravascular congestion, but these approaches carry risk, additional expense and are not universally available. More importantly, such procedures cannot be performed serially at the bedside to inform clinical decisions such as the initiation or discontinuation of volume resuscitation, diuresis or renal replacement therapy.6,7 Even indwelling pulmonary artery catheters, which can be utilized for serial monitoring over time, are associated with added cost and risk of complications.8,9 As we move towards the personalization of care for critically ill patients with conditions such as septic shock and decompensated heart failure, it is increasingly pressing to develop a standardized point-of-care method for evaluating volume status that can inform clinical decision-making in real-time.10–17
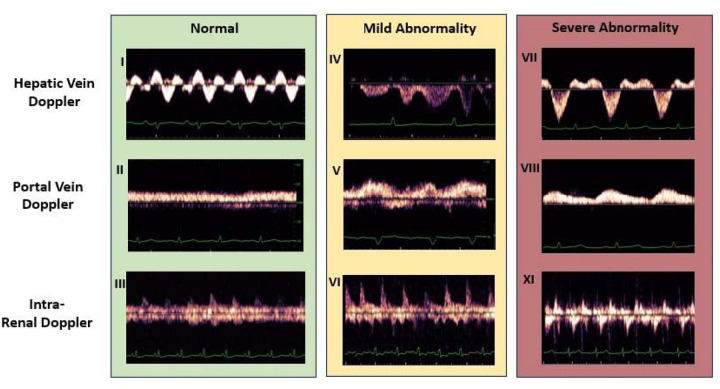
Venous excess ultrasound score (VExUS) is an emerging volume assessment tool that utilizes inferior vena cava (IVC) diameter and pulse-wave Doppler waveforms of the portal, hepatic and renal veins to evaluate venous congestion (Fig. 1). A point-of-care ultrasound exam initially developed by Beaubein-Souligny et al., VExUS represents a reproducible, non-invasive and accurate means of assessing venous congestion.18 This four-point examination has been shown to surpass the limits of other bedside ultrasound exams (e.g., measurement of IVC diameter alone) and to correlate with clinically relevant outcomes, such as the development of acute kidney injury (AKI) in post-cardiac surgery patients and patients with acute coronary syndrome.18–20
Figure 1. Representative pulse-wave Doppler waveforms for each VExUS component.
Any patient whose IVC diameter is <2 cm is assigned a VExUS score of 0, indicating no venous congestion. For any patient who has an IVC diameter >2 cm, the scores of the component parts are totaled to contrive a composite score. Any combination of normal and mildly abnormal scores is assigned a VExUS score of 1, indicating mild venous congestion. Any patient with one severely abnormal waveform is assigned a VExUS score of 2, indicating moderate congestion. Two or more severely abnormal waveforms results in a VExUS grade of 3, indicating severe congestion.
More recently, VExUS has been validated against RHC.20,21 While VExUS scores were shown to correlate with elevated cardiac filling pressures (i.e., right atrial pressure (RAP) and pulmonary capillary wedge pressure (PCWP)) at a static point in time, the ability of VExUS to capture dynamic changes in volume status has yet to be elucidated. We hypothesized that paired VExUS examinations performed before and after hemodialysis (HD) would reflect changes in venous congestion in a diverse patient population.
METHODS
Enrollment and image acquisition
Inpatients scheduled to undergo intermittent HD at two tertiary medical centers near Denver, CO were screened for enrollment in this study from May to December 2023. Inclusion criteria were inpatient admission, age > 18 years, scheduled HD and ability to provide informed consent. Exclusion criteria included pregnancy, continuous renal replacement therapy, presence of an open abdominal wound, invasive mechanical ventilation, incarceration and inability to provide informed consent.
Enrolled patients were evaluated with two transabdominal VExUS and lung ultrasonography exams—one before and one after same-day HD. VExUS exams were performed using the standardized protocol previously described.18,20 In patients who had received a prior renal transplant, either of the native kidneys was scanned, but the transplanted kidney was not. Lung ultrasonography was performed using a standardized protocol that involved ultrasonographic examination of eight lung zones—four anterior zones, and two lateral zones on each side—to evaluate for the presence of B-lines (i.e., vertical sonographic lines that can indicate the presence of interstitial edema).22 A lung zone that contained > 3 B-lines was considered positive for B-lines.23 Sonographers recorded the number and distribution of lung zones with positive B-lines while performing both pre-HD and post-HD ultrasound exams. The sonographers were internal medicine resident physicians who had undergone remote and in-person training by physicians with experience performing the VExUS exam. Members of the research team were not part of the patients’ clinical team, and study data was not made available to enrolled patients or to clinical team members. Acquired images were anonymized and uploaded to a secure database.
