Abstract
Human endogenous retroviruses (HERVs) are repetitive, noninfectious chromosomal elements degenerated from exogenous retroviruses. The HERV-H family is composed of approximately 1,000 elements which are dispersed throughout the human genome. We have shown previously that an HERV-H element splices into a downstream locus, termed PLA2L, which has a large open reading frame (ORF) containing two domains with phospholipase A2 homology. Over half of the putative 5′ untranslated region (5′ UTR) of the resulting fusion transcript is derived from HERV-H long-terminal-repeat and internal sequences. As 5′ UTRs are known to modulate translation initiation, we tested for possible effects upon gene expression at the translation level due to the 5′ fusion with HERV-H sequences. No PLA2L protein was detected in teratocarcinoma cell lines in which PLA2L mRNA is abundantly expressed. In addition, despite a high level of transcription, no protein synthesis was detected when the full-length PLA2L cDNA was expressed in COS cells. Upon removal of the 5′-terminal HERV-H sequences, PLA2L protein was seen in transfectants. The 5′ UTR contains both small ORFs and a strong predicted RNA secondary structure, both of which have been shown to contribute to translation suppression. The HERV-H sequences, combined with a unique PLA2L 5′ UTR sequence, form a predicted RNA stem-loop that has a stability greater than that proposed to negatively affect translation. Interestingly, this stem-loop is abolished when the HERV-H sequences are removed. We hypothesize that the PLA2L 5′ HERV-H sequences function as an abnormally long and complex 5′ UTR, resulting in suppression of translation in both teratocarcinoma cell lines and full-length cDNA transfectants. This is the first known example of a endogenous retrovirus integration affecting expression of a heterologous human gene at the translational level.
Human endogenous retroviruses (HERVs) have been estimated to compose approximately 2% of the human genome by mass (23) and are structurally similar to, and presumably degenerated from, exogenous retroviruses. While the retroviral structural genes of most HERVs have largely become nonfunctional through mutation, the long terminal repeats (LTRs) of many HERV elements are active and can promote, enhance, splice into, and polyadenylate adjacent cellular genes (28). The HERV-H family of elements is most homologous to murine C-type retroviruses such as murine leukemia virus, and members are found on all chromosomes, with copy numbers of approximately 1,000 and a similar number of solitary LTRs per haploid human genome (17).
HERV elements can potentially affect cellular genes by promoting deletions or translocations due to interelement recombination or by retrotransposition. Depending upon the location, HERV insertions have the potential to cause alterations in gene expression such as those causing alternate tissue specificity, inappropriate promoter activity, premature truncation of a reading frame via introduction of a frameshift or alternate polyadenylation (4, 6, 15, 22, 24, 28). By far the most common effects exerted by HERVs upon cellular genes are at the transcriptional level, not the translational level. Because the insertion of an HERV into a transcriptional unit is usually a very disruptive event, the damage is often realized at the immediate level of transcription, whereas to effect a translational alteration, the damage must be much more subtle and allow transcription to occur.
In order to elucidate the effects that HERV-H elements may exert upon proximal genes, we have studied a transcript, isolated from a human teratocarcinoma cell line, that reflects fusion between an HERV-H element and a novel human locus containing two domains with similarity to the sequence of secreted phospholipase A2. The HERV-H LTR promotes this fusion transcript, termed the PLA2L transcript (phospholipase A2-like transcript), which contains a short segment of the LTR and leader region of HERV-H sequence spliced to downstream exons. As this transcript possesses HERV- derived sequences at its 5′ terminus, and as 5′ untranslated regions (5′ UTRs) of vertebrate genes are known to regulate the initiation of translation (21), the regulation of protein synthesis of PLA2L was studied. Here we report that HERV-H sequences, acting as a 5′ UTR, serve to suppress translation of the PLA2L transcript in both the original teratocarcinoma cell line and in a heterologous expression system.
PLA2L is a fusion between HERV-H sequences and a novel human locus whose sequence is similar to that of cellular phospholipase A2.
