Abstract
For active specific immunotherapy of cancer patients, we designed the autologous virus–modified tumor cell vaccine ATV-NDV. The rationale of this vaccine is to link multiple tumor-associated antigens (TAAs) from individual patient-derived tumor cells with multiple danger signals (DS) derived from virus infection (dsRNA, HN, IFN-α). This allows activation of multiple innate immune responses (monocytes, dendritic cells, and NK cells) as well as adaptive immune responses (CD4 and CD8 memory T cells). Preexisting antitumor memory T cells from cancer patients could be activated by antitumor vaccination with ATV-NDV as seen by augmentation of antitumor memory delayed-type hypersensitivity (DTH) responses. In a variety of phase II vaccination studies, an optimal formulation of this vaccine could improve long-term survival beyond what is seen in conventional standard therapies. A new concept is presented which proposes that a certain threshold of antitumor immune memory plays an important role (1) in the control of residual tumor cells which remain after most therapies and (2) for long-term survival of treated cancer patients. This immune memory is T-cell based and most likely maintained by persisting TAAs from residual dormant tumor cells. Such immune memory was prominent in the bone marrow in animal tumor models as well as in cancer patients. Immunization with a tumor vaccine in which individual TAAs are combined with DS from virus infection appears to have a positive effect on antitumor immune memory and on patient survival.
Keywords: Immunotherapy, Memory T cells, Newcastle disease virus, Tumor-antigen, Tumor vaccine
Introduction
The host immune response to foreign challenge requires the coordinated action of both the innate and acquired arms of the immune system. The innate immune response not only provides the first line of defense against microorganisms but also the biological context—the “danger signal” (DS)—that instructs the adaptive immune system to mount a response [1]. The adaptive response is mediated by T and B lymphocytes that have undergone germline gene rearrangements of their antigen-specific receptors. This second line of defense is characterized by exquisite specificity and long-lasting memory.
This review summarizes our concept and experiences with a particular type of tumor vaccine. We exploit the use of virus infection to introduce DS into tumor cells in order to activate, via antitumor vaccination, innate immune responses and adaptive antitumor immune responses in connection with long-term immunological memory. The review includes a summary of results from many clinical studies. The survival data are interpreted as resulting from activation and maintenance of long-term immune memory.
Development of a new concept in animal tumor models
About 20 years ago, we started this work in the murine ESb lymphoma animal tumor model. The ESb lymphoma is a very aggressive animal tumor. It metastasizes to visceral organs, in particular to the liver, and kills syngeneic hosts within about 12 days. We used it as a challenge to design new antimetastatic therapy strategies. Treatment with cytostatic drugs was not effective and even reduced the overall survival time. In contrast, postoperative vaccination with a virus-modified—but not with unmodified—ESb cells was able to give protection from metastases in about 50% of syngeneic mice [2]. The surviving animals developed long-lasting protective immunity, which was specific for the ESb tumor line and did not cross-react with other syngeneic or allogeneic tumor lines. It was based on tumor-specific immune T-cell memory.
The effectiveness of this approach of postoperative active specific immunotherapy (ASI) with virus-modified autologous live tumor cell vaccine was thereafter confirmed in other metastasizing animal tumors such as murine B16 melanoma [3], 3LL Lewis lung carcinoma [4], and guinea pig L10 hepatocarcinoma [5] (for review, see also [6]).
For the purpose of virus infection, we selected an avian RNA paramyxovirus, namely Newcastle disease virus (NDV). NDV is an enveloped virus 150–300 nm in size containing a nonsegmented negative-stranded RNA of 15 Kb. Virulent strains of NDV are important pathogens in the poultry industry and are widely distributed in naturally occurring bird populations. The NDV genome contains six genes encoding for the following six gene products listed in order from the 3′-end: nucleocapsid protein (NP, 55 kDa), phosphorprotein (P, 53 kDa), matrix protein (M, 40 kDa), fusion protein (F, 67 kDa), hemagglutinin-neuraminidase (HN, 74 kDa), and large protein (L, 200 kDa). The F glycoprotein is synthesized as an inactive precursor (Fo, 67 kDa), which undergoes proteolytic cleavage to yield the biologically active protein consisting of the disulfide-linked chains F1 (55 kDa) and F2 (12.5 kDa) [7]. We selected NDV not only because of its tumor-selective replication properties but also because we wanted to avoid the use of viruses that had the chance to adapt to mammalian hosts during evolution. Instead of immune escape and immune suppression, NDV shows strong immunostimulatory properties in cancer patients and has never caused severe side effects or viremia.
Human infections are mild and can cause symptoms of conjunctivitis or laryngitis. There is an extensive safety database for NDV, primarily from low-dose human tumor vaccine trials. Even replication-competent, oncolytic NDV is well tolerated in humans in doses of at least 3×109 infectious units by the intravenous route and at least 4×1012 infectious units by the intratumoral route [8, 9]. By infecting patient-derived tumor cells with NDV, we developed the autologous virus-modified tumor vaccine ATV-NDV [10]. Human tumor cell modification by NDV infection was found to be efficient and safe, and produced a cancer vaccine with pleiotropic immune stimulatory properties [10].
The NDV can replicate up to ×10,000 better in human cancer cells than in most normal human cells [8–10]. This finding has prompted much interest in this virus as a potential anticancer agent. The resistance of normal cells to infection by NDV may have to do with their strong interferon-α response. Table 1 shows the interferon-α response of human normal peripheral blood mononuclear cells (PBMCs) and of human tumor cells. While normal PBMCs produce IFN-α immediately after surface contact with inactivated NDV, tumor cells produce IFN-α only after true virus infection. IFN-α is an antiviral factor, which can quickly induce a state of virus resistance in other cells.
Table 1.
IFN-α response to NDV of human normal cells and tumor cells
| Representative example | IFN-α (pg/ml)a | |
|---|---|---|
| Normal cells (adherent PBMCs)b | Tumor cells (MCF-7)c | |
| +UV-inactivated NDV Ulster | 86,431 | <10 |
| +Live NDV Ulster | 77,210 | 55 |
| Effects | Independent on viral replication | Dependent on viral replication |
| IFN-α titers | High | Low |
aCells (PBMCs or MCF-7) were coincubated for 3 days with 60 HU/107 cells of either live or UV-inactivated NDV, and then supernatants were tested by ELISA for IFN-α content
bThe major cell type producing IFN-α in PBMCs is the NIPC
cA human breast carcinoma cell line
The NDV has been labeled as a complementary and alternative medicine (CAM), and much detailed information can be found on a Web page of the US National Cancer Institute (http://www.nci.nih.gov/cancerinfo/pdq/cam/NDV ). The first hints of the potential anticancer benefit of this virus were given over 30 years ago. Three different conceptual uses of NDV in cancer treatment can be distinguished: (1) use for tumor selective cytolysis (= oncolysis) [8, 9], (2) use of NDV as an adjuvant and DS in a tumor vaccine for stimulation of CTL and delayed-type hypersensitivity (DTH) responses after antitumor vaccination [10, 11], and (3) use of NDV for nonspecific immune stimulation and induction of cytokines and interferons [6, 12].
