Abstract
Tertiary lymphoid organs (TLOs), also known as tertiary or ectopic lymphoid structures or tissues, are accumulations of lymphoid cells in sites other than canonical lymphoid organs, that arise through lymphoid neogenesis during chronic inflammation in autoimmunity, microbial infection, cancer, aging, and transplantation, the focus of this review. Lymph nodes and TLOs are compared regarding their cellular composition, organization, vascular components, and migratory signal regulation. These characteristics of posttransplant TLOs (PT-TLOs) are described with individual examples in a wide range of organs including heart, kidney, trachea, lung, artery, skin, leg, hand, and face, in many species including human, mouse, rat, and monkey. The requirements for induction and maintenance of TLOs include sustained exposure to autoantigens, alloantigens, tumor antigens, ischemic reperfusion, nephrotoxic agents, and aging. Several staging schemes have been put forth regarding their function in organ rejection. PT-TLOs most often are associated with organ rejection, but in some cases contribute to tolerance. The role of PT-TLOs in cancer is considered in the case of immunosuppression. Furthermore, TLOs can be associated with development of lymphomas. Challenges for PT-TLO research are considered regarding staging, imaging, and opportunities for their therapeutic manipulation to inhibit rejection and encourage tolerance.
INTRODUCTION
Tertiary lymphoid organs (TLOs), also known as tertiary or ectopic lymphoid structures or tissues, are accumulations of lymphoid cells in sites other than canonical lymphoid organs that arise during chronic inflammation in autoimmunity, microbial infection, cancer, aging, organ damage, and transplantation through lymphoid neogenesis. In this review, we use the term, TLO, since this is the convention in transplantation, the emphasis of this article (they tend to be called tertiary lymphoid structures in the tumor literature). TLOs bear striking cellular and organizational similarities to secondary lymphoid organs (SLOs) such as lymph nodes (LNs), spleen, and Peyer’s patches. Many excellent reviews have been written about TLOs and their relationship to lymphoid organs1-7 and to transplantation.1,8 Here, we concentrate on posttransplant TLOs (PT-TLOs) in allotransplantation and consider how they are similar or distinct from TLOs in other contexts, analyze their functions in facilitating rejection and/or maintenance of transplanted organs, and probe their roles in cancer.
COMPARISON OF LNs AND TLOs
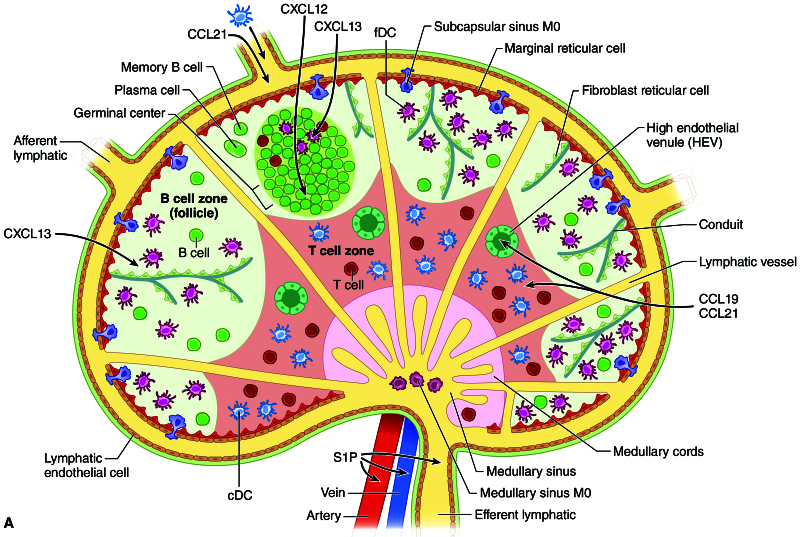
LNs (Figure 1), examples of SLOs (in contrast to primary lymphoid organs such as the thymus and bone marrow), are kidney bean shaped entities located in discrete and reproducible areas throughout the body. They are connected to each other by lymphatic vessels (LVs), are arranged like beads on a string, and consist of cells that are organized in a fashion to allow maximal interaction of antigen with naive T and B lymphocytes. They develop in locations that are predetermined before birth. Their structure is ideal for their function of maximizing interaction of antigen-inexperienced lymphocytes with cognate antigen, permitting proliferation and differentiation into effector T (T helper 1 [Th1], Th2, Th17, regulatory T cell [Treg], or B [cytokine, antigen presenting, antibody producing]) and memory cells. SLOs are relatively constant in that the structure itself remains, although it can undergo extensive remodeling during an immune response.9 Naive L-selectin+ T (C-C chemokine receptor type 7 [CCR7]+) and B (CXC motif chemokine receptor [CXCR]4+CXCR5+CCR7+) cells enter LNs via specialized blood vessels called high endothelial venules (HEVs) that express adhesion molecules such as intercellular adhesion molecule 1 and peripheral node addressin (PNAd) (recognized by MECA 79 antibody) in peripheral LNs and mucosal addressin cell adhesion molecule-1 (recognized by MECA 367 antibody) in mucosal LNs and Peyer’s patches. CCR7+ dendritic cells (DCs) carrying antigen enter through afferent LVs. T cells and DCs are directed to the paracortical region of LNs by C-C chemokine ligand (CCL) 19 and CCL21, the ligands for CCR7 that are produced by stromal cells, HEVs, and LVs; B cells are directed to the follicle by CXCL13, the ligand for CXCR5. A series of conduits (fibroblastic-lined channels) and LVs allows antigen and cells to percolate through the LN. B cells encounter soluble antigen and antigen-presenting follicular dendritic cells (FDCs) in the follicle where they can differentiate into memory cells or plasma cells through the benefit of activation-induced cytidine deaminase, which allows class switch recombination and hypermutation. Activated lymphocytes exit the LN through efferent LVs by their interaction with sphingosine 1-phosphate (S1P) expressed in that vessel. The LNs are connected one to another through their LVs, which join the thoracic duct. Entrance into the blood stream occurs through the left subclavian vein for the trunk and right subclavian vein for the head region.
FIGURE 1.
LN organization. The LN is organized into T- and B-cell zones with antigen-presenting cells, stromal cells forming conduits, high endothelial venules, and lymphatic vessels (reproduced with permission from Flajnik ME, Singh NJ, Holland SM. Paul’s Fundamental Immunology. 8th ed. Wolters Kluwer; 2022). CCL, C-C chemokine ligand; cDC, classical dendritic cell; CXCL, CXC motif chemokine ligand; fDC, follicular dendritic cell; HEV, high endothelial venule; LN, lymph node; S1P, sphingosine 1-phosphate.
