Abstract
Introduction
Gastrointestinal symptoms correlate poorly with cancer diagnosis. A faecal immunochemical test (FIT) result of ≥10 µg has high sensitivity and negative predictive value for colorectal cancer (CRC) detection. An FIT-based diagnostic pathway may lead to more effective resource utilisation. We aimed to use National Endoscopy Database (NED) data to create a new colonoscopy performance measure, cancer detection rate (CDR) to assess the appropriate identification of target populations for colonoscopy; then to use CDR to assess the impact of implementing an FIT-based referral pathway locally.
Methods
NED data were analysed to compare local diagnostic colonoscopic CDR in 2019 (prepathway revision) and 2021 (postpathway revision), benchmarked against overall national CDR for the same time frames.
Results
1, 123, 624 NED diagnostic colonoscopies were analysed. Locally, there was a significant increase in CDR between 2019 and 2021, from 3.01% (2.45%–3.47%) to 4.32% (3.69%–4.95%), p=0.003. The CDR increase was due to both a 10% increase in the number of CRCs detected and a 25% reduction in the number of diagnostic colonoscopies performed. Nationally, there was a smaller, but significant, increase in CDR from 2.02% (1.99%–2.07%) to 2.33% (2.29%–2.37%), p<0.001. The rate of increase in CDR% between 2019 and 2021 was significantly different locally compared with nationally.
Conclusion
Our study indicates that the introduction of a robustly vetted FIT-based algorithm to determine whether diagnostic colonoscopy is required, is effective in increasing the colonoscopic CDR. Moreover, CDR appears to be a meaningful performance metric that can be automatically calculated through NED, enabling monitoring of the quality of referral and vetting pathways.
Keywords: colorectal cancer
WHAT IS ALREADY KNOWN ON THIS TOPIC
Symptoms correlate poorly with cancer diagnosis. Faecal immunochemical testing (FIT) has high sensitivity and negative predictive value in colorectal cancer diagnosis.
WHAT THIS STUDY ADDS
A FIT-based colorectal cancer diagnosis pathway may diagnose more cancers, while submitting fewer patients to colonoscopy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
A FIT-based colorectal cancer diagnostic pathway can help focus colonoscopy resources to patients who need it the most. Cancer detection rate (CRD) appears to be a meaningful performance metric, enabling monitoring of the quality of referral and vetting pathways.
Introduction
Colorectal cancer is the second most lethal malignancy in the UK.1 The majority of colorectal cancers are diagnosed following referral from primary care for endoscopic investigation of gastrointestinal symptoms. However, symptoms and age stratification alone are not specific enough to allow rapid and targeted cancer diagnosis. Performance analyses of the symptoms-based referral criteria for colorectal cancer (CG27) published by the National Institute of Health and Care Excellence (NICE) in 2005, suggested a positive predictive value for colorectal cancer of 3%–4%.2 3
It is important to target resources towards the right patients. The early COVID-19 pandemic, which brought elective endoscopy services to a near-halt in the UK,4 highlighted the importance of this. Even once endoscopy services reopened, capacity has been constrained and substantial waiting lists have developed: in England, 35.5% of patients wait more than 6 weeks for their endoscopy.5
The faecal immunochemical test (FIT) detects human haemoglobin using antibodies to globin. FIT was initially recommended by NICE in 2017 in the DG30 guidance,6 aiming to identify patients with potentially serious lower GI pathology who did not fulfil NG12 criteria for cancer pathway referral, but the approach was not widely implemented at that time. Large-scale research is now available, suggesting that a diagnostic strategy based on an FIT of ≥10 µg Hb/g has a sensitivity of greater than 87% for cancer detection in symptomatic patients, with a negative predictive value of 99.5% and a number needed to scope of 10 to detect one cancer under the NG12 criteria.7 When non-NG12 cohorts are included, the number of cancers missed is as low as 1 per 1000 FITs performed.7
Subsequently, in 2022, the British Society of Gastroenterology and the Association of Coloproctology of Great Britain and Ireland issued a joint guideline on the use of FIT for patients with symptoms suspicious of colorectal cancer.7 That guideline broadly aligns with our regional approach, apart from recommending the use of FIT to risk-stratify patients with iron-deficiency anaemia (IDA), in contrast to our local pathway where we accept previously uninvestigated IDA irrespective of the FIT result.