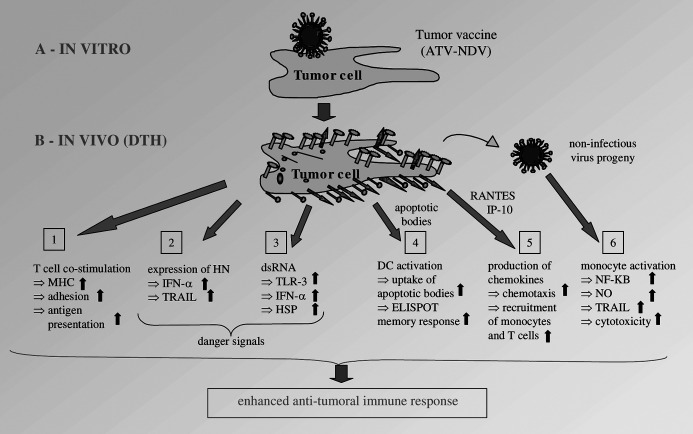
Figure 1 shows a diagram of the immunological consequences of tumor cell infection by NDV. Six different mechanisms are distinguished which lead to activation of innate [2, 3, 6] and adaptive [1, 4, 5] immune responses. As a consequence of infection of tumor cells by NDV, tumor cells can be perceived by the immune system as “dangerous” because of the following DS molecules which we have identified: (1) viral HN molecules [11, 12], (2) double-stranded RNA [13], and (3) interferon-α and interferon-β [12, 14] (Fig. 1, mechanisms 2 and 3).
Fig. 1.
The immunological consequences of tumor cell infection by NDV. a The tumor vaccine ATV-NDV is produced by coincubation of 107 200-Gy irradiated tumor cells with 32 HU of NDV Ulster for 1 h at 37°C. b After intradermal application of the vaccine, virus replication occurs in situ and leads within 24–48 h in the skin to delayed-type hypersensitivity (DTH) reactivity. This may involve distinct innate (4–6) and adaptive (1–3) immune mechanisms as analyzed in vitro.
The NDV-induced release of high amounts of IFN-α by PBMCs is mostly due to natural interferon-producing cells (NIPCs) such as plasmacytoid dendritic cells (DCs). These cells of the innate immune system have pattern recognition receptors for bacteria and viruses to sense “danger” for the body. Upon activation, such cells produce ×1,000 more IFN-α than other blood cells, migrate to inflamed lymph nodes, activate macrophage scavengers and natural killer (NK) cells, and promote the survival of activated T cells [15]. IFN-α has an important adjuvant function in the immune response [16]. It activates DCs [17], induces TRAIL in NK cells [18] and the IL-12 receptor β chain in T cells [19]. Together with IL-12, IFN-α polarizes the T cell toward a cell-mediated Th1 response characterized by DTH and CTL activity. In addition, IFN-α induces the up-regulation of molecules which are important for antigen recognition (e.g., HLA [20]), cell–cell interaction (e.g., cell adhesion molecules [CAM] [20]), and cytotoxicity (e.g., TRAIL [12]).
Experiments with HN transfectants showed that this molecule, when expressed at cell surfaces could function to induce IFN-α in PBMCs [14]. In addition, HN transfectants showed an increased capacity for T-cell binding and T-cell costimulation [11]. Many more CD4 T lymphocytes bound to HN transfectants than to wild-type fibroblasts or neogene-transfected control cells [21]. Viral HN molecules when expressed on peptide MHC class I–presenting stimulator cells augmented the induction of peptide-specific CD8 cytolytic T lymphocytes (CTLs). A greater than sixfold increase in peptide-specific CTL response was observed in cultures restimulated with peptide-pulsed fibroblasts, which coexpressed viral HN due to either infection or transfection [11]. In a human melanoma-specific CD4 T- helper clone, NDV infection of autologous melanoma cells induced a B7-1/B7-2 independent T-cell costimulatory activity, thereby breaking tolerance [22] (Fig. 1, mechanism 1).
The avirulent, lentogenic strains Ulster and La Sota of NDV were shown to be capable of inducing antitumor cytotoxicity in mouse macrophages [23] and in human monocytes [24]. NDV activated NF-κB in mouse macrophages and stimulated nitric oxide (NO) production [25]. The anticancer activity of murine NDV-activated macrophages could be attributed to the production and release of TNF-α [23], while the antitumor cytotoxicity of NDV-activated human monocytes was attributed to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) [24] (Fig. 1, mechanism 6).
Dendritic cells are the most important professional antigen-presenting cells (APCs) and were recently shown to play an important role in tumor antigen processing and initiation of specific T-lymphocyte responses. We evaluated the effects of infecting human tumor cells with NDV on the antigen-presentation capacity of DCs and on their ability to stimulate autologous breast cancer reactive memory T cells. DCs from breast cancer patients were pulsed with lysates from the MCF-7 breast cancer line (Tu-L) or from NDV-infected MCF-7 cells (TuN-L, viral oncolysates) and compared for stimulatory capacity in an ELISpot response of autologous memory T cells from the bone marrow of the same patients [26]. DCs pulsed with viral oncolysates showed increased expression of costimulatory molecules and induced significantly higher ELISpot responses in comparison to Tu-L–pulsed DCs (Fig. 1, mechanism 4). Supernatants from cocultures of memory T cells and TuN-L–pulsed DCs contained increased titers of IFN-α and IL-15. IL-15 supports memory CD8 T-cell proliferation and maintenance through interaction with IL-15 receptor α chains on these cells [27].
Upon infection by NDV, human tumor cells also produce the chemokines RANTES and IP-10 [20]. These molecules increase chemotaxis and lead to the recruitment of monocytes and T cells to the site of vaccine application. Such cellular inflammation can be quantified 24–48 h later by measuring the diameter of induration at the vaccination site. The DTH reaction then disappears, and the vaccine cells undergo apoptosis [20] (Fig. 1, mechanism 5).
Memory T cells and tumor dormancy
Tumor dormancy in general, and in clinical cancer in particular, can be due to different growth constrain mechanisms [28]. One involves macrometastatic lesions that fail to induce angiogenic activity, which is necessary for their expansion. Local hypoxia and undernourishment is insufficient to eradicate the cells but limits their expansion by inducing apoptotic death. Angiogenesis inhibitors such as angiostatin and endostatin can keep small tumor foci dormant over extended periods of time. Another form of tumor dormancy consists of solitary cancerous cells that reside after resection of the primary tumor (PT), at distinct sites such as bone marrow or other tissues.
In our animal tumor studies, we noticed an interactive balance between residual tumor cells and immune memory [28]. Persistence of tumor cells and thus persistence of the tumor-associated antigens (TAAs) derived thereof, correlated with long-term protective immune memory [29, 30]. Following injection of live or irradiated tumor cells into the external ear pinna (e.g.) of mice, a site where the tumor cells could not grow—comparable numbers of X-Gal stained cells were detectable in the bone marrow (BM) of host animals 1 week after inoculation. Live tumor cells persisted at a similar level (about 30 cells/106 bone marrow cells) in the BM for follow-up periods of up to 2 months while the number of BM-derived irradiated cells declined within 3 weeks. The persistence or nonpersistence of tumor cells in BM seen with injection of either live or irradiated tumor cells correlated with the presence or absence of long-term memory in these respective groups. Thus, when the tumor challenge was made later than 4 weeks after vaccination, only the live tumor vaccine could effectively protect the mice. The tumor-dormant mice were fully protected even when challenged with parental tumor cells 6 months after primary—i.e., tumor cell—injection.