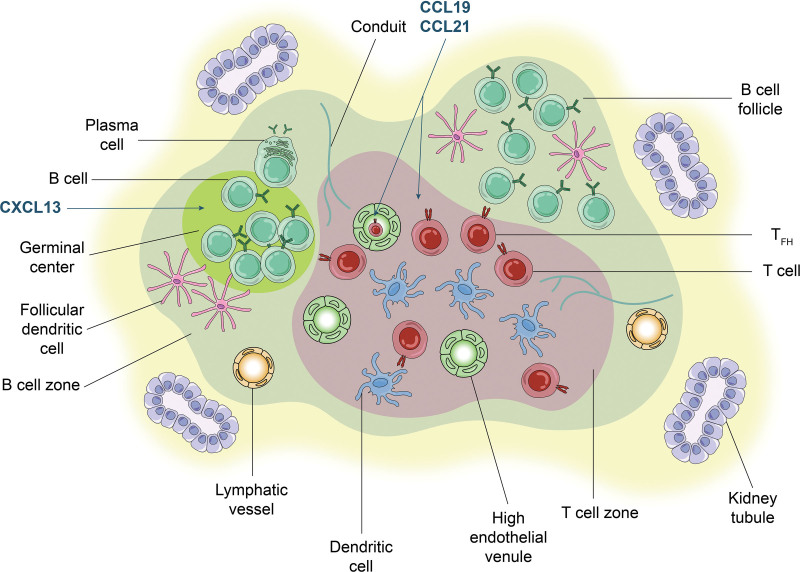
The many examples of TLOs, including PT-TLOs (Figure 2), share several SLO characteristics, though there are some differences. Similarities include cellular composition, LVs, conduits, organization into T- and B-cell compartments, dictated by lymphoid chemokines, CCL19, CCL21, and CXCL13, and germinal centers (GCs) with expression of activation-induced cytidine deaminase.7 In the case of PT-TLOs, allopeptides are presented to naive T cells by antigen presenting cells (APCs), including those of the host.10 B cells recognize alloantigens and can differentiate into plasma cells producing alloantibody.11 HEVs can be critical components of TLOs in that they allow the entrance of naive cells that can be activated by APCs, and some authors even use the presence of HEVs as a criterion for TLO identification (eg, rejecting murine cardiac allografts).12
FIGURE 2.
TLO organization. A diagram of an organized accumulation of lymphoid cells in the PT-TLO of a transplanted kidney. Note extensive similarities in cellular content and organization to that of a lymph node. This is a diagram of components that have been described in PT-TLOs. It is not meant to indicate that all features depicted are present in every PT-TLO. T cells can include effector T cells, resident memory, and regulatory T cells depending on the context and Tfh depending on the location. CCL, C-C chemokine ligand; CXCL, CXC motif chemokine ligand; PT-TLO, posttransplant tertiary lymphoid organ; TFH, T follicular helper cell; TLO, tertiary lymphoid organ.
Even the signals for cellular migration out of LNs, that is, the role of S1P, have been noted in TLOs in the nonobese diabetic (NOD) mouse model of type I diabetes, in which treatment with FTY720 (fingolimod), an S1P inhibitor prevents egress from the pancreatic TLOs with stabilization of the lymphoid accumulations of lymphoid accumulations and diabetes prevention.13 FTY720 treatment of aged mice undergoing ischemic reperfusion injury also results in an increased size of renal TLOs, although an effect on kidney function was only seen if treatment was begun before their appearance.14 It remains to be seen if PT-TLOs will also be sensitive to S1P inhibition. TLOs are considerably more plastic than SLOs in that they resolve if the impetus for their development is removed (see below). Such is the case in PT-TLOs of the transplanted heart once the rejection episode has been resolved,15 in type 1 diabetes wherein the TLO resolves once the beta cells are destroyed,13 and in TLOs in kidneys of aged mice after treatment with dexamethasone.16
An important difference between SLOs and TLOs is the absence of a capsule in the latter. Thus, TLOs lack the organizational structure of an LN with its subcapsular APCs. The absence of a capsule results in an intimate interaction with the microenvironment in which they are located—the transplanted organ, tumor, or organ undergoing an autoimmune response. How do individual TLOs in a particular rejecting organ interact with each other? Do cells circulate between one TLO and another in an individual organ in the manner that cells traffic from one LN to another via LVs, eventually rejoining the blood circulation, or are they confined to one area? How do they interact with nearby LNs? Is there movement from LN to TLO and back? In general, it is assumed that there is interaction between draining LNs and the organ in which a TLO resides. For example, in the case of type 1 diabetes, antigens are initially presented by migrating DCs from the islet to T cells in pancreatic LNs.17 Once those T cells migrate back to the islet and set up conditions for a TLO with the development of HEVs that allow access of naive lymphocytes, is there migration of cells back to the pancreatic LN? The fact that unique T-cell receptor and B-cell receptor usage is apparent in TLOs compared with the peripheral circulation would suggest such interaction is minimal and may be limited to the draining LN. On the other hand, continual seeding of tumors (and likely their TLOs) is apparent from the observations of “stem like” precursor CD8+ T cells in tumor (but not nondraining) LNs18 and in tumor TLO HEVs.19 These studies support the notion of transit between draining LNs and TLOs.
PARTICULAR CHARACTERISTICS OF POSTTRANSPLANT TLOs
Solid organ and skin transplantation have a long experimental and clinical history. Early forays into clinical organ replacement were plagued by immediate rejection, mediated by antibodies due to prior sensitization or by acute rejection mediated by CD8 T cells. With the advent of more precise tissue matching and immunosuppressant agents, greater success in solid organ transplantation has been realized. Even so, rejection can occur. When this does happen, TLOs can arise.
Rejecting heart and kidney grafts with PNAd+ vessels (HEVs) were the earliest descriptions of posttransplant infiltrates that had characteristics of TLOs.15,20 These vessels were associated with acute rejection; the cellular infiltrates were not further analyzed. Fully developed PT-TLOs were described by Baddoura et al21 in acute, chronic, and mixed acute and chronically rejecting murine cardiac allografts. Although TLOs were seen in all instances of rejection, they were most frequently seen in chronic rejection and the authors conclude that TLOs correlate with that process. These cellular accumulations had lymphoid follicles, T- and B-cell compartmentalization, GCs, and plump PNAd+ HEVs. TLOs, although not invariably seen, have been described in many different transplanted organs. As noted above, PT-TLOs are most often associated with chronic rejection, but in some instances, HEVs have also been noted in acute rejection,15,20,21 although in the case of rejecting heart transplants they correlate with the severity of rejection.15 Just a few examples of PT-TLOs, in a variety of organs from multiple species are provided in Table 1 and include kidney, heart, lung, skin, aorta, and vascularized composite allotransplantation of hand and face.
TABLE 1.