Image interpretation and data analysis
VExUS exams were graded by readers blinded to the patient’s clinical status. The interpretation team was comprised of internal medicine resident physicians who were proficient in the VExUS exam and had completed a 4-video imaging course on VExUS interpretation. The interpretation team did not participate in image acquisition. Images were interpreted and scored according to the standardized protocol previously described by Beaubien-Souligny et al.18 and utilized in the RHC validation study by Longino et al.20 Graders assigned a score to each component of the VExUS exam in addition to determining a composite score and an image quality score. Image quality was assessed using an ordinal scale from 1 (“uninterpretable”) to 5 (“unambiguously high quality”). Images with a quality score < 3 were excluded from the analysis. A sample of images was reviewed and interpreted by two graders to ensure consistency in image scoring.
Clinical characteristics of included patients were obtained via chart review and stored in a secure database. End-stage renal disease (ESRD) etiology was determined by reviewing clinical documentation from nephrology advanced practice providers, fellow physicians or attending physicians. Etiologies were not considered mutually exclusive, and more than one etiology was recorded for patients with multiple contributors to their renal disease. Left ventricular ejection fraction (LVEF) was determined from transthoracic echocardiogram (TTE) reports, considering only TTEs obtained within the past five years. If more than one TTE report was available, the LVEF value from the most recent report was used. Recent TTE reports and LVEF data were available for 93% of patients included in the final analysis.
Image graders were blinded to recorded clinical characteristics. Descriptive characteristics of the study cohort were summarized as mean ± standard deviation for continuous variable and frequency and percentage for categorical variables. The minimum IVC diameter (IVCmin) (i.e., IVC diameter measured on inspiration) and maximum IVC diameter (IVCmax) were measured as part of each VExUS exam and used to calculate IVC collapsibility indices (ICI) using the formula ICI = (IVCmax - IVCmin)/IVCmax.24 Paired t-tests were conducted to detect differences between pre-HD and post-HD VExUS scores (including both composite and component scores), B-line scores, patient-reported dyspnea scores, and pre-HD and post-HD IVC diameters and ICIs. VExUS grade was treated as a categorical variable throughout. Paired t-test analyses were also performed for prespecified subgroups (i.e., pre-HD VExUS score of 0 versus > 0, volume removal of < 1 L with HD versus volume removal of > 1 L with HD). All calculations were performed in R (version 4.3.2, R Foundation for Statistical Computing, Vienna, Austria) and RStudio (version 2023.12.1 + 402, RStudio, Inc., Boston, MA). A predetermined p-value of < 0.05 was considered statistically significant for all calculations.
RESULTS
Study population
Fifty-six patients were screened for inclusion in the study, and ten were excluded due to insufficient image quality or incomplete exams. Ninety-two paired VExUS exams were included in the final analysis. The mean age of all subjects was 60. Twenty-six (57%) were male, and twenty (43%) were female. The most common comorbidity was pulmonary hypertension (26%), followed by heart failure with reduced ejection fraction (22%). Patients with both AKI with unknown renal recovery and diagnosed ESRD were screened for inclusion in this study. All forty-six patients included in the final analysis carried a diagnosis of ESRD, and the most common ESRD etiology was diabetes mellitus (32.6%). The mean Charlson comorbidity index for all included patients was 5 (range 3–7) (Table 1). None of the patients enrolled in this study were undergoing positive pressure ventilation.