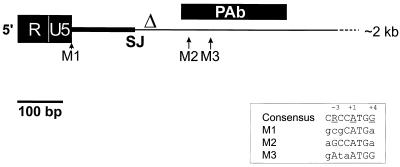
The PLA2L transcript was originally isolated from an NTera2D1 human teratocarcinoma cell line cDNA library by a differential hybridization screening procedure. This screen was designed to detect transcripts that initiated in an HERV-H LTR and subsequently spliced into downstream cellular genes (5). The PLA2L cDNA isolated from this screen is 2.4 kb in length, has 251 bp of HERV-H-derived sequence at the 5′ end, and encodes a 584-amino-acid product of an open reading frame (ORF). The ORF is composed primarily of anonymous sequence, relieved only by two distinct domains with sequences similar to that of the secreted form of phospholipase A2. Consistent with an LTR-promoted transcript, the cDNA possesses R and U5 regions of an LTR at the 5′ terminus, followed by the HERV-H leader region, which ends in the conserved splice donor site (Fig. 1). In the 5′-terminal 500 bp of the transcript, there are three potential initiating methionine residues within the same reading frame which possess Kozak consensus sequences of varied quality. The context of the first two AUG codons (nucleotides [nt] 101 and 416, respectively) is suboptimal, especially with regard to the crucial −3 position, which is a purine in almost all true initiating codons (12). The last AUG codon (nt 455) matches the Kozak consensus to a much greater extent, including both the −3 A and the +4 G, and we consider it to be the most likely initiation codon.
FIG. 1.
Schematic of the 5′ region of the PLA2L fusion transcript. HERV-H-encoded sequences, which end at the splice junction site (SJ; nt 251) are shown as a thick black line. The HincII site used to delete the 5′ HERV-H sequences to create pPLA2L- del is indicated with a Δ. Possible initiating methionine residues are indicated as M1, M2, and M3. The region of the PLA2L cDNA which was expressed as a GST fusion and used to generate rabbit polyclonal antibody (PAb) is shown as a black rectangle. The Kozak consensus sequence aligned with the three potential start codons is shown within a box. The complete map and sequence of this cDNA have been published previously (5).
Endogenous expression of PLA2L in teratocarcinoma cells.
We showed previously by Northern blot analysis that HERV-H-promoted PLA2L transcripts are present in NTera2D1, the cell line from which the original cDNA was isolated, and in an independent teratocarcinoma cell line, Tera1 (5). The level of PLA2L mRNA is at least 10-fold higher in Tera1 than in NTera2D1. In Tera1 and NTera2D1, we have no evidence of PLA2L being transcribed by a promoter other than that of the HERV-H LTR. Transcription of PLA2L was not detected in other cell lines by Northern blot analysis or by reverse transcription-PCR. Interestingly, these results mimic what has been observed for the population of HERV-H elements in general. HERV-H elements with an LTR structure like that of the PLA2L element are highly transcribed in teratocarcinoma cell lines, and of the cell lines tested, Tera1 has the highest level of HERV-H mRNA (27).
To determine the regulatory role that the HERV-H element may play at the PLA2L locus, polyclonal antiserum against PLA2L was raised. A PLA2L–glutathione S-transferase (GST) fusion protein was generated by amplifying bases 391 to 584 of the original PLA2L AF-5 cDNA by PCR. This PCR product was cloned into the SmaI site of pGEX2T (Pharmacia), in frame to GST. Log-phase bacteria containing this construct were induced for 5 h at 37°C with 70 μM fresh isopropyl-β-d-thiogalactopyranoside (IPTG; Fisher Biotech) and subsequently lysed by sonication. Affinity chromatography with glutathione-agarose beads (Sigma) was performed upon the cleared lysate. Agarose beads containing purified PLA2L- GST fusion protein were repeatedly washed and were used, in conjunction with Freund’s incomplete adjuvant, to directly immunize a New Zealand White rabbit (20). Clarified rabbit anti-PLA2L antiserum was used in all subsequent Western blotting at a dilution of approximately 1:750. Prior to the primary boost of PLA2L- GST protein, preimmune sera was taken and was determined to be negative for anti-PLA2L reactivity. Mammalian cell lysates and Western blotting were performed as previously described (16).