Our National Healthcare Service (NHS) Trust comprises two District General Hospitals, with a catchment area of approximately 400 000 people. We incorporated FIT into NG12 primary care referral criteria early during the pandemic, to identify at-risk patients and offer timely endoscopic investigation, while avoiding unnecessary procedures for low-risk people who do not need investigating for cancer exclusion purposes. Recent national Cancer Alliance data have demonstrated that our trust has one of the highest use of FIT gatekeeping for colonoscopy.8
We felt it is meaningful for patients and services to assess the impact of this pathway change and to identify a potential national performance measure to monitor it, using the National Endoscopy Database (NED), which was created in 2013 with the purpose of accumulating data from all Endoscopy Practices across the UK, to facilitate research and improve quality assurance.9 As of April 2023, 515 out of the 520 Joint Advisory Group-registered sites were uploading to NED. NED has the ability to generate key endoscopy performance indicators automatically, based on data uploaded from individual units.
The aims of the study were:
To use NED to create a new automated colonoscopy performance measure, cancer detection rate (CDR) to assess the appropriate identification of high-risk populations for colonoscopy.
To use CDR to assess the benefit of implementing an enhanced, FIT-based vetting process of the NG12 referral pathway in our Trust (a ‘revised’ pathway), by comparing two time periods (before (2019) and after pathway implementation (2021)), and to compare this against overall national CDR for the same periods.
Materials and methods
The revised referral pathway
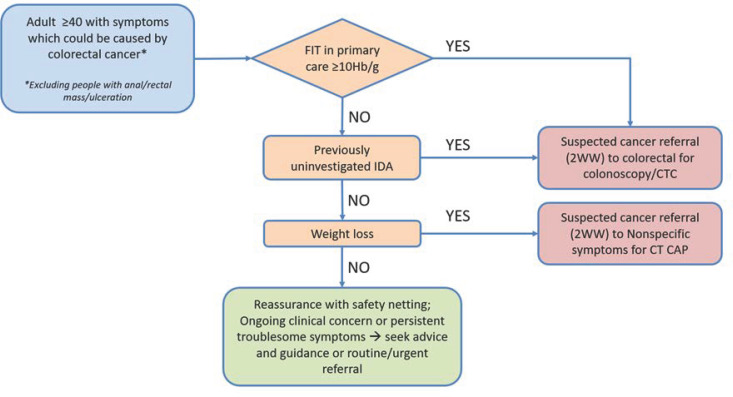
In 2020, as part of a regional multidisciplinary initiative involving both primary and secondary care, under the auspices of the Northern Cancer Alliance (NCA) and the North East and North Cumbria Integrated Care System (NENC ICS) Endoscopy Network, we developed a revised gastrointestinal referral pathway based on symptoms/laboratory findings traditionally linked with a risk of colorectal cancer (NG12 guidance), incorporating FIT in primary and, where required, secondary care (figure 1).
Figure 1.
Simplified lower gastrointestinal (GI) investigation subalgorithm of the NCA combined GI referral pathway. CTC, computed tomography colonoscopy scan. CT CAP, computed tomography scan of the chest, abdomen and pelvis; NCA, Northern Cancer Alliance.
This revised pathway was implemented in May 2020 and applied to all referral priorities from primary and secondary care, including routine, urgent potential cancer (the ‘2-week wait’ (2ww) pathway) and non-2ww urgent referrals. Referral forms were revised to align with the pathway. Referrals were vetted against the pathway, initially by two consultant gastroenterologists, then subsequently by implementing a process whereby experienced non-medical endoscopists vetted against a standard operating policy, escalating complex cases to the two consultant gastroenterologists. A key component of the pathway was that where key data on symptoms/FIT results were missing, further information was sought from the referring colleagues, in order to fully inform management plans.
Creation of CRD within NED
We extracted NED data on colonoscopies undertaken in the years 2019 and 2021, and their outcomes. We included diagnostic procedures performed following referrals under the NG12 and non-NG12 (ie, urgent or routine diagnostic referrals, outside a cancer pathway) criteria. We excluded colonoscopies performed under the Bowel Cancer Screening Programme, procedures performed for polyp, polyposis or family history surveillance, assessment of known cancer, emergency/inpatient procedures and those planned for therapeutic interventions. For the purposes of this study, we refer to the remaining procedures as ‘diagnostic’.