TLOs in a variety of organs posttransplant and can be associated with acute and chronic rejection
| Organ | Species | TLO characteristics | Notable features | Time posttransplant | Acute or chronic rejection | References |
|---|---|---|---|---|---|---|
| Heart | Human | HEVs | Early example of HEVs; other characteristics not noted | N.I. | Chronic | Toppila et al15 |
| Human | T/B compart, FDCs, HEVs, GCs, CXCL13, CXCR5 | Early examples of human PT-TLO | N.I. | Chronic | Thaunat et al11 and Di Carlo et al22 | |
| Rat | T/B compart, HEVs | Early example of murine PT-TLO | Median: 95 d | Some acute, but mostly correlated with chronic | Baddoura et al21 | |
| Mouse | T/B compart, FDCs, HEVs, LVs | Blocking LTβR blocks TLO | Identified in 3 of 5 at day 20 and all at days 40 and 100 | Chronic | Motallebzadeh et al12 | |
| Kidney | Human | HEVs | Early example of HEVs; other characteristics not noted | N.I. | Acute | Kirveskari et al20 |
| Human | T/B compart, CCR7+ T cells, DCs, GCs, CCL21, LVs | First description of LVs in PT-TLO | Biopsies with infiltrates correlate with rejection | Mainly chronic | Kerjaschki et al23 | |
| Human | T/B compart, FDCs, HEVs, GCs | Early example of human PT-TLO | N.I. | Chronic | Thaunat et al11 | |
| Mouse | Nodular infiltrates, tissue resident memory cells | TLO? | 4 wk | Chronic | Abou-Daya et al24 | |
| Mouse | CD4, CD8, Treg, DC, macrophages, HEV | Graft acceptance | 13.5 wk | Chronic | Brown et al10 | |
| DBA/2 to C57BL/6 | ||||||
| Mouse | Progression CD8, B, CD4, CD4, Treg, no HEVs | Graft acceptance | 1–60 wk | Chronic | Rosales et al25 | |
| DBA/2 to C57BL/6 | ||||||
| TLO designation suggested | ||||||
| Trachea | Rat | T/B compart, HEVs | 56 d | Chronic | Sato et al26 | |
| Lung | Human | T/B not compart, HEVs, no FDCs | Obliterative bronchiolitis | 52.9 ± 10.4 mo | Chronic | Sato et al26 |
| Artery | Rat | T/B compart, HEVS | Antibody | HEVs apparent at 10 d; later resolved | Acute | Thaunat et al11 |
| Mouse | GCs and multiple B-cell types | Transcriptomic analysis of inflammation progress | 4 wk | Chronic | Cai et al27 | |
| Skin | Mouse | T/B compart, HEVs | Generation of effector and memory cells | N.A. transgenic model | Nasr et al28 | |
| Leg | Rat | Lymphocytes HEVs | Correlation with late rejection | Acute chronic | Hautz et al29 | |
| Hand | Human | T, B, HEVs | Correlation between PNAd staining and late rejection | All time points | Acute chronic | Hautz et al29 |
| Face | Monkey | Lymphocytes HEVs | 15, 36, and 332 d | Acute chronic | Hautz et al29 |
CCL, C-C chemokine ligand; CCR7, C-C chemokine receptor type 7; CD, cluster of differentiation; CXCL13, CXC motif chemokine ligand 13; CXCR5, CXC motif chemokine receptor 5; DC, dendritic cell; FDC, follicular dendritic cell; GC, germinal center; HEV, high endothelial venule; LTβR, lymphotoxin beta receptor; LV, lymphatic vessel; N.A., not applicable; N.I., not indicated; PNAd, peripheral node addressin; PT-TLO, posttransplant tertiary lymphoid organ; T/B compart, T/B compartmentalization; TLO, tertiary lymphoid organ; Treg, regulatory T cell.
LVs have been noted in many TLOs.3 They were first described in CCR7+ lymphocytic infiltrates in chronically rejecting human kidneys.23 Lymphangiogenesis can occur as early as 10 d posttransplant and is not necessarily correlated with TLOs.30 T follicular helper cell (Tfh) cells have been noted in acutely rejecting human kidneys31 and Tfh, tissue resident memory cells, and plasma cells have been noted in the infiltrates of chronically rejecting mouse kidney grafts.24 The process of effector to memory cell transition occurs in rejecting kidneys themselves,24 consistent with the concept that the TLO itself acts as an immunocompetent site (see below). Host-derived DCs interacting with antigen-specific T cells invade the chronically rejecting kidney graft, setting up the process of activation of host T cells in the graft.24,32
INDUCTION AND MAINTENANCE OF TLOs
SLO development, which occurs in precise anatomical locations during ontogeny, is dictated by coordination of cytokines and chemokines, orchestrated by a group of cells that include fibroblast activation protein-α+33 stromal lymphoid tissue organizer (LTo) cells and CD4+CD3–interleukin (IL)-7 receptor+RAR-related orphan receptor gamma t+ hematopoietic lymphoid tissue inducer (LTi) cells that express the lymphotoxin αβ (LTαβ) complex and drive expression of lymphoid chemokines.7,34,35 TLOs, in contrast to SLOs, arise postnatally in the context of inflammation. The impetus for their origin, rather than the precise, highly coordinated dance of LTi and LTo cells, varies, but frequently includes the same cytokines and chemokines that drive SLO development. However, the nature of the stromal cells that act as organizers is variable, in part, depending on the tissue in which the TLO arises.36 Fibroblast activation protein positive cells are present in adult LNs33 and in salivary gland TLOs in Sjogren’s syndrome37 and it is likely that they and other stromal cells provide the function of LTo cells in PT-TLOs. Various stromal cells can substitute for LTo cells in PT-TLOs. Lymphatic endothelial cells provide CCL21 in chronically rejecting human kidney grafts23; CXCL13, CCL21, and LTβR are expressed in chronically rejecting kidney allografts.38 CXCL13 is produced by CD68+ (monocyte lineage) cells in rejecting heart transplants,22 and fibroblasts produce CXCL13 and CCL19, serving as LTo cells in aged injured kidney TLOs.39
The mechanism of TLO induction, rather than the precise antigen-independent coordination of LTo and LTi cells is usually, but not always, the response of the immune system to sustained expression of an antigen and production of the same cytokines crucial for SLO development. The early experimental examples of TLOs were transgenic mice that ectopically expressed members of the LT family40,41 or lymphoid chemokines.42 Although LTi cells have been invoked in the formation of TLOs43 and there is evidence for their continued existence44-46 and ability to induce TLOs in the adult,43 several other sources of LTαβ are available postnatally including T cells, B cells, natural killer cells,47 and DCs,48 which can substitute for classical LTi cells1,49 in PT-TLOs. LTαβ has been invoked in TLO development via signaling through the lymphotoxin beta receptor (LTβR) in TLOs in the aged aorta50 and cardiac allografts.12 In the latter case, C57BL/6 recipients of bm12 cardiac allografts were treated with LTβR-immunoglobulin, which blocks LTβR signaling. There are slightly fewer lymphoid aggregates, T/B compartmentalization is lost, and there is a significant decrease in the number of HEVs and LVs. FDC networks are reduced, as is alloantibody production and grafts are maintained significantly longer than in control-treated mice. However, in a transgenic TLO model induced by lymphotoxin alpha (LTα), signaling occurs through the tumor necrosis factor receptor 1 with no requirement for lymphotoxin beta.51 IL-17 producing cells that share some characteristics of LTi cells, including the transcription factor, RAR-related orphan receptor gamma t, have been noted in TLOs in chronically rejecting human kidney allografts52 and can substitute for LTi cells. Thus, several instances indicate that the rigid requirements of SLO development are somewhat more flexible when it comes to TLOs in general including PT-TLOs.