Translation of the PLA2L mRNA was initially examined by attempting to detect PLA2L protein in human teratocarcinoma cell lines. Both NTera2D1 and Tera1 were assayed for the presence of PLA2L protein synthesis. However, despite the high level of PLA2L RNA (5), no evidence of specific immunoreactive PLA2L protein was seen on Western blots of lysates of Tera1 (Fig. 2) and NTera2D1 (data not shown) or in immunoprecipitations of Tera1 lysates with rabbit polyclonal antiserum (data not shown). Thus, it appears that PLA2L is either not translated or translated at a much lower level than the abundance of mRNA suggests.
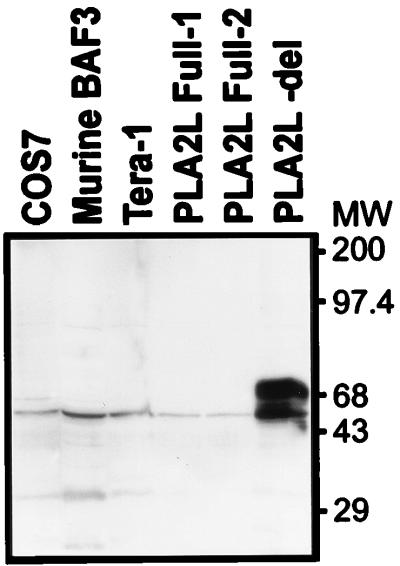
FIG. 2.
Anti-PLA2L Western blot of lysates of various cell lines and transfectants. The Western blot was blocked overnight at 4°C in 2% BLOTTO (5% skim milk powder in phosphate-buffered saline), washed three times for 1 h each time in TBST (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% Tween 20 [Sigma]), and hybridized to rabbit antiserum in 2% BLOTTO for 1 h. All washing and hybridization steps, unless noted otherwise in the text, were carried out at room temperature with constant agitation. The Western blot was then washed four times for 30 min each time in TBST. The secondary antibody consisted of horseradish peroxidase-conjugated AffiniPure goat anti-rabbit immunoglobulin G (Jackson Immunoresearch Laboratories), which was used at an approximately 1:8,000 dilution in TBST for a 50-min incubation. The Western blot was then washed four times for 30 min each time in TBST and visualized with a Renaissance enhanced-chemiluminescence kit (Dupont, NEN). Molecular weights (MW) are noted in thousands.
HERV-H sequences affect translation of the PLA2L mRNA.
The lack of detectable PLA2L protein in teratocarcinoma cells which express the PLA2L mRNA raises the possibility that the presence of HERV-H sequences in the 5′ UTR might inhibit translation. To test this possibility, translation of different PLA2L cDNA constructs was examined after transfection into COS cells. The mammalian expression vector pCDNA3 (Invitrogen) containing the cytomegalovirus early promoter-enhancer was used as a backbone for two PLA2L constructs. To construct pPLA2L- Full, the complete 2.4-kb AF-5 cDNA containing HERV-H sequences at the 5′ end (Fig. 1) was cloned into the EcoRI site of pCDNA3. Since our laboratory has previously noticed occasional recombination and instability of HERV-H sequences within the DH5α Escherichia coli strain, this ligation was transformed into XL2-Blue (Clontech) and STBL2 (Life Technologies), two independent E. coli strains known to suppress some recombinations. The constructs derived from XL2-Blue and STBL2 bacteria were termed pPLA2L- Full1 and -Full2, respectively. A 5′-deletion construct, lacking all HERV-H-derived sequences and termed pPLA2L- del, was generated by inserting the 2,166-bp HincII fragment of the PLA2L cDNA in pBluescript into the EcoRV site of pCDNA3. The vector-insert junctions