From this national dataset, we abstracted local (our Trust’s) data. Thus the national dataset did not include our local Trust data. In both this (reduced) national and local datasets, CDR was calculated as the number of colonoscopies in which a cancer was detected, divided by the total number of colonoscopies, expressed as a percentage. CRD was calculated separately for 2019 and 2021.
Statistical analyses
We first compared CDR in 2019 and 2021 in both local and national datasets. Then, to assess whether any change over time observed in our Trust reflected our change in pathway, or whether it might reflect a more general change in referral behaviour or cancer prevalence post-COVID-19 pandemic, we compared Trust CDR against national CDR for the same years.
Outcomes were analysed as rates and are expressed with 95% CIs. Two-way associations were analysed using the χ2 test and the three-way interaction of region×year×CDR% with log linear analysis. In all cases, the effect sizes of associations are expressed as ORs with 95% CIs. An alpha level of p<0.05 (two sided) was used throughout as the cut-off for significance. Analysis was performed by using IBM SPSS V.28.0.0.0.
Results
Colonoscopy procedures in 2019 and 2021
Overall, we analysed 1 123 624 diagnostic colonoscopies in the years 2019 and 2021. In 2019, 505 739 diagnostic colonoscopies were performed, comprising 495 457 negative procedures and 10 282 procedures with an endoscopic diagnosis of cancer (2.03%). In 2021, 617 885 procedures were analysed, comprising 603 377 negative diagnostic procedures and 14 508 procedures detecting cancer (2.35%).
In our Trust, 9248 diagnostic procedures were undertaken in the years 2019 and 2021. In 2019, 5245 colonoscopies were performed, comprising 5087 negative diagnostic procedures and 158 procedures revealing colorectal cancer (3.01%). In 2021, 4003 diagnostic colonoscopies were performed; the number of negative diagnostic procedures decreased by 25% to 3830, while the number of cancers detected increased by 10% to 173 (4.32%).
Nationally (excluding our Trust), there were 1 114 376 diagnostic procedures in the years 2019 and 2021. In 2019, 500 494 colonoscopies were performed, comprising 490 370 negative diagnostic colonoscopies and 10 124 procedures revealing cancer (2.02%). In 2021, 613 882 procedures were performed, comprising 599 547 negative diagnostic procedures and 14 335 procedures detecting cancer (2.33%).
CDR in 2019 and 2021, locally and nationally
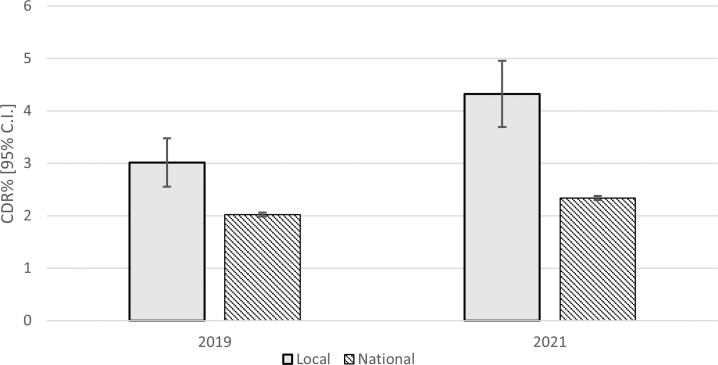
Locally, there was a significant increase in CDR between 2019 and 2021, from 3.01% (2.45%–3.47%) to 4.32% (3.69%–4.95%), χ2(1)=8.76, p=0.003, OR 1.43 (95% CI 1.15 to 1.79). Nationally (excluding our Trust), there was also a significant increase in CDR between 2019 and 2021, from 2.02% (1.99%–2.07%) to 2.33% (2.29%–2.37%), χ2(1)=125.87, p<0.001, OR 1.16 (95% CI 1.13 to 1.19), although this was smaller in magnitude that the increase observed locally (figure 2). There was a significant difference in the rate of increase in CDR% between 2019 and 2021 locally compared with nationally, χ2(1)=4.12, p=0.042, z=2.02 and, in addition, each of the underlying two-way effects was also significant, (year×area χ2(1)=515.56, p<0.001, z=6.51; year×CDR% χ2(1)=127.76, p<0.001, z=4.48 and area×CDR% χ2(1)=69.47, p<0.001, z=9.05).