TLOs differ from SLOs in that, in general, continuation of the impetus for their formation is required for their maintenance. As noted above, TLOs can arise as the result of T-cell recognition of a local antigen. In the case of cancer, it is a tumor antigen; in infection, a microbe; in PT-TLO, the impetus is the expression of an alloantigen in the transplanted organ. In many cases, continued expression of an inciting antigen is necessary for induction and maintenance of a TLO. Once the induction signal is removed, the TLO dissipates as noted above.
TLOs can be associated with acute kidney injury induced by ischemic reperfusion,16 nephrotoxic agents,39 or infection such as polyomavirus-induced nephritis in mice,25 hepatitis C infection of human liver,53 or latent cytomegalovirus infection of rat heart.54 TLOs with T and B cells, HEVs, LVs, and fibroblasts expressing lymphoid chemokines CCL19, CCL21, and CXCL13 arise in the kidney following these insults, particularly in aged mice (12 mo old).39 Inflammatory infiltrates in the renal cortex with some characteristics of TLOs, termed urinary tract-associated lymphoid structures, have been described in humans and in a noninfectious mouse model of chronic nephritis.55 In this case, the authors implicate the contribution of urine to compromising urothelial barrier integrity and induction of lymphoid chemokines as contributing factors to these lymphoid accumulations. It is likely that these TLOs are associated with reactivity to an insult-exposed self-antigen or with molecular mimicry between the insult and a self-antigen.56,57
Aging is associated with “spontaneous” development of TLOs. Lymphoid accumulations with all the characteristics of TLOs (T-, B-cell compartmentalization, HEVs, LVs) are found in the kidneys,39 salivary glands,58 and liver59 of “super aged” mice (>23 mo old) without an overt stimulus. Fat-associated lymphoid clusters, a type of TLO that is apparent in young mice, becomes more prominent with age with an increase in aged adipose B cells and Nlrp3 inflammasome activation.60 The stimuli that induce the various TLOs associated with aging are not completely understood and could be manifestations of autoimmune processes and/or responses to low-level chronic stress induced by external agents (microbes, tissue damage).
TLO STAGES
It is important to determine whether particular PT-TLOs are associated with acute rejection, and/or chronic rejection, or tolerance. As indicated in Table 1, most, but not all, are associated with chronic rejection. TLOs can be characterized by cellular composition and classified into stages that indicate their “maturity.” Various authors attribute different characteristic to these stages and a consensus is yet to be reached regarding their classification. Staging schema have been put forward in autoimmunity in the pancreas of the NOD mouse,13 atherosclerosis,50,61 colorectal cancer,62 and other tumors.5 There are 3 stages of pancreatic TLO development and dissolution in the NOD mouse, Initially, the cellular infiltrate is disorganized (stage I), then the cells organize into T- and B-cell compartments and HEVs appear as a typical mature TLO, with no apparent beta cell destruction (stage II). Later, the cells in the TLO are activated, the beta cells destroyed, and the TLO disappears to be replaced by a fibrotic accumulation (stage III). TLO maintenance depends on the presence of the beta cell antigen. Once it is removed, the TLO disperses. Sato et al16 put forth a staging scheme for TLOs of human kidneys with pyelonephritis, those from young mice undergoing ischemic injury, or spontaneously developing TLOs in very old mice. Stage I consists of B cells and CXCL13, stage II includes B-cell infiltrates accompanied by FDCs, and stage III consists of organized GCs with FDCs. In this publication, the figures include compartmentalized T and B cells and HEVs, but the authors do not consider either of these characteristics in their staging scheme. Lee et al63 used a slightly different staging scheme (I-TLOs lacking FDCs and GCs; II-TLOs with FDCs but no GC; and III-TLOs with FDCs and GCs was used recently to evaluate the correlation of TLOs and kidney rejection). Stage II, particularly the presence of FDCs, was associated with a less favorable outcome.
It is important to determine whether PT-TLOs are associated with acute rejection and/or chronic rejection or tolerance. In one of the earliest examples of PT-TLOs, HEV antibody staining intensity correlated with rejection grade.15 Staining was most intense during early stages of rejection, remaining elevated throughout the episode and leveling off as rejection resolved. It seems logical that the Banff criteria for kidney rejection should be standardized to consider TLO staging as was the case in the study by Lee et al63 in correlating TLO stage and Banff score.
FUNCTIONS OF PT-TLOs
Determination of the function of PT-TLOs and, in fact, all TLOS, will allow their manipulation when they are detrimental as in autoimmunity or helpful as in cancer. The key question is do they function in a manner like LNs in serving as sites of antigen presentation and generation of effector and memory cells and antibody-producing cells? Are PT-TLOs detrimental serving as sites of rejection? Or beneficial serving as sites of tolerance or even simply epiphenomena?
It is likely that certain PT-TLOs act as sites of antigen presentation and maturation of immune responses. They can have the cells (lymphocytes, APCs, stromal cells) vasculature (HEVs and LVs), and organization (T and B cells with respective APCs) to generate an immune response. Their HEVs can serve as entrance points for naive T and B cells, APCs, and sources of antigen either intrinsic to the TLO itself in its microenvironment (graft, autoimmune site, tumor) and LVs. Recipient APCs expressing donor peptides are present in transplanted kidneys.10 There is much evidence from transgenic and autoimmune TLO models to support TLOs’ ability to generate an immune response with evidence of antibody-producing plasma cells,41,64 rearranged V genes and evidence of somatic hypermutation in the TLO,65 distinct Vκ usage,66 and evidence of distinct TLO B-cell clones.67 Evidence that B-cell maturation occurs in PT-TLOs is apparent from the minimal overlap of B-cell alloantibody repertoires in human kidney grafts and peripheral blood38 and the fact that B-cell clones in TLOs of rejecting human kidneys exhibit somatic mutations and differ from those in peripheral blood,67 consistent with similar earlier studies of TLOs in transgenic models41,64 and autoimmunity.65,66 Nevertheless, the absence of defined dark and light zones typical of SLOs, the generation of autoantibodies,1 and an apparent defect in the ability to expand Tfh cells in rejecting kidney lymphoid infiltrates68 suggest that, although events typical of SLO GCs occur in TLOs, some differences exist.
T cells are important components of acute and chronic graft rejection, through their CD8 T cell–mediated cytotoxicity, their production of cytokines, such as LT, that contribute to TLO organization, and their provision of B-cell help. Determinant spreading, that is the generation of new T-cell reactivities, occurs in TLOs in autoimmunity.69,70 Intramolecular and intermolecular determinant T-cell spreading also occurs in graft rejection71 and is likely occurring directly in PT-TLOs, thus contributing to chronic inflammation and exacerbating graft rejection.