As already mentioned, postoperative vaccination in the murine ESb tumor model with ESb-NDV vaccine resulted in about 50% long-term survivors which had established systemic and tumor-specific immune memory [31]. A detailed analysis later revealed the existence of residual tumor cells in such mice in the bone marrow. These cells were never completely eradicated and were kept under immune control at a low level. Therapeutic antitumor vaccination in these animals had thus caused the establishment not only of immunological memory but also of tumor dormancy. This was confirmed in prophylactic immunization experiments. In immunocompetent animals, we saw a correlation between the persistence of dormant tumor cells at low levels in the bone marrow and long-term protective immune memory [29]. In immunocompetent tumor-dormant mice, 21% of the BM-derived tumor cells were positive for the proliferation marker Ki67. When immune CD8 T cells were depleted from tumor dormancy animals, the frequency of BM-residing tumor cells increased by two orders of magnitude before the animals died [30]. These findings suggested that tumor dormancy in this model was due to active immune control of tumor cells by CD8 T cells in the bone marrow, keeping them at a low level without elimination [28].
Long-term persistence of tumor cells in a dormant state is suggested also from clinical observations in patients with cancer, notably breast cancer [32]. Some patients develop secondary tumors at distant sites many years after successful therapy of the PTs; others do not develop recurrencies in spite of disseminated tumor cells at the time of diagnosis. The longest duration of breast cancer dormancy, i.e., the longest interval between primary treatment and tumor recurrence, was calculated in a retrospective study, involving 1,547 patients, to be between 20 and 25 years [32]. In breast cancer, 25–43% of primary operated patients exhibit micrometastatic tumor cells in their bone marrow. Detection levels are in the range of 1–10 cancer cells per 106–107 BM-derived mononuclear cells. Successful enrichment and reliable identification and molecular profiling are key issues of ongoing and future studies [33].
Memory T-cell stimulation by antitumor vaccination
While naïve T cells can only be primed by antigens that are cross-presented by professional host APCs such as DCs, memory T cells can also be activated directly by a tumor vaccine presenting TAA together with costimulatory signals. Secondary immune responses by memory T cells are faster and stronger than primary responses. Their requirements for activation are less strict (lower dependency on costimulation), and they release a broader spectrum of cytokines and are multifunctional after reactivation [34]. Thus, memory T cells are superior to naïve T cells [35] for protective immunity. There is a programmed development of effector and memory CD8 T cells, and different subsets of memory T cells, namely “central” and “effector” memory cells, have recently been distinguished. Of special interest are also the stem cell–like properties of memory T cells: Upon response to homeostatic signals they have a self-renewal capacity [35].
We recently established a novel tumor model system for the study of long-term protective immunity and immune T-cell memory [36]. In this adoptive memory T-cell transfer system involving as recipients nude (nu/nu) mice, we were able to study (1) characteristics of a potent tumor vaccine–induced secondary antitumor T-cell response [37], (2) the role of persisting antigen for long-term maintenance of peptide epitope–specific CD8 memory T cells, and (3) the longevity of the cells.
The conclusion that ATV-NDV vaccine can present TAA directly to memory T cells and stimulate them is supported by the following results: (1) in a coculture with a TAA-specific memory T-cell clone, ATV-NDV stimulated T-cell proliferation and IL-2 production while ATV without NDV infection induced tolerance [22], (2) viability of the irradiated vaccine was important for CTL activation [38] and for clinical efficacy [39], and (3) APCs transfected with the viral HN cDNA showed increased CTL stimulatory capacity [11]. The same was true for NDV infection of tumor-stimulatory cells [40].
Memory T cells in cancer patients
To test for the presence of memory T cells in cancer patients, we investigated bone marrow of breast cancer patients with respect to tumor cell content, immune activation status, and memory T-cell content [41]. BM-derived cells from primary operated breast cancer patients (n=90) were compared with those from healthy donors (n=10) and also with cells from respective blood samples. Cytokeratine 19–positive tumor cells were detected by nested PCR. Three-color flow cytometry was used to identify numbers and activation state of T cells, NK cells, monocytes/macrophages, and subsets by a panel of monoclonal antibodies (mAbs). The proportion of memory T cells among the CD4 and CD8 T cells was found to be much higher in BM of cancer patients than in healthy donors (p<0.001). The extent of memory T-cell increase was related to the size of the PT. The highest relative memory content was detected in patients with a T2 tumor stage, and these were significantly different from both patients with T1 and those with T3/4 stages. Thus, with tumor progression, the memory content in T-cell populations first steadily increased and then decreased again. Patients with disseminated tumor cells in their BM had more memory CD4 T cells than patients with tumor cell–negative BM [41]. Our proposition from animal studies that BM is a special compartment for immunological memory and tumor dormancy [29, 30] had thus been supported by our clinical findings.
The calculated frequency of memory T cells in ELISpot among total T lymphocytes from BM of patients responding to autologous TAA was rather high and varied from 1/200 to 1/11,000. We could also induce antitumor CTL activity in reactivated BM-derived memory T cells. Their immunotherapeutic competence in vivo was finally demonstrated by showing regression of human breast cancer in NOD/SCID mice after transfer of autologous reactivated BM-derived memory T cells [42]. Following such cell transfer, human T cells were found to infiltrate the human breast cancer tissue. Most infiltrating T cells had the memory marker CD45 RO. CD8 T cells were found in close contact with CD4 T cells and within the tumor close to necrotic areas, where in serial tumor sections apoptotic cells could be detected [42]. Cognate interactions between BM-derived memory T cells and TAA presenting DCs led to bidirectional cell stimulation, survival and antitumor activity in vivo [43].
Development of an autologous virus–modified tumor vaccine (ATV-NDV) for clinical studies
The question of how to define and select cancer antigens suitable as targets of immunotherapy is important but still unresolved. A major aspect relates to the choice between common (shared) or unique (individual) TAAs. The latter may be more important for tumor rejection responses because corresponding T-cell receptors are expected to have higher affinities and because T cells with specificity for unique mutant peptides were found to dominate the immune response in comparison to T cells recognizing shared antigens. The logical extrapolation from this is to use an individualized approach for tumor vaccine generation. We furthermore decided to use autologous tumor cells because we had found that protective antitumor immunity in animal tumor models was highly specific for the autologous tumor [2, 31] and because autologous tumor cells represent the closest possible match to a patient’s tumor. Autologous tumor cells might express common TAAs as well as individually unique TAAs derived from mutations or other genetic alterations. The use of whole tumor cells simulates a physiological situation and eliminates the need to first identify the respective TAA.
Thus, the specific component of the vaccine that we developed is patient-derived (autologous) live tumor cells (ATV). These are infected by the avirulent strain Ulster of NDV and inactivated by 200 Gy γ-irradiation to produce the vaccine ATV-NDV. Tumor cells can be isolated from freshly resected tumor specimens by mechanical dissection and enzymatic dissociation, and can be stored after controlled freezing in liquid nitrogen. While the first clinical studies employed total dissociated cells from a tumor, we later introduced a further purification procedure to remove tumor-infiltrating lymphocytes (TILs) by immunomagnetic beads [39]. In recent studies, cell culture–adapted autologous tumor cells are being used. All studies were approved beforehand by local ethical commissions.
We selected the nonlytic strain Ulster for reasons of safety during application in cancer patients and also because we intended to develop a virus-infected whole cell cancer vaccine consisting of intact viable irradiated cancer cells. NDV Ulster has a monocyclic abortive replication cycle in tumor cells [10, 11]. This replication involves the formation of double-stranded (ds) RNA which functions as a DS and activates toll-like receptor 3 [13]. Thus we tried to link the expression of TAAs with DS [1, 44].