Paired pre-HD and post-HD VExUS scores
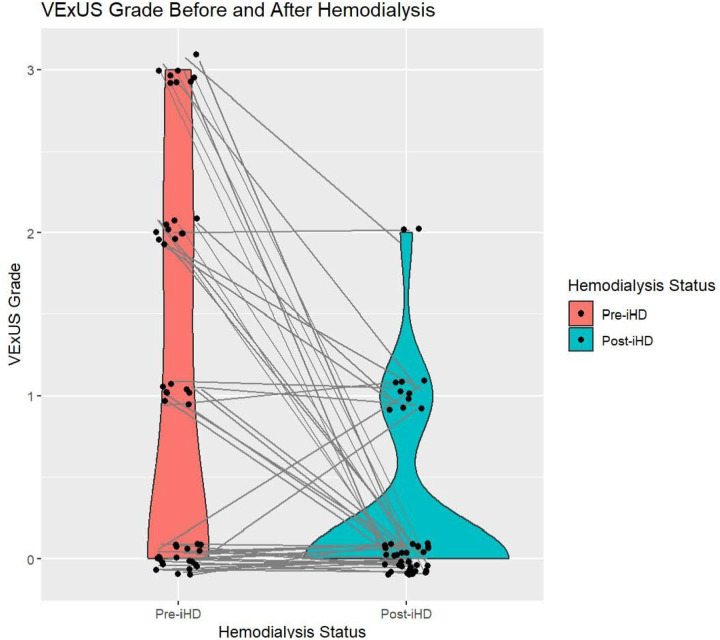
The volume of fluid removed with HD ranged from zero to four liters, with a mean fluid removal of 1.9 L. The mean VExUS score before HD was 1.1, and the mean VExUS score after HD was 0.28 (Fig. 2). Nearly half of patients (46% or n = 21) had a VExUS grade of 0 prior to HD, indicating an absence of venous congestion. A smaller portion of patients demonstrated mild or moderate congestion, with 17% having a pre-HD VExUS grade of 1 and 20% having a pre-HD VExUS grade of 2. Only 17% of patients had severe venous congestion (as indicated by a VExUS grade of 3) before HD. After HD, 76% of patients had a VExUS grade of 0, 20% had a VExUS grade of 1 and 4.3% had a VExUS grade of 2. There were no patients with a VExUS grade of 3 (i.e., severe venous congestion) following HD (Table 2).
Figure 2. VExUS grades before and after HD.
Matched pre-HD and post-HD VExUS grades. Paired t-test analysis of pre-HD and post-HD VExUS scores revealed a mean VExUS grade change of 0.82 (p<0.001).
Paired t-test analysis of pre-HD and post-HD VExUS scores revealed a statistically significant difference in VExUS grade before and after HD, with a mean VExUS grade change of 0.82 (p < 0.001). While there were significant differences in IVC diameter before and after HD, there was not a significant change in ICI. The mean pre-HD IVCmin was 1.46 cm compared to a mean post-HD IVCmin of 1.27 cm (p = 0.01), and the mean pre-HD IVCmax was 2.01 cm compared to a mean post-HD IVCmax of 1.88 cm (p = 0.048). ICI was similar pre-HD and post-HD (0.30 and 0.33, respectively, with p = 0.21). The individual components of hepatic vein VExUS score and portal vein VExUS score signicantly decreased after HD, with mean pre-HD scores of 0.87 and 0.87 for both components, and mean post-HD scores of 0.37 (p < 0.001) and 0.3 (p < 0.001), respectively. Interestingly, there was not a statistically significant decrease in renal vasculature VExUS score before and after HD. Rather, the mean renal vasculature score increased from 0.33 pre-HD to 0.83 post-HD (p = 0.01). Pre-HD and post-HD component and composite VExUS scores are further outlined in Table 2. The mean difference in B-line score following HD was 0.8 (p = 0.001). There was no statistically significant difference in subjective dyspnea score before and after HD (p = 0.41).
For patients who had a pre-HD VExUS score > 0 (indicating some degree of venous congestion), the mean VExUS grade change was 1.56 (p < 0.001), and for patients with a pre-HD VExUS score of 0 (indicating an absence of venous congestion), there was no statistically significant change in VExUS score following HD (p = 0.16). Similarly, the delta VExUS score (i.e., pre-HD VExUS score minus post-HD VExUS score) was higher in patients who had a greater volume of fluid removed with HD. For patients who had > 1 L of volume removed during HD, the mean delta VExUS was 1.12 (p < 0.001), yet there was no statistically significant change in VExUS grade in patients who had < 1 L removed with HD (p = 0.07).
DISCUSSION
Large-volume fluid removal with HD was represented by changes in VExUS score, highlighting the utility of the VExUS exam to capture dynamic shifts in volume status. This multicenter study is the first interrogation of the ability of VExUS to appraise rapid changes in a patient’s hemodynamic state, particularly venous congestion.