of all constructs were subsequently sequenced to confirm correct orientation relative to that of the cytomegalovirus promoter. These constructs were transiently transfected into COS cells with DEAE-dextran (7). Transfected cells were grown for 48 h and then lysed in Nonidet P-40 lysis buffer as previously described (16). Following centrifugation, the concentration of the supernatant was determined by the Bradford assay (Bio-Rad) and approximately 10 μg of COS transfectant lysates and 20 μg of Tera1 and BaF3 lysates (an irrelevant murine cell line) were electrophoresed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, electroblotted onto a polyvinylidene difluoride membrane, and subjected to Western blotting with anti-PLA2L antiserum. Figure 2 shows immunoreactive products between 65 and 85 kDa in the pPLA2L- del transfectant and the lack of specific immunoreactive bands in both the pPLA2L- Full transfectants and the Tera1 human teratocarcinoma cell line lysates. The faint 43-kDa band seen in the COS7, BAF3, and Tera1 cell lysates is nonspecific and was seen in all lysates tested, including ones negative for PLA2L transcription as assayed by reverse transcription-PCR. In addition, no specific immunoreactivity was detected in a negative control murine hemopoietic cell line, BaF3 (a gift of G. Krystal), or in the transfectant host cell line, COS. The presence of two to three immunoreactive bands in the pPLA2L- del transfectant likely signifies either the use of alternative AUGs to initiate translation or differential glycosylation or modification of the PLA2L protein by COS cell systems. Proteolytic degradation does not seem to be the cause, as similar patterns of bands are seen in multiple, independent transfections (data not shown).
HERV-H sequences suppress PLA2L translation, not transcription.
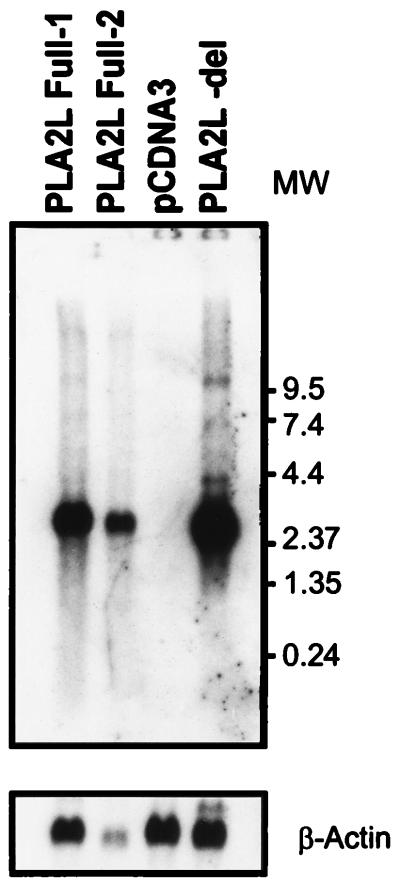
To ensure that the observed inhibition of PLA2L protein synthesis was due to HERV-H sequences inhibiting translation and not transcription, total RNA was prepared from all transfectants with Trizol (Life Technologies). RNA formaldehyde gel electrophoresis and Northern blotting were carried out as previously described (14) with a 410-bp BbsI fragment of the PLA2L cDNA containing the first phospholipase A2-like domain (5) (Fig. 3). Intact, full-length PLA2L message was seen only in the PLA2L transfectant RNAs and not in the vector control. Figure 3 shows that there is a slight increase (approximately twofold) in the level of the pPLA2L- del mRNA relative to the level of pPLA2L- Full1 mRNA. This implies either that the deletion of the HERV-H 5′ UTR modestly increases the heterologous transcription of PLA2L or that transfection of the pPLA2L- del was slightly more efficient. However, this modest increase cannot account for the absence of detectable PLA2L protein in the pPLA2L- Full constructs. It is more likely that the deletion of HERV-H sequences in pPLA2L- del enables efficient translation of PLA2L protein.
FIG. 3.