Figure 2.
Mean yearly local and national (excluding local) CDR% (95% CI) for 2019 and 2021. CDR, cancer detection rate.
Looking solely at the number of negative tests performed, these decreased from 5087 to 3830 locally while they increased from 490 370 to 5 99 832 nationally. This is a significant, χ2(1)=520.34, p<0.001, OR 1.62 (95% CI 1.56 to 1.69) with examination of standardised residuals revealing that the majority of this difference is due to a large reduction in the number of negative tests performed locally coupled with a small rise in the number performed nationally.
Discussion
Our study, using NED data from over 1 million diagnostic colonoscopies, indicates that the full implementation of an FIT-based algorithm to determine whether diagnostic colonoscopy is required has significantly increased the colonoscopic CDR and the number of colorectal cancers detected.
Even prior to the pandemic, services in the UK were struggling to cope with increased demand, driven both by an ageing population and the laudable desire to lower the threshold for colonoscopic investigation to reduce diagnostic delay and improve cancer outcomes. However, the poor correlation between common bowel symptoms and colorectal cancer incidence, coupled with increased pressure on referrers ‘not to miss a cancer’ led to a predicable increase in referrals, and an increasing capacity-demand mismatch, compounded further by the pandemic.
Before 2020, FIT was rarely used for decision-making in secondary care, including in our Trust. However, consistent and compelling evidence was emerging that FIT testing was an effective and superior means to stratify a patient’s risk of colorectal cancer compared with using symptoms alone.2 7 10 11 We incorporated FIT into NG12 symptoms criteria in May 2020, although it took a few months for FIT to become embedded into everyday practice.
Although our Trust had a higher-than-average CDR prior to pathway revision, our study highlights a significant increase in CDR following FIT pathway implementation. Our data demonstrate that this came about through a 10% increase in the number of colorectal cancers detected, despite a 25% reduction in the number of diagnostic colonoscopies performed. It seems plausible that this is due to the introduction of the FIT-based revised pathway leading to better identification of at-risk patients and a reduction in low-risk (FIT-negative) referrals, resulting in a reduction in the number of colonoscopies required to detect one cancer.
The successful introduction of the new pathway required combination of multidisciplinary pathway redesign overseen by our Cancer Alliance and the NENC ICS Endoscopy Network, supportive education and communication with primary and secondary care clinicians and robust ongoing vetting against the new pathway. NCA primary care FIT utilisation data from 2022/2023 demonstrate that more than 80% of urgent suspected colorectal cancer referrals were accompanied by an FIT result. Vetting (review of referrals within the hospital team) is an essential component but is time-consuming and has resource implications. In the future, it might be possible to use robotic process automation or artificial intelligence to increase the efficiency of this aspect.
As suggested by the analyses here, the use of FIT in referral pathways, further strengthened by high-level vetting of referrals in secondary care, is likely to have a positive impact on cancer identification and resource utilisation (‘scope less-find more’). However, it is possible that the reduction in colonoscopy workload might be transient, as general practitioners lower their threshold for using FIT tests on patients with more minor GI symptoms; further research will be required to assess whether this will reduce future CDR (more colonoscopies but proportionally fewer additional cancers), or identify a larger cohort of high-risk patients in a more timely fashion (more colonoscopies and commensurately more additional cancers, detected in earlier stages).
CDR appears to be a meaningful performance metric that can be automatically calculated through NED, enabling monitoring of the quality of referral and vetting pathways. The ability to calculate the metric automatically is important, as it does not place an additional burden on already busy endoscopy services; it also means the methodology is standardised across all endoscopy services, permitting national benchmarking. We would suggest that national implementation of CDR as a service quality metric is feasible and potentially highly valuable. NHS England is planning to use the metric to monitor performance of endoscopy services. Further research is required to understand what the minimum and target CDR levels should be.