It has been the experience of many tumor immunologists that whole cell vaccines can stimulate the immune system more efficiently than oncolysates [45]. Tumor cell membrane integrity was found to be important for CTL activation by cancer vaccines [38]. NDV Ulster is first adsorbed to the tumor cells in vitro (1-h binding). Then the virus-modified tumor vaccine is injected intradermally thus allowing for virus replication in vivo at the site of vaccine application. Vaccine consisting of tumor cells infected with NDV Ulster should remain in the body long enough to generate effective immune responses which are mostly based on T-cell–mediated immunity. Viral replication in the tumor cells takes about 6–40 h [10–12], a time sufficient to generate DTH skin responses, which are dependent on antigen-specific memory T cells. During this time period of 6–40 h after vaccine application, the infected tumor cells can produce progeny NDV virus particles. Although these are noninfectious due to uncleaved Fo molecules, they are able to stimulate innate immune responses (Fig. 1).
Phase I clinical studies
Much of this pioneering work of establishing a human NDV-modified tumor vaccine was performed by my clinical colleague Dr T. Ahlert, who now has over 10 years of experience with vaccine preparation and application in cancer patients.
After having optimized a technical procedure for isolating live tumor cells from fresh resection specimens and having calculated average yields and stability parameters [46, 47], we started to perform phase I clinical studies [46–51]. In the ESb animal tumor model we had described that an optimal vaccine composition which yielded 50% survival benefit after a single inoculation was composed of 107 irradiated tumor cells infected by 32 hemagglutinating units (HU) of NDV Ulster. The first systematic optimization studies for the vaccine ATV-NDV were performed in breast carcinoma [48], colorectal carcinoma [49–52], and in renal cell carcinoma [53, 54] patients. After 2–3 weeks of tumor operation, we applied vaccine intradermally at the upper thigh at different sites. The vaccine was composed of different numbers of tumor cells and of different doses of virus. After 24–48 h, the local skin reactions to the vaccine were measured by determining the mean diameter of induration. Optimal skin reactions were observed with 1×107 tumor cells infected with 32 HU NDV Ulster. With this vaccine formulation, 85% of colorectal carcinoma patients and 90% of renal carcinoma patients showed about 7- to 11-mm skin indurations at the vaccination site.
The fact that the optimal vaccine composition was similar in the ESb mouse tumor model and in cancer patients can perhaps best be explained on the basis of a local memory immune response, which requires only low amounts of antigen.
Next, we evaluated skin responsiveness in patients not only to the vaccine ATV-NDV but also to autologous tumor cells without virus (ATV) and to various controls. In nonvaccinated patients, skin reactivity to ATV was much smaller than to ATV-NDV, both in terms of mean induration and in terms of percentage of responder patients. Reactions to the virus alone were either absent or very small. For specificity control of the DTH test we performed either the Mérieux test consisting of several recall antigens or we used normal cells from either kidney epithelium (in renal carcinoma patients) or from colon mucosa (in colorectal carcinoma patients), which were separated under the same conditions as the tumor cells. In 40% of colorectal carcinoma patients, we saw some reactivity to normal colon mucosa [49, 52], while in none of the 20 renal carcinoma patients did we detect reactivity to normal kidney epithelium cells [54]. Whether the weak reactivity to normal colon mucosa is due to cross-reactions or represents true autoimmune responses remains to be investigated. We concluded from these studies that DTH responses to TAAs can be distinguished from responses to recall antigens and from autoimmune responses.
In a separate study, we demonstrated that the recall antigen responses of patients did not correlate with, or allow any prediction of, their responsiveness to autologous tumor cells [52]. It was thus not a better general immunocompetence that distinguished immunological responder from nonresponder patients. This was recently confirmed with ELISpot results demonstrating distinct respective memory responses to recall antigens and to TAAs in individual patients [43]. The similarity to memory ELISpot responses suggests a strong involvement of TAA-specific memory cells in the DTH test. With the ELISpot assay, we also found out that DCs, when pulsed with viral oncolysates from the ATV-NDV vaccine, stimulate antitumor memory T-cell responses from cancer patients more strongly than when pulsed with ATV-derived tumor lysate [26].
Effects of repeated vaccinations
We next tested the effect of repeated vaccinations on local skin reactivity to the vaccine ATV-NDV. A positive skin response was defined as being above 2-mm diameter induration. While in tests of various tumor types [46, 48] we had seen 23% nonresponders at the first ATV-NDV inoculation, virtually all patients became responders at the third vaccination. This was also true for studies in colorectal carcinoma patients, who were vaccinated with vaccine derived either from the PT [55] or from liver metastases (LM) [49–52]. There was not only an increase in the percentage of responder patients but also of the induration intensity. While at the first vaccination (1°), patients showed intensities of 3–5 or 5–10 mm, at the third vaccination (3°), many showed an induration intensity of 10–20 mm [55].
We were able to induce anti-(ATV) DTH reactivity in immunological nonresponders (Table 2). Among 90 cancer patients tested who did not react to ATV before vaccination, 31% changed from a nonresponder to a responder phenotype after vaccination with ATV-NDV (at least three times) (Table 2). Thus, in these 31% of patients, we were able by vaccination to induce a specific antitumor DTH memory response.
Table 2.
Induction of new antitumor DTH reactivity (change from nonresponder to responder). ATV-NDV induced DTH reactivity to ATV in patients who were negative to ATV at first test
| Breast carcinomaa | Colorectal carcinoma with LMb | Various tumor typesc | |
|---|---|---|---|
| Patients (%) | 43 | 9 | 30 |
| Patients (n)d | 44 | 23 | 23 |
bIn two patients the reaction increased from 0 to 10 mm and from 0 to 15 mm [50, 52]
cMelanoma, colon carcinoma, rectal carcinoma, stomach carcinoma, ovarian carcinoma, breast carcinoma, renal cell carcinoma, and acute myeloblastic leukemia [46]
dIn total, among 90 patients tested, 31% showed induction of new DTH reactivity
Next, we investigated the effect of antitumor vaccination with ATV-NDV in original responder patients who had already reacted to ATV challenge before vaccination. Table 3 summarizes the results obtained from studies in breast carcinoma [39], ovarian carcinoma [39, 56], colorectal carcinoma [49–52, 55], renal carcinoma [54], and other tumor types [46–48]. Among 264 patients tested, 44% showed significantly increased DTH reactivity to ATV (without NDV) after the course of vaccination. A significant increase of DTH reactivity to ATV was defined by an increase of diameter greater than 3 mm. Thus, in these 44% of patients, we had increased the level of systemic antitumor immune memory reactivity.
Table 3.
Potentiation of antitumor DTH reactivity in immunological responders following vaccination with ATV-NDV. Potentiation of DTH responses to ATV (comparison of last to first reaction), percentage of patients with increases ≥3 mm. PT Primary tumor, LM liver metastasis, n number of patients
| Breast carcinoma | Ovarian carcinoma | Colorectal carcinoma | Renal carcinomaa | Other tumor typesb | ||
|---|---|---|---|---|---|---|
| PT | LM | |||||
| Patients (%) | 46 | 39 | 57 | 42 | 35 | 45 |
| Patients (n)c | 67 | 18 | 14 | 24 | 20 | 24 |
aIn this tumor type, increased DTH reactions were observed only when the tumor vaccine ATV-NDV was supplemented with IL-2 [53, 54]
bMelanoma, colon carcinoma, rectal carcinoma, stomach carcinoma, ovarian carcinoma, breast carcinoma, renal cell carcinoma, and acute myeloblastic leukemia [46]
cIn total, among 264 patients tested, 44% showed increased DTH reactivity to ATV after vaccination
The great majority of cancer patients did not show a detrimental response after vaccination [52, 55]. This is reassuring and supports the present strategy of vaccination. The fraction of patients with a decrease was less than 10%.