Northern blot of total RNAs from COS cells transfected with full-length PLA2L cDNA (PLA2L- Full1 and PLA2L- Full2, respectively), a vector-only control (pCDNA3), and a deletion construct lacking all HERV-H sequences (PLA2L- del). The lower panel shows the blot rehybridized to a β-actin probe. A 410-bp phospholipase A2 domain probe was labeled with [32P]dCTP (Amersham) and hybridized to the Northern blot at approximately 3 × 106 dpm/ml for 16 h at 42°C. This Northern blot was subsequently washed twice for 20 min each time at 55°C in 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])–0.3% SDS, twice for 20 min each time at 55°C in 1× SSPE–0.5% SDS, and twice for 20 min each time at 60°C in 0.3× SSPE–1% SDS and then exposed to Kodak X-Omat AR film for 48 h at −70°C. A chicken β-actin cDNA probe to control for RNA loading was labeled, hybridized, and washed as described above but was exposed to X-ray film for only 36 h. Molecular weights (MW) are noted in thousands.
The results described here led us to hypothesize that the 5′ fusion of HERV-H sequences to the cellular PLA2L transcript functions as an aberrantly long and complex 5′ UTR, explaining the concomitant inhibition of PLA2L protein synthesis. 5′ UTRs are known to be the primary modulators of translation efficiency because they control the initiation of the 43S preinitiation complex, which contains the scanning 40S ribosome, on the 5′ end of the mRNA. The 40S ribosome scans the 5′ UTR until an AUG codon in the correct context (13) is found, when the 60S subunit binds the 40S subunit and translation is initiated. Although the method by which the 40S ribosome scans the 5′ UTR is unknown, certain structures within the 5′ UTR can greatly repress or inhibit translation (9). 5′ UTRs which suppress translation generally possess some or all of the following features: stable RNA secondary structures such as stem- loops, length greater than the average of 100 to 140 bp, high GC content, and AUG codons with small or micro-ORFs (μORFs) upstream of the correct start codon, especially if the μORF lacks a termination codon (12).
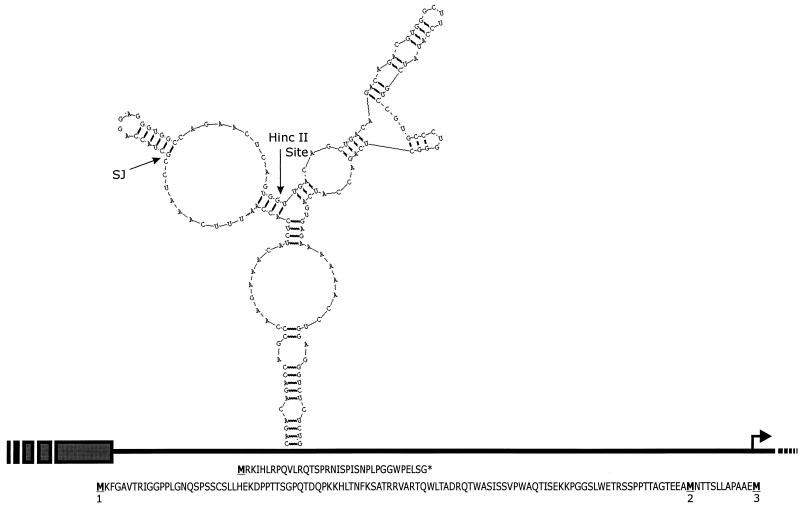
If we assume that the AUG codon with the best Kozak consensus is the true initiation codon, the PLA2L fusion transcript possesses a 454-bp 5′ UTR, with 252 bases being HERV-H derived. This 5′ UTR contains three μORFs, two of which are in the same reading frame (+3) as the correct AUG codon and lack a stop codon. The third μORF exists in the +1 frame and contains a stop codon (Fig. 4). μORFs are hypothesized to inhibit translation by causing stalling of the scanning ribosome, while lack of a subsequent stop codon causes inefficient reinitiation of the 43S ribosome downstream at the correct AUG codon (13).
FIG. 4.