PT-TLOs likely serve as sites of T-cell antigen recognition and memory generation. Skin from rat insulin promoter-LTα mice that contains TLOs is rejected when transplanted to histoincompatible aly/aly mice that are totally devoid of lymphoid tissue, whereas skin from wild type mice is accepted.28 Furthermore, naive lymphocytes transferred into aly/aly mice carrying rat insulin promoter-LTα skin TLOs differentiate into effector and memory cells28 and in TLOs in renal allografts.24 These studies and others demonstrate that TLOs in transplants “can” serve as sites of antigen presentation and differentiation, but it is unclear from these data whether they are the major sites of perpetuation of graft rejection. However, this is answered, the data are consistent with the interpretation that host APCs present antigen in TLOs and that both T-cell and B-cell differentiation occur at the local site.
TLOs likely can contribute to chronic organ transplant rejection11 and are thus considered to be detrimental to graft survival. A considerable amount of literature demonstrates a correlation between TLOs and rejection in mice, rats, and humans. Alloantibody production at the graft site is one possible mechanism, as demonstrated in several studies including one with human kidney grafts.38 An early study correlated B-cell infiltrates with human kidney rejection,72 whereas another found no such correlation.73 More recently, the staging scheme described by Sato et al16 was used to evaluate progressive human kidney graft dysfunction.63 Poor function correlated with highly differentiated TLOs, characterized as stage II, with T- and B-cell compartmentalization and FDCs (but not GCs).
The role of B cells in PT-TLOs has been a subject of continual scrutiny from the first studies that showed a local humoral response in rejecting rat aortas11 and has been recently reviewed in some detail.1 In general, as noted above, the emphasis has been on their role in alloantibody production and graft rejection, but B cells can also function as APCs and producers of cytokines, such as LT, which can contribute to TLO development and maintenance. On the other hand, B cells can produce IL-10, a suppressive cytokine, which has been reported to be a biomarker of enhanced renal graft survival.74
An alternative interpretation of the roles of PT-TLOs derives from analysis of a fully allogeneic murine renal graft model between DBA/2 donors and C57BL/6 recipients that results in variable acute rejection, chronic rejection, or indefinite acceptance.10 TLOs with HEVs are seen and correlate with superior graft function (acceptance) whereas podoplanin+ LVs are more likely seen in infiltrates of kidneys with poor function. Forhead box P3 protein (Foxp3) (a marker of Treg) is noted in kidney TLOs. Miyajima et al75 also noted the presence of Foxp3 cells in the kidney TLOs in this model. Foxp3 depletion resulted in graft rejection, indicating that these Treg are functional. Treg are not unique to PT-TLOs, as they have been noted in prediabetic TLOs in NOD mice76 and in tumor TLOs.77 In fact, removal of the Treg results in tumor rejection, indicating their function. In a later study of tolerant DBA/2 kidney grafts in C57BL/6 mice the lymphoid accumulations are designated as Treg-rich organized lymphoid structures (TOLSs).25 The TOLSs are located around an artery or arteriole, exhibit a progression from an early mixed CD8, B, CD4 cell, and CD4 Foxp3 infiltrate to a later predominance of Foxp3+ cells. The authors posit that TOLSs differ from classic TLOs in that they lack T- and B-cell compartmentalization, GCs, and HEVs, and lack a requirement for LTβR signaling.25 The absence of LTβR signaling is not unique to TOLSs, as this has been noted in other TLOs.51,78 LTβR signaling is frequently,9,40,79,80 but not always81 required for PNAd+ HEVs. The difference between the presence of HEVs in one study and their absence in another could be due to the time of analysis posttransplant (13.5 versus 60 wk) of the lymphoid accumulations. At the later time, the function of HEVs (ie, entrance of naive cells) might be less important. Thus far, the clinical significance of Treg in human PT-TLOs has not been determined, but it would be important to evaluate whether they are associated with long-term graft acceptance. At any rate, these studies demonstrate the importance of carefully categorizing the various kinds of lymphoid accumulations in grafts—those associated with acute rejection, chronic rejection, and tolerance to understand their differing functions to harness them for optimal clinical outcome.
THE ROLE OF PT-TLOs IN CANCER
The literature is replete with excellent articles and reviews concerning the roles of TLOs in cancer. These include summaries of early articles indicating that the higher the number of TLOS (as measured by HEVs and/or GCs), the more favorable the prognosis in breast cancer.82 Later studies emphasized the role of intratumoral B cells in TLOs in cancer defense,83-85 and a recent review summarizes the field in detail.5 What role do TLOs play in malignancies in organ transplantation? It is well established that there is an increased incidence of several types of cancers after solid organ transplant, variously estimated between 4% and 18%.86 These include colorectal cancer, nonmelanoma skin cancer, and non-Hodgkin’s lymphoma. The conventional understanding of the increase in malignancies posttransplant is that the immunosuppressive regimens to prevent rejection also reduce overall immunosurveillance. Posttransplant patients with cancer have a poor prognosis. A comparison of tumors from transplant patients with those from nonimmunosuppressed patients with cancer showed a much-reduced number and size of TLOs86—pointing to an advantage of TLOs in tumor rejection, although likely a disadvantage in the transplanted organ where they can exacerbate the antigraft immune response.
Lymphoproliferative disorders can occur posttransplant. The pathogenesis of this complication of predominately proliferating B cells that can include B lymphomas has been attributed to 3 factors that reflect the fact that 50%–80% of these disorders are positive for Epstein-Barr virus (EBV). These include: a reduced defense after immunosuppressive therapies, the presence of EBV, and a derangement of molecular signaling and DNA repair mechanisms as a direct effect of immunosuppressant agents.87 However, it is possible that the chronic immunostimulation and B-cell proliferation in PT-TLOs could also contribute to the development of such disorders and malignancies. This logic is consistent with the observation of an increase in lymphomas in individuals with Sjogren’s syndrome,88 an autoimmune condition of the salivary and lacrimal glands in which TLOs are a prominent feature. Similarly, there is an increase in gastric lymphomas in the chronic TLOs of Helicobacter pylori infection.89 Is any aspect of posttransplant lymphoproliferative disorder attributable to the chronically proliferating and mutating B cells in the TLO in addition to the 3 factors noted above? The best evidence derives from non-EBV positive disorders. Support for such a role would derive from data from patients (or animals) with long-term grafts with TLOs and EBV-negative lymphomas. It is quite possible that the chronic B-cell proliferation and mutation in the TLOs could predispose to lymphomas even in the absence of overt immunosuppression, perhaps with well-matched donor recipient pairs.