A positive correlation between increase of DTH responsiveness to ATV and prognosis was seen by us [54, 55] and others [57, 58]. A strong increase of antitumor DTH reactivity (>5 mm) to tumor challenge after vaccination (in comparison to the first reactivity) predicted a survival advantage (35 vs 14 months), a correlation that was significant by Wilcoxon test [54]. It has been assumed that such a correlation just reflects a better general immunocompetence and thus better prognosis in this subgroup of patients [59]. This is, however, unlikely because we and others did not see a correlation between response to general recall antigens (Mérieux test) and response to autologous tumor cells [43, 57, 60, 61]. Since there was no DTH reactivity to normal kidney epithelial cells, the correlation seen in renal carcinoma patients [54] is likely due to activation of specific antitumor immune memory.
Side effects
The phase I clinical studies were also performed to evaluate possible side effects. The intradermal vaccinations were well tolerated and could be repeated many times without causing serious problems. A few patients developed mild fever and/or mild headache for 1–2 days. There was no evidence of autoimmune phenomena such as vasculitis, hematoid arthritis, or lymphatic disorders.
Phase II clinical studies: evaluation of efficacy
Having seen that antitumor vaccination of cancer patients with ATV-NDV could lead to a significant increase of systemic memory DTH responses to autologous tumor cells (ATV), we next engaged in phase II clinical vaccination studies to evaluate the clinical efficacy of this approach. Tables 4, 5, and 6 summarize the most important results from a variety of phase II studies performed over the last 10 years. Since the majority of studies have been published, I want to focus here only on the most important aspect, namely the survival data.
Table 4.
Two-year survival rates after postoperative vaccination with ATV-NDV. n Number of patients
| Tumor type | Stage | Two-year survival rate | Increase of survival rate (%) | Significance (p) | |
|---|---|---|---|---|---|
| Colorectal carcinoma | |||||
| Median follow-up 22 months | (a) Locally advanced d | Controla (n=601, 74%) | Vaccinated (n=48, 98%) | 24 | |
| Median follow-up 18 months | (b) Solitary livere metastases, RO resectable | Controlb (n=23, 61%) | Vaccinated (n=23, 87%) | 26 | 0.05 |
| Glioblastoma multiforme | |||||
| Median follow-up 59 months | Grade IVf | Controlb (n=87, 11%) | Vaccinated (n=23, 39%) | 28 | <0.001 |
| Malignant melanoma | |||||
| Median follow-up 18 months | Recurrent resectableg | Vaccinated c (a) ATV-NDV <3×106 (n=20, 45%) | Vaccinated (b) ATV-NDV >3×106 (n=21, 65%) | 20 | |
aHistorical control from the same clinic
bPair-matched controls
cThis control group was vaccinated with a vaccine containing less than the indicated number of tumor cells and less than 33% cell viability. It is not historical but an actual internal control
dFrom [55]
eRecurrence-free survival data from [52, 60]
fFrom [62]
gFrom [6]
Table 5.
Five-year survival rates after postoperative vaccination with ATV-NDV. a Group of patients vaccinated with a vaccine containing less than the indicated number of tumor cells and less than 33% cell viability; as in Table 4 this is an actual internal control with an insufficient formulation of the same vaccine; a historical control is included in addition in the colorectal carcinoma study. b Test group which received a high-quality formulation of the vaccine. n Number of patients
| Tumor type | Stage | Five-year survival rate | Increase of survival rate (%) (b−a) | Significance (p) | ||
|---|---|---|---|---|---|---|
| Control (a) | Verum (b) | |||||
| Breast carcinoma | Locally advanceda | ATV-NDV <1.5×106 (n=31, 48%) | ATV-NDV >1.5×106 (n=32, 84%) | 36 | 0.004 | |
| Colorectal carcinoma | Locally advancedb | ATV-NDV <3×106 (n=18, 60%) | ATV-NDV >3×106 (n=20, 85%) | Historical control (n=48, 50%) | 25 | ≤0.05 |
Table 6.
Exceptionally long-term survivors among patients treated with ATV-NDV vaccine from autologous cell cultures
| Tumor type | Median survival time expected (months) | Median survival observeda |
|---|---|---|
| Ovarian carcinoma | ~12 | 30 months DFS in 15/24 (63%)b |
| Glioblastoma multiforme | ~12 | >36 months in 2/23 (9%)c |
| Pancreatic carcinoma | ~12 | >36 months in 5/9 (55%)d |
| Stomach carcinoma | ~12 | >36 months in 3/7 (43%)e |
Table 4 lists the 2-year survival rates observed in four clinical studies. The survival rate in the group of patients who received postoperative vaccinations with ATV-NDV, was compared to that of nonvaccinated control groups specified in the legend. Two studies were performed in patients with colorectal carcinoma with either locally advanced tumor [55] or with resected solitary LM [52]. There was about a 25% increase of the 2-year survival rate in both studies. Results from a recent study in patients with glioblastoma multiforme [62] revealed a 28% increase of the 2-year survival rate. In a fourth study of patients with recurrent resectable malignant melanoma [6], there was a 20% increase of survival rate in a group of 21 patients. In this study, the comparison was made between a group treated with a vaccine containing at least 3×106 tumor cells and having greater than 33% viable cells (b) and another group (a) treated with a vaccine in which both vaccine parameters were below that threshold. This internally controlled study suggests that the observed vaccination effect is not due to a placebo effect because both groups were vaccinated. Instead, the better survival was dependent on the dose (>3×106 tumor cells) and quality (>33% cell viability) of the vaccine product.
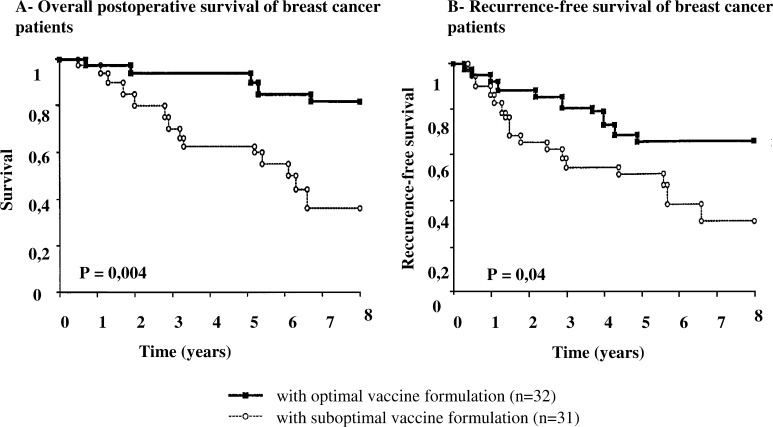
Table 5 shows the 5-year survival benefits after postoperative vaccination with ATV-NDV in two studies with (1) locally advanced primary breast cancer and (2) locally advanced colorectal carcinoma. The results are derived from follow-up evaluations of earlier, published phase II studies [37, 55]. In the colorectal carcinoma study, we saw a 25% increase of the 5-year survival rate in a group of 20 patients who received a high-quality ATV-NDV vaccine [6]. In the breast carcinoma study, the follow-up revealed a 36% increase of the 5-year survival rate in a group of 33 vaccinated patients for whom the vaccine contained a least 1.5×106 tumor cells and greater than 33% viability (Fig. 2).