Various structural elements within the PLA2L 5′ UTR. The 5′ region of PLA2L cDNA is shown as a black line, HERV-H LTR sequences are shown as gray boxes, and the junction between HERV-H and cellular sequences is denoted SJ on the stem-loop. Upstream μORFs are shown below the line. Potential initiating methionines are underlined and numbered according to Fig. 1. The putative start of translation is shown by a bent arrow. The strongest and most stable predicted RNA stem-loop is shown above the line, from nt 209 to 369, with a ΔG of −52.1 kcal/mol. The HincII site used to construct pPLA2L- del is shown on the RNA stem-loop.
Additional potential encumbrances to translation which the PLA2L 5′ UTR possesses are predicted secondary RNA structures such as stem-loops. These structural elements may be the most potent translational inhibitory element found in 5′ UTRs (8, 29). 5′ UTRs containing secondary structures with a free energy of greater than −30 kcal/mol are known to obstruct the scanning of an mRNA by the 43S preinitiation ribosome (11). While prediction methods differ, the energy minimization method of Zuker (30) used by the RNAStructure program (19) predicts a strong stem-loop with a free energy of −52.1 kcal/mol, in the PLA2L 5′ UTR, between nt 209 and 369. The probability that this predicted stem-loop functions in the observed translational suppression of PLA2L is supported by the observation that the HincII site used to delete HERV-H sequences and construct pPLA2L- del occurs in the center of this stem-loop. Removal of sequences 5′ to the site would destroy the predicted stem-loop (Fig. 4). The GC nucleotide content of the PLA2L 5′ UTR does not appear to differ from the average.
Although deletion of the HERV-H-encoded 5′-terminal 251 bases releases the PLA2L transcript from translational suppression, allowing efficient heterologous protein synthesis (Fig. 2), unique 5′ UTR sequences proximal to the junction with HERV-H also play a crucial role in translational control. Insertion of the 251-bp HERV-H fragment into the 5′ UTR of an unrelated reporter gene (the human cell surface molecule Thy-1/CD90 [3]) within the same vector resulted in no change in protein expression, relative to that of controls (data not shown). This indicates that the HERV-H fragment does not adversely affect the translation of all genes. These results suggest that both the juxtaposition of proximal sequences unique to the PLA2L locus and HERV-H sequences are necessary for the inhibition of PLA2L protein synthesis. This phenomenon is predicted by the RNA stem-loop seen in Fig. 4, which is composed of both HERV-H and unique PLA2L sequences.
Transcriptional effects of endogenous retroviruses on cellular genes are common, with numerous examples being reported for mice and some for humans (1, 26, 28). At the PLA2L locus, we demonstrated previously that the HERV-H element appears to have assumed transcriptional control of the region in teratocarcinoma cells where HERV-H LTRs are highly active (5). In addition, we have recent evidence suggesting that the PLA2L transcripts produced in these cells are actually fusions of HERV-H with two unrelated downstream genes (unpublished data). Translational effects of retroviruses on cellular genes are much less common, but a few cases have been reported. In a murine lymphoma line, it has been found that an exogenous Moloney murine leukemia retrovirus inserts into the 5′ UTR of the lck proto-oncogene, leading to downregulation of translation (18). Similar to our PLA2L results, the suppression is removed upon deletion of the retroviral sequences from the 5′ UTR. In contrast to what is known about suppression, two examples of translational activation due to 5′ UTR insertion of exogenous retroviruses are known. In the first, the only other known example for a human cell line, interleukin 15 protein synthesis is increased in a T-cell leukemia line due to human T-cell leukemia virus type 1 integration in the 5′ UTR of the interleukin 15 gene (2). A similar event in a murine leukemia line, due to a 5′ UTR murine leukemia virus insertion, results in upregulated translation of the c-akt proto-oncogene (25).