Importantly, the VExUS exam has previously been shown to outperform both ICI and the measurement of IVC diameter—two metrics routinely incorporated into standard echocardiogram protocols—in the estimation of RAP. Our group recently published a study validating VExUS against RHC, an analysis that found that the area under the curve (AUC) for VExUS grade as a predictor of RAP > 10 mmHg was 0.9, whereas the AUC for ICI and IVC diameter as predictors of RAP > 10 mmHg were 0.65 and 0.77, respectively. Similarly, the AUC for VExUS grade as predictor of RAP < 7 mmHg was 0.79, while the AUC for ICI and IVC diameter as predictors of RAP < 7 mmHg were 0.62 and 0.74, respectively, highlighting the fact that VExUS was superior to ICI or IVC diameter for the detection of euvolemia or hypovolemia in addition to venous congestion. These findings hold true in the current study as well. Whereas the VExUS exam was able to detect significant changes in volume status, ICI was not. There was no statistically significant change in paired ICI scores before and after HD, underscoring the limitations of this unidimensional metric.25,26 In fact, the American Society of Echocardiography’s (ASE) guidelines for the estimation of RAP via ICI (i.e., an ICI < 50% is indicative of an RAP 10–20 mmHg) would suggest that both the pre-HD and post-HD cohort trended towards hypervolemia given their ICIs of 0.30 and 0.33, respectively.27 This interpretation does not reflect the fact that a mean volume change of nearly negative 2 L occurred between paired pre-HD and post-HD ultrasound exams. While there was a statistically significant difference in pre-HD and post-HD IVC diameter, an interpretation of IVC diameter utilizing ASE guidelines would yield a similarly static conclusion. In the current analysis, the mean pre-HD IVCmax was 2.01 cm, and the mean post-HD IVCmax was 1.88 cm. According to ASE criteria, an IVC diameter of < 2.1 cm is suggestive of a RAP < 5 mmHg.27 Utilizing this schema, both the pre-HD and post-HD cohorts would have been considered to be uncongested, but—similar to ICI—there would have been no indication that a significant volume loss occurred between paired pre-HD and post-HD exams, as an IVC diameter of 2.01 cm and an IVC diameter of 1.88 cm both signal a RAP < 5 mmHg.
Overall, these findings underscore the constraints of binary metrics (e.g., IVC size > 2.1 cm versus < 2.1cm or ICI > 50% versus < 50%) for the estimation of a patient’s degree of venous congestion and highlight an opportunity to expand on the IVC evaluation that is integrated into current echocardiographic protocols. VExUS represents an objective, validated, multidimensional, and more nuanced appraisal of hemodynamic status that moves beyond the assessment of IVC alone to incorporate data from multiple encapsulated abdominal organs in addition to the IVC. The tiered scoring system utilized by VExUS (i.e., scores 0–3, representing no venous congestion, mild congestion, moderate congestion and severe congestion, respectively) expands on current schema for the estimation of venous hypertension and allows for more gradation in the valuation of a patient’s volume status. The supplement of three additional views (i.e., the hepatic vein, portal vein and intralobar renal vasculature) with pulse-wave Doppler wave forms has the potential to augment the already marked clinical utility of volume assessment via ultrasound exam, either as part of full echocardiographic exam or a stand-alone limited protocol.
Beyond the integration of VExUS into formal sonographic procedures, there also lies an opportunity to incorporate VExUS into routine clinical care as an easily accessible, point-of-care exam. VExUS has the unique advantage of being a non-invasive, inexpensive and reproducible ultrasound protocol, making it highly accessible to bedside clinicians. As such, the VExUS exam can be used to serially to assess changes in a patient’s volume status over time, marking it as an ideal tool for evaluating rapid changes in a patient’s hemodynamic status in real-time, such as diuresis in hypervolemic states (e.g., decompensated heart failure) or volume resuscitation in rapidly evolving clinical scenarios such as sepsis. Future studies should evaluate change in VExUS grade with intravenous fluid or diuretic administration in various patient populations, with the ultimate goal of evaluating the capacity of a standardized bedside ultrasound protocol to guide inpatient volume optimization.