Fig. 2.
Survival curves of vaccinated patients. Primary operated breast cancer patients received postoperative vaccinations with the autologous virus-modified tumor vaccine ATV-NDV in two different formulations given to two cohorts of more than 30 patients with similar risk factors [37]. The figure shows the Kaplan-Meier estimates after a prolonged median observation period of 5.2 years for long-term survival (a) and recurrence-free survival (b). The analysis was performed by FOCUS (Düsseldorf) with the financial support of the Dietmar Hopp-Stiftung (Walldorf). There was a highly significant long-term survival benefit and also a significant benefit in recurrence-free survival in the group which received a vaccine formulation of >1.5×106 viable tumor cells with at least 33% overall cell viability. There were only 5 patients dead in this group as compared to 16 in the other group, which could only receive a suboptimal vaccine formulation. The survival in the latter group corresponds to expectation of patients with similar risk factors under standard therapy without vaccination.
In four different tumor types in which antitumor vaccinations were performed with ATV-NDV vaccine from autologous tumor cell cultures we observed exceptionally long-term survivors [6, 56, 62]. Table 6 shows the median survival time which was expected from standard treatment and the median survival observed. It can be seen that a significant fraction of treated patients, varying from 9% to 63%, consisted of exceptionally long-term survivors. In a few long-term survivor patients, we recently succeeded in demonstrating the presence of antitumor immune memory T-cell reactivity in peripheral blood [62]. This was observed even several years after the last vaccination.
Positive results have recently also been reported from randomized trials of antitumor vaccination of patients with (1) stage II colon cancer [63], (2) tumors of the digestive tract [64], and (3) renal cell carcinoma [65].
Conclusions from survival data of ATV-NDV immunotherapy studies
We would like to critically review the results obtained and ask three relevant questions which from a biometrical point of view [59] should be addressed to any cancer therapy:
Has the therapy the potential to cause antitumor effects? This question can clearly be answered positively. We were able to demonstrate in various tumor models as well as in studies with human cells that the vaccine can increase the activation of a variety of antitumor killer cells including NK cells, macrophages [23, 24], and CTLs [11, 12, 40]. The HN molecule of the virus was proven to play an important role in the activation of killer activity by induction of a strong IFN-α response [12, 14], by up-regulation of TRAIL on monocytes [24] and T cells [12], and by increasing costimulatory signals in CTL precursors [11] and T-helper cells [21, 22].
Has this therapy a promising antitumoral activity in patients? Since most of our vaccination studies were performed in the postoperative adjuvant situation without residual tumor mass, no direct tumor responses could be determined. In these studies, an antitumor activity can only be deduced from the survival data. There were, however, singular cases of tumor responses in some studies. From 40 evaluable renal carcinoma patients treated with ATV-NDV and systemic IFN-α, 5 exhibited a complete response (CR), 6 partial remission (PR), and 12 stable disease (SD; median 25 months); 23/40 (57.5%) patients (CR, PR, and SD) appeared to have a significant survival advantage compared with patients with progressive disease during the treatment period and to a historical reference group [53]. In a glioblastoma patient with residual disease after operation, we saw complete remission several months after vaccination with ATV-NDV [62]. In addition, a subgroup of seven glioblastoma patients with residual tumor mass showed after vaccination with ATV-NDV a median survival which was twice that of a nonvaccinated comparable group [62]. The therapy therefore has a promising antitumoral activity in patients.
Does this therapy improve survival of treated patients in comparison to standard treatment or to nontreated patients, and what is the effect on quality of life? Since the side effects of this treatment are negligible, we conclude that the quality of life of treated patients is not affected in a negative way. The evidence level for survival benefit reached so far is that of phase II studies. In some studies, we used pair-matched controls from patients treated at the same clinic during the same time period without vaccination, in others we used historical controls, and in several studies we used actual internal controls with a different formulation of the same vaccine. Prospective randomized controlled studies are needed to validate these findings. The observed improvements in survival rates, between 20% and 36%, the observed median survival prolongation by about 100%, together with significant fractions of exceptionally long-term survivors, however, speak in favor of the clinical effectiveness of this type of therapy.
Acknowledgements
I would like to acknowledge the help of many clinical colleagues without whom this translational research would not have been possible. While their names can be seen from the respective publications, I would like to thank here in particular P. Schlag and D. Ockert (colorectal carcinoma), T. Ahlert and G. Bastert (breast carcinoma), S. Pomer (renal carcinoma), V. Möbus (ovarian carcinoma), and H.H. Steiner and C. Herold-Mende (glioblastoma).
Note added in proof: Recently we observed also in patients with Head and Neck Squamous Cell Carcinoma (HNSCC) that antitumor vaccination with ATV-NDV caused improvement of antitumor immune memory and improvement of patient long-term survival (Karcher et al., Antitumor vaccination with HNSCC with autologous virus-modified tumor cells. Cancer Research, in press November 2004).
References
- 1.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114. doi: 10.1016/S0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 2.Heicappell R, Schirrmacher V, von Hoegen P, et al. Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. I. Parameters for optimal therapeutic effects. Int J Cancer. 1986;37:569. doi: 10.1002/ijc.2910370416. [DOI] [PubMed] [Google Scholar]
- 3.Plaksin D, Progador A, Vadai E, et al. Effective anti metastatic melanoma vaccination with tumor cells transfected with MHC genes and/or infected with Newcastle Disease Virus (NDV) Int J Cancer. 1994;59:796. doi: 10.1002/ijc.2910590615. [DOI] [PubMed] [Google Scholar]
- 4.Shoham J, Hirsch R, Zakay-Rones Z, et al. Augmentation of tumor cell immunogenicity by viruses—an approach to specific immunotherapy of cancer. Nat Immunol Cell Growth Regul. 1990;9:165. [PubMed] [Google Scholar]
- 5.Bier H, Armonat G, Bier J, et al. Postoperative active-specific immunotherapy of lymph node micrometastasis in a Guinea pig tumor model. Otorhinopharyngology. 1989;51:197. doi: 10.1159/000276059. [DOI] [PubMed] [Google Scholar]
- 6.Schirrmacher V, Ahlert T, Pröbstle T, Steiner HH, Herold-Mende C, Gerhards R, Hagmüller E. Immunization with virus modified tumor cells. Semin Oncol. 1998;25:677. [PubMed] [Google Scholar]