In this study we have shown that HERV-H sequences suppress translation at the PLA2L locus. To our knowledge, this appears to be the first description of a retroviral insertion (endogenous or exogenous) affecting the translation of a human transcript. Interestingly, the transcriptional and translational effects mediated by the HERV-H element are presumably not detrimental to the species, since this particular HERV-H insertion has been fixed in the primate germ line for 15 to 20 million years (10). The PLA2L fusion transcript studied here has been detected only in teratocarcinoma cells where the LTR promoter is most active (5). The HERV-H element appears to be within an intron, so it is possible that a native promoter located 5′ to the retroviral element is active in other cell types, resulting in removal of the entire HERV-H element by splicing. Unfortunately, we do not know the function of the gene into which the HERV-H element has inserted, since it has no strong similarity to known genes and no part of the PLA2L transcript is yet represented in the human Expressed Sequence Tag database (as of March 1998). However, while the functional significance of the finding reported here remains unknown at this time, it illustrates a novel way in which retroviral insertions can affect gene expression.
Acknowledgments
We thank Robert Kay, Gerald Krystal, and Mark Ware (Terry Fox Laboratory) for kind assistance with protein and fluorescence-activated cell sorting techniques and Patrik Medstrand for critically reading the manuscript.
This work was supported by a grant from the Medical Research Council of Canada.
REFERENCES
- 1.Amariglio N, Rechavi G. Insertional mutagenesis by transposable elements in the mammalian genome. Environ Mol Mutagen. 1993;21:212–218. doi: 10.1002/em.2850210303. [DOI] [PubMed] [Google Scholar]
- 2.Bamford R N, Battiata A P, Burton J D, Sharma H, Waldmann T A. Interleukin (IL) 15/IL- T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region/IL- 15 fusion message that lacks many upstream AUGs that normally attenuate IL- 15 mRNA translation. Proc Natl Acad Sci USA. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig W, Kay R, Cutler R L, Lansdorp P M. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177:1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Cristofano A, Strazullo M, Longo L, La Mantia G. Characterization and genomic mapping of the ZNF80 locus: expression of this zinc-finger gene is driven by a solitary LTR of ERV9 endogenous retroviral family. Nucleic Acids Res. 1995;23:2823–2830. doi: 10.1093/nar/23.15.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feuchter-Murthy A E, Freeman J D, Mager D L. Splicing of a human endogenous retrovirus to a novel phospholipase A2 related gene. Nucleic Acids Res. 1993;21:135–143. doi: 10.1093/nar/21.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodchild N L, Wilkinson D A, Mager D L. A human endogenous long terminal repeat provides a polyadenylation signal to a novel, alternatively spliced transcript in normal placenta. Gene. 1992;121:287–294. doi: 10.1016/0378-1119(92)90133-a. [DOI] [PubMed] [Google Scholar]
- 7.Hammarskjold M L, Wang S C, Klein G. High-level expression of the Epstein-Barr virus EBNA1 protein in CV1 cells and human lymphoid cells using a SV40 late replacement vector. Gene. 1986;43:41–50. doi: 10.1016/0378-1119(86)90006-5. [DOI] [PubMed] [Google Scholar]
- 8.Horvath P, Suganuma A, Inaba M, Pan Y B, Gupta K C. Multiple elements in the 5′ untranslated region down-regulate c-sis messenger RNA translation. Cell Growth Differ. 1995;6:1103–1110. [PubMed] [Google Scholar]
- 9.Jansen M, de Moor C H, Sussenbach J S, van den Brande J L. Translational control of gene expression. Pediatr Res. 1995;37:681–686. doi: 10.1203/00006450-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kowalski P E, Freeman J D, Nelson D T, Mager D L. Genomic structure and evolution of a novel gene (PLA2L) with duplicated phospholipase A2-like domains. Genomics. 1997;39:38–46. doi: 10.1006/geno.1996.4471. [DOI] [PubMed] [Google Scholar]