While this analysis demonstrates the ability of the VExUS exam to capture changes in venous congestion that occur in the setting of large-volume fluid removal in patients undergoing HD, it does not assess the ability of the VExUS exam to detect volume loss in other clinical settings (e.g., diuresis, gastrointestinal losses or hemorrhage), nor does it assess the ability of the VExUS exam to detect rapid volume expansion. Moreover, this study included only patients with ESRD, and the effect of chronic renal disease on the renal component of the VExUS exam has not been directly investigated, leaving open the possibility that ESRD necessitating HD may impair the interpretation of renal vasculature pulse-wave Doppler waveforms. It is important to note, however, that the VExUS exam was validated in diverse patient populations with multiple compounding comorbidities and is intended as a tool to interrogate venous congestion across a spectrum of critical illness.20,21 Beaubien-Souligny et al.’s seminal study, for instance, investigated the ability of VExUS to predict AKI in patients who had recently undergone cardiac surgery with the use of cardiopulmonary bypass, and Longino et al.’s recent analyses interrogating the utility of VExUS to estimate RAP occurred in patients undergoing RHC, overall suggesting that the multivariate nature of the exam confers robustness and validity even in the setting of chronic disease.18,20,21 In the current analysis, the renal vasculature score did not decrease between paired pre-HD and post-HD exams, but the composite VExUS score decreased concordantly with the remaining component scores, suggesting that the redundancy of the overall VExUS exam is effective at mitigating variation between patients and disease states. Future studies are warranted to expressly validate VExUS in specific subpopulations, including patients with ESRD.
CONCLUSIONS
Large-volume fluid removal with HD was represented by changes in VExUS score, highlighting the utility of the VExUS exam to capture dynamic shifts in volume status. VExUS holds potential as an addition to formal sonographic protocols and as a point-of-care bedside tool that can be utilized to detect changes in venous congestion in real-time. Future studies should evaluate change in VExUS grade with intravenous fluid or diuretic administration in various patient populations, with the ultimate goal of evaluating the capacity of a standardized bedside ultrasound protocol to guide inpatient volume optimization.
Funding:
Leyba: NHBLI 5 R38 HL 143511-4
Abbreviations
- AKI
acute kidney injury
- ASE
American Society of Echocardiography
- AUC
area under the curve
- ESRD
end-stage renal disease
- HD
hemodialysis
- IVC
inferior vena cava
- ICI
IVC collapsibility index
- LVEF
left ventricular ejection fraction
- PCWP
pulmonary capillary wedge pressure
- RAP
right atrial pressure
- RHC
right heart catheterization
- TTE
transthoracic echocardiogram
- VExUS
venous excess ultrasounds score
Funding Statement
Leyba: NHBLI 5 R38 HL 143511-4
Footnotes
Ethical approval: This study had approval from the local Colorado Multiple Institutional Review Board (#22–2024).
Competing interests: None of the authors have any competing interests to report.
Additional Declarations: No competing interests reported.
Table 1 and 2 are available in the Supplementary Files section.
Supplementary Files
Contributor Information
Katarina Leyba, University of Colorado.
August Longino, University of Colorado.
Ryen Ormesher, University of Colorado.
Mary Krienke, University of Colorado.
Natalie Van Ochten, University of Colorado.
Katherine Zimmerman, University of Colorado.
Luke McCormack, University of Colorado.
Katharine Martin, University of Colorado School of Medicine.
Theresa Thai, University of Colorado.
Seth Furgeson, Denver Health Medical Center.
Isaac Teitelbaum, University of Colorado.
Joseph Burke, University of Colorado.
Ivor Douglas, Denver Health Medical Center.
Edward Gill, University of Colorado.
Data Availability
All original ultrasound images and datasets are available upon request to the corresponding author.
References
- 1.Kearney D, Reisinger N, Lohani S. Integrative Volume Status Assessment. POCUS J. 2022;7(Kidney):65–77. doi: 10.24908/pocus.v7iKidney.15023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elhassan MG, Chao PW, Curiel A. The Conundrum of Volume Status Assessment: Revisiting Current and Future Tools Available for Physicians at the Bedside. Cureus. May 26 2021;13(5):e15253. doi: 10.7759/cureus.15253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. Nov 1987;83(5):905–8. doi: 10.1016/0002-9343(87)90649-8 [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie DC, Noble VE. Assessing volume status and fluid responsiveness in the emergency department. Clin Exp Emerg Med. Dec 2014;1(2):67–77. doi: 10.15441/ceem.14.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein JH, Neumann A, Marcus RH. Comparison of estimates of right atrial pressure by physical examination and echocardiography in patients with congestive heart failure and reasons for discrepancies. Am J Cardiol. Dec 15 1997;80(12):1615–8. doi: 10.1016/s0002-9149(97)00776-5 [DOI] [PubMed] [Google Scholar]