- 7.Phillips RJ, Samson AC, Emmerson PT. Nucleotide sequence of the 5′-terminus of Newcastle disease virus and assembly of the complete genomic sequence: agreement with the “rule of six”. Arch Virol. 1998;143:1993. doi: 10.1007/s007050050435. [DOI] [PubMed] [Google Scholar]
- 8.Lorence RM, Roberts, MS, Groene, WS, Rabin H (2001) Replication-competent, oncolytic Newcastle disease virus for cancer therapy. In: Hernáiz Driever P, Rabkin SD (eds) Replication-competent viruses for cancer therapy monograph virology, vol 22. Karger, Basel, p 160
- 9.Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL, Goldberg S, Gross P, O’Neil JD, Groene WS, Roberts MS, Rabin H, Bamat MK, Lorence RM. Phase I trial of intravenous administration of PV701, an oncolytic virus, patients with advanced solid cancers. J Clin Oncol. 2002;1(20):2251. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Schirrmacher V, Haas C, Bonifer R, Ahlert T, Gerhards R, Ertel C. Human tumor cell modification by virus infection: an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle disease virus. Gene Therapy. 1999;6:63. doi: 10.1038/sj.gt.3300787. [DOI] [PubMed] [Google Scholar]
- 11.Ertel C, Millar NS, Emmerson PT, Schirrmacher V, von Hoegen P. Viral hemagglutinin augments peptide specific cytotoxic T-cell responses. Eur J Immunol. 1993;23:2592. doi: 10.1002/eji.1830231032. [DOI] [PubMed] [Google Scholar]
- 12.Zeng J, Fournier P, Schirrmacher V. Induction of interferon α and TRAIL in human blood mononuclear cells by HN but not F protein of Newcastle disease virus. Virology. 2002;297:19. doi: 10.1006/viro.2002.1413. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappa B by toll-like receptor. Nature. 2001;413:732. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 14.Zeng J, Fournier P, Schirrmacher V. Stimulation of human natural interferon-α response via paramyxo-virus hemagglutinin lectin-cell interaction. J Mol Med. 2002;80:443. doi: 10.1007/s00109-002-0339-1. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulendran B, Banchereau J, Maraskovsky E, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22:41. doi: 10.1016/S1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 17.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461. doi: 10.1016/S1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 18.Sato K, Hida S, Takayanagi H, Yokochi T, Kayagaki N, Takeda K, Yagita H, Okumura K, Tanaka N, Taniguchi T, Ogasawara K. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol. 2001;31:3138. doi: 10.1002/1521-4141(200111)31:11<3138::AID-IMMU3138>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler Sinigaglia E. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washburn B, Schirrmacher V. Human tumor cell infection by Newcastle disease virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int J Oncol. 2002;21:85. doi: 10.3892/ijo.21.1.85. [DOI] [PubMed] [Google Scholar]
- 21.Haas C, Ertel C, Gerhards R, Schirrmacher V. Introduction of adhesive and costimulatory immune functions into tumor cells by infection with Newcastle disease virus. Int J Oncol. 1998;13:1105. doi: 10.3892/ijo.13.6.1105. [DOI] [PubMed] [Google Scholar]
- 22.Termeer CC, Schirrmacher V, Bröcker EB, Becker JC. Newcastle disease virus infection induces B7-1/B7-2 independent T-cell costimulatory activity in human melanoma cells. Cancer Gene Ther. 2000;7:316. doi: 10.1038/sj.cgt.7700109. [DOI] [PubMed] [Google Scholar]
- 23.Schirrmacher V, Bai L, Umansky V, Yu L, Xing Y, Qian Z. Newcastle disease virus activates macrophages for anti-tumor activity. Int J Oncol. 2000;16:363. [PubMed] [Google Scholar]
- 24.Washburn B, Weigand MA, Grosse-Wilde A, Janke M, Stahl H, Rieser E, Sprick MR, Schirrmacher V, Walczak H. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J Immunol. 2003;170:1814. doi: 10.4049/jimmunol.170.4.1814. [DOI] [PubMed] [Google Scholar]
- 25.Umansky V, Shatrov VA, Lehmann V, Schirrmacher V. Induction of nitric oxide synthesis in macrophages by Newcastle disease virus is associated with activation of nuclear factor κB. Int Immunol. 1996;8:491. doi: 10.1093/intimm/8.4.491. [DOI] [PubMed] [Google Scholar]
- 26.Bai L, Koopmann J, Fiola C, Fournier P, Schirrmacher V. Dendritic cells pulsed with viral oncolysates potently stimulate autologous T cells from cancer patients. Int J Oncol. 2002;21:685. doi: 10.3892/ijo.21.4.685. [DOI] [PubMed] [Google Scholar]
- 27.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15 R alpha signals are required for bystander proliferation. J Exp Med. 2001;194:1187. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schirrmacher V. T-cell immunity in the induction and maintenance of a tumor dormant state. Semin Cancer Biol. 2001;11:285. doi: 10.1006/scbi.2001.0384. [DOI] [PubMed] [Google Scholar]
- 29.Khazaie K, Prifti S, Beckhove P, Griesbach A, Russell S, Collins M, Schirrmacher V. Persistence of dormant tumor cells in the bone marrow of tumor-cell-vaccinated mice correlates with long term immunological protection. Proc Natl Acad Sci U S A. 1994;91:7430. doi: 10.1073/pnas.91.16.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller M, Gounari F, Prifti S, Hacker HJ, Schirrmacher V, Khazaie K. Eb-lacZ tumor dormancy in bone marrow and lymph nodes: active control of proliferating tumor cells by CD8+ immune T cells. Cancer Res. 1998;58:5439. [PubMed] [Google Scholar]
- 31.SchirrmacherV R. Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. II. Establishment of specific systemic anti tumor immunity. Clin Exp Metastasis. 1987;5:147. doi: 10.1007/BF00058060. [DOI] [PubMed] [Google Scholar]
- 32.Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst. 1999;91:80. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- 33.Pantel K, Otte M. Occult micrometastasis: enrichment, identification and characterization of single disseminated tumor cells. Cancer Biol. 2001;11:327. doi: 10.1006/scbi.2001.0388. [DOI] [PubMed] [Google Scholar]
- 34.Veiga-Fernandes U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 35.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T cell differentiation: implications for vaccine development. Nature Rev. 2002;2:251. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 36.Mahnke Y, Schirrmacher V. A novel tumor model system for the study of long-term protective immunity and immune T cell memory. Cellular Immunol. 2003;221:89. doi: 10.1016/S0008-8749(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 37.Mahnke Y, Schirrmacher V. Characteristics of a potent tumor vaccine-induced secondary anti-tumor T cell response. Int J Oncol. 2004;24:1427. [PubMed] [Google Scholar]
- 38.Schirrmacher V, von Hoegen P. Importance of tumor cell membrane integrity and viability for CTL activation by cancer vaccines. Vaccine Res. 1993;2:183. [Google Scholar]
- 39.Ahlert T, Sauerbrei W, Bastert G, Ruhland S, Bartik B, Simiantonaki N, Schumacher J, Häcker B, Schumacher M, Schirrmacher V. Tumor cell number and viability as quality and efficacy parameters of autologous virus modified cancer vaccines. J Clin Oncol. 1997;15:1354. doi: 10.1200/JCO.1997.15.4.1354. [DOI] [PubMed] [Google Scholar]
- 40.Von Hoegen P, Zawatzky R, Schirrmacher V. Modification of tumor cells by a low dose of Newcastle disease virus: III. Potentiation of tumor specific cytolytic T cell activity via induction of interferon α, β cell. Immunology. 1990;126:80. doi: 10.1016/0008-8749(90)90302-8. [DOI] [PubMed] [Google Scholar]