The main limitation of our study is that we cannot be certain that the change in CDR has arisen solely from the introduction of FIT testing into our pathway. Alternative explanations could be other internal or external changes. Internally, apart from FIT incorporation, our pathway also introduced changes such as cross-sectional imaging of patients with weight loss, meaning that the increased CDR may not be entirely attributable to incorporation of FIT; however, the use of FIT was the dominant change in our revised pathway. Externally, it is possible that the change in our Trust’s CDR related to broader national changes such as altered referral practices due to the pandemic—we benchmarked our Trust’s CDR against the national CDR change over time to explore this: the national change in CDR was substantially lower than in our Trust, meaning that it is plausible that our Trust’s increase in CDR reflects local changes, although we cannot entirely exclude that other Trusts were implementing similar pathways.
A further limitation is that we did not have access to histological cancer diagnoses, hence were reliant on the endoscopic diagnosis. However, while this might underestimate (or less likely overestimate) the true number of cancers detected, these differences are likely to be reasonably consistent between time periods and Trusts, hence would be unlikely to introduce bias.
In addition, the number of sites uploading data to NED is likely to have been smaller in 2019 compared with 2021, which may have potentially affected the analyses.
In conclusion, our nationally benchmarked study indicates that the introduction of a robustly vetted FIT-based algorithm to determine whether diagnostic colonoscopy is required, is effective in increasing the colonoscopic CDR. Moreover, colonoscopic CDR appears to be a meaningful performance metric that can be automatically calculated through NED, enabling monitoring of the quality of referral and vetting pathways.
Footnotes
@iosifbeintaris, @Rutter_Matt
Contributors: MR and IB conceived the local FIT-based diagnostic pathway, later developed into regional practice with input by KE, under the auspices of the Northern Cancer Alliance. JR performed statistical analyses for the project. All authors contributed to authorship and critical review of the manuscript. IB is the article guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. UK BC . Facts and figures on bowel cancer. 2023.
- 2. Quyn AJ, Steele RJ, Digby J, et al. Application of NICE guideline Ng12 to the initial assessment of patients with lower gastrointestinal symptoms: not FIT for purpose Ann Clin Biochem 2018;55:69–76. 10.1177/0004563217707981 [DOI] [PubMed] [Google Scholar]
- 3. Jellema P, van der Windt DAWM, Bruinvels DJ, et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ 2010;340:c1269. 10.1136/bmj.c1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris EJA, Goldacre R, Spata E, et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol 2021;6:199–208.:S2468-1253(21)00005-4. 10.1016/S2468-1253(21)00005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NHS England . Diagnostic Waiting Times and Activity Report 2022. [Google Scholar]
- 6. NICE . Quantitative Faecal Immunochemical tests to guide referral for colorectal cancer in primary care. 2017. [PubMed]
- 7. Monahan KJ, Davies MM, Abulafi M, et al. Faecal Immunochemical testing (FIT) in patients with signs or symptoms of suspected colorectal cancer (CRC): a joint guideline from the Association of Coloproctology of great Britain and Ireland (ACPGBI) and the British society of Gastroenterology (BSG). Gut 2022;71:1939–62. 10.1136/gutjnl-2022-327985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alliance NC. National cancer alliance Q1-Q4 colonoscopy FIT data 2022/2023. 2023.
- 9. Lee TJ, Siau K, Esmaily S, et al. Development of a national automated Endoscopy database: the United Kingdom national Endoscopy database (NED). United European Gastroenterol J 2019;7:798–806. 10.1177/2050640619841539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Souza N, Georgiou Delisle T, Chen M, et al. Faecal Immunochemical test is superior to symptoms in predicting pathology in patients with suspected colorectal cancer symptoms referred on a 2Ww pathway: a diagnostic accuracy study. Gut 2021;70:1130–8. 10.1136/gutjnl-2020-321956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Booth R, Carten R, D’Souza N, et al. Role of the Faecal Immunochemical test in patients with risk-stratified suspected colorectal cancer symptoms: A systematic review and meta-analysis to inform the ACPGBI/BSG guidelines. Lancet Reg Health Eur 2022;23:100518. 10.1016/j.lanepe.2022.100518 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.