CHALLENGES FOR PT-TLO RESEARCH
Staging Quantification
Although TLOs have been studied for many years, challenges and opportunities exist. One is the lack of a consensus regarding standardization of classification of the criteria for their identification and staging. As indicated above, various authors have put forth a variety of staging schemes, mostly based on immunohistochemical or fluorescent identification of cellular populations by their expression of various markers. Multiplex immunostaining combined with density and spatial distribution allows a quantitative approach to evaluating the nature of TLOs in primary and metastatic melanomas.90 A recent publication presents an entirely different approach using hematoxylin and eosin staining and density assessment to evaluating TLOs in a variety of lung cancers.91 The advantages of speed, minimal reagent use (eg, multiple antibodies), and automation could be useful clinically but lack the granular specificity of identification of TLO stages of maturation and function. Access to TLOs is another issue as their presence can be quite variable within the context of, for example, an individual organ undergoing rejection or responding to therapy. A biopsy can miss the full complement of TLOs in a large organ. The development of noninvasive biomarkers that could be detectable in serum or peripheral blood to assess the existence and nature of TLOs in, for example, a transplanted organ would be useful in designing therapies to encourage or prevent them.
Imaging
Imaging TLOs, 3D in real time could address the nature of their interactions with the rest of the immune system and with other TLOs in the same organ, and reveal how they develop over time, and how they interact with and are influenced by their microenvironment. The increased vascular permeability in the inflammatory setting of TLOs can be exploited by using magnetic nanoparticle MRI. Early studies in NOD mice demonstrate in vivo in real time uptake of monocrystalline iron oxide nanoparticles by macrophages in inflammatory lesions.92 A positive correlation of decreased inflammation with response to anti-CD3 therapy was apparent in these studies. This concept was further refined in studies of humans with type I diabetes.93 It was possible with the use of ferumoxytol nanoparticle uptake by macrophages, to generate MRI 3D high-resolution pancreas maps. Although these studies did not evaluate the TLO per se, they provide insight in real time into the inflammatory process and should be readily applicable to the analysis of PT-TLOs.
Technical problems need to be solved to accomplish the goals of in vivo imaging. Several transgenic mouse lines including those with green fluorescent HEVs94 and red fluorescent LVs95 that have been used successfully in 2-photon microscopic analysis of LNs could be useful in such studies. One difficulty is the inaccessibility of target organs. Exteriorizing rejecting murine kidney transplants allowed in vivo imaging by 2-photon microscopy of interactions between DCs and antigen-specific T cells24 and demonstrated that T resident memory cells do not circulate outside of the PT-TLO. These studies indicate that analysis of TLOs in real time is possible. Advanced intravital time-lapse microscopy was used to evaluate inflammation in the exteriorized pancreas of the NOD mouse over the course of several hours. By evaluating early (3–5 wk) or late (10–12 wk), insight was obtained into interactions between CD4 and CD8 cells with DCs and macrophages around the islet.96 Interestingly, these authors did not observe any clear cellular organization or TLOs, possibly because of the fact they did not image B cells or HEVs. The time of peak TLO organization is crucial in their evaluation as indicated in previous studies.13,97 At the present time, exteriorizing organs and evaluating multiple time points in the same animal is not practical for evaluating progression of PT-TLOs, although useful for short-term evaluation, and is not realistic for a clinical setting.
Manipulating TLOs Therapeutically
TLOs need to be accessed for therapeutic purposes, ideally without affecting the rest of the immune system. Once it has been determined that the TLO is detrimental to graft survival, it would be ideal to eliminate it or even switch it to a tolerogenic function. It was possible to prevent TLOs in a model of BALB/c mouse aortic segment transplantation into the carotid of C57BL/6 mice, where intimal hyperplasia resembles typical TLOs with Tfh, GCs, and plasma cells. Application of CCL21- and CXCR3-neutralizing antibodies to the local graft region at early times (before TLOs appear) significantly delays the progression of arteriosclerosis.27 It would be interesting to determine if an increase in Treg in a PT-TLO could be generated by such a treatment. In a murine model of rheumatoid arthritis, IL-27 inhibits TLO development.98 NOD mice develop a Sjögren’s-like syndrome with TLOs in lacrimal and salivary glands. Treatment of such mice beginning at 9 wk with an LTβR-Fc fusion protein, which antagonizes LTαβ signaling, results in dramatic effects on the TLOs in salivary glands with reduction of lymphoid chemokines CXCL13 and CCL19, FDC networks, HEVs, and B and T cells.99 LNs are affected only minimally, and salivary gland function is partially restored. LPR mice develop renal perivascular cell clusters of age with typical TLOs, with lymphoid chemokines, Tfh, and GCs. If the mice are treated with dexamethasone at 3 mo, when TLOs are in the process of developing, the lymphoid accumulations are drastically reduced in size as is the expression of lymphoid chemokines,100 although the effect on the systemic immune response was not evaluated, and as noted above, dexamethasone treatment reduces TLOs in kidneys of aged mice.16 Pretreatment of renal transplant patients with rituximab affects the composition of TLOs in that there are fewer B cells than in aged kidneys or those from patients with chronic kidney disease.63 These studies, taken together, demonstrate that manipulation with external agents is possible, even when TLOs are well underway in their development, and provide hope for such treatments in inhibiting rejection and enhancing tolerance to transplanted organs.
Footnotes
The author declares no conflict of interest.
Funding was previously provided by grants from the National Institutes of Health, the Juvenile Diabetes Foundation, and the National Multiple Sclerosis Society.
N.H.R. is responsible for all aspects of this contribution.