- 6.Del Rio-Pertuz G, Nugent K, Argueta-Sosa E. Right heart catheterization in clinical practice: a review of basic physiology and important issues relevant to interpretation. Am J Cardiovasc Dis. 2023;13(3):122–137. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Shlofmitz E, Khalid N, et al. Right Heart Catheterization-Related Complications: A Review of the Literature and Best Practices. Cardiol Rev. Jan/Feb 2020;28(1):36–41. doi: 10.1097/CRD.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 8.Bossert T, Gummert JF, Bittner HB, et al. Swan-Ganz catheter-induced severe complications in cardiac surgery: right ventricular perforation, knotting, and rupture of a pulmonary artery. J Card Surg. May- Jun 2006;21(3):292–5. doi: 10.1111/j.1540-8191.2006.00235.x [DOI] [PubMed] [Google Scholar]
- 9.Hadian M, Pinsky MR. Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit Care. 2006;10 Suppl 3(Suppl 3):S8. doi: 10.1186/cc4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. Oct 20 2013;17(5):R246. doi: 10.1186/cc13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. Feb 2011;39(2):259–65. doi: 10.1097/CCM.0b013e3181feeb15 [DOI] [PubMed] [Google Scholar]
- 12.Legrand M, Dupuis C, Simon C, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. Nov 29 2013;17(6):R278. doi: 10.1186/cc13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanji HD, McCallum J, Sirounis D, MacRedmond R, Moss R, Boyd JH. Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care. Oct 2014;29(5):700–5. doi: 10.1016/j.jcrc.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 14.Mekontso Dessap A, Roche-Campo F, Kouatchet A, et al. Natriuretic peptide-driven fluid management during ventilator weaning: a randomized controlled trial. Am J Respir Crit Care Med. Dec 15 2012;186(12):1256–63. doi: 10.1164/rccm.201205-0939OC [DOI] [PubMed] [Google Scholar]
- 15.Myburgh JA. Fluid resuscitation in acute illness--time to reappraise the basics. N Engl J Med. Jun 30 2011;364(26):2543–4. doi: 10.1056/NEJMe1105490 [DOI] [PubMed] [Google Scholar]
- 16.Caille V, Amiel JB, Charron C, Belliard G, Vieillard-Baron A, Vignon P. Echocardiography: a help in the weaning process. Crit Care. 2010;14(3):R120. doi: 10.1186/cc9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porapakkham P, Porapakkham P, Zimmet H, Billah B, Krum H. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch Intern Med. Mar 22 2010;170(6):507–14. doi: 10.1001/archinternmed.2010.35 [DOI] [PubMed] [Google Scholar]
- 18.Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. Apr 9 2020;12(1):16. doi: 10.1186/s13089-020-00163-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viana-Rojas JA, Argaiz E, Robles-Ledesma M, et al. Venous excess ultrasound score and acute kidney injury in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. Jul 7 2023;12(7):413–419. doi: 10.1093/ehjacc/zuad048 [DOI] [PubMed] [Google Scholar]
- 20.Longino A, Martin K, Leyba K, et al. Prospective Evaluation of Venous Excess Ultrasound (VExUS) for Estimation of Venous Congestion. Chest. Oct 7 2023;doi: 10.1016/j.chest.2023.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longino A, Martin K, Leyba K, et al. Correlation between the VExUS score and right atrial pressure: a pilot prospective observational study. Crit Care. May 26 2023;27(1):205. doi: 10.1186/s13054-023-04471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhoil R, Ahluwalia A, Chopra R, Surya M, Bhoil S. Signs and lines in lung ultrasound. J Ultrason. Aug 16 2021;21(86):e225–e233. doi: 10.15557/JoU.2021.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murali A, Prakash A, Dixit R, Juneja M, Kumar N. Lung ultrasound for evaluation of dyspnea: a pictorial review. Acute Crit Care. Nov 2022;37(4):502–515. doi: 10.4266/acc.2022.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaptein MJ, Kaptein EM. Inferior Vena Cava Collapsibility Index: Clinical Validation and Application for Assessment of Relative Intravascular Volume. Adv Chronic Kidney Dis. May 2021;28(3):218–226. doi: 10.1053/j.ackd.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 25.Di Nicolo P, Tavazzi G, Nannoni L, Corradi F. Inferior Vena Cava Ultrasonography for Volume Status Evaluation: An Intriguing Promise Never Fulfilled. J Clin Med. Mar 13 2023;12(6)doi: 10.3390/jcm12062217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. Jul 2016;42(7):1164–7. doi: 10.1007/s00134-016-4357-9 [DOI] [PubMed] [Google Scholar]
- 27.Porter TR, Shillcutt SK, Adams MS, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Echocardiogr. Jan 2015;28(1):40–56. doi: 10.1016/j.echo.2014.09.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All original ultrasound images and datasets are available upon request to the corresponding author.