- 41.Feuerer M, Rocha M, Bai L, Umansky V, Solomayer EF, Bastert G, Diel IJ, Schirrmacher V. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer. 2001;92(1):96. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1152>3.3.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 42.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Heep J, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient derived re-activated memory T cells from bone marrow. Nature Med. 2001;7:452. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 43.Bai L, Beckhove P, Feuerer M, Umansky V, Choi C, Schütz F, Solomayer E-F, Diel IJ, Schirrmacher V. Cognate interactions between memory T cells and tumor antigen-presenting dendritic cells from bone marrow of breast cancer patients: bidirectional cell stimulation, survival and antitumor activity in vivo. Int J Cancer. 2003;103:73. doi: 10.1002/ijc.10781. [DOI] [PubMed] [Google Scholar]
- 44.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi H, Sendo F, Shirai T, Kaji H, Kodama T, Saito H. Modification in growth of transplantable rat tumors exposed to Friend virus. J Natl Cancer Inst. 1969;42:413. [PubMed] [Google Scholar]
- 46.Schirrmacher V, von Hoegen P, Schlag P, Liebrich W, Lehner B, Schumacher K, Ahlert T, Bastert G. Active specific immunotherapy with autologous tumor cell vaccines modified by Newcastle disease virus: experimental and clinical studies. In: Schirrmacher V, Schwartz-Albiez R, editors. Cancer metastasis. Berlin Heidelberg New York: Springer; 1989. p. 157. [Google Scholar]
- 48.Ahlert T, Bastert G, Schirrmacher V. Mamma- und Ovarialkarzinom mit autologen virusmodifizierten Tumorzellen, aktiv-spezifische Immuntherapie (ASI) Theorie, Praxis, Perspektiven T.W. Gynäkologie. 1989;2:359. [Google Scholar]
- 48.Ahlert T, Gremm B, Kohler S, Rexin M, Hoffmann R, Terinde R, Rethfeld E, Schirrmacher V, Kaufmann M, Heinrich H, Meisenbacher G, Bastert G (1994) Aktiv spezifische Immuntherapie (ASI), 9: Arbeitsgespräch der klinischen Tumorimmunologie in der Gynäkologie (in German). In: Koldovski U, Kreienberg R (eds) Aktuelle Onkologie, band 79, vol 61. Munich, p 236
- 49.Lehner B, Schlag P, Liebrich W, Schirrmacher V. Postoperative active specific immunization in curatively resected colorectal cancer patients with virus-modified autologous tumor cell vaccine. Cancer Immunol Immunther. 1990;32:173. doi: 10.1007/BF01771453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manasterski M, Liebrich W, Möller P, Schirrmacher V, Schlag P. Active specific immunotherapy in colorectal cancer and melanoma. In: Klapdor R, editor. Recent results in tumor diagnosis and therapy. München: ZuckschwerdtVerlag; 1990. pp. 499–504. [Google Scholar]
- 51.Lehner B, Liebrich W, Mechtersheimer G, Schirrmacher V, Schlag P, Herfarth C (1989) Charakterisierung und erste Ergebnisse einer aktiven spezifischen Immuntherapie bei Patienten mit colorectalem Carcinom (in German). Langenbeck’s Arch Chir [Suppl Chir Forum], S513–S517
- 52.Schlag P, Manasterski M, Gerneth T, Hohenberger P, Dueck M, Herfarth C, Liebrich W, Schirrmacher V. Active specific immunotherapy with NDV modified autologous tumor cells following liver metastases resection in colorectal cancer: First evaluation of clinical response of a Phase II trial. Cancer Immunol Immunother. 1992;35:325. doi: 10.1007/BF01741145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pomer S, Thiele R, Staehler G, Löhrke H, Schirrmacher V. Tumor vaccination in renal cell carcinoma with and without interleukin-2 (IL-2) as adjuvant. A clinical contribution to the development of effective active specific immunization. Urologe-A. 1995;34:215. [PubMed] [Google Scholar]
- 54.Pomer S, Schirrmacher V, Thiele R, Löhrke H, Staehler G. Tumor response and 4 year survival data of patients with advanced renal cell carcinoma treated with autologous tumor vaccine and subcutaneous r-IL-2 and IFN-Alpha 2b. Int J Oncol. 1995;6:947. doi: 10.3892/ijo.6.5.947. [DOI] [PubMed] [Google Scholar]
- 55.Ockert D, Schirrmacher V, Beck N, Stoelben E, Ahlert T, Flechtenmacher J, Hagmuller E, Bucheik R, Nagel M, Saeger HD. Newcastle disease virus infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin Cancer Res. 1996;2:21. [PubMed] [Google Scholar]
- 56.Möbus V, Horn S, Stock M, Schirrmacher V. Tumor cell vaccination for gynecological tumors. Hybridoma. 1993;12:543. doi: 10.1089/hyb.1993.12.543. [DOI] [PubMed] [Google Scholar]
- 57.McCune CS, O’Donnell RW, Marquis DM, Saharrabudhe DM. Renal cell carcinoma treated by vaccines for active specific immunotherapy: correlation of survival with skin testing by autologous tumor cells. Cancer Immunol Immunother. 1990;32:62. doi: 10.1007/BF01741726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, Hanna MG, Jr, Pinedo HM. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 59.Abel U. Grundlagen der Biometrie. In: Beuth J, editor. Grundlagen der Komplementäronkologie. Stuttgart: Hippokrates Verlag; 2002. p. 51. [Google Scholar]
- 60.Bohle W, Schlag P, Liebrich W, Hohenberger P, Manasterski M, Möller P, Schirrmacher V. Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumor cell vaccine: first clinical results with tumor cell vaccines modified with live but avirulent Newcastle disease virus. Cancer. 1990;66:1517. doi: 10.1002/1097-0142(19901001)66:7<1517::aid-cncr2820660714>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 61.Liebrich W, Schlag P, Manasterski M, Lehner B, Stöhr M, Möller P, Schirrmacher V. In vitro and clinical characterization of a Newcastle disease virus-modified autologous tumor cell vaccine for treatment of colorectal cancer patients. Eur J Cancer. 1991;27:703. doi: 10.1016/0277-5379(91)90170-I. [DOI] [PubMed] [Google Scholar]
- 62.Steiner HH, Bonsanto MM, Beckhove P, Brysch M, Schuele-Freyer R, Geletneky K, Kremer P, Golamrheza R, Bauer H, Kunze S, Schirrmacher V, Herold-Mende C. Anti-tumor vaccination of patients with glioblastoma multiforme in a case-control study: feasibility, safety and clinical benefit. J Clin Oncol. 2004:4272. doi: 10.1200/JCO.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 63.Vermorken JB, Claessen AM, van Tinteren H, et al. Active specific immunotherapy for stage I and stage II human colon cancer: a randomized trial. Lancet. 1999;353:345. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 64.Liang W, Wang H, Sun TM, Yao WQ, Chen LL, Jin Y, Li CL, Meng FJ. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J Gastroenterol. 2003;9(3):495. doi: 10.3748/wjg.v9.i3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jocham D, Richter A, Hoffmann L, Iwig K, Fahlenkamp D, Zakrzewski G, Schmitt E, Dannenberg T, Lehmacher W, von Wietersheim J, Doehn C. Adjuvant autologous renal tumor cell vaccine and risk of tumor progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004;363:594. doi: 10.1016/S0140-6736(04)15590-6. [DOI] [PubMed] [Google Scholar]
- 66.Ahlert T, Striffler H, Bastert G, Kaufmann M, Schirrmacher V (1989) Aktueller Stand gynäkologischer Studien zur aktiv-spezifischen Immuntherapie mit virusmodifizierten autologen Tumorzellen (in German). In: Melchert, Neuses, Wischink (eds) Aktuelle Onkologie, 60 Klinische Tumorimmunologie in der Gynäkologie, 8 Arbeitsgespräch Mannheim, 20–21 October 1989. S196–S204