REFERENCES
- 1.Alsughayyir J, Pettigrew GJ, Motallebzadeh R. Spoiling for a fight: B lymphocytes as initiator and effector populations within tertiary lymphoid organs in autoimmunity and transplantation. Front Immunol. 2017;8:1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drayton DL, Liao S, Mounzer RH, et al. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. [DOI] [PubMed] [Google Scholar]
- 3.Ruddle NH. High endothelial venules and lymphatic vessels in tertiary lymphoid organs: characteristics, functions, and regulation. Front Immunol. 2016;7:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sautes-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–325. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375:eabf9419. [DOI] [PubMed] [Google Scholar]
- 6.Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: parallels with lymph node stroma. Front Immunol. 2012;3:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruddle NH. Basics of inducible lymphoid organs. Curr Top Microbiol Immunol. 2020;426:1–19. [DOI] [PubMed] [Google Scholar]
- 8.Koenig A, Thaunat O. Lymphoid neogenesis and tertiary lymphoid organs in transplanted organs. Front Immunol. 2016;7:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. [DOI] [PubMed] [Google Scholar]
- 10.Brown K, Sacks SH, Wong W. Tertiary lymphoid organs in renal allografts can be associated with donor-specific tolerance rather than rejection. Eur J Immunol. 2011;41:89–96. [DOI] [PubMed] [Google Scholar]
- 11.Thaunat O, Field AC, Dai J, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci U S A. 2005;102:14723–14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motallebzadeh R, Rehakova S, Conlon TM, et al. Blocking lymphotoxin signaling abrogates the development of ectopic lymphoid tissue within cardiac allografts and inhibits effector antibody responses. FASEB J. 2012;26:51–62. [DOI] [PubMed] [Google Scholar]
- 13.Penaranda C, Tang Q, Ruddle NH, et al. Prevention of diabetes by FTY720-mediated stabilization of peri-islet tertiary lymphoid organs. Diabetes. 2010;59:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo R, Cheng Y, Chang D, et al. Tertiary lymphoid organs are associated with the progression of kidney damage and regulated by interleukin-17A. Theranostics. 2021;11:117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toppila S, Paavonen T, Nieminen MS, et al. Endothelial L-selectin ligands are likely to recruit lymphocytes into rejecting human heart transplants. Am J Pathol. 1999;155:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato Y, Boor P, Fukuma S, et al. Developmental stages of tertiary lymphoid tissue reflect local injury and inflammation in mouse and human kidneys. Kidney Int. 2020;98:448–463. [DOI] [PubMed] [Google Scholar]
- 17.Turley S, Poirot L, Hattori M, et al. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly KA, Kuchroo M, Venkat A, et al. A reservoir of stem-like CD8(+) T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci Immunol. 2021;6:eabg7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua Y, Vella G, Rambow F, et al. Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1(+) T lymphocyte niches through a feed-forward loop. Cancer Cell. 2022;40:1600–1618.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirveskari J, Paavonen T, Hayry P, et al. De novo induction of endothelial L-selectin ligands during kidney allograft rejection. J Am Soc Nephrol. 2000;11:2358–2365. [DOI] [PubMed] [Google Scholar]
- 21.Baddoura FK, Nasr IW, Wrobel B, et al. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant. 2005;5:510–516. [DOI] [PubMed] [Google Scholar]
- 22.Di Carlo E, D’Antuono T, Contento S, et al. Quilty effect has the features of lymphoid neogenesis and shares CXCL13-CXCR5 pathway with recurrent acute cardiac rejections. Am J Transplant. 2007;7:201–210. [DOI] [PubMed] [Google Scholar]
- 23.Kerjaschki D, Regele HM, Moosberger I, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. [DOI] [PubMed] [Google Scholar]
- 24.Abou-Daya KI, Tieu R, Zhao D, et al. Resident memory T cells form during persistent antigen exposure leading to allograft rejection. Sci Immunol. 2021;6:eabc8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosales IA, Yang C, Farkash EA, et al. Novel intragraft regulatory lymphoid structures in kidney allograft tolerance. Am J Transplant. 2022;22:705–716. [DOI] [PubMed] [Google Scholar]
- 26.Sato M, Hirayama S, Hwang DM, et al. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J Immunol. 2009;182:7307–7316. [DOI] [PubMed] [Google Scholar]
- 27.Cai J, Deng J, Gu W, et al. Impact of local alloimmunity and recipient cells in transplant arteriosclerosis. Circ Res. 2020;127:974–993. [DOI] [PubMed] [Google Scholar]
- 28.Nasr IW, Reel M, Oberbarnscheidt MH, et al. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant. 2007;7:1071–1079. [DOI] [PubMed] [Google Scholar]
- 29.Hautz T, Zelger BG, Nasr IW, et al. Lymphoid neogenesis in skin of human hand, nonhuman primate, and rat vascularized composite allografts. Transpl Int. 2014;27:966–976. [DOI] [PubMed] [Google Scholar]
- 30.Stuht S, Gwinner W, Franz I, et al. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. Am J Transplant. 2007;7:377–384. [DOI] [PubMed] [Google Scholar]
- 31.de Leur K, Clahsen-van Groningen MC, van den Bosch TPP, et al. Characterization of ectopic lymphoid structures in different types of acute renal allograft rejection. Clin Exp Immunol. 2018;192:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang Q, Liu Q, Divito SJ, et al. Graft-infiltrating host dendritic cells play a key role in organ transplant rejection. Nat Commun. 2016;7:12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denton AE, Carr EJ, Magiera LP, et al. Embryonic FAP(+) lymphoid tissue organizer cells generate the reticular network of adult lymph nodes. J Exp Med. 2019;216:2242–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Togni P, Goellner J, Ruddle NH, et al. Pillars article: abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994. 264: 703-707. J Immunol. 2014;192:2010–2014. [PubMed] [Google Scholar]
- 35.Koni PA, Sacca R, Lawton P, et al. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. [DOI] [PubMed] [Google Scholar]
- 36.Barone F, Gardner DH, Nayar S, et al. Stromal fibroblasts in tertiary lymphoid structures: a novel target in chronic inflammation. Front Immunol. 2016;7:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayar S, Campos J, Smith CG, et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc Natl Acad Sci U S A. 2019;116:13490–13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thaunat O, Patey N, Caligiuri G, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185:717–728. [DOI] [PubMed] [Google Scholar]
- 39.Sato Y, Mii A, Hamazaki Y, et al. Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight. 2016;1:e87680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drayton DL, Ying X, Lee J, et al. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kratz A, Campos-Neto A, Hanson MS, et al. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luther SA, Lopez T, Bai W, et al. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. [DOI] [PubMed] [Google Scholar]
- 43.Meier D, Bornmann C, Chappaz S, et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–654. [DOI] [PubMed] [Google Scholar]
- 44.Kim HJ, Kammertoens T, Janke M, et al. Establishment of early lymphoid organ infrastructure in transplanted tumors mediated by local production of lymphotoxin alpha and in the combined absence of functional B and T cells. J Immunol. 2004;172:4037–4047. [DOI] [PubMed] [Google Scholar]
- 45.Kim MY, McConnell FM, Gaspal FM, et al. Function of CD4+CD3- cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109:1602–1610. [DOI] [PubMed] [Google Scholar]
- 46.Scandella E, Bolinger B, Lattmann E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. [DOI] [PubMed] [Google Scholar]
- 47.Ware CF, Crowe PD, Grayson MH, et al. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol. 1992;149:3881–3888. [PubMed] [Google Scholar]
- 48.Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–546. [DOI] [PubMed] [Google Scholar]
- 49.Marinkovic T, Garin A, Yokota Y, et al. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116:2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grabner R, Lotzer K, Dopping S, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice. J Exp Med. 2009;206:233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacca R, Cuff CA, Lesslauer W, et al. Differential activities of secreted lymphotoxin-alpha3 and membrane lymphotoxin-alpha1beta2 in lymphotoxin-induced inflammation: critical role of TNF receptor 1 signaling. J Immunol. 1998;160:485–491. [PubMed] [Google Scholar]