- 11.Kozak M. Features in the 5′ non-coding sequences of rabbit alpha- and beta-globin mRNAs that affect translational efficiency. J Mol Biol. 1994;235:95–110. doi: 10.1016/s0022-2836(05)80019-1. [DOI] [PubMed] [Google Scholar]
- 12.Kozak M. Interpreting cDNA sequences: some insights from studies on translation. Mamm Genome. 1996;7:563–574. doi: 10.1007/s003359900171. [DOI] [PubMed] [Google Scholar]
- 13.Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- 14.Krosl J, Damen J E, Krystal G, Humphries R K. Erythropoietin and interleukin-3 induce distinct events in erythropoietin receptor-expressing BA/F3 cells. Blood. 1995;85:50–56. [PubMed] [Google Scholar]
- 15.Liu A Y, Abraham B A. Subtractive cloning of a hybrid human endogenous retrovirus and calbindin gene in the prostate cell line PC3. Cancer Res. 1991;51:4107–4110. [PubMed] [Google Scholar]
- 16.Liu L, Damen J E, Cutler R L, Krystal G. Multiple cytokines stimulate the binding of a common 145-kilodalton protein. Mol Cell Biol. 1994;14:6926–6935. doi: 10.1128/mcb.14.10.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mager D L, Henthorn P S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci USA. 1984;81:7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marth J D, Overell R W, Meier K E, Krebs E G, Perimutter R M. Translational activation of the lck proto-oncogene. Nature. 1988;332:171–173. doi: 10.1038/332171a0. [DOI] [PubMed] [Google Scholar]
- 19.Mathews, D. H., T. C. Andre, J. Kim, D. H. Turner, and M. Zuker. An updated recursive algorithm for RNA secondary structure: prediction with improved free energy parameters. In N. B. Leontis and J. SantaLucia (ed.), Molecular modelling of nucleic acids, in press. American Chemical Society, Washington, D.C.
- 20.Oettinger H F, Pasqualini R, Bernfield M. Recombinant peptides as immunogens: a comparison of protocols for antisera production using the pGEX system. BioTechniques. 1992;12:544–549. [PubMed] [Google Scholar]
- 21.Sachs A B, Sarnow P, Hentze M W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 22.Schulte A M, Lai S, Kurtz A, Czubayko F, Riegel A T, Wellstein A. Human trophoblast and choriocarcinoma expression of the growth factor pleiotrophin attributable to germ-line insertion of an endogenous retrovirus. Proc Natl Acad Sci USA. 1996;93:14759–14764. doi: 10.1073/pnas.93.25.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smit A F. The origin of interspersed repeats in the human genome. Curr Opin Genet Dev. 1996;6:743–748. doi: 10.1016/s0959-437x(96)80030-x. [DOI] [PubMed] [Google Scholar]
- 24.Ting C N, Rosenberg M P, Snow C M, Samuelson L C, Meisler M H. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev. 1992;6:1457–1465. doi: 10.1101/gad.6.8.1457. [DOI] [PubMed] [Google Scholar]
- 25.Wada H, Matsuo M, Uenaka A, Shimbara N, Shimizu K, Nakayama E. Rejection antigen peptides on BALB/c RL male 1 leukemia recognized by cytotoxic T lymphocytes: derivation from the normally untranslated 5′ region of the c-akt proto-oncogene activated by long terminal repeat. Cancer Res. 1995;55:4780–4783. [PubMed] [Google Scholar]
- 26.Wang X Y, Steelman L S, McCubrey J A. Abnormal activation of cytokine gene expression by intracisternal type A particle transposition: effects of mutations that result in autocrine growth stimulation and malignant transformation. Cytokines Cell Mol Ther. 1997;3:3–19. [PubMed] [Google Scholar]
- 27.Wilkinson D A, Freeman J D, Goodchild N L, Kelleher C A, Mager D L. Autonomous expression of RTVL- H endogenous retroviruslike elements in human cells. J Virol. 1990;64:2157–2167. doi: 10.1128/jvi.64.5.2157-2167.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson D A, Mager D L, Leong J C. Endogenous human retroviruses. In: Levy J A, editor. The Retroviridae. Vol. 3. New York, N.Y: Plenum Press; 1994. pp. 465–535. [Google Scholar]
- 29.Wood M W, VanDongen H M, VanDongen A M. The 5′-untranslated region of the N-methyl-d-aspartate receptor NR2A subunit controls efficiency of translation. J Biol Chem. 1996;271:8115–8120. doi: 10.1074/jbc.271.14.8115. [DOI] [PubMed] [Google Scholar]
- 30.Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]