- 52.Deteix C, Attuil-Audenis V, Duthey A, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5344–5351. [DOI] [PubMed] [Google Scholar]
- 53.Murakami J, Shimizu Y, Kashii Y, et al. Functional B-cell response in intrahepatic lymphoid follicles in chronic hepatitis C. Hepatology. 1999;30:143–150. [DOI] [PubMed] [Google Scholar]
- 54.Orloff SL, Hwee YK, Kreklywich C, et al. Cytomegalovirus latency promotes cardiac lymphoid neogenesis and accelerated allograft rejection in CMV naive recipients. Am J Transplant. 2011;11:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichii O, Hosotani M, Masum MA, et al. Close association between altered urine-urothelium barrier and tertiary lymphoid structure formation in the renal pelvis during nephritis. J Am Soc Nephrol. 2022;33:88–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652–658. [DOI] [PubMed] [Google Scholar]
- 58.Liao S, Bentley K, Lebrun M, et al. Transgenic LacZ under control of Hec-6st regulatory sequences recapitulates endogenous gene expression on high endothelial venules. Proc Natl Acad Sci U S A. 2007;104:4577–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh P, Coskun ZZ, Goode C, et al. Lymphoid neogenesis and immune infiltration in aged liver. Hepatology. 2008;47:1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camell CD, Gunther P, Lee A, et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 2019;30:1024–1039.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akhavanpoor M, Gleissner CA, Akhavanpoor H, et al. Adventitial tertiary lymphoid organ classification in human atherosclerosis. Cardiovasc Pathol. 2018;32:8–14. [DOI] [PubMed] [Google Scholar]
- 62.Posch F, Silina K, Leibl S, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7:e1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee YH, Sato Y, Saito M, et al. Advanced tertiary lymphoid tissues in protocol biopsies are associated with progressive graft dysfunction in kidney transplant recipients. J Am Soc Nephrol. 2022;33:186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Picarella DE, Kratz A, Li CB, et al. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci U S A. 1992;89:10036–10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stott DI, Hiepe F, Hummel M, et al. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren’s syndrome. J Clin Invest. 1998;102:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gause A, Gundlach K, Carbon G, et al. Analysis of VH gene rearrangements from synovial B cells of patients with rheumatoid arthritis reveals infiltration of the synovial membrane by memory B cells. Rheumatol Int. 1997;17:145–150. [DOI] [PubMed] [Google Scholar]
- 67.Cheng J, Torkamani A, Grover RK, et al. Ectopic B-cell clusters that infiltrate transplanted human kidneys are clonal. Proc Natl Acad Sci U S A. 2011;108:5560–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Cavazzoni CB, Hanson BL, et al. Transcriptionally distinct B cells infiltrate allografts after kidney transplantation. Transplantation. 2023;107:e47–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuerten S, Schickel A, Kerkloh C, et al. Tertiary lymphoid organ development coincides with determinant spreading of the myelin-specific T cell response. Acta Neuropathol. 2012;124:861–873. [DOI] [PubMed] [Google Scholar]
- 70.McMahon EJ, Bailey SL, Castenada CV, et al. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. [DOI] [PubMed] [Google Scholar]
- 71.Suciu-Foca N, Harris PE, Cortesini R. Intramolecular and intermolecular spreading during the course of organ allograft rejection. Immunol Rev. 1998;164:241–246. [DOI] [PubMed] [Google Scholar]
- 72.Moreso F, Seron D, O’Valle F, et al. Immunephenotype of glomerular and interstitial infiltrating cells in protocol renal allograft biopsies and histological diagnosis. Am J Transplant. 2007;7:2739–2747. [DOI] [PubMed] [Google Scholar]
- 73.Scheepstra C, Bemelman FJ, van der Loos C, et al. B cells in cluster or in a scattered pattern do not correlate with clinical outcome of renal allograft rejection. Transplantation. 2008;86:772–778. [DOI] [PubMed] [Google Scholar]
- 74.Cherukuri A, Salama AD, Mehta R, et al. Transitional B cell cytokines predict renal allograft outcomes. Sci Transl Med. 2021;13:eabe4929. [DOI] [PubMed] [Google Scholar]
- 75.Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herman AE, Freeman GJ, Mathis D, et al. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joshi NS, Akama-Garren EH, Lu Y, et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. 2015;43:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peske JD, Thompson ED, Gemta L, et al. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun. 2015;6:7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Browning JL, Allaire N, Ngam-Ek A, et al. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–550. [DOI] [PubMed] [Google Scholar]
- 80.Hemmerich S, Bistrup A, Singer MS, et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15:237–247. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez AB, Parriott G, Engelhard VH. Tumor necrosis factor receptor regulation of peripheral node addressin biosynthetic components in tumor endothelial cells. Front Immunol. 2022;13:1009306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinet L, Garrido I, Filleron T, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–5687. [DOI] [PubMed] [Google Scholar]
- 83.Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. [DOI] [PubMed] [Google Scholar]
- 84.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petitprez F, de Reynies A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. [DOI] [PubMed] [Google Scholar]
- 86.Datta RR, Schran S, Persa OD, et al. Post-transplant malignancies show reduced T-cell abundance and tertiary lymphoid structures as correlates of impaired cancer immunosurveillance. Clin Cancer Res. 2022;28:1712–1723. [DOI] [PubMed] [Google Scholar]
- 87.Asleh R, Alnsasra H, Habermann TM, et al. Post-transplant lymphoproliferative disorder following cardiac transplantation. Front Cardiovasc Med. 2022;9:787975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165:2337–2344. [DOI] [PubMed] [Google Scholar]
- 89.Mazzucchelli L, Blaser A, Kappeler A, et al. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999;104:R49–R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Werner F, Wagner C, Simon M, et al. A standardized analysis of tertiary lymphoid structures in human melanoma: disease progression- and tumor site-associated changes with germinal center alteration. Front Immunol. 2021;12:675146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barmpoutis P, Di Capite M, Kayhanian H, et al. Tertiary lymphoid structures (TLS) identification and density assessment on H&E-stained digital slides of lung cancer. PLoS One. 2021;16:e0256907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turvey SE, Swart E, Denis MC, et al. Noninvasive imaging of pancreatic inflammation and its reversal in type 1 diabetes. J Clin Invest. 2005;115:2454–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaglia JL, Harisinghani M, Aganj I, et al. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc Natl Acad Sci U S A. 2015;112:2139–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bentley KL, Stranford S, Liao S, et al. High endothelial venule reporter mice to probe regulation of lymph node vasculature. Adv Exp Med Biol. 2011;691:35–44. [DOI] [PubMed] [Google Scholar]
- 95.Truman LA, Bentley KL, Smith EC, et al. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol. 2012;180:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mohan JF, Kohler RH, Hill JA, et al. Imaging the emergence and natural progression of spontaneous autoimmune diabetes. Proc Natl Acad Sci U S A. 2017;114:E7776–E7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu W, Wojtkiewicz G, Weissleder R, et al. Early window of diabetes determinism in NOD mice, dependent on the complement receptor CRIg, identified by noninvasive imaging. Nat Immunol. 2012;13:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones GW, Bombardieri M, Greenhill CJ, et al. Interleukin-27 inhibits ectopic lymphoid-like structure development in early inflammatory arthritis. J Exp Med. 2015;212:1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gatumu MK, Skarstein K, Papandile A, et al. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjogren’s syndrome in salivary glands of non-obese diabetic mice. Arthritis Res Ther. 2009;11:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masum MA, Ichii O, Elewa YHA, et al. Vasculature-associated lymphoid tissue: a unique tertiary lymphoid tissue correlates with renal lesions in lupus nephritis mouse model. Front Immunol. 2020;11:595672. [DOI] [PMC free article] [PubMed] [Google